Abstract
Epithelial tissues facing the external environment are essential to combating microbial infection. In addition to providing a physical barrier, epithelial tissues mount chemical defenses to prevent invasion of internal tissues by pathogens. Here, we describe that the melanization reaction implicated in host defense is activated in the respiratory system, the trachea, of Drosophila. Tracheal melanization can be activated by the presence of microorganisms but is normally blocked by Spn77Ba, a protease inhibitor in the serpin family. Spn77Ba inhibits a protease cascade involving the MP1 and MP2 proteases that activates phenol oxidase, a key enzyme in melanin biosynthesis. Unexpectedly, we found that tracheal melanization resulting from Spn77Ba disruption induces systemic expression of the antifungal peptide Drosomycin via the Toll pathway. Such signaling between local and systemic immune responses could represent an alarm mechanism that prepares the host in case a pathogen breaches epithelial defenses to invade internal tissues.
INTRODUCTION
Innate immunity is an ancient system of host defense against pathogens used by a wide range of organisms (Medzhitov and Janeway, 2000). In humans, innate immunity is the front line of host defense that acts before the adaptive immune system. Whereas the adaptive immune system can be programmed to recognize an almost infinite number of specific antigens, the innate immune system is hardwired to recognize a limited number of common determinants found in infectious agents. Nonetheless, the innate immune system involves a sophisticated repertoire of humoral and cellular responses, acting at local as well as systemic levels, which together provide an effective barrier to infection by pathogens (Medzhitov, 2007). In recent years, many fundamental mechanisms and principles of innate immunity have been revealed from studies with the fruit fly Drosophila (Lemaitre and Hoffmann, 2007).
A key innate immune mechanism found in both humans and Drosophila involves signaling by receptors in the Toll family. In humans, upon microbial infection, Toll-like receptors (TLRs) activate the synthesis of cytokines as well as other molecules that stimulate induction of the adaptive immune system (Akira et al., 2006). In Drosophila, Toll activates the synthesis of antimicrobial peptides (AMPs) by the fat body, the functional equivalent of human liver and adipose tissue (Lemaitre et al., 1996). Since AMPs synthesized by the fat body are secreted into the blood-like hemolymph that bathe all internal tissues and organs, they provide systemic protection against pathogens that may invade the body cavity. In humans, pathogens are directly sensed by TLRs, but in Drosophila, they are detected by circulating pattern recognition receptors (PRRs) that trigger protease cascades, which lead to cleavage of the Spätzle protein to generate the ligand that activates Toll signaling. In Drosophila, the Toll pathway is mainly responsive to infection by Gram-positive bacteria and fungi, whereas the Imd pathway activates AMP synthesis in response to infection by Gram-negative bacteria. Both pathways, as well as human TLRs, trigger immune gene expression by activating a transcription factor in the NF-κB family (Ferrandon et al., 2007).
Another major immune response in Drosophila, and more generally in insects and other arthropods, is the melanization reaction (Cerenius and Soderhall, 2004). Melanization involves the rapid synthesis of melanin at the site of infection and injury in order to contain a microbial pathogen, as well as to facilitate wound healing. A key enzyme in melanin biosynthesis is phenol oxidase (PO), which catalyzes the oxidation of phenols to quinones that then polymerize into melanin. A by-product of PO activity is reactive oxygen species (ROS), a potent antimicrobial agent. PO is synthesized as an inactive zymogen called proPO (PPO), which is released by crystal cells that circulate in the hemolymph and cleaved to generate active PO at the end of a protease cascade (Cerenius and Soderhall, 2004). The proteases in this cascade also exist as inactive zymogens in the hemolymph, analogous to components of the complement pathway found in human blood and tissues. Thus, like the human complement pathway, the melanization reaction functions as a component of the humoral immune response to contain pathogens that invade internal body spaces.
As illustrated by the Toll pathway and the melanization reaction in Drosophila but also by the complement pathway in humans, a recurring theme in innate immunity is the use of protease cascades to activate immune responses. Such protease cascades, because of their potential for enormous signal amplification, are advantageous for detecting minute levels of pathogens. However, tight regulation must exist to prevent their activation under normal conditions and to localize their action both temporally and spatially. Indeed, C1 inhibitor, a member of the serpin family of protease inhibitors, is an essential regulator of the complement pathway (Wagenaar-Bos and Hack, 2006). Moreover, genetic studies in Drosophila have defined important roles for serpins in inhibiting the protease cascades that activate Toll and the melanization reaction (De Gregorio et al., 2002; Levashina et al., 1999; Ligoxygakis et al., 2002b).
Epithelial tissues exposed to microorganisms in the environment play an important role in innate immunity. In addition to providing a physical barrier, such epithelia secrete chemical defenses to prevent pathogen penetration of internal tissues. Recent studies have shown that epithelia in Drosophila, as in humans, mount local immune responses. In particular, the respiratory and reproductive tracts express the antifungal peptide, Drosomycin (Drs), in response to contact with pathogens, but interestingly this response depends on the Imd pathway, not the Toll pathway utilized by the fat body during systemic antimicrobial responses (Ferrandon et al., 1998; Tzou et al., 2000). Moreover, reactive oxygen species generated by a dual oxidase is essential for the antimicrobial activity of the gastrointestinal tract (Ha et al., 2005).
Here, we show that the melanization reaction can be activated in the trachea, the respiratory system, of Drosophila. In an RNAi screen to investigate the function of serpins in Drosophila, we discovered that the serpin Spn77Ba is a trachea-specific inhibitor of melanization. As in other tissues, melanization of the trachea is strongly enhanced by the presence of microorganisms. Surprisingly, however, tracheal melanization induces a systemic response (Drs expression in the fat body) via the Toll pathway, which may represent a mechanism for alerting and preparing the host for possible infection of internal tissues. Our data suggest that melanization is an intrinsic immune response of respiratory epithelium under tight negative control by a serpin.
RESULTS
Serpin Spn77Ba Inhibits Tracheal Melanization and is Essential for Organism Viability
As part of an RNAi screen to investigate the biological function of serpins in Drosophila, we generated transgenic flies bearing an inverted repeat construct of serpin Spn77Ba under control of the UAS element recognized by the Gal4 transcriptional activator (UAS-Spn77Ba-IR). These flies were crossed to Gal4 “driver” flies in order to activate RNAi of Spn77Ba in the progeny (Duffy, 2002). When the act-Gal4 driver was used to activate Spn77Ba RNAi in virtually all tissues during development, the result was a striking phenotype in larva: melanization of the tracheal system, the respiratory organ (Figure 1A). Tracheal melanization was first visible during the second or third instar larval stage, and increased in intensity until the affected larva died within a few days before reaching the pupal stage. This phenotype was observed in experiments with two different inverted repeat constructs targeting different regions of the Spn77Ba gene and with multiple independently generated transgenic lines for each construct. By quantitative RT-PCR analysis, we estimated that RNAi was able to knock down Spn77Ba expression to 20-30% of the wild-type level (data not shown). These results suggest that Spn77Ba is required for inhibiting melanization in the tracheal system and for larval viability.
Figure 1. Serpin Spn77Ba is Required to Prevent Tracheal Melanization.
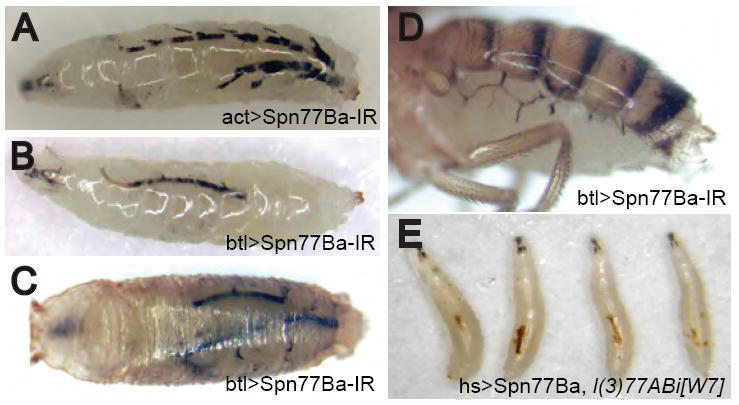
(A) Melanization of larval trachea resulted when Spn77Ba RNAi was broadly activated with the act-Gal4 driver and a UAS construct encoding an inverted repeat of Spn77Ba sequence.
(B-D) Spn77Ba RNAi with tracheal-specific btl-Gal4 driver resulted in tracheal melanization in larvae (B), pupae (C), and adults (D).
(E) Embryonic lethality caused by a spn77Ba null mutation was rescued by Spn77Ba expression resulting from leaky activity of the hs-Gal4 driver at 18°C. The larval survivors showed tracheal melanization.
We identified a spn77Ba null mutation, l(3)77ABi[W7], that causes early lethality (Lukinova et al., 1999), which was rescued by over-expression of the Spn77Ba protein using the act-Gal4 driver (see Supplemental Data). To bypass this early essential requirement for Spn77Ba function, we over-expressed Spn77Ba in the l(3)77ABi[W7] mutant background, using the hs-Gal4 driver. At 18°C leaky expression of Spn77Ba was sufficient for some larvae to grow to the 2nd instar stage, and these survivors had melanized trachea and in most cases died before reaching the 3rd instar stage (Figure 1E). At 22-25°C some larvae survived to 3rd instar and even grew to pupae. These larvae and pupae also had melanized trachea, which was similar to that induced by Spn77Ba RNAi (data not shown). These results confirm that l(3)77ABi[W7] is a mutation of the spn77Ba gene and that the phenotype induced by RNAi is due to specific knock down of the spn77Ba gene. They also suggest that low level of Spn77Ba activity, such as left by RNAi, was sufficient to fulfill Spn77Ba’s essential function during early development but not its role in inhibiting tracheal melanization during the larval stage.
Inhibition of Tracheal Melanization Involves Spn77Ba Expressed by Tracheal Cells
To determine if Spn77Ba gene function is required in tracheal cells to prevent tracheal melanization, we used the tracheal-specific btl-Gal4 driver to activate Spn77Ba RNAi. In btl-Gal4/UAS-Spn77Ba-IR larvae, we observed tracheal melanization, albeit at a weaker level than seen in act-Gal4/UAS-Spn77Ba-IR larvae (Figure 1B). All of the btl-Gal4/UAS-Spn77Ba-IR larvae were able to develop to the prepupal stage. Around 35% of btl-Gal4/UAS-Spn77Ba-IR prepupae or pupae showed severe melanization, defined as intense melanization of a significant portion of the tracheal system (Figure 1C). The remaining 65% of btl-Gal4/UAS-Spn77Ba-IR pupae were able to develop to adults, of which 96% showed tracheal melanization (Figure 1D). Moreover, l(3)77ABi[W7], the spn77Ba null mutation, enhanced the phenotype seen with Spn77Ba RNAi. At 18°C, where 81% of btl-Gal4/UAS-Spn77Ba-IR flies were viable, all btl-Gal4/UAS-Spn77Ba-IR; l(3)77ABi[W7]/+ flies died before the adult stage. These results suggest that Spn77Ba gene function is required in tracheal cells to prevent melanization of the tracheal system.
To confirm that Spn77Ba is expressed in tracheal cells, we made transgenic flies in which the 5’ transcriptional control region of the spn77Ba gene is used to express the Gal4 protein. Combining this Spn77Ba-Gal4 driver with a UAS-GFP construct resulted in strong GFP expression in tracheal cells throughout the larval tracheal system (Figure 2A). Weak GFP expression was also visible in other tissues, including the gut and the epidermis. When the Spn77Ba-Gal4 driver was used to activate Spn77Ba RNAi, the result was tracheal melanization (data not shown). These results demonstrate that Spn77Ba is expressed in tracheal cells.
Figure 2. Spn77Ba is Expressed by Tracheal Cells and is Localized at Apical Surface of Tracheal Epithelium Where Melanization is Initiated.
(A) A Gal4 driver containing 2.2 kbp of DNA from 5’end of Spn77Ba gene was used to activate GFP expression from a UAS-GFP construct. GFP signal is seen in trachea and at lower levels in gut and epidermis.
(B) When expressed by transfected S2 cells, HA-tagged Spn77Ba protein was detected mainly in the culture medium by Western blot using anti-HA antibody.
(C-D) When Spn77Ba-GFP fusion protein was expressed under control of the da-Gal4 driver, GFP signal (C and D, green) was seen on apical surface of tracheal epithelium, toward luminal side of apical F-actin and co-localized with chitin. F-actin of tracheal cells was stained with Alexa Fluor 633–phalloidin (C’, red) and chitin was visualized with a rhodamine-conjugated chitin-binding probe (D’, red). N: nucleus.
(E) Melanization (arrow) resulting from Spn77Ba RNAi can be initially detected between tracheal cuticle and epithelial cells. Cells of tracheal epithelium were visualized by expression of cytoplasmic GFP under control of the btl-Gal4 driver (E’). N: nucleus.
(F) Non-melanized and melanized sections of trachea in btl>Spn77Ba-IR larva were compared for integrity of the basement membrane, which was visualized with a vkg-GFP reporter (Morin et al., 2001). F-actin staining with Alexa Fluor 633–phalloidin was used to visualize the epithelial cells.
Having a potential N-terminal signal peptide, Spn77Ba protein is likely secreted by tracheal cells. Indeed, Spn77Ba protein was mainly detected in the culture medium when expressed by transfected S2 cells (Figure 2B). To investigate the subcellular localization of the Spn77Ba protein in vivo, we made transgenic flies that can express a fusion protein of Spn77Ba and a C-terminal GFP under Gal4/UAS control. When da-Gal4 was used to drive the expression of the fusion protein, GFP signal was detected on the apical surface of tracheal cells facing the airway lumen (Figure 2C). The GFP signal was predominantly associated with the chitinous cuticle lining the tracheal epithelium (Figure 2D). These results suggest that Spn77Ba is localized in the extracellular space facing the airway lumen.
Consistent with the localization of the Spn77Ba-GFP fusion protein, the melanin resulting from Spn77Ba RNAi was first detected at a low level between tracheal cells and the overlying cuticle (Figure 2E). As the amount of melanin generated by the melanization reaction increased, it spread to encompass the epithelial layer occupied by the tracheal cells. Surprisingly, despite such massive melanization of the tracheal epithelium, the underlying basement membrane appeared to remain intact (Figure 2F).
Spn77Ba Inhibits Phenol Oxidase Activity Required for Melanin Biosynthesis
A key enzyme in the melanin biosynthetic pathway is phenol oxidase (PO), which catalyzes the oxidation of phenols to quinones that then polymerize into melanin. To test if the melanization resulting from Spn77Ba RNAi is due to an increase in PO activity, we developed an ex vivo assay for tracheal PO activity. We dissected dorsal tracheal trunks from btl-Gal4/UAS-Spn77Ba-IR larvae and incubated them on filter paper pre-soaked in L-DOPA, a PO substrate. After 1 hour of incubation, trachea from Spn77Ba RNAi larvae, but not control larvae, were strongly melanized, indicating a higher than normal level of PO activity (Figure 3A-B). These results suggest that Spn77Ba is required to inhibit PO activity in trachea.
Figure 3. Spn77Ba Inhibits the MP1/MP2 Protease Cascade that Activates Phenol Oxidase.

(A-D) Phenol oxidase (PO) activity was assayed ex vivo using dissected larval trachea incubated in L-DOPA solution. Trachea from wild-type (wt) larva showed no detectable melanization (A), but trachea from btl>Spn77Ba-IR larva showed significant melanization, indicating a high level of PO activity (B). Trachea in which MP1 protease was over-expressed showed weak melanization (C), which was suppressed by simultaneously over-expressing Spn77Ba (D).
(E) Spn77Ba regulates tracheal melanization by inhibiting the MP1/MP2 protease cascade. The percentage of adults of various genotypes showing tracheal melanization was measured.
(F) Suppression of tracheal melanization rescues semi-lethality caused by Spn77Ba RNAi in trachea. Adult flies of various genotypes were counted within one day of eclosion. Siblings carrying the CyO balancer were regarded as 100% viable. Data represent the average of at least three independent experiments ± SDV.
We wondered if the PO involved in tracheal melanization is endogenously made by tracheal cells like Spn77Ba or is imported. The Bc1 mutation in the Black cells gene, encoding a phenol oxidase stored in hemolymph crystal cells, suppresses hemolymph melanization and lethality caused by loss of serpin Spn27A (De Gregorio et al., 2002). To check if Bc is also involved in tracheal melanization controlled by Spn77Ba, we activated Spn77Ba RNAi with the tracheal-specific btl-Gal4 driver in a Bc1 mutant background. We found that Bc1 did not suppress melanization or lethality caused by Spn77Ba RNAi (Figure 3E-F), which suggests that the PO involved in tracheal melanization is not from crystal cells.
Spn77Ba Inhibits a Protease Cascade, Involving the MP1 and MP2 Proteases, that Activates Phenol Oxidase
MP1 and MP2 were previously identified as two proteases that function in a melanization cascade, with MP1 acting downstream of MP2, to activate PO in the hemolymph (Castillejo-Lopez and Hacker, 2005; Leclerc et al., 2006; Tang et al., 2006). To check if these two proteases are also involved in activating tracheal melanization, we generated flies in which both Spn77Ba RNAi and MP1 or MP2 RNAi were activated with the act-Gal4 driver. RNAi of MP1 or MP2 completely suppressed tracheal melanization and larval lethality caused by Spn77Ba RNAi (data not shown). These data suggest that Spn77Ba inhibits the MP1/MP2 melanization cascade, and that melanization is the cause of lethality, perhaps by compromising the respiratory function of the trachea.
We then checked if tracheal melanization involves the MP1 protease expressed by tracheal cells. If so, then disrupting MP1 specifically in tracheal cells should suppress melanization induced by Spn77Ba RNAi. Indeed, using the btl-Gal4 driver, we found that MP1 RNAi rescued the adult lethality and melanization caused by Spn77Ba RNAi. The adult survival rate increased from 65% to 96%, and the adult melanization rate decreased from 95% to 0% (Figure 3E-F). These results suggest that MP1 gene activity is required in tracheal cells in order to activate tracheal melanization.
Since MP1 is required to activate tracheal melanization, over-expression of this protease would be predicted to result in similar phenotypes as caused by Spn77Ba RNAi. Surprisingly, over-expressing pre-activated MP1 using the btl-Gal4 driver did not result in significant tracheal melanization or larval lethality. However, PO activity was detected in dissected trachea from these larvae using the ex vivo assay, and this PO activity resulting from MP1 over-expression was suppressed by simultaneously over-expressing Spn77Ba (Figure 3C-D). We therefore wondered if endogenous Spn77Ba is repressing the activity of over-expressed MP1 protease such that melanization was not observed in trachea in situ. To check this possibility, we over-expressed MP1 in the background of Spn77Ba RNAi using the btl-Gal4 driver. In this case, over-expression of pre-activated MP1enhanced the lethality, from 35% to 85%, caused by Spn77Ba RNAi (Figure 3F). Similar results were obtained with pre-activated MP2 (data not shown). All together, these results suggest that Spn77Ba regulates the melanization cascade involving the MP1 and MP2 proteases in the tracheal system.
The MP1/MP2 melanization cascade is inhibited by the serpin Spn27A in the hemolymph (Tang et al., 2006). To check if Spn27A can replace Spn77Ba in the tracheal system, we over-expressed Spn27A in the background of Spn77Ba RNAi using the btl-Gal4 driver. We found that Spn27A over-expression suppressed the lethality and melanization resulting from Spn77Ba RNAi, with 96% of the flies being viable and only 4% of the adults showing melanization (Figure 3E-F). As a control, over-expression of another serpin Spn47C did not significantly suppress the phenotypes resulting from Spn77Ba RNAi (data not shown).
Tracheal Melanization Induces Drosomycin Expression both Locally and Systemically
We found that in act-Gal4/UAS-Spn77Ba-IR larvae the antifungal peptide Drosomycin (Drs) was strongly induced in trachea and fat body, as assessed with a GFP reporter driven by the Drs promoter (Figure 4B). Quantitative RT-PCR revealed that the observed Drs expression level was comparable to the level in larvae infected with M. luteus (data not shown). Loss of Spn77Ba function in trachea was sufficient to induce Drs, as RNAi knockdown of Spn77Ba with btl-Gal4 resulted in Drs-GFP expression in trachea as well as fat body of larvae, pupae, and adults (Figure 4C-E). The Drs level in Spn77Ba RNAi larvae and adults was about 50 fold and 6-7 fold higher, respectively, than in the control (Figure 4H-I). As assessed with GFP reporters for other AMPs, no Metchnikowin, Diptericin (Dpt), and Drosocin, and only a very low level of Attacin A and Cecropin A1, was detected in btl-Gal4/Spn77Ba-IR flies. Knockdown of Spn77Ba using the c564-Gal4 driver active in the fat body and hemocytes, two major cell types involved in the systemic immune response, did not result in a high expression level of Drs or Dpt (data not shown). In addition, the c564-Gal4/UAS-Spn77Ba-IR flies had a survival rate similar to that of wild-type flies after microbial infection, and over-expression of Spn77Ba with the da-Gal4 driver did not affect the expression level of Drs or Dpt after infection. Altogether, these results indicate that, while Spn77Ba does not seem to play a major role in the fat body or hemocytes to control systemic expression of AMPs, loss of Spn77Ba function in trachea induces both local and systemic Drs expression.
Figure 4. Tracheal Melanization Induces Drs Expression.
(A-F) Flies carrying a Drs-GFP construct, in which GFP is expressed under the Drs transcriptional control region, were used to assay Drs expression in larvae, pupae and adults with designated genotypes. Note the darkened regions in the two dorsal tracheal trunks where intense melanization excluded GFP signal (B).
(G and G’) Drs expression was detected on the trachea that had melanization (arrow) but not the other trachea lacking visible melanization.
(H) Quantitative RT-PCR was used to monitor the expression level of Drs in 1-2 day old adult males of various genotypes. Drs expression level in control flies having btl-Gal4 driver alone was defined as 1.
(I) Quantitative RT-PCR was used to monitor the expression level of Drs in late third instar larvae of various genotypes. Drs expression level in wild-type larvae was defined as 1. Data represent average of at least three independent experiments ± SDV.
We also examined if melanization is required for the induction of Drs expression by Spn77Ba RNAi. When we inactivated the MP1/MP2 melanization cascade in trachea, by knocking down MP1 or over-expressing Spn27A with btl-Gal4, Spn77Ba RNAi did not induce Drs-GFP expression (Figure 4H). Bc1, which suppresses hemolymph but not tracheal melanization (see above), did not block Drs-GFP expression resulting from Spn77Ba RNAi (Figure 4H). Thus, activation of melanization in the trachea induces Drs expression.
Consistent with a role for tracheal melanization in inducing Drs expression, the overall Drs expression level correlated with the degree of tracheal melanization, as flies in which Spn77Ba RNAi and MP1 over-expression were simultaneously activated in tracheal cells showed a higher level of Drs expression and more severe melanization than Spn77Ba RNAi flies (Figure 4H). Moreover, in larvae in which only one of the two dorsal tracheal trunks was visibly melanized, 75% of the larvae showed Drs expression only on the melanized tracheal trunk (Figure 4G and G’). The remaining 25% showed Drs expression on both tracheal trunks, probably due to the non-melanized tracheal trunk having a very low level of melanization that is not visible. However, we never saw a larva that showed Drs expression only on the apparently non-melanized tracheal trunk. These results support the idea that tracheal melanization induces Drs expression.
A Product of the Melanization Reaction Induces Fat Body Drosomycin Expression through the Toll Signaling Pathway
To investigate if the melanization reaction itself or a final product of the reaction induces Drs expression, we controlled the timing of Spn77Ba RNAi using a temperature sensitive form of the Gal80 protein that inhibits Gal4. We generated flies of the genotype T80-Gal4, tubP-Gal80ts, UAS-GFP, UAS-Spn77Ba-IR in which the broadly expressed T80-Gal4 driver can activate expression of both GFP and Spn77Ba RNAi only when the Gal80 inhibitor is inactivated at 29°C but not at 18°C (McGuire et al., 2003). As expected, when larvae of this genotype were raised at 18°C for 48 hours after egg hatching, they showed no detectable GFP expression (data not shown) or melanization and had wild-type levels of Spn77Ba RNA as well as Drs. If the larvae were then transferred to 29°C for 48 hours, they showed strong GFP expression and had a reduced level of Spn77Ba RNA, about 40% of the wild-type level. Most of these larvae showed melanization, and their Drs expression level was 17 fold higher than the wild-type level. After an additional 48 hours at 29°C, melanization had increased in intensity, Spn77Ba RNA was further lowered to less than 30% of the wild-type level, and the Drs level had increased to 60 fold higher than the control. However, if the larvae were transferred back to 18°C after 48 hours at 29°C, melanization only increased slightly in intensity over the next 24 hours but not beyond that time (data not shown). Moreover, even though Spn77Ba RNA had returned to almost the wild-type level such that tracheal melanization should not be triggered, Drs expression had increased to about 60 times the wild-type level, similar to the case in which the larvae were kept at 29°C during the entire 96-hour period (Figure 5A-B). These results suggest that a product of the melanization reaction, rather than the reaction itself, induces Drs expression.
Figure 5. A Product of the Melanization Reaction Induces Drs Expression through the Toll Signaling Pathway.

(A-B) Larvae and prepupae with the genotype of T80-Gal4, tubP-Gal80ts, UAS-Spn77Ba-IR, UAS-GFP were used to control the activation of Spn77Ba RNAi at different developmental times. At 18°C, the temperature-sensitive Gal80 prevents Gal4 from activating Spn77Ba RNAi, whereas at 29°C, Gal80 is inactivated, thereby allowing activation of Spn77Ba RNAi. Quantitative RT-PCR was used to monitor Spn77Ba (A) and Drs (B) expression levels. The gene expression levels of wild-type larvae or prepupae at the corresponding developmental stages were used as controls to define 100% and 1 for Spn77Ba and Drs, respectively, in samples 1 to 4.
(C and C’) Melanized trachea from act-Gal4/Spn77Ba-IR larva or non-melanized trachea from wild-type larva was transplanted to a recipient fly carrying a Drs-GFP reporter. GFP signal was observed in most flies after transplantation, but the signal persisted for at least 120 hours in flies receiving Spn77Ba trachea while it began fading away 48 hours later in flies receiving wild-type trachea. Recipient flies are shown at 96 hours after transplantation, when 75% and 15% of flies (n>20) receiving Spn77 RNAi and wild-type trachea, respectively, were GFP positive. Wild-type trachea became melanized after transplantation.
(D) Quantitative RT-PCR was used to monitor the expression level of Drs in 1-2 day old adult males. Drs expression level in male flies carrying the btl-Gal4 driver alone was defined as 1. The higher than control level of Drs seen in the experiment with the seml mutation may be largely due to the associated yellow (y) mutation, which results in an accumulation of dopachrome intermediates of the melanization reaction (Wittkopp et al., 2003). Data represent average of at least three independent experiments ± SDV.
As a further test of the idea that a tracheal melanization product induces Drs, we transplanted melanized trachea from Spn77Ba RNAi larva to 1-2 day old virgin female adults carrying the Drs-GFP reporter. Melanized trachea from Spn77Ba RNAi larva, but not non-melanized trachea from wild-type larva, resulted in persistent Drs expression in the recipient flies (Figure 5C and C’). These results argue that a tracheal melanization product induces Drs expression.
Excessive melanization may cause cell death. To test whether indirect effects of cell death are sufficient to account for the ability of tracheal melanization to induce Drs expression, we examined Drs expression in third instar larvae over-expressing the pro-apoptotic gene reaper. Larvae of the genotype btl-Gal4, tubP-Gal80ts, UAS-GFP, UAS-Reaper or T80-Gal4, tubP-Gal80ts, UAS-GFP, UAS-Reaper were raised at 22°C until early third instar stage and then transferred to 29°C for 48 hours in order to induce Reaper and thus cell death in the tracheal system or in multiple tissues. In either case, no significant Drs expression was detected by quantitative RT-PCR, suggesting that apoptotic cell death is not sufficient to induce Drs expression (Figure 4I).
The Toll pathway is the major signaling pathway that regulates Drs synthesis in response to infection in Drosophila. We therefore checked if it is required for tracheal melanization to induce fat body Drs expression. The spzrm7 mutation, which disrupts the ligand Spätzle that activates Toll, strongly suppressed overall Drs expression (Figure 5D) but not tracheal-specific Drs expression (Figure 4F) or melanization (Figure 3E) resulting from Spn77Ba RNAi. Suppression of Drs expression but not melanization was also observed with psh5 (Ligoxygakis et al., 2002a), a mutation inactivating the Persephone (Psh) protease involved in activating Spätzle (Figure 5D). However, the seml mutation that disrupts PGRP-SA, a pattern recognition receptor for Gram-positive bacteria acting upstream of Toll, did not suppress Drs expression (Figure 5D) or melanization (Figure 3E) resulting from Spn77Ba RNAi. In addition, over-expression of Spn77Ba with the da-Gal4 driver did not block constitutive Drs-GFP expression in flies caused by over-expressing either Persephone or PGRP-SA/GNBP1 (data not shown). These results argue that Spn77Ba does not directly regulate the activation of Toll, and that tracheal melanization resulting from Spn77Ba RNAi activates the Toll pathway through a serine protease cascade that involves Psh.
Tracheal Melanization Can Be Induced by the Presence of Microorganisms
An important question is what role microorganisms play in inducing tracheal melanization. To address this question, we first examined whether microorganisms present in the culture medium are responsible for the tracheal melanization in Spn77Ba RNAi flies. We therefore raised btl-Gal4/UAS-Spn77Ba-IR larvae under sterile or normal conditions or in the presence of exogenous bacteria (a mixture of E. coli and M. luteus) and then examined the flies for melanization at the larval and pupal stages. In all 3 conditions, nearly all flies showed melanization, either mild or severe; however, the percentage of flies showing severe melanization increased with greater microbial exposure, from 16% to 35% to 53% (Figure 6A-B). The Drs level in these flies correlated better with severity of melanization than with degree of microbial exposure (Figure 6C), consistent with Drs being largely induced by tracheal melanization rather than directly by microbial infection. These results suggest that, in the absence of normal levels of Spn77Ba, tracheal melanization is at least partially caused by microorganisms.
Figure 6. Tracheal Melanization can be Induced by Microorganisms.
(A) Larvae in which Spn77Ba RNAi was activated with btl-Gal4 were raised under 3 different conditions: sterile, normal, or + bacteria. The percentage of larvae and pupae showing no, mild, or severe melanization as seen in (B) was visually recorded. At least 200 larvae and pupae were counted for each condition.
(B) Representative examples of pupae with no melanization, mild melanization, and severe melanization. Arrow indicates melanization.
(C) Quantitative RT-PCR was used to monitor Drs expression level of prepupae showing either mild or severe melanization when cultured under sterile or non-sterile conditions. Data represent the average of at least three independent experiments ± SDV.
(D-G) Tracheal PO activity was assayed ex vivo in the presence or absence of bacteria (see Experimental Procedures). A mixture of E. coli and M. luteus induced in wild-type trachea a high level of PO activity, which was suppressed by either Spn77Ba over-expression or MP1 RNAi.
(H and H’) Exposure of wild-type larvae to B. bassiana spores induced melanization on cuticle (arrows) and Drs expression in both trachea and fat body.
(I) Cuticle melanization induced by B. bassiana was partially suppressed by Spn77Ba over-expression or MP1 RNAi. The percentage of larvae that showed melanization 24 hours after infection was counted.
(J) Drs expression induced by B. bassiana was partially suppressed by Spn77Ba over-expression or MP1 RNAi. The percentage of larvae that showed Drs-GFP expression 24 hours after infection was counted. *: p<0.05 (paired T-test).
To test directly whether microorganisms can induce tracheal melanization, we used the ex vivo tracheal PO assay to expose larval trachea to a mixture of E. coli and M. luteus. Trachea from wild-type larva was strongly melanized after 2 hours of incubation with bacteria, which was blocked by either Spn77Ba over-expression or MP1 RNAi (Figure 6E-G). While E. coli and M. luteus are not natural pathogens of Drosophila, they could induce tracheal melanization by mimicking the ability of a natural pathogen to penetrate the tracheal cuticle or by exploiting an opening made by spontaneous injury to the trachea. These results indicate that microorganisms can trigger in the trachea the activation of PO and the melanization reaction under control of Spn77Ba.
To examine whether a more natural mode of infection could induce tracheal melanization, we exposed larvae to the entomopathogenic fungus B. bassiana whose spores can penetrate the host cuticle (Braun et al., 1998). Such treatment resulted in about 60% and 65% of wild-type larvae showing, respectively, melanization on the cuticle and Drs expression in the tracheal system and fat body (Figure 6H and H’). A significant level of tracheal melanization or ex vivo tracheal PO activity was not detected, perhaps because the spores do not efficiently access the tracheal system under our experimental conditions. Nonetheless, ubiquitous over-expression of Spn77Ba or knock down of MP1 reduced the percentage of larvae showing cuticle melanization and Drs expression to about 30% and 15%, respectively (Figure 6I and J). Thus, although we did not observe tracheal melanization being induced by B. bassiana, the results suggest that Spn77Ba-regulated melanization plays an intermediary role in inducing Drs expression after natural fungal infection.
DISCUSSION
We have identified serpin Spn77Ba as a negative regulator of melanization in the respiratory system, the trachea, of Drosophila. We showed that Spn77Ba inhibits a protease cascade involving the MP1 and MP2 proteases, previously implicated in activating melanization in the hemolymph. Our data suggest that tracheal melanization regulated by Spn77Ba is a local immune response, which in turn induces systemic expression of the antifungal peptide Drosomycin via the Toll pathway.
Spn77Ba regulates tracheal melanization by inhibiting a protease cascade that activates PO, a key enzyme in melanin biosynthesis. This protease cascade involves the proteases MP1 and MP2, which previously were shown to regulate the melanization reaction in the hemolymph where it is inhibited by the serpin Spn27A (Tang et al., 2006). While hemolymph melanization involves MP1 and MP2 made by fat body cells and/or hemocytes, tracheal melanization involves the same proteases locally synthesized by tracheal cells. We also showed that Spn77Ba is required, as well as expressed, in tracheal cells to prevent tracheal melanization. A previous study showed that the cuticular PO involved in host defense and wound healing in the silkworm is transported from the hemolymph (Ashida and Brey, 1995). In contrast, our results obtained with the Bc1 mutation indicate that tracheal melanization is not mediated by hemolymph PO. In preliminary experiments, tracheal-specific RNAi of each of several PO genes (CG2952, CG5779, CG8193, CG10484) did not suppress tracheal melanization resulting from Spn77Ba RNAi (unpublished data), perhaps indicating that multiple PO genes are responsible for tracheal melanization. Interestingly, the RelE20 mutation in Relish, a transcription factor in the Imd pathway, suppressed tracheal melanization and overall Drs expression resulting from Spn77Ba RNAi (unpublished data), which suggests that Rel could be important for the expression of a component required for tracheal melanization.
We showed that tracheal melanization is inducible by microorganisms. Thus, tracheal melanization may be a local immune response analogous to the local synthesis of reactive oxygen species and antimicrobial peptides by epithelia facing the external environment. Such a role is consistent with our observation that Spn77Ba is localized in the extracellular space facing the tracheal lumen. Melanization could be used to encapsulate pathogens killed by these other local immune responses, and reactive oxygen species generated during the melanization reaction may aid in killing pathogens as well. Melanization could also create a physical barrier to impede pathogen penetration of the tracheal epithelium, as in the case where melanin is deposited at the site of entry in defense against entomopathogenic fungi that infect by crossing the insect cuticle (Vey and Gotz, 1986). We found that Spn77Ba over-expression or MP1 RNAi did not make larvae more susceptible to natural B. bassiana infection (unpublished data). However, testing of more pathogens will be needed to determine the importance of melanization in combating infection.
An issue that remains to be addressed is the mechanism by which microorganisms activate the melanization cascade in the tracheal system. Over-expression of PGRP-LE, a PRR expressed in tracheal epithelia, does not lead to constitutive tracheal melanization (Takehana et al., 2002; Takehana et al., 2004), suggesting that this PRR is not a tracheal-specific activator of melanization. Interestingly, under axenic conditions, Spn77Ba RNAi flies still showed tracheal melanization, although at a lower strength than seen under normal conditions or in the presence of bacteria. This low level of melanization could reflect activation of the melanization cascade by dead microorganisms present in the culture food medium. Alternatively, even in the absence of microbial infection, the target protease of Spn77Ba could exist in the tracheal system already activated rather than entirely as inactive zymogen, but is prevented from activating the whole cascade and thus melanization by the presence of Spn77Ba. In this case, microbial infection could trigger tracheal melanization by either reducing the level of Spn77Ba or increasing the amount of activated target protease. Defining the actual mechanism may require identifying a natural pathogen that strongly induces tracheal melanization.
Regardless of how it is activated, tracheal melanization appears to play a key intermediary role in inducing systemic expression of the antifungal peptide gene, Drosomycin (Drs). It had previously been reported that melanotic “tumors” resulting from melanization of aberrant host tissue are associated with systemic Drs expression, but the mechanism linking melanization and Drs expression has been obscure (Ligoxygakis et al., 2002b; Scherfer et al., 2006). Our data suggest that melanization induces Drs expression, rather than the two immune responses being triggered in parallel, and that a product of the melanization reaction is involved in inducing Drs expression. However, as melanization does not necessarily activate Drs expression in all other contexts, this product may not be melanin itself but rather a melanin metabolite or a secondary signal induced by melanin when melanization is activated in the trachea. Indeed, the mechanical and ROS-related insults resulting from tracheal melanization may themselves induce a stress response that contributes to the systemic induction of Drs. In any case, it appears that hemocytes are not involved in transmitting signals that connect tracheal melanization to fat body Drs expression, as l(3)hem larvae lacking hemocytes retain the systemic response (unpublished data).
Whatever the molecular nature of this product, it is presumably a diffusible molecule that can pass through the tracheal system basement membrane into the hemolymph, where it activates the Toll pathway to induce Drs synthesis by the fat body. Moreover, it apparently acts upstream or at the level of the Persephone (Psh) protease involved in cleaving Spätzle to generate the Toll ligand. Interestingly, it was recently shown that Psh, which specifically functions to activate Toll in response to fungal infection, is proteolytically activated by a secreted fungal protease and thus acts as a direct sensor of this virulence factor (Gottar et al., 2006). Our data suggest that local melanization is involved in inducing Drs expression after fungal infection and that Psh can be activated by an alternative mechanism in which a host factor arising from melanization triggers Psh activation, which could be analogous to a danger signal from damaged tissue that activates the immune system (Matzinger, 2002). We therefore speculate that induction of Drs synthesis by tracheal melanization represents signaling between local and systemic immune responses that alerts and prepares the host for potential invasion of internal tissues by pathogens such as entomopathogenic fungi. Such an alarm system could be advantageous for organisms in which pathogens are naturally first encountered at epithelial surfaces.
EXPERIMENTAL PROCEDURES
Information about fly stocks and RNA analysis used in this study can be found in Supplemental Experimental Procedures.
DNA Constructs
We used 2 different UAS-Spn77Ba-IR constructs. One contained 2 inverted copies of a 551 bp fragment (nucleotides 200-750) of the Spn77Ba mRNA in the pWIZ vector (Lee and Carthew, 2003). The other construct, containing 2 inverted copies of a different region of the Spn77Ba transcript in the pUAST-R57 vector, was obtained from R. Ueda (National Institute of Genetics, Mishima, Japan). The UAS-Spn77Ba construct contains the full-length Spn77Ba open reading frame with a C-terminal HA tag in the pUASP vector (Rorth, 1998). The UAS-Spn77Ba-eGFP fusion protein construct was made in the pPWG vector (Drosophila Genomics Resource Center, Bloomington, IN). The Spn77Ba-Gal4 driver was made by cloning 2.2 kbp of genomic DNA immediately upstream of the Spn77Ba gene into the pG4PN vector (Dobritsa et al., 2003). Results from all experiments with transgenic flies in this study were representative of at least 2 independently generated lines.
Ex Vivo Trachea Assay for PO Activity
Dorsal tracheae of third instar larvae were dissected and, after washing in PBS, placed on a piece of filter paper pre-soaked in a saturated solution of L-DOPA in PBS. The tracheae were submerged in a thin layer of L-DOPA solution by this procedure and thus kept in the dark at room temperature for one to two hours before observation. In the experiments involving bacteria, trachea were incubated with a concentrated mixture of E. coli and M. luteus, prepared from an overnight culture of each bacterium in LB medium that was harvested, washed in PBS, and re-suspended in PBS with saturated L-DOPA at a final OD600 of about 10 for each bacterium.
Culturing Larvae Under Sterile and Non-Sterile Conditions
Larvae were raised under axenic conditions as described previously (Tzou et al., 2001). Briefly, embryos were collected from apple juice plates and effectively sterilized by incubation in 50% bleach for 5 min to remove the chorion and then washing in 60% ethanol for 3 min. These embryos were then transferred to 50 mm plates containing autoclaved apple juice medium, and the larvae that developed from these embryos were fed with autoclaved yeast paste. For larvae grown under normal conditions, the apple juice medium and yeast paste were not autoclaved. To culture larvae with exogenous bacteria, E. coli and M. luteus were each grown in LB medium overnight to an O.D. of ~ 2 and then concentrated by centrifugation to an O.D. of ~100. 0.5 ml of each concentrated culture was spread on an apple juice plate to which was added about 100 early third instar larvae. The severity of melanization was visually recorded at the pre-pupal to pupal stage. B. bassiana natural infection was performed as described (Braun et al., 1998).
Chitin and F-actin Imaging
Third instar larvae were dissected in PBS and fixed in 4% paraformaldehyde in PBS plus 0.1% Tween-20 (PBTw). Isolated trachea from these fixed larvae was then incubated in PBTw with 1:500 rhodamine-conjugated chitin-binding probe (New England Biolabs) or 1:500 Alexa Fluor 633–phalloidin (Invitrogen) at room temperature for overnight or 2 hours, respectively, before microscopic examination.
Trachea Transplantation
A small piece of trachea was dissected in PBS from third instar wild-type or act-Gal4/Spn77Ba-IR larvae and transplanted into recipient 1-2 day old virgin adult females under anesthesia. The transplant was placed just under the abdominal cuticle with minimal disturbance of internal organs through an incision made with MicroPoint scissors. Recipient flies were allowed to recover at room temperature.
Supplementary Material
Figure 7. Epithelial melanization and the induction of a systemic immune response.

Spn77Ba inhibits a protease cascade involving the MP1 and MP2 proteases that activates phenol oxidase (PO), a key enzyme in melanin biosynthesis. Melanization is activated in extracellular space between cuticle lining the tracheal lumen and the apical surface of tracheal cells. The MP1/MP2 cascade also activates melanization in the hemolymph where Spn27A inhibits it. Tracheal melanization induces Drs expression in tracheal epithelium as well as in the fat body. The mechanism for signaling from trachea to fat body involves a yet-to-be-identified product of tracheal melanization that passes through basement membrane to hemolymph, where it activates the Persephone (Psh) protease involved in cleaving Spätzle to generate the ligand for the receptor Toll, which in turn initiates signaling leading to induction of Drs expression.
Acknowledgments
We thank Ryu Ueda for providing RNAi lines and Tian Xu for generous sharing of equipment. H.T. was supported by a Yale University fellowship. The laboratory of B.L. was funded by Agence National de la Recherche, the Schlumberger Foundation, and the Bettencourt Foundation. This work was supported by NIH grant GM49370.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Ashida M, Brey PT. Role of the integument in insect defense: pro-phenol oxidase cascade in the cuticular matrix. Proc Natl Acad Sci USA. 1995;92:10698–10702. doi: 10.1073/pnas.92.23.10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Hoffmann JA, Meister M. Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc Natl Acad Sci USA. 1998;95:14337–14342. doi: 10.1073/pnas.95.24.14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillejo-Lopez C, Hacker U. The serine protease Sp7 is expressed in blood cells and regulates the melanization reaction in Drosophila. Biochem Biophys Res Commun. 2005;338:1075–1082. doi: 10.1016/j.bbrc.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Cerenius L, Soderhall K. The prophenoloxidase-activating system in invertebrates. Immunol Rev. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Han SJ, Lee WJ, Baek MJ, Osaki T, Kawabata S, Lee BL, Iwanaga S, Lemaitre B, Brey PT. An immune-responsive Serpin regulates the melanization cascade in Drosophila. Dev Cell. 2002;3:581–592. doi: 10.1016/s1534-5807(02)00267-8. [DOI] [PubMed] [Google Scholar]
- Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- Duffy JB. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Jung AC, Criqui M, Lemaitre B, Uttenweiler-Joseph S, Michaut L, Reichhart J, Hoffmann JA. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 1998;17:1217–1227. doi: 10.1093/emboj/17.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, Butt TM, Belvin M, Hoffmann JA, Ferrandon D. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–1437. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Leclerc V, Pelte N, El Chamy L, Martinelli C, Ligoxygakis P, Hoffmann JA, Reichhart JM. Prophenoloxidase activation is not required for survival to microbial infections in Drosophila. EMBO Rep. 2006;7:231–235. doi: 10.1038/sj.embor.7400592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Carthew RW. Making a better RNAi vector for Drosophila: use of intron spacers. Methods. 2003;30:322–329. doi: 10.1016/s1046-2023(03)00051-3. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA, Reichhart JM. Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science. 1999;285:1917–1919. doi: 10.1126/science.285.5435.1917. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science. 2002a;297:114–116. doi: 10.1126/science.1072391. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Ji C, Leclerc V, Duvic B, Belvin M, Jiang H, Hoffmann JA, Reichhart JM. A serpin mutant links Toll activation to melanization in the host defence of Drosophila. EMBO J. 2002b;21:6330–6337. doi: 10.1093/emboj/cdf661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukinova NI, Roussakova VV, Fortini ME. Genetic characterization of cytological region 77A-D harboring the presenilin gene of Drosophila melanogaster. Genetics. 1999;153:1789–1797. doi: 10.1093/genetics/153.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci USA. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- Scherfer C, Qazi MR, Takahashi K, Ueda R, Dushay MS, Theopold U, Lemaitre B. The Toll immune-regulated Drosophila protein Fondue is involved in hemolymph clotting and puparium formation. Dev Biol. 2006;295:156–163. doi: 10.1016/j.ydbio.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Takehana A, Katsuyama T, Yano T, Oshima Y, Takada H, Aigaki T, Kurata S. Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc Natl Acad Sci USA. 2002;99:13705–13710. doi: 10.1073/pnas.212301199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana A, Yano T, Mita S, Kotani A, Oshima Y, Kurata S. Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J. 2004;23:4690–4700. doi: 10.1038/sj.emboj.7600466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Kambris Z, Lemaitre B, Hashimoto C. Two proteases defining a melanization cascade in the immune system of Drosophila. J Biol Chem. 2006;281:28097–28104. doi: 10.1074/jbc.M601642200. [DOI] [PubMed] [Google Scholar]
- Tzou P, Meister M, Lemaitre B. Methods for studying infection and immunity in Drosophila. Methods Microbiol. 2001;31:507–529. [Google Scholar]
- Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, Lemaitre B, Hoffmann JA, Imler JL. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity. 2000;13:737–748. doi: 10.1016/s1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- Vey A, Gotz P. Antifungal cellular defense mechanisms in insects. In: Gupta AP, editor. Hemocytic and Humoral Immunity in Arthropods. New York, USA: John Wiley and Sons Inc; 1986. pp. 89–115. [Google Scholar]
- Wagenaar-Bos IG, Hack CE. Structure and function of C1-inhibitor. Immunol Allergy Clin North Am. 2006;26:615–632. doi: 10.1016/j.iac.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Wittkopp PJ, Carroll SB, Kopp A. Evolution in black and white: genetic control of pigment patterns in Drosophila. Trends Genet. 2003;19:495–504. doi: 10.1016/S0168-9525(03)00194-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





