Abstract
It is widely (although not universally) accepted that organismal aging is the result of two opposing forces: (i) processes that destabilize the organism and increase the probability of death, and (ii) longevity assurance mechanisms that prevent, repair, or contain damage. Processes of the first group are often chemical and physico-chemical in nature, and are either inevitable or only under marginal biological control. In contrast, protective mechanisms are genetically determined and are subject to natural selection. Life span is therefore largely dependent on the investment into protective mechanisms which evolve to optimize reproductive fitness. Recent data indicate that toxicants, both environmental and generated endogenously by metabolism, are major contributors to macromolecular damage and physiological dysregulation that contribute to aging; electrophilic carbonyl compounds derived from lipid peroxidation appear to be particularly important. As a consequence, detoxification mechanisms, including the removal of electrophiles by glutathione transferase-catalyzed conjugation, are major longevity assurance mechanisms. The expression of multiple detoxification enzymes, each with a significant but relatively modest effect on longevity, is coordinately regulated by signaling pathways such as insulin/insulin-like signaling, explaining the large effect of such pathways on life span. The major aging-related toxicants and their cognate detoxification systems are discussed in this review.
Keywords: aging, detoxification, electrophiles, 4-hydroxynonenal, lipid peroxidation, glutathione transferase
1. Introduction
It is not surprising that aging holds a deep fascination for any sentient being that is subject to the process. The fascination is both intellectual and emotional. In fact, much of human culture revolves around aging and death. The philosophical and theological effort to understand and to come to terms with mortality engendered attempts to control and delay aging. Clearly, for most of human history, such interventions were beyond the reach of the available tools. An understanding of aging requires the convergence of several distinct areas of biology, in particular theory of evolution, genetics, and molecular and cell biology and biochemistry. Relative to the time scale of human civilization, these disciplines emerged only recently and are still not mature. Therefore, past attempts to postpone senescence and death were for the most part futile, and – worse – gave the study of aging a somewhat unsavory reputation. This reputation persists even today: the mention of aging research frequently evokes either exaggerated expectations or deep skepticism about tackling a process widely assumed to be inescapable, immutable, and largely unknowable. Rapid progress during the past decade or two has demonstrated that aging is neither unknowable nor immutable, at least in non-human model organisms. The initial dramatic evidence that life span can be significantly extended by an experimental intervention came from genetics (Friedman and Johnson, 1988; Kenyon et al., 1993). While the identification of genes that modulate life span launched the modern era of aging research, in itself it provided only indirect information on the molecular mechanisms that modulate aging. At their root, living organisms are biochemical and biophysical machines, albeit enormously complex ones. Therefore, a true functional understanding of a biological process requires knowledge of the underlying biochemical reactions, in addition to information on the regulatory circuits that affect these reactions. The characterization of metabolic processes that affect aging is perhaps the most active area of gerontology at the present time. This review is an attempt to summarize the current status of research on one particular type of metabolism that is relevant to aging, the detoxification reactions.
2. Basic biology of aging
Multiple reviews that address the process of aging have been recently published, among them (Antebi, 2007; Bishop and Guarente, 2007; Greer and Brunet, 2008; Guarente, 2008; Kenyon, 2005; Kirkwood, 2005, 2008a, b; Murphy and Partridge, 2008; Partridge and Gems, 2007; Piper et al., 2008). The above list, albeit partial and largely arbitrarily selected, illustrates the availability of excellent sources that discuss the complex and often controversial topic of aging. Therefore, the following summary is not intended to be a comprehensive review of aging theories. Instead, I will present a conceptual framework that is based on an emerging (although not universally accepted!) consensus on the nature of aging. This framework will be used in the remainder of the present article.
Even though gerontology suffers from the lack of a precise definition of its fundamental terms (Hayflick, 2007; Lithgow, 2006), aging is usually understood as an increase of the probability of death with time. If “failure” is substituted for “death”, this definition applies not only to biological organisms but also to many inanimate objects – with the passage of time (or time in use), machines tend to malfunction more often, and eventually fail. On the other hand, there is evidence that not all organisms are subject to aging as defined above (I will discuss non-aging organisms later). Also, the definition of aging does not specify the mathematical relationship between age and survivorship. Benjamin Gompertz noticed almost two centuries ago that an exponential function describes well the mortality in human populations (Gompertz, 1825). This formalism withstood the test of time and is widely used today, at least to model the “middle” part of the survivorship of a population (i.e., excluding both early mortality and the tail of the curve due to a small number of very long-lived individuals). It is important to note that neither the fact that mortality increases with age nor the particular shape of this increase shed much light on the underlying mechanism of aging.
Significant insight into aging can be gained through genetics. The observations that birds and bats typically live longer than mice or rats (Brunet-Rossinni and Austad, 2004; Pamplona et al., 2002), even after correction for body mass and after elimination of most extrinsic sources of mortality such as predators, accidents, disease, or harsh environment, indicates that life span is subject to evolution, and thus, is under genetic control. Genetic control of life span was forcefully confirmed by the already mentioned identification of single-gene mutations that extend life (Friedman and Johnson, 1988; Kenyon et al., 1993), in an extreme case, by a factor of ten (Ayyadevara et al., 2008).
In the absence of molecular information on the underlying mechanisms, genetic control of longevity could be interpreted in two ways. First, there could be a deterministic genetic program that specifies aging, in analogy to the genetic program that orchestrates the development of an organism. Second, aging could be due to a failure of genetically controlled reactions to counteract and limit a spontaneous aging process. The first possibility, that of an aging program, appears unlikely for several reasons. (i) Such program would be maladaptive to the affected individual, making it difficult to rationalize how, in the course of evolution, such a program could be selected for. (ii) Unlike development which is very precise, there are very significant (several-fold) differences in life span between individuals belonging to a genetically uniform cohort maintained in an identical environment (Kirkwood et al., 2005; Rea et al., 2005), an observation not readily reconcilable with a deterministic aging program. (iii) Disruption by mutations of a hypothetical pro-aging program should abolish aging. However, in contrast to mutations that delay aging, no mutations have been found that eliminate it. (iv) Finally, the strongest argument against a genetic program for aging is an evolutionary one. In the wild, most individuals do not live long enough to age: the vast majority die early from already mentioned extrinsic causes (including accidents, infections, predators, or cold temperatures). For example, the average life span of mice in a natural habitat is approximately 100 to 130 days (reviewed by Austad and Kristan, 2003; Berry and Bronson, 1992), versus an average of 700 to 1000 days for wild-derived mice kept in the protective environment of the laboratory (Miller et al., 2002). Selection can act only on carriers of a genetic allele that, through its phenotype, improves the reproductive success of the individual, thus increasing the likelihood that the gene will be passed on to progeny. If only a small fraction of the population survives to an age at which the hypothetical aging program becomes phenotypically manifest, the selection for (or against) the genes specifying such program would be very weak, and unlikely to prevent the loss of the genes through random mutations. This implies that a genetic program for aging would be very unlikely to evolve.
The arguments listed above, rooted in the early work of Medawar (1952), Williams (1957) and Kirkwood (1977) and further developed by several authors including Kirkwood (2005, 2008b), Hayflick (2007), de Grey (2007), Austad (2004), Gems (2005) and many others, make the existence of an active genetic program for aging most implausible. This focuses attention on the previously mentioned alternative interpretation for genetic modulation of life span. According to this interpretation, aging happens by default, but can be at least partially prevented or repaired by genetically controlled longevity assurance mechanisms. Failure of these protective mechanisms results in progressive loss of cell and tissue function, loss of homeostasis, and eventual death. This model will be described in the next section, with emphasis on aspects relevant to detoxification reactions.
It is important to note that what I would like to call the "standard hypothesis of aging" (that aging results from an imbalance between non-regulated, largely stochastic damage on one side, and genetically controlled protective longevity assurance mechanisms on the other side) is widely accepted by gerontologists, but that the acceptance is not universal. Models that incorporate programmed aging (Bredesen, 2004; Goldsmith, 2004; Goldsmith, 2008; Longo et al., 2005; Mitteldorf, 2006) or at least some elements of it (Blagosklonny, 2007a, b) continue to be proposed, and spirited discussions ensue (Melov, 2004, and associated references). These "counter-proposals" merit special attention. Even though, in my opinion, the standard hypothesis will eventually accommodate the "counter-examples" that are being raised, it would be too dogmatic to rule out a priori the possibility that an aging program may exist after all, perhaps limited to exceptional ecological niches or to a species with an unusual life history. Even if this were not the case, the analysis of the "counter-examples" will certainly contribute to a refinement of the standard hypothesis.
3. "Standard hypothesis" of aging
Thermodynamically, a living system is far from equilibrium. Therefore, maintaining a steady-state (or near-steady-state) that is compatible with life requires work, realized through a flux of energy and matter between the organism (thermodynamically an open system) and its environment. Metabolic reactions utilize energy and matter to maintain the organism in a functional state (in addition to enabling other activities such as mobility or reproduction). In part, the work necessary to keep the organism away from thermodynamic equilibrium is due to the need to prevent destabilization caused by external and internal damage. An organism is essentially a chemical machine, and – being an open system – is susceptible to physical and chemical influences of the environment. In addition, metabolism itself generates reactive intermediates and/or products able to degrade the function of cells and, as a consequence, the fitness of the organism.
Mechanisms that protect an organism against time-dependent loss of homeostasis clearly exist; otherwise, a near-steady-state lasting for most of adulthood (many years in long-lived species) would not be possible. Still, we age, even though aging is clearly maladaptive for the affected individual. Thus, the general question "Why do we age?" can be recast as the more specific "Why are the protective mechanisms not capable of indefinitely maintaining the organism in an essentially unchanged steady-state?". Any answer must take into account the fact that, in principle, such a feat is possible. The germ line of multicellular organisms is maintained indefinitely (a state that could be called "functional immortality" but not true immortality, because of accidents, predation, infections etc.). Similarly, symmetrically dividing unicellular organisms are by definition functionally immortal because each of the two identical daughter cells must be sufficiently rejuvenated to reach its own subsequent division in no worse condition than that of its parent. Any accumulation of damage from generation to generation, however small, would lead to a demise of the species. It should be noted that the demonstration that cell division in Escherichia coli is functionally asymmetric and that the bacterium ages may indicate that truly symmetric division does not occur, even in microorganisms (Desnues et al., 2003; Stewart et al., 2005). If so, this would mean that bacteria resemble us (and other multicellular organisms) more closely than previously imagined: the repeatedly rejuvenated daughter cells would be the functional equivalent of the germ line, whereas the mother cell that accumulates damaged macromolecules and dies after a finite number of divisions would correspond to the soma. A similar mechanism is operative in the budding yeast Saccharomyces cerevisiae (Jazwinski, 2002; Tissenbaum and Guarente, 2002). Whether mechanistically related to the continuity of a germ line or based on symmetrical division, functional immortality in unicellular organisms constitutes another example that indefinite maintenance is biologically possible.
At the level of evolutionary biology, an initial answer to the question why protective mechanisms fail to prevent aging is provided by the realization that selective force diminishes with age of an organism (Medawar, 1952). Genes that encode traits linked to increased reproductive fitness have a higher probability of being passed on to the next generation, and are thus favored by natural selection. Some of the parameters relevant to the optimization of reproductive fitness define how early in life reproduction begins and how long it continues. Generally, in environments in which extrinsic mortality is high, e.g. because of predation, it is advantageous to reproduce early and copiously, whereas low extrinsic mortality permits later onset and more protracted reproduction. This has been well documented (Austad, 1993; Chapuisat and Keller, 2002; Holmes and Austad, 1994) and illustrates that an extended reproductive period can evolve and be sustained provided that a sizable fraction of the late-reproducing individuals actually survives to an old age. Only then will the genes that specify late reproduction confer a benefit when transmitted to the next generation. If most individuals die early of extrinsic causes, having the capacity to reproduce late will be rarely expressed, will not be selected, and will be eventually lost either through random genetic drift (Griffiths et al., 1996, p. 807) or through antagonistic pleiotropy (Williams, 1957). Late reproduction obviously entails the capacity for at least equally long life. Therefore, genetic variants that delay aging will co-evolve with alleles that permit late reproduction; in fact, these two set of genes may overlap.
The general principle that, because of extrinsic mortality, the force of selection diminishes with age and therefore is not able to fix life-extending or eliminate life-shortening genes that are phenotypically expressed only late in life, provides a very useful conceptual framework but does not identify the genes or processes involved. Several more specific, mutually non-exclusive hypotheses have been put forward. In his mutation accumulation hypothesis, Medawar (1952) proposed that mutations which become phenotypically manifest only late in life will not be subject to significant selection. Since most mutations are detrimental, such late-expressing mutations will tend to destabilize the organismal homeostasis and thus contribute to aging. The subsequently proposed antagonistic pleiotropy hypothesis of Williams (1957) postulates that some gene alleles produce adaptive phenotypes early in life but become maladaptive late in life. Such alleles will become fixed in a population because the positive selection for the early benefit is not balanced by elimination of the late detrimental phenotype of the same genetic allele because that detrimental phenotype is only expressed at an age when selection becomes weak.
The disposable soma theory of Kirkwood (1977) emphasizes the role of metabolism over that of evolutionary constraints. If aging is the result of incomplete prevention and/or repair of damage, could the protective anti-aging processes evolve to be more effective? Kirkwood's answer is that they certainly could, but at a metabolic cost that would make the organism less competitive in comparison with organisms that spend fewer resources on longevity assurance. This is a special case of a very general “parsimony principle” in biology. Another example is provided by the fidelity of macromolecule synthesis. During DNA replication, errors are introduced at a rate of 10−8 to 10−10 per nucleotide (Lewin, 1997, p. 472), whereas the error rate of translation (protein synthesis) is much higher, 10−3 to 10−4 per amino acid (Kurland, 1992). Whereas fidelity can never be absolute, by addition of layers of proofreading mechanisms it can be improved asymptotically, but at an ever-increasing cost in free energy. For each process, there is a selective optimum, reflecting a trade-off that minimizes energy cost while maintaining the error rate at a tolerable level. What is tolerable depends on the circumstances: fewer errors are permissible in the replication of genetic material than in the synthesis of a protein that is used for a limited time and degraded, and in which a misincorporated amino acid is rarely critical (Kurland, 1992). The trade-off between accuracy and fitness has been elegantly demonstrated by experimentally increasing the fidelity of translation in bacteria (Kurland, 1992). The resulting bacterial cells produced proteins with fewer errors but were at a growth disadvantage relative to wild-type cells, not only because of the greater inherent cost of proofreading, but mainly due to the propensity of hyperaccurate ribosomes to terminate translation prematurely. The resulting truncated proteins were either degraded, wasting all resources invested in their synthesis, or were outright toxic.
The extension to aging of the principle of cost/benefit ratio optimization appears straightforward: an organism would do best investing in maintenance just enough to assure that its reproductive period matches the window of opportunity constrained by extrinsic mortality, and also investing just enough to assure that the majority of individuals do not die from intrinsic causes before the end of the reproductive period. Thus, the level of maintenance would be optimized but not maximized. However, the problem with imperfect maintenance of the genome is that each subsequent generation would inherit the damage accumulated by their parents. The compounded damage would increase the “cost of selection”, in the extreme, risking extinction of the species. The solution to this problem is specialization: the distinction that evolved between the germline and the soma.
The division of functions between germline and somatic cells has wide-ranging consequences. For example, it permitted differentiation of somatic cells into tissues, many of them post-mitotic, with highly specialized functions, a development that made possible complex, multicellular and multi-tissue organisms, including humans. In the context of aging, specialization allows an investment of resources into the maintenance of the germline that is sufficient to prevent its deterioration from generation to generation, thus assuring the survival of the species. Given the already discussed competitive constraint on the total resources that can be spent on maintenance, a preferential treatment of the germline means that somatic cells will be less protected. Indeed, the disposable soma theory postulates that somatic tissues are maintained only to an extent that allows them to survive just beyond the reproductive period (with some "reserve capacity" to assure that only a few individuals die while still reproductively active).
The dichotomy of highly protected germline and only adequately protected soma has a functional, albeit not morphological, analogy in the asymmetric division of some unicellular organisms, or even in the differences in fidelity of DNA replication and protein synthesis within a cell. In all of these cases, there is a distinction between an element (germline, rejuvenated daughter cell, information in genetic material) that is propagated indefinitely and thus needs to be highly protected, and another element (soma, mother cell, proteins and most RNA in a cell) that plays an essential but transient role, and is therefore maintained only for a limited time.
4. Challenges to the "standard hypothesis" of aging
A recurrent criticism of the standard hypothesis of aging is that some biological phenomena are best explained by an aging program, and that even a single instance of such program falsifies the standard hypothesis. However, in my opinion, these concerns are not as critical as they seem. First, phenomena interpreted as a the result of an aging program may in fact have alternative explanations. This will be discussed later on the example of semelparous fish. Second, even if an aging program could be conclusively demonstrated, perhaps in a niche situation, this would not mean that such program is the sole cause of aging. If it were, a disabling mutation in the program would completely eliminate aging, an outcome that has never been observed in species that normally age. Thus, a hypothetical identification of an aging program (unlikely in my opinion) would not disprove the existence of aging that can be explained without such program, and that accounts for the majority of known life histories.
Semelparous organisms reproduce only once, and die shortly thereafter. The most widely known and dramatic example of semelparity is that of Pacific salmon which spawn in a stream, migrate to the ocean where they spend several years, return to their natal stream to reproduce, and die within weeks due to massive degeneration of tissues, loss of immunity, and infection. These effects are caused by very high levels of the "stress hormone" cortisol (Austad, 2004). Secretion of excessive amounts of cortisol is clearly detrimental to the organism, and it could be interpreted as self-sacrifice benefiting the group (Wingfield and Sapolsky, 2003). However, elevated cortisol may improve the ability of the salmon to return to their natal stream (Carruth et al., 2002). This return (homing) depends on initial imprinting of young fish on the chemical composition of the stream, and the ability to recall the memory and register the composition through olfaction during homing. Cortisol may improve memory recall and olfaction in salmon (Carruth et al., 2002), and could therefore be highly adaptive prior to reproduction. The subsequent rapid tissue degeneration may be thus an (admittedly extreme) example of antagonistic pleiotropy.
Other life histories also appear to contradict the standard hypothesis of aging, or parts of it. For example, the premise that somatic maintenance and thus longevity should not evolve to last much beyond the reproductive period appears to be violated in some social species. However, genes that enable a contribution of older individuals to the fitness of relatives are certainly adaptive and will be selected for, except that selection acts not only at the level of the individual but also its kin (Bourke, 2007; Kirkwood, 2008b). At the other extreme, situations have been described in which individuals appear to commit “altruistic suicide” for the benefit of their descendants or even unrelated members of the group; examples include certain marsupials, perhaps the Pacific salmon (by contributing nutrients to the natal stream or lake, Larkin and Slaney, 1997; Merz and Moyle, 2006), as well as some plants and insects (Longo et al., 2005; Wingfield and Sapolsky, 2003). These arguments typically invoke group selection and therefore do not readily fit into classical theory of evolution. Even if these interpretations withstand the test of time, the rapid mortality does not need to be triggered by an aging program. “Evolved neglect”, or lack of protection, remains a viable explanation that is compatible with the standard hypothesis of aging.
5. Large life span extensions by mutation of single genes
Paradoxically, the finding that arguably launched modern gerontology, namely the discovery that mutations in single genes can greatly extend life span (Friedman and Johnson, 1988; Kenyon et al., 1993), also presented a serious challenge to the standard hypothesis of aging. The latter hypothesis posits that aging is due to incomplete protection from, or repair of, molecular damage that – if left unchecked – would compromise organismal homeostasis. There are many types of damage affecting an organism, leading to the prediction that multiple defense mechanisms evolved, each contributing only incrementally to longevity assurance. If protection against aging is a polygenic trait, mutations in any single gene should have at best a minor effect. Moreover, the types of damage that are relevant to aging were thought to differ between species, depending on the particulars of the organism's physiology and the characteristics of its typical environment. Thus, longevity assurance was predicted to be not only polygenic but also idiosyncratic (Shmookler Reis, 1989). Therefore, the discovery that hypomorphic alleles of certain genes, in particular those encoding components of the insulin/insulin-like signaling (IIS) pathway in the nematode Caenorhabditis elegans (Friedman and Johnson, 1988; Kenyon et al., 1993), extend life span several-fold, caused considerable excitement but also some consternation. It also revived hypotheses of an active aging program that can be turned off by a single mutation. In hindsight, the explanation of this surprising result appears relatively straightforward. Mechanisms evolved that sense the environmental conditions of an organism and adjust its physiology in response to external stimuli. Such mechanisms regulate adaptive behavior (including the timing and rate of reproduction) and modulate metabolism. The latter function permits, among others, the adjustment of longevity assurance processes according to environmental conditions such as crowding, lack of food, toxic chemicals, etc. (Kirkwood, 2008b). To achieve a broadly adaptive response, sensing/regulatory pathways such as IIS affect, in a coordinated way, a large number of target genes and processes. Mutations in IIS lead to remarkable effects on life span (Arantes-Oliveira et al., 2003; Ayyadevara et al., 2008) probably because they partly mimic normal IIS signaling, and thus affect a substantial subset of the normal IIS targets. However, the fascination with the huge life span gains achievable in C. elegans deflected attention from the fact that the basis of these longevity effects remains polygenic, with modest and probably partially redundant contributions from the individual effector genes. In effect, IIS constitutes an intervening regulatory layer that coordinates the actual anti-aging processes in response to metabolic and environmental cues. At the risk of being provocative, one could even claim that studies of IIS and other high-level regulatory processes are primarily concerned not with aging but with signaling circuits; longevity assurance is only one of several aspects of physiology and biochemistry modulated by IIS.
Does this diminish, in the context of aging, the importance of studying IIS and other regulatory processes such as caloric restriction, hormonal signaling from gonads, and others? Certainly not; fundamental insights have been, and will continue to be gained from such work. I will argue, however, that parallel studies of downstream effector processes are equally important. Such studies present formidable technical and conceptual challenges: effects on life span are modest, perhaps 15 to 25%, while partial redundancy of reactions complicates the interpretation of results. Given the large number of effector processes, it is difficult to decide a priori which are relevant. However, there are several compelling reasons to carry out such studies. First, downstream effector reactions act directly on compounds which would otherwise cause damage leading to aging. Thus, an understanding of these reactions may provide unique insights into the chemical basis of the aging process. Second, signaling pathways such as IIS are conserved between species in their overall logic (Kenyon, 2005) but not in functional details. For example, mutations in IIS can extend life span ten-fold in C. elegans (Ayyadevara et al., 2008) but less than two-fold in Drosophila melanogaster (Clancy et al., 2001; Tatar et al., 2001) and less still in mice (Selman et al., 2008) – although a significantly greater life span extension may result from expression of phosphoenolpyruvate carboxykinase (PEPCK-C) in murine muscle (Hakimi et al., 2007; Hanson and Hakimi, 2008), an intervention that could lower the activity of IIS. More importantly, nematodes have a single insulin/insulin-like receptor but close to forty ligands, whereas mammals have separate insulin and insulin growth factor receptors but only a few known ligands. Thus, results from invertebrate model systems are not directly transferable to mammals. In contrast, a metabolic reaction, for example a specific type of detoxification, is likely to be very similar in even distantly related species. Finally, compared with regulatory pathways such as IIS, the downstream reactions are narrower in their biochemical scope. This limits the magnitude of their individual contributions to longevity, but also minimizes side effects resulting from experimental changes of the expression of the gene. This is important in view of possible future anti-aging interventions in humans. For example, experimental alterations of insulin growth factor signaling in mice lead to multiple phenotypes (Bartke, 2008), some of them undesired – as expected for modulation of a signaling system that has multiple targets. In contrast, experimental augmentation of a detoxification reaction could (moderately) extend mammalian life span without serious side effects.
6. Is aging universal?
Aging is commonly assumed to be inevitable and to affect every individual. This is certainly true for our own species, but is not restricted to it. In fact, the universality of aging has been claimed to be formally proven for every conceivable organism (Hamilton, 1966). Therefore, reports of species that do not age represent important conceptual challenges to the field of gerontology.
It should be stressed that lack of aging does not equal immortality. Extrinsic events will eventually lead to the death of each organism, even a member of a non-aging species. Absence of aging only means that the probability of death does not increase with the age of an individual. Using this specific definition, certain species have been identified that exhibit negligible or no aging. These include the naked mole rat (Buffenstein, 2008), some turtles (Miller, 2001), rockfish and other deep ocean-dwelling organisms, both vertebrate and invertebrate (Cailliet et al., 2001; Finch, 1990), and a number of other species, in particular plants (reviewed by Baudisch, 2008, chapter 3.3.3). Most of the putative non-aging species are not as well-characterized as standard laboratory organisms used in aging research, e.g., C. elegans, D. melanogaster, or the mouse. Nevertheless, even if only some of the examples stand up to further scrutiny, the startling and, for humans, deeply fascinating finding of negligible aging requires an explanation.
Does the existence of non-aging species falsify the standard hypothesis of aging? It appears that not only there is no contradiction, but that the hypothesis can readily accommodate the data. The basic premise of the standard hypothesis of aging is that natural selection maximizes reproductive fitness. In most cases (including humans), this results in a reproductive period limited by extrinsic mortality characteristic for the environment, with longevity tracking the reproductive period. However, combinations of organismal properties and environments can be envisioned for which this “typical” life history is not optimal (Baudisch, 2005; Baudisch, 2008; Vaupel et al., 2004). In such situations, other life histories can evolve to optimize fitness, and this may include lack of aging or even negative senescence (i.e., a decrease of probability of death with age). Such outcomes have been mathematically modeled and demonstrated to be possible (Baudisch, 2005; Baudisch, 2008; Vaupel et al., 2004). For a rigorous treatment, the reader is referred to the original publications. I will present only a qualitative example to illustrate one possible scenario.
Many non-aging organisms exhibit life-long growth, in contrast to more common life histories in which growth ceases approximately at the beginning of the reproductive period. Continuous growth has several implications. One of them is a possible molecular mechanism of longevity assurance that will be discussed in section 8.3. In addition, larger body size can be of competitive advantage, at least in some environments. Considering the example of a deep-sea fish, a small individual is more likely to fall prey to a predator than a larger individual. Moreover, a larger (older) fish may have access to food sources that are out of range for a smaller individual. In fact, the metabolic reserves of rockfish, measured as liver size and amount of mesenteric fat, increase faster with body length than does body volume (Berkeley et al., 2004). Together with cellular rejuvenation due to constant growth, the additional reserves probably explain not only the greater resilience of older fish, but also the higher reproductive efficiency of older females of some fish species (Berkeley et al., 2004; Longhurst, 2008). These factors indicate that, as the fish gets older and larger, its extrinsic mortality will decrease and reproductive success will increase, leading to a positive selection for genes specifying a long reproductive period and long life. However, according to the disposable soma theory, long-term maintenance of the body may exact a prohibitive cost in resources. Thus, lack of aging would require a situation in which maintenance costs are low (Baudisch, 2008). For a deep-sea fish, an environment characterized by low temperature and low oxygen content could minimize molecular damage (Vetter and Lynn, 1997), and thus reduce the need for resources necessary for maintenance. The combination of an extrinsic mortality that decreases with age, at least in part due to life-long growth, with an efficient, low-cost damage repair may result in a life history characterized by absent or even negative senescence. Because of fragmentary data and a bias introduced to the age distribution of fish populations by human fishing activities (Berkeley et al., 2004; Cailliet et al., 2001), it is not known whether non-senescence can persist until accidental (extrinsic) death, or whether it is time-limited. A cessation of aging that affects only a specific phase of life has been identified (aging slows or may be suspended in very old individuals; discussed by Rose et al., 2006). Additional scenarios resulting in slow or absent aging have been modeled; interestingly, not all involve benign environments (Baudisch, 2008). For example, an unstable (but non-lethal) environment may favor selection for a long reproductive period and, thus, long life. This is because, in an unpredictably changing environment, reproductive success is also highly variable (Berkeley et al., 2004; Longhurst, 2008). However, in all cases the resulting mortality trajectories can be rationalized in terms of an evolutionary fitness optimization within the conceptual framework of the standard hypothesis of aging.
7. Molecular mechanisms that contribute to loss of homeostasis in aging
Historically, aging has been depicted either as a consequence of wear and tear, a manifestation of increasing entropy, an effect of random damage, i.e., spontaneous and undirected deterioration – or, longevity has been considered to be an evolved, genetically precisely controlled trait. In light of the discussion presented so far, it should be obvious that the contradiction between these two views is only apparent. In fact, both statements are true, as they apply to complementary aspects of the same phenomenon. As succinctly stated by Hayflick: "Entropy explains aging, genetic determinism explains longevity, and undefined terminology explains misunderstanding both" (Hayflick, 2007). In other words, there is damage, much of it physical or chemical in nature and, from the biological perspective, only marginally or not at all regulated, that causes a gradual loss of function and a diminished capacity to withstand environmental and internal (physiological) challenges. On the other side, there are evolved and tightly regulated processes that prevent, repair, or contain the damage. The balance of these two forces determines the life span of an individual and the mortality trajectory of a population. Therefore, it is useful to review the processes that cause damage and drive aging. This will be followed, in the next section, by a discussion of protective (longevity assurance) mechanisms, including detoxification.
7.1. Entropy-driven decay
As mentioned previously (section 3), a living system is far removed from equilibrium. If isolated, such system would maximize its entropy. Although it would be inappropriate to equate the increase of entropy with an only intuitively defined idea of disorder or chaos (Michaelides, 2008), it is still useful to conceptualize it as a loss of structures, leveling of gradients (whether concentration or energy), and thus diminished ability to do work (Michaelides, 2008). A constant flux of matter and energy, mediated by metabolism, is necessary to maintain the organism in a functional state. Thus, even without any external destabilizing forces, the mere fact that an organism is a highly organized structure far from thermodynamic equilibrium indicates that it would decay, or age, if not maintained.
7.2. Stochastic events
Genetically identical individuals diverge phenotypically even if they experience an identical environment. For example, a cohort of such individuals has a rather wide distribution of life spans (Kirkwood et al., 2005; Kirkwood and Finch, 2002; Rea et al., 2005). The phenotypic heterogeneity is in part caused by residual differences in environmental conditions that are experimentally impossible to eliminate. However, most of the heterogeneity is thought to result from random events that cause even isogenic individuals to be non-identical. The fundamental biochemical and cell biological processes that are the basis of life often involve a relatively small number of individual molecules or other functional entities. For example, many regulatory proteins are present in a cell in as few as tens or hundreds of copies (McAdams and Arkin, 1999), and mitochondria are present in a few thousands of copies. This could be compared with a glass of water which contains 1025 molecules. Positions, velocities, or orientations of individual water molecules average out over this large number, resulting in a statistically highly predictable system. Not so in a cell where chance will dictate where the few molecules of a protein are, how they associate with other cell components, and thus how they function; even slight shifts in the number can substantially change the effective concentration. Similarly, mitochondrial heteroplasmy may distribute unequally between daughter cells (Khaidakov and Shmookler Reis, 2005). These subtle fluctuations, occurring at an important place or time, may result in large macroscopic consequences (see Losick and Desplan, 2008, for a discussion of conditions under which stochastic noise can determine cell fate). In spite of tight genetic control, stochastic events prevent developmental processes from being fully deterministic, leading to a distribution of outcomes. For example, site-specific methylation status is subject to stochastic drift (Shmookler Reis et al., 1990); phenotypically, adult organ sizes, and thus function, can differ substantially between genetically identical organisms, as can the timing of development (reviewed by Kirkwood, 2008b). Effects of random fluctuations, whether developmental or acquired later in life, will generate organisms of different robustness and different life spans. Stochastic effects may be even more immediate in unicellular organisms where each daughter cell will inherit different numbers of low-abundance macromolecules, and which lack functional redundancy provided by other cells in a metazoan tissue.
7.3. Mutations
In a multicellular organism with separate germline and soma, three types of mutation can be distinguished. According to Medawar (1952), the gradual decline with age of the force of selection allows the accumulation of heritable detrimental mutations only if they are late-acting; early-acting detrimental mutations will be eliminated by natural selection. Distinct from this is the accumulation of somatic mutations (Martin et al., 1996) which affect lineages of somatic cells but are not heritable from one generation of organism to the next. Finally, mutations in mitochondrial DNA (Kujoth et al., 2005) have the potential to affect essential functions such as cellular bioenergetics and apoptosis. The role of somatic mutation accumulation in aging is a subject of debate (results reviewed by Kirkwood, 2005; Vermulst et al., 2007; Wiesner et al., 2006), and a discussion of this complex question is beyond the scope of this review. However, insofar as they play a role in aging, mutations could facilitate the formation of destabilizing metabolites, including but not limited to oxidants and radicals (Harman, 1956), or they could interfere with the defense against endo- and xenobiotic damage. The latter effect could be due to loss-of-function mutations in protective enzymes, or to destabilization of regulatory pathways that control such enzymes. Thus, a potential progeric role of mutations is compatible with the standard hypothesis of aging.
7.4. Oxidative stress
Significantly more free energy (usable to do metabolic work) can be derived from full oxidation of food, for example oxidation of glucose to CO2 and H2O, than from fermentative processes, such as conversion of glucose to ethanol and CO2 during which parts of the starting molecule are reduced and other parts are oxidized in a balanced reaction. Full oxidation requires an external electron acceptor, oxygen for most organisms. Evolutionary acquisition of the ability to use oxygen for this purpose made possible the variety and complexity of extant life (Lane, 2002), but carried with it the risk of cell damage. Metabolism involving oxygen, particularly aerobic respiration but also other processes, generate as by-products several oxidants and radicals, known collectively as “reactive oxygen species” or ROS. Radicals centered on atoms other than oxygen, especially on nitrogen, are also formed (“reactive nitrogen species” or RNS). These reactive compounds assumed a variety of biologically essential regulatory functions. Thus, if present in excess, ROS and RNS not only cause untargeted damage to cellular macromolecules, also disrupt signaling and regulatory pathways (Packer and Cadenas, 2007). Detrimental oxidation and radical-mediated reactions have been proposed to be at the core of the aging process. The early formulation of the free radical theory of aging (Harman, 1956) has been later refined (for example Balaban et al., 2005; Beckman and Ames, 1998). In particular, it is now widely accepted that the hypothesis that oxidative and other damage is a simple consequence of total aerobic metabolism integrated over the lifetime ("rate of living" theory, Pearl, 1928) is an oversimplification. Although oxidative damage is unavoidable, regulated biochemical mechanisms can limit it (see, for example, Echtay and Brand, 2007) or, in some cases, reverse it (Stadtman, 2006). Thus, there is no linear relationship between total mitochondrial metabolism and oxidative damage (Hulbert et al., 2004). However, even in its refined form, the oxidative stress theory of aging remains one of the most controversial topics in gerontology. On one hand, ample evidence has been presented, both within and between species, that longevity is associated with lower production of ROS and/or lower oxidative damage to macromolecules (Barja, 2004; Pletcher et al., 2007; Sanz et al., 2006; Sohal et al., 1993b; Sohal et al., 1993a; Sohal et al., 2002). However, counter-examples abound. The long-lived naked mole rat does not have elevated antioxidant defenses (Andziak et al., 2005) and accumulates significantly higher levels of oxidative damage than mice that have a much shorter life span (Andziak et al., 2006). Long-lived ant queens have lower SOD levels than short-lived workers (Parker et al., 2004). Similarly, a comparison of gene expression between honey bee queens and workers indicates that queen longevity is due to factors other than antioxidant defenses (Corona et al., 2005). Although the list could be expanded, the above examples are sufficient to illustrate the lack of consensus in this area of research.
More than most research areas, gerontology is plagued by correlations that are not helpful in inferring causation, and may even be misleading. Direction of causality can usually be established by experimental manipulation of the variables of interest. However, even this powerful approach failed to produce unequivocal answers regarding oxidative damage and aging. For example, administration of SOD mimetics increased life span of C. elegans in some (Kim et al., 2008; Melov et al., 2000) but not in other experiments (Keaney et al., 2004), and had no effect in the housefly (Bayne and Sohal, 2002). Deletion of SODs in C. elegans demonstrated that resistance to oxidative stress and longevity are not necessarily coupled (Honda et al., 2008). In human clinical trials that used incidence of chronic diseases rather than aging as the endpoint, a variety of antioxidants had variable and unpredictable effects, and some were actually harmful (Bjelakovic et al., 2007; Howes, 2006). Similarly, results obtained by transgenic expression of antioxidant enzymes were inconsistent. In early experiments, transgenic expression in D. melanogaster of Cu,Zn-SOD, alone or together with catalase, was reported to extend life span (Orr and Sohal, 1994; Parkes et al., 1998; Phillips et al., 2000; Sun and Tower, 1999), but more recent data showed no effect (Orr et al., 2003; Orr and Sohal, 2003). Targeting catalase to mitochondria extended the life span of mice (Schriner et al., 2005), as did ubiquitous overexpression of thioredoxin (Mitsui et al., 2002) or of metallothionein (Yang et al., 2006). However, ubiquitous expression of SOD1 actually shortened life span, albeit only slightly (Huang et al., 2000).
The interpretation of the seemingly contradictory results is not straightforward, but several relevant aspects begin to emerge. One is the complexity of oxidative stress, a phenomenon due to a variety of chemical species that have distinct functions and effects. As already mentioned, this includes signaling (Packer and Cadenas, 2007), but also random damage to cell constituents. Thus, experimental lowering of oxidative stress will have the beneficial effect of containing damage but may, at the same time, disrupt signaling. The overall outcome could be destabilizing or protective, depending on the physiological context. Therapeutic administration of antioxidant carries with it another risk. An "antioxidant" is a compound capable of being oxidized, sparing a biologically important molecule in the process. However, the now oxidized form of the drug may be capable of oxidizing other cell components, thus reversing its intended role. It is therefore more fruitful to think of antioxidants in terms of their chemical nature: compounds capable of entering redox reactions. The direction of the reaction will be determined by the redox potential of the drug and of its biological reaction partners, as well as by the concentrations of both, in turn affected by the hydrophobicity of the antioxidant and thus its partitioning among the various cellular compartments. Consequently, the outcome is a complex function of the biological context and of pharmacokinetic and chemical properties of the antioxidant. Yet another complicating factor is the variety of compounds contributing to oxidative stress. Superoxide, H2O2, the hydroxyl radical, and singlet oxygen are all examples of ROS, but they have vastly different chemical and biological properties. In specific physiological contexts and at specific sites, ROS have protective functions which may be attenuated by antioxidants (Salganik, 2001); such attenuation could contribute to aging-related damage accumulation. Finally, as will be discussed in more detail in section 8.6, moderate levels of ROS contribute to adaptive (hormetic) upregulation of protective enzymes that are part of the longevity assurance mechanism. Overall, supplementation with an antioxidant is a rather blunt tool applied to an exceedingly complex system, perhaps explaining the failure of most such interventions (Bjelakovic et al., 2007; Howes, 2006); the wrong compound at the wrong level, wrong time, and wrong site can be quite harmful, or at least ineffective. Many of the same considerations apply to antioxidant enzymes. The classical example is overexpression of SOD, an antioxidant enzyme that converts superoxide to H2O2 and O2. In the absence of a sufficient capacity to metabolize the resulting H2O2, toxicity could result because the biological properties of H2O2 are very different from those of superoxide. In fact, cytosolic Cu,Zn-SOD is also present in the intermembrane space of mitochondria where it can be detrimental (Goldsteins et al., 2008)
Complete disruption of SOD1 expression in D. melanogaster leads to serious deleterious effects, including a drastic reduction of life span. Strikingly, transgenic restoration of as little as 5% of wild-type SOD activity rescues life span, indicating that SOD is normally present in excess with regard to the longevity phenotype (Mockett et al., 2003a). It could be hypothesized that such excess capacity is not eliminated by natural selection because it provides protection against spikes of oxidative stress that may be infrequent or absent under laboratory conditions, but would be lethal were they to occur in a more challenging environment. Such interpretation could have far-reaching consequences for the understanding of the role of oxidative damage in aging, or at least in determination of life span. The prediction would be that antioxidant defenses are crucial, and limiting, in preventing acute injury or in rescuing the effects of pathologies, such as may be present in short-lived strains of experimental animals. This prediction is supported by experimental results (Magwere et al., 2006; Mockett et al., 2003b; Muller et al., 2007; Orr et al., 2003, and others). It can be further predicted that, because of the high antioxidant capacity that evolved to deal with rare but potentially serious episodes of oxidative stress, oxidative damage would accumulate only slowly under protected laboratory conditions, and would be largely irrelevant to aging in such environment. Moreover, even relatively large experimental shifts, up or down, of antioxidant defenses would not be expected to have a significant effect on life span. Thus, the conspicuous lack of strong experimental evidence in favor of the oxidative/free radical theory of aging may have evolutionary reasons, in addition to our relative ignorance as to the types, sites, and timing of oxidative damage that could truly contribute to aging.
7.5. Electrophilic stress
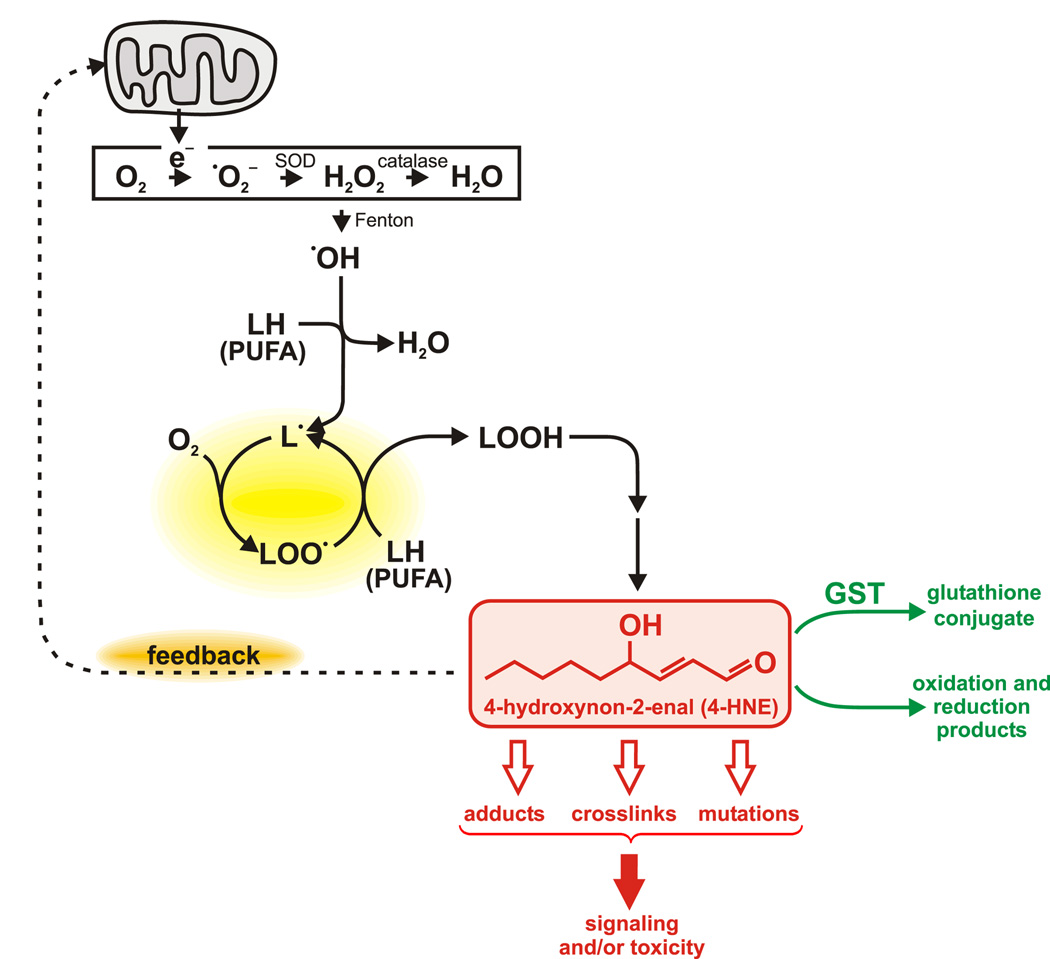
The reaction of ROS with polyunsaturated fatty acids (PUFAs) results in a chain reaction (Gutteridge and Halliwell, 1990) that amplifies the original event (Fig. 1). The products of the process, lipid hydroperoxides, can be converted (Schneider et al., 2004, 2008; Sun and Salomon, 2004) into a variety of α,β-unsaturated aldehydes of which 4-hydroxynon-2-enal (4-HNE) (Poli et al., 2007) is the most abundant and prototypical example (Fig. 1). Specifically, n-6 PUFA give rise to 4-HNE, whereas n-3 PUFA are the precursors of other α,β-unsaturated aldehydes (Guichardant et al., 2006; Long et al., 2008). At physiological concentrations, 4-HNE is a signaling molecule that modulates a variety of fundamental biological processes (reviewed by Barrera et al., 2008; Dwivedi et al., 2007; Leonarduzzi et al., 2004; Petersen and Doorn, 2004). The compound exerts its biological effects mainly by forming adducts at nucleophilic sites in proteins (Fig. 2). At higher levels of 4-HNE, more proteins form adducts at a wider range of target sites (Uchida and Stadtman, 1992; Uchida et al., 1993; Vila et al., 2008), leading to toxicity (Esterbauer et al., 1991). In addition, 4-HNE can be mutagenic (Singh et al., 2005).
Fig. 1.
Schematic representation of the formation and functions of the lipid peroxidation product 4-HNE. Electrons leaking from the mitochondrial respiratory chain to molecular oxygen lead to the formation of superoxide which can be converted to other ROS. ROS can be generated by other processes (e.g., Krause, 2006) but mitochondria are their major source. Via the Fenton reaction, transition metals catalyze the conversion of H2O2 to the hydroxyl radical which can abstract a hydrogen from a polyunsaturated fatty acid (PUFA) molecule (denoted as LH), generating a carbon-centered radical. The latter initiates a chain reaction provided there is a supply of oxygen and additional PUFAs (yellow highlight). The resulting lipid hydroperoxides (LOOH) can be converted to electrophilic aldehydes such as 4-HNE. These aldehydes are metabolized (green lettering) by glutathione conjugation or redox reactions (reduction of the aldehyde group or of the carbon-carbon double bond, or oxidation of the aldehyde group). Otherwise, 4-HNE can react with cellular macromolecules, chiefly proteins (Fig. 2), changing their activity. This can modulate signaling or cause toxicity. Targets of 4-HNE include mitochondrial proteins, leading to either a negative or positive feedback loop (orange highlight): activation of mitochondrial uncoupling proteins by 4-HNE decreases ROS production (Echtay and Brand, 2007; Echtay et al., 2003; Wolkow and Iser, 2006), but damage to components of the respiratory chain increases generation of ROS (Lee et al., 2006; Uchida, 2003). The latter process could additionally amplify the action of 4-HNE by triggering a higher level of lipid peroxidation. The concentration of 4-HNE may determine whether negative or positive feedback predominates.
Fig. 2.
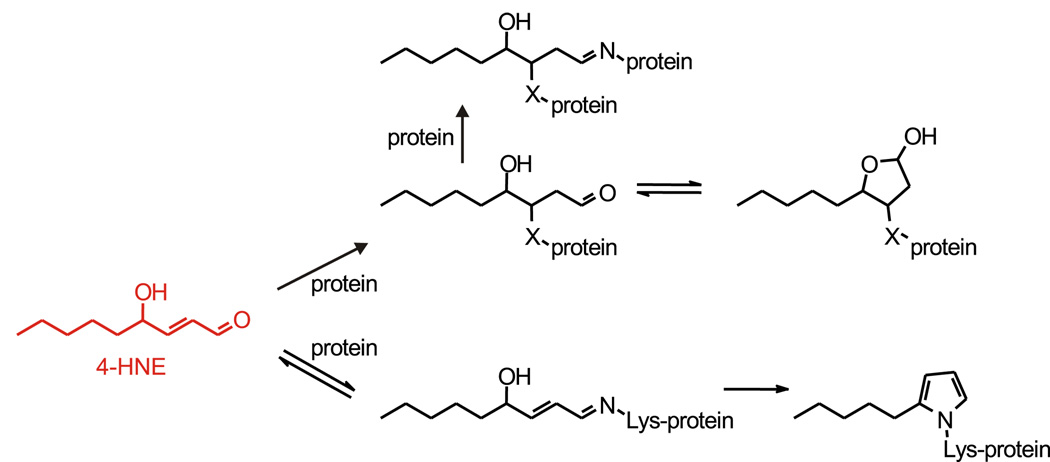
Major reactions of 4-HNE with proteins. Upper part of scheme: the predominant reaction is a Michael addition of a nucleophilic center (side chain of cysteine, histidine, or lysine) in a protein to the double bond of 4-HNE. Subsequently, the resulting adduct can cyclize to a hemiacetal, or the aldehyde group of the 4-HNE moiety of the adduct can form a Schiff base with an amino group on the same or another protein, causing protein cross-linking. Lower part of scheme: a less common reaction is the formation of a Schiff base of the aldehyde group of 4-HNE with a lysine side chain in a protein, followed by dehydration and cyclization to a pentylpyrrole adduct. The scheme is based on (Liu et al., 2003).
Although adduct formation on lysine side chains can be chemically reversible, that on histidines and cysteines is not (Liu et al., 2003). No enzymatic mechanisms are known for the removal of 4-HNE covalently bound to proteins. Proteins that are moderately derivatized by 4-HNE can be turned over more rapidly than native proteins (Carbone et al., 2004), but proteins more extensively derivatized or crosslinked by 4-HNE inhibit the proteasome (Friguet, 2006; Friguet and Szweda, 1997; Grune and Davies, 2003), as does 4-HNE itself by direct modification of the proteasome (Farout et al., 2006). Thus, proteins that carry 4-HNE adducts combine an altered function with resistance to degradation. Modification by 4-HNE is therefore an attractive candidate for damage that contributes to aging. It is likely, however, that the importance of 4-HNE transcends its ability to inflict generalized, non-targeted damage. As already mentioned, 4-HNE is a signaling molecule that conveys the information that oxidative damage, and hence lipid peroxidation, has occurred. 4-HNE is well-suited for such signaling function because its chemical properties are very different from those of ROS. Unlike most oxidants and radicals, 4-HNE reacts with a protein fairly selectively and permits a specific and reproducible change in the protein’s properties. 4-HNE adducts could be thus viewed not as damage but as posttranslational modifications that regulate function. In fact, some proteins carrying 4-HNE adducts gain, rather than lose, activity; phosphoinositide-specific phospholipase C (Maggiora and Rossi, 2003; Rossi and Dianzani, 2000) and L-type Ca2+ channels (Akaishi et al., 2004) may serve as examples. The specific regulation of individual proteins suggests that 4-HNE, in addition to causing undirected damage to large sets of targets, may modulate signaling pathways, including those relevant to aging.
If the formation of 4-HNE is a consequence of lipid peroxidation, it could be surmised that 4-HNE concentrations should be directly proportional to the level of oxidative stress. If so, the arguments laid out in the preceding section that life span is probably not limited by the accumulation of oxidative damage, at least under laboratory conditions, should also apply to electrophilic modifications caused by 4-HNE and similar lipid peroxidation products. This would negate a role of 4-HNE in aging. I will, however, argue that this is not the case because of the unique chemistry of 4-HNE formation.
The near-saturating amount of antioxidants means that any further increase in antioxidants, even if substantial, would have only an incremental effect on the residual ROS levels. However, even a low concentration of ROS will initiate the lipid peroxidation chain reaction which will propagate as long as PUFAs and oxygen are present (Fig. 1). In other words, a small “seed” amount of ROS, likely to persist even in the presence of additional antioxidants, could be sufficient to generate a substantial amount of lipid peroxidation products such as 4-HNE. The level of resulting peroxidation products would depend strongly on the availability and quantities of PUFAs, but is expected to be less sensitive to the amount of initiating ROS. In this context it is worth noting that life span among vertebrates correlates not only with ROS production, but also with the degree of lipid unsaturation and the position of the double bonds (Barja, 2004; Hulbert, 2005; Hulbert et al., 2007; Pamplona et al., 2002; Sanz et al., 2006). Wild-derived mice live longer in a protected laboratory environment than “domesticated” but genetically heterogeneous animals (laboratory mice interbred to prevent inbreeding depression) (Miller et al., 2002). The shorter-lived laboratory mice have a higher content of n-3 PUFAs in membrane phospholipids than the longer-lived wild-derived animals (Hulbert et al., 2006). The naked mole rat, which lives for almost 30 years in spite of a substantial accumulation of oxidative damage (Andziak et al., 2006), has a significantly lower level of the n-3 PUFA, DHA (docosahexaenoic acid) than mice (Mitchell et al., 2007) which live one-tenth as long. In addition, naked mole rats have a higher abundance of ether-linked phospholipids (plasmalogens) in their membranes (Mitchell et al., 2007). The vinyl ether linkage in plasmalogens is highly susceptible to an attack by ROS; therefore, these lipids can act as radical scavengers (Brosche and Platt, 1998; Gorgas et al., 2006; Kuczynski and Reo, 2006) and thus prevent the lipid peroxidation chain reaction. Honey bee queens, which share their genome with workers but outlive them by a factor of ten or more (Heinze and Schrempf, 2008), have membranes that contain less PUFAs and are thus less susceptible to peroxidation than membranes of worker bees (Haddad et al., 2007). Similarly, offspring of human nonagenarians have a lower content of PUFAs in erythrocyte membranes than control subjects matched with respect to age and other variables (Puca et al., 2008). The above examples are persuasive but still correlative in nature. However, direct evidence has been obtained for a cause-effect relationship between fatty acid unsaturation and molecular damage that could contribute to aging. The fatty acid composition of mitochondria was altered in rats by dietary intervention, resulting in a higher double bond index (a measure of unsaturation; Pamplona et al., 2004). This led to elevated lipid peroxidation and increased formation of aldehyde-protein adducts, protein carbonyls, and other forms of molecular damage in mitochondria of heart (Herrero et al., 2001) as well as brain and liver (Pamplona et al., 2004). In a complementary experiment, damage caused by the lipid peroxidation product malondialdehyde was prevented in Arabidopsis thaliana plants in which the synthesis of PUFAs was eliminated by genetic disruption of fatty acid desaturases (Mene-Saffrane et al., 2007).
Unsaturated fatty acids have multiple functions. Some fatty acids, including arachidonic (an n-6 PUFA) (Funk, 2001) and others (Watts and Browse, 2006), are specific precursors for biosynthetic pathways that lead to potent regulatory molecules. Competition between individual fatty acids for the enzymes that convert them to signaling compounds may account for the antagonistic biological roles of n-6 and n-3 PUFAs in processes such as inflammatory signaling, lipid metabolism, metabolic syndrome, and others (Schmitz and Ecker, 2008). Stearoyl-CoA desaturase SCD2 (and thus presumably one or more species of unsaturated fatty acids) is required for PPARγ induction and adipogenesis in the murine 3T3-L1 adipocyte cell line (Christianson et al., 2008). An unrelated function of unsaturated fatty acids that are part of membrane phospholipids is the modulation of membrane fluidity, a parameter essential for membrane function (Los and Murata, 2004; Marguet et al., 2006). In this context, the molecular shape is of primary importance. The first double bond introduced into a fatty acid molecule is near the center of the chain. A double bond in this position causes a packing defect that increases membrane fluidity. The contribution to fluidity of additional double bonds (located closer to the ends of the chain) becomes progressively smaller (Brenner, 1984; Pamplona et al., 2002).
A variety of unsaturated fatty acids is synthesized or obtained from the diet (species differ in their capacity to elongate and desaturate fatty acids, Hashimoto et al., 2008) to satisfy the sometimes conflicting biochemical and physiological needs. For example, membrane fluidity must be well-controlled to assure proper membrane function; this requires a high content of unsaturated fatty acids. However, unsaturated fatty acids, especially PUFAs, are prone to peroxidation. The ensuing damage may be tolerated in short-lived species but could become limiting in longer-lived organisms. Such organisms may evolve other strategies that assure an appropriate membrane fluidity, for example a shift from PUFAs to mono-unsaturated fatty acids, to shorter-chain fatty acids, or changes in the level of other relevant components such as sterols. Similar trade-offs may occur in other areas of metabolism in which unsaturated fatty acids participate. Thus, within the framework of the standard hypothesis of aging, a shift of lipids to a lower double bond index, or perhaps more importantly, a shift to a lower membrane peroxidation index (Hulbert et al., 2007), constitutes a longevity-assurance mechanism that slows down the accumulation of damage relevant to aging. The role of unsaturated fatty acids in aging, and their relationship to other life span-limiting factors, is the topic of a recent review (Hulbert et al., 2007).
Interestingly, n-3 PUFAs are more susceptible to peroxidation than n-6 PUFAs (Hulbert, 2005), suggesting a case of “antagonistic pleiotropy” on the metabolic rather than genetic level: the protective role of n-3 PUFAs (Calder, 2006; Fritsche, 2006; Lombardo and Chicco, 2006) during most of life span may be partially offset by accelerated aging due to increased lipid peroxidation.
In the context of aging mechanisms, the data summarized above could be interpreted in terms of a partial loss of function of a biological membrane due to peroxidation of unsaturated fatty acids. For example, altered ion permeability of the membrane could disturb tissue homeostasis and eventually contribute to aging. It is equally possible that increased formation of lipid peroxidation products, particularly electrophilic aldehydes such as 4-HNE, leads – via modification of cellular proteins – to untargeted damage, changes in signal transduction, lipofuscin-mediated inhibition of autophagy (section 7.6), or a combination of these mechanisms. Evidence in favor of a pro-aging role of 4-HNE will be discussed in section 8.5.
7.6. Other endogenously generated destabilizing factors
Lipofuscin
A conspicuous correlate of aging is the accumulation in tissues of typically brown pigments (Sulzer et al., 2008), collectively known as "age pigments". Although age pigments are a highly heterogeneous mixture, two types can be distinguished: advanced glycation end-products (AGE), formed by reaction of amino acid side chains in proteins with reactive intermediates derived from carbohydrates (a subset of the Maillard or browning reaction, Baynes, 2001), and lipofuscin (reviewed by Beckman and Ames, 1998). Lipofuscin is the result of adduct formation and crosslinking of proteins by lipid peroxidation products, including 4-HNE, malondialdehyde, and others (Itakura et al., 2000; Schutt et al., 2003; Tsai et al., 1998; Uchida, 2006). Many age pigments are fluorescent. Their heterogeneity results in a broad emission spectrum; for example, in C. elegans, fluorescence emission is centered at 430 nm but covers a range of 350 to almost 600 nm (Fig. S1 and Table S1 in Gerstbrein et al., 2005). This "autofluorescence" creates major technical problems in the experimental use of fluorescent probes, but provides a conveniently measurable and important marker of aging. The question remains unresolved whether lipofuscin is a consequence or a cause of aging. Traditionally, it has been assumed that that the pigment is a byproduct of the aging process (reviewed by Beckman and Ames, 1998). However, the "lysosomal-mitochondrial axis" hypothesis (Kurz et al., 2007; Terman, 2006) postulates that lipofuscin causally contributes to aging. According to this model, lipofuscin forms in lysosomes (Brunk et al., 1992) but is refractory to degradation by these organelles; the accumulating lipofuscin-loaded, dysfunctional lysosomes constitute a sink for newly synthesized lysosomal enzymes, and thus prevent efficient autophagy of mitochondria and other cellular components. Autophagy is an important longevity assurance mechanism (Bergamini et al., 2007; Hansen et al., 2008), indicating that lipofuscin may be causally linked to aging. In this context, the involvement of 4-HNE in lipofuscin generation (e.g., Schutt et al., 2003) suggests that the lysosomal mechanism could contribute to the pro-aging affect of 4-HNE.
Lipofuscin consists of proteins modified and crosslinked by lipid peroxidation products. Accordingly, lipofuscin accumulation has been thought to be associated with oxidative stress that triggers lipid peroxidation (reviewed by Beckman and Ames, 1998). However, studies in C. elegans revealed that mutants that overproduce ROS do not accumulate more lipofuscin than a wild-type strain (Gerstbrein et al., 2005). This apparent inconsistency can be resolved by the argument laid out in section 7.5: if antioxidant defenses are present at levels that are sufficient or in excess of what is needed under all but the most extreme conditions, the levels of 4-HNE – the compound that is relevant to lipofuscin formation – will be relatively insensitive to changes in ROS concentration.
Protein aggregation
Biological synthetic processes typically attract more and earlier attention of researchers than degradation reactions. For example, development was studied well before gerontology became a serious branch of biology, and cell proliferation was researched prior to apoptosis. Similarly, the elucidation of protein synthesis became an early cornerstone of modern molecular biology, with the study of the mechanisms and regulation of protein degradation lagging behind. The latter example illustrates the working and preferences of the human mind more than the relative scientific importance of the topics: it is obvious that, at a steady-state as present in post-mitotic cells, overall protein synthesis must approximately equal protein degradation, and failure of either is highly detrimental. Mechanisms by which unfolded proteins are shielded from aggregation, stabilized, and refolded, and – if beyond repair – proteins and even entire organelles are degraded, mostly by the proteasome and by autophagy, are of fundamental importance for aging. The discussion of these processes is beyond the scope of the present article; the reader is referred to numerous excellent reviews covering the relevance to aging of heat shock proteins and their regulation, autophagy, and proteasomal function. However, two points are relevant in the context of the role of detoxification in aging. One is that in vivo aggregation of proteins may lead to toxicity. Prions, amyloid β peptide, proteins containing polyglutamine tracts, and mutant SOD are examples of proteins that are prone to aggregation as a consequence of a conformational change or partial unfolding. The aggregates are frequently refractory to degradation, form persistent fibrils or plaques, and cause severe neuropathologies. The second point is that the net stabilization free energy of most proteins is as low as 5 to 20 kcal/mol (Somero, 1995). This could be compared with the free energy of ATP hydrolysis under typical conditions within a cell, -12 kcal/mol (Nelson and Cox, 2000, p. 501), illustrating that the hydrolysis of a single ATP molecule may suffice to unfold a protein molecule, given an appropriate mechanism. The relatively low stability of proteins evolved for functional reasons (Somero, 1995), but it also means that even mild stimuli such a moderate shift in temperature (heat shock), interaction with other macromolecules, binding of heavy metals, or covalent modifications are able to change conformation/unfold a protein at least partially and initiate aggregation. Adduct formation with 4-HNE could serve as an example of such a destabilizing modification. In fact, reaction of 4-HNE with the amyloid β peptide induces fibril formation (Liu et al., 2008). Given the large number of different proteins in a cell, it appears likely that many if not most reactive compounds (toxicants) will change the properties of some proteins in a way leading to a detrimental outcome. This could manifest itself as acute toxicity, or as a gradual accumulation of damage that results in aging. The wide spectrum of toxicants acting on a variety of targets suggests that there may not be a single, or even predominant, cause of damage that is relevant to aging. Instead, there could be a wide range of mechanisms with additive effects.
Reactive compounds of intermediary metabolism
Life is a complex but basically chemical process. Therefore, it must have evolved to make use of metabolites that are able to enter into the required chemical reactions. In fact, it has been postulated that extant metabolism is not one of many possible models that happened to have been fixed by evolution, but that its emergence is a largely determinate consequence of the constraints of organic chemistry in an aqueous environment (Morowitz et al., 2000; Smith and Morowitz, 2004). Some metabolic reactions require rather high intrinsic reactivity of the compounds involved. The examples of ROS, RNS, and electrophiles have been already discussed. In special situations, reactivity can be further increased or modified: neutrophils generate ROS via NADPH oxidase (Krause, 2006) and use ROS to form hypochlorite (Mikkelsen and Wardman, 2003; Winterbourn and Kettle, 2000), the active compound in household bleach – for a purpose similar to that desired at home, namely killing of invading microorganisms. Nevertheless, it could be argued that the reactive compounds mentioned above originally formed as by-products or end-products of biological reactions. Even though some acquired secondary functionality, they could be viewed as being outside of "core" metabolism. Similarly, the damaging presence of unbound iron or copper ions could be considered an accident rather than part of normal metabolism. However, many compounds that participate in intermediary metabolism are highly reactive because this is required by their function. Examples include redox-active sulfhydryl compounds and coenzyme Q, thioesters, mixed anhydrides of organic acids with phosphoric acid, sugars with free keto but especially aldehyde groups, and some prosthetic groups of enzymes. Certain metabolites are reactive not because of their function but because they are unavoidable in a metabolic pathway. For example, tyrosinemia type I, a defect in tyrosine catabolism, causes accumulation of fumarylacetoacetate and is associated with early childhood hepatocellular carcinomas (Ashorn et al., 2006; Nakamura et al., 2007). Fumarylacetoacetate and related metabolites are α,β-unsaturated carbonyl compounds that may undergo a Michael addition much like 4-HNE, and therefore have the capacity to damage proteins. In a wild-type background, fumarylacetoacetate will not reach high levels but will be still present at a finite concentration, possibly causing cumulative perturbations that contribute to aging. Reactive intermediates are also formed in the course of detoxification. For example, polycyclic aromatic hydrocarbons are converted by cytochrome P-450/epoxide hydrolase action to reactive diol epoxides which are detoxified by conjugation (Hu et al., 1999; Hu et al., 1997). Diol epoxides that escape conjugation may form adducts with DNA, accounting for their carcinogenicity. In addition to specific chemical reactivity, metabolism-linked microenvironments such as low pH in lysosomes or in the lumen of the stomach may contribute to damage accumulation and aging.
The balance between metabolite reactivities that are sufficient to assure efficient metabolism but low enough to prevent destabilizing side reactions involves trade-offs. In the context of aging, damage resulting from such side reactions will be tolerated as long as most individuals survive throughout a reproductive period that was evolutionarily optimized given the prevalent extrinsic mortality. Should metabolism-inflicted damage become life-limiting, mechanisms can evolve to contain it. Examples of such mechanisms include channeling of metabolites between enzymes, shielding reaction intermediates from bulk solution, or creating microenvironments and specialized reaction compartments within organelles. I propose, however, that these mechanisms evolve not to minimize damage, but to optimize the balance between investment of resources and the resulting protective effect. Thus, to a certain extent, metabolism will remain self-destructive. This is a broader reformulation of the rate-of-living theory, embracing not only oxidative damage but core intermediary metabolism itself.
7.7. Xenobiotics
Many external toxicants are man-made. Pervasive environmental pollution may result in concentrations of these compounds that are sub-toxic but nevertheless significant in the context of aging. However, the number of industrial chemicals is probably dwarfed by "natural" xenobiotics, i.e., compounds of biological origin. This has a good reason. Plants, many microorganisms, and some animals compete (in the Darwinian sense) using chemistry rather than behavior. Therefore, these organisms produce a mind-numbing variety of toxicants, noxious chemicals, odorants, and other compounds that evolved specifically to harm, repel, or otherwise modify the behavior of predators and competitors. Darwinian selection led to substances that act by a variety of mechanisms, often involving highly specific interference with a particular target, such as an enzyme or a receptor, although the broad chemical classes of oxidants, free radical generators, and electrophiles found among endogenously produced toxicants are also represented among xenobiotics (Gregus, 2008). To achieve their protective purpose, at least vis-à-vis animals, most xenobiotics have to act acutely. At lower concentrations some of these compounds could lead to an accumulation of damage that, in the long term, may contribute to aging. However, it appears most likely that destabilizing compounds that may limit life span act via the broader mechanisms of covalent modification of macromolecules involving electrophilic attack and perhaps redox and free radical-mediated reactions. In this mechanistic respect and in the context of aging, there is little functional difference between endogenously produced compounds and xenobiotics.
7.8. Proximal causes of death
As mentioned at the outset of this section, death results from accumulation of uncompensated damage that compromises homeostasis severely enough to cause the failure of an essential vital function. A question of considerable mechanistic interest is whether death is the consequence of a stressor, such as a disease pathology or a toxicant, that acts on the weakened organism and "pushes it over the edge", or whether, if left alone in a protected environment, the organism will nevertheless die "from natural causes". A distinction has been made between age-related diseases and "natural aging" (reviewed by Kirkwood, 2008a). I would argue that such a distinction is artificial. Obviously, extreme situations are easy to classify. It would be entirely unreasonable to attribute to aging, a death caused by a direct hit by a meteorite: organismal robustness and homeostasis are irrelevant in this context. However, getting hit by a car is less straightforward. Quite possibly, cognitive or motor dysfunction increases the probability of a person stepping in front of a moving vehicle. Similarly, dying from an infection may indicate that the affected individual lost part of immune or other protective function and is more susceptible to disease. The loss of cognitive abilities or of immune protection with age could be attributed to accumulating tissue damage, and any resulting deaths could be subsumed under the "aging-related" category. Nevertheless, such deaths are usually classified as due to a road accident or to infectious disease because the contribution of the external cause is perceived to be greater than that of intrinsic susceptibility. At the other end of the spectrum, an aged and very frail organism may succumb to a challenge so subtle that it may be difficult to identify. In this view, both destabilizing stimuli and organismal robustness form continua; death always requires a stimulus, but occurs only if the stimulus exceeds resistance. This would mean that the experimental gold standard, namely a "natural" life span limited only by intrinsic properties of an organism and not curtailed by pathology or accident, can be asymptotically approached by providing increasingly sheltered environments, but never attained. The problem is compounded by uncertainty as to what constitutes a sheltered environment. For example, individually housed mice are deprived of social interactions but are shielded from stress and potential fighting; germ-free animals are protected from infections but can have a drastically altered physiology (Backhed et al., 2007; Bajzer and Seeley, 2006). This indicates that life span, a surrogate measure of aging, can only be determined in an arbitrarily chosen environment and will be influenced by that environment. This does not negate the value of life span measurements but affects their interpretation.
8. Longevity assurance: mechanisms for prevention, repair, or containment of damage
The evolutionary and genetic considerations summarized in the opening sections of this review provide essential information on the logic of the biological processes that modulate aging, and on their theoretical constraints. The basic tenets of the standard hypothesis of aging are derived from evolutionary considerations, in particular, the conclusion that aging is not driven by an active genetic program but is the consequence of imperfect maintenance that, in turn, is a by-product of evolutionary optimization of reproductive fitness. As important as these conclusions are in providing a conceptual framework to the field, they afford only limited insight into the molecular mechanisms of aging. Elucidation of these mechanisms requires a combination of evolutionary and genetic approaches with molecular biology and, in particular, with biochemistry. The resulting synergism is at the root of recent exciting advances in gerontology, and promises to yield further insights.
Destabilizing processes and reactions that negatively impact homeostasis and fitness of an organism, and thus shorten life span, have been summarized in the preceding section of this review. In this section, I will discuss mechanisms that have the capacity to prevent such damage or minimize its effects on life span. These longevity assurance mechanisms are genetically determined and evolutionarily selectable. Their elucidation is therefore not only of fundamental importance in the understanding of the aging process, but also it opens the possibility of life span-extending interventions.
8.1. Replacement of damaged macromolecules
Modifications of proteins (by oxidation of amino acid side chains, covalent adduct formation, non-covalent but strong binding of heavy metals, or even thermal motion that can lead to partial unfolding) are potentially detrimental. These harmful effects can be, however, minimized by replacing the affected proteins. Although catabolism of 4-HNE-modified GAPDH by cathepsin G has been reported (Tsuchiya et al., 2007), the major pathways for degradation of damaged proteins appear to be the ubiquitin-proteasome pathway and autophagy. Both are major longevity assurance processes (Ghazi et al., 2007; Hansen et al., 2008)
It is tempting to think that because of turnover, modified proteins become a non-issue. This could be true in terms of acute toxicity and cell death, but may not apply to aging. In the steady-state resulting from ongoing damage to proteins and concomitant turnover, a finite concentration of modified proteins will be present in cells. In the case of some modifications, e.g., 4-HNE adduct formation, the partial inhibition of degradative pathways (see section 7.5) will increase the steady-state level of the modified protein, although perhaps still below the toxicity threshold. However, even sub-toxic levels are likely to have an impact on cell performance. In keeping with the previous discussion, this detrimental effect is more likely to be caused by the presence of a partially unfolded or otherwise changed protein than by a simple loss of function; therefore, higher synthesis of the protein would be unable to compensate. Suboptimal performance of a critical tissue may lower organismal fitness and increase the probability of death. In dividing cells, modifications of low-abundance regulatory proteins would increase the "stochastic noise" and, by mechanisms summarized in section 7.2, may give rise to clones of deficient cells, again increasing the likelihood of death. Thus, damage to proteins can accelerate aging even in the absence of long-term accumulation of persistent adducts.
8.2. Repair of damaged macromolecules
Although counter-examples exist such as reduction of methionine sulfoxide (Friguet, 2006; Moskovitz, 2005; Stadtman, 2006) or reduction of disulfide bonds, covalently modified proteins are rarely repaired. The reason for this probably lies in the chemical diversity of amino acid side chains, and thus a multiplicity of possible modifications. A separate enzyme system would be needed to repair each kind of damage, even assuming that the information required to reverse the defect were available. The metabolic cost of maintaining extensive protein repair systems would likely exceed the (still appreciable) cost of replacing the affected proteins. Moreover, as discussed above (section 3), proteins tolerate well the variability that results from amino acid misincorporation, and are probably similarly insensitive to most chemical modifications. Finally, changes to proteins are not heritable and thus of limited impact. In contrast, consequences of damage to DNA are more serious, and multiple DNA repair mechanisms exist. DNA repair is an essential longevity assurance mechanism (for example, see Vijg, 2008). However, the topic is beyond the scope of the present review.
8.3. Sorting of damaged macromolecules
It has been recently shown that E. coli divides asymmetrically, sorting oxidatively damaged proteins into a "mother" cell which eventually ceases to reproduce (Desnues et al., 2003; Nystrom, 2007; Stewart et al., 2005). A similar mechanism is active in the yeast S. cerevisiae where protein carbonyls as well as toxic rDNA circles are preferentially retained by the mother cell (Aguilaniu et al., 2003; Sinclair and Guarente, 1997). Such segregation of damaged macromolecules permits a re-setting of the physiological age of the progeny, and thus constitutes, for the daughter cell, an efficient longevity assurance mechanism which, in this particular case, obviates the need for macromolecule repair or replacement, but requires cell division. Obviously, the process is consistent with the logic of the disposable soma theory (Kirkwood, 1977), and may be its evolutionary predecessor. Once such a strategy was established, it would confer similar (arguably, greater) benefits to multicellular organisms, including mammals, where the germline is functionally immortal. A different mechanism has been proposed, however, for the rejuvenation of mammalian offspring (Hernebring et al., 2006). According to this model, gametes contain damaged macromolecules at a level characteristic of the adult (parental) organism, but early embryonic development includes a phase of intense proteasomal degradation of such macromolecules. It is not clear to what extent the proposed macromolecule replacement mechanism accounts for the re-setting of the biological age of mammalian offspring. Embryogenesis involves rapid cell division as well as widespread apoptosis, suggesting that sorting into cells destined for elimination could also contribute.
Sorting of damaged molecules could play a role in non-aging species that continue to grow throughout life (section 6). A tissue that contains dividing cells could maintain at least some of these cells in a rejuvenated state through damage sorting. Aged “mother cells” may undergo apoptosis, assuring undiminished functionality of the tissue.
8.4. Minimizing molecular damage
The replacement, repair, or sorting mechanisms described above apply to situations in which macromolecular damage has already occurred. An alternative strategy of longevity assurance would be to prevent the formation of potentially damaging toxicants, or to intercept (detoxify) them before they modify cellular macromolecules.
The conceptual impact of the discoveries that dietary restriction and inhibition of IIS extend life span derives in large measure from the realization that genetically determined mechanisms can limit molecular damage. In other words, accumulation of molecular damage is not a passive and inevitable consequence of metabolism (as originally postulated by the rate-of-living hypothesis), but is subject to regulation. The genetic switches that up- or downregulate such protective mechanisms on an organismal, rather than evolutionary, level (Kirkwood, 2005) respond to sensory inputs that report the status of the environment, and to metabolic and hormonal inputs that reflect the state of the organism itself. The switches are thought to determine the allocation of limiting resources between reproduction and maintenance (Kirkwood, 2005; Kirkwood, 1977). This interpretation has been recently refined in D. melanogaster: relative to shorter-lived, fully-fed flies, animals with a life span extended by caloric restriction actually invested fewer carbon and nitrogen resources to the soma in absolute terms, but had a higher ratio of resources devoted to the soma relative to those invested in egg production (O'Brien et al., 2008). This corroborates previous evidence indicating that egg production is a major detrimental factor in insect longevity (Mockett and Sohal, 2006), but does not alter the conclusion that life span will be extended as long as somatic maintenance outweighs reproductive damage, irrespective of the absolute levels of both.
What is the mechanistic meaning of devoting more resources (whether in absolute or relative terms) to somatic maintenance? In other words, how can an organism minimize molecular damage? Obviously, there are many strategies that could be deployed to achieve that end. Limiting the supply of toxicants is an attractive option. Activation of uncoupling proteins has been postulated to decrease the production of ROS (Echtay and Brand, 2007; Echtay et al., 2003; Parker et al., 2008; Wolkow and Iser, 2006) (Fig. 1), although this mechanism has been questioned (Bezaire et al., 2007; Shabalina et al., 2006). A strategy of major importance is to lower the content of unsaturated fatty acids, especially PUFAs, in membranes (section 7.5), and it appears that evolved differences in fatty acid composition may explain a substantial component of the disparate life spans of species. Acute responses, e.g., to caloric restriction, on an organismal rather than evolutionary time scale, do not seem to utilize this mechanism; short-term shifts in fatty acid composition are more likely aimed at stabilizing membrane fluidity, especially in poikilotherms. The limited potential to restrict the supply of toxicants or their precursors emphasizes the importance of intercepting them once they are formed, i.e., detoxification.
Detoxification, or detoxication, denotes a large set of reactions that typically lower the toxicity and increase water solubility of a wide range of endogenous and xenobiotic compounds. Traditionally, these reactions have been grouped in phases. Phase I entails the introduction of a functional group, often by cytochrome P450-catalyzed hydroxylation, that may decrease or actually increase toxicity. In phase II, the newly-introduced or a pre-existing functional group is conjugated with a polar moiety to reduce toxicity and increase solubility. Phase III involves transport of the conjugate out of the cell, typically via an ABC pump. However, it has been pointed out (Josephy et al., 2005; Parkinson and Ogilvie, 2008) that the above categorization has too many exceptions to be useful. Moreover, such classification would incorrectly imply that the reactions are unique to detoxification, and that detoxifying enzymes are distinct from those participating in general metabolism. In reality, many enzymes catalyze transformations of both endogenous core metabolites and toxic xenobiotics. To avoid an artificial separation of detoxification from metabolism, it is more useful to think of detoxifying enzymes in terms of the usual biochemical convention, i.e., to group them according to the reaction they catalyze (Josephy et al., 2005). Selected reactions relevant to detoxification are summarized in Table 1. Only the major reactions are shown, and the classification can be ambiguous. For example, the assignment of peroxidases depends on the point of view of the reader. Peroxidases could be listed in the "oxidation" category if the reaction of interest is, e.g., the oxidation of chloride to hypochlorite, or in the "reduction" category if the focus is on reduction of lipid hydroperoxides. Similarly, the distinction between oxidants and electrophiles is somewhat arbitrary. An electrophile seeks and is able to accept electrons, which makes it by definition an oxidant. Nonetheless, in a biological setting, the roles of electrophiles and oxidants differ. Imprecisely, electrophiles could be defined as electron-deficient compounds/functional groups that form a chemical bond with a nucleophilic center, whether covalent (as in 4-HNE adducts) or non-covalent (as in glutathione-heavy metal ion complexes). In contrast, oxidants exchange electrons and perhaps hydrogen with a reductant, but no lasting chemical bond is formed. Many of the proteins listed in Table 1, in particular some members of the CYP and GST groups but also some SDRs, act not only in detoxification but also participate in a wide range of biosynthetic processes. Finally, only categories of enzymes are shown in Table 1. Each category consists of multiple proteins or even classes of proteins (Hayes et al., 2005; Hoffmann and Maser, 2007; Parkinson and Ogilvie, 2008; Zimniak and Singh, 2006). The reasons are not clear for the existence of multiple detoxifying enzymes (for example, as many as 44 GSTs in C. elegans) with overlapping but nevertheless non-identical substrate specificities. A possible interpretation is based on the physiological role of these enzymes: functionally, they constitute a "chemical immune system" that must be able to deal with a wide spectrum of substrates, including those it has not previously encountered, without interfering with non-toxic endogenous metabolites. A single, versatile enzyme would increase the latter danger, whereas many specific ones would be too costly to maintain. The evolved optimal number of enzymes in each category is probably co-determined by the prevalence of xenobiotics in the typical environment of each species. Rapid gene duplication (actually stimulated by toxicant stresses, Schimke, 1984), and subsequent adaptive evolution of the replicated genes, is initially a divergent process, but may ultimately result in multiple isozymes assuming specificity for a given substrate by convergent evolution rather than via common ancestry. This seems to be the case for catalytic efficiency for 4-HNE in GSTs of mammals (murine versus human GSTA4-4, Zimniak, 2006), C. elegans (Ayyadevara et al., 2007), and D. melanogster (Sigma and Delta-class GSTs, Sawicki et al., 2003; Singh et al., 2001), and may be a general phenomenon. However, in spite its simplification and ambiguities, Table 1 may serve as a guide to the most common detoxification processes.
Table 1.
Major types of biochemical reactions relevant to detoxification. The examples of enzymes and, in particular, of reactions are for illustration only; the list is not meant to be complete. Based on (Hoffmann and Maser, 2007; Josephy et al., 2005; Parkinson and Ogilvie, 2008; Zimniak, 2006).
| Process | Enzyme | Common acronym | Example reactions |
|---|---|---|---|
| Oxidation | cytochrome P450 | CYP | Hydroxylation of aromatic rings; hydroxylation of steroids |
| alcohol dehydrogenase | ADH | Conversion of ethanol to acetaldehyde | |
| aldehyde dehydrogenase | ALDH | Conversion of acetaldehyde to acetic acid | |
| peroxidase | Oxidation of an organic, e.g., glutathione, or inorganic compound with concomitant reduction of an organic hydroperoxide or of H2O2 | ||
| Reduction | aldo-keto reductase | AKR | Reduction of aldehydes, e.g., aldoses or 4-HNE, to alcohols |
| short chain dehydrogenase/reductase | SDR | Reduction of keto group in doxorubicin; conversion of cortisone to cortisol | |
| Hydrolysis | epoxide hydrolase | Conversion of naphthalene epoxide to naphthalene Diol | |
| Conjugation of nucleophiles | UDP-glucuronosyltransferase | UGT | Glucuronidation of naphthol (on hydroxyl group), of aniline (on amino group), of thiophenol (on sulfhydryl group), of bilirubin (on carboxyl group) |
| sulfotransferase | SULT | Sulfonation (sulfation) of phenols and aliphatic alcohols | |
| methyltransferase | Methylation of hydroxyl group in L-DOPA; of imidazole nitrogen in histamine | ||
| acetyltransferase | Acetylation of aromatic amines and of substituted hydrazine | ||
| Conjugation of electrophiles | glutathione transferase | GST | Glutathione conjugation at electrophilic carbon atom in: 1-chloro-2,4-dinitrobenzene (halogen displacement), 4-HNE (Michael addition), epoxide (ring opening) |
8.5. Which detoxification reactions are relevant to longevity assurance?
The detoxification reactions discussed in the preceding section have been originally characterized in the context of classical toxicology (Gregus, 2008; Parkinson and Ogilvie, 2008). A priori, it is not obvious which, if any, of these reactions are relevant to longevity assurance. In fact, initial emphasis in gerontology was on antioxidant defenses, a category that, toxicologically, is of some but not of central interest. The question of gerontological relevance of detoxification can be addressed in two ways: (i) via modulating, by biochemical or genetic intervention, individual reactions and recording the effects on life span, and (ii) through a global, unbiased examination of shifts in gene expression between a normal- and long-lived states of an organism, i.e., between physiological situations in which the investment of resources into somatic maintenance is expected to be low and high, respectively. The latter approach was taken by Gems and colleagues in a series of elegant studies. Initially, they identified classes of genes whose transcription was altered in two long-lived forms of C. elegans: the dauer larva, and hypomorphic daf-2 mutants (Gems and McElwee, 2005; McElwee et al., 2004). More recently, this approach was broadened to include C. elegans, D. melanogaster, and two strains of mice (McElwee et al., 2007). For all three organisms, physiological states were examined in which impaired IIS signaling results in life span extension. The cross-species comparison has the power to identify "public" (widespread, perhaps universal), as opposed to "private" (species-specific), determinants of longevity.
In the context of toxicology, the striking result of the above studies was that extended life span correlated with a significantly higher transcript level of certain, but not all, categories of genes encoding detoxification enzymes (McElwee et al., 2007). For GSTs, transcript levels were increased in all four experimental systems (nematodes, flies, and two mouse strains), with group P < 0.0005 for all systems except worms where P < 0.005. The evidence was almost as strong for short chain dehydrogenases/reductases (SDRs), although their transcripts did not reach significance in the Ames mouse. Finally, cytochromes P450 were increased with high significance in long-lived worms and flies, but less so in mice (P < 0.05 for the Ames mouse, and not significant in the Little mouse). No other group of detoxification genes was reported to be consistently elevated in the longer-lived state. In particular, SOD and catalase, major antioxidant enzymes involved in decomposition of ROS, were increased in some but not all of the species that were compared (McElwee et al., 2007). These data suggest two generalizations. One is based on the observation that, among the enzymes listed in Table 1, only cytochromes P450, GSTs, and SDRs are significantly involved (besides detoxification) in the synthesis of endogenous metabolites, particularly steroid hormones and signaling molecules derived from arachidonic acid. Therefore, changes in the levels of these enzymes could affect the hormonal status of the organism, and thus longevity. The second generalization is biochemical. The strongest correlate of longevity is the increased expression of GSTs, the only category of detoxification enzymes that evolved specifically to remove electrophiles. Even though UDP-glucuronosyltransferases were highly expressed in long-lived nematodes (McElwee et al., 2004), none of the several groups of nucleophile-conjugating enzymes (Table 1) are consistently elevated in all three species. Together, these data indicate that it is not general toxicity, and in particular not oxidative stress, but specifically electrophilic toxicity that may shorten life span. This conclusion is consistent with the increase in SDR expression. The latter enzymes are able to reduce carbonyl groups, and many carbonyl-containing compounds are electrophilic; the strongly electrophilic aldehyde 4-HNE can be reduced by aldo-keto reductases (Srivastava et al., 1999) which are functionally (albeit not structurally) related to SDRs.
The most abundant intracellular electrophiles (Marnett et al., 2003), such as 4-HNE, are derived from peroxidation of PUFAs. High levels of PUFAs in membranes correlate with short life span (section 7.5), but strong anti-electrophile defenses are associated with longevity (McElwee et al., 2007). The above information suggests a causal link leading from high PUFA to high lipid peroxidation, to high 4-HNE, and to shortened life span. A key role of lipid peroxidation in limiting life span is also consistent with the lack of a clear correlation of longevity with antioxidant enzymes (McElwee et al., 2007). As discussed in section 7.5, lipid peroxidation appears to be primarily dependent on the availability of PUFAs but relatively insensitive to the amount of initiating ROS.
Conclusions based on transcript levels, such as determined by microarray analysis, may be weakened by the imperfect correlation between steady-state transcript and protein concentrations. However, the microarray-based hypothesis that GSTs modulate life span (McElwee et al., 2007) is fully consistent with results of targeted assays of the amount and function of relevant proteins. The level of CeGSTP2-2 (product of the gst-10 gene), one of several C. elegans GSTs with appreciable catalytic efficiency for 4-HNE (Ayyadevara et al., 2007; Ayyadevara et al., 2005b), strongly correlates with the median life span of a series of daf-2 hypomorphic mutants (Ayyadevara et al., 2005a). Moreover, glutathione conjugating activity for 4-HNE also correlates with life span in several congenic lines of C. elegans (Ayyadevara et al., 2005b), and extremely long-lived age-1 null mutants are resistant to acute toxicity of 4-HNE (Ayyadevara et al., 2008), suggesting that they have an increased capacity to metabolize the compound.
The results summarized above are highly suggestive, but nevertheless are based on correlative data. Direct evidence for a cause-effect relationship between 4-HNE and life span can be obtained by experimental manipulation of the organism so as to increase or lower the endogenous concentration of 4-HNE. We have done this by RNAi silencing of gst-10 (Ayyadevara et al., 2005a), or by transgenic overexpression of the gst-10 gene product or of the murine mGSTA4-4 (Ayyadevara et al., 2005b), an enzyme that also has high conjugating activity for 4-HNE (Zimniak et al., 1994). A composite of the results is shown in Fig. 3. As expected, enzymatic activity of 4-HNE conjugation was reduced as a consequence of gst-10 silencing, but was increased when the GSTs were overexpressed (Fig. 3A). The increment of 4-HNE-conjugating activity was greater for CeGSTP2-2 than for mGSTA4-4, in spite of the higher catalytic efficiency of the latter. This was probably due to a much lower expression level of the heterologous protein. The expression of both GSTs was driven by the gst-10 promoter, and both proteins assumed native tissue distribution (Ayyadevara et al., 2005b). The amount of 4-HNE-protein adducts was inversely proportional to enzymatic activity (Fig. 3B). This is important because the biological functions of 4-HNE are thought to be mediated mainly via covalent modification of proteins. Finally, the median life span was directly proportional to enzyme activity and inversely proportional to adduct levels (Fig. 3C), confirming a role of 4-HNE in modulating longevity. It is worth noting that the above results at least partially conform to the recent postulate that the contribution of specific variables to aging be evaluated using the concept of metabolic control analysis (Murphy and Partridge, 2008). This approach requires that the variable in question is changed in a series of small steps, and that the effect of these changes on mortality is determined. Moreover, the variable should remain within a range permitted by normal physiology because unreasonable extremes may cause mortality that does not reflect normal aging. We feel that these criteria are largely satisfied by the four-point “dose-response” curve presented in Fig. 3, and that this curve provides more information than the more typical binary intervention experiment in which the comparison is, for example, between the presence and absence of a protein.
Fig. 3.
Composite figure illustrating the effect of 4-HNE on median life span of C. elegans. Worms were either subjected to RNAi against gst-10 (Ayyadevara et al., 2005a) or made transgenic for murine mGsta4 or for gst-10 (Ayyadevara et al., 2005b). Panel A shows 4-HNE-conjugating activity in worm homogenates; panel B, the level of 4-HNE-protein adducts; and panel C, the median life span of the lines. Asterisks denote statistical significance versus control.
The experiments, described above, in which the tissue level of 4-HNE was experimentally modulated, establish that there is a causal link between 4-HNE and life span. However, the mechanism of this effect remains to be characterized. As mentioned previously, 4-HNE could inflict untargeted electrophilic damage to multiple proteins, causing general deterioration and loss of homeostasis. Alternatively or in addition, 4-HNE modification of a specific regulatory protein, e.g., a component of IIS, may be most relevant to aging. Characterization of the underlying mechanisms will require a careful biochemical dissection of the system.
8.6. Hormesis
In the preceding discussion, it was tacitly assumed that more of a potentially toxic compound, for example an electrophile, leads to more damage and destabilization, and thus to accelerated aging. Although this chain of events is probably true in most cases, it is modulated and blunted by adaptive responses or, according to the recommended nomenclature (Calabrese et al., 2007), "chemical conditioning hormesis". In a hormetic response, a low (sub-toxic) dose of a potentially harmful compound, which can be endogenous or external, elicits a defensive response, e.g., induction of a detoxification enzyme. The presence of such enzyme affords protection against a subsequent high, or even lethal, dose of the toxicant. The concept of hormesis was originally developed in the context of toxicology (reviewed by Calabrese and Baldwin, 2003) but has been also applied to aging (for example, Lithgow et al., 1995) in which a hormetic response affords protection not against a lethal single dose of a poison, but against a constant, low-level exposure. In this sense, hormesis is a protective mechanism that can minimize damage relevant to aging. The concept of hormesis has become prominent in the recent gerontological literature (Cypser and Johnson, 2002; Cypser et al., 2006; Gems and Partridge, 2008; Hercus et al., 2003; Olsen et al., 2006; Parsons, 2007; Rattan et al., 2004; Schulz et al., 2007).
The well-documented finding that mild stressors can extend life span indicates that maximal longevity may be achieved more readily by optimization of many (but not all) toxicants and stressors –rather than by their elimination (Gems and Partridge, 2008). This further compounds the previously discussed problem of defining "normal life span" (section 7.8): should this be determined under extremely artificial conditions that minimize stress as much as possible, or does a protected environment run the risk of curtailing life by preventing hormesis? How much and what kind of stress maximizes life span?
9. Conclusions
Aging is a multifactorial and complex phenomenon. However, a broad consensus is emerging that describes the major features of aging. According to this consensus, stochastic events as well as chemical damage that can originate from the environment or from the organism's own metabolism, and that can be essentially random or affecting specific regulatory targets, contribute to a gradual deterioration of organismal function which increases the probability of death. The destabilizing forces are essentially chemical or physical in nature and cannot be biologically controlled. However, even in the face of these perturbations, organisms can delay the loss of homeostasis by several mechanisms that are genetically controlled, subject to evolutionary selection, and that present potential targets for life-extending interventions.
Various types of destabilizing forces, and of cognate protective mechanisms, have been proposed. A major question in current gerontology is which of the many possible types of damage are most relevant to aging. Mounting evidence points to toxicants, i.e., compounds that are sufficiently reactive to interfere with the metabolism and the structures of an organism. Former emphasis on oxidative stress as the most relevant of these toxicants has lessened with the realization that important toxicants include electrophiles, largely derived from peroxidation of polyunsaturated lipids but also from other types of metabolism, as well as other reactive carbonyl compounds. This shift of emphasis highlights metabolic detoxification enzymes as essential longevity assurance mechanisms. In particular, glutathione transferases that conjugate electrophiles, enzymes capable of reducing carbonyl groups, and cytochromes P450 have been implicated as components of a fundamental (perhaps universal) longevity-assurance strategy. The key regulatory systems that modulate longevity, including insulin/insulin-like signaling and dietary restriction, may in part work by inducing the expression of broad classes of the above detoxifying enzymes.
Many theories of aging have been proposed, and most suffer from an overly narrow view resulting in an emphasis on a single mechanism thought to explain a very complex biological process. It is important to avoid repeating this error by assuming that detoxification will provide a universal answer. However, mounting evidence indicates that it is a significant and underappreciated contributor to longevity assurance.
Acknowledgment
The author is supported by NIH grants R01 AG18845 (to P.Z.) and P01 AG20641 (to Robert J. Shmookler Reis). The author is a recipient of a VA Research Career Scientist Award. The work discussed in this review and the conceptual framework of the field originated in laboratories too numerous to identify specifically; I apologize for any omissions. I thank Drs. John C. Field, Robert J. Shmookler Reis, and Ludwika Zimniak for critical reading of the manuscript and for comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- Akaishi T, Nakazawa K, Sato K, Saito H, Ohno Y, Ito Y. Modulation of voltage-gated Ca+ current by 4-hydroxynonenal in dentate granule cells. Biol. Pharmaceut. Bull. 2004;27:174–179. doi: 10.1248/bpb.27.174. [DOI] [PubMed] [Google Scholar]
- Andziak B, O'Connor TP, Buffenstein R. Antioxidants do not explain the disparate longevity between mice and the longest-living rodent, the naked mole-rat. Mech. Ageing Dev. 2005;126:1206–1212. doi: 10.1016/j.mad.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Andziak B, O'Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Antebi A. Genetics of aging in Caenorhabditis elegans. PLoS Genet. 2007;3:e129. doi: 10.1371/journal.pgen.0030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Berman JR, Kenyon C. Healthy animals with extreme longevity. Science. 2003;302:611. doi: 10.1126/science.1089169. [DOI] [PubMed] [Google Scholar]
- Ashorn M, Pitkanen S, Salo MK, Heikinheimo M. Current strategies for the treatment of hereditary tyrosinemia type I. Pediatr. Drugs. 2006;8:47–54. doi: 10.2165/00148581-200608010-00004. [DOI] [PubMed] [Google Scholar]
- Austad SN. Retarded senescence in an insular population of Virginia opossums (Didelphis virginiana) J. Zool. 1993;229:695–708. [Google Scholar]
- Austad SN. Is aging programed? Aging Cell. 2004;3:249–251. doi: 10.1111/j.1474-9728.2004.00112.x. [DOI] [PubMed] [Google Scholar]
- Austad SN, Kristan DM. Are mice calorically restricted in nature? Aging Cell. 2003;2:201–207. doi: 10.1046/j.1474-9728.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell. 2008;7:13–22. doi: 10.1111/j.1474-9726.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Dandapat A, Singh SP, Beneš H, Zimniak L, Shmookler Reis RJ, Zimniak P. Lifespan extension in hypomorphic daf-2 mutants of Caenorhabditis elegans is partially mediated by glutathione transferase CeGSTP2-2. Aging Cell. 2005a;4:299–307. doi: 10.1111/j.1474-9726.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Dandapat A, Singh SP, Siegel ER, Shmookler Reis RJ, Zimniak L, Zimniak P. Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal. Mech. Ageing Dev. 2007;128:196–205. doi: 10.1016/j.mad.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyadevara S, Engle MR, Singh SP, Dandapat A, Lichti CF, Beneš H, Shmookler Reis RJ, Liebau E, Zimniak P. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell. 2005b;4:257–271. doi: 10.1111/j.1474-9726.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajzer M, Seeley RJ. Physiology: Obesity and gut flora. Nature. 2006;444:1009–1010. doi: 10.1038/4441009a. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Barrera G, Pizzimenti S, Dianzani MU. Lipid peroxidation: control of cell proliferation, cell differentiation and cell death. Mol. Aspects Med. 2008;29:1–8. doi: 10.1016/j.mam.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Bartke A. Impact of reduced IGF-1/insulin signaling on aging in mammals: Novel findings. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2008.00387.x. in press. [DOI] [PubMed] [Google Scholar]
- Baudisch A. Hamilton's indicators of the force of selection. Proc. Natl. Acad. Sci. USA. 2005;102:8263–8268. doi: 10.1073/pnas.0502155102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudisch A. Inevitable aging?: contributions to evolutionary-demographic theory. Berlin: Springer; 2008. http://www.springer.com/economics/population/book/978-3-540-76655-1; http://www.demogr.mpg.de/books/drm/004/ [Google Scholar]
- Bayne ACV, Sohal RS. Effects of superoxide dismutase/catalase mimetics on life span and oxidative stress resistance in the housefly, Musca domestica. Free Radic. Biol. Med. 2002;32:1229–1234. doi: 10.1016/s0891-5849(02)00849-3. [DOI] [PubMed] [Google Scholar]
- Baynes JW. The role of AGEs in aging: causation or correlation. Exp. Gerontol. 2001;36:1527–1537. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Bergamini E, Cavallini G, Donati A, Gori Z. The role of autophagy in aging: its essential part in the anti-aging mechanism of caloric restriction. Ann. N.Y. Acad. Sci. 2007;1114:69–78. doi: 10.1196/annals.1396.020. [DOI] [PubMed] [Google Scholar]
- Berkeley SA, Hixon MA, Larson RJ, Love MS. Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries. 2004;29:23–32. [Google Scholar]
- Berry RJ, Bronson FH. Life history and bioeconomy of the house mouse. Biol. Rev. 1992;67:519–550. doi: 10.1111/j.1469-185x.1992.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Bezaire V, Seifert EL, Harper ME. Uncoupling protein-3: clues in an ongoing mitochondrial mystery. FASEB J. 2007;21:312–324. doi: 10.1096/fj.06-6966rev. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat. Rev. Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. J. Am. Med. Assoc. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Paradoxes of aging. Cell Cycle. 2007a;6:2997–3003. doi: 10.4161/cc.6.24.5124. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Program-like aging and mitochondria: instead of random damage by free radicals. J. Cell. Biochem. 2007b;102:1389–1399. doi: 10.1002/jcb.21602. [DOI] [PubMed] [Google Scholar]
- Bourke AFG. Kin selection and the evolutionary theory of aging. Annu. Rev. Ecol. Evol. Syst. 2007;38:103–128. [Google Scholar]
- Bredesen DE. The non-existent aging program: how does it work? Aging Cell. 2004;3:255–259. doi: 10.1111/j.1474-9728.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- Brenner RR. Effect of unsaturated acids on membrane structure and enzyme kinetics. Prog. Lipid Res. 1984;23:69–96. doi: 10.1016/0163-7827(84)90008-0. [DOI] [PubMed] [Google Scholar]
- Brosche T, Platt D. The biological significance of plasmalogens in defense against oxidative damage. Exp. Gerontol. 1998;33:363–369. doi: 10.1016/s0531-5565(98)00014-x. [DOI] [PubMed] [Google Scholar]
- Brunet-Rossinni AK, Austad SN. Ageing studies on bats: a review. Biogerontology. 2004;5:211–222. doi: 10.1023/B:BGEN.0000038022.65024.d8. [DOI] [PubMed] [Google Scholar]
- Brunk UT, Jones CB, Sohal RS. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat. Res. 1992;275:395–403. doi: 10.1016/0921-8734(92)90042-n. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B. 2008 doi: 10.1007/s00360-007-0237-5. in press. [DOI] [PubMed] [Google Scholar]
- Cailliet GM, Andrews AH, Burton EJ, Watters DL, Kline DE, Ferry-Graham LA. Age determination and validation studies of marine fishes: do deep-dwellers live longer? Exp. Gerontol. 2001;36:739–764. doi: 10.1016/s0531-5565(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu. Rev. Pharm. Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu S-Z, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SIS, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Doorn JA, Petersen DR. 4-hydroxynonenal regulates 26S proteasomal degradation of alcohol dehydrogenase. Free Radic. Biol. Med. 2004;37:1430–1439. doi: 10.1016/j.freeradbiomed.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Carruth LL, Jones RE, Norris DO. Cortisol and Pacific salmon: a new look at the role of stress hormones in olfaction and home-stream migration. Integr. Comp. Biol. 2002;42:574–581. doi: 10.1093/icb/42.3.574. [DOI] [PubMed] [Google Scholar]
- Chapuisat M, Keller L. Division of labour influences the rate of ageing in weaver ant workers. Proc. R. Soc. London Ser. B. 2002;269:909–913. doi: 10.1098/rspb.2002.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JL, Nicoloro S, Straubhaar J, Czech MP. Stearoyl-CoA desaturase 2 is required for peroxisome proliferator-activated receptor γ expression and adipogenesis in cultured 3T3-L1 cells. J. Biol. Chem. 2008;283:2906–2916. doi: 10.1074/jbc.M705656200. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Corona M, Hughes KA, Weaver DB, Robinson GE. Gene expression patterns associated with queen honey bee longevity. Mech. Ageing Dev. 2005;126:1230–1238. doi: 10.1016/j.mad.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J. Geront. Ser. A: Biol. Sci. & Med. Sci. 2002;57:B109–B114. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans. Exp. Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Grey AD. Calorie restriction, post-reproductive life span, and programmed aging: a plea for rigor. Ann. N.Y. Acad. Sci. 2007;1119:296–305. doi: 10.1196/annals.1404.029. [DOI] [PubMed] [Google Scholar]
- Desnues B, Cuny C, Gregori G, Dukan S, Aguilaniu H, Nystrom T. Differential oxidative damage and expression of stress defence regulons in culturable and non-culturable Escherichia coli cells. EMBO Rep. 2003;4:400–404. doi: 10.1038/sj.embor.embor799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi S, Sharma A, Patrick B, Sharma R, Awasthi YC. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep. 2007;12:4–10. doi: 10.1179/135100007X162211. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Brand MD. 4-hydroxy-2-nonenal and uncoupling proteins: an approach for regulation of mitochondrial ROS production. Redox Rep. 2007;12:26–29. doi: 10.1179/135100007X162158. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, Brand MD. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Farout L, Mary J, Vinh J, Szweda LI, Friguet B. Inactivation of the proteasome by 4-hydroxy-2-nonenal is site specific and dependant on 20S proteasome subtypes. Arch. Biochem. Biophys. 2006;453:135–142. doi: 10.1016/j.abb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Finch CE. Longevity, senescence, and the genome. Chicago: University of Chicago Press; 1990. [Google Scholar]
- Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006;580:2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Friguet B, Szweda LI. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett. 1997;405:21–25. doi: 10.1016/s0014-5793(97)00148-8. [DOI] [PubMed] [Google Scholar]
- Fritsche K. Fatty acids as modulators of the immune response. Annu. Rev. Nutr. 2006;26:45–73. doi: 10.1146/annurev.nutr.25.050304.092610. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Gems D, McElwee JJ. Broad spectrum detoxification: the major longevity assurance process regulated by insulin/IGF-1 signaling? Mech. Ageing Dev. 2005;126:381–387. doi: 10.1016/j.mad.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Gems D, Partridge L. Stress-response hormesis and aging: "That which does not kill us makes us stronger". Cell Metabol. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Gerstbrein B, Stamatas G, Kollias N, Driscoll M. In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell. 2005;4:127–137. doi: 10.1111/j.1474-9726.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- Ghazi A, Henis-Korenblit S, Kenyon C. Regulation of Caenorhabditis elegans lifespan by a proteasomal E3 ligase complex. Proc. Natl. Acad. Sci. USA. 2007;104:5947–5952. doi: 10.1073/pnas.0700638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith TC. Aging as an evolved characteristic - Weismann's theory reconsidered. Med. Hypoth. 2004;62:304–308. doi: 10.1016/S0306-9877(03)00337-2. [DOI] [PubMed] [Google Scholar]
- Goldsmith TC. Aging, evolvability, and the individual benefit requirement; medical implications of aging theory controversies. J. Theor. Biol. 2008 doi: 10.1016/j.jtbi.2008.02.035. in press. [DOI] [PubMed] [Google Scholar]
- Goldsteins G, Keksa-Goldsteine V, Ahtoniemi T, Jaronen M, Arens E, Akerman K, Chan PH, Koistinaho J. Deleterious role of superoxide dismutase in the mitochondrial intermembrane space. J. Biol. Chem. 2008;283:8446–8452. doi: 10.1074/jbc.M706111200. [DOI] [PubMed] [Google Scholar]
- Gompertz B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. Lond. 1825;115:513–585. doi: 10.1098/rstb.2014.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgas K, Teigler A, Komljenovic D, Just WW. The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim. Biophys. Acta. 2006;1763:1511–1526. doi: 10.1016/j.bbamcr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. Signaling networks in aging. J. Cell Sci. 2008;121:407–412. doi: 10.1242/jcs.021519. [DOI] [PubMed] [Google Scholar]
- Gregus Z. Mechanisms of toxicity. In: Klaassen CD, editor. Casarett and Doull's Toxicology: The Basic Science of Poisons. 7th ed. New York: McGraw-Hill Medical; 2008. pp. 45–106. [Google Scholar]
- Griffiths AJF, Miller JH, Suzuki DT, Lewontin RC, Gelbart WM. An introduction to genetic analysis. 6th ed. New York: W.H. Freeman; 1996. [Google Scholar]
- Grune T, Davies KJA. The proteasomal system and HNE-modified proteins. Mol. Aspects Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- Guarente L. Mitochondria - a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichardant M, Bacot S, Moliere P, Lagarde M. Hydroxy-alkenals from the peroxidation of n-3 and n-6 fatty acids and urinary metabolites. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:179–182. doi: 10.1016/j.plefa.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Gutteridge JMC, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. (TIBS) 1990;15:129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Haddad LS, Kelbert L, Hulbert AJ. Extended longevity of queen honey bees compared to workers is associated with peroxidation-resistant membranes. Exp. Gerontol. 2007;42:601–609. doi: 10.1016/j.exger.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Hakimi P, Yang J, Casadesus G, Massillon D, Tolentino-Silva F, Nye CK, Cabrera ME, Hagen DR, Utter CB, Baghdy Y, Johnson DH, Wilson DL, Kirwan JP, Kalhan SC, Hanson RW. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J. Biol. Chem. 2007;282:32844–32855. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD. The moulding of senescence by natural selection. J. Theor. Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C. A role for autophagy genes in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson RW, Hakimi P. Born to run; the story of the PEPCK-C(mus) mouse. Biochimie. 2008 doi: 10.1016/j.biochi.2008.03.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Yoshizawa AC, Okuda S, Kuma K, Goto S, Kanehisa M. The repertoire of desaturases and elongases reveals fatty acid variations in 56 eukaryotic genomes. J. Lipid Res. 2008;49:183–191. doi: 10.1194/jlr.M700377-JLR200. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu. Rev. Pharm. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Entropy explains aging, genetic determinism explains longevity, and undefined terminology explains misunderstanding both. PLoS Genet. 2007;3:e220. doi: 10.1371/journal.pgen.0030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze J, Schrempf A. Aging and reproduction in social insects. Gerontology. 2008 doi: 10.1159/000122472. in press. [DOI] [PubMed] [Google Scholar]
- Hercus MJ, Loeschcke V, Rattan SI. Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology. 2003;4:149–156. doi: 10.1023/a:1024197806855. [DOI] [PubMed] [Google Scholar]
- Hernebring M, Brolen G, Aguilaniu H, Semb H, Nystrom T. Elimination of damaged proteins during differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:7700–7705. doi: 10.1073/pnas.0510944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A, Portero-Otin M, Bellmunt MJ, Pamplona R, Barja G. Effect of the degree of fatty acid unsaturation of rat heart mitochondria on their rates of H2O2 production and lipid and protein oxidative damage. Mech. Ageing Dev. 2001;122:427–443. doi: 10.1016/s0047-6374(01)00214-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann F, Maser E. Carbonyl reductases and pluripotent hydroxysteroid dehydrogenases of the short-chain dehydrogenase/reductase superfamily. Drug Metabol. Rev. 2007;39:87–144. doi: 10.1080/03602530600969440. [DOI] [PubMed] [Google Scholar]
- Holmes DJ, Austad SN. Fly now, die later: life-history correlates of gliding and flying in mammals. J. Mammalogy. 1994;75:224–226. [Google Scholar]
- Honda Y, Tanaka M, Honda S. Modulation of longevity and diapause by redox regulation mechanisms under the insulin-like signaling control in Caenorhabditis elegans. Exp. Gerontol. 2008 doi: 10.1016/j.exger.2008.02.009. in press. [DOI] [PubMed] [Google Scholar]
- Howes RM. The free radical fantasy: a panoply of paradoxes. Ann. N.Y. Acad. Sci. 2006;1067:22–26. doi: 10.1196/annals.1354.004. [DOI] [PubMed] [Google Scholar]
- Hu X, Herzog C, Zimniak P, Singh SV. Differential protection against benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide-induced DNA damage in HepG2 cells stably transfected with allelic variants of π class human glutathione S-transferases. Cancer Res. 1999;59:2358–2362. [PubMed] [Google Scholar]
- Hu X, Xia H, Srivastava SK, Herzog C, Awasthi YC, Ji X, Zimniak P, Singh SV. Activity of four allelic forms of glutathione S-transferase hGSTP1-1 for diol epoxides of polycyclic aromatic hydrocarbons. Biochem. Biophys. Res. Commun. 1997;238:397–402. doi: 10.1006/bbrc.1997.7311. [DOI] [PubMed] [Google Scholar]
- Huang TT, Carlson EJ, Gillespie AM, Shi Y, Epstein CJ. Ubiquitous overexpression of CuZn superoxide dismutase does not extend life span in mice. J. Gerontol. Series A Biol. Sci. Med. Sci. 2000;55:B5–B9. doi: 10.1093/gerona/55.1.b5. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. On the importance of fatty acid composition of membranes for aging. J. Theor. Biol. 2005;234:277–288. doi: 10.1016/j.jtbi.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Usher MJ, Wallman JF. Food consumption and individual lifespan of adults of the blowfly, Calliphora stygia: a test of the 'rate of living' theory of aging. Exp. Gerontol. 2004;39:1485–1490. doi: 10.1016/j.exger.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Faulks SC, Harper JM, Miller RA, Buffenstein R. Extended longevity of wild-derived mice is associated with peroxidation-resistant membranes. Mech. Ageing Dev. 2006;127:653–657. doi: 10.1016/j.mad.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K, Oya-Ito T, Osawa T, Yamada S, Toyokuni S, Shibata N, Kobayashi M, Uchida K. Detection of lipofuscin-like fluorophore in oxidized human low-density lipoprotein. 4-hydroxy-2-nonenal as a potential source of fluorescent chromophore. FEBS Lett. 2000;473:249–253. doi: 10.1016/s0014-5793(00)01539-8. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM. Growing old: metabolic control and yeast aging. Annu. Rev. Microbiol. 2002;56:769–792. doi: 10.1146/annurev.micro.56.012302.160830. [DOI] [PubMed] [Google Scholar]
- Josephy PD, Guengerich FP, Miners JO. "Phase I" and "phase II" drug metabolism: terminology that we should phase out? Drug Metabol. Rev. 2005;37:575–580. doi: 10.1080/03602530500251220. [DOI] [PubMed] [Google Scholar]
- Keaney M, Matthijssens F, Sharpe M, Vanfleteren J, Gems D. Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard aging in the nematode Caenorhabditis elegans. Free Radic. Biol. Med. 2004;37:239–250. doi: 10.1016/j.freeradbiomed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Khaidakov M, Shmookler Reis RJ. Possibility of selection against mtDNA mutations in tumors. Molecular Cancer. 2005;4:36. doi: 10.1186/1476-4598-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Takahashi M, Shimizu T, Shirasawa T, Kajita M, Kanayama A, Miyamoto Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech. Ageing Dev. 2008 doi: 10.1016/j.mad.2008.02.011. in press. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. A systematic look at an old problem. Nature. 2008a;451:644–647. doi: 10.1038/451644a. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding ageing from an evolutionary perspective. J. Int. Med. 2008b;263:117–127. doi: 10.1111/j.1365-2796.2007.01901.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Feder M, Finch CE, Franceschi C, Globerson A, Klingenberg CP, Lamarco K, Omholt S, Westendorp RG. What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mech. Ageing Dev. 2005;126:439–443. doi: 10.1016/j.mad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Finch CE. Ageing - the old worm turns more slowly. Nature. 2002;419:794–795. doi: 10.1038/419794a. [DOI] [PubMed] [Google Scholar]
- Krause KH. Aging: A revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp. Gerontol. 2006;42:256–262. doi: 10.1016/j.exger.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Kuczynski B, Reo N. Evidence that plasmalogen is protective against oxidative stress in the rat brain. Neurochem. Res. 2006;31:639–656. doi: 10.1007/s11064-006-9061-7. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Kurland CG. Translational accuracy and the fitness of bacteria. Annu. Rev. Genet. 1992;26:29–50. doi: 10.1146/annurev.ge.26.120192.000333. [DOI] [PubMed] [Google Scholar]
- Kurz T, Terman A, Brunk UT. Autophagy, ageing and apoptosis: The role of oxidative stress and lysosomal iron. Arch. Biochem. Biophys. 2007;462:220–230. doi: 10.1016/j.abb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Lane N. Oxygen: the molecule that made the world. New York, NY: Oxford University Press; 2002. [Google Scholar]
- Larkin GA, Slaney PA. Implications of trends in marine-derived nutrient influx to south coastal British Columbia salmonid production. Fisheries. 1997;22:16–24. [Google Scholar]
- Lee JY, Jung GY, Heo HJ, Yun MR, Park JY, Bae SS, Hong KW, Lee WS, Kim CD. 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicol. Lett. 2006;166:212–221. doi: 10.1016/j.toxlet.2006.07.305. [DOI] [PubMed] [Google Scholar]
- Leonarduzzi G, Robbesyn F, Poli G. Signaling kinases modulated by 4-hydroxynonenal. Free Radic. Biol. Med. 2004;37:1694–1702. doi: 10.1016/j.freeradbiomed.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Lewin B. Genes VI. Oxford: Oxford University Press; 1997. [Google Scholar]
- Lithgow GJ. Why aging isn't regulated: A lamentation on the use of language in aging literature. Exp. Gerontol. 2006;41:890–893. doi: 10.1016/j.exger.2006.06.051. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Komatsu H, Murray IV, Axelsen PH. Promotion of amyloid beta protein misfolding and fibrillogenesis by a lipid oxidation product. J. Mol. Biol. 2008;377:1236–1250. doi: 10.1016/j.jmb.2008.01.057. [DOI] [PubMed] [Google Scholar]
- Liu Z, Minkler PE, Sayre LM. Mass spectroscopic characterization of protein modification by 4-hydroxy-2-(E)-nonenal and 4-oxo-2-(E)-nonenal. Chem. Res. Toxicol. 2003;16:901–911. doi: 10.1021/tx0300030. [DOI] [PubMed] [Google Scholar]
- Lombardo YB, Chicco AG. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. J. Nutr. Biochem. 2006;17:1–13. doi: 10.1016/j.jnutbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Long EK, Murphy TC, Leiphon LJ, Watt J, Morrow JD, Milne GL, Howard JR, Picklo MJ., Sr Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation. J. Neurochem. 2008 doi: 10.1111/j.1471-4159.2007.05175.x. in press. [DOI] [PubMed] [Google Scholar]
- Longhurst A. The sustainability myth; Proceedings, Western Groundfish Meeting; Santa Cruz, CA. 2008. [5–8 February, 2008]. [Google Scholar]
- Longo VD, Mitteldorf J, Skulachev VP. Programmed and altruistic ageing. Nat. Rev. Genet. 2005;6:866–872. doi: 10.1038/nrg1706. [DOI] [PubMed] [Google Scholar]
- Los DA, Murata N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta. 2004;1666:142–157. doi: 10.1016/j.bbamem.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Losick R, Desplan C. Stochasticity and cell fate. Science. 2008;320:65–68. doi: 10.1126/science.1147888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiora M, Rossi MA. Experimental researches on the role of phosphoinositide-specific phospholipase C in 4-hydroxynonenal induced exocytosis. Cell Biochem. Funct. 2003;21:155–160. doi: 10.1002/cbf.1013. [DOI] [PubMed] [Google Scholar]
- Magwere T, West M, Riyahi K, Murphy MP, Smith RA, Partridge L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech. Ageing Dev. 2006;127:356–370. doi: 10.1016/j.mad.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Marguet D, Lenne PF, Rigneault H, He HT. Dynamics in the plasma membrane: how to combine fluidity and order. EMBO J. 2006;25:3446–3457. doi: 10.1038/sj.emboj.7601204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Invest. 2003;111:583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM, Ogburn CE, Colgin LM, Gown AM, Edland SD, Monnat RJ., Jr Somatic mutations are frequent and increase with age in human kidney epithelial cells. Hum. Mol. Genet. 1996;5:215–221. doi: 10.1093/hmg/5.2.215. [DOI] [PubMed] [Google Scholar]
- McAdams HH, Arkin A. It's a noisy business! Genetic regulation at the nanomolar scale. Trends Genet. 1999;15:65–69. doi: 10.1016/s0168-9525(98)01659-x. [DOI] [PubMed] [Google Scholar]
- McElwee J, Schuster E, Blanc E, Piper M, Thomas J, Patel D, Selman C, Withers D, Thornton J, Partridge L, Gems D. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007;8:R132. doi: 10.1186/gb-2007-8-7-r132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J. Biol. Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- Medawar PB. An unsolved problem of biology. London: H. K. Lewis; 1952. [Google Scholar]
- Melov S. Introduction - Head-to-head debate: is there a program for aging? Aging Cell. 2004;3:247–247. [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Mene-Saffrane L, Davoine C, Stolz S, Majcherczyk P, Farmer EE. Genetic removal of tri-unsaturated fatty acids suppresses developmental and molecular phenotypes of an arabidopsis tocopherol deficient mutant: whole-body mapping of malondialdehyde pools in a complex eukaryote. J. Biol. Chem. 2007;282:35749–35756. doi: 10.1074/jbc.M706838200. [DOI] [PubMed] [Google Scholar]
- Merz JE, Moyle PB. Salmon, wildlife, and wine: marine-derived nutrients in human-dominated ecosystems of central California. Ecol. Appl. 2006;16:999–1009. doi: 10.1890/1051-0761(2006)016[0999:swawmn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Michaelides EE. Entropy, order and disorder. Open Thermodynamics Journal. 2008;2:7–11. [Google Scholar]
- Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- Miller JK. Escaping senescence: demographic data from the three-toed box turtle (Terrapene carolina triunguis) Exp. Gerontol. 2001;36:829–832. doi: 10.1016/s0531-5565(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harper JM, Dysko RC, Durkee SJ, Austad SN. Longer life spans and delayed maturation in wild-derived mice. Exp. Biol. Med. 2002;227:500–508. doi: 10.1177/153537020222700715. [DOI] [PubMed] [Google Scholar]
- Mitchell TW, Buffenstein R, Hulbert AJ. Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): A comparative study using shotgun lipidomics. Exp. Gerontol. 2007;42:1053–1062. doi: 10.1016/j.exger.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, Ishizaki-Koizumi S, Hirakawa T, Inoue T, Yodoi J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid. Redox Signal. 2002;4:693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- Mitteldorf J. How evolutionary thinking affects people's ideas about aging interventions. Rejuvenation Res. 2006;9:346–350. doi: 10.1089/rej.2006.9.346. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Sohal RS. Temperature-dependent trade-offs between longevity and fertility in the Drosophila mutant, methuselah. Exp. Gerontol. 2006;41:566–573. doi: 10.1016/j.exger.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Radyuk SN, Benes JJ, Orr WC, Sohal RS. Phenotypic effects of familial amyotrophic lateral sclerosis mutant Sod alleles in transgenic Drosophila. Proc. Natl. Acad. Sci. USA. 2003a;100:301–306. doi: 10.1073/pnas.0136976100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett RJ, Bayne AC, Kwong LK, Orr WC, Sohal RS. Ectopic expression of catalase in Drosophila mitochondria increases stress resistance but not longevity. Free Radic. Biol. Med. 2003b;34:207–217. doi: 10.1016/s0891-5849(02)01190-5. [DOI] [PubMed] [Google Scholar]
- Morowitz HJ, Kostelnik JD, Yang J, Cody GD. The origin of intermediary metabolism. Proc. Natl. Acad. Sci. USA. 2000;97:7704–7708. doi: 10.1073/pnas.110153997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim. Biophys. Acta. 2005;1703:213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Partridge L. Toward a control theory analysis of aging. Annu. Rev. Bioch. 2008;77 doi: 10.1146/annurev.biochem.77.070606.101605. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Tanaka Y, Mitsubuchi H, Endo F. Animal models of tyrosinemia. J. Nutr. 2007;137:1556S–1560S. doi: 10.1093/jn/137.6.1556S. discussion 1573S–1575S. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 3rd ed. New York, NY: Worth Publishers; 2000. [Google Scholar]
- Nystrom T. A bacterial kind of aging. PLoS Genet. 2007;3:e224. doi: 10.1371/journal.pgen.0030224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien DM, Min KJ, Larsen T, Tatar M. Use of stable isotopes to examine how dietary restriction extends Drosophila lifespan. Curr. Biol. 2008;18:R155–R156. doi: 10.1016/j.cub.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Olsen A, Vantipalli MC, Lithgow GJ. Lifespan extension of Caenorhabditis elegans following repeated mild hormetic heat treatments. Biogerontology. 2006;7:221–230. doi: 10.1007/s10522-006-9018-x. [DOI] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Does overexpression of Cu,Zn-SOD extend life span in Drosophila melanogaster? Exp. Gerontol. 2003;38:227–230. doi: 10.1016/s0531-5565(02)00263-2. [DOI] [PubMed] [Google Scholar]
- Orr WC, Mockett RJ, Benes JJ, Sohal RS. Effects of overexpression of copper-zinc and manganese superoxide dismutases, catalase, and thioredoxin reductase genes on longevity in Drosophila melanogaster. J. Biol. Chem. 2003;278:26418–26422. doi: 10.1074/jbc.M303095200. [DOI] [PubMed] [Google Scholar]
- Packer L, Cadenas E. Oxidants and antioxidants revisited. New concepts of oxidative stress. Free Radic. Res. 2007;41:951–952. doi: 10.1080/10715760701490975. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Barja G, Portero-Otin M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? Ann. N.Y. Acad. Sci. 2002;959:475–490. doi: 10.1111/j.1749-6632.2002.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Portero-Otin M, Sanz A, Requena J, Barja G. Modification of the longevity-related degree of fatty acid unsaturation modulates oxidative damage to proteins and mitochondrial DNA in liver and brain. Exp. Gerontol. 2004;39:725–733. doi: 10.1016/j.exger.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Parker JD, Parker KM, Sohal BH, Sohal RS, Keller L. Decreased expression of Cu-Zn superoxide dismutase 1 in ants with extreme lifespan. Proc. Natl. Acad. Sci. USA. 2004;101:3486–3489. doi: 10.1073/pnas.0400222101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker N, Vidal-Puig AJ, Brand MD. Stimulation of mitochondrial proton conductance by hydroxynonenal requires a high membrane potential. Biosci. Reports. 2008 doi: 10.1042/BSR20080002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat. Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Ogilvie BW. Biotransformation of xenobiotics. In: Klaassen CD, editor. Casarett and Doull's Toxicology: The Basic Science of Poisons. 7th ed. New York: McGraw-Hill Medical; 2008. pp. 161–304. [Google Scholar]
- Parsons PA. The ecological stress theory of aging and hormesis: an energetic evolutionary model. Biogerontology. 2007;8:233–242. doi: 10.1007/s10522-007-9080-z. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Benchmarks for ageing studies. Nature. 2007;450:165–167. doi: 10.1038/450165a. [DOI] [PubMed] [Google Scholar]
- Pearl R. The rate of living. London: University of London Press; 1928. [Google Scholar]
- Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic. Biol. Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Phillips JP, Parkes TL, Hilliker AJ. Targeted neuronal expression and longevity in Drosophila. Exp. Gerontol. 2000;35:1157–1164. doi: 10.1016/s0531-5565(00)00117-0. [DOI] [PubMed] [Google Scholar]
- Piper MD, Selman C, McElwee JJ, Partridge L. Separating cause from effect: how does insulin/IGF signalling control lifespan in worms, flies and mice? J. Int. Med. 2008;263:179–191. doi: 10.1111/j.1365-2796.2007.01906.x. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Kabil H, Partridge L. Chemical complexity and the genetics of aging. Annu. Rev. Ecol. Evol. Syst. 2007;38:299–326. doi: 10.1146/annurev.ecolsys.38.091206.095634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Schaur RJ, Siems WG, Leonarduzzi G. 4-Hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med. Res. Rev. 2007 doi: 10.1002/med.20117. in press. [DOI] [PubMed] [Google Scholar]
- Puca AA, Andrew P, Novelli V, Anselmi CV, Somalvico F, Cirillo NA, Chatgilialoglu C, Ferreri C. Fatty acid profile of erythrocyte membranes as possible biomarker of longevity. Rejuvenation Res. 2008;11:63–72. doi: 10.1089/rej.2007.0566. [DOI] [PubMed] [Google Scholar]
- Rattan SI, Gonzalez-Dosal R, Roge Nielsen E, Kraft DC, Weibel J, Kahns S. Slowing down aging from within: mechanistic aspects of anti-aging hormetic effects of mild heat stress on human cells. Acta Biochim. Pol. 2004;51:481–492. [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat. Genet. 2005;37:894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MR, Rauser CL, Mueller LD, Benford G. A revolution for aging research. Biogerontology. 2006;7:269–277. doi: 10.1007/s10522-006-9001-6. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Dianzani MU. Action of 2-nonenal and 4-hydroxynonenal on phosphoinositide-specific phospholipase C in undifferentiated and DMSO-differentiated HL-60 cells. Cell Biochem. Funct. 2000;18:209–214. doi: 10.1002/1099-0844(200009)18:3<209::AID-CBF874>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Salganik RI. The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J. Am. Coll. Nutr. 2001;20:464S–472S. doi: 10.1080/07315724.2001.10719185. [DOI] [PubMed] [Google Scholar]
- Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid. Redox Signal. 2006;8:582–599. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- Sawicki R, Singh SP, Mondal AK, Beneš H, Zimniak P. Cloning, expression, and biochemical characterization of one Epsilon-class (GST-3) and ten Delta-class (GST-1) glutathione S-transferases from Drosophila melanogaster, and identification of additional nine members of the Epsilon class. Biochem. J. 2003;370:661–669. doi: 10.1042/BJ20021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke RT. Gene amplification in cultured animal cells. Cell. 1984;37:705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schneider C, Porter NA, Brash AR. Autoxidative transformation of chiral ω6 hydroxy linoleic and arachidonic acids to chiral 4-hydroxy-2E-nonenal. Chem. Res. Toxicol. 2004;17:937–941. doi: 10.1021/tx049913n. [DOI] [PubMed] [Google Scholar]
- Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: Fundamental issues in the mechanisms of lipid peroxidation. J. Biol. Chem. 2008 doi: 10.1074/jbc.R800001200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metabol. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Schutt F, Bergmann M, Holz FG, Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2003;44:3663–3668. doi: 10.1167/iovs.03-0172. [DOI] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Petrovic N, Kramarova TV, Hoeks J, Cannon B, Nedergaard J. UCP1 and defence against oxidative stress: 4-hydroxy-2-nonenal effects on brown-fat mitochondria are uncoupling protein 1-independent. J. Biol. Chem. 2006;281:13882–13893. doi: 10.1074/jbc.M601387200. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis RJ. Model systems for aging research: syncretic concepts and diversity of mechanisms. Genome. 1989;31:406–412. doi: 10.1139/g89-061. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis RJ, Finn GK, Smith K, Goldstein S. Clonal variation in gene methylation: c-H-ras and α-hCG regions vary independently in human fibroblast lineages. Mutat. Res. 1990;237:45–57. doi: 10.1016/0921-8734(90)90031-l. [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 1997;91:1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- Singh SP, Coronella JA, Beneš H, Cochrane BJ, Zimniak P. Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1-1 (GST-2) in conjugation of lipid peroxidation end products. Eur. J. Bioch. 2001;268:2912–2923. doi: 10.1046/j.1432-1327.2001.02179.x. [DOI] [PubMed] [Google Scholar]
- Singh SP, Chen T, Chen L, Mei N, McLain E, Samokyszyn V, Thaden JJ, Moore MM, Zimniak P. Mutagenic effects of 4-hydroxynonenal triacetate, a chemically protected form of the lipid peroxidation product 4-hydroxynonenal, as assayed in L5178Y/Tk+/− mouse lymphoma cells. J. Pharmacol. Exp. Ther. 2005;313:855–861. doi: 10.1124/jpet.104.080754. [DOI] [PubMed] [Google Scholar]
- Smith E, Morowitz HJ. Universality in intermediary metabolism. Proc. Natl. Acad. Sci. USA. 2004;101:13168–13173. doi: 10.1073/pnas.0404922101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S. Biochemical correlates of longevity in two closely related rodent species. Biochem. Biophys. Res. Commun. 1993a;196:7–11. doi: 10.1006/bbrc.1993.2208. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Dubey A, Orr WC. Protein oxidative damage is associated with life expectancy of houseflies. Proc. Natl. Acad. Sci. USA. 1993b;90:7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero GN. Proteins and temperature. Annu. Rev. Physiol. 1995;57:43–68. doi: 10.1146/annurev.ph.57.030195.000355. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Watowich SJ, Petrash JM, Srivastava SK, Bhatnagar A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Free Radic. Res. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- Stewart EJ, Madden R, Paul G, Taddei F. Aging and death in an organism that reproduces by morphologically symmetric division. PLoS Biol. 2005;3:e45. doi: 10.1371/journal.pbio.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Mosharov E, Talloczy Z, Zucca FA, Simon JD, Zecca L. Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J. Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05385.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol. Cell. Biol. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Salomon RG. Oxidative fragmentation of hydroxy octadecadienoates generates biologically active γ-hydroxyalkenals. J. Am. Chem. Soc. 2004;126:5699–5708. doi: 10.1021/ja038756w. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Terman A. Catabolic insufficiency and aging. Ann. N.Y. Acad. Sci. 2006;1067:27–36. doi: 10.1196/annals.1354.005. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Model organisms as a guide to mammalian aging. Dev. Cell. 2002;2:9–19. doi: 10.1016/s1534-5807(01)00098-3. [DOI] [PubMed] [Google Scholar]
- Tsai L, Szweda PA, Vinogradova O, Szweda LI. Structural characterization and immunochemical detection of a fluorophore derived from 4-hydroxy-2-nonenal and lysine. Proc. Natl. Acad. Sci. USA. 1998;95:7975–7980. doi: 10.1073/pnas.95.14.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y, Okuno Y, Hishinuma K, Ezaki A, Okada G, Yamaguchi M, Chikuma T, Hojo H. 4-Hydroxy-2-nonenal-modified glyceraldehyde-3-phosphate dehydrogenase is degraded by cathepsin G. Free Radic. Biol. Med. 2007;43:1604–1615. doi: 10.1016/j.freeradbiomed.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Uchida K. Lipofuscin-like fluorophores originated from malondialdehyde. Free Radic. Res. 2006;40:1335–1338. doi: 10.1080/10715760600902302. [DOI] [PubMed] [Google Scholar]
- Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc. Natl. Acad. Sci. USA. 1992;89:4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Szweda LI, Chae H-Z, Stadtman ER. Immunochemical detection of 4-hydroxynonenal protein adducts in oxidized hepatocytes. Proc. Natl. Acad. Sci. USA. 1993;90:8742–8746. doi: 10.1073/pnas.90.18.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel JW, Baudisch A, Dolling M, Roach DA, Gampe J. The case for negative senescence. Theor. Popul. Biol. 2004;65:339–351. doi: 10.1016/j.tpb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA, Loeb LA. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat. Genet. 2007;39:540–543. doi: 10.1038/ng1988. [DOI] [PubMed] [Google Scholar]
- Vetter RD, Lynn EA. Bathymetric demography, enzyme activity patterns, and bioenergetics of deep-living scorpaenig fishes (genera Sebastes and Sebastolobus): paradigms revisited. Mar. Ecol. Prog. Ser. 1997;155:173–188. [Google Scholar]
- Vijg J. The role of DNA damage and repair in aging: New approaches to an old problem. Mech. Ageing Dev. 2008 doi: 10.1016/j.mad.2008.02.009. in press. [DOI] [PubMed] [Google Scholar]
- Vila A, Tallman KA, Jacobs AT, Liebler DC, Porter NA, Marnett LJ. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chem. Res. Toxicol. 2008;21:432–444. doi: 10.1021/tx700347w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JL, Browse J. Dietary manipulation implicates lipid signaling in the regulation of germ cell maintenance in C. elegans. Develop. Biol. 2006;292:381–392. doi: 10.1016/j.ydbio.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner RJ, Zsurka G, Kunz WS. Mitochondrial DNA damage and the aging process - facts and imaginations. Free Radic. Res. 2006;40:1284–1294. doi: 10.1080/10715760600913168. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic. Biol. Med. 2000;29:403–409. doi: 10.1016/s0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- Wolkow CA, Iser WB. Uncoupling protein homologs may provide a link between mitochondria, metabolism and lifespan. Ageing Res. Rev. 2006;5:196–208. doi: 10.1016/j.arr.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Doser TA, Fang CX, Nunn JM, Janardhanan R, Zhu M, Sreejayan N, Quinn MT, Ren J. Metallothionein prolongs survival and antagonizes senescence-associated cardiomyocyte diastolic dysfunction: role of oxidative stress. FASEB J. 2006;20:1024–1026. doi: 10.1096/fj.05-5288fje. [DOI] [PubMed] [Google Scholar]
- Zimniak P. Substrates and reaction mechanisms of GSTs. In: Awasthi YC, editor. Toxicology of glutathione transferases. Boca Raton, FL: CRC Press; 2006. pp. 71–101. [Google Scholar]
- Zimniak P, Singh SP. Families of glutathione transferases. In: Awasthi YC, editor. Toxicology of glutathione transferases. Boca Raton, FL: CRC Press; 2006. pp. 11–26. [Google Scholar]
- Zimniak P, Singhal SS, Srivastava SK, Awasthi S, Sharma R, Hayden JB, Awasthi YC. Estimation of genomic complexity, heterologous expression, and enzymatic characterization of mouse glutathione S-transferase mGSTA4-4 (GST 5.7) J. Biol. Chem. 1994;269:992–1000. [PubMed] [Google Scholar]