Abstract
The acetylcholine receptor ganglionic (G-AchR) antibody is a very specific serologic test for autoimmune autonomic ganglionopathy. The spectrum of autoimmune (or presumed to be autoimmune) autonomic disorders, however, is quite broad and positivity to this antibody has been reported in a variety of other conditions, albeit infrequent and with low titer.
This review describes the autonomic neuropathies most frequently encountered in clinical practice in which an autoimmune etiology is suspected. They include a chronic form (pure autonomic failure) and limited autonomic neuropathies with predominant involvement of one neurotransmitter type (i.e., cholinergic vs. adrenergic) or one system (such as the gastrointestinal system) or a distal small fiber dysfunction. In each of these conditions, occasional positivity to the G-AchR antibody has been found, but the pathogenetic significance of such finding is still uncertain. Other antigens and antibodies yet to be identified are more likely to be responsible in these disorders.
The identification of the acetylcholine receptor ganglionic (G-AchR) antibody by Vernino et al. in 1998 and especially following the comprehensive study in 2000 opened a new era in the diagnosis, evaluation, and treatment of the autonomic neuropathies.
The antibody is detected with a radioimmunoprecipitation assay similar to the one used to detect muscle AchR antibodies in myasthenia gravis (Vernino et al., 1998) which uses solubilized membranes from a human neuroblastoma cell line (IMR-32) complexed with a high affinity ligand for ganglionic AChR, 125I-labeled epibatidine. High-titer ganglionic antibody is associated with a specific phenotype, with clinical features and anatomic localization that indicates major involvement of autonomic ganglia, hence the term autoimmune autonomic ganglionopathy (AAG) has replaced the more generic term autoimmune autonomic neuropathy (AAN) (Suarez et al., 1994; Vernino et al., 1998). The autoimmune disorder was suspected in the original description (Young et al., 1969, 1975) when it was considered as an autonomic variant of Guillain-Barre' syndrome. The view was sustained in the Mayo Clinic experience, where we demonstrated selective involvement of C fibers, an inflammatory, presumed immune attack of nerve and possible response to immunotherapy (Low et al., 1983; Suarez et al., 1994). The antigen and the site of autoantibody attack, however, were not known.
Vernino et al. (2003) clearly proved the pathogenetic role of the G-AchR antibodies thus confirming the target is the α3 subunit of the acetylcholine receptor at the autonomic ganglia level. Antibodies that specifically bind to the G-AChR are detectable in about 50% of patients with subacute AAG. G-AChR autoantibodies are not found in healthy control subjects or in patients with myasthenia gravis.
G-AChR antibody in high titers is highly specific and this antibody provides a tool for the quantitative and sensitive detection of this variety of autoimmune autonomic neuropathy. Furthermore, there is a robust relationship between antibody levels and clinical autonomic severity (Vernino et al., 2000). However, the antibody will only detect 50% of cases of severe autoimmune antonomic neuropathy. Presumably, the antibody negative cases of pandysautonomia, which can have identical phenotype and respond similarly to immunotherapy, are due to antibodies directed at different targets in nerve, including ganglion.
The heterogeneous manifestations of various disorders of autonomic function support the notion that more than one mechanism is involved in the pathogenesis and more than one target may be the site of attack in different autoimmune variants.
The prototypical AAG case is a previously healthy young or middle-aged subject, more likely to be a female, presenting with a severe panautonomic failure that evolves within days to 1-2 weeks, similarly to the somatic counterpart GBS. The course is generally monophasic with slow, often incomplete recovery. The clinical picture is dominated by orthostatic hypotension, widespread anhidrosis, dry mouth, dry eyes, sexual dysfunction, urinary retention, impaired pupillary responses, reduced heart rate variability and gastrointestinal symptoms ranging from gastroparesis (manifesting as early satiety, postprandial abdominal pain, bloating and vomiting), diarrhea, constipation and in the most severe cases intestinal pseudoobstruction.
As in GBS, an antecedent event, such as a viral syndrome, recent immunizations or surgical procedures, is often reported. Patients with AAG often have high antibody levels (>0.5 nmol/L). Serum levels of G-AChR binding antibody are significantly correlated with severity of autonomic dysfunction. Patients with high antibody levels have the most severe and widespread autonomic failure and are most likely to present with the “classic” AAG phenotype. Improvement in autonomic function is associated with a decline in antibody levels.
However, the existence of a broad spectrum of autoimmune autonomic syndromes has been recognized very early since the identification of G-AchR antibodies. Lower antibody titers are often associated with either subacute-chronic variants of autonomic neuropathy or limited forms of autonomic neuropathy.
Klein, Sandroni et al. (2003, 2004) reported a high antibody titer was often associated with more acute-subacute onset, more severe dysautonomia and prominent cholinergic dysfunction (i.e., sicca complex, prominent gastrointestinal dysmotility and pupillary abnormality), while lower titers were often seen in more indolent, chronic phenotypes. The strongest correlation was not with the temporal profile, as originally thought, but with the degree of cholinergic involvement. When comparing patients with dysautonomia who were antibody positive vs. those who were antibody negative, once again cholinergic dysfunction was more prominent in the first group, but temporal profile was also different, with the antibody positive cases being 3 times more likely to have subacute onset.
The chronic form of AAG usually presents with longstanding orthostatic hypotension (OH) without evidence of somatic neuropathy or central nervous system involvement. Autonomic testing and more detailed review of symptoms typically reveal evidence of more widespread autonomic dysfunction. This chronic form of AAG may be clinically indistinguishable from pure autonomic failure (PAF).
At Mayo Clinic, we have a large patient population that routinely undergoes extensive serologic testing looking for evidence of autoimmunity, either secondary to occult cancer or idiopathic. The G-AchR antibodies are also tested as part of the panel. Thus over the years, we have accumulated a significant number of data in various neurological syndromes, but the quest for a complete definition and characterization of the spectrum of autoimmune autonomic disorders is far from over.
We cannot emphasize enough the fact the full spectrum of phenotypes associated with ganglionic antibody is not known. Also unknown is the strength of association between antibody and phenotype, outside of AAG. On this background, especially with the phenotypes where the prevalence, incidence and strength of association are less well defined, we want to provide the reader with illustrative case reports, for them to be able to recognize such conditions (some more common than others) and make the judgment about the nature of them. We do not claim that these phenotypes are due to the antibody, but provide the cases to support the hypothesis.
PAF
Traditionally, pure autonomic failure (PAF) (a.k.a., Bradbury-Eggleston syndrome or idiopathic orthostatic hypotension) has been considered a degenerative postganglionic autonomic disorder in contrast to the preganglionic pathology that characterizes multiple system atrophy (MSA). Indeed, biochemical [i.e., low plasma cathecolamines, reduced norepinephrine release, and clearance (Polinsky et al., 1985)], postganglionic sudomotor testing (Cohen et al., 1987) and studies of sympathetic cardiac innervation and norepinephrine spillover (Goldstein, 2000) confirms the postganglionic dysfunction in PAF.
More recently, an underlying neuropathologic basis for PAF has been proposed (Kaufmann 2000, 2001, 2003). The underlying basis of this postganglionic disorder appears to be a synucleinopathy, with Lewy bodies affecting primarily the autonomic ganglia neurons with some evidence of Lewy bodies in central nervous system nuclei but with insignificant neuronal loss. The early findings have been confirmed in subsequent autopsy studies (Kaufmann, personal communication). Of interest is that this phenotype could remain unchanged or evolve into a central synucleinopathy (such as MSA) over time (Kaufmann 2000).
One of the key findings of the paper from Klein et al. (2003) was to recognize that some patients with AAG and positive antibody titer had a clinical course that resembled a degenerative condition, like PAF, characterized by slow onset and continuous progression. The paper showed 50% of cases with clinical diagnosis of PAF were antibody positive, but it was based on only 4 cases, so the frequency of positivity is not truly known. One can speculate patients are more likely diagnosed as AAG if early in their course, while long-standing cases may be more likely diagnosed as PAF, which may in fact represent at least in some cases, a slower variant of AAG or a residual, burnt out form.
Such data further question the identity of PAF as a specific disease and rather raise the possibility this is a syndrome with various etiologies (i.e., autoimmune as well as degenerative) and how many cases could be attributed to one form versus the other is unknown at this point.
Illustrative Case of PAF
A 65 yo man presented with chief complaint of orthostatic intolerance and lightheadedness of 15 yrs. duration, with progressive worsening resulting in severe orthostatic hypotension. He also reported progressive difficulties with impotence, urinary retention and heat intolerance during the same period of time. He had no other significant neurological or medical problems. His evaluations demonstrated profound, generalized autonomic failure, global anhidrosis, very low plasma catecholamines and low G-AChR antibody titer (0.23 nmol/L). We diagnosed him as suffering from PAF and managed him symptomatically. His condition continued to deteriorate gradually, making him less and less responsive to the available symptomatic treatment strategies.
Postural Tachycardia Syndrome (POTS)
POTS is arguably the most common syndrome of orthostatic intolerance, characterized by excessive tachycardic response upon standing as well as multiple symptoms in other autonomic (particularly gastrointestinal) and somatic domains. POTS is a syndrome, not a specific disease and a major subtype is the neuropathic variant, associated with impaired sudomotor function (Schondorf et al., 1993; Thieben et al., 2007) and altered peripheral adrenergic vasomotor tone. There is considerable heterogeneity of pathogenetic mechanisms, with venous pooling and abnormal beta-2-receptor sensitivity likely to play key roles.
Approximately 50% of cases report an acute or subacute onset, often following a viral illness, suggesting an immune-mediated process. In a recent review (Thieben, 2007) of 152 cases of POTS seen consecutively by Sandroni and Low, 6 of 42 patients (who had ganglionic antibody measured) had increased levels of antibody. These were consistently of low titer. In our ongoing prospective study, the percent of positive cases we have found a positivity of 25% (unpublished data). Hence we do not provide a firm percent in Table 1. There is no obvious clinical characteristic that seem to differentiate antibody positive from the antibody negative cases, although the issue has not been formally studied.
Table 1.
Possible clinical phenotypes of G-AchR antibody autoimmunity
| Disorder | Key clinical manifestations | Antibody + frequency | Antibody titers |
|---|---|---|---|
| AAG | Acute/subacute onset of pandysautonomia with prominent cholinergic abnormalities; often monophasic; may respond well to immunotherapy | 50% | Usually high |
| PAF | Gradual, progressive generalized autonomic failure | ? | low |
| POTS | Often subacute onset of orthostatic intolerance and inappropriate postural tachycardia; patients often polysyntomatic for various autonomic domains; fatigue can be a refractory symptom | 15-25% | low |
| CIA | Variable onset of heat intolerance due to inadequate sweat production. Loss can be complete. May respond to immunotherapy | ? | low |
| GI dysmotility | Variable onset, often subacute, of upper and/or lower GI tract motility disorder: can manifest as gastroparesis, pseudoobstruction or both | ? | low |
| DSFN | Variable onset of burning discomfort in the distal limbs; neurological exam and EMG often normal, but abnormal sweat found in >90% of cases | ? | low |
Illustrative Case of POTS
A 21 yo woman presented with symptoms of orthostatic intolerance and fatigue following a flu-like (? Lyme) illness 1 year earlier. Her autonomic studies revealed excessive orthostatic tachycardia, mild distal sudomotor dysfunction and beta-supersensitivity (as suggested by a large Valsalva ratio and exaggerated phase IV). She had no other autonomic or neurologic symptoms. The patient was therefore diagnosed as suffering from the neuropathic variant of POTS. Her G-AChR antibody titer was 0.44 nmol/L (0.16 nmol/L and 0.26 nmol/L at recheck). At the last visit, she appeared to be fairly stable, still symptomatic from orthostatic intolerance (OI) and fatigue.
Chronic Idiopatahic Anhidrosis (CIA)
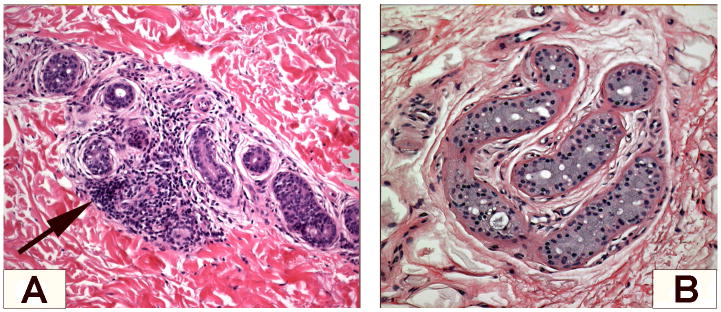
Chronic idiopathic anhidrosis is a rare syndrome, although possibly under diagnosed, at least in the less severe forms. The affected patients are typically heat intolerant and have significant vasomotor reactions when exposed to heat, but they are unable to sweat or do so to a degree that is inadequate to control body temperature. They often become lightheaded and short of breath in a hot environment. Although anhidrosis can be part of generalized autonomic failure, CIA is an isolated clinical syndrome and these patients have no other symptoms or signs of dysautonomia. This restricted dysautonomia renders their prognosis much more favorable, provided they avoid hot environment when they are at risk of heat stroke, and their quality of life may be affected by their inability to sweat. The site of the lesion may be at the end organ itself, as reported by Low et al., 1985, with inflammatory infiltrates surrounding the sweat glands (Fig. 1). CIA may be heterogeneous as well, and indeed the original cases included 4 with preganglionic (or ganglionic) dysfunction. Treatment with immunosuppressive agents may reverse this syndrome. There is also anecdotal report of spontaneous resolution. Therefore, an autoimmune mechanism has been postulated to be involved in the pathogenesis of CIA. We have been studying these patients more extensively in recent years and the G-AchR antibody seemed a potential good candidate: however only 1 case of the 15 tested had a positive test at low titer.
Figure 1.
Skin biopsies showing perieccrine inflammatory cells infiltration around sweat glands in anhidrotic skin (A) and normal sweat gland coils from sweating skin (B) in the same patient with classic CIA. Courtesy of Dr. Robert D. Fealey.
Illustrative CIA Case
A 45 yo woman presented with the complaint of sweating abnormalities (both hypo- and hyperhydrosis in different part of her body) of 2 yrs. duration. She carried the diagnosis of Adies' pupil for over 20 yrs, but had no other neurologic or medical symptoms.
Her autonomic study and thermoregulatory sweat test (TST) confirmed segmental anhidrosis and her clinical evaluation was consistent with Ross' syndrome. All her studies were negative, except for a low positive G-AchR antibody (0.03 nmol/L).
Distal Small Fiber Neuropathy (DSFN)
Small fiber involvement can variably occur in any somatic peripheral neuropathy, autonomic neuropathy and even in association with certain central degenerative disorders.
The clinical isolated syndrome of distal small fiber neuropathy, often also called the “burning feet” syndrome is perhaps the most common presentation of small-fiber neuropathy (DSFN) in clinical practice. These patients have distal involvement with burning, prickling, and some stabbing discomfort, with variable allodynia. By definition, they have completely normal motor function, intact tendon reflexes, and normal nerve conduction studies. Autonomic fibers are usually involved as well, since these patients commonly have vasomotor symptoms, manifesting as excessive coldness or warmth, skin discoloration, and sudomotor symptoms (either hyper- or hypohidrosis) can also be present.
As small unmyelinated nerve fibers comprise 2 subpopulations, i.e., somatic and autonomic fibers, the clinical picture can be variable, but there is generally good correlation between symptoms, signs and test results for both somatic and autonomic functions (Novak et al., 2001). Our group showed sudomotor testing to be highly sensitive (98%) in detecting this entity (Low et al., 2006).
DFSN, as many other neuropathies, has multiple causes, but in up to 40% of cases no specific etiology can be identified even after extensive testing. As in some cases patients report an event, such as a viral illness or a surgical procedure, after which the symptoms appeared, an autoimmune cause has been speculated for some cryptogenic forms. In our center, these patients undergo extensive evaluations which include a complete paraneoplastic panel. Of the hundreds of patients screened with clinical diagnosis of DSFN, only 1 had a low positivity to the G-AchR antibody.
Illustrative DSFN Case
A 73 yo woman with no significant past medical history presented for evaluation of burning discomfort affecting her feet that had started 7-8 yrs prior. Clinically, she appeared to suffer from a mild distal small fiber irritation without deficits.
Her evaluation included nerve conduction studies and electromyogram, quantitative sensory testing, autonomic reflex screen (ARS), and TST as well as extensive blood and urine studies, all of which were unrevealing except for a low G-AChR antibody titer (0.05 nmol/L).
Chronic intestinal pseudo obstruction (CIP)
CIP is a dysmotility syndrome that presents with symptoms and signs of intestinal obstruction and radiographic evidence of dilated bowels, but no anatomic obstruction can be found. It primarily is a disorder of small bowel motility, but it can occur anywhere in the gastrointestinal tract. The etiology of such condition is not fully elucidated. As for many dysmotility syndromes, more than one cause may be responsible and thus this population is heterogeneous. As for many other idiopathic isolated syndromes, an autoimmune etiology has been postulated as well.
However, no specific antigen or antibody has been to date identified. As patients with G-AchR antibody positivity have generally prominent cholinergic dysfunction and hence gastrointestinal symptoms, it seemed plausible CIP could be yet another autonomic variant of AAG. But only 2 patients of the many tested had a low positivity (Dr. Farrugia, personal communication).
In a recent paper by Dhamija et al. (2008), the authors identified 24 patients who presented with gastrointestinal dysmotility as major symptom and were found to have 1 or more autoantibody on serologic testing by the neuroimmunology laboratory. Eleven of the 24 had positivity for the G-AchR. Serologic positivity was present either in paraneoplastic or idiopathic forms. Unfortunately we do not know how many patients with similar symptoms were tested negative to provide an estimate of prevalence. Early identification and treatment of autoimmune gastrointestinal dysmotility may avoid the need for invasive procedures and aid in more specific and successful management.
Illustrative Pseudo Obstruction Case
A 37 yo woman presented for evaluation of complex constellation of GI symptoms suggesting of generalized dysmotility that had began 4 yrs prior following a viral illness. Her symptoms suggested gastroparesis and colonic inertia. She also reported fatigue and mild orthostatic intolerance. Her upper GI symptoms had improved since the onset, but her constipation persisted. Her studies showed presence of mild, limited autonomic neuropathy, with features of POTS and DSFN and prolonged transit time. Her G-AchR antibody titer was 0.18 nmol/L when measured 8 yrs later. At that point, she continued to have symptoms but her studies had improved, suggesting more a functional syndrome.
Comments
The spectrum of AAG is likely to continue to expand as we gather more data. We can conclude at present we have no definite evidence G-AchR antibody has a pathogenetic role in POTS, CIA, or DSFN. The low titer detected in a few cases, without specific clinical characteristics differentiating them from the seronegative ones, is more likely indicative of an underlying autoimmune response (even one directly responsible for the clinical syndromes) and may suggest another auto-antibody may be involved, however one yet to be identified.
As for CIP and gastrointestinal dysmotility in general, recent data suggest G-AchR may indeed be pathogenetic, alone or in association with other autoantibodies.
Some individual seronegative patients respond to immunotherapy as well (P.A.L., unpublished observations). This observation suggests that the clinical phenotype of AAG, persistent severe autonomic failure, unassociated with G-AChR antibodies could be caused by antibodies other than G-AChR antibody and could respond to immunotherapy. However the numbers are still very small and the decision to treat with expensive and not risk-free therapies should not be taken lightly.
The take home messages from this brief overview of conditions other than AAG in which G-AchR positivity may be found are:
the spectrum of autoimmune autonomic disorders is broad and still expanding
clinical suspicion of an autoimmune pathogenesis is higher whenever an antecedent event, subacute onset, evidence of multiple (albeit sometimes subtle) organ/system involvement or of other autoimmune disorder is present
the decision to treat with costly therapies requiring careful monitoring (such as long-term immunosuppressive agents) should probably be entertained whenever the index of suspicion of an autoimmune pathogenesis is high, regardless of the low titer seropositivity or seronegativity, whenever a patient fails to respond adequately to symptomatic therapies. Such decision should be taken in a case by case approach as there is no large study at present to justify otherwise
seronegativity does not exclude autoimmune pathogenesis: it may simply mean the responsible autoantibody has not been yet identified
similar phenotypes may have very different pathogenetic mechanisms and “idiopathic” should not equate “autoimmune”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cohen J, et al. Somatic and autonomic function in progressive autonomic failure and multiple system atrophy. Ann Neurol. 1987;22:692–699. doi: 10.1002/ana.410220604. [DOI] [PubMed] [Google Scholar]
- Dhamija R, et al. Serologic profiles aiding the diagnosis of autoimmune gastrointestinal dysmotility. Clin Gastroenterol Hepatol. 2008;6:988–992. doi: 10.1016/j.cgh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D, et al. Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- Kaufmann H. Primary autonomic failure: three clinical presentations of one disease? Ann Intern Med. 2000;133:382–384. doi: 10.7326/0003-4819-133-5-200009050-00014. [DOI] [PubMed] [Google Scholar]
- Kaufmann H, et al. Accumulation of alpha-synuclein in autonomic nerves in pure autonomic failure. Neurology. 2001;56:980–981. doi: 10.1212/wnl.56.7.980. [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Biaggioni I. Autonomic failure in neurodegenerative disorders. Semin Neurol. 2003;23:351–363. doi: 10.1055/s-2004-817719. [DOI] [PubMed] [Google Scholar]
- Klein C, et al. The spectrum of autoimmune autonomic neuropathies. Ann Neurol. 2003;53:752–758. doi: 10.1002/ana.10556. [DOI] [PubMed] [Google Scholar]
- Low P, et al. Acute panautonomic neuropathy. Ann Neurol. 1983;13:412–417. doi: 10.1002/ana.410130407. [DOI] [PubMed] [Google Scholar]
- Low P, et al. Chronic idiopathic anhidrosis. Ann Neurol. 1985;18:344–348. doi: 10.1002/ana.410180312. [DOI] [PubMed] [Google Scholar]
- Low V, et al. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34:57–61. doi: 10.1002/mus.20551. [DOI] [PubMed] [Google Scholar]
- Novak V, et al. Autonomic impairment in painful neuropathy. Neurology. 2001;56:861–868. doi: 10.1212/wnl.56.7.861. [DOI] [PubMed] [Google Scholar]
- Polinsky R, et al. Decreased sympathetic neuronal uptake in idiopathic orthostatic hypotension. Ann Neurol. 1985;18:48–53. doi: 10.1002/ana.410180109. [DOI] [PubMed] [Google Scholar]
- Sandroni P, et al. Idiopathic autonomic neuropathy: comparison of cases seropositive and seronegative for ganglionic acetylcholine receptor antibody. Arch Neur. 2004;61:44–48. doi: 10.1001/archneur.61.1.44. [DOI] [PubMed] [Google Scholar]
- Schondorf R, Low P. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- Suarez G, et al. Idiopathic autonomic neuropathy: clinical, neurophysiologic, and follow-up studies on 27 patients. Neurology. 1994;44:1675–1682. doi: 10.1212/wnl.44.9.1675. [DOI] [PubMed] [Google Scholar]
- Thieben M, et al. Postural orthostatic tachycardia syndrome - Mayo Clinic experience. Mayo Clin Proc. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- Vernino S, et al. Neuronal nicotinic ACh receptor antibody in subacute autonomic neuropathy and cancer-related syndromes. Neurology. 1998;50:1806–1813. doi: 10.1212/wnl.50.6.1806. [DOI] [PubMed] [Google Scholar]
- Vernino S, et al. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- Vernino S, et al. Experimental autoimmune autonomic neuropathy. J Neurophysiol. 2003;90:2053–2059. doi: 10.1152/jn.00408.2003. [DOI] [PubMed] [Google Scholar]
- Young R, et al. Pure pan-dysautonomia with recovery. Trans Am Neurol Assoc. 1969;94:355–357. [PubMed] [Google Scholar]
- Young R, et al. Pure pan-dysautonomia with recovery. Description and discussion of diagnostic criteria. Brain. 1975;98:613–636. doi: 10.1093/brain/98.4.613. [DOI] [PubMed] [Google Scholar]