Abstract
CD4+CD25+ regulatory T cells (Tregs) play a critical role in preventing immune aggression. One way in which Tregs exert immune surveillance activities is by modifying the function of antigen presenting cells (APCs) such as dendritic cells, macrophages, and B cells. Tregs can induce apoptosis of APCs or inhibit their activation and function, thereby regulating subsequent innate and adaptive immune responses. These actions of Tregs are mediated by both soluble factors (interleukin [IL]-10, transforming growth factor-β, perforins, granzymes) and cell-associated molecules (cytotoxic T lymphocyte antigen 4, lymphocyte activation gene-3, CD18, neuropilin-1, LFA-1/CD11a, CD39), of which cytotoxic T lymphocyte antigen 4 has a key role. However, in autoimmunity, chronically activated APCs under the influence of intracellular signaling pathways, such as phosphatidyl inositol 3 kinase, JAK-STAT, MAPK, and nuclear factor-κB pathways, can escape surveillance by Tregs, leading to the activation of T cells that are refractory to suppression by Tregs. Moreover, APCs and APC-derived inflammatory cytokines such as tumor necrosis factor, IL-6, IL-1β, and IL-23 can render Tregs defective and can also reciprocally enhance the activity of the IL-17-producing pathogenic Th17 T cell subset. Emerging knowledge of the importance of APC-Treg interactions in maintaining immune tolerance and aberrations in this cross talk in autoimmune diseases provides a rationale for therapeutic approaches specifically targeting this axis of the immune system.
The immune system is constantly subjected to regulatory mechanisms. Apart from central tolerance, several mechanisms operate in the periphery to control undesired pathogenic immune responses to self-antigens (autoimmune diseases) and to prevent excessive inflammatory responses to foreign antigens. One level of control involves immune regulation by T cells with suppressive activity. Several prominent suppressive T cell subtypes have been characterized, including CD4+CD25+ regulatory T cells (Tregs), interleukin (IL)-10-producing T regulatory 1 cells, transforming growth factor (TGF)-β-producing Th3 cells, CD8+ T suppressor cells, and natural killer (NK)T cells. Among these populations, CD4+CD25+ Tregs have been extensively studied in recent years. This population includes those cells that acquire regulatory potential during differentiation in the thymus, termed ‘natural’ Tregs, and a population induced from naïve T cells in the periphery, called ‘induced’ or ‘adaptive’ Tregs.
Natural Tregs express glucocorticoid-induced tumor necrosis factor receptor, CCR4 (chemokine receptor for CCL17 and CCL22), CD62L (lymph node homing receptor), cytotoxic T lymphocyte antigen 4 (CTLA-4, a CD28-family receptor that binds to CD80 and CD86 on antigen-presenting cells), and the lineage-specific transcription factor forkhead box protein FoxP3, and most Tregs display markers of previous activation (eg, CD45RO in human and CD45RBlow in mouse). While induced Tregs share similar phenotype markers, the induced Tregs are functionally unstable and regulatory regions of the Foxp3 gene are more widely dimethylated in natural Tregs than in induced Tregs.1,2 In this review, we focus on natural Tregs as these are hitherto the best characterized population of regulatory T cells, and the mechanisms of maintenance of immune tolerance and the therapeutic strategies to target these Tregs are being explored in detail.
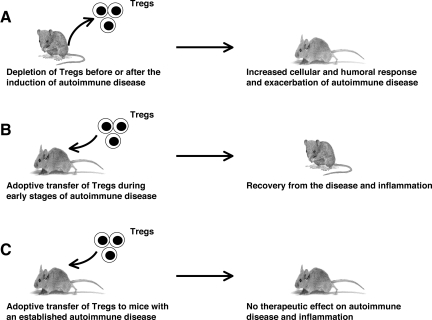
The central role of natural Tregs in maintaining self-tolerance has been shown clearly by the fact that their deficiency leads to fatal autoimmune diseases and inflammatory conditions with dysregulated lymphoproliferation. Thus, deficiency of Tregs in experimental animals results in either the appearance or exacerbation of autoimmune disease, whereas adoptive transfer of Tregs either before or during the early phase of induction of autoimmune disease cures the disease (Figure 1). In humans, IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome is a severe autoimmune inflammatory disorder that results from a deficiency of Tregs due to mutations in FoxP3.3,4,5 A naturally occurring mutation in FoxP3 similarly results in systemic autoimmunity in mice,6,7 as does ablation of Tregs in healthy adult mice.8 These observations indicate that Tregs actively regulate autoimmunity throughout life.
Figure 1.
Role of Tregs in the pathogenesis of autoimmune diseases: lessons from experimental models. A: Using several experimental animal models, it has been demonstrated that depletion of Tregs in animals before or after the induction of autoimmune disease leads to increased cellular and humoral responses and to an exacerbation of disease. B: Adoptive transfer of Tregs at the time of induction of disease has been shown to decrease the severity of disease and inflammation, suggesting that Tregs have a potential therapeutic application in the early stages of an autoimmune disease. C: Transfer of Tregs appears unable to cure established chronic inflammatory process, in part due to the influence of chronically activated APCs.
The population of natural Tregs is polyclonal and potentially able to recognize various self-antigens. Natural Tregs develop in the thymus, and their selection process is similar to conventional T cells except that natural Tregs are positively selected through recognition of self-peptides with a special range of avidities presented by thymic stromal cells. The signaling during these interactions likely imparts anergic status to Tregs and induces anti-apoptotic molecules, such as glucocorticoid-induced tumor necrosis factor receptor, that protect Tregs from negative selection.1 However, several aspects of the cellular basis of Treg maturation, including the role of antigen presentation, T cell receptor diversity, and interactions with thymic microenvironment are still not completely understood.
Once generated in the thymus, Tregs migrate to the periphery where they receive appropriate signals to survive. Mature naïve T cells in the periphery apparently require repeated contact with self-peptide-major histocompatibility complexes (MHCs) in the T-cell zones of secondary lymphoid organs, identical or similar to those on which they were originally selected in the thymus. However, several lines of evidence suggest that Tregs do not require such contacts, but that cytokines such as IL-2 and co-stimulatory pathway B7/CD28 may be involved in their maintenance in the periphery.1 On exposure to antigens in secondary lymphoid organs, Tregs become activated and exert suppressive functions at a much lower concentration of antigen than naive T cells, suggesting that Tregs can be activated even by immature dendritic cells (DCs), which express levels of co-stimulatory molecules such as CD80/86 and self-peptide/MHCs that are too low to activate naive self-reactive T cells.1
Several molecular and cellular mechanisms have been described to explain the process by which Tregs contribute to the maintenance of immune tolerance.2,9,10 Tregs inhibit the proliferation and cytokine production by conventional T cells in vitro and in vivo. In vitro studies have generally indicated that suppression of T cell responses requires direct cell–cell contact between the responding T cells and Tregs, but soluble factors, particularly TGF-β and IL-10, have also been implicated in Treg activity. In addition, Tregs can target several other cell types, including natural killer cells, natural killer T cells, and antigen presenting cells (APCs) such as DCs, macrophages, and B cells. Overall, the cumulative data suggest that the Treg-mediated surveillance mechanism might depend on several pathways that target multiple cell types at different anatomical sites. Given the crucial role of APCs in initiating, regulating, and maintaining immune responses, elucidating the nature of Treg-APC interactions is of great importance for the understanding of the pathogenesis of autoimmune and inflammatory diseases. Moreover, the ability to modify such regulatory mechanisms could provide novel therapeutic opportunities in these disorders. Here we discuss how Tregs exert immune tolerance by monitoring the activation and functions of APCs and the processes by which these cells can escape immune suppression by Tregs.
Modulation of Dendritic Cell Function by Tregs
DCs are professional APCs at the interface of innate and adaptive immunity and have several features which make them unique among APCs.11 Because of their capacity to stimulate naive T cells, DCs have a central role in the initiation of primary immune responses. However, this T cell stimulatory activity is not constitutive. Resting, or “immature” DCs have a high capacity to capture and internalize antigens but are poor stimulators of T cells. To acquire T cell stimulatory ability, DCs must undergo a maturation and activation process that involves up-regulation of surface expression of MHC class II and of co-stimulatory molecules, secretion of immunoregulatory cytokines, and migration into T cell areas of secondary lymphoid tissues. In addition to T cell stimulation, DCs also regulate B cell growth and secretion of immunoglobulins.
The functional plasticity of DCs is also related to the existence of heterogeneity within this cell population, which appears to be independent of maturation stage. Thus, several subpopulations of DCs with distinct features have been identified in human and mouse in lymphoid and nonlymphoid tissues.12 The major subtypes of human DCs are Langerhans cells, interstitial dermal DCs, monocyte-derived inflammatory DCs (Mo-DCs), BDCA-1+ and BDCA-3+ myeloid DC subsets, and plasmacytoid DCs. The major subsets of mouse DCs include CD4−CD8αhiCD205hiCD11b− DCs, CD4+CD8α−CD205−CD11b+ DCs, CD4−CD8α−CD205− CD11b+ DCs, CD4−CD8α−CD205+CD11b+ DCs, plasmacytoid DCs, and Langerhans DCs.
Several recent reports demonstrate that Tregs modulate the maturation, activation, and function of various subsets of human and murine DCs both in vitro and in vivo.13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30 Interaction of immature or activated human Mo-DCs or circulating myeloid DCs, murine CD11c+splenic, or bone marrow-derived DCs (BM-DCs) in vitro with Tregs has been shown to result in the down-regulation of the co-stimulatory molecules CD80, CD86, and CD40, and the number of MHC-peptide complexes on DCs, as well as up-regulation of the inhibitory B7-H3 molecule (a member of the programmed death ligand family that comprises B7-H1, B7-DC, B7-H3, and B7-H4), leading to impaired T cell stimulatory function of DCs. Furthermore, Tregs have been shown to reduce significantly the production of several DC-associated inflammatory cytokines such as IL-12, IL-1β, IL-6, and IL-8, but to enhance expression of the anti-inflammatory cytokine IL-10. These Treg “educated” DCs were found to be poor stimulators of T cell proliferation and induce lower amounts of T-cell cytokines such as IL-2, interferon (IFN)-γ, IL-4, and IL-5.
Consistent with in vitro results, in vivo results in experimental models show that Tregs inhibit T cell immune responses mediated by CD11c+ splenic and lymph node DCs, BM-DCs, pulmonary myeloid DCs, and airway mucosal DCs.23,31,32,33 Tregs can exert an early effect on the immune responses by attenuating the establishment of stable contacts during priming of naive T cells by CD8α+ DCs, CD11b+ DCs, and BM-DCs both in vitro and in vivo and by forming synapses and aggregation with DCs more frequently than naive T cells.18,19,34 These prolonged interactions of Tregs with DCs are mediated by virtue of high expression of neuropilin-1 and adhesion molecules such as LFA-1 (CD11a) on Tregs.18,28,34 Visualization of adoptively transferred Tregs in the lymph nodes of mice revealed that Tregs form stable associations with CD8α+ and CD11b+ DCs that in turn prevents subsequent strong interactions between DCs and autoreactive effector T cells. Significant contact between Tregs and effector T cells was not observed, thus implying that DCs are the primary targets of Tregs in vivo.18,19
Modulation of Cross Talk between DCs and NK Cells by Tregs
NK cells are a type of cytotoxic lymphocyte that constitute a major component of the innate immune system. DCs can prime resting NK cells, which, in turn, after activation, might induce DC maturation. However, NK cells also negatively regulate the function of DCs by killing immature DCs in peripheral tissues. Interestingly several reports demonstrate that Tregs modulate the interactions between DCs and NK cells. Thus, in one study, Tregs were shown to interfere with IL-15-mediated proliferation of NK cells by suppressing the expression of IL-15Rα, on DCs, which normally “trans-presents” bound IL-15 to NK cells.35 In another report, Tregs were shown to control mature NK cell number in lymph nodes, in the process indirectly modulating the proportion of CD8α+ immature DCs in these locations.36 Thus, it was demonstrated that depletion or adoptive transfer of Tregs in vivo leads to increased or decreased numbers of mature NK cells and decreased or increased proportions CD8α+ immature DCs, respectively, in the lymph nodes (ie, increased Tregs → decreased NK cells → increased CD8α+ immature DC, and vice versa). Since immature DCs maintain steady state tolerance to peripheral self-antigens, the ability of Tregs to regulate the number of these cells could represent part of the mechanism by which Tregs act in vivo.
Mechanisms of Modulation of DC Functions by Tregs
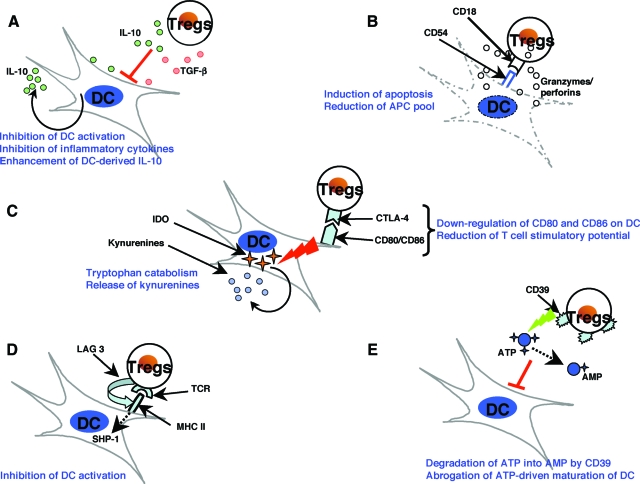
Several mutually nonexclusive mechanisms have been proposed to describe the modulation of DCs by Tregs (Figure 2). While for many of these, the significance remains to be verified in vivo, the implication is that Treg modulation of DCs involves multiple mechanisms; several of the reported mechanisms are described briefly below. It remains to be determined whether these mechanisms represent components of the same pathway or of parallel pathways that converge into a common point.
Figure 2.
The mechanisms of suppression of dendritic cells by Tregs. Several mutually nonexclusive mechanisms have been proposed to account for the modulation of DCs by Tregs. The suppression of DCs by Tregs is mediated by both soluble factors and cell-associated molecules. However, recent reports suggest that CTLA-4 molecule has a key role in Treg-mediated suppressive functions. A: Treg-derived IL-10 and TGF-β induce a suppressive phenotype in DCs and inhibit inflammatory cytokine expression by DCs while enhancing the secretion of the anti-inflammatory cytokine IL-10. B: Activated Tregs express high levels of granzyme A and perforin and exert CD18/CD54 adhesive interaction-dependent cytotoxicity against both immature and mature DCs, thus reducing the antigen-presenting and co-stimulatory pool. C: CTLA-4 interaction with B7 molecules transduces a negative signal for DCs CTLA-4 diminishes the co-stimulatory molecules CD80 and CD86 and converts DCs into less potent APCs with reduced T cell stimulatory potential. CTLA-4 can also induce indoleamine 2,3-dioxygenase (IDO) expression in DCs. IDO catalyzes the conversion of tryptophan into kynurenines, which are potent immunosuppressive metabolites. D: Tregs can interact with MHC class II on DCs via lymphocyte activation gene-3 (LAG-3), a CD4-related transmembrane protein that inhibits DC activation by a mechanism involving ERK-mediated recruitment of SHP-1. E: Tregs express CD39 (nucleoside triphosphate diphosphohydrolase-1), an ectoenzyme that degrades ATP to AMP. Thus, Tregs reduce ATP-mediated activation of DCs and the adjuvant activities of ATP. Some of these mechanisms are also reported to be critical in Treg-mediated regulation of functions of macrophages and B cells.
Tregs constitutively express high levels of CTLA-4 that binds to CD80 (B7–1) and CD86 (B7–2) with higher affinity. In addition to blocking CD28-B7 co-stimulatory signaling between effector T cells and DCs, the interaction of CTLA-4 on Tregs with CD80 and CD86 molecules on CD11c+ splenic DCs transduces a negative signal to DCs, and reduces the expression of these co-stimulatory molecules on DCs in vitro.28,30 Since co-stimulatory molecules provide intense stimuli for activation of lymphocytes, inhibition of these molecules by CTLA-4 affects the capacity of CD11c+ splenic and BM-DCs to activate lymphocytes and thus renders them less competent APCs.21,30 Interestingly, deficiency of CTLA-4 abolishes the capacity of Tregs to induce tolerance. Furthermore, CTLA-4 interaction with B7 molecules may induce indoleamine 2,3-dioxygenase (IDO) expression in CD11c+ splenic DCs in vitro and prime DCs for tolerogenic antigen presentation in vivo.15,37 IDO catalyzes the conversion of tryptophan into kynurenines that act as potent immunosuppressive metabolites in the local environment of DCs by means of either cytotoxicity or by inducing de novo generation of Tregs from naïve CD4+CD25− T cells. These data thus indicate that CTLA-4 engagement of B7 molecules on DCs represents a critical mechanism of tolerance induction in vivo.15,30
Treg-derived IL-10 and TGF-β confer a suppressive phenotype on human Mo-DCs, circulating myeloid DCs, and murine BM-DCs and inhibit expression of inflammatory cytokines in vitro.16,20,22,25 Tregs also induce production of the anti-inflammatory cytokine IL-10 by DCs, which further spreads the immunosuppression by acting on both DCs and effector T cells.16,17 DC-derived IL-10 can also induce T regulatory 1 cells, which can inhibit antigen-specific T cell responses.
In addition, activated Tregs express high levels of granzyme A and can display perforin-dependent cytotoxicity in vitro against both immature and mature human Mo-DC.38 This cytotoxicity is dependent on CD18/CD54 adhesive interactions but is independent of Fas/FasL. These findings suggest that the perforin/granzyme pathway is one of the mechanisms that Tregs can use to control immune responses by reducing the antigen-presenting and co-stimulatory pool.
Tregs have been reported to interact with MHC class II on human Mo-DCs, murine BM-DCs and CD11c+ splenic DCs via lymphocyte activation gene-3 (LAG-3), a CD4-related transmembrane protein, the expression of which is regulated by heme oxygenase-1.24,39,40 It was shown that during Treg-DC interactions, LAG-3 engagement with MHC class II molecules inhibits Mo-DC activation and generates semimature DCs with the potential to migrate into secondary lymphoid organs. Indeed, MHC class II cross-linking by agonistic antibodies in vitro recapitulates the activity of LAG-3, characterized by the induction of an ITAM-mediated inhibitory signaling pathway, and involves FcγR and ERK-mediated recruitment of SHP-1, which suppresses BM-DC maturation and immunostimulatory capacity. This novel, ITAM-mediated inhibitory signaling pathway in DCs triggered by MHC class II engagement of LAG-3 provides a molecular mechanism by which Tregs may down-modulate immune responses via modulating DC function.
Tregs express CD39 (nucleoside triphosphate diphosphohydrolase-1 [NTPDase 1]), an ectoenzyme that degrades ATP to AMP.41 In the immune system, extracellular ATP functions as a “natural adjuvant” that exhibits multiple pro-inflammatory effects. By virtue of the expression of CD39, activated Tregs are able to abrogate in vitro ATP-related effects such as P2 receptor-mediated cell toxicity and ATP-driven maturation of BM-DC.41
The Physiological Significance of Treg-Mediated Inhibition of DC Function
Accumulating evidence suggests that DCs attain a semimature tolerogenic phenotype on interaction with Tregs and gain an ability to migrate to secondary lymphoid tissues, where presentation of self-antigenic peptides to effector T cells in the context of reduced co-stimulatory signaling and inflammatory cytokines results in down-regulation of T cell activation, proliferation, and intensity of immune response. Thus, in addition to directly suppressing autoreactive T cells, Tregs maintain immune tolerance via modulating DC function.
Tregs have also been shown to participate in the control of immune responses against infection, helping to avoid pathology and tissue damage that could be associated with excessive responses. Indeed, inhibition of Treg activity either by their depletion by monoclonal antibodies or by blocking their migration results in markedly superior immune responses to pathogens and vaccines.27,42,43 The inhibitory effects of Tregs on DCs exposed to infection-associated molecules, such as lipopolysaccharide, CpG, and Poly I:C, could involve the generation of tolerogenic DCs with low levels of expression of co-stimulatory molecules and inflammatory cytokines, and the generation of IDO, which compromises effector function of T cells by affecting their tryptophan metabolism.15,17,20,22,24,31,44 Thus, modulation of DCs by Tregs in the context of infection may provide a fail-safe mechanism; should presentation of self antigens predominate on an activated DC, Tregs could help prevent induction of autoimmunity during the context of infection.
Modulation of Functions of Monocytes/Macrophages by Tregs
Monocytes/macrophages play a critical role in both innate and adaptive immunity that is linked to several key functions of these cells: (1) recognition of foreign antigens via pattern-recognition receptors, (2) phagocytosis of antigens and antigen clearance, (3) processing and presenting antigen-derived peptides in the context of MHC class II molecules to CD4+ T cells, and (4) secretion of a wide range of immunoregulatory cytokines and chemokines. At present, macrophages are classified as belonging to two major subtypes based on their functional properties and mode of generation.45 The initiation of the inflammatory process is mediated by M1, or classically activated, macrophages, which produce elevated amounts of pro-inflammatory cytokines and reactive oxygen species. This subset can be generated in vitro by activation with IFN-γ/lipopolysaccharide. On the contrary, M2, or alternatively activated macrophages, are associated with the resolution phase of inflammation. The M2 macrophages mainly produce anti-inflammatory cytokines, express high levels of the endocytic macrophage-mannose receptor CD206 and/or the hemoglobin scavenger receptor CD163, and have high phagocytic activity. This subset of macrophages can be obtained by stimulating macrophages with IL-4/IL-13 or IL-10 or glucocorticoids.45,46,47
Several in vitro and in vivo experiments suggest that Tregs exert direct suppressive effects on monocytes/macrophages, thereby affecting subsequent innate and adaptive immune responses. Co-culture of monocytes with Tregs results in reduced expression of MHC class II, CD40, CD80, and CD86, and reduced inflammatory cytokine production as compared with stimulation with effector T cells.48,49,50 On the other hand, Tregs steer monocyte differentiation toward alternatively activated (M2-like) macrophages, which produce increased amounts of CCL18.
Treg-conditioned monocytes and macrophages inefficiently produce inflammatory cytokines and chemokines such as IL-1β, IL-6, IL-8, tumor necrosis factor (TNF), and macrophage inflammatory protein-1α, even on activation with toll-like receptor-ligands.48,50 In addition, Tregs also inhibit lipopolysaccharide-induced survival of monocytes through a pro-apoptotic mechanism involving the Fas/FasL pathway.51 While in vivo experiments provide evidence that Treg-derived IL-10 and TGF-β can suppress innate immune responses,49,52 Tregs can also trigger high levels of IL-10 production by monocytes and macrophages. This IL-10 can act in an autocrine manner to stimulate expression of B7-H4 on monocytes/macrophages.53 Importantly, Treg-conditioned monocytes and macrophages are poor stimulators of T cells, and high expression of B7-H4 on monocytes/macrophages partly contributes to reduced T cell activation.48,53
Thus, these data provide evidence that Tregs promote the induction of alternatively activated monocytes/macrophages, which will help to maintain tissue homeostasis and prevent local tissue damage. These findings may help us to understand the pathogenesis of many autoimmune diseases and may also provide tools to manipulate local immune responses.
Modulation of Functions of B Lymphocytes by Tregs
The function of B lymphocytes in the immune response is not only restricted to the production of antibodies; B cells also mediate multifaceted functions essential for dynamic immune equilibrium. B cells are required for optimal CD4+ T cell activation and, through specific B cell receptor, can internalize, process, and then present antigenic peptides to T cells in an MHC class II-restricted manner. B cells also produce cytokines and chemokines that promote the organization of lymphatic architecture.54 Therefore, a harmony in the activation and function of B cells is a critical factor in maintaining immune tolerance.
Regulation of B cell functions appears to be another important factor in the maintenance of tolerance by Tregs. Tregs have been reported to regulate the antigen-presenting pool of B cells both in vitro and in vivo by directly inducing apoptosis of proliferating antigen-specific B cells via perforin and granzymes.55,56 In addition, administration of Tregs into autoimmune animals significantly reduces autoantibody responses.57 Recruitment of Tregs to B cell areas of secondary lymphoid tissues by the chemokine CCL4 can suppress T cell-dependent B cell humoral responses in vivo to both self and foreign antigens, whereas a defect in Treg recruitment leads to autoimmune activation and excessive humoral immune response to target antigens.58,59,60 Interestingly, Lim and colleagues have shown that Tregs that are found in T cell-B cell area borders and within germinal centers of human lymphoid tissues can suppress directly the B cell immunoglobulin response without the need to first suppress T cells.61 The direct suppression of B cell immunoglobulin production by Tregs is accompanied by inhibition of immunoglobulin class switch recombination.
There is evidence that Tregs exert immunosuppression on T cell-dependent B cell immunoglobulin production by a cell-cell interaction involving cell surface TGF-β1.60,62 It has also been suggested that Tregs maintain human B cell tolerance via CD40L-CD40 and T cell receptor-MHC class II interactions.63 Further, Fields et al report that Tregs inhibit the maturation, rather than the initiation, of autoantibody responses in vivo. This function of Tregs is associated with an inhibition of production of Th cell cytokines IL-10 and IFN-γ.64
Role of APCs in Autoimmune and Inflammatory Diseases
A breakdown in immune tolerance is central to the initiation and progression of autoimmune disease. APCs play a crucial role in the pathogenesis of autoimmune and inflammatory disorders. In human leukocyte antigen-restricted autoimmune diseases, DC priming of the immune response in lymphoid organs is a requisite first step that leads to the activation of T cells and the production of pathogenic autoantibodies and the ensuing chronic inflammatory process.65,66 Unabated induction of DC maturation by IFN-α has been proposed to drive the autoimmune responses in systemic lupus erythematosus.67 By coordinating the recruitment and/or activation of other immune cells, DCs can also drive the generation of ectopic lymphoid tissues, as in the case of inflamed synovia in rheumatoid arthritis (RA) and systemic lupus erythematosus.68
Monocytes/macrophages, in addition to presenting auto-antigens in the context of MHC class II molecules, produce a broad range of cytokines and chemokines that influence the migration of immune cells at the site of inflammation. Macrophages have destructive and remodeling capabilities that determine the course and intensity of the immune response in inflammation and autoimmune diseases. The abundance and activation state of macrophages frequently correlates with the severity of inflammatory process.
B lymphocytes contribute to the pathogenesis of autoimmune diseases by producing autoantibodies, but a broader role of B cells is becoming recognized from the somewhat unexpected efficacy of B cell-targeted therapies in autoimmune diseases not thought to be primarily antibody-mediated. One other important function of B cells is their ability to act as APCs for T cells; in the context of autoimmunity, this can contribute to the activation of autoreactive T cells. In addition, reciprocal “help” from the activated T cells further leads to the expansion of autoreactive B cell clones. Extra-follicular germinal center-like structures and a disturbance of peripheral blood B cell homeostasis are characteristic features of patients with autoimmune diseases,54 and chronic activation of B cells in inflammatory settings such as RA and systemic lupus erythematosus may also lead to B cell lymphoproliferative disorders.69
Tregs in Autoimmune and Inflammatory Diseases
An imbalance in immune regulation due to Treg deficiency or defective function can lead to autoimmunity.8,70 Analysis of the frequency of peripheral blood CD4+CD25+ Tregs in autoimmune patients has, however, yielded contradictory results. While some studies have reported an increased frequency of peripheral blood Tregs, others have demonstrated either no difference in the frequency of Tregs, as compared with healthy donors, or a decreased level of peripheral blood Tregs (see review by Brusko et al).70 In part, this may reflect the imprecision in identifying Tregs phenotypically, particularly in using CD25, which is also expressed on recently activated conventional T cells, as a Treg marker. This is clearly a potential confounding factor in disease settings in which T cell activation figures prominently. In addition, although FoxP3 is required for and closely associated with Treg development and activity, it too can be transiently expressed by activated human non-Treg T cells.71 Therefore, these conflicting results might be due in part to the different methodologies used to analyze the Treg populations or be related to the analysis of Tregs in patients with different stages of pathogenesis, the past history of the patients, and the modes of immunosuppressive treatment they receive.
On the contrary, several reports have demonstrated an increase in the frequency of Tregs at the sites of inflammation. This could be due to preferential homing of Tregs to inflammatory sites and/or local expansion of a Treg population as a compensatory mechanism to reduce the inflammation. However, the persistence of inflammation despite an increased number of Tregs at the site of inflammation indicates that these cells are ineffective in controlling the inflammatory response. While this may again reflect the lack of definitive phenotypic markers for Tregs, it also highlights that analyzing the frequency of Tregs alone is not sufficient to decipher the relevance of Tregs in autoimmune diseases.72 Ultimately, assessing Treg function is essential.
Notwithstanding these caveats, there is now a widely held view that defective regulation of the immune system by Tregs contributes to the pathogenesis of several autoimmune diseases. Patients with autoimmune diseases such as multiple sclerosis, autoimmune polyglandular syndrome type II, systemic lupus erythematosus, type 1 diabetes, psoriasis, myasthenia gravis, RA, and chronic immune thrombocytopenia appear to possess Tregs with compromised functional activity.70,73,74,75,76,77,78,79,80,81,82,83,84 Thus, putative Tregs isolated from patients with autoimmune diseases show functional defects such as reduced expression of FoxP3, CTLA-4, impaired survival, inability to suppress the proliferation of effector T cells, inability to suppress pro-inflammatory cytokine secretion from activated T cells and monocytes, and an inability to confer a suppressive phenotype on conventional T cells. One possible explanation for these observations is that the “Treg” population, defined phenotypically, actually contains a significant proportion of activated conventional T cells in autoimmune disease, which would confound in vitro assays of suppressive activity. Hence, the defect could lie in Treg numbers rather than function. However, there is also clear evidence that exposure to inflammatory cytokines released by APCs can render Tregs defective in mediating their suppressive, anti-inflammatory functions.
Chronically Activated APCs Can Escape Surveillance by Tregs
Adoptive transfer of Tregs fails to cure the established autoimmune disease in various experimental models (Figure 1). These results indicate that under conditions of chronic inflammation or in a situation where disease is already established, Tregs are unable to exert their functions. This appears to be due in part to the influence of chronically activated APCs on Tregs. APCs can render Treg-mediated immune regulation defective by several mechanisms.
APCs receive and respond to environmental stimuli provided by co-stimulatory molecules (mediated, for example, by increased co-stimulation through CD40 or ICOSL) and inflammatory cytokines via several distinct intracellular signal transduction pathways. The major intracellular signaling pathways that are implicated in chronic activation of APCs and inflammatory response include phosphoinositide-3 kinase (PI3K), Janus protein tyrosine kinase family (JAK)-signal transducers and activators of transcription (STAT), Ras-mitogen-activated protein kinase (MAPK), and nuclear factor (NF)-κB pathways. Chronically activated APCs can escape suppression by Tregs and generate activated T cells that are refractory to the suppression by Tregs. In several experimental models, it has been demonstrated that CD154-CD40 interactions and strong innate stimuli can release immature DCs and macrophages from suppression by Tregs.14,26,44,85 This escape of DCs and macrophages from Tregs suppressor activity is dependent in part on IL-6 induced by toll-like receptors on recognition of microbial products.
Although Tregs can suppress the production of inflammatory cytokines by recently activated APCs, high amounts of pro-inflammatory cytokines that are released by APCs due to constant and prolonged activation can render Tregs functionally defective. For instance, prolonged exposure to TNF can inhibit the function of Tregs by signaling through TNF receptor II, which is constitutively expressed on Tregs and the expression of which is up-regulated by TNF.78,80 TNF-mediated inhibition of Tregs function was shown to associate with a decrease in the expression of FoxP3 mRNA and protein by CD4+CD25high Tregs. Further, IL-6, IL-12, and IL-1β may also promote autoimmunity by interfering with the regulatory functions of Tregs and promoting activation and expansion of effector T cells.44,86,87,88 These findings highlight an interaction between the innate and adaptive immune systems, in which inflammatory cytokines, products of the innate immune compartment, could promote immune reactivity by limiting the action of Tregs.
In addition to directly suppressing the functions of Tregs, APC-derived cytokines such as IL-6, IL-1β, and IL-23 can reciprocally regulate the populations of induced Tregs and IL-17-producing pathogenic Th17 cells.89 Th-17 cells are characterized by the expression of transcription factor RORC2+ (in human) or RORγt+ (in mice), require STAT3 signaling for differentiation, and produce IL-17 as well as other cytokines including IL-17F and IL-22. Th17 cells are critical for protection against several extra-cellular pathogens but are also implicated in the induction and progression of many autoimmune diseases such as RA, multiple sclerosis, systemic scleroderma, and psoriasis.89,90 In humans, IL-1β and IL-6 promote IL-17 and IL-21 production from activated and memory T cells. IL-21 further supports the differentiation of RORC2+ Th17 cells from naïve CD4 T cells in the presence of TGF-β. Notably, TGF-β typically functions as an anti-inflammatory cytokine; it can induce FoxP3+ Tregs from non-Tregs populations and is required for the maintenance of induced Treg in the periphery. IL-23 may further expand or stabilize differentiated Th17 cells.91,92,93,94,95,96 Conversely, in mice, TGF-β contributes to the generation of RORγt+ Th17 cells in the presence of IL-6,97,98 while IL-21 amplifies and IL-23 stabilizes the differentiated Th17 cells.99,100,101 IL-6 attenuates the FoxP3 function and hence the activity of Tregs by down-regulating FoxP3 binding to chromatin in the presence of TGF-β and by inhibiting the recruitment of FoxP3 on the human IL-2 promoter.102
Therapeutic Strategies to Render Tregs Functionally Fit in Vivo
The emerging knowledge of the importance of APCs, APC-derived cytokines, and intracellular signal transduction pathways in creating an inflammatory environment in autoimmune diseases provides a rationale for pursuing strategies to block these inhibitory pathways as a means to render Tregs functionally competent and restore tolerance mechanisms in vivo.103 In fact, evidence to date suggests that therapies aimed at APCs and APC-derived cytokines do have positive effects on Tregs.
Targeting Inflammatory Cytokines by Monoclonal Antibodies
In accordance with the negative effect of TNF on Treg function, treatment of RA patients with anti-TNF monoclonal antibodies has been reported to restore the function of Tregs, increase FoxP3 mRNA and protein expression, enhance survival of Tregs, and decrease expression of TNF receptor II.78 Further investigation in patients with RA has suggested that anti-TNF (infliximab) therapy leads to de novo production of a differentiated population of Tregs that exerts its suppressive function via TGF-β and IL-10.79 Unlike natural Tregs, these de novo-generated CD4+CD25highFoxP3+ Tregs lack CD62L expression and hence are phenotypically distinct. Interestingly, despite the fact that these de novo-differentiated Tregs possess potent suppressor activity, the natural CD62L+ Tregs remained defective in patients treated with infliximab. Of note, clinical studies have demonstrated that anti-TNF therapy, in addition to neutralizing TNF, can also suppress the production of other cytokines, such as IL-6 and IL-1β, that are also implicated in rendering Tregs defective.104 Such a broad range of anti-inflammatory effects of anti-TNF therapy may explain the restoration of Treg functions, although future studies will be required to investigate directly the effects on Tregs of individual neutralizing monoclonal antibodies to IL-6 and IL-1β. In addition, considering the role of IL-23 in stabilizing the Th17 population, neutralization of IL-23 by monoclonal antibody specific for the p40 subunit of IL-12 and IL-23 (anti-IL-12p40) might also allow Treg function105,106; data on this question are yet to be reported.
Depletion of APCs
There is evidence that targeting APCs can improve Treg-mediated tolerance mechanisms. In particular, depletion of B cells by anti-CD20 monoclonal antibody (rituximab) in patients with mixed cryoglobulinemia vasculitis and systemic lupus erythematosus results in a significant increase in the Treg population.107,108 Thus, while restoration of Treg function has thus far not been a direct goal of these therapeutic interventions, it may contribute to the benefit that has been observed.
Targeting Intracellular Signal Transduction Pathways
Distinct intracellular signaling pathways regulate diverse cellular responses, such as activation, proliferation, secretion of soluble factors, survival, migration, and cellular differentiation in response to stimuli. Therefore these signaling pathways are possible therapeutic targets to inhibit inflammatory response and hence we can expect that these therapeutic approaches may also improve the functions of Tregs.
PI3K signaling pathway: PI3K is activated by multiple signals including inflammatory cytokines, the B7/CD28 co-stimulatory pathway and adhesive molecules. Of particular interest, TNF, one of the major inflammatory cytokines that inhibits the functions of Tregs, activates PI3K through Akt and NF-κB.109 Interestingly, the PI3K/Akt pathway is dispensable in cytokine-mediated survival of Tregs, and reduced activity of this pathway is necessary for the suppressive function of human Tregs.110,111 Therefore, small molecule inhibitors of PI3K pathways are possible targets to improve the functions of Tregs.112 Alternatively, generation of small molecule agonists to phosphatase and tensin homologue deleted on chromosome 10, a molecule expressed highly in Tregs and implicated in negative regulation of the PI3K signaling pathway,110 may also be of benefit. In fact, mice that are haploinsufficient for or have a T cell-specific deletion of phosphatase and tensin homologue deleted on chromosome 10 develop autoimmune disease.113
JAK-STAT signaling pathways: Several inflammatory cytokines mediate immune responses via JAK-STAT signaling pathways. JAKs are receptor-associated protein tyrosine kinases and contain four members: JAK1, JAK2, JAK3, and TYK2. STATs are latent cytoplasmic transcription factors that are activated by tyrosine phosphorylation and there are seven STAT proteins: STAT1–4, 5A, 5B, and 6. In general, cytokine stimulation involves the ligation of at least two different receptor subunits, and association of a pair of different JAKs.114 For example, IL-6 activates JAK1 and JAK2, and IL-15 activates JAK1 and JAK3. Although individual STATs may be activated by multiple ligands, some cytokines preferentially activate particular STATs. Thus, IL-6 preferentially activates STAT3, and IFN-γ preferentially activates STAT1.114 Therefore by targeting JAK-STAT pathways, inflammatory responses mediated by selective cytokines can be inhibited and hence can improve the functions of Tregs.
Interestingly, selective inhibitors of JAK kinases have been discovered in recent years with promising results in experimental models and clinical trials.114 For example, clinical trials for a small molecule antagonist (CP690550) that targets the JAK-3 pathway, and hence blocks signals of IL-2, IL-4, IL-7, and IL-15, are ongoing in patients with RA.115 On the contrary, small molecule inhibitors of STATs have not yet been successfully developed. The pleiotropic nature of STATs, which can have divergent activities on the pathogenesis of autoimmune disease, depending on cell type and stage of the disease, may be a confounding factor.114 Therefore STATs might have both pathogenic and protective roles and hence caution should be exercised while targeting these pathways.
MAPK signaling pathways: The MAPKs are group of signaling molecules that relay pro-inflammatory signals to the nucleus via a cascade of phosphorylation events. Among the members of MAPKs, ERK, c-Jun N-terminal kinase, and MAPKp38 have received particular attention. Inflammatory cytokines regulate activation, proliferation, survival, and differentiation of immune cells by signaling via these molecules. In fact, these signaling pathways are known to be activated in several inflammatory disorders such as RA.115
Inhibition of MAPKp38 or c-Jun N-terminal kinase with specific small-molecule inhibitors has been tested in experimental arthritis. The blockade of these signaling pathways was associated with a reduction in the expression of numerous inflammatory cytokines such as TNF, IL-1β, and IL-6.116,117 Considering the direct inhibitory effect of these inflammatory cytokines on Tregs and their role in reciprocal regulation of the populations of induced Tregs and Th17 subsets, it is tempting to speculate that blocking MAPKs would render Tregs functionally competent and restore tolerance mechanisms in vivo. However, considering the fact that MAPKs are involved in diverse physiological processes and due to overlapping functions of the different MAPKs, highly selective therapeutic molecules with a fine balance between therapeutic effects and safety should be considered.
NF-κB signaling pathways: The transcription factor NF-κB is widely recognized as a critical mediator of immune and inflammatory responses. In nonstimulated cells, NF-κB is bound to inhibitor of nuclear factor-κB (IκB) and is thereby sequestered in the cytoplasm. Signaling by inflammatory cytokines and toll-like receptors promotes phosphorylation of IκB by IκB kinase. The phosphorylated IkB then dissociates from NF-κB in the cytosol and activated NF-κB then translocates to nucleus.118 In the nucleus, NF-κB regulates genes encoding cytokines, cytokine receptors, cell adhesion, and co-stimulatory molecules, and genes involved in cell-growth, differentiation, and the inflammatory process. Therefore, therapeutic molecules that inhibit phosphorylation of IκB (for eg, BMS-345541) and thus inhibit the activation of NF-κB and inflammation119 may prove to be very promising candidates to improve Treg functions.
Conclusion and Perspectives
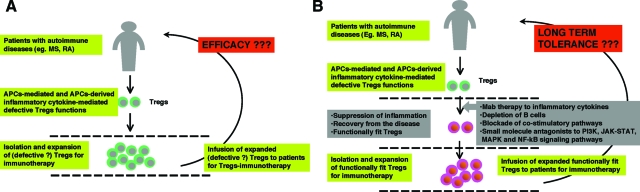
Enhancing or restoring Treg function appears to be a promising avenue for therapeutic immunointervention in autoimmune and inflammatory diseases. For this reason, recent years have witnessed a flurry of proposed approaches for Treg immunotherapy, through adoptive transfer of Tregs, to treat such disorders.120 In favor of such a strategy, several experimental models have demonstrated that prophylactic Treg therapy or therapy during the early stages of disease can be of benefit. However, there are currently numerous obstacles to the use of Treg therapy in patients. First, isolation and expansion of antigen-specific Treg populations from patients will require highly specialized facilities and the procedure will be extremely costly. Second, identifying and isolating relevant antigen-specific Treg populations remains a serious challenge. Third, chronic exposure of Tregs to an inflammatory environment appears to result in irreversible defects in Treg function. Therefore, Treg-immunotherapy based on isolating and expanding potentially defective Tregs might not be efficacious if such defects are not reversed (Figure 3). Furthermore, ongoing inflammation within the host could also render Tregs defective after adoptive transfer.
Figure 3.
Neutralization of inflammatory cytokines and blocking APC function are critical for the success of Treg immunotherapy in chronic autoimmune and inflammatory diseases. A: Tregs are reported to be defective in several autoimmune diseases such as multiple sclerosis (MS) and rheumatoid arthritis (RA). Therefore, Treg-immunotherapy, by isolating and expanding these ‘defective’ Tregs, might not be efficacious in inducing tolerance. Further, ongoing inflammation within the host could also render Tregs defective after adoptive transfer. B: In such conditions, therapeutic restoration of the Treg compartment in vivo either by targeting inflammatory cytokines or B cells, blocking co-stimulatory pathways, or targeting intracellular signal transduction pathways (PI3K, JAK-STAT, MAPK, or NF-κB signaling pathways) might constitute a primary goal. Once conditions are established in which Treg function is restored, Treg-immunotherapy by isolating, expanding, and re-infusing these competent cells could be considered as a second step for inducing long-term immune tolerance.
In light of these challenges, therapeutic restoration or boosting of the Treg compartment in vivo by direct or indirect (ie, targeting factors that inhibit Treg function) approaches might constitute a better option (Figure 3). The amenability of these approaches to small molecule or biopharmaceutical therapeutics would allow for such treatments to be more affordable and more widely available than customized Treg therapy. However, it is also unclear whether current transient anti-inflammatory approaches can lead to long-term restoration of Treg-mediated tolerance. Therapies that preferentially target Treg enhancement or regeneration might prove more efficacious. Ultimately, combining Treg-immunotherapy with an anti-inflammatory approach might offer an improved opportunity for maintaining long-term immune tolerance.
Footnotes
Address reprint requests to Jagadeesh Bayry, DVM, PhD, INSERM U 872, Equipe 16, Centre de Recherche des Cordeliers, 15 rue de l’Ecole de Médicine, Paris, F-75006, France. E-mail: jagadeesh.bayry@crc.jussieu.fr.
Supported by grants from Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), UPMC-Paris VI, and Agence Nationale de la Recherche (ANR-07-JCJC-0100-01 and ANR-07-RIB-002-02), France.
References
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Serra P, Amrani A, Yamanouchi J, Han B, Thiessen S, Utsugi T, Verdaguer J, Santamaria P. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J Immunol. 2006;176:6202–6210. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houot R, Perrot I, Garcia E, Durand I, Lebecque S. Human CD4+CD25high regulatory T cells modulate myeloid but not plasmacytoid dendritic cells activation. J Immunol. 2006;176:5293–5298. doi: 10.4049/jimmunol.176.9.5293. [DOI] [PubMed] [Google Scholar]
- Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayry J, Triebel F, Kaveri SV, Tough DF. Human dendritic cells acquire a semimature phenotype and lymph node homing potential through interaction with CD4+CD25+ regulatory T cells. J Immunol. 2007;178:4184–4193. doi: 10.4049/jimmunol.178.7.4184. [DOI] [PubMed] [Google Scholar]
- Mahnke K, Ring S, Johnson TS, Schallenberg S, Schonfeld K, Storn V, Bedke T, Enk AH. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: role of B7–H3 expression and antigen presentation. Eur J Immunol. 2007;37:2117–2126. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- Bayry J, Kaveri SV, Tough DF. Do activated human dendritic cells diminish the suppressive functions of CD4+, CD25+ regulatory T cells? Arthritis Rheum. 2007;56:3874–3876. doi: 10.1002/art.23028. [DOI] [PubMed] [Google Scholar]
- Larmonier N, Marron M, Zeng Y, Cantrell J, Romanoski A, Sepassi M, Thompson S, Chen X, Andreansky S, Katsanis E. Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-β and IL-10. Cancer Immunol Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanig J, Lutz MB. Suppression of mature dendritic cell function by regulatory T cells in vivo is abrogated by CD40 licensing. J Immunol. 2008;180:1405–1413. doi: 10.4049/jimmunol.180.3.1405. [DOI] [PubMed] [Google Scholar]
- Bayry J, Tchilian EZ, Davies MN, Forbes EK, Draper SJ, Kaveri SV, Hill AV, Kazatchkine MD, Beverley PC, Flower DR, Tough DF. In silico identified CCR4 antagonists target regulatory T cells and exert adjuvant activity in vaccination. Proc Natl Acad Sci USA. 2008;105:10221–10226. doi: 10.1073/pnas.0803453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit A, Gebhardt F, Lahl K, Neuenhahn M, Schmitz F, Anderl F, Wagner H, Sparwasser T, Busch DH, Kastenmuller K. Circumvention of regulatory CD4(+) T cell activity during cross-priming strongly enhances T cell-mediated immunity. Eur J Immunol. 2008;38:1585–1597. doi: 10.1002/eji.200737966. [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Oldenhove G, de Heusch M, Urbain-Vansanten G, Urbain J, Maliszewski C, Leo O, Moser M. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature dendritic cells in vivo. J Exp Med. 2003;198:259–266. doi: 10.1084/jem.20030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, Holt PG. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J Exp Med. 2006;203:2649–2660. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terme M, Chaput N, Combadiere B, Ma A, Ohteki T, Zitvogel L. Regulatory T cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T cells. J Immunol. 2008;180:4679–4686. doi: 10.4049/jimmunol.180.7.4679. [DOI] [PubMed] [Google Scholar]
- Giroux M, Yurchenko E, St-Pierre J, Piccirillo CA, Perreault C. T regulatory cells control numbers of NK cells and CD8α+ immature dendritic cells in the lymph node paracortex. J Immunol. 2007;179:4492–4502. doi: 10.4049/jimmunol.179.7.4492. [DOI] [PubMed] [Google Scholar]
- Feunou P, Vanwetswinkel S, Gaudray F, Goldman M, Matthys P, Braun MY. Foxp3+CD25+ T regulatory cells stimulate IFN-γ-independent CD152-mediated activation of tryptophan catabolism that provides dendritic cells with immune regulatory activity in mice unresponsive to staphylococcal enterotoxin B. J Immunol. 2007;179:910–917. doi: 10.4049/jimmunol.179.2.910. [DOI] [PubMed] [Google Scholar]
- Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S, Tybulewicz V, Vignali D, Clynes R. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, Atkinson MA, Agarwal A, Kapturczak MH. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol. 2008;173:154–160. doi: 10.2353/ajpath.2008.070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'Acqua ML, Rossini PM, Battistini L, Rotzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook JD, Zink MC, Klein SL. Regulatory T cells enhance persistence of the zoonotic pathogen Seoul virus in its reservoir host. Proc Natl Acad Sci USA. 2007;104:15502–15507. doi: 10.1073/pnas.0707453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Ehrchen J, Steinmuller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, Nordhues U, Sorg C, Sunderkotter C, Roth J. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109:1265–1274. doi: 10.1182/blood-2006-02-001115. [DOI] [PubMed] [Google Scholar]
- Weaver LK, Pioli PA, Wardwell K, Vogel SN, Guyre PM. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J Leukoc Biol. 2007;81:663–671. doi: 10.1189/jlb.0706428. [DOI] [PubMed] [Google Scholar]
- Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan D, Wang Y, Qin X, Zheng G, Wang YM, Alexander SI, Harris DC. CD4+CD25+ regulatory T cells protect against injury in an innate murine model of chronic kidney disease. J Am Soc Nephrol. 2006;17:2731–2741. doi: 10.1681/ASN.2005080842. [DOI] [PubMed] [Google Scholar]
- Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Powell WS, Monneret G. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J Immunol. 2006;177:6540–6547. doi: 10.4049/jimmunol.177.9.6540. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, Chen L, Zou W. Cutting edge: induction of B7–H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Portugall I, Hamilton-Williams EE, Gottschalk C, Kurts C. Cutting Edge: cD25+ regulatory T cells prevent expansion and induce apoptosis of B cells specific for tissue autoantigens. J Immunol. 2008;181:4447–4451. doi: 10.4049/jimmunol.181.7.4447. [DOI] [PubMed] [Google Scholar]
- Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, Caton AJ, Erikson J. The impact of T helper and T regulatory cells on the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114:1640–1649. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahri F, Oldenhove G, Denanglaire S, Urbain J, Leo O, Andris F. CD4+ CD25+ regulatory T cells control the magnitude of T-dependent humoral immune responses to exogenous antigens. Eur J Immunol. 2006;36:855–863. doi: 10.1002/eji.200535500. [DOI] [PubMed] [Google Scholar]
- Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor β. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve M, Isnardi I, Ng YS, Bussel JB, Ochs HD, Cunningham-Rundles C, Meffre E. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007;204:1583–1593. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields ML, Hondowicz BD, Metzgar MH, Nish SA, Wharton GN, Picca CC, Caton AJ, Erikson J. CD4+ CD25+ regulatory T cells inhibit the maturation but not the initiation of an autoantibody response. J Immunol. 2005;175:4255–4264. doi: 10.4049/jimmunol.175.7.4255. [DOI] [PubMed] [Google Scholar]
- Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med. 1998;188:1493–1501. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O, Conrad C, Tonel G, Gilliet M, Nestle FO. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol. 2007;28:51–57. doi: 10.1016/j.it.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002;168:5333–5341. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- Hansen A, Lipsky PE, Dorner T. B-cell lymphoproliferation in chronic inflammatory rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:561–569. doi: 10.1038/ncprheum0620. [DOI] [PubMed] [Google Scholar]
- Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- Bayry J, Siberil S, Triebel F, Tough DF, Kaveri SV. Rescuing CD4+CD25+ regulatory T-cell functions in rheumatoid arthritis by cytokine-targeted monoclonal antibody therapy. Drug Discov Today. 2007;12:548–552. doi: 10.1016/j.drudis.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004;199:1285–1291. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–741. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- Yu J, Heck S, Patel V, Levan J, Yu Y, Bussel JB, Yazdanbakhsh K. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood. 2008;112:1325–1328. doi: 10.1182/blood-2008-01-135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venigalla RK, Tretter T, Krienke S, Max R, Eckstein V, Blank N, Fiehn C, Ho AD, Lorenz HM. Reduced CD4+, CD25- T cell sensitivity to the suppressive function of CD4+, CD25high, CD127−/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008;58:2120–2130. doi: 10.1002/art.23556. [DOI] [PubMed] [Google Scholar]
- Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- Zozulya AL, Wiendl H. The role of regulatory T cells in multiple sclerosis. Nat Clin Pract Neurol. 2008;4:384–398. doi: 10.1038/ncpneuro0832. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- King IL, Segal BM. Cutting edge: iL-12 induces CD4+CD25- T cell activation in the presence of T regulatory cells. J Immunol. 2005;175:641–645. doi: 10.4049/jimmunol.175.2.641. [DOI] [PubMed] [Google Scholar]
- O'Sullivan BJ, Thomas HE, Pai S, Santamaria P, Iwakura Y, Steptoe RJ, Kay TW, Thomas R. IL-1β breaks tolerance through expansion of CD25+ effector T cells. J Immunol. 2006;176:7278–7287. doi: 10.4049/jimmunol.176.12.7278. [DOI] [PubMed] [Google Scholar]
- Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuran B, Abraham DJ. Possible implication of the effector CD4+ T-cell subpopulation TH17 in the pathogenesis of systemic scleroderma. Nat Clin Pract Rheumatol. 2007;3:682–683. doi: 10.1038/ncprheum0618. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Chen Z, Tato CM, Muul L, Laurence A, O'Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-β are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Pallone F, MacDonald TT. Interleukin-21: a critical regulator of the balance between effector and regulatory T-cell responses. Trends Immunol. 2008;29:290–294. doi: 10.1016/j.it.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta A, Li B, Song X, Bembas K, Zhang G, Katsumata M, Saouaf SJ, Wang Q, Hancock WW, Shen Y, Greene MI. TGF-β and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3. Proc Natl Acad Sci USA. 2008;105:14023–14027. doi: 10.1073/pnas.0806726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Monoclonal antibody and intravenous immunoglobulin therapy for rheumatic diseases: rationale and mechanisms of action. Nat Clin Pract Rheumatol. 2007;3:262–272. doi: 10.1038/ncprheum0481. [DOI] [PubMed] [Google Scholar]
- Ulfgren AK, Andersson U, Engstrom M, Klareskog L, Maini RN, Taylor PC. Systemic anti-tumor necrosis factor α therapy in rheumatoid arthritis down-regulates synovial tumor necrosis factor α synthesis. Arthritis Rheum. 2000;43:2391–2396. doi: 10.1002/1529-0131(200011)43:11<2391::AID-ANR3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Toichi E, Torres G, McCormick TS, Chang T, Mascelli MA, Kauffman CL, Aria N, Gottlieb AB, Everitt DE, Frederick B, Pendley CE, Cooper KD. An anti-IL-12p40 antibody down-regulates type 1 cytokines, chemokines, and IL-12/IL-23 in psoriasis. J Immunol. 2006;177:4917–4926. doi: 10.4049/jimmunol.177.7.4917. [DOI] [PubMed] [Google Scholar]
- Krueger GG, Langley RG, Leonardi C, Yeilding N, Guzzo C, Wang Y, Dooley LT, Lebwohl M. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- Saadoun D, Rosenzwajg M, Landau D, Piette JC, Klatzmann D, Cacoub P. Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood. 2008;111:5334–5341. doi: 10.1182/blood-2007-11-122713. [DOI] [PubMed] [Google Scholar]
- Vallerskog T, Gunnarsson I, Widhe M, Risselada A, Klareskog L, van Vollenhoven R, Malmstrom V, Trollmo C. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol. 2007;122:62–74. doi: 10.1016/j.clim.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- Ivashkiv LB, Hu X. The JAK/STAT pathway in rheumatoid arthritis: pathogenic or protective? Arthritis Rheum. 2003;48:2092–2096. doi: 10.1002/art.11095. [DOI] [PubMed] [Google Scholar]
- Tarner IH, Muller-Ladner U, Gay S. Emerging targets of biologic therapies for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2007;3:336–345. doi: 10.1038/ncprheum0506. [DOI] [PubMed] [Google Scholar]
- Mbalaviele G, Anderson G, Jones A, De Ciechi P, Settle S, Mnich S, Thiede M, Abu-Am Y, Portanova J, Monahan J. Inhibition of p38 mitogen-activated protein kinase prevents inflammatory bone destruction. J Pharmacol Exp Ther. 2006;317:1044–1053. doi: 10.1124/jpet.105.100362. [DOI] [PubMed] [Google Scholar]
- Medicherla S, Ma JY, Mangadu R, Jiang Y, Zhao JJ, Almirez R, Kerr I, Stebbins EG, O'Young G, Kapoun AM, Luedtke G, Chakravarty S, Dugar S, Genant HK, Protter AA. A selective p38α mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J Pharmacol Exp Ther. 2006;318:132–141. doi: 10.1124/jpet.105.098020. [DOI] [PubMed] [Google Scholar]
- Sun SC, Ley SC. New insights into NF-κB regulation and function. Trends Immunol. 2008;29:469–478. doi: 10.1016/j.it.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre KW, Shuster DJ, Gillooly KM, Dambach DM, Pattoli MA, Lu P, Zhou XD, Qiu Y, Zusi FC, Burke JR. A highly selective inhibitor of IκB kinase. BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum. 2003;48:2652–2659. doi: 10.1002/art.11131. [DOI] [PubMed] [Google Scholar]
- Roncarolo M-G, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]