Abstract
Epithelial-mesenchymal transition (EMT) describes a series of rapid changes in cellular phenotype. During EMT, epithelial cells down-modulate cell-cell adhesion structures, alter their polarity, reorganize their cytoskeleton, and become isolated, motile, and resistant to anoikis. The term EMT is often applied to distinct biological events as if it were a single conserved process, but in fact EMT-related processes can vary in intensity from a transient loss of cell polarity to the total cellular reprogramming, as found by transcriptional analysis. Based on clinical observations, it is more appropriate in most cases to describe the emergence of an EMT-like phenotype during tumor progression. Although EMT implies complete trans-differentiation, EMT-like emphasizes the intermediary phenotype associated with tumor cell renewal and adaptation to specific microenvironments. Here, we categorize the various EMT-like phenotypes found in human carcinomas that, depending on the tumor type, may or not represent analogous stages in tumor progression. We based these categories on the global tumor phenotype. The tumor microenvironment, which is associated with stromal reactions, hypoxia, paucity of nutrients, impaired differentiation, and activation of various EMT-associated pathways, modulates overall tumor phenotype and leads to tumor heterogeneity.
The Epithelial-Mesenchymal Transition (EMT) Concept
EMT describes a rapid and often reversible change of cell phenotype. EMT was originally defined in the context of the cellular remodeling that occurs during heart morphogenesis,1 but has been applied to a range of events, including mesoderm and neural crest formation.2 The reverse process, mesenchymal-epithelial transition also occurs during development.3 During EMT, epithelial cells lose their characteristic cell-cell adhesion structures, change their polarity, modulate the organization of their cytoskeletal systems, switch expression from keratin- to vimentin-type intermediate filaments, and become isolated, motile, and resistant to anoikis.4,5
EMT bears at least a superficial resemblance to the metastatic transition of normal cells during epithelial tumor progression. The relevance of EMT to tumor progression was refined and explored in vitro using epithelial cell models, most notably by Greenburg and Hay.6 Greenburg and Hay transformed epithelial cells into individualized motile cells through manipulations with growth factors or extracellular matrix components.6 Early EMT models include the transformation of lens epithelial cells growing in collagen gels,7 NBT-II carcinoma cells responding to fibroblast growth factor,8 and MDCK cells responding to hepatocyte growth factor/scatter factor.9,10,11 Throughout the years, many more models have been described, and a number of genes have been implicated in EMT-like behavior of tumor cells.12 As is often the case, genes active (and activated) during the course of carcinoma progression and metastasis are also active in other processes, specifically during early embryogenesis, tissue morphogenesis, and wound healing.2,13,14,15 This has lead to the idea that EMT, as it occurs in the course of developmental events, is reactivated during tumor progression. The popularity of this idea is suggested by the fact that a recent search on epithelial-mesenchymal transition and cancer using PubMed (NCBI, NIH) yielded more than 463 references (accessed 11/26/2008). On the other hand, a number of pathologists have objected to the assumption that EMT is relevant to cancer progression because of the lack of direct clinical evidence and unambiguous molecular markers.16 A re-evaluation of the literature suggests that there are good reasons to make a clear distinction between EMT sensu stricto and the EMT-like phenotype observed in carcinoma.
Relevance of EMT in the Cancer Environment
By definition, the term EMT applies only to epithelial-derived cancers (carcinomas), which account for 90% of human tumors. To intravasate and metastasize, epithelial cells must, at least transiently, down-regulate their cell-cell adhesion structures.14 The loss of cell-cell adhesion, however, does not make an EMT! Perhaps the major obstacle in identifying EMT-specific events is the lack of definitive markers for post-EMT cells. For example, keratin expression is routinely used by pathologists to identify epithelial-derived carcinoma cells,17 but cannot be used to identify post-EMT cells because these cells should (if an actual EMT event were taking place) no longer express keratin. In fact, post-EMT epithelial cells will be phenotypically similar to (normal) vimentin-expressing stromal cells.
Several groups have attempted to decipher the clonal origins of the epithelial and mesenchymal components of tumors by comparative genotypic analysis using microsatellite markers.18,19,20,21 In fact, some less common tumors such as phyllodes tumors of the prostate were found to combine stromal and epithelial components with distinct clonal origins.22 This strategy has been problematic, however, when applied to more prevalent mammary invasive ductal carcinomas: transformed stroma cells share chromosomal rearrangements with transformed epithelial cells, suggesting common origin, but also contain stroma-specific rearrangements.23,24,25 A clearer situation is found in carcinosarcoma, rare tumors mostly described in uterus and lung, which display a mix of epithelial and mesenchymal cell types. Most studies indicate that these tumors are derived from a common epithelial progenitor that gives rise to a mesenchymal subpopulation presumably through an EMT-like process.
EMT-Like Phenotypes in Cancer
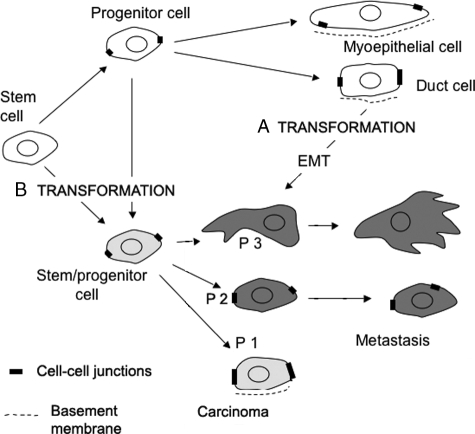
An important distinction arises because of the poorly differentiated state of many carcinoma cells. Their phenotype may reflect a dedifferentiation rather than a trans-differentiation process, such as occurs during EMT. From this perspective, transformed epithelial progenitors/stem cells may simply fail to differentiate normally, rather than differentiate into mesenchymal cells. If this is the case, the use of the term EMT is inappropriate and confusing, because it is misleading about the origin of the cells (Figure 1).
Figure 1.
EMT, EMT-like phenotype, and tumor progression in breast carcinoma. An EMT-like phenotype can be interpreted in several ways. A: It can result from the transformation of normally differentiated epithelial cells followed by an EMT process, generating tumor cells with poorly differentiated features. Conversely, an EMT-like phenotype can result from a lack of differentiation by stem/progenitor cells, resulting in cells expressing a partially differentiated phenotype: P1 (most tumor cells characterized by loss of cell polarity but retaining cohesive cell-cell contacts and keratin expression), P2 (loss of cell-cell adhesion in most tumor cells, still expressing keratins), or P3 (loss of keratin and substantial expression of vimentin). B: These early progenitors express a metastable phenotype but do not reach an epithelial differentiated state. In both situations, the tumor microenvironment controls cell phenotype through several tumor-specific pathways, which are currently under investigation.
To address this issue, we suggest using the term EMT-like as a more descriptive and accurate term to describe the phenotypes of epithelial cancers, because it does not imply a specific mechanism or the origin of the cells described. We propose three functional criteria to define EMT-like phenotypes in human carcinomas: i) state of cell polarization, ii) state of cell cohesiveness, and iii) intermediate filament protein expression. Based on these criteria we define the following four EMT-like phenotypes: phenotype 0: differentiated tumor cells with preserved epithelial structure and cell polarity; phenotype 1: most tumor cells display loss of cell polarity,15 but retain cohesive cell-cell contacts and keratin expression; phenotype 2: there is loss of cell-cell adhesion in most tumor cells, but the cells still express keratins; phenotype 3: there is loss of keratin expression and substantial expression of vimentin in most tumor cells.
Considering the phenotypic heterogeneity of most human tumors, this classification does not preclude that some tumor area may show distinct phenotypic features. That said, most tumors display a dominant phenotype that can be used by pathologists for typing. We, therefore, applied this scheme to human carcinoma types based on reference works,26,27 original articles,28,29,30 and our personal observations on breast and colon carcinomas typed and identified by the pathology department at CRLC Val d’Aurelle (Montpellier, France) (Table 1). Some of the criteria we suggest here for EMT-like phenotyping are routinely used by pathologists. For example, expression of E-cadherin, a major player in epithelial cell-cell adhesion, is used to differentiate breast invasive ductal carcinomas that express E-cadherin protein and invasive lobular carcinomas that do not.31,32 In summary, there are limited but therapeutically significant numbers of carcinomas with phenotypes 2 and 3, involving poorly differentiated cells that may remain partially cohesive.
Table 1.
EMT-Like Phenotypes in Human Carcinomas
| General tumor type | Tumor subtype | EMT-like stage |
|---|---|---|
| Bladder cancer | Transitional cell carcinoma, papillary | 3 |
| Transitional cell carcinoma, solid | 3 | |
| Carcinosarcoma | 1 | |
| Breast cancer | Invasive ductal carcinoma | 3 |
| Infiltrating lobular carcinoma | 2 | |
| Carcinosarcoma | 1 | |
| Colon and rectal cancer | Adenocarcinomas | 3a |
| Carcinosarcoma | 1 | |
| Anal cancer | Epidermoid carcinoma | 3 |
| Endometrial cancer | Adenocarcinomas | 3 |
| Carcinosarcoma | 1 | |
| Ovarian cancer | Serous and mucinous carcinoma | 3 |
| Testicular cancer | Embryonal carcinoma, teratocarcinoma | 1 |
| Gastric cancer | Diffuse gastric cancer | 2 |
| Intestinal gastric cancer | 3 | |
| Head and neck cancer | Carcinoma (squamous cell carcinoma) | 3 |
| Kidney (renal cell) cancer | Wilms | 2 |
| Renal cell carcinoma | 3 | |
| Liver cancer | Hepatocellular carcinoma | 3 |
| Lung cancer | Squamous cell carcinomas | 3 |
| Adenocarcinoma | 3 | |
| Small cell lung carcinoma | 3b | |
| Spindle-cell carcinoma | 2 | |
| Carcinosarcoma | 1 | |
| Pancreatic cancer | Adenocarcinomas | 3 |
| Prostate cancer | Adenocarcinomas | 3 |
| Carcinosarcoma | 1 | |
| Skin cancer | Basal cell cancer | 3 |
| Squamous cell cancer | 3 | |
| Spindle cell squamous cell | 1 | |
| Melanoma | 3 | |
| Thyroid cancer | Adenocarcinoma | 3 |
| Anaplastic carcinomas | 2 | |
| Malignant mesothelioma | 1 |
We selected three functional criteria to define EMT-like phenotypes in human carcinomas, ranging from partial to total EMT-like phenotype: a) state of cell polarization, b) state of cell cohesiveness, and c) intermediate filament expression. Combination of these criteria helped us define the following four EMT-like phenotypes. Phenotype 0: differentiated tumor cells with preserved epithelial structure and cell polarity; phenotype 1: most tumor cells characterized by cellular depolarization; however, they retain cohesive cell-cell contacts and the expression of keratins; phenotype 2: loss of cell-cell adhesion in most tumor cells still expressing keratins; phenotype 3: loss of keratin and substantial expression of vimentin.
EMT-Like Behavior and Tumor Heterogeneity
Most carcinomas display histological heterogeneity. We and others14,33 have suggested that only a small percentage of tumor cells ever undergo a total transition (corresponding to phenotype 3) and that it is these cells that are presumably the source of actively metastatic cells. Perhaps the best-documented example of this is the invasive front in colorectal carcinomas. Cells in this region usually express a less differentiated progenitor/stem cell phenotype34 together with nuclear β-catenin, indicative of active canonical Wnt signaling. They are thought to play a critical role in the tumor invasion mechanism.
A link between progenitor/stem cells and EMT-like grade is expected based on the immature epithelial phenotype of precursor cells.34 Many molecules involved in progenitor/stem cell maintenance also appear to be involved in the regulation of cell motility, EMT, and EMT-like phenotypes. Among these proteins CD24, CD44, CD49/integrin (6, CD29/integrin β1), and Slug (Snail2) constitute good examples.35,36,37,38,39 Recently, work from R. Weinberg’s laboratory40 has emphasized molecular links between EMT process and emergence of stemness. This is interesting because stemness is distinct from mesenchymal behavior.
Epithelial cells initiate migration by becoming activated. Activation is considered a metastable phenotype,41 which combines incomplete differentiation, cell motility, and some cell-cell cohesiveness. It can be considered EMT-like. For example, during wound healing, immature basal keratinocytes are activated by changes in the local microenvironment and express such a metastable phenotype.38 Their migration involves cohort migration, in which cells migrate as groups, not isolated cells. Similarly, during mammary gland tubulogenesis, cells located at the tip of growing tubule terminal end buds (cap cells) migrate as a group entity and express specific cadherins (P-cadherin). Interfering with cell-cell adhesion disrupts rather than enhances the migratory pattern.42 This type of behavior is similar to that displayed by carcinoma cells expressing a metastable phenotype; it explains the cellular chords and partially differentiated tubules found in mammary invasive ductal carcinoma. Total lack of cohesiveness between carcinoma cells, more representative of EMT, represents a distinct mode of invasion present, for example, in infiltrating lobular carcinoma. This type of invasion is more insidious: although not inherently more invasive than invasive ductal carcinomas, it tends to be detected later during tumor progression, resulting in a poorer prognosis.
Old and New Pathways: What Happened to EMT Master Genes?
The onset of both EMT and EMT-like events is associated with loss of cellular polarity, partial to total destabilization of cell-cell junctions, remodeling and replacement of cytoskeletal components, the onset of cell motility, and the suppression of apoptosis. In vitro and in vivo model systems have allowed the characterization of various pathways leading to EMT and EMT-like phenotypes. Such pathways are referred to as EMT pathways in this review, without assuming functional specificity. Five main pathways have been found to trigger EMT-associated processes13,14,43: tyrosine kinase receptors (epidermal growth factor, fibroblast growth factor, hepatocyte growth factor, platelet-derived growth factor, insulin-like growth factor), integrins, Wnt, NF-κB, and TGF-β pathways. These pathways involve Akt, GSK3, Rho-GTPases, and SMAD signaling pathways. A distinct EMT pathway has also been recently described involving the protein tyrosine phosphatase Pez.44 In direct association with cancer progression, several molecules including ILEI,12,45 RKIP,46 and CXCR447 appear to control EMT-like phenotypes and tumor metastasis in mouse models.13,14
Transcriptional down-regulation of junctional components accompanies the EMT process in several systems13,48 and may be either a cause or an effect of EMT-like events. Down-regulation of E-cadherin is linked to cell-cell dissociation and invasion in pancreas, prostate, and mammary gland mouse cancer models.31,49 Specific transcription factors, in particular Snail (Snail1), Slug (Snail2), Twist, SIP1/Zeb, and E47, negatively regulate E-cadherin expression,50,51 and presumably display overlapping functional redundancy, in part through their common recognition of E-box sequences. These factors appear to be involved in most physiological EMT situations, and their overexpression in epithelial cell lines usually induces an EMT.48,50,51,52 At the same time, detailed mechanism(s) of their effects remain unclear; cellular co-expression of Snail and E-cadherin has been described in breast and colon carcinomas by several groups.33,53 In addition, specificity of these transcription factors is clearly not restricted to E-cadherin regulation and the EMT process. For example, members of the Snail family have been shown to be involved in cell motility, proliferation control, differentiation, and apoptotic regulation in vivo and in cell models.54,55,56,57,58,59
Distinct pathways inducing EMT have been uncovered recently, emphasizing functional links between EMT-like phenotypes and inductive pathways specifically activated during tumor growth and progression (Figure 2). Tumor cell growth requires an increase in local vasculature to provide metabolites and oxygen. Cells adjust to a nutritionally impoverished and hypoxic environment by activating specific pathways associated with hypermetabolism,60 glycolysis and resistance to acidosis-induced toxicity, and neoangiogenesis. Hypoxia genes have been found to be expressed locally within solid tumors, probably contributing to tumor heterogeneity.61 The link between hypoxia and EMT has been recently strengthened by the observed activation of Snail and Twist expression by HIF-1, a key hypoxia effector.62,63,64 Another hypoxia-related gene, lysyl oxydase, was found to interact directly with Snail, but the functional significance of this interaction has been disputed.65,66
Figure 2.
Tumor-specific changes converge in inducing EMT phenotype. Most pathways controlling EMT-like behavior in cancer cells can be linked to specific environmental changes that occur during tumor growth and progression. Tumor cells adapt to an impoverished and hypoxic environment by activating alternative pathways to adjust to hypoxia and by adopting hypermetabolism. Tumor cells typically express a partially differentiated phenotype, suggesting impairment in differentiation pathways. Finally, another specific feature of the tumor microenvironment is the stromal reaction. Stromal and inflammatory cells play a major role in secreting activating factors and controlling tumor cell motility.
Another specific feature of tumor microenvironment is the stromal reaction through which epithelial-mesenchymal interactions activate or regulate several pathways involving integrins, cytokines, and growth factors that are critical for tumor growth and metastasis.67 Inflammatory cells play a major role in secreting activating factors, and NF-κB, a key regulator of the inflammatory response, has been found to regulate Slug, Snail, and Twist (unpublished observations).67 A putative role of macrophages in supporting the movement of individualized cells from mammary tumors into the bloodstream has recently been suggested in striking movies.68
In conclusion, EMT has often been tacitly assumed to be a single conserved process, but this is by no means unambiguously established. What is clear is that EMT and EMT-like processes are influenced by the origin of the cells under consideration. Based on clinical observations, it appears more appropriate in most cases to describe the emergence of an EMT-like phenotype during tumor progression. This descriptive term does not necessarily imply an active dedifferentiation process but emphasizes an intermediary phenotype resulting from tumor cell renewal and adaptation to specific microenvironments. It clearly emphasizes the importance of better understanding cell population kinetics, survival, and differentiation mechanisms during tumor growth and metastasis.
Footnotes
Address reprint requests to Pierre Savagner, IRCM, INSERM U896, Montpellier, F-34298, France. E-mail: psavagner@valdorel.fnclcc.fr.
Supported by the National Institutes of Health (grant GM58004 to M.W.K.), the American Cancer Society (seed grant through the University of Colorado Cancer Center to M.W.K.), the Ligue Regionale Contre le Cancer (Languedoc-Roussillon) (to P.S.), and the Groupement des Entreprises Francaises dans la Lutte Contre le Cancer (Languedoc-Roussillon) (to P.S.).
References
- Bolender D, Markwald R. Epithelial-mesenchymal transformation in chick atrioventricular cushion morphogenesis. Scan Electron Microsc. 1979;3:313–321. [PubMed] [Google Scholar]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- Baum B, Settleman J, Quinlan M. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. J Cell Biol. 2001;154:1185–1196. doi: 10.1083/jcb.200104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés F, Alvarez A, Locascio A, Vega S, Herrera B, Fernández M, Benito M, Nieto M, Fabregat I. The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor beta in fetal rat hepatocytes. Mol Cancer Res. 2002;1:68–78. [PubMed] [Google Scholar]
- Greenburg G, Hay E. Cytodifferentiation and tissue phenotype change during transformation of embryonic lens epithelium to mesenchyme-like cells in vitro. Dev Biol. 1986;115:363–379. doi: 10.1016/0012-1606(86)90256-3. [DOI] [PubMed] [Google Scholar]
- Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer B, Tucker G, Valles A, Franke W, Thiery J. Rearrangements of desmosomal and cytoskeletal proteins during the transition from epithelial to fibroblastoid organization in cultured rat bladder carcinoma cells. J Cell Biol. 1989;109:1495–1509. doi: 10.1083/jcb.109.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Joseph A, Bhargava M, Rosen E, Nakamura T, Goldberg I. Effect of scatter factor and hepatocyte growth factor on motility and morphology of MDCK cells. In Vitro Cell Dev Biol. 1992;28:364–368. doi: 10.1007/BF02877060. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Kitamura N. Expression of a human hepatocyte growth factor/scatter factor cDNA in MDCK epithelial cells influences cell morphology, motility, and anchorage-independent growth. J Cell Biol. 1992;117:889–894. doi: 10.1083/jcb.117.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner K, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–154. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waerner T, Alacakaptan M, Tamir I, Oberauer R, Gal A, Brabletz T, Schreiber M, Jechlinger M, Beug H. ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell. 2006;10:227–239. doi: 10.1016/j.ccr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Berx G, Raspe E, Christofori G, Thiery JP, Sleeman JP. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clin Exp Metastasis. 2007;24:587–597. doi: 10.1007/s10585-007-9114-6. [DOI] [PubMed] [Google Scholar]
- Dow LE, Humbert PO. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6001. doi: 10.1158/0008-5472.CAN-05-0699. [DOI] [PubMed] [Google Scholar]
- Barak V, Goike H, Panaretakis KW, Einarsson R. Clinical utility of cytokeratins as tumor markers. Clin Biochem. 2004;37:529–540. doi: 10.1016/j.clinbiochem.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Halachmi S, DeMarzo A, Chow N, Halachmi N, Smith A, Linn J, Nativ O, Epstein J, Schoenberg M, Sidransky D. Genetic alterations in urinary bladder carcinosarcoma: evidence of a common clonal origin. Eur Urol. 2000;37:350–357. doi: 10.1159/000052369. [DOI] [PubMed] [Google Scholar]
- Dacic S, Finkelstein S, Sasatomi E, Swalsky P, Yousem S. Molecular pathogenesis of pulmonary carcinosarcoma as determined by microdissection-based allelotyping. Am J Surg Pathol. 2002;26:510–516. doi: 10.1097/00000478-200204000-00015. [DOI] [PubMed] [Google Scholar]
- Fujii H, Yoshida M, Gong Z, Matsumoto T, Hamano Y, Fukunaga M, Hruban R, Gabrielson E, Shirai T. Frequent genetic heterogeneity in the clonal evolution of gynecological carcinosarcoma and its influence on phenotypic diversity. Cancer Res. 2000;60:114–120. [PubMed] [Google Scholar]
- Thompson L, Chang B, Barsky S. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol. 1996;20:277–285. doi: 10.1097/00000478-199603000-00003. [DOI] [PubMed] [Google Scholar]
- McCarthy R, Zhang S, Bostwick D, Qian J, Eble J, Wang M, Lin H, Cheng L. Molecular genetic evidence for different clonal origins of epithelial and stromal components of phyllodes tumor of the prostate. Am J Pathol. 2004;165:1395–1400. doi: 10.1016/S0002-9440(10)63397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukino K, Shen L, Matsumoto S, Morrison C, Mutter G, Eng C. Combined total genome loss of heterozygosity scan of breast cancer stroma and epithelium reveals multiplicity of stromal targets. Cancer Res. 2004;64:7231–7236. doi: 10.1158/0008-5472.CAN-04-2866. [DOI] [PubMed] [Google Scholar]
- Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60:2562–2566. [PubMed] [Google Scholar]
- Howe JR, Roth S, Ringold JC, Summers RW, Jarvinen HJ, Sistonen P, Tomlinson IP, Houlston RS, Bevan S, Mitros FA, Stone EM, Aaltonen LA. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280:1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- Rosai J, Ackerman L. New York: Mosby; Surgical Pathology. (ed 4) 2004 [Google Scholar]
- Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Jr, Gansler TS, Holland JF, Frei E., III Hamilton: BC Decker; Cancer Medicine. 2003 [Google Scholar]
- Hu L, Lau S, Tzang C, Wen J, Wang W, Xie D, Huang M, Wang Y, Wu M, Huang J, Zeng W, Sham J, Yang M, Guan X. Association of vimentin overexpression and hepatocellular carcinoma metastasis. Oncogene. 2004;23:298–302. doi: 10.1038/sj.onc.1206483. [DOI] [PubMed] [Google Scholar]
- Ngan C, Yamamoto H, Seshimo I, Tsujino T, Mani M, Ikeda J, Konishi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer. 2007;96:986–992. doi: 10.1038/sj.bjc.6603651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernat W, Kordek R, Liberski P, Woźniak L. Carcinosarcoma of the skin. Case report and literature review. Am J Dermatopathol. 1996;18:614–619. doi: 10.1097/00000372-199612000-00012. [DOI] [PubMed] [Google Scholar]
- Derksen PWB, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink J, Krimpenfort P, Peterse JL, Cardiff RD, Berns A, Jonkers J. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Moll R, Mitze M, Frixen U, Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993;143:1731–1742. [PMC free article] [PubMed] [Google Scholar]
- Côme C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, Savagner P. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12:5395–5402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Fassler R. Beta 1 integrin function in vivo: adhesion, migration and more. Cancer Metastatsis Rev. 2005;24:403–411. doi: 10.1007/s10555-005-5132-5. [DOI] [PubMed] [Google Scholar]
- Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yagita H, Sleeman JP. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005;65:10783–10793. doi: 10.1158/0008-5472.CAN-05-0619. [DOI] [PubMed] [Google Scholar]
- Hamilton SR, Fard SF, Paiwand FF, Tolg C, Veiseh M, Wang C, McCarthy JB, Bissell MJ, Koropatnick J, Turley EA. The hyaluronan receptors CD44 and Rhamm (CD168) form complexes with ERK1,2 that sustain high basal motility in breast cancer cells. J Biol Chem. 2007;282:16667–16680. doi: 10.1074/jbc.M702078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savagner P, Kusewitt DF, Carver EA, Magnino F, Choi C, Gridley T, Hudson LG. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- Storci G, Sansone P, Trere D, Tavolari S, Taffurellii M, Ceccarelli C, Guarnieri T, Paterini P, Pariali M, Montanaro L, Santini D, Chieco P, Bonafe M. The basal-like breast carcinoma phenotype is regulated by SLUG gene expression. J Pathol. 2008;214:25–37. doi: 10.1002/path.2254. [DOI] [PubMed] [Google Scholar]
- Mani S, Guo W, Liao M, Eaton E, Ayyanan A, Zhou A, Brooks M, Reinhard F, Zhang C, Shipitsin M, Campbel IL, Polyak K, Brisken C, Yang J, Weinberg R. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel CW, Strickland P, Friedmann Y. Expression and functional role of E- and P-cadherins in mouse mammary ductal morphogenesis and growth. Dev Biol. 1995;169:511–519. doi: 10.1006/dbio.1995.1165. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin C-H. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt L, Wadham C, Crocker LA, Lardelli M, Khew-Goodall Y. The protein tyrosine phosphatase Pez regulates TGFbeta, epithelial-mesenchymal transition, and organ development. J Cell Biol. 2007;178:1223–1235. doi: 10.1083/jcb.200705035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie NC, Raz E. Found in translation: a new player in EMT. Dev Cell. 2006;11:434–436. doi: 10.1016/j.devcel.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Beach S, Tang H, Park S, Dhillon AS, Keller ET, Kolch W, Yeung KC. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene. 2008;27:2243–2248. doi: 10.1038/sj.onc.1210860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Liang Z, Zhang X, Choe M, Zhu A, Cho HT, Shin DM, Goodman MM, Chen ZG, Shim H. CXC chemokine receptor-4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Res. 2007;67:7518–7524. doi: 10.1158/0008-5472.CAN-06-2263. [DOI] [PubMed] [Google Scholar]
- Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Bolós V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116:499–511. doi: 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani S, Donaher J, Ramaswamy S, Itzykson R, Come C, Savagner P, Gitelman I, Richardson A, Weinberg R. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Becker KF, Rosivatz E, Blechschmidt K, Kremmer E, Sarbia M, Hofler H. Analysis of the E-cadherin repressor Snail in primary human cancers. Cells Tissues Organs. 2007;185:204–212. doi: 10.1159/000101321. [DOI] [PubMed] [Google Scholar]
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- Castro Alves C, Rosivatz E, Schott C, Hollweck R, Becker I, Sarbia M, Carneiro F, Becker KF. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007;211:507–515. doi: 10.1002/path.2138. [DOI] [PubMed] [Google Scholar]
- Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Mancera PA, Gonzelez-Herrero I, Perez-Caro M, Gutiirrez-Cianca N, Flores T, Gutierrez-Adan A, Pintado B, Sanchez-Martin M, Sanchez-Garcia I. SLUG in cancer development. Oncogene. 2005;24:3073–3082. doi: 10.1038/sj.onc.1208505. [DOI] [PubMed] [Google Scholar]
- Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Zhang C, Carl TF, Trudeau ED, Simmet T, Klymkowsky MW. An NF-kappaB and slug regulatory loop active in early vertebrate mesoderm. PLoS ONE. 2006;1:e106. doi: 10.1371/journal.pone.0000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias JI, Aller MA, Arias J. Cancer cell: using inflammation to invade the host. Mol Cancer. 2007;6:29. doi: 10.1186/1476-4598-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, Konishi I. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163:1437–1447. doi: 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AJ, Russell RC, Roche O, Burry TN, Fish JE, Chow VW, Kim WY, Saravanan A, Maynard MA, Gervais ML, Sufan RI, Roberts AM, Wilson LA, Betten M, Vandewalle C, Berx G, Marsden PA, Irwin MS, Teh BT, Jewett MA, Ohh M. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol Cell Biol. 2007;27:157–169. doi: 10.1128/MCB.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Wu K. TWIST activation by hypoxia inducible factor-1 (HIF-1): implications in metastasis and development. Cell Cycle. 2008;7:2090–2096. doi: 10.4161/cc.7.14.6324. [DOI] [PubMed] [Google Scholar]
- Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min C, Kirsch KH, Zhao Y, Jeay S, Palamakumbura AH, Trackman PC, Sonenshein GE. The tumor suppressor activity of the lysyl oxidase propeptide reverses the invasive phenotype of Her-2/neu-driven breast cancer. Cancer Res. 2007;67:1105–1112. doi: 10.1158/0008-5472.CAN-06-3867. [DOI] [PubMed] [Google Scholar]
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]