Abstract
The vertical transmission of a prion disease from infected mothers to their offspring is believed to be one of the routes for the natural spread of animal prion diseases. Supporting this notion is the observation that prion infectivity occurs in the placenta of infected ewes. Furthermore, the prion protein (PrP), both in its cellular form (PrPC) and its pathological isoform (PrPSc), has been observed at the fetal-maternal interface of scrapie-infected sheep. However, whether these features of prion infectivity also hold true for human prion diseases is currently unknown. To begin to address such an important question, we examined PrP in the uterus as well as gestational tissues, including the placenta and amniotic fluid, in a pregnant woman with sporadic Creutzfeldt-Jakob disease (CJD). Although the proteinase K (PK)-resistant prion protein, PrP27-30, was present in the brain tissues of the mother, the PrP detected in the uterus, placenta, and amniotic fluid was sensitive to PK digestion. Unlike PrPC in the brain and adjacent cerebrospinal fluid, the predominant PrP species in the reproductive and gestational tissues were N-terminally truncated, similar to urine PrP. Our study did not detect abnormal PrP in the reproductive and gestational tissues in this case of CJD. Nevertheless, examination by a highly sensitive bioassay is ongoing to ascertain possible prion infectivity from CJD in the amniotic fluid.
The transmissible prion diseases affecting both humans and animals are characterized by the accumulation of an infectious prion protein particle (PrPSc) mainly in the central nervous system (CNS) and occasionally in the peripheral tissues.1,2,3,4 Animal prion diseases such as scrapie in sheep and goats, chronic wasting disease in deer and elks, and bovine spongiform encephalopathy in cattle are believed to spread naturally by oral transmission, close contact between animals, and maternal transmission. Indeed, Western blot analysis and bioassays have demonstrated that PrPSc and prion infectivity are present not only in the CNS, but also in many peripheral tissues including the tonsils, spleen, lymph nodes, nasal mucosa, distal colon, ovaries, uterus, skeletal muscle, placenta, and amniotic fluid of affected animals.2
In humans, the transmission of prion diseases has been observed in the acquired form of the disease including kuru, iatrogenic Creutzfeldt-Jakob disease (iCJD), and variant CJD (vCJD).5 Kuru is associated with cannibalistic rituals,6,7 whereas iCJD is caused by prion exposure in the course of medical or surgical procedures, and vCJD has been attributed to the consumption of prion-contaminated meat.8,9 In addition to CNS, PrPSc has also been detected in the tonsils, spleen, lymph node, retina, optic nerve, rectum, adrenal gland, and thymus of vCJD and in the spleen and skeletal muscles of sporadic CJD (sCJD).3,4 However, it remains unknown whether human prion diseases are vertically transmitted in pregnancy. For instance, none of four offspring born to four gravid women with CJD had reportedly developed the disease when they reached the respective ages of 22, 10, 7, and 3 years.10 In addition, no reports are available concerning examination of PrP in the uterus and gestational tissues from prion-affected patients.
We examined PrPSc distribution in CNS, uterus, and gestational tissues from a woman affected with prion disease, who had become pregnant and delivered a baby boy during the time she had the disease. Typical PK-resistant PrP core fragments and neuropathological changes, characteristic of sCJD (with PrPSc type 1 carrying a valine/valine polymorphism at codon 129 of PrP gene), were detected in the brain tissues obtained at either biopsy or autopsy. Although PrP was detectable in the uterus, placenta, and amniotic fluid, it was PK-sensitive. Moreover, neither conventional nor enrichment-based Western blot analysis revealed the presence of abnormal PrP species. In contrast to the PrP in the brain and cerebrospinal fluid (CSF), the PrP detected in the uterus and gestational tissues, including placenta and amniotic fluid, was N-terminally truncated, similar to that normally found in the urine. Although the presence of prion infectivity in tissue remains to be determined by highly sensitive bioassay, our current study suggests that abnormal PrP species, including both PK-resistant and PK-sensitive forms, are undetectable in the uterus and gestational tissues in sporadic CJD.
Materials and Methods
Reagents and Antibodies
Sodium phosphotungstic acid (NaPTA), proteinase K (PK), and phenylmethyl sulfonyl fluoride were purchased from Sigma Chemical Co. (St. Louis, MO). Peptide N-glycosidase F (PNGase F) was purchased from New England Biolabs (Beverly, MA) and used according to the manufacturer’s protocol. Reagents for enhanced chemiluminescence (ECL Plus) were from Amersham Pharmacia Biotech, Inc. (Piscataway, NJ). Magnetic beads (Dynabeads M-280, tosyl-activated) were from Dynal Co. (Oslo, Norway). Anti-PrP antibodies, including rabbit anti-C-terminal antiserum immunoreactive to human PrP residues 220 to 231, mouse monoclonal antibody 3F4 against human PrP residues 109 to 112, mouse monoclonal antibody 1E4 against human PrP 97 to 108 (Cell Sciences, Canton, MA), and mouse monoclonal antibody 6H4 against human PrP 145 to 152 (Prionics AG, Zurich, Switzerland) were used.11,12,13,14
Human Tissues
Consent to use autopsy and biopsy material for research purposes was obtained for all samples. The neuropathological examination of biopsy and autopsy specimens of the proband was performed at the Indiana Alzheimer Disease Center, Indianapolis, IN, and at the National Prion Disease Pathology Surveillance Center, Cleveland, OH (http://www.cjdsurveillance.com/). Biopsy brain tissues were immediately frozen in liquid nitrogen and stored at −80°C. The autopsy was performed within 20 hours of death. Clinical data and relevant hospital records were examined. Brains, CSF, and urine were from patients free of neurological disorders and PrP mutations indicated by neurohistology, immunohistochemistry, Western blotting, and genetic analysis at National Prion Disease Pathology Surveillance Center, Cleveland, OH. The normal uterine and placental tissues and amniotic fluid were provided by the Pathology Department Human Tissue Procurement Facility, Case Western Reserve University/University Hospital Case Medical Center, Cleveland, OH.
Preparation of Tissue Homogenates
The 10% (w/v) brain, uterus, and placenta homogenates were prepared in 9 vol of lysis buffer (10 mmol/L Tris, 150 mmol/L NaCl, 0.5% Nonidet P-40, 0.5% deoxycholate, 5 mmol/L ethylenediaminetetraacetic acid, pH 7.4) with pestle on ice. When required, the tissue homogenates were centrifuged at 1000 × g for 10 minutes at 4°C to collect supernatants (S1).
Preparation of Gene 5 Protein
The recombinant g5p was isolated from Escherichia coli, transformed with an Ff gene 5-containing plasmid and purified using DNA cellulose affinity plus Sephadex G75 sizing columns as described.15 The purity was >99% as determined by quantitation of Coomassie Blue-stained bands on sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Molecular Genetic Analysis
Genomic DNA was extracted from frozen brain tissue. The human prion protein gene (PRNP) coding region sequence and polymorphism at codon 129 were determined as previously described.16,17
Histopathology and PrP Immunohistochemistry of Brain Tissues
Histopathology and PrP immunohistochemistry were performed as described.18 The sections were deparaffinized, rehydrated, and immersed in 98% formic acid for 1 hour at room temperature. Endogenous peroxidase was blocked by immersion in 8% H2O2 in methanol for 10 minutes. Sections were completely immersed in 1.5 mmol/L hydrochloric acid and microwaved for 10 minutes. After rinsing, they were incubated with 3F4 at 1:600, washed, and incubated with secondary antibody (goat anti-mouse, 1:50; Cappel, Durham, NC) followed by incubation with mouse PAP complex (1:250; Sternberger, Meyer Immunocytochemicals, Jarrettsville, MD). Diaminobenzidine tetrahydrochloride was used to visualize the immunoreactivity.
Enrichment of PrP by G5p and 6H4 Beads
The g5p molecule and 6H4 antibody (100 μg) was conjugated to 7 × 108 tosyl-activated magnetic beads in 1 ml of phosphate-buffered saline (PBS) at 37°C for 20 hours. The g5p- or 6H4 -conjugated beads were incubated with 0.1% bovine serum albumin in PBS to block nonspecific binding. The prepared g5p or 6H4 beads were stable for at least 3 months at 4°C. The specific capture of PrP by g5p or 6H4 was performed as described incubating S1 fractions from the total brain homogenates, CSF, or amniotic fluid and g5p- or 6H4-conjugated beads (10 μg mAb/6 × 107 beads) in 1 ml of binding buffer (3% Tween-20 and 3% Nonidet P-40 in PBS, pH 7.5).19,20 After incubation with constant rotation for 3 hours at room temperature, the PrP-containing g5p or 6H4 beads were attracted to the sidewall of Eppendorf tubes by external magnetic force, allowing easy removal of all unbound molecules in the solution. After three washes in wash buffer (2% Tween-20 and 2% Nonidet P-40 in PBS, pH 7.5), the g5p and 6H4 beads were collected and heated at 95°C for 5 minutes in sodium dodecyl sulfate sample buffer (3% sodium dodecyl sulfate, 2 mmol/L ethylenediaminetetraacetic acid, 10% glycerol, 50 mmol/L Tris-HCl, pH 6.8).
Enrichment of PrP by NaPTA
Precipitation of PrP aggregates by NaPTA was conducted as described with minor modification.3,14 Briefly, 10% (w/v) homogenates from brain, uterus, or placenta tissues were prepared in PBS lacking Ca2+ and Mg2+. The samples were centrifuged at 1000 × g for 10 minutes at 4°C. A 500-μl aliquot of supernatant was mixed with an equal volume of 4% (w/v) Sarcosyl prepared in PBS, pH 7.4, and incubated for 10 minutes at 37°C with constant agitation. Samples were adjusted to final concentrations of 50 U/ml Benzonase (Benzon nuclease; Merck & Co., Whitehouse Station, NJ) and 1 mmol/L MgCl2 and incubated for 30 minutes at 37°C with constant agitation. Subsequently, the samples were adjusted with 81.3 μl of a stock solution containing 4% (w/v) NaPTA and 170 mmol/L MgCl2 at a final concentration in the sample of 0.3% (w/v) NaPTA. Samples were incubated at 37°C for 30 minutes with constant agitation before centrifugation at 16,000 × g for 30 minutes. After careful isolation of the supernatant, the pellet was resuspended in 1× lysis buffer for Western blotting.
Western Blots
Samples were resolved on 15% Tris-HCl Criterion precast gels (Bio-Rad, Hercules, CA) for one-dimensional gel electrophoresis. The proteins on the gels were transferred to Immobilon-P membrane (PVDF, Millipore, Bedford, MA) for 2 hours at 70 V. For probing the PrP molecule, the membranes were incubated for 2 hours at room temperature with either 3F4 (1:40,000), 6H4 (1:10,000), or anti-C-terminal antibody (1:4000) as primary antibody. After incubation with horseradish peroxidase-conjugated sheep anti-mouse IgG or donkey anti-rabbit IgG at 1:3000, the PrP bands were visualized on Kodak film (Eastman-Kodak, Rochester, NY) by the ECL Plus (GE Healthcare, Fairfield, CT) as described by the manufacturer.
Results
Clinical History
The patient was a 41-year-old pregnant Caucasian woman who suffered from rapidly progressive behavioral regression and eventually failed to perform simple activities of daily living. Shortly after the onset of her symptoms she became pregnant. Concurrently, she had evidence of executive cognitive dysfunction followed by weight loss, insomnia, aggressive behavior, and inability to care for personal hygiene. The patient had not traveled outside of the United States nor had she had a blood transfusion, corneal transplant, or treatment with human growth hormone. There was no family history of dementia or ataxia.
The history, clinical course, and MRI abnormalities were suggestive of CJD. As the disease progressed, the patient developed myoclonus and ataxia. The definitive diagnosis of sCJD was made by neuropathological, molecular genetic, and Western blot analyses performed at the Indiana Alzheimer Disease Center, Indianapolis, IN, and confirmed by the National Prion Disease Pathology Surveillance Center, Cleveland, OH.
As the clinical neurological symptoms progressed rapidly to a state of severe dementia, a cesarean delivery was performed at 35 weeks of gestation, during which samples of the amniotic fluid, umbilical cord blood, placenta, a full thickness biopsy of the uterine wall, and a biopsy of the uterus in the location of the placental bed were collected for storage and research. Tissues from the uterus and placenta and ∼60 ml of the amniotic fluid were sent to the National Prion Disease Pathology Surveillance Center, Cleveland, OH, for PrP analyses (see below).
After delivery of a vital baby weighing 2.61 kg and 45.5 cm in length, the patient was closely followed by her neurologists. Five months after the delivery, the patient was admitted to the hospital for dehydration. On examination, she was obtunded and had frequent myoclonic jerks. The patient died at age 42 years and 1 month, 6 months after the birth of her child.
Histology and Immunohistochemistry of Brain Tissue
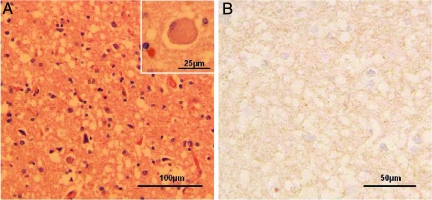
There were severe spongiform degeneration, astrogliosis, occasional ballooned neurons, and possible neuronal loss throughout the neocortex (Figure 1A). The hippocampal formation and the basal ganglia were also severely affected. The cerebellum showed minimal spongiform degeneration and no atrophy, and kuru plaques, while the brain stem was normal (Figure 1A). Immunostaining for PrP was weak and consistently showed a fine granular or synaptic pattern throughout the cerebral cortex (Figure 1B). The neuropathological changes observed in the brain biopsy samples were similar to those observed in the autopsy samples.
Figure 1.
Histology and immunohistochemistry of brain tissues. A: Widespread spongiform degeneration, often with large vacuoles, was observed in the cortex. Reactive astrocytes also were prominent. Inset: Ballooned neuron. B: Immunostaining for the prion protein demonstrated very minute positive granules, some of which are indicated with circles, in the cortical regions with severe spongiform degeneration using antibody 3F4.
PRNP Analysis
To determine whether there was a mutation in the PRNP, DNA was extracted from brain tissues and assessed by genetic analysis. The polymorphism at codon 129 of PRNP was of valine/valine and no mutations were observed in the coding region of the PRNP in this patient.
Detection of PrPSc in Brain Tissues Obtained at Biopsy and Autopsy Using Western Blot
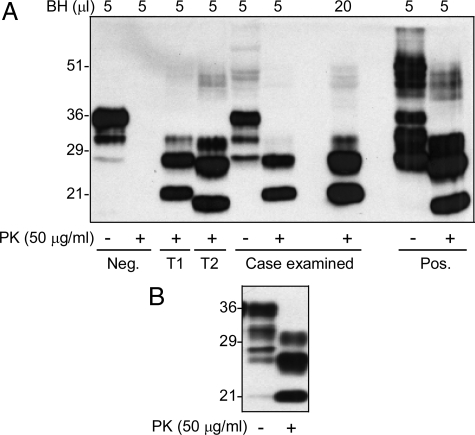
A piece of brain tissue (∼77 mg) obtained at biopsy was homogenized in lysis buffer. The samples treated with PK at 50 μg/ml were subjected to Western blot analysis with 3F4 antibody. Proteinase K (PK)-resistant PrP 27-30 (rPrPSc) was detected; the unglycosylated rPrPSc fragment migrated at ∼21 kDa, identifying the rPrPSc as type 1 (Figure 2A). The brain samples received at autopsy were also examined and the rPrPSc was confirmed to have a migration pattern similar to the rPrPSc detected in the biopsy tissue (Figure 2B).
Figure 2.
Detection of rPrPSc in brain tissues obtained at biopsy and autopsy. A: rPrPSc in brain tissue obtained at biopsy was detected with Western blot analysis. Ten percent of brain homogenates from the current case, negative (Neg), or positive (Pos) CJD cases were treated with or without PK. A 5 μl or 20 μl of sample each was used for Western blotting. PK-resistant PrP was detected in either 5 μl or 20 μl of samples of the current case. The gel mobility of the PK-resistant PrP fragments from this case matches that of rPrPSc type 1 control. T1, rPrPSc type 1 control; T2, rPrPSc type 2 control. B: The rPrPSc in brain sample obtained at autopsy was also examined with Western blotting. Blots in A and B were probed with 3F4.
Detection of PrP in the Uterus, Placenta, and Amniotic Fluid
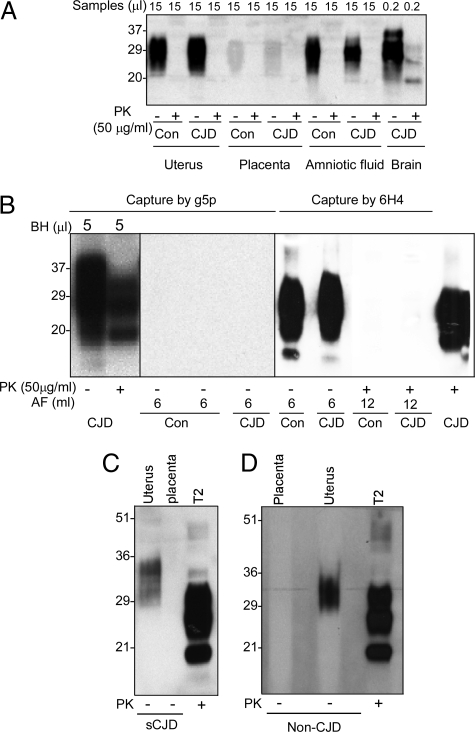
Western blot analysis showed that although PrPC was easily detectable in both amniotic fluid and uterus, rPrPSc was not observed in the CJD and non-CJD control samples using an antibody termed anti-C, raised against the carboxyl terminus of human PrP 220-231 (Figure 3A). Only faint PrP bands from both CJD and non-CJD placenta samples were observed in overexposed films and they were all PK-sensitive (Figure 3A).
Figure 3.
Detection of PrP in the uterus, placenta, and amniotic fluid. A: PrP in the uterus, placenta, amniotic fluid, and brain from the present case was detected with conventional Western blotting. Tissue homogenate (15 μl) from uterus and placenta, or 15-μl of amniotic fluids from the patient were examined. As controls, uterine and placenta tissues and amniotic fluid from nonprion disease patients (Con) were also examined. The blot was probed with antibody against the PrP C-terminal domain 220-231 (anti-C antibody). B: PrP detection in amniotic fluid (AF) with Western blotting after enrichment. PrP was captured by the g5p-conjugated beads from 5 μl of brain homogenate from the present case and the truncated PrP fragments were detectable after PK treatment of the sample precipitated by the g5p beads. In contrast, PrP was not captured by the g5p beads from 6 ml of amniotic fluid of normal control (Con) or the present case. Although a large amount of PrP was immunoprecipitated by the 6H4-conjugated beads from 6 ml of AF of both control and the present case, No PrP was detectable in the patient’s amniotic fluid after PK treatment. The blots were probed with anti-C antibody. C and D: PrP enrichment from the uterus and placenta by NaPTA. The PrP molecule was precipitated by NaPTA from 500 μl of 10% tissue homogenate from the uterus or placenta of normal (Non-CJD) (D) or present case (sCJD) (C) before Western blot analysis with 6H4 antibody. Small amounts of PrP were recovered from the uterus but not from the placenta of controls and the present case by NaPTA. T2, control rPrPSc type 2 directly loaded onto the gel.
To determine whether there is a small amount of PrPSc in these tissues, a large amount of samples (6 ml of amniotic fluid) was subjected to a gene 5 protein (g5p)-based enrichment of PrPSc before Western blot analysis. G5p is capable of capturing both rPrPSc and PK-sensitive PrPSc (sPrPSc) species.14,20,21 Although the g5p beads precipitated the abnormal PrPSc from 5 μl of CJD brain homogenate, PrP was not detectable in samples captured by g5p from 6 ml of amniotic fluid and 6 ml of uterine and placental homogenates each in both CJD and non-CJD controls (Figure 3B and data not shown). PrP was immunoprecipitable by 6H4-conjugated beads (Figure 3B). However, the PrP species precipitated from 12 ml of amniotic fluid of both CJD and non-CJD control was sensitive to PK digestion, suggesting that it is PrPC (Figure 3B). There were faint bands migrating between 20 kDa and 35 kDa in the PK-treated amniotic fluid from both non-CJD and CJD in the blots probed with1E4 against human PrP 97-108 and anti-C (Figure 3B and data not shown). These bands should not represent PrPSc because they were detected in both non-CJD and CJD samples (data not shown). Using sodium phosphotungstate (NaPTA), another reagent that has been widely used for precipitating abnormal PrP, a small amount of PrP was also precipitated from the uterus but not the placenta of both CJD and non-CJD (Figure 3, C and D). However, the PrP species precipitated by NaPTA from CJD and non-CJD uterus was sensitive to PK digestion (data not shown).
Characteristics of PrP from the Uterine and Gestational Tissues
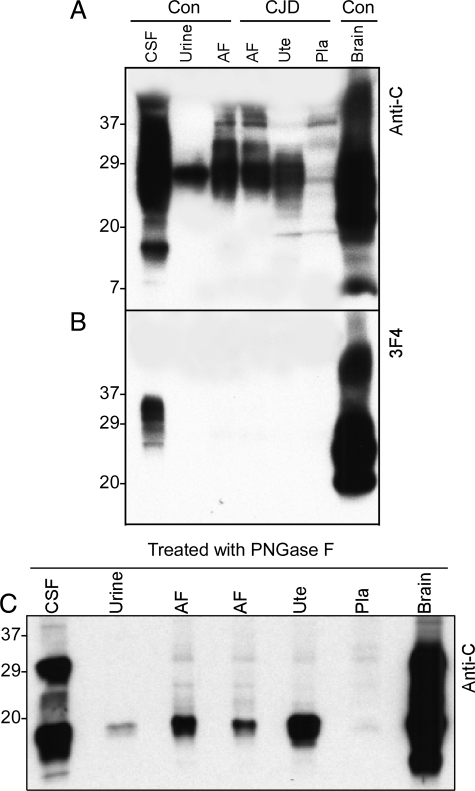
We compared the electrophoretic mobility of PrP obtained from a variety of samples including amniotic fluid, uterus, placenta, brain, CSF, and urine. Using the anti-C to residues PrP 220 to 231, PrP was detected in all samples despite the very small amount in the placenta (Figure 4A). The mobility of most PrP species from the brain and CSF was between ∼38 kDa and ∼20 kDa. In contrast, the migration of most PrP species from the amniotic fluid, uterus, and placenta was between 35 kDa and 26 kDa. Moreover, using 3F4 antibody against PrP 109-112, PrP was only detected in the brain and CSF but not in the peripheral tissues (Figure 4B), indicating that predominant PrP species from the amniotic fluid, uterus, and placenta are N-terminally truncated, similar to the urine PrP. This is consistent with previous observations.22,23,24
Figure 4.
Comparison of profiles of PrP from different tissues. A: The profile of PrP from the uterus, placenta, and amniotic fluid (AF) was compared with that of PrP from brain, CSF, and urine using Western blot analysis with anti-C antibody. The migration of most PrP species from the uterus, placenta, and AF is between 24 and 34 kDa, significantly different from that of PrP from the brain and CSF but similar to that of urine PrP. B: PrP from CSF, urine, amniotic fluid (AF), uterus (Ute), placenta (Pla), and brain was probed with 3F4. PrP was detectable in CSF and brain samples but not in urine, AF, uterus, and placenta. C: Samples from CSF, urine, AF, uterus, placenta, and brain were deglycosylated with PNGase F before Western blot analysis with anti-C antibody. As expected, PrP from CSF and brain shows two major bands corresponding to the ∼28- to 29-kDa full-length and ∼18- to 19-kDa N-terminally truncated PrP whereas PrP from urine, AF, uterus, and placenta shows only one major band corresponding to the ∼18- to 19-kDa N-terminally truncated PrP fragment.
To further confirm that the dominant PrP species from the uterine and gestational tissues are N-terminally truncated, the samples were subjected to deglycosylation with PNGase F. After treatment with PNGase F, by using the anti-C antibody two major PrP fragments were detected in brain tissues and CSF migrating at ∼28 kDa and ∼18 kDa. In contrast, only one major PrP band was detected in the amniotic fluid, uterus, placenta, and urine migrating at ∼18 kDa (Figure 4C). As expected, the PrP band migrating at ∼28 kDa, corresponding to the full-length PrP, was only detected in brain tissue and CSF, but not in the uterine and gestational tissues and urine (Figure 4C).
Discussion
It is widely thought that vertical transmission constitutes one route for the natural spread of prion diseases in animals, including scrapie in sheep and goats and bovine spongiform encephalopathy in cattle. However, the precise mechanism underlying the transmission of prion infectivity from ewe to lamb remains poorly understood. Since it has been demonstrated that prion infectivity is harbored in the blood of sheep with scrapie,25 vertical transmission could simply result from the large accumulation of blood in the placenta. Indeed, PrPSc has been observed at the fetal-maternal interface of infected sheep.22,26 As for human prion diseases, to date eight gravid women with CJD (including the current case) have been documented through the completion of pregnancy and delivery of their neonates.10,27,28,29 In addition, an 11-month-old girl born to a mother with vCJD was suspected to have the disease, inasmuch as she presented with convulsions and stiff limbs and had difficulty swallowing.30 However, to our knowledge, the diagnosis of CJD in this case has never been established. In fact, no case of vCJD has ever been reported in a baby to date. The youngest patient (a 12-year-old girl) with vCJD had no maternal history of the disease.31 Prion disease has been transmitted by inoculation to BALB/c mice with placenta and colostrum as well as leukocytes from umbilical cord blood samples, but not from amniotic fluid, from only one of the eight pregnant women.28 The finding of human-to-human transmission of vCJD through blood transfusion32 further implies the possibility that transmission from infected gravid women to neonate could occur through the placenta, a part of fetoplacental blood reservoir. But, neither the presence nor the individual features of PrP have been reported in the reproductive and gestational tissues in CJD and nonprion disease cases.
Genetic analysis revealed homozygosity valine at codon 129 and no mutation in the coding region of the PrP gene. Western blot of brain tissues obtained at biopsy and autopsy demonstrated the presence of rPrPSc type 1 (Figure 2), whereas neuropathological examination revealed severe spongiform degeneration, astrogliosis, and the presence of ballooned neurons associated with weak PrP immunostaining, displaying a fine granular pattern (Figures 1 and 2).33 Cumulatively, these characteristics unquestionably define this case as sCJDVV1.33
Our study indicated that most of the PrP in the uterus, placenta, and amniotic fluid is truncated at the N-terminal and shares gel mobility (∼18 kDa) with C1, a C-terminal PrP fragment generated during normal metabolism of PrPC in the brain.12 It is noteworthy that the gel mobility of the PrP fragment present in these tissues is similar to that of the urine PrP, but different from that of CSF and brain, in agreement with earlier observations.22,23,24 The difference in the PrP profile between CNS and peripheral organs suggests that PrP may be subject to distinct metabolic pathways in the brain and peripheral organs. But the molecular mechanism associated with the generation of different PrP profiles needs to be further investigated. No significant differences in the amount and profile of PrP from these peripheral tissues were observed between normal controls and this CJD case. The rPrPSc type 1 was detected in the brain from the patient but not in the uterus, placenta, and amniotic fluid even after using g5p enrichment procedure. Although a small amount of PrP was precipitated from the uterus of the present case by NaPTA, a similar amount of PrP was also precipitated from the control (Figure 3C). Thus the captured PrP by NaPTA is unlikely to be associated with CJD.
PrPC and PrPSc in the reproductive and gestational tissues have been well characterized in normal and scrape-infected sheep using Western blot analysis and immunohistochemistry.26,22,34 N-terminally truncated and glycosylated PrPC that migrates at ∼23 to 37 kDa, has been observed in cotyledonary chorioallantois, allantoic fluid, endometrium, myometrium, oviduct, and ovary of pregnant and nonpregnant uninfected ewes.22 In addition, PrPC in low or undetectable amounts was observed in intercotyledonary chorioallantois, amnion, and amniotic fluid. When detectable, PrPC had an electrophoretic mobility of ∼18 to 22 kDa after deglycosylation. The rPrPSc fragments were detected in enriched tissue homogenates of uterine caruncular endometrium and placental cotyledonary chorioallantois but not in other parts of the uterus or placenta, nor in amniotic fluid from pregnant scrapie-infected ewes.22 By immunohistochemistry, PrPSc staining was observed mainly in the uterine endometrial epithelium, trophoblast cells, and multinucleate cells in zone A of the maternal endometrial crypts of scrapie-infected ewes.34 Interestingly, the rPrPSc bands present in sheep placenta had lower molecular mass than did corresponding bands from the brain.26 It has been proposed that the differences in the size and glycosylation patterns of the PK-resistant PrP in the placenta and brain represent unique PrPSc processing in different tissues.26,35
Although our current study did not detect abnormal PrP species in the uterus and gestational tissues, including the placenta and amniotic fluid from this CJD case, it must be emphasized that only portions of the uterine and placental tissues were examined. Given localization of PrPSc only in certain areas of the uterus and placenta of the infected ewes,22,34 additional studies on more areas of these organs from gravid women with CJD are needed. Moreover, whether these tissues are infectious remains to be further determined by bioassay. Because the amniotic fluid is continually being swallowed and inhaled by the fetus during pregnancy, it is important to determine whether or not amniotic fluid harbors prion infectivity. Although close long-term follow-up of the offspring of the eight pregnant women with CJD is necessary, a definitive negative result of the transmissibility studies by direct bioassay or by amplification-based detection of reproductive and gestational tissues including the amniotic fluid would greatly relieve these children and their families from discomfort and anguish.
Acknowledgments
We thank Francine Epperson for autopsy coordination; Brenda Dupree and Rose Richardson for technical support in histological and immunohistochemical analyses of the brain biopsy and autopsy specimens at the Indiana Alzheimer Disease Center; Kay Edmonds for tissue coordinating; Diane Kofskey for technical support in studies of histology and immunohistochemistry; and Kai Jing for technical support in diagnostic Western blot analysis of the autopsy brain sample at the National Prion Disease Pathology Surveillance Center.
Footnotes
Address reprint requests to Wen-Quan Zou, M.D., Ph.D., Institute of Pathology, Case Western Reserve University, 2085 Adelbert Rd., Cleveland, OH 44106. E-mail: wenquan.zou@case.edu.
Supported in part by the CJD Foundation; the National Institutes of Health (grants P30AG010133, AG-14359, AG-08702, and the Center for Disease Control and Prevention contract UR8/CCU515004); the Britton Fund; and the Department of Neurological and Behavioral Sciences, University of Siena, Siena, Italy (to S.S.).
References
- Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB, Williams E, Laplanche JL, Shinagawa M. Scrapie, chronic wasting disease, and transmissible mink encephalopathy. Prusiner SB, editor. New York: Woodbury; Prion Biology and Diseases. 2004:pp 545–594. [Google Scholar]
- Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, Collinge J. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358:171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- Glatzel M, Abela E, Maissen M, Aguzzi A. Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. N Engl J Med. 2003;349:1812–1820. doi: 10.1056/NEJMoa030351. [DOI] [PubMed] [Google Scholar]
- Will RG. Acquired prion disease: iatrogenic CJD, variant CJD, kuru. Br Med Bull. 2003;66:255–265. doi: 10.1093/bmb/66.1.255. [DOI] [PubMed] [Google Scholar]
- Gajdusek DC, Zigas V. Degenerative disease of the central nervous system in New Guinea: the endemic occurrence of “kuru” in the native population. N Engl J Med. 1957;257:974–978. doi: 10.1056/NEJM195711142572005. [DOI] [PubMed] [Google Scholar]
- Alpers MP, Gajdusek DC. Changing patterns of kuru: epidemiological changes in the period of increasing contact of the Fore people with western civilization. Am J Trop Med Hyg. 1965;14:852–879. doi: 10.4269/ajtmh.1965.14.852. [DOI] [PubMed] [Google Scholar]
- Brown P, Brandel JP, Preese M, Sato T. Iatrogenic Creutzfeldt–Jakob disease: the waning of an era. Neurology. 2006;67:389–393. doi: 10.1212/01.wnl.0000231528.65069.3f. [DOI] [PubMed] [Google Scholar]
- Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith PG. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- Berrebi A, Cohen M, Ayoubi JM. [Creutzfeldt-Jakob disease and pregnancy.] J Gynecol Obstet Biol Reprod (Paris) 1997;26:755–759. [PubMed] [Google Scholar]
- Monari L, Chen SG, Brown P, Parchi P, Petersen RB, Mikol J, Gray F, Cortelli P, Montagna P, Ghetti B, Goldfarb LG, Gajdusek DC, Lugaresi E, Gambetti P, Autilio-Gambetti L. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: different prion proteins determined by a DNA polymorphism. Proc Natl Acad Sci USA. 1994;91:2839–2842. doi: 10.1073/pnas.91.7.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SG, Teplow DB, Parchi P, Teller JK, Gambetti P, Autilio-Gambetti L. Truncated forms of the human prion protein in normal brain and in prion diseases. J Biol Chem. 1995;270:19173–19180. doi: 10.1074/jbc.270.32.19173. [DOI] [PubMed] [Google Scholar]
- Kascsak RJ, Rubenstein R, Merz PA, Tonna-DeMasi M, Fersko R, Carp RI, Wisniewski HM, Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Xiao X, McGeehan J, Dong Z, Cali I, Fujioka H, Kong Q, Kneale G, Gambetti P, Zou WQ. Insoluble aggregates and protease-resistant conformers of prion protein in uninfected human brains. J Biol Chem. 2006;281:34848–34858. doi: 10.1074/jbc.M602238200. [DOI] [PubMed] [Google Scholar]
- Oliver AW, Bogdarina I, Schroeder E, Taylor IA, Kneale GG. Preferential binding of fd gene 5 protein to tetraplex nucleic acid structures. J Mol Biol. 2000;301:575–584. doi: 10.1006/jmbi.2000.3991. [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Petersen RB, Tabaton M, Brown P, LeBlanc AC, Montagna P, Cortelli P, Julien J, Vital C, Pendelbury WW, Haltia M, Willis PR, Hauw JJ, McKeever PE, Monari L, Schrank B, Swergold GD, Autilio-Gambetti L, Gajdusek C, Lugaresi E, Gambetti P. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: disease phenotype determined by a DNA polymorphism. Science. 1992;258:806–808. doi: 10.1126/science.1439789. [DOI] [PubMed] [Google Scholar]
- Parchi P, Castellani R, Capellari S, Ghetti B, Young K, Chen SG, Farlow M, Dickson DW, Sima AA, Trojanowski JQ, Petersen RB, Gambetti P. Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 1996;39:767–778. doi: 10.1002/ana.410390613. [DOI] [PubMed] [Google Scholar]
- Pastore M, Chin SS, Bell KL, Dong Z, Yang Q, Yang L, Yuan J, Chen SG, Gambetti P, Zou WQ. Creutzfeldt-Jakob disease (CJD) with a mutation at codon 148 of prion protein gene: relationship with sporadic CJD. Am J Pathol. 2005;167:1729–1738. doi: 10.1016/S0002-9440(10)61254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou WQ, Cashman NR. Acidic pH and detergents enhance in vitro conversion of human brain PrPC to a PrPSc-like form. J Biol Chem. 2002;277:43942–43947. doi: 10.1074/jbc.M203611200. [DOI] [PubMed] [Google Scholar]
- Zou WQ, Zheng J, Gray DM, Gambetti P, Chen SG. Antibody to DNA detects scrapie but not normal prion protein. Proc Natl Acad Sci USA. 2004;101:1380–1385. doi: 10.1073/pnas.0307825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambetti P, Dong Z, Yuan J, Xiao X, Zheng M, Alshekhlee A, Castellani R, Cohen M, Barria MA, Gonzalez-Romero D, Belay ED, Schonberger LB, Marder K, Harris C, Burke JR, Montine T, Wisniewski T, Dickson DW, Soto C, Hulette CM, Mastrianni JA, Kong Q, Zou WQ. A novel human disease with abnormal prion protein sensitive to protease. Ann Neurol. 2008;63:697–708. doi: 10.1002/ana.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo W, Zhuang D, Knowles DP, Cheevers WP, Sy MS, O'Rourke KI. Prp-c and Prp-Sc at the fetal-maternal interface. J Biol Chem. 2001;276:18229–18234. doi: 10.1074/jbc.M008887200. [DOI] [PubMed] [Google Scholar]
- Wong BS, Green AJ, Li R, Xie Z, Pan T, Liu T, Chen SG, Gambetti P, Sy MS. Absence of protease-resistant prion protein in the cerebrospinal fluid of Creutzfeldt-Jakob disease. J Pathol. 2001;194:9–14. doi: 10.1002/path.872. [DOI] [PubMed] [Google Scholar]
- Narang HK, Dagdanova A, Xie Z, Yang Q, Chen SG. Sensitive detection of prion protein in human urine. Exp Biol Med (Maywood) 2005;230:343–349. doi: 10.1177/153537020523000508. [DOI] [PubMed] [Google Scholar]
- Houston F, McCutcheon S, Goldmann W, Chong A, Foster J, Sisó S, González L, Jeffrey M, Hunter N. Prion diseases are efficiently transmitted by blood transfusion in sheep. Blood. 2008;112:4739–4745. doi: 10.1182/blood-2008-04-152520. [DOI] [PubMed] [Google Scholar]
- Race R, Jenny A, Sutton D. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis. 1998;178:949–953. doi: 10.1086/515669. [DOI] [PubMed] [Google Scholar]
- Bernoulli C, Siegfried J, Baumgartner G, Regli F, Rabinowicz T, Gajdusek DC, Gibbs CJ., Jr Danger of accidental person-to-person transmission of Creutzfeldt-Jakob disease by surgery. Lancet. 1977;1:478–479. doi: 10.1016/s0140-6736(77)91958-4. [DOI] [PubMed] [Google Scholar]
- Tamai Y, Kojima H, Kitajima R, Taguchi F, Ohtani Y, Kawaguchi T, Miura S, Sato M, Ishihara Y. Demonstration of the transmissible agent in tissue from a pregnant woman with Creutzfeldt-Jakob disease. N Engl J Med. 1992;327:649. doi: 10.1056/NEJM199208273270918. [DOI] [PubMed] [Google Scholar]
- Lane KL, Brown P, Howell DN, Crain BJ, Hulette CM, Burger PC, DeArmond SJ. Creutzfeldt-Jakob disease in a pregnant woman with an implanted dura mater graft. Neurosurgery. 1994;34:737–740. doi: 10.1227/00006123-199404000-00026. [DOI] [PubMed] [Google Scholar]
- Ashraf H. The first case of mother-to child transmission of vCJD? Lancet. 2000;356:1085. [Google Scholar]
- Verity CM, Nicoll A, Will RG, Devereux G, Stellitano L. Variant Creutzfeldt-Jakob disease in UK children: a national surveillance study. Lancet. 2000;356:1224–1227. doi: 10.1016/s0140-6736(00)02785-9. [DOI] [PubMed] [Google Scholar]
- Llewelyn CA, Hewitt PE, Knight RS, Amar K, Cousens S, Mackenzie J, Will RG. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004;363:417–421. doi: 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- Gambetti P, Kong Q, Zou WQ, Parchi P, Chen SG. Sporadic and familial CJD: classification and characterisation. Br Med Bull. 2003;66:213–239. doi: 10.1093/bmb/66.1.213. [DOI] [PubMed] [Google Scholar]
- Tuo W, O'Rourke KI, Zhuang D, Cheevers WP, Spraker TR, Knowles DP. Pregnancy status and fetal prion genetics determine PrPSc accumulation in placentomes of scrapie-infected sheep. Proc Natl Acad Sci USA. 2002;99:6310–6315. doi: 10.1073/pnas.072071199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B, Race RE, Ernst D, Buchmeier MJ, Chesebro B. Prion protein biosynthesis in scrapie-infected and uninfected neuroblastoma cells. J Virol. 1989;63:175–181. doi: 10.1128/jvi.63.1.175-181.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]