Abstract
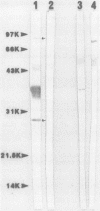
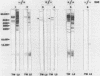
Recent studies suggest that a group of Chlamydia strains known as TWAR, which are now proposed to be a new species called Chlamydia pneumoniae, may be a frequent cause of respiratory disease in the United States and many other countries. Current serotesting methods do not allow rapid screening of large numbers of samples to distinguish C. trachomatis exposure from C. pneumoniae exposure. We developed an enzyme immunoassay to decrease cross-reactivity between immunoglobulin G antibodies reactive with C. trachomatis and C. pneumoniae. Elementary bodies of C. trachomatis or C. pneumoniae were treated with a detergent-chelating solution to decrease the reactivity of the common lipopolysaccharide antigens. Sera from four groups of patients, totaling 143 persons, were tested by this assay. The prevalences of titers of greater than or equal to 128 to C. trachomatis and C. pneumoniae, respectively, were as follows: (i) for 23 women seropositive for C. trachomatis by the microimmunofluorescence test, 21 (91%) and 18 (78%); (ii) for 50 adult blood donors, 13 (26%) and 39 (78%); (iii) for 40 sexually transmitted disease clinic patients, 20 (50%) and 32 (80%); (iv) for 30 healthy children 5 to 7 years old, 0 (0%) and 8 (27%). Western blots (immunoblots) of each antigen corroborated the differential reactivity of C. trachomatis-positive, C. pneumoniae-negative and C. trachomatis-negative, C. pneumoniae-positive serum samples. Western blots of serum samples from rabbits immunized with either C. trachomatis or C. pneumoniae elementary bodies revealed at least two protein bands (30 and 80 kilodaltons) which appeared to represent unique C. pneumoniae antigens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kuo C. C., Kenny G. E. Antigenic analysis of Chlamydiae by two-dimensional immunoelectrophoresis. I. Antigenic heterogeneity between C. trachomatis and C. psittaci. J Immunol. 1975 Oct;115(4):963–968. [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Thissen R. W., Grayston J. T. Isolation of a gene encoding a Chlamydia sp. strain TWAR protein that is recognized during infection of humans. Infect Immun. 1989 Jan;57(1):71–75. doi: 10.1128/iai.57.1.71-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darougar S., Forsey T., Brewerton D. A., Rogers K. L. Prevalence of antichlamydial antibody in London blood donors. Br J Vener Dis. 1980 Dec;56(6):404–407. doi: 10.1136/sti.56.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir S. P., Hakomori S., Kenny G. E., Grayston J. T. Immunochemical studies on chlamydial group antigen (presence of a 2-keto-3-deoxycarbohydrate as immunodominant group). J Immunol. 1972 Jul;109(1):116–122. [PubMed] [Google Scholar]
- Evans R. T., Taylor-Robinson D. Development and evaluation of an enzyme-linked immunosorbent assay (ELISA), using chlamydial group antigen, to detect antibodies, to Chlamydia trachomatis. J Clin Pathol. 1982 Oct;35(10):1122–1128. doi: 10.1136/jcp.35.10.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsey T., Darougar S., Treharne J. D. Prevalence in human beings of antibodies to Chlamydia IOL-207, an atypical strain of chlamydia. J Infect. 1986 Mar;12(2):145–152. doi: 10.1016/s0163-4453(86)93608-x. [DOI] [PubMed] [Google Scholar]
- Forsey T., Stainsby K., Hoger P. H., Ridgway G. L., Darougar S., Fischer-Brugge U. Comparison of two immunofluorescence tests for detecting antibodies to C. trachomatis. Eur J Epidemiol. 1986 Jun;2(2):163–164. doi: 10.1007/BF00157029. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Harrison H. R., Alexander E. R., Weinstein L., Lewis M., Nash M., Sim D. A. Cervical Chlamydia trachomatis and mycoplasmal infections in pregnancy. Epidemiology and outcomes. JAMA. 1983 Oct 7;250(13):1721–1727. [PubMed] [Google Scholar]
- Kleemola M., Saikku P., Visakorpi R., Wang S. P., Grayston J. T. Epidemics of pneumonia caused by TWAR, a new Chlamydia organism, in military trainees in Finland. J Infect Dis. 1988 Feb;157(2):230–236. doi: 10.1093/infdis/157.2.230. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ladany S., Sarov I. Recent advances in Chlamydia trachomatis. Eur J Epidemiol. 1985 Dec;1(4):235–256. doi: 10.1007/BF00237099. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahony J. B., Schachter J., Chernesky M. A. Detection of antichlamydial immunoglobulin G and M antibodies by enzyme-linked immunosorbent assay. J Clin Microbiol. 1983 Aug;18(2):270–275. doi: 10.1128/jcm.18.2.270-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Rietschel E. T., Brade H. Chemical characterization of Chlamydia trachomatis lipopolysaccharide. Infect Immun. 1985 May;48(2):573–575. doi: 10.1128/iai.48.2.573-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway G. L. Chlamydial infections in man. Postgrad Med J. 1986 Apr;62(726):249–253. doi: 10.1136/pgmj.62.726.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikku P., Wang S. P., Kleemola M., Brander E., Rusanen E., Grayston J. T. An epidemic of mild pneumonia due to an unusual strain of Chlamydia psittaci. J Infect Dis. 1985 May;151(5):832–839. doi: 10.1093/infdis/151.5.832. [DOI] [PubMed] [Google Scholar]
- Schachter J. Chlamydia psittaci--"reemergence" of a forgotten pathogen. N Engl J Med. 1986 Jul 17;315(3):189–191. doi: 10.1056/NEJM198607173150311. [DOI] [PubMed] [Google Scholar]
- Schmeer N., Krauss H. Purification of genus-specific chlamydial antigen and its separation into several components by ion-exchange chromatography. J Clin Microbiol. 1982 May;15(5):830–834. doi: 10.1128/jcm.15.5.830-834.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stamm W. E., Koutsky L. A., Benedetti J. K., Jourden J. L., Brunham R. C., Holmes K. K. Chlamydia trachomatis urethral infections in men. Prevalence, risk factors, and clinical manifestations. Ann Intern Med. 1984 Jan;100(1):47–51. doi: 10.7326/0003-4819-100-1-47. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J Infect Dis. 1974 Oct;130(4):388–397. doi: 10.1093/infdis/130.4.388. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Grayston J. T. Formalinized Chlamydia trachomatis organisms as antigen in the micro-immunofluorescence test. J Clin Microbiol. 1979 Aug;10(2):259–261. doi: 10.1128/jcm.10.2.259-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]