Abstract
Here we used the Met-1 cell line in an orthotopic transplantation model in FVB/N mice to dissect the role of the Cav-1(P132L) mutation in human breast cancer. Identical experiments were performed in parallel with wild-type Cav-1. Cav-1(P132L) up-regulated the expression of estrogen receptor-α as predicted, because only estrogen receptor-α-positive patients have been shown to harbor Cav-1(P132L) mutations. In the context of primary tumor formation, Cav-1(P132L) behaved as a loss-of-function mutation, lacking any tumor suppressor activity. In contrast, Cav-1(P132L) caused significant increases in cell migration, invasion, and experimental metastasis, consistent with a gain-of-function mutation. To identify possible molecular mechanism(s) underlying this invasive gain-of-function activity, we performed unbiased gene expression profiling. From this analysis, we show that the Cav-1(P132L) expression signature contains numerous genes that have been previously associated with cell migration, invasion, and metastasis. These include i) secreted growth factors and extracellular matrix proteins (Cyr61, Plf, Pthlh, Serpinb5, Tnc, and Wnt10a), ii) proteases that generate EGF and HGF (Adamts1 and St14), and iii) tyrosine kinase substrates and integrin signaling/adapter proteins (Akap13, Cdcp1, Ddef1, Eps15, Foxf1a, Gab2, Hs2st1, and Itgb4). Several of the P132L-specific genes are also highly expressed in stem/progenitor cells or are associated with myoepithelial cells, suggestive of an epithelial-mesenchymal transition. These results directly support clinical data showing that patients harboring Cav-1 mutations are more likely to undergo recurrence and metastasis.
We and others have shown that the caveolin-1 (Cav-1) gene is commonly mutated in human breast cancers.1,2 Up to one-third of estrogen receptor (ER)-α-positive breast cancers harbor Cav-1 mutations,2 indicating that it may be a very common initiating or early event in the development of breast cancers in humans. In accordance with these human genetic studies, Cav-1 (−/−)-null mice show numerous mammary gland phenotypes, including progressive mammary intraductal hyperplasia,3,4 and are more susceptible to mammary tumorigenesis and metastasis, when crossed with established mouse models that spontaneously develop mammary tumors.5,6,7 Thus, Cav-1 is thought to function as a tumor suppressor or modifier gene in mammary epithelia.8,9
Although eight Cav-1 breast cancer-associated mutations have been described to date, the most common mutation is a proline to leucine change at position 132 within its putative transmembrane domain.2 The P132L mutation accounts for more than half of the breast cancer cases with Cav-1 mutations, indicating that this residue is a hot-spot for sporadic mutation in the genome, akin to the Ras (G12V) mutation. Interestingly, this proline residue is critical because it is 1 of 12 invariant caveolin residues that are conserved from worms (Caenorhabditis elegans) to humans.10 The analogous proline residue is also mutated in Cav-3 (P104L), a muscle-specific caveolin-related protein, and gives rise to an autosomal dominant form of muscular dystrophy, termed limb-girdle muscular dystrophy (LGMD-type 1C).11 However, unlike Cav-1 (P132L), which does not undergo significant degradation, Cav-3 (P104L) is rapidly degraded by a ubiquitin/proteosomal-dependent pathway.12
Little is known about how the Cav-1 (P132L) mutation mediates its effects. Based on biochemical studies, P132L appears to represent a loss-of-function mutation, that can also act in a dominant-negative manner, by inactivating the WT Cav-1 protein product. These initial studies showed that when the P132L mutant is transiently expressed in fibroblastic cell types, it is misfolded, forms high-molecular mass aggregates, and is retained in a perinuclear ER/Golgi-like compartment.3 It is not efficiently targeted to the plasma membrane. Interestingly, when Cav-1 (P132L) is co-expressed with WT Cav-1, both are retained intracellularly, indicating that Cav-1 (P132L) behaves in a dominant-negative manner.3 Thus, because wild-type (WT) Cav-1 is normally localized to plasmalemmal caveolae, the functional effects of the Cav-1 (P132L) mutant may be explained in part by its mislocalization and intracellular retention.
Studies in NIH-3T3 fibroblasts have shown that expression of the Cav-1 (P132L) mutant is sufficient to mediate cell transformation.1 Similarly, knock-down of Cav-1 protein expression in NIH-3T3 cells, using an anti-sense cDNA approach, also drives cell transformation and tumor formation in immune-deficient mice.13 Thus, these studies also provide evidence that the P132L mutation represents a loss-of-function mutation. However, this may be an oversimplification because human breast cancer patients that harbor Cav-1 mutations are more likely to undergo recurrence and metastasis, despite the good prognosis that is usually associated with ER positivity.2
Thus, we developed an orthotopic transplantation model in mice to study the behavior of the Cav-1 (P132L) mutation in vivo. For this purpose, we chose Met-1 cells, which are a mouse luminal mammary epithelial cell line derived from an MMTV-PyMT mammary tumor.14,15 Although Met-1 cells have been selected for their capacity to undergo metastasis, they also form primary mammary tumors when orthotopically implanted in FVB/N mice. We have previously shown that Cav-1 (WT) behaves as a tumor suppressor in this system,7 indicating that Met-1 cells may also be a useful model for studying the properties of Cav-1 mutants associated with human breast cancer.
Here, using the Met-1 cell system, we show that the Cav-1 (P132L) mutation behaves as a loss-of-function mutation in the context of primary tumor formation. However, we also show that the Cav-1 (P132L) mutation acts as a gain-of-function mutation in the context of cell migration, invasion, and experimental metastasis. Global genome-wide expression profiling studies reveal a Cav-1 (P132L)-specific expression signature that contains numerous genes previously shown to be associated with breast cancer metastasis, cell motility, or invasiveness. As such, these studies may provide a mechanistic basis for understanding why breast cancer patients with Cav-1 mutations are more prone to disease recurrence. In further support of the validity of this model, we also show that the Cav-1 (P132L) mutant induces ER-α protein expression (as seen by Western blotting) and activates ER-α signaling (as seen by gene expression profiling), consistent with the association of this mutation with ER positivity in human breast cancers.
Materials and Methods
Materials
Antibodies and their sources were as follows: anti-Cav-1 (N-20) rabbit polyclonal antibody (pAb) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-β-actin mouse monoclonal antibody (mAb) AC-15 (Sigma, St. Louis, MO), anti-Ki-67 rabbit pAb (Abcam, Inc., Cambridge, MA), anti-cyclin D1 rabbit pAb (Lab Vision, Inc., Fremont, CA), anti-ER-α rabbit pAbs (MC-20 and H-184) (Santa Cruz Biotechnology, Inc.), anti-Cyr61 rabbit pAb (H-78, Santa Cruz Biotechnology, Inc), anti-Foxf1a rabbit pAb (Abcam), anti-Krt14 rabbit pAb (Covance, Princeton, NJ), anti-Wnt10a rat mAb (R&D Systems, Inc., Minneapolis, MN), anti-Gab2 rabbit pAb (26B6; Cell Signaling Technology, Beverly, MA), and anti-Usp34 mouse mAb (clone 2E2; Abnova Corp., Ann Arbor, MI). Rabbit polyclonal antibodies to MLN64 were from Abcam (for Western blotting) or were as previously described (for immunohistochemistry).16,17,18,19,20,21,22 Antibodies to Cab45, an established Golgi marker protein,23 were the generous gift of Dr. Philipp E. Scherer (University of Texas Southwestern, Dallas, TX). Inhibitors of EGF-R/ErbB2 (GW-583340), the c-MET receptor tyrosine kinase (PHA-665752), and the transforming growth factor (TGF)-β type I receptor (LY-364947) were purchased from Tocris Biosciences, Ellisville, MO. Met-1 cells were the generous gift of Dr. Robert D. Cardiff (University of California–Davis); it is important to note that in the current studies we used an earlier passage (less aggressive/less metastatic) version of the cell line. Our previously published studies, which did not examine the effects of the Cav-1 (P132L) mutant, used the later passage/more metastatic Met-1 cells.7
Retroviral Transduction and Cell Culture
Met-1 stable cell lines were produced by retroviral-mediated transduction (using the vector pBABE-puro), essentially as we previously described.7 Briefly, C-terminally Myc-tagged cDNAs encoding WT Cav-1 and Cav-1 (P132L) were generated by polymerase chain reaction (PCR) and subcloned into the pBABE vector using the BamH1/EcoR1 restriction site. The correctness of intended base substitutions and the absence of unwanted mutations were verified by DNA sequencing. After retroviral transduction, Met-1 cells were selected for 5 to 7 days in 10 μg/ml of puromycin. Met-1 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Immunoblot Analysis
Met-1 cells were lysed in 800 μl of lysis buffer (10 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 1% Triton X-100, 60 mmol/L octyl glucoside), containing protease (Roche Applied Science, Indianapolis, IN) and phosphatase inhibitors (Sigma). Cell lysates were then centrifuged at 12,000 × g for 10 minutes to remove insoluble debris. Protein concentrations were analyzed using the BCA reagent (Pierce, Rockford, IL) and the volume required for 50 μg of protein was determined. Cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8 to 10% acrylamide) and transferred to nitrocellulose. The nitrocellulose membranes were stained with Ponceau S (to visualize protein bands), followed by immunoblot analysis. Subsequent wash buffers contained 10 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 0.05% Tween-20 (TBS-Tween), which was supplemented with 1% bovine serum albumin (BSA) and 4% nonfat dry milk (Carnation, Wilkes-Barre, PA) for the blocking solution and 1% BSA for the antibody diluent. For phospho-antibody analysis, the blocking solution contained only 5% BSA in TBS-Tween (without nonfat milk). Primary antibodies were used at a 1:100 to 1:500 dilution. Horseradish peroxidase-conjugated secondary antibodies [anti-mouse, 1:6000 dilution (Pierce) or anti-rabbit 1:5000 (BD Pharmingen, San Diego, CA)] were used to visualize bound primary antibodies, with the Supersignal chemiluminescence substrate (Pierce).
Immunofluorescence Microscopy
Met-1 cells were grown on sterile glass coverslips, washed three times in phosphate-buffered saline (PBS), and fixed for 30 minutes at room temperature with 2% paraformaldehyde in PBS. After fixation, cells were permeabilized with 0.1% Triton X-100/0.2% BSA/PBS for 10 minutes. Cells were then treated with 25 mmol/L NH4Cl in PBS for 10 minutes at room temperature to quench free aldehyde groups. After rinsing with PBS, cells were incubated with primary antibody diluted in 0.1% Triton X-100/0.2% BSA/PBS, overnight at 4°C. The day after, three washes with PBS for 5 minutes each were done before the secondary antibody incubation (with a rhodamine-conjugated anti-mouse or anti-rabbit antibody) for 30 minutes at room temperature. Finally, cells were washed three times with PBS (10 minutes each wash), and mounted on a glass slide with slow-fade anti-fade reagent (Molecular Probes, Eugene, OR).
Animal Studies
All animals were housed and maintained in a barrier facility at the Kimmel Cancer Center at Thomas Jefferson University. All WT mice used in this study were virgin female in the FVB/N genetic background. Animal protocols used for this study were pre-approved by the institutional animal care and use committee.
Primary Mammary Tumor Formation
For orthotopic implantation, 0.5 × 105 cells were resuspended in 5 μl of PBS and injected through the nipple of the inguinal (no. 4) mammary gland into 2-month-old FVB/N female mice using a Hamilton syringe with a 26-gauge needle.24 Met-1 cells are syngeneic to the FVB/N strain. At 6 weeks after injection, mice were sacrificed, and the tumors were carefully excised and weighed.
Immunohistochemistry
Immunostaining of slides containing deparaffinized formalin-fixed mammary tumor sections was performed essentially as we described.2,7 Briefly, paraffin-embedded tumors were sectioned at 5 μm. Sections were then deparaffinized first by treatment with xylene and rehydrated by passage through a graded series of ethanol. Antigen retrieval was performed by microwaving the slides in 100 mmol/L sodium citrate buffer for 15 minutes. Endogenous peroxide activity was quenched by incubating the slides for 10 minutes in 3% H2O2. Slides were then washed in phosphate-buffered saline (PBS) and blocked with a solution containing 10% goat serum in PBS for 1 hour at room temperature. Samples were washed with PBS and incubated with the primary antibody in blocking solution for 12 to 16 hours at 4°C. Slides were then washed with PBS (three washes, 5 minutes each) and incubated with a biotinylated secondary antibody in blocking solution for 30 minutes at room temperature. Slides were further washed in PBS (three washes, 5 minutes each) and incubated with the avidin/biotin-horseradish peroxidase reagent for 30 minutes at room temperature. Next, samples were washed in PBS and incubated with the 3,3′-diaminobenzidine reagent until color production developed. Finally, the slides were washed in PBS to remove excess diaminobenzidine, counterstained with hematoxylin, dehydrated, and mounted with coverslips.
Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick-End Label (TUNEL) Staining
TUNEL-positive cells were identified using the ApopTag peroxidase in situ apoptosis detection kit (Millipore, Temecula, CA) per kit instructions. Briefly, paraffin sections were deparaffinized in xylene, rehydrated in ethanol, and washed with PBS. The tissue was treated with 20 μg/ml of proteinase K (Roche) diluted in PBS for 15 minutes at room temperature, washed, and blocked with 3% hydrogen peroxide for 5 minutes. The sections were then incubated with equilibration buffer briefly, followed by working strength TdT enzyme for 1 hour at 37°C. After washing, the sections were incubated with anti-digoxigenin horseradish peroxidase conjugated antibody for 30 minutes at room temperature, washed, and TUNEL-positive cells were detected using 3,3′-diaminobenzidine. TUNEL-positive cells were enumerated in 8 to 12 random ×40 fields from each group and the mean number of TUNEL-positive cells per field was calculated. For quantitation purposes, focal areas of necrosis were omitted and random viable areas were used.
Experimental Metastasis (Lung Colonization Assay)
To study cell invasive capacity in vivo, 5 × 105 cells suspended in 0.1 ml of PBS were injected through the tail vein of six WT FVB/N female mice for each cell line. After 4 weeks, the lungs were removed and insufflated with 2 ml of 15% India Ink, washed in water for 5 minutes, and bleached in Fekete’s solution (70% ethanol, 3.7% paraformaldehyde, 0.75 mol/L glacial acetic acid).7 Surface lung colonies were counted in a blinded manner under low power using a Nikon (Tokyo, Japan) SMZ-1500 stereomicroscope. P values were determined by applying the Mann-Whitney statistical analysis parameters, which does not assume a Gaussian distribution (nonparametric test).
Cell Migration and Invasion Assays
The invasive potential of Met-1 cell lines was measured via an in vitro modified Boyden chamber assay.25,26 Briefly, Met-1 cells in 0.5 ml of serum-free Dulbecco’s modified Eagle’s medium were added to the wells of 8-μm-pore membrane modified Boyden chambers, either coated with (for invasion assays; catalog no. 354483) or without (for migration assays; catalog no. 354578) Matrigel (Transwells; BD Biosciences, San Jose, CA). The lower chambers contained 10% fetal bovine serum in Dulbecco’s modified Eagle’s medium to serve as a chemoattractant. Cells were incubated at 37°C and allowed to migrate or invade, respectively, throughout the course of 6 and 18 hours. Noninvasive cells were removed from the upper surface of the membrane by scrubbing with cotton swabs. Chambers were stained in 0.5% crystal violet diluted in 100% methanol for 30 to 60 minutes, rinsed in water, and examined under a bright-field microscope. Values for invasion and migration were obtained by counting five fields per membrane (×20 objective) and represent the average of three independent experiments performed throughout multiple days. For inhibitor studies, the inhibitor was placed in both the lower and upper chambers.
Gene Expression Profiling
Total RNA (5 μg) was reverse transcribed using the Superscript III first-strand synthesis system (Invitrogen, Carlsbad, CA) using a HPLC purified T7-dT24 primer (Sigma Genosys, St. Louis, MO) which contains the T7 polymerase promoter sequence. The single-stranded cDNA was converted to double-stranded cDNA using DNA polymerase I (Promega, Madison, WI) and purified by cDNA spin column purification using GeneChip Sample Cleanup Module (Affymetrix, Santa Clara, CA). The double-stranded cDNA was used as a template to generate biotinylated cRNA using the Bioarray high-yield RNA transcription labeling kit (Enzo, New York, NY) and the labeled cRNA purified by GeneChip Sample Cleanup Module (Affymetrix). Fifteen μg of cRNA was fractionated to produce fragments of between 35 to 200 bp using 5× fragmentation buffer provided in the Cleanup Module. The sample was hybridized to mouse 430 2.0 microarray (Affymetrix) representing more than 39,000 transcripts. The hybridization and washing steps were performed in accordance with Affymetrix protocols for eukaryotic arrays. The arrays were scanned at 570 nm with a confocal scanner from Affymetrix.
Analysis of the arrays was performed using the R statistics package and the limma library of the Bioconductor software package. Arrays were normalized using robust multiarray analysis (RMA), and P value of 0.05 was applied as criteria for statistically differentially expressed genes. GO tree analysis of gene function was analyzed by Webgestalt (Vanderbilt University, Nashville, TN). Myo-epithelial and luminal gene sets were defined as previously described by gene expression profiling.27
Pathway Analysis
Global pathway analysis of our gene expression profiling studies was performed using ASSESS (analysis of sample set enrichment scores), a computer-based gene set enrichment algorithm.28
Results
Cav-1 (P132L) Behaves as a Loss-of-Function Mutation in the Context of Primary Tumor Formation
Derivation of Met-1 Cells Expressing Cav-1 (WT and P132L)
Here, we have used the mammary epithelial cell line, termed Met-1, as an orthotopic transplantation model to study the role of the Cav-1 (P132L) mutation in the pathogenesis of human breast cancers. Met-1 cells are a highly-transfectable cell line derived from a MMTV-PyMT mammary tumor and were selected for their metastatic capacity, although they also form primary tumors.14,15 Stable Met-1 lines expressing WT Cav-1, Cav-1 (P132L), or the vector alone (pBABE-puro) were derived by retroviral-mediated transduction, thereby avoiding the problems often associated with selecting individual clones.
Figure 1 shows that both Cav-1 (WT) and Cav-1 (P132L) were well-expressed in these transfected Met-1 stable cell lines, as seen by Western blot analysis with anti-Cav-1 IgG. Note that the P132L mutation slightly retards the mobility of Cav-1 in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, as we have previously described.3 Immunofluorescence analysis reveals that Cav-1 (WT) is properly targeted to the plasma membrane, whereas Cav-1 (P132L) is retained intracellularly in a perinuclear Golgi-like compartment (Figure 2A). Similar results have been previously obtained by transient transfection of Cos-7 cells.3 This is consistent with the idea that the Cav-1 (P132L) protein is misfolded.
Figure 1.
Derivation of Met-1 mammary cell lines expressing Cav-1 (WT and P132L). Met-1 cells were retrovirally transduced with pBABE-puro (empty vector), pBABE-Cav-1 (WT), or pBABE-Cav-1 (P132L). After selection with puromycin, pooled stable cell lines were generated and subjected to Western blot analysis. Immunoblots were probed with an anti-Cav-1 IgG (pAb N-20) to detect both endogenous (bottom band) and transfected (recombinant, top band) forms of Cav-1. As expected, recombinant Myc-tagged Cav-1 migrates slightly slower than endogenous Cav-1 (arrow). Note that both Cav-1 (WT and P132L) proteins were expressed at higher levels than endogenous Cav-1. Equal loading was assessed by immunoblotting with anti-β-actin IgG.
Figure 2.
Expression of Cav-1 (P132L) in Met-1 cells up-regulates ER-α levels. A: Immunofluorescence. To evaluate ER-α expression and localization, Met-1 cells were fixed and subjected to immunofluorescence analysis with an antibody directed against ER-α. Note that Met-1 cells expressing Cav-1 (P132L) exhibit intense ER-α nuclear staining, consistent with ER-receptor activation, as compared with the pBABE (empty vector) and Cav-1 (WT). Also, immunostaining of Met-1 cells with anti-Cav-1 IgG (pAb, N-20) was performed to visualize the cellular distribution of Cav-1. Note that Cav-1 (P132L) is primarily retained intracellularly in a perinuclear compartment and did not reach the plasma membrane to a significant extent. In contrast, Cav-1 (WT) is efficiently targeted to the plasma membrane. Met-1 cells transfected with vector alone (pBABE) are shown for comparison. B: Western blot analysis. Met-1 cell lysates were subjected to Western blot analysis with anti-ER-α-specific IgG (MC-20). Note that cells expressing Cav-1 (P132L) exhibit increased expression levels of ER-α, as compared with vector alone and Cav-1 (WT)-transfected counterparts. Equal loading was assessed by immunoblotting with anti-β-actin IgG.
In humans, the P132L mutation is exclusively associated with ER-α-positive breast cancers.2 To determine whether there is a cause-effect relationship, we also examined the expression of ER-α in transfected Met-1 cells. As shown in Figure 2A, vector-alone control Met-1 cells express a modest amount of ER-α, and this expression is suppressed in Cav-1 (WT) transfected cells. In contrast, Met-1 cells expressing Cav-1 (P132L) show a dramatic increase in ER-α expression, which was tightly localized to the nucleus, suggesting nuclear receptor activation. Such dramatic up-regulation of ER-α expression by Cav-1 (P132L) was also directly confirmed by Western blot analysis (Figure 2B). Thus, it appears that the Met-1 cell system is a valid model because the P132L mutation confers ER-α up-regulation, as seen in P132L-positive human breast cancers. Finally, we confirmed the Golgi localization of the Cav-1 (P132L) mutant by performing double-labeling experiments using a well-established Golgi marker protein, namely Cab45.23 Note the strict co-localization of Cav-1 (P132L) with Cab45 within the perinuclear region of the cell (Figure 3).
Figure 3.
Cav-1 (P132L) is mislocalized to the Golgi apparatus. Met-1 cells expressing Cav-1 (P132L) were subjected to double labeling with rabbit polyclonal antibodies directed against Cab45 (a Golgi marker protein, shown in red)23 and a mouse monoclonal antibody (mAb 9E10) directed against the Myc-epitope tag [to detect Cav-1 (P132L), shown in green]. Note the strict co-localization of Cav-1 (P132L) with Cab45 within the perinuclear region of the cell (arrows). N, nucleus.
Primary Tumor Formation
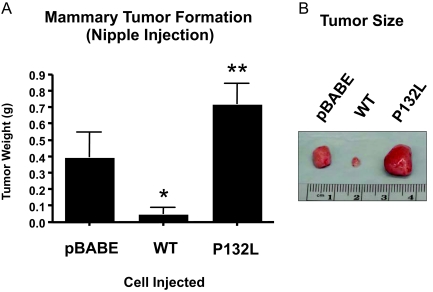
Next, we orthotopically implanted these Met-1 cell lines in FVB/N mice via nipple injection. It is important to note that Met-1 cells are syngeneic to the FVB/N strain. At 6 weeks after injection, mammary tumors were harvested and subjected to a detailed analysis. Figure 4, A and B, shows the results of this analysis. Expression of Cav-1 (WT) leads to a dramatic reduction in tumor growth, as expected based on its known tumor suppressor properties. In contrast, Met-1 cells harboring Cav-1 (P132L) formed large tumors of approximately the same size as Met-1 cells transfected with the vector alone (pBABE).
Figure 4.
Cav-1 (P132L) behaves as a loss-of-function mutation in the context of primary tumor formation. Met-1 cell lines were injected through the inguinal (no. 4) nipple of 2-month-old female FVB/N mice (n = 6 mice per cell line) and allowed to form tumors. A: At 6 weeks after injection, mammary tumors were harvested and subjected to a detailed analysis. Note that Met-1 cells expressing Cav-1 (WT) formed very small tumors with a near 10-fold reduction in tumor weight, as compared with cells transfected with vector alone (pBABE). In contrast, cells expressing Cav-1 (P132L) formed slightly larger tumors than the pBABE vector-alone control, but this did not reach statistical significance. However, Cav-1 (P132L) tumors were significantly larger (∼14-fold) that Cav-1 (WT) tumors, indicating a clear loss-of-function. Bars indicate the SE. *P < 0.05, pBABE versus Cav-1 (WT) and **P < 0.01, Cav-1 (WT) versus Cav-1 (P132L); Student’s t-test. B: Representative gross images of the mammary tumors are shown.
We also characterized these tumors by immunohistochemistry with known markers normally associated with increased cell proliferation, such as Ki-67 and cyclin D1. Expression of Cav-1 (WT) dramatically reduced the intensity of both Ki-67 and cyclin D1 immunostaining, whereas Cav-1 (P132L) had little or no effect (Figure 5, A–C). Quantitation of Ki-67-positive nuclei also directly supported these qualitative observations (Figure 5A). However, no differences were observed in tumor apoptotic rates, as visualized by TUNEL staining (see Supplemental Figure S1 at http://ajp.amjpathol.org). Thus, we conclude that in the context of primary tumor formation that Cav-1 (P132L) behaves as a loss-of-function mutation.
Figure 5.
Cav-1 (P132L) does not suppress the expression of proliferative markers, such as Ki-67 and cyclin D1. Mammary tumors described in Figure 3 were subjected to immunohistochemical analysis with two pro-proliferative markers, Ki-67 and cyclin D1. A and B: For Ki-67, the number of positive tumor cells per field was quantitated (A). Note that expression of Cav-1 (WT) significantly represses Ki-67 expression (*P < 0.05, Student’s t-test). However, P132L and the vector alone control (pBABE) do not show a significant difference. Nevertheless, Cav-1 (P132L) tumors showed significantly more Ki-67 staining, as compared with Cav-1 (WT) tumors, indicating a loss-of-function (**P < 0.01, Student’s t-test). These findings directly support our conclusions from the study of tumor weights. Representative images are shown in B. C: Virtually identical results were obtained with cyclin D1 immunostaining. Representative images are shown.
Cav-1 (P132L) Increases Mammary Cell Migration, Invasion, and Experimental Metastasis
Experimental Metastasis (Lung Colonization)
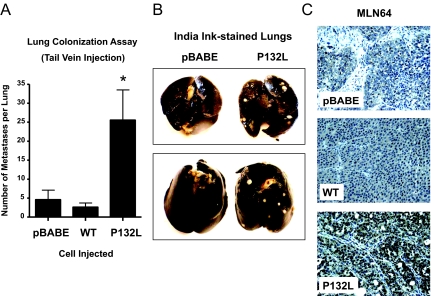
To study the potential role of the Cav-1 (P132L) mutation in the pathogenesis of metastatic disease, we next used an in vivo experimental metastasis approach via tail vein injection of FVB/N mice. This assay reflects the ability of cancer cells to colonize the lung because of their invasive capacity. At 4 weeks after injection, the lungs were harvested and insufflated with India Ink to allow visualization of the colonies on the surface of the lung, which negatively stain white. Remarkably, expression of Cav-1 (P132L) dramatically accentuated the potential metastatic capacity of Met-1 cells by approximately fivefold, as compared with vector-alone control cells. Quantitation is shown in Figure 6A and representative examples are shown in Figure 6B. Consistent with these findings, expression of Cav-1 (P132L) in Met-1 primary tumors led to the up-regulation of MLN64 (metastatic lymph node 64), a known marker of poor clinical outcome (usually attributable to metastasis) (Figure 6C).29,30 Similar results were also observed by Western blot analysis.
Figure 6.
Cav-1 (P132L) increases mammary cell metastasis. FVB/N female mice (n = 6 mice per cell line) were injected with Met-1 cells via the tail vein. A: At 4 weeks after injection, mice were sacrificed and the lungs were insufflated with India Ink. Then, the lungs were removed, washed, and bleached in Fekete’s solution before counting colonies. Note that expression of Cav-1 (P132L) significantly increases experimental metastasis ∼5-fold compared with vector alone (pBABE) and ∼10-fold compared with Cav-1 (WT). *P < 0.05, Mann-Whitney test; Cav-1 (P132L) versus Cav-1 (WT) or pBABE. Bars indicate the SE. B: Representative gross images of India Ink-stained lungs are shown. Lung colonies of Met-1 cells negatively stain white. C: Note that Cav-1 (P132L) expression in Met-1 primary tumors up-regulates MLN64 (metastatic lymph node 64), a known marker of poor clinical outcome. The observed nuclear staining actually represents perinuclear staining at higher magnification.
Cell Migration and Invasion
We speculated that these increases in metastatic capacity might reflect increased cellular motility or cell invasiveness. Thus, we subjected these Met-1 cell lines to modified Boyden chamber (Transwell) assays to test this hypothesis directly. Figure 7 shows that, as predicted, Met-1 cells harboring the Cav-1 (P132L) mutation have an increased capacity to invade a monolayer of Matrigel (Figure 7A). Virtually identical results were obtained in migration assays, suggesting that the mutation also increases cell motility (Figure 7B). As compared with vector-alone control cells, this represents an approximately threefold increase in cell invasiveness, and a near fourfold increase in cell migration. The behavior of Met-1 cells transfected with Cav-1 (WT) is also shown for comparison. Thus, in the context of cell migration, invasion, and experimental metastasis, Cav-1 (P132L) behaves as a gain-of-function mutation.
Figure 7.
Cav-1 (P132L) increases mammary cell invasiveness and motility. Modified Boyden chambers (Transwells) were used to quantitatively measure Met-1 cell invasion (A) and migration (B). A: Cell invasion. Transwells (8-μm pore size) coated with Matrigel were used to measure cell invasiveness in response to a chemoattractant (serum). Invasive cells migrated through the thin layer of Matrigel matrix throughout an 18-hour period. Then, the membrane was processed for crystal violet staining and migrating cells were counted. Note that Cav-1 (P132L)-expressing cells showed an approximately threefold increase in invasion, as compared with vector-alone control cells (pBABE), and an approximately sixfold increase as compared with Cav-1 (WT). *P < 0.05, pBABE versus Cav-1 (WT) or Cav-1 (P132L); Student’s t-test. B: Cell migration. Transwells (8-μm pore size, noncoated) were used to measure cell migration in response to a chemoattractant (serum). Met-1 cells migrated through the 8-μm filter throughout a 6-hour period. Interestingly, the motility of Met-1 cells expressing Cav-1 (P132L) was increased by ∼3.5-fold compared with cells transfected with pBABE alone. Similarly, migration of Cav-1 (P132L) cells was increased by fivefold compared with cells expressing Cav-1 (WT). *P < 0.05, pBABE versus Cav-1 (P132L); Student’s t-test.
Mechanistic Insights from Transcriptome Analysis: Cav-1 (P132L) Activates Multiple Signaling Pathways Associated with Metastasis
To understand the mechanism(s) underlying the ability of the Cav-1 (P132L) mutation to confer an increased capacity toward migration, invasion, and metastasis, we subjected these cultured Met-1 cell lines to gene expression profiling (DNA microarray analysis). Cav-1 (WT) and Cav-1 (P132L) cells were first individually compared pair-wise with vector-alone control cells (pBABE). Then, the gene sets altered by expression of Cav-1 (WT) and Cav-1 (P132L) were intersected. Remarkably, there was little overlap in the gene profiles that were affected by WT and the P132L mutant (See Supplemental Table S1 at http://ajp.amjpathol.org).
In accordance with its behavior as a gain-of-function mutation, Cav-1 (P132L) uniquely up-regulated 62 genes, as compared with Cav-1 (WT), which uniquely up-regulated 12 genes. Only 11 genes were commonly up-regulated by both Cav-1 WT and P132L. Interestingly, the 11 genes that were commonly up-regulated are mainly genes that are known to be expressed in epithelial cell types. Thus, Cav-1 (P132L) generates a unique gene expression signature. Venn diagrams summarizing these results are shown in Figure 8A. Supplemental Tables S2 and S3 (available at http://ajp.amjpathol.org) list all of the genes that were up-regulated and down-regulated, respectively. All of the genes that were changed by ≥1.5-fold and achieved statistical significance (P < 0.05) are listed.
Figure 8.
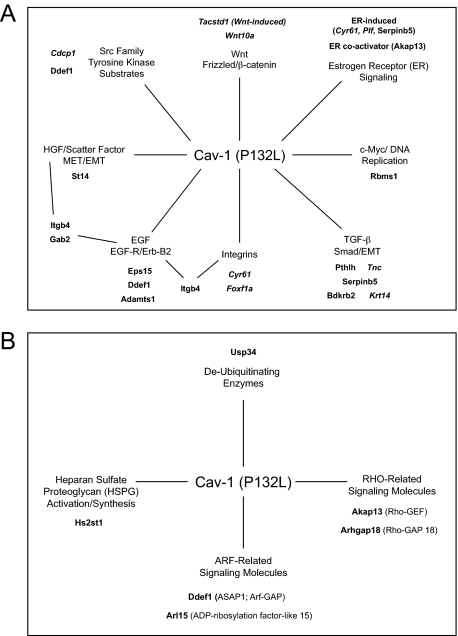
Gene expression profiling of cells expressing Cav-1 (WT and P132L). We subjected Met-1 cell lines to gene expression profiling (DNA microarray analysis). Cav-1 (WT) and Cav-1 (P132L) cells were first individually compared pair-wise with vector-alone control cells (pBABE). Then, the gene sets altered by expression of Cav-1 (WT) and Cav-1 (P132L) were intersected. Remarkably, there was little overlap in the gene profiles that were affected by WT and the P132L mutant. A: Venn diagrams. In accordance with its behavior as a gain-of-function mutation, Cav-1 (P132L) uniquely up-regulated 62 genes, as compared with Cav-1 (WT), which uniquely up-regulated 12 genes. Only 11 genes were commonly up-regulated by both Cav-1 WT and P132L. Thus, Cav-1 (P132L) generates a unique gene expression signature. Supplemental Tables S2 and S3 (available at http://ajp.amjpathol.org) list all of the genes that were up-regulated and down-regulated, respectively. All of the genes were changed by ≥1.5-fold and achieved statistical significance (P < 0.05). B: Signaling pathways affected. Expression of Cav-1 (WT) led to the up-regulation of four genes (Plagl1, Peg3, Ngfr, Ndrg4) that are known to be pro-apoptotic or associated with tumor suppression. Sulf2 (sulfatase 2) was up-regulated, which leads to degradation of the sulfate moieties attached to HSPGs (heparan sulfate proteglycans), and could inactivate HSPG-dependent signaling events. In contrast, Cav-1 (P132L) led to the up-regulation of genes normally associated with the activation of multiple growth factor signaling pathways These include HGF-, TGF-β-, EGF, Wnt-, integrin-, and ER-α-based signaling pathways. Also, the levels of Hs2st1 (heparan sulfate 2-O-sulfotransferase 1) are elevated leading to increased synthesis of HSPGs. HSPGs function as co-receptors and positively regulate HGF/MET, EGF-R/Erb-B2, integrin, Wnt, and TGF-β signaling.
Interestingly, expression of Cav-1 (WT) led to the up-regulation of four genes (Plagl1, Peg3, Ngfr, Ndrg4) that are known to be pro-apoptotic or associated with tumor suppression,31,32,33,34 providing mechanistic insights into Cav-1’s tumor suppressor function (Figure 8B; and see Supplemental Figure S2 available at http://ajp.amjpathol.org). Similarly, Sulf2 (sulfatase 2) was up-regulated, which leads to degradation of the sulfate moieties attached to HSPGs (heparan sulfate proteoglycans), and could inactivate HSPG-dependent signaling events. HSPGs are known to function as co-receptors for multiple growth factor signaling pathways. For example, HSPGs, such as CD44 and syndecan-1, bind HGF and promote HGF/MET signaling.
Conversely, expression of Cav-1 (P132L) led to the up-regulation of genes normally associated with the activation of multiple growth factor signaling pathways (Figure 8B; 9, A and B). These include HGF-, TGF-β-, EGF-, Wnt-, integrin-, and ER-α-based signaling pathways. Also, the levels of Hs2st1 (heparan sulfate 2-O-sulfotransferase 1) are elevated leading to increased synthesis of HSPGs. These HSPGs function as co-receptors and positively regulate HGF/MET, EGF-R/Erb-B2, integrin, Wnt, and TGF-β signaling, by affecting ligand recruitment and receptor binding.
Figure 9.
Summary of the major signaling pathways predicted to be affected by Cav-1 (P132L). A and B: Key genes induced by Cav-1 (P132L). Genes up-regulated by the Cav-1 (P132L) mutation can be divided into three major categories: i) secreted growth factors and extracellular matrix proteins (Cyr61, Plf, Pthlh, Serpinb5, Tnc, Wnt10a); ii) proteases that generate EGF and HGF (Adamts1, St14); as well as iii) tyrosine kinase (EGFR, ErbB2, MET, Src) substrates and integrin signaling/adapter proteins (Akap13, Cdcp1, Ddef1, Eps15, Foxf1a, Gab2, Hs2st1, Itgb4). Several of the P132L-specific genes are also highly expressed in stem/progenitor cells (Cdcp1, Cyr61, Foxf1a, Krt14, Plf, Tacstd1, Tnc, Wnt10a) and are shown in italics. Some of their functions are summarized in Supplemental Table S4 (available at http://ajp.amjpathol.org).
Interestingly, one of the Cav-1 (P132L)-induced genes encodes a deubiquitinating enzyme (ubiquitin-specific peptidase 34; Usp34) the up-regulation of which could mechanistically explain why the misfolded Cav-1 (P132L) mutant protein is not degraded. Importantly, we validated the up-regulation of six of these gene products (Cyr61, Foxf1a, Krt14, Wnt10a, Gab2, and Usp34) by Western blot analysis (Figure 10).
Figure 10.
Validation of Cav-1 (P132L)-induced gene products by Western blot analysis. Extracts from Met-1 cell lines were immunoblotted with antibodies to Cyr61, Foxf1a, Krt14, Wnt10a, Gab2, and Usp34. Note that their expression levels are increased in the cells expressing Cav-1 (P132L), as compared with vector alone (pBABE) and Cav-1 (WT). The expression of MLN64 is shown for comparison. β-Actin is shown as a control for equal loading.
The genes up-regulated by the Cav-1 (P132L) mutation can be divided into three major categories: 1) secreted growth factors and extracellular matrix proteins (Cyr61, Plf, Pthlh, Serpinb5, Tnc, Wnt10a), 2) proteases that generate EGF and HGF (Adamts1, St14), as well as 3) tyrosine kinase (EGFR, ErbB2, MET, Src) substrates and integrin signaling/adapter proteins (Akap13, Cdcp1, Ddef1, Eps15, Foxf1a, Gab2, Hs2st1, Itgb4). See Table 1 for details.
Table 1.
Genes Selectively Up-Regulated by Cav-1 (P132L) that Are Associated with Stem Cells, Invasiveness, and Metastasis
| Symbol | Name | Fold increase |
|---|---|---|
| 1) Secreted growth factors and extracellular matrix proteins | ||
| Cyr61 | Cysteine-rich protein 61 | 1.5 |
| Plf | Proliferin | 1.5 |
| Pthlh | Parathyroid hormone-like peptide | 1.7 |
| Serpinb5 | Serine (or cysteine) peptidase inhibitor, clade B, member 5 | 1.9 |
| Tnc | Tenascin C | 1.7 |
| Wnt10a | Wingless related MMTV integration site 10a | 1.9 |
| 2) Proteases that generate EGF and HGF | ||
| Adamts1 | A disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 1 | 1.6 |
| St14 | Suppression of tumorigenicity 14 (colon carcinoma) | 1.6 |
| 3) Tyrosine kinase (EGFR, Erb-B2, MET/HGF, Src) and integrin signaling/adapter proteins | ||
| Akap13 | A kinase (PRKA) anchor protein 13 | 1.5 |
| Cdcp1 | CUB domain containing protein 1 | 1.5 |
| Ddef1 | Development and differentiation enhancing | 1.6 |
| Eps15 | Epidermal growth factor receptor pathway substrate 15 | 1.6 |
| Foxf1a | Forkhead box F1a | 2.2 |
| Gab2 | Growth factor receptor bound protein 2-associated protein 2 | 1.7 |
| Hs2st1 | Heparan sulfate 2-O-sulfotransferase 1 | 1.6 |
| Itgb4 | Integrin β 4 | 1.6 |
| 4) Other | ||
| Bdkrb2 | Bradykinin receptor, β 2 | 1.5 |
| Krt14 | Keratin 14 | 2.1 |
| Rbms1 | RNA binding motif, single-stranded interacting protein 1 | 1.7 |
| Tacstd1 | Tumor-associated calcium signal transducer 1 | 1.7 |
| Usp34 | Ubiquitin-specific peptidase 34 | 1.5 |
Several of the P132L-specific genes are also highly expressed in stem/progenitor cells (Cdcp1, Cyr61, Foxf1a, Krt14, Plf, Tacstd1, Tnc, Wnt10a) or are associated with mammary myoepithelial cells (Adamts1, Bdkrb2, Dsg2, Dst, Irf6, Itgb4, Krt14, Lhfp, Pthlh, Serpinb5, Tnc), suggestive of an epithelial-mesenchymal transition. Finally, some of these genes are also associated with luminal mammary epithelial cells (Cyr61, Eps15, Foxf1a, Tacstd1).
Most interestingly, many of these gene products have already been shown to be associated with increased cell migration, invasion, and metastasis. These functional associations are summarized in detail in Supplemental Table S4 (available at http://ajp.amjpathol.org). Thus, the novel gene profile that we describe here that is characteristic of the Cav-1 (P132L) mutation may be viewed as a new metastasis-associated gene signature.
To independently verify that many of these signaling pathways are indeed activated, we performed pathway analysis using ASSESS (analysis of sample set enrichment scores), a computer-based gene enrichment algorithm (Figure 11). Based on this type of analysis, ER-α-, Wnt-, TGF-β-, and Rho GTPase-dependent signaling pathways all appear to be activated, among others.
Figure 11.
Results from pathway analysis with ASSESS. We also performed pathway analysis using ASSESS (analysis of sample set enrichment scores). Note that ER-α-, Wnt-, TGF-β-, and Rho GTPase-dependent signaling pathways all appear to be activated by Cav-1 (P132L), among others.
Cav-1 (P132L): Involvement of EGF, HGF, and TGF-β Signaling Pathways
Multiple signaling pathways appear to be activated by the Cav-1 (P132L) mutant, and could account for the observed increases in cell migration. To test their functional involvement, we used an inhibitor-based approach, by using specific inhibitors for EGF-R/ErbB2 (GW-583340),35 the c-MET receptor tyrosine kinase (PHA-665752),36 and the TGF-β type I receptor (LY-364947).37 All of these kinase inhibitors were administered at a relatively low concentration (0.1 μmol/L) to avoid possible cytotoxicity.
Figure 12A shows the results of this inhibitor analysis. Interestingly, all three inhibitors significantly reduced the migration of Met-1 cells expressing Cav-1 (P132L). GW-583340, PHA-665752, and LY-364947 resulted in ∼6.5-fold, ∼2.5-fold, and ∼7.5-fold reductions in cell migration, respectively. Thus, all three of these signaling pathways may significantly contribute to the migratory phenotype induced by expression of Cav-1 (P132L), providing independent functional validation for our gene expression profiling studies.
Figure 12.
Cav-1 (P132L): functional involvement of EGF, HGF, and TGF-β signaling pathways. A: Cell migration. Multiple signaling pathways appear to be activated by the Cav-1 (P132L) mutant. To test this hypothesis, we used an inhibitor-based approach, by using relatively specific inhibitors for EGF-R/ErbB2 (GW-583340), the c-MET receptor tyrosine kinase (PHA-665752), and the TGF-β type I receptor (LY-364947). All of these kinase inhibitors were administered at a relatively low concentration (0.1 μmol/L). Interestingly, all three inhibitors significantly reduced the migration of Met-1 cells expressing Cav-1 (P132L). Thus, all three of these signaling pathways may significantly contribute to the migratory phenotype induced by expression of Cav-1 (P132L). *P < 0.001, no inhibitor versus EGF, HGF, or TGF-β inhibitor; Student’s t-test. B: Cell invasiveness. Similar studies were also conducted to investigate the effects of these inhibitors on P132L-induced cell invasiveness. In these experiments, the inhibitors were used at a concentration of 1 μmol/L because little or no effect was observed at 0.1 μmol/L. Note that GW-583340 had no effect, whereas treatment with PHA-665752 and LY-364947 resulted in approximately twofold and approximately fourfold reductions in cell invasiveness, respectively. *P < 0.05, no inhibitor versus HGF or TGF-β inhibitor; Student’s t-test. Note that inhibition of the TGF-β type I receptor with LY-364947 had the most significant effect on both P132L-induced cell migration and invasiveness (arrows).
Similar studies were also conducted to investigate the effects of these inhibitors on P132L-induced cell invasiveness. In these experiments, the inhibitors were used at a concentration of 1 μmol/L because little or no effect was observed at 0.1 μmol/L. Figure 12B shows that GW-583340 had no effect, whereas treatment with PHA-665752 and LY-364947 resulted in approximately twofold and approximately fourfold reductions in cell invasiveness, respectively. Thus, inhibition of the TGF-β type I receptor with LY-364947 had the most significant effect on P132L-induced cell invasiveness.
Discussion
Several different models have been presented to explain how the cells of a primary breast tumor become disseminated and colonize distant organ sites.38,39,40 One involves selection pressure in which metastatic cells acquire new properties, such as invasiveness, cell motility, and growth-factor independence, and may also involve an epithelial-mesenchymal transition, invoking signaling pathways related to EGF, HGF, and TGF-β signaling. Another view involves the tumor microenvironment,41,42 where stromal cells provide the necessary growth factors or cues, via paracrine interactions with tumor epithelial cells, thereby allowing the tumor cells to become invasive and motile.
Here, we present another possible mechanism involving the mutation of a single tumor suppressor gene. Remarkably, in different phases of oncogenesis, this mutation either behaves as a loss-of-function or a gain-of-function mutation (summarized in Figure 13). However, gene expression profiling of the isolated cells harboring the mutant gene reveals that this mutant has the capacity to induce a metastatic genetic program, long before the onset of metastasis. Thus, the Cav-1 (P132L) mutation may be thought of as a metastasis mutation, defining a novel class of tumor suppressor mutations that lead to a gain-of-function, behaving like an activated oncogene. Notably, the signaling pathways that are activated have been implicated both in stem cell signaling and the epithelial-mesenchymal transition, suggesting that these events may be somehow interrelated.39 In accordance with this hypothesis, the Cav-1 (P132L) mutant induces numerous genes that are highly expressed in stem/progenitor cells (Cdcp1, Cyr61, Foxf1a, Krt14, Plf, Tacstd1, Tnc, Wnt10a) or are associated with mammary myoepithelial cells (Adamts1, Bdkrb2, Dsg2, Dst, Irf6, Itgb4, Krt14, Lhfp, Pthlh, Serpinb5, Tnc)—indicative of an epithelial-mesenchymal transition.
Figure 13.
Cav-1 (P132L): Phenotypic and genotypic analysis. Summarizes the behavior of the Cav-1 (P132L) mutation in the context of primary tumor formation and metastasis, and relates these findings with gene expression profiling data.
Interestingly, based on unbiased genome-wide expression profiling, the Cav-1 (P132L) mutation activates numerous signaling pathways that have already been implicated in cell migration, invasion, and metastasis, including EGF, HGF, and TGF-β signaling. One interpretation of these findings in that the Cav-1 (P132L) mutation allows the resulting misfolded and mislocalized protein product to interact with new signaling protein partners that do not normally interact with Cav-1 (WT). Future studies will be necessary to identify these Cav-1 (P132L)-specific interacting partner proteins. In this regard, it is important to note that inhibition of the TGF-β type I receptor with LY-364947 had the most significant effect on P132L-induced cell migration and invasiveness. These findings also have potentially important implications for the diagnostic and therapeutic stratification of human breast cancer patients because it could help clinicians to identify a subset of patients that are more likely to undergo recurrence and/or metastasis, simply based on the genotyping of the primary tumor for Cav-1 mutations. Similarly, certain p53 mutations show either a loss-of-function, gain-of-function, or dominant-negative activity.43 Thus, there is a precedent for the occurrence of oncogenic or activating mutations in more established tumor suppressor genes, such as p53.44,45
Footnotes
Address reprint requests to Dr. Gloria Bonuccelli or Dr. Michael P. Lisanti, Department of Cancer Biology, Kimmel Cancer Center, Thomas Jefferson University, 233 South 10th St., Philadelphia, PA, 19107. E-mail: gloria.bonuccelli@jefferson.edu and michael.lisanti@kimmelcancercenter.org.
Supported by the National Institutes of Health (National Cancer Institute grants R01-CA-80250, R01-CA-098779, and R01-CA-120876 to M.P.L.; R01-CA-70896, R01-CA-75503, R01-CA-86072, and R01-CA-107382 to R.G.P.; and a cancer center core grant P30-CA-56036 to the Kimmel Cancer Center), the American Association for Cancer Research (to M.P.L.), the Department of Defense (Breast Cancer Research Program synergistic idea award to M.P.L.), the Susan G. Komen Breast Cancer Foundation (investigator-initiated grant to M.P.L., a postdoctoral fellowship to I.M., and a career catalyst award to P.G.F.), the Elsa U. Pardee Foundation (to F.S.), the W.W. Smith Charitable Trust (to F.S. and P.G.F.), the American Cancer Society (research scholar grant to F.S.), the Breast Cancer Alliance (young investigator award to F.S.), the American Heart Association (to F.C), “La Ligue Contre le Cancer comité du Haut-Rhin” (to F.A., M.-C.R., and C.T.), and the Pennsylvania Department of Health (to M.P.L.) (the Department specifically disclaims responsibility for any analyses, interpretations or conclusions).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University or Albert Einstein College of Medicine was involved in the peer review process or final disposition for this article.
References
- Hayashi K, Matsuda S, Machida K, Yamamoto T, Fukuda Y, Nimura Y, Hayakawa T, Hamaguchi M. Invasion activating caveolin-1 mutation in human scirrhous breast cancers. Cancer Res. 2001;61:2361–2364. [PubMed] [Google Scholar]
- Li T, Sotgia F, Vuolo MA, Li M, Yang WC, Pestell RG, Sparano JA, Lisanti MP. Caveolin-1 mutations in human breast cancer: functional association with estrogen receptor alpha-positive status. Am J Pathol. 2006;168:1998–2013. doi: 10.2353/ajpath.2006.051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Park DS, Razani B, Russell RG, Pestell RG, Lisanti MP. Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: caveolin-1 (P132L) behaves in a dominant-negative manner and caveolin-1 (−/−) null mice show mammary epithelial cell hyperplasia. Am J Pathol. 2002;161:1357–1369. doi: 10.1016/S0002-9440(10)64412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Lee H, Frank PG, Razani B, Nguyen AV, Parlow AF, Russell RG, Hulit J, Pestell RG, Lisanti MP. Caveolin-1-deficient mice show accelerated mammary gland development during pregnancy, premature lactation, and hyperactivation of the Jak-2/STAT5a signaling cascade. Mol Biol Cell. 2002;13:3416–3430. doi: 10.1091/mbc.02-05-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Cheung MW, Park DS, Razani B, Cohen AW, Muller WJ, Di Vizio D, Chopra NG, Pestell RG, Lisanti MP. Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol Biol Cell. 2003;14:1027–1042. doi: 10.1091/mbc.E02-08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Lee H, Cheung MW, Cohen AW, Razani B, Iyengar P, Scherer PE, Pestell RG, Lisanti MP. Combined loss of INK4a and caveolin-1 synergistically enhances cell proliferation and oncogene-induced tumorigenesis. J Biol Chem. 2004;279:24745–24756. doi: 10.1074/jbc.M402064200. [DOI] [PubMed] [Google Scholar]
- Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo: role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol. 2005;288:C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- Bouras T, Lisanti MP, Pestell RG. Caveolin-1 in breast cancer. Cancer Biol Ther. 2004;3:931–941. doi: 10.4161/cbt.3.10.1147. [DOI] [PubMed] [Google Scholar]
- Tang Z, Okamoto T, Boontrakulpoontawee P, Katada T, Otsuka AJ, Lisanti MP. Identification, sequence, and expression of an invertebrate caveolin gene family from the nematode Caenorhabditis elegans: implications for the molecular evolution of mammalian caveolin genes. J Biol Chem. 1997;272:2437–2445. doi: 10.1074/jbc.272.4.2437. [DOI] [PubMed] [Google Scholar]
- Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M, Masetti E, Mazzocco P, Egeo A, Donati MA, Volonte' D, Galbiati F, Cordone G, Bricarelli FD, Lisanti MP, Zara F. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet. 1998;18:365–368. doi: 10.1038/ng0498-365. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Minetti C, Bregman DB, Lisanti MP. Limb-girdle muscular dystrophy (LGMD-1C) mutants of caveolin-3 undergo ubiquitination and proteasomal degradation. Treatment with proteasomal inhibitors blocks the dominant negative effect of LGMD-1C mutanta and rescues wild-type caveolin-3. J Biol Chem. 2000;275:37702–37711. doi: 10.1074/jbc.M006657200. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 1998;17:6633–6648. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky AD, Namba R, Young LJ, Hunter KW, Hodgson JG, Tepper CG, McGoldrick ET, Muller WJ, Cardiff RD, Gregg JP. Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clin Exp Metastasis. 2005;22:47–59. doi: 10.1007/s10585-005-2908-5. [DOI] [PubMed] [Google Scholar]
- Namba R, Young LJ, Abbey CK, Kim L, Damonte P, Borowsky AD, Qi J, Tepper CG, MacLeod CL, Cardiff RD, Gregg JP. Rapamycin inhibits growth of premalignant and malignant mammary lesions in a mouse model of ductal carcinoma in situ. Clin Cancer Res. 2006;12:2613–2621. doi: 10.1158/1078-0432.CCR-05-2170. [DOI] [PubMed] [Google Scholar]
- Alpy F, Boulay A, Moog-Lutz C, Andarawewa KL, Degot S, Stoll I, Rio MC, Tomasetto C. Metastatic lymph node 64 (MLN64), a gene overexpressed in breast cancers, is regulated by Sp/KLF transcription factors. Oncogene. 2003;22:3770–3780. doi: 10.1038/sj.onc.1206500. [DOI] [PubMed] [Google Scholar]
- Alpy F, Latchumanan VK, Kedinger V, Janoshazi A, Thiele C, Wendling C, Rio MC, Tomasetto C. Functional characterization of the MENTAL domain. J Biol Chem. 2005;280:17945–17952. doi: 10.1074/jbc.M500723200. [DOI] [PubMed] [Google Scholar]
- Alpy F, Stoeckel ME, Dierich A, Escola JM, Wendling C, Chenard MP, Vanier MT, Gruenberg J, Tomasetto C, Rio MC. The steroidogenic acute regulatory protein homolog MLN64, a late endosomal cholesterol-binding protein. J Biol Chem. 2001;276:4261–4269. doi: 10.1074/jbc.M006279200. [DOI] [PubMed] [Google Scholar]
- Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- Alpy F, Wendling C, Rio MC, Tomasetto C. MENTHO, a MLN64 homologue devoid of the START domain. J Biol Chem. 2002;277:50780–50787. doi: 10.1074/jbc.M208290200. [DOI] [PubMed] [Google Scholar]
- Degot S, Le Hir H, Alpy F, Kedinger V, Stoll I, Wendling C, Seraphin B, Rio MC, Tomasetto C. Association of the breast cancer protein MLN51 with the exon junction complex via its speckle localizer and RNA binding module. J Biol Chem. 2004;279:33702–33715. doi: 10.1074/jbc.M402754200. [DOI] [PubMed] [Google Scholar]
- Kedinger V, Alpy F, Tomasetto C, Thisse C, Thisse B, Rio MC. Spatial and temporal distribution of the traf4 genes during zebrafish development. Gene Expr Patterns. 2005;5:545–552. doi: 10.1016/j.modgep.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Lederkremer GZ, Williams S, Fogliano M, Baldini G, Lodish HF. Cab45, a novel (Ca2+)-binding protein localized to the Golgi lumen. J Cell Biol. 1996;133:257–268. doi: 10.1083/jcb.133.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Sotgia F, Lee H, Hassan G, Di Vizio D, Bonuccelli G, Capozza F, Mercier I, Rui H, Pestell RG, Lisanti MP. Stromal and epithelial caveolin-1 both confer a protective effect against mammary hyperplasia and tumorigenesis: caveolin-1 antagonizes cyclin d1 function in mammary epithelial cells. Am J Pathol. 2006;169:1784–1801. doi: 10.2353/ajpath.2006.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- Hulit J, Suyama K, Chung S, Keren R, Agiostratidou G, Shan W, Dong X, Williams TM, Lisanti MP, Knudsen K, Hazan RB. N-cadherin signaling potentiates mammary tumor metastasis via enhanced extracellular signal-regulated kinase activation. Cancer Res. 2007;67:3106–3116. doi: 10.1158/0008-5472.CAN-06-3401. [DOI] [PubMed] [Google Scholar]
- Grigoriadis A, Mackay A, Reis-Filho JS, Steele D, Iseli C, Stevenson BJ, Jongeneel CV, Valgeirsson H, Fenwick K, Iravani M, Leao M, Simpson AJ, Strausberg RL, Jat PS, Ashworth A, Neville AM, O'Hare MJ. Establishment of the epithelial-specific transcriptome of normal and malignant human breast cells based on MPSS and array expression data. Breast Cancer Res. 2006;8:R56. doi: 10.1186/bcr1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E, Porrello A, Guinney J, Balakumaran B, Bild A, Febbo PG, Mukherjee S. Analysis of sample set enrichment scores: assaying the enrichment of sets of genes for individual samples in genome-wide expression profiles. Bioinformatics. 2006;22:e108–e116. doi: 10.1093/bioinformatics/btl231. [DOI] [PubMed] [Google Scholar]
- Moog-Lutz C, Tomasetto C, Regnier CH, Wendling C, Lutz Y, Muller D, Chenard MP, Basset P, Rio MC. MLN64 exhibits homology with the steroidogenic acute regulatory protein (STAR) and is over-expressed in human breast carcinomas. Int J Cancer. 1997;71:183–191. doi: 10.1002/(sici)1097-0215(19970410)71:2<183::aid-ijc10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Tomasetto C, Regnier C, Moog-Lutz C, Mattei MG, Chenard MP, Lidereau R, Basset P, Rio MC. Identification of four novel human genes amplified and overexpressed in breast carcinoma and localized to the q11-q21.3 region of chromosome 17. Genomics. 1995;28:367–376. doi: 10.1006/geno.1995.1163. [DOI] [PubMed] [Google Scholar]
- Abdollahi A, Pisarcik D, Roberts D, Weinstein J, Cairns P, Hamilton TC. LOT1 (PLAGL1/ZAC1), the candidate tumor suppressor gene at chromosome 6q24-25, is epigenetically regulated in cancer. J Biol Chem. 2003;278:6041–6049. doi: 10.1074/jbc.M210361200. [DOI] [PubMed] [Google Scholar]
- Deng Y, Wu X. Peg3/Pw1 promotes p53-mediated apoptosis by inducing Bax translocation from cytosol to mitochondria. Proc Natl Acad Sci USA. 2000;97:12050–12055. doi: 10.1073/pnas.97.22.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis-Filho JS, Steele D, Di Palma S, Jones RL, Savage K, James M, Milanezi F, Schmitt FC, Ashworth A. Distribution and significance of nerve growth factor receptor (NGFR/p75NTR) in normal, benign and malignant breast tissue. Mod Pathol. 2006;19:307–319. doi: 10.1038/modpathol.3800542. [DOI] [PubMed] [Google Scholar]
- Kovacevic Z, Richardson DR. The metastasis suppressor, Ndrg-1: a new ally in the fight against cancer. Carcinogenesis. 2006;27:2355–2366. doi: 10.1093/carcin/bgl146. [DOI] [PubMed] [Google Scholar]
- Gaul MD, Guo Y, Affleck K, Cockerill GS, Gilmer TM, Griffin RJ, Guntrip S, Keith BR, Knight WB, Mullin RJ, Murray DM, Rusnak DW, Smith K, Tadepalli S, Wood ER, Lackey K. Discovery and biological evaluation of potent dual ErbB-2/EGFR tyrosine kinase inhibitors: 6-thiazolylquinazolines. Bioorg Med Chem Lett. 2003;13:637–640. doi: 10.1016/s0960-894x(02)01047-8. [DOI] [PubMed] [Google Scholar]
- Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R, Lipson KE, Ramphal J, Do S, Cui JJ, Cherrington JM, Mendel DB. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- Sawyer JS, Anderson BD, Beight DW, Campbell RM, Jones ML, Herron DK, Lampe JW, McCowan JR, McMillen WT, Mort N, Parsons S, Smith EC, Vieth M, Weir LC, Yan L, Zhang F, Yingling JM. Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. J Med Chem. 2003;46:3953–3956. doi: 10.1021/jm0205705. [DOI] [PubMed] [Google Scholar]
- Gentile A, Trusolino L, Comoglio PM. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev. 2008;27:85–94. doi: 10.1007/s10555-007-9107-6. [DOI] [PubMed] [Google Scholar]
- Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- Mazzone M, Comoglio PM. The Met pathway: master switch and drug target in cancer progression. FASEB J. 2006;20:1611–1621. doi: 10.1096/fj.06-5947rev. [DOI] [PubMed] [Google Scholar]
- Radisky ES, Radisky DC. Stromal induction of breast cancer: inflammation and invasion. Rev Endocr Metab Disord. 2007;8:279–287. doi: 10.1007/s11154-007-9037-1. [DOI] [PubMed] [Google Scholar]
- Sleeman JP, Cremers N. New concepts in breast cancer metastasis: tumor initiating cells and the microenvironment. Clin Exp Metastasis. 2007;24:707–715. doi: 10.1007/s10585-007-9122-6. [DOI] [PubMed] [Google Scholar]
- Strano S, Dell'Orso S, Di Agostino S, Fontemaggi G, Sacchi A, Blandino G. Mutant p53: an oncogenic transcription factor. Oncogene. 2007;26:2212–2219. doi: 10.1038/sj.onc.1210296. [DOI] [PubMed] [Google Scholar]
- Li Y, Prives C. Are interactions with p63 and p73 involved in mutant p53 gain of oncogenic function? Oncogene. 2007;26:2220–2225. doi: 10.1038/sj.onc.1210311. [DOI] [PubMed] [Google Scholar]
- Lozano G. The oncogenic roles of p53 mutants in mouse models. Curr Opin Genet Dev. 2007;17:66–70. doi: 10.1016/j.gde.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Menéndez JA, Mehmi I, Griggs DW, Lupu R. The angiogenic factor CYR61 in breast cancer: molecular pathology and therapeutic perspectives. Endocr Relat Cancer. 2003;10:141–152. doi: 10.1677/erc.0.0100141. [DOI] [PubMed] [Google Scholar]
- Chen Y, Du XY. Functional properties and intracellular signaling of CCN1/Cyr61. J Cell Biochem. 2007;100:1337–1345. doi: 10.1002/jcb.21194. [DOI] [PubMed] [Google Scholar]
- Schütze N, Schenk R, Fiedler J, Mattes T, Jakob F, Brenner RE. CYR61/CCN1 and WISP3/CCN6 are chemoattractive ligands for human multipotent mesenchymal stroma cells. BMC Cell Biol. 2007;8:45. doi: 10.1186/1471-2121-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote K, Salguero G, Ballmaier M, Dangers M, Drexler H, Schieffer B. The angiogenic factor CCN1 promotes adhesion and migration of circulating CD34+ progenitor cells: potential role in angiogenesis and endothelial regeneration. Blood. 2007;110:877–885. doi: 10.1182/blood-2006-07-036202. [DOI] [PubMed] [Google Scholar]
- Corbacho AM, Martinez De La Escalera G, Clapp C. Roles of prolactin and related members of the prolactin/growth hormone/placental lactogen family in angiogenesis. J Endocrinol. 2002;173:219–238. doi: 10.1677/joe.0.1730219. [DOI] [PubMed] [Google Scholar]
- Wang XY, Yin Y, Yuan H, Sakamaki T, Okano H, Glazer RI. Musashi1 modulates mammary progenitor cell expansion through proliferin-mediated activation of the Wnt and Notch pathways. Mol Cell Biol. 2008;28:3589–3599. doi: 10.1128/MCB.00040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise TA. Parathyroid hormone-related protein and bone metastases. Cancer. 1997;80:1572–1580. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1572::aid-cncr7>3.3.co;2-d. [DOI] [PubMed] [Google Scholar]
- Smid M, Wang Y, Klijn JG, Sieuwerts AM, Zhang Y, Atkins D, Martens JW, Foekens JA. Genes associated with breast cancer metastatic to bone. J Clin Oncol. 2006;24:2261–2267. doi: 10.1200/JCO.2005.03.8802. [DOI] [PubMed] [Google Scholar]
- Jahkola T, Toivonen T, Nordling S, von Smitten K, Virtanen I. Expression of tenascin-C in intraductal carcinoma of human breast: relationship to invasion. Eur J Cancer. 1998;34:1687–1692. doi: 10.1016/s0959-8049(98)00215-9. [DOI] [PubMed] [Google Scholar]
- Jahkola T, Toivonen T, Virtanen I, von Smitten K, Nordling S, von Boguslawski K, Haglund C, Nevanlinna H, Blomqvist C. Tenascin-C expression in invasion border of early breast cancer: a predictor of local and distant recurrence. Br J Cancer. 1998;78:1507–1513. doi: 10.1038/bjc.1998.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahkola T, Toivonen T, von Smitten K, Blomqvist C, Virtanen I. Expression of tenascin in invasion border of early breast cancer correlates with higher risk of distant metastasis. Int J Cancer. 1996;69:445–447. doi: 10.1002/(SICI)1097-0215(19961220)69:6<445::AID-IJC4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- von Holst A, Egbers U, Prochiantz A, Faissner A. Neural stem/progenitor cells express 20 tenascin C isoforms that are differentially regulated by Pax6. J Biol Chem. 2007;282:9172–9181. doi: 10.1074/jbc.M608067200. [DOI] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–4829. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- Veltmaat JM, Van Veelen W, Thiery JP, Bellusci S. Identification of the mammary line in mouse by Wnt10b expression. Dev Dyn. 2004;229:349–356. doi: 10.1002/dvdy.10441. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Xu Y, Yu Q. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene. 2006;25:2452–2467. doi: 10.1038/sj.onc.1209287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY, Dolled-Filhart M, Ocal IT, Singh B, Lin CY, Dickson RB, Rimm DL, Camp RL. Tissue microarray analysis of hepatocyte growth factor/Met pathway components reveals a role for Met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node-negative breast cancer. Cancer Res. 2003;63:1101–1105. [PubMed] [Google Scholar]
- Rubino D, Driggers P, Arbit D, Kemp L, Miller B, Coso O, Pagliai K, Gray K, Gutkind S, Segars J. Characterization of Brx, a novel Dbl family member that modulates estrogen receptor action. Oncogene. 1998;16:2513–2526. doi: 10.1038/sj.onc.1201783. [DOI] [PubMed] [Google Scholar]
- Wirtenberger M, Tchatchou S, Hemminki K, Klaes R, Schmutzler RK, Bermejo JL, Chen B, Wappenschmidt B, Meindl A, Bartram CR, Burwinkel B. Association of genetic variants in the Rho guanine nucleotide exchange factor AKAP13 with familial breast cancer. Carcinogenesis. 2006;27:593–598. doi: 10.1093/carcin/bgi245. [DOI] [PubMed] [Google Scholar]
- Bühring HJ, Kuci S, Conze T, Rathke G, Bartolovic K, Grunebach F, Scherl-Mostageer M, Brummendorf TH, Schweifer N, Lammers R. CDCP1 identifies a broad spectrum of normal and malignant stem/progenitor cell subsets of hematopoietic and nonhematopoietic origin. Stem Cells. 2004;22:334–343. doi: 10.1634/stemcells.22-3-334. [DOI] [PubMed] [Google Scholar]
- Uekita T, Jia L, Narisawa-Saito M, Yokota J, Kiyono T, Sakai R. CUB domain-containing protein 1 is a novel regulator of anoikis resistance in lung adenocarcinoma. Mol Cell Biol. 2007;27:7649–7660. doi: 10.1128/MCB.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper JD, Zijlstra A, Aimes RT, Liang H, Claassen GF, Tarin D, Testa JE, Quigley JP. Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen. Oncogene. 2003;22:1783–1794. doi: 10.1038/sj.onc.1206220. [DOI] [PubMed] [Google Scholar]
- Bharti S, Inoue H, Bharti K, Hirsch DS, Nie Z, Yoon HY, Artym V, Yamada KM, Mueller SC, Barr VA, Randazzo PA. Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol Cell Biol. 2007;27:8271–8283. doi: 10.1128/MCB.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazioli F, Minichiello L, Matoskova B, Wong WT, Di Fiore PP. eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol Cell Biol. 1993;13:5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichenko VV, Gusarova GA, Kim IM, Shin B, Yoder HM, Clark J, Sapozhnikov AM, Whitsett JA, Costa RH. Foxf1 haploinsufficiency reduces Notch-2 signaling during mouse lung development. Am J Physiol. 2004;286:L521–L530. doi: 10.1152/ajplung.00212.2003. [DOI] [PubMed] [Google Scholar]
- Malin D, Kim IM, Boetticher E, Kalin TV, Ramakrishna S, Meliton L, Ustiyan V, Zhu X, Kalinichenko VV. Forkhead box F1 is essential for migration of mesenchymal cells and directly induces integrin-beta3 expression. Mol Cell Biol. 2007;27:2486–2498. doi: 10.1128/MCB.01736-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Wu D, Princen F, Nguyen T, Pang Y, Lesperance J, Muller WJ, Oshima RG, Feng GS. Role of Gab2 in mammary tumorigenesis and metastasis. Oncogene. 2007;26:4951–4960. doi: 10.1038/sj.onc.1210315. [DOI] [PubMed] [Google Scholar]
- Bentires-Alj M, Gil SG, Chan R, Wang ZC, Wang Y, Imanaka N, Harris LN, Richardson A, Neel BG, Gu H. A role for the scaffolding adapter GAB2 in breast cancer. Nat Med. 2006;12:114–121. doi: 10.1038/nm1341. [DOI] [PubMed] [Google Scholar]
- van der Voort R, Taher TE, Wielenga VJ, Spaargaren M, Prevo R, Smit L, David G, Hartmann G, Gherardi E, Pals ST. Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J Biol Chem. 1999;274:6499–6506. doi: 10.1074/jbc.274.10.6499. [DOI] [PubMed] [Google Scholar]
- Derksen PW, Keehnen RM, Evers LM, van Oers MH, Spaargaren M, Pals ST. Cell surface proteoglycan syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma. Blood. 2002;99:1405–1410. doi: 10.1182/blood.v99.4.1405. [DOI] [PubMed] [Google Scholar]
- Abdel-Ghany M, Cheng HC, Elble RC, Pauli BU. The breast cancer beta 4 integrin and endothelial human CLCA2 mediate lung metastasis. J Biol Chem. 2001;276:25438–25446. doi: 10.1074/jbc.M100478200. [DOI] [PubMed] [Google Scholar]
- Carraway KL, III, Sweeney C. Co-opted integrin signaling in ErbB2-induced mammary tumor progression. Cancer Cell. 2006;10:93–95. doi: 10.1016/j.ccr.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell. 2001;107:643–654. doi: 10.1016/s0092-8674(01)00567-0. [DOI] [PubMed] [Google Scholar]
- Bertotti A, Comoglio PM, Trusolino L. Beta4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth. J Cell Biol. 2006;175:993–1003. doi: 10.1083/jcb.200605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco S, Elia MG, Muscella A, Romano S, Storelli C, Marsigliante S. Bradykinin stimulates cell proliferation through an extracellular-regulated kinase 1 and 2-dependent mechanism in breast cancer cells in primary culture. J Endocrinol. 2005;186:291–301. doi: 10.1677/joe.1.06052. [DOI] [PubMed] [Google Scholar]
- Greco S, Muscella A, Elia MG, Romano S, Storelli C, Marsigliante S. Mitogenic signalling by B2 bradykinin receptor in epithelial breast cells. J Cell Physiol. 2004;201:84–96. doi: 10.1002/jcp.20052. [DOI] [PubMed] [Google Scholar]
- Fulford LG, Reis-Filho JS, Ryder K, Jones C, Gillett CE, Hanby A, Easton D, Lakhani SR. Basal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survival. Breast Cancer Res. 2007;9:R4. doi: 10.1186/bcr1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedcke J, Traub F, Milde S, Wilkens L, Stan A, Ostertag H, Christgen M, von Wasielewski R, Kreipe HH. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol. 2007;20:864–870. doi: 10.1038/modpathol.3800830. [DOI] [PubMed] [Google Scholar]
- Niki T, Izumi S, Saegusa Y, Taira T, Takai T, Iguchi-Ariga SM, Ariga H. MSSP promotes ras/myc cooperative cell transforming activity by binding to c-Myc. Genes Cells. 2000;5:127–141. doi: 10.1046/j.1365-2443.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- Watson MA, Ylagan LR, Trinkaus KM, Gillanders WE, Naughton MJ, Weilbaecher KN, Fleming TP, Aft RL. Isolation and molecular profiling of bone marrow micrometastases identifies TWIST1 as a marker of early tumor relapse in breast cancer patients. Clin Cancer Res. 2007;13:5001–5009. doi: 10.1158/1078-0432.CCR-07-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choesmel V, Anract P, Hoifodt H, Thiery JP, Blin N. A relevant immunomagnetic assay to detect and characterize epithelial cell adhesion molecule-positive cells in bone marrow from patients with breast carcinoma: immunomagnetic purification of micrometastases. Cancer. 2004;101:693–703. doi: 10.1002/cncr.20391. [DOI] [PubMed] [Google Scholar]
- Spizzo G, Went P, Dirnhofer S, Obrist P, Simon R, Spichtin H, Maurer R, Metzger U, von Castelberg B, Bart R, Stopatschinskaya S, Kochli OR, Haas P, Mross F, Zuber M, Dietrich H, Bischoff S, Mirlacher M, Sauter G, Gastl G. High Ep-CAM expression is associated with poor prognosis in node-positive breast cancer. Breast Cancer Res Treat. 2004;86:207–213. doi: 10.1023/B:BREA.0000036787.59816.01. [DOI] [PubMed] [Google Scholar]