Abstract
Hypoxia-inducible transcription factors (HIFs) play important roles in the response of the kidney to systemic and regional hypoxia. Degradation of HIFs is mediated by three oxygen-dependent HIF-prolyl hydroxylases (PHDs), which have partially overlapping characteristics. Although PHD inhibitors, which can induce HIFs in the presence of oxygen, are already in clinical development, little is known about the expression and regulation of these enzymes in the kidney. Therefore, we investigated the expression levels of the three PHDs in both isolated tubular cells and rat kidneys. All three PHDs were present in the kidney and were expressed predominantly in three different cell populations: (a) in distal convoluted tubules and collecting ducts (PHD1,2,3), (b) in glomerular podocytes (PHD1,3), and (c) in interstitial fibroblasts (PHD1,3). Higher levels of PHDs were found in tubular segments of the inner medulla where oxygen tensions are known to be physiologically low. PHD expression levels were unchanged in HIF-positive tubular and interstitial cells after induction by systemic hypoxia. In rat models of acute renal injury, changes in PHD expression levels were variable; while cisplatin and ischemia/reperfusion led to significant decreases in PHD2 and 3 expression levels, no changes were seen in a model of contrast media-induced nephropathy. These results implicate the non-uniform expression of HIF-regulating enzymes that modify the hypoxic response in the kidney under both regional and temporal conditions.
In the kidney total blood flow is physiologically high, which is a prerequisite for adequate glomerular filtration and solute clearance. Nevertheless, due to shunt diffusion of oxygen and counter current mechanisms of pre- and postglomerular vessels, physiological oxygen tensions in the kidney are much lower than those in the renal vein.1,2 Various pathophysiological conditions, like a fall in systemic blood pressure or decreases in the systemic oxygen saturation, can thus easily lead to a critical undersupply with oxygen. Lack of oxygen is not only a state of reduced energy supply but induces complex cellular transcriptional changes, which are believed to adapt cells to reduced oxygen availability and to enable cellular survival. A key mediator of these processes is the transcription factor hypoxia-inducible factor (HIF). HIF is stabilized by low oxygen tensions and controls transcription of more than 100 genes, including vascular endothelial growth factor, platelet-derived growth factor, Glucose Transporter-1, erythropoietin (EPO), thereby stimulating angiogenesis, anaerobic glycolysis, erythropoiesis, and other adaptive processes.3,4,5,6,7
HIF consists of an oxygen regulated α- and a constitutive ß-subunit and belongs to the basic helix–loop–helix PAS (Per, Arnt, and Sim) domain proteins.5,6 Three α-chains have been identified and HIF-1α and -2α are regarded as the main functional proteins with overlapping but partly distinct transcriptional specificities. In the presence of oxygen, two prolyl- and one asparagyl-residue(s) of the continuously synthesized α-chains are hydroxylated by three HIF-prolyl hydroxylases (prolyl hydroxylase domain proteins, PHD1-3) and by the factor inhibiting HIF (FIH-1), respectively.8,9,10,11 Hydroxylation of the prolyl residues leads to ubiquitination of the HIF-α proteins by an E3 VHL ubiquitin ligase complex, which targets HIF for proteasomal degradation.12 Under hypoxia, when molecular oxygen is not available for hydroxylation, HIF-α can accumulate in the cell and promotes transcription of its target genes.13,14
The PHDs 1-3 are oxygen- and 2-oxoglutarate dependent enzymes that are expressed at different levels in all tissues.15,16,17,18 In cell-free biochemical assays, all three PHDs were able to hydroxylate the HIF-α subunits.19 Their activity is dependent on the abundance of O2, ferrous iron, ascorbate, and 2-oxoglutarate, and is modified in a dose-dependent manner by Krebs cycle intermediates.19,20 Using small interfering RNA techniques, only PHD2, but not PHD1 and 3 knock down has been demonstrated to induce HIF protein accumulation in vitro under normoxia.21,22 In genetic targeting experiments, knockout of PHD2 resulted in severe placental and heart defects and led to embryonic death. On the other hand, PHD1 and PHD3 knockout mice showed no apparent phenotype.23
Where the PHDs are expressed in the kidney and how they are regulated is not yet known in detail. Li et al used rat kidney extracts to show that PHD expression was more pronounced in medullary than in cortical regions.24 In a further immunohistological study, using new PHD and FIH antibodies, expression in tubular, but not in glomerular cells, was described.25
HIF-1α and -2α have been shown to be differentially expressed in the kidney following systemic hypoxia. Whereas HIF-1α is predominantly found in tubular cells and in particular in medullary collecting ducts, HIF-2α is expressed in glomerular cells, peritubular endothelial cells, and interstitial fibroblasts.26 Preconditional induction of HIF-1α and of HIF-2α by hypoxia has been shown to protect against different forms of renal injury.27,28,29,30 New HIF-prolyl hydroxylase inhibitors are under clinical development for treatment of renal anemia and are promising candidates to induce tissue protection.29,31,32
In view of PHDs evolving as putative therapeutic targets and the lack of knowledge about their cellular expression in the kidney, we investigated: (1) the cellular expression of the three PHDs in the rat kidney, (2) the hypoxic regulation of PHDs in isolated primary proximal and distal tubular cells, and (3) the PHD expression patterns under systemic hypoxia and in models of acute renal injury.
Materials and Methods
The M-1 mouse collecting duct cell line33 was obtained from American Type Culture Collection (2038-CRL, Rockville, MD). M-1 cells were maintained in PC-1 culture medium (Lonza Verviers Sprl, Verviers, Belgium). Other cell culture reagents were from Invitrogen (Karlsruhe, Germany) and Biochrom (Berlin, Germany). All other chemicals were from Sigma (Taufkirchen, Germany), unless indicated otherwise.
Cell Culture
M-1 cells were seeded onto Millicell-HA culture plate inserts (Millipore, Bedford, MA) and were allowed to grow to confluence for 9 to 11 days postseeding. Transepithelial voltage (Vte) and resistance (Rte) were routinely checked using a commercially available epithelial volt-ohm-meter to confirm that the cells had formed a tight epithelial monolayer and performed electrogenic transepithelial sodium transport characteristic for collecting duct principal cells.34,35 For analysis of PHD or HIF expression cells were exposed to normoxia or hypoxia (1% O2, 5% CO2) for 16 hours.
Isolation of Murine Proximal Tubular Cells
Primary murine proximal tubular cells were isolated from the kidney cortex of 4- to 5-week-old mice, essentially as described36 and cultured in serum-free Dulbecco’s Modified Eagle Medium/Ham’s F-12 supplemented with 2 mmol/L l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium. Cells were used for experiments 4 to 5 days after plating. For analysis of PHD or HIF expression, cells were exposed to normoxia or hypoxia (1% O2, 5% CO2) for 16 hours. Cells were routinely checked for NaPi-IIa expression as a marker for proximal tubular cells (see Supplemental Figure S1 at http://ajp.amjpathol.org).
Protein Extraction and Immunoblotting
Preparation of cell lysates and immunoblotting was performed as described previously.37 Proteins were transferred onto polyvinylidene difluoride membranes (Bio-Rad, Munich, Germany) and probed with PHD antibodies (PHD1 NB 100-310, PHD2 NB 100-2219, PHD3 NB 100-303, Novus Biologicals, Littleton, CO), HIF-1α antibody (Cayman Chemical, Ann Arbor, MI) or HIF-2α antibody (NB 100-122, Novus Biologicals). Equal loading was verified by staining the blot with a diazo dye (amido black).
RNA Preparation, Real-Time PCR, and RNase Protection Assay
Total RNA from cell culture experiments was extracted with RNAzol B (Biozol, Eching, Germany) according to the manufacture’s instruction and analyzed by reverse transcription (RT)- or real-time RT-PCR as indicated. 1 μg of total RNA was reverse transcribed with MULV-reverse transcriptase (Fermentas, St. Leon-Rot, Germany). Real-time PCR was performed using the SYBR-green method. Expression was measured on an ABI Prism cycler (Applied Biosystems, Foster City, CA) and data were analyzed using the ΔΔCT method. Hypoxanthine-guanine phosphoribosyltransferase (HPRT) served as internal control. Primers used for murine hypoxanthine-guanine phosphoribosyltransferase HPRT and PHD1-3: PHD1 forward 5′-CATCAATGGGCGCACCA-3′, PHD1 reverse 5′-GATTGTCAACATGCCT-3′; PHD2 forward 5′-TAAACGGCCGAACGAAAGC-3′, PHD2 reverse 5′-GGGTTATCAACGTGACGGACA-3′; PHD3 forward 5′-CTATGTCAAGGAGCGGTCCAA-3′, PHD3 reverse 5′-GTCCACATGGCGAACATAACC-3′; HPRT forward 5′-GTTGGATACAGGCCAGACTTTGT-3′, HPRT reverse 5′-CCACAGGACTAGAACACCTGC-3′; and EPO forward 5′-CATCTGCGACAGTCGAGTTCTG-3′, EPO reverse 5′-CACAACCCATCGTGACATTTTC-3′. Primers for rat HPRT and PHD1-3: PHD1 forward 5′-GCTGCTGCGTTGGTTAC-3′, PHD1 reverse 5′-GCCTCCTGGTTCTCTTG-3′; PHD2 forward 5′-CTGGGACGCCAAGGTGA-3′, PHD2 reverse 5′-CAATGTCAGCAAACTGG-3′; PHD3 forward 5′-GTTCAGCCCTCCTATGC-3′, PHD3 reverse 5′-ACCACCGTCAGTCTTTA-3′; and HPRT forward 5′-CTCCTCAGACCGCTTTTCCC-3′, HPRT reverse 5′-ACCTGGTTCATCATCACTAATCACG-3′.
Specificity and length of PCR products were verified by melting point analyses and agarose gel electrophoresis, respectively. For mouse NaPi-IIa the following primers were used: NaPi-IIa forward 5′-GTCCAGAGCAGCTCCGTGTT-3′, NaPi-IIa reverse 5′-CAGCAAACCAGCGGTACTTG-3′, product length: 301 bp.
From tissues total RNA was extracted with RNAzol B (Biozol, Eching, Germany) and analyzed by RNase protection assay essentially as described previously.37 [32P]-labeled antisense RNA probes were transcribed with SP6 or T7 polymerase (Roche, Mannheim, Germany) from linearized plasmids containing cDNA fragments of PHD1-3 and U6 small nuclear RNA. 40 μg of total RNA was hybridized to the radiolabeled probes and 1 μg total RNA to the U6sn probe, which was used as an internal control for each sample.
Animals
Animal experiments were approved by the local institutional review board for the care of animal subjects and were performed in accordance with National Institutes of Health guidelines. Male Sprague-Dawley rats (Charles River, Sulzfeld, Germany) were used at weights of 200 to 230 g. For the isolation of proximal tubular cells and in vivo hypoxia (8% O2 for 6 hours) or carbon monoxide (0.1% for 6 hours) experiments C57BL/6 mice were purchased from Jackson Laboratories, Sulzfeld, Germany. Animals were hosted in standard cages, fed a standard diet, and had free access to tap water.
Microdissection of Rat Nephron Segments
Nephron segments from male Sprague-Dawley rats were microdissected essentially as described.38 All procedures were conducted at 20% oxygen (room air). From the cortex we collected glomeruli with and without arterioles, proximal and distal convoluted tubules, cortical thick ascending limbs, and connecting and cortical collecting ducts. In the outer medulla, we differentiated between proximal straight tubules, descending thin limbs, medullary thick ascending limbs, and outer medullary collecting ducts. In the inner medulla, we collected descending/ascending thin limbs of Henle and inner medullary collecting ducts. The segments were identified by their morphological appearance and classification was reconfirmed with marker primers as shown in the study by Vitzthum et al.39 Collected tubules were dissolved in 400 μl guanidine thiocyanate solution (4 mol/L) at −80°C for RNA extraction according to the protocol of Chomczynski.40
Experimental Protocol of Cisplatin-Induced Acute Renal Injury
To induce acute renal injury, Sprague-Dawley rats were injected with 8 mg/kg cisplatin intraperitoneally and followed-up for 24 hours, 72 hours, or 168 hours. Serum creatinine and urea were determined at baseline (0 hours) and at the end of the experiments. To determine the expression of the PHDs at different time points, animals were sacrificed at 24 hours, 72 hours, or 168 hours after cisplatin administration, respectively, and the kidneys were processed for immunohistochemistry and RNA analysis.
Experimental Protocol of Ischemia Reperfusion-Induced Acute Renal Injury
Animals were anesthetized with ketamine (100 mg/kg body wt) and pentobarbital sodium (50 mg/kg body wt, Nembutal; Abbott, Wiesbaden, Germany). A constant body temperature of 37°C was maintained using a heated table with temperature feedback by a rectal probe (Heating Controller type 861; Hugo Sachs Electronic-Harvard Apparatus, March, Germany). The abdomen was shaved, and a midline incision was made. The left renal artery was clamped with an arterial clamp (B-2; S&T, Neuhausen, Switzerland) to induce ischemia, which was verified by the change of the renal color. During ischemia, the abdomen was closed. After 40 minutes, the clamp was removed and reperfusion was started. The abdomen was closed in two layers, and the rat was kept on the heating table until awakening after anesthesia. After 24 hours, 72 hours, and 168 hours, the animals were sacrificed by cervical dislocation, and the kidneys were removed for analysis.
Experimental Protocol of Contrast Media-Induced Acute Renal Injury
To induce acute renal failure, a combination of l-nitro-arginine methyl ester, iothalamate, and indomethacin was sequentially administered at 15-minute intervals as previously described.41 After 24 hours or 120 hours animals were sacrificed and kidneys were removed and processed for immunohistochemistry or RNA isolation.
Immunohistochemistry
Kidneys were fixed by perfusion or immersion with 4% paraformaldehyde in PBS (pH 7.4) at 4°C for 24 hours and processed for paraffin embedding. Paraffin sections (2 to 4 μm) were dewaxed in xylene and rehydrated in a series of ethanol washes. For detection of HIF isoforms, monoclonal mouse anti-human HIF-1α (α67, Novus Biologicals, Littleton, CO, 1:10.000), polyclonal rabbit anti-human HIF-1α (Cayman Cemicals, Ann Arbor, MI 1:20.000), polyclonal rabbit anti-mouse HIF-2α (PM9, 1:10.000), and polyclonal rabbit anti PHD1-3 antibodies (1:10.000, PHD1 NB 100-310, PHD2 NB 100-2219, PHD3 NB 100-139, and NB 100-303, Novus Biologicals) were used. Additional primary antibodies were used: rabbit anti-rat NaCl cotransporter antibody (1:500, a kind gift from D.J. Fife, Portland), a sheep anti- rat β-hydroxy-steroid-dehydrogenase II (1:200, Chemicon International, Temecula, CA), a mouse anti-rat synaptopodin (1:500, Progen, Heidelberg, Germany), a rabbit anti-rat NaPi-IIa antibody (1:500, a kind gift from J. Biber, and H. Murer, Zürich), a goat anti-Tamm-Horsfall protein antibody (1:500, ICN, Aurora, OH), and a mouse anti-rat endothelial cell antibody (RECA-1, 1:50, Serotec, Oxford, UK). Biotinylated or fluorescence labeled secondary anti-mouse, anti-sheep, or anti-rabbit antibodies (Dako, Hamburg, Germany) were used. For signal amplification of HIF-1α, HIF-2α and PHDs, a catalyzed signal amplification system (Dako, Hamburg, Germany) was used as described previously.26
Statistics
All data given represent the means ± SD (SD). A P value <0.05 was considered significant. Statistical analyses were performed using student’s t-test calculated with Sigma Stat-Software for Windows.
Results
Hypoxic Regulation of PHD Expression in Isolated Proximal and Distal Tubular Cells
To test for the presence of the three PHDs in renal tubular cells, we first analyzed isolated proximal and distal tubular epithelial cells from the mouse. Primary proximal tubular cells (mPT) were isolated and tested for the expression of NaPi-IIa, which is located in S1-3 segments of the proximal tubular epithelium (see Supplemental Figure S1 at http://ajp.amjpathol.org). For comparison we used the murine M-1 cell line that retains and expresses many of the differentiated morphological and functional properties of collecting duct principal cells in vivo, including the amiloride-sensitive epithelial sodium channel.34,35
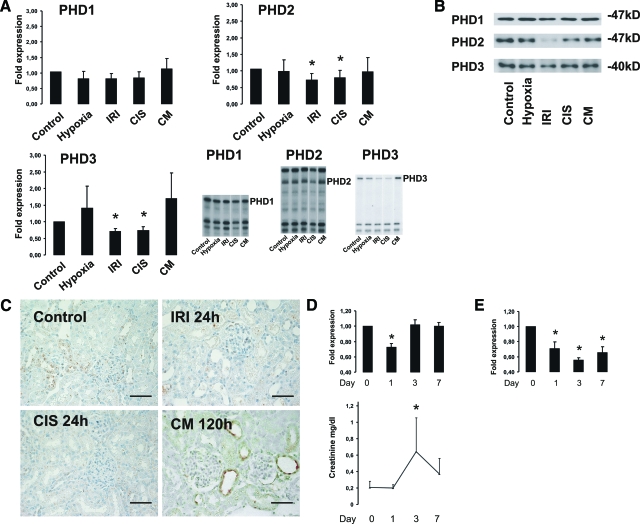
Using real-time PCR, we first screened for the occurrence of the three PHDs in each cell type: Under basal conditions, M-1 cells had significantly higher levels of PHD2 and PHD3 mRNA than mPT cells. In both cell types PHD1 mRNA was expressed at the lowest levels (Figure 1A). When both cell types were exposed to hypoxia (1% O2) a marked increase of PHD2 and in particular of PHD3 mRNA in mPT, but not in M-1 cells, was observed (Figure 1B). Immunoblotting of protein extracts paralleled results obtained in mRNA analysis, except for PHD1 protein levels, which showed strong bands in immunoblotting compared with low mRNA levels detectable by PCR (Figure 1C). We therefore determined the protein stability of the different PHDs in mPT cells using cycloheximide. PHD1 protein was still detectable after 30 hours, which indicated a higher stability for PHD1 than for PHD2 and 3. This may explain the discrepancy in PHD1 mRNA and protein levels (Figure 1D). Altogether, proximal tubular cells had lower basal, but hypoxia-inducible PHD levels, whereas distal tubular cells had higher basal levels, which were not induced by hypoxia anymore. Because PHD2 and 3 have been shown to be HIF target genes, we also performed immunoblots for HIF protein in the same extracts. Exposure to 1% oxygen for 16 hours led to a marked accumulation of HIF-1α, but not HIF-2α protein in both cell types (Figure 1E), indicating that the lack of hypoxic PHD induction in M-1 cells was not due to missing HIF-1 induction.
Figure 1.
A: Relative PHD1-3 mRNA levels in murine collecting duct (M-1) cells and murine proximal tubular cells (mPT) under normoxic conditions. Results were obtained in triplicates from three different cell preparations and normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression. Values are means ± SD; *P < 0.05 as compared with PHD1 expression in M-1 and mPT cells, respectively. B: Regulation of PHD expression by hypoxia in mPT and M-1 cells. mRNA was isolated after exposure of the cells to 1% O2 or normoxia for 16 hours. Real-time PCR was performed in triplicates from three different cell preparations. Results were normalized to the HPRT signal and related to results from normoxic cells. Values are means ± SD; *P < 0.05. C: Hypoxic regulation of PHD2 and 3 protein expression. Western blot analyses revealed hypoxic induction of PHD2 and 3 in proximal tubular cells, whereas no induction was seen in M-1 cells. PHD1 protein levels were not altered by hypoxia in both cell types. D: Protein stability of the different PHDs in mPT cells determined by treatment of the cells with cycloheximide for the indicated times. PHD1 had the longest half life in mPT cells. E: In both cell types, hypoxia for 16 hours leads to HIF-1α accumulation. No significant HIF-2α induction was seen.
PHD mRNA Levels in Rat Nephron Segments
Previous studies have demonstrated that PHD2 is the quantitatively predominant isoform in whole kidney extracts, followed by PHD1 and 3.16,24 Concordantly, in our analyses of whole rat kidney extracts PHD2 mRNA levels in ribonuclease protection assays exceeded PHD1 and PHD3 levels (Figure 2A). To determine PHD mRNA expression in different tubular segments in vivo, we microdissected single nephron segments from rat kidneys. Real time PCR analyses revealed the presence of PHD1-3 mRNA in all nephron segments examined with significant differences in expression levels (Figure 2B). The abundance of PHD1 mRNA varied up to threefold, that of PHD2 mRNA up to sevenfold, and that of PHD3 mRNA up to 2.2-fold, as compared with glomerular RNA. Although differences across the tubule were most pronounced for PHD2, a comparable variation pattern was seen also for PHD1 and 3. Compared with glomerular mRNA levels that served as a reference level, mRNA levels that were at least twofold higher were found in the PST for PHD1 and PHD2 and for all PHDs in the thick ascending limb (TAL), and the outer medullary collecting ducts. A particular prominent expression in inner medullary collecting ducts was observed for PHD2. In addition, PHD2 mRNA levels were 2.5-fold above reference mRNA levels in the DCT (Figure 2B).
Figure 2.
A: PHD mRNA levels from whole kidney lysates as determined by ribonuclease protection assay. Values were obtained from RNA of four rats and normalized to the internal control U6sn probe. *P < 0.05 as compared with PHD1 expression. B: Real-time PCR for PHD mRNA expression in microdissected nephron segments of the rat kidney. Results are expressed relative to PHD expression in the glomeruli with arterioles (Glom+). HPRT was used as an internal control. Values are means ± SD of three different nephron segment preparations, real-time PCR was carried out in duplicates. *P < 0.05 as compared with Glom+. Schematic illustration depicts PHD2 expression along the rat nephron (bottom right). Expression >two–fold over glomerular expression levels is shown in bold lines. Glom+: glomeruli with arterioles; Glom−: glomeruli without arterioles; PCT: proximal convoluted tubules; PST: proximal straight tubules; dTL: descending thin limbs; TL: descending/ascending limbs; mTAL: medullary thick ascending limbs; cTAL: cortical thick ascending limbs; DCT: distal convuluted tubules; CCD: connecting and cortical collecting ducts; OMCD: outer medullary collecting ducts; IMCD: inner medullary collecting ducts. C: Western blot analyses of whole kidney lysates from normoxic rats using isoform specific antibodies for PHD1-3 (Novus Biol.). Specific protein bands for PHD1 (approx. 47kDa), PHD2 (approx. 43kDa), and PHD3 (approx. 43kDa) were seen (asterisks mark specific bands). Additional bands were detected in immunoblots of PHD2 and 3.
Immunohistochemistry for the Three PHDs in Rat Kidney
Using isoform-specific antibodies in crude rat kidney lysates, immunoblotting revealed specific bands for PHD1, 2, and 3 proteins at the predicted molecular weight. Additional, weaker bands were found in immunoblots using PHD2 and 3 antibodies (Novus Biologicals) (Figure 2C). To further characterize PHD protein localization within the rat kidney, immunostaining for PHD1-3 was performed. Similar results were obtained with a panel of available PHD antibodies, including for PHD1 (Novus Biologicals), for PHD2 (two antibodies from Novus Biologicals, one antibody from Zymed, San Francisco, CA), for PHD3 (Novus Biologicals) and a monoclonal serum raised against PHD3 (188b)16. For further immunohistochemical studies we used only one set of antibodies (Novus Biologicals).
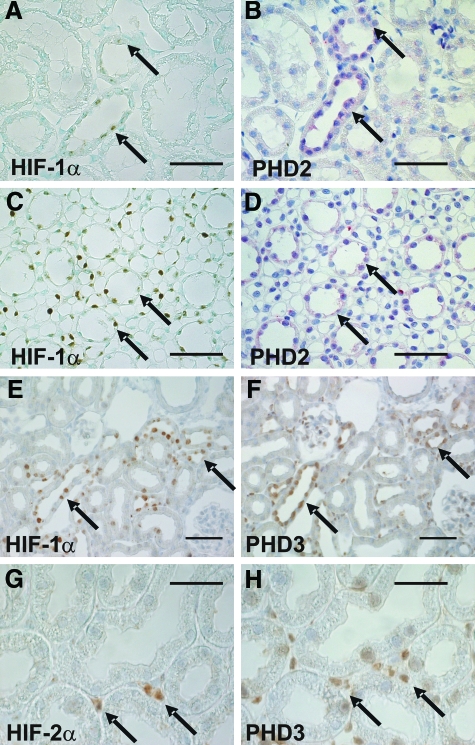
With respect to different cell types and structures within the kidney, staining for all three PHDs was remarkably similar, with predominant presence in distal tubules of the cortex and tubules of the medulla, as well as in glomeruli and in interstitial cells of the peritubular interstitium (Figure 3 A–F). In concordance with previous reports describing the intracellular distribution of the PHDs in cell culture42 and in the heart,16 PHD1 localized in the nucleus, PHD2 in the cytoplasm, and PHD3 in both compartments of renal epithelia (Figure 3G).
Figure 3.
Immunohistological staining of PHD1 (A, B), PHD2 (C, D) and PHD3 (E, F) in cortical and medullary regions of the rat kidney. Staining of all three isoforms was predominantly found in the medulla (B, D, F) and in distal tubules of the cortex (A, C, E). Furthermore, PHD1 and 3 protein was found in glomeruli and in peritubular interstitial cells (A and E). Scale bar = 50 μm. G: Higher magnification images of PHD expression in the rat kidney cortex indicate different intracellular distribution of the three 3 PHDs. PHD1 showed a nuclear, PHD2 a cytoplasmic, whereas PHD3 showed both a nuclear and a cytoplasmic staining. Scale bar = 25 μm.
To determine precisely, which cells express the different PHDs, we performed immunostaining on consecutive sections for PHDs and known markers for subsets of tubular and glomerular renal cells (Figure 4 A–H and Figure 5, A and B).
Figure 4.
Colocalization of PHD3 with markers for different nephron segments in serial sections of the cortex from rat kidneys. Low PHD3 (B) expression was detected in proximal tubules that stained positive for NaPi-IIa (A). PHD3 (D, F, H) was predominantly expressed in distal nephron segments as shown by colocalization with Tamm-Horsfall-Protein (C, THP, thick ascending limb), NaCl cotransporter (E, NCC, distal convoluted tubule), and 11(β)-hydroxysteroid dehydrogenase II (G, 11-β-HSD, distal convoluted tubule and collecting duct). Arrows either mark missing (A, B) or positive correlation of PHD3 and tubular markers in consecutive sections (C–H). Scale bar = 50 μm. I: PHD2 expression overall increased from the cortex toward the medulla. Adjacent images of the kidney section were overlayed using an image processing program.
Figure 5.
A: In glomeruli, PHD3 colocalized with synaptopodin, a marker for podocytes (arrows: Synaptopodin). B: PHD3 protein signals in the glomeruli and the tubulointerstitium (large arrows) did not correlate to RECA-1, a marker for endothelial cells (small arrows), indicating that PHD3 positive cells in the interstitium were not endothelial cells but most likely interstitial fibroblasts. V: vessel.
Using tubular segment markers, PHD expression corresponded to Tamm-Horsfall protein, NaCl cotransporter, and β-hydroxy-steroid-dehydrogenase II-positive cells, indicating that PHD expression localized to the TAL, the cortical DCT, the connecting tubule, and to all parts of the collecting duct (CD), respectively. No significant PHD signals were detected in the S1-S3 segments of the proximal tubule, (positive for the sodium phosphate cotransporter IIa [NaPi-IIa]). Tubular expression toward the medulla appeared overall to be more pronounced and homogeneous for all three PHDs (shown for PHD2 in Figure 4I). No specific nephron segment was detected that stained exclusively for one of the PHDs (summary Table 1).
Table 1.
Synopsis of PHD mRNA and Immunohistochemical Protein Detection in the Rat Kidney
| PHD1
|
PHD2
|
PHD3
|
||||
|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | mRNA | Protein | |
| Glomerulus | + | ++ | + | < dl | + | ++ |
| PCT | + | < dl | + | < dl | + | < dl |
| PST | ++ | < dl | +++ | < dl | + | < dl |
| TL | + | < dl | + | < dl | + | < dl |
| TAL | ++ | ++ | ++ | ++ | ++ | ++ |
| DCT | + | ++ | ++ | ++ | + | ++ |
| Cortical CD | + | ++ | + | ++ | + | ++ |
| Medullary CD | ++ | ++ | ++ | ++ | ++ | ++ |
| Endothelial cells | na | + | na | < dl | na | + |
| Interstitial fibroblasts | na | ++ | na | < dl | na | ++ |
Note. Endothelial and interstitial cells could not be microdissected and analyzed separately for mRNA expression. mRNA data in microdissected nephrons: + reference level obtained from glomerular mRNA expression (onefold); ++ twofold above reference level; +++ >fourfold above reference level; na: not available. Protein data in immunohistochemistry: < dl: below detection limit; + significant visible signals (×400); ++ strong visible signals (×100 and ×400).
In comparison, in glomeruli PHD1 and 3 showed significantly stronger expression signals than PHD2. Using synaptopodin as a podocyte marker on consecutive sections, PHD positive cells within the glomerulum were identified as podocytes (Figure 5A).
Like podocytes, interstitial cells were also positive only for PHD1 and 3. Using RECA-1 as a marker for endothelial cells, PHD3-positive cells in the interstitium were negative for RECA-1 (Figure 5B), implying that these cells are most likely interstitial fibroblasts.
In summary, mRNA data from microdissected nephrons and immunohistochemistry corresponded well for PHD1-3 in the TAL, the DCT, and the CD (Table 1). In contrast, a significant mRNA expression in the PST did not translate into protein levels detectable by immunohistochemistry. Whether posttranscriptional modifications prevent PHD protein synthesis in the PST remains unclear. The expression in glomeruli was more prominent using immunohistochemistry than mRNA analysis, which can probably be explained by the selective expression in podocytes and their limited contribution to total glomerular mRNA.
Regulation of PHDs in Vivo under Hypoxia and Correlation to HIF Expression Patterns
Previous studies showed that HIF-1α and -2α have different expression patterns when stabilized under hypoxic conditions in the rat kidney. Because prolyl hydroxylating enzymes are non-equilibrium enzymes, i.e., they do not catalyze the reverse reaction, their abundance is predicted to correlate to HIF degradation capacities. Therefore, in theory, high levels of PHD should lead to lower levels of HIF accumulation. Vice versa HIF itself has been shown to induce PHD2 and 3 mRNA in vitro,21 which establishes a feedback loop that limits HIF induction.43 To directly compare HIF with PHD expression we performed immunohistochemical HIF and PHD staining on consecutive sections of the rat kidney. Under normoxic conditions virtually no cells stained positive for HIF-1α (not shown). In contrast, significant staining was seen for PHD1-3, as described above. To test if hypoxia alters PHD expression patterns, we exposed rats to 0.1% carbon monoxide. After 6 hours exposure HIF-1α signals became detectable, as described previously.26 But PHD protein signals in immunohistochemistry remained unchanged (Figure 6). Because PHD2 and 3 were induced by hypoxia in isolated mouse proximal cells (Figure 1), we also exposed mice to hypoxia (8%) or carbon monoxide (0.1%) to test for potential species differences. We found strong induction of EPO mRNA in both experimental settings, which confirmed activation of the HIF system in these animals. In contrast to the rat, we found significant increases of PHD3 mRNA and protein in mouse kidneys exposed to carbon monoxide (see Supplemental Figure S2 at http://ajp.amjpathol.org).
Figure 6.
HIF-1α (A, C) expression colocalized with PHD2 signals (B, D) in serial sections of rat kidney cortex (A, B) or medulla (C, D). Similarly, PHD3 colocalized with HIF-1α in the kidney cortex (E, F) and medulla (not shown). In cortical interstitial cells that stained positive for HIF-2α (G) PHD3 (H) was detectable. Arrows show regions of colocalization. HIF-1α and HIF-2α were stabilized after exposure of the rats to 0.1% CO for 6 hours. Scale bar = 50 μm in A–F; = 25 μm in (G and H).
Interestingly, cortical and medullary tubules that stained positive for HIF-1α protein under hypoxia consistently showed strong PHD2 and 3 signals (Figure 6, A–F). Interstitial cells, which showed HIF-2α signals and which have been previously identified as interstitial fibroblasts,26 were also positive for PHD1 and 3 (Figure 6, G–H), whereas no significant signals were seen for PHD2. Compared with previous data on HIF expression in the hypoxic kidney,26 those tubules that stained positive for HIF were positive for PHD as well (Table 2).
Table 2.
Synopsis of PHD Protein Expression as Seen in Immunohistochemistry and HIF Signals in the Rat Kidney after 6 Hours 0.1% Carbon Monoxide Exposure
| PHD1 | PHD2 | PHD3 | HIF1 | HIF2 | |
|---|---|---|---|---|---|
| Glomerulus | ++ | ++ | + | ||
| PCT | ++ | ||||
| PST | + | ||||
| TAL | ++ | ++ | ++ | ++ (mTAL) | |
| DCT | ++ | ++ | ++ | ||
| Cortical CD | ++ | ++ | ++ | ++ | |
| Medullary CD | ++ | ++ | ++ | ++ | |
| Endothelial cells | + | + | + | ++ | |
| Interstitial fibroblasts | ++ | ++ | ++ |
PHD Expression in Animal Models of Acute Renal Failure
To further investigate how the PHDs are affected under conditions of renal injury we analyzed PHD mRNA and protein levels in different models of acute tubular necrosis. Ischemia/reperfusion and contrast media induced nephropathy were chosen as models of ischemic injury. Cisplatin nephropathy was chosen as a model of toxic injury. In the ischemia reperfusion model and the cisplatin model, preconditional activation of HIF has been demonstrated to be protective.27,29,30
In the cisplatin nephropathy model, after 1 day, a significant decrease of PHD2 and 3 mRNA (ribonuclease protection assays; Figure 7A) and protein (Western blot and immunohistochemistry) (Figure 7, B and C) was observed. Also after ischemia/reperfusion injury a significant down-regulation of PHD2 and PHD3 mRNA was seen after 24 hours. In comparison, contrast media nephropathy led to no significant changes of PHD mRNA and protein levels 24 hours (data not shown) or 120 hours after injury (Figure 7, A, B, and C).
Figure 7.
A: Regulation of PHD mRNA expression in the rat kidney in different models of acute renal failure. Normoxic and hypoxic (8% O2 for 6 hours) animals served as controls. In the cisplatin (CIS) induced nephropathy and in the ischemia reperfusion injury (IRI) a significant decrease of PHD2 and 3 mRNA was seen. CM: contrast media induced nephropathy. For consistency reasons lanes for CIS and CM of the same blot were rearranged for illustration. n = 4 to 8 per model. Shown are means ± SD; *P < 0.05. B: Western blot analysis for PHD1-3 of whole kidney extracts from the different injury models. PHD2 and PHD3 expression decreased in the cisplatin nephropathy (CIS) and in ischemia reperfusion injury (IRI), whereas no change of PHD protein expression was seen under hypoxia and contrast media nephropathy (CM). C: Immunohistochemical staining of PHD3 in cortical kidney sections of the different models showed down-regulation of PHD3 protein in cisplatin nephropathy (CIS) and ischemia reperfusion injury (IRI). In contrast media nephropathy (CM) no significant increase of PHD3 protein was seen. Scale bar = 50 μm D: Time course of PHD3 mRNA expression in cisplatin induced acute renal failure. Ribonuclease protection assay revealed diminished PHD3 mRNA expression on day 1. Serum creatinine levels rose until day 3 and kidney function begun to recover the following days. Shown are means ± SD; *P < 0.05, as compared with values from day 0. E: Time course of PHD3 expression in ischemia reperfusion injury. PHD3 mRNA was depressed in ischemia/reperfusion injury over the whole follow-up period. n = 2 to 4 per time point. Values are normalized to controls. Shown are means ± SD; *P < 0.05. Serum creatinine levels in rats from IRI experiments did not change due to unilateral injury and are not shown.
To test for PHD3 expression levels in the course of the cisplatin and ischemia reperfusion injuries, we performed time course experiments (day 0, 1, 3, and 7). A recovery of PHD3 expression was seen in parallel to improving kidney function in the cisplatin model (Figure 7D). In contrast, in the ischemia reperfusion model PHD3 levels remained depressed even after 7 days (Figure 7E). In all cases reduced PHD levels did not lead to increased HIF levels in the injury models, suggesting that reduced PHD levels in these models were not sufficient to stabilize HIF in the presence of oxygen.
Discussion
Oxygen dependent prolyl hydroxylation implies a straightforward mechanism that controls cellular HIF levels, depending on regional oxygen availability. However, complexity arises from the fact that there are at least three PHD isoforms and two HIFα isoforms, which have been demonstrated to have different regulatory effects and targets. In the present study we have investigated the expression of PHDs in the rat kidney in vivo and in isolated tubular cells and thereby demonstrate that further complexity of this system arises from uneven distribution of these enzymes and heterogeneous regulation. The main findings are that PHD1-3 are expressed with variable abundance at the mRNA level in glomeruli and all tubular segments and that at the protein level, PHD1, 2, and 3 are primarily present in distal tubules, while PHD1 and PHD3 also occur in podocytes and peritubular cells. Moreover, in models of acute tubular injury PHD2 and PHD3 were down-regulated.
Within the tubular system we found PHDs to be predominantly expressed in medullary nephron segments, both at the mRNA and the protein level. Since local oxygen tensions are physiologically low in the medulla, and the genes of PHD2 and PHD3 are inducible by hypoxia,44,45 this expression could in part be hypoxia-driven. However, in M-1 cells, which have characteristics of differentiated collecting duct principal cells, a high level of PHD expression was found in vitro, which was not inducible by exposure to a low-oxygen environment, suggesting that local hypoxia is not the primary determinant of PHD levels in the distal nephron. In line with this conclusion, a marked, albeit less pronounced expression of PHDs was also observed in cortical tubules. Irrespective of the mechanisms controlling PHD levels, the comparatively higher expression in the renal medulla might explain why, despite a chronic hypoxic environment, no constitutive HIF stabilization is found in the medulla under physiological conditions.26 Also in the distal convoluted tubule, where PHD expression was comparatively high, previous studies have shown that HIF-1α was only expressed at very low levels under conditions of systemic hypoxia.26
On the other hand, a coincident HIF and PHD expression was seen in tubular epithelial cells under systemic hypoxia. Cells in which HIF-1α signals were detectable were usually also positive for PHD signals. An explanation for the colocalization of PHDs with HIF under these conditions may be that PHD2 and 3 are themselves target genes of HIF,44,45 which in turn then targets HIF protein for degradation in a negative feedback loop.21,43,46 In vivo results of time course experiments of myocardial infarction in the rat revealed that early HIF accumulation was followed by PHD2 and 3 induction, which persisted for many days, in contrast to HIF signals that disappeared.16 Concordant induction of HIF and PHD could thus implicate a mutual regulatory loop between HIF proteins and PHD enzymes.
Coincident with the results in isolated mouse cells, proximal tubules of mice exposed to systemic hypoxia became also positive for PHD3. Overall, inducibility of PHD3 in mice, but not in rats in our study, suggests a species difference. Hypothetically this could also be linked to the 116 amino acid N-terminal extension of the rat protein including inter alia a mitochondrial recognition sequence, which is not present in the mouse.18 In respect to PHD2, where we measured high levels of PHD2 mRNA in the PST, which did not translate into high protein levels in immunohistochemistry, data are in contrast to the protein data from Li et al.24 This discrepancy cannot be fully explained, but may lay in different protocols used for PHD2 immunohistochemistry.
While tubular cells expressed all three PHDs, a more selective expression pattern was observed in the tubulointerstitial space and glomeruli, where only PHD1 and PHD3 were detectable, whereas PHD2, which is the only isoform, which has been identified to destabilize HIF-1α in isolated cells21,22 remained very low. PHD expression in the tubulointerstitium is of particular relevance due to the synthesis of EPO in peritubular fibroblasts.47 Interestingly the EPO producing peritubular fibroblasts were found to express HIF-2α only26 and additional evidence confirms that HIF-2α plays a dominant role in EPO regulation.48,49,50 Moreover, PHD3 has been suggested to target in particular HIF-2α in cell lines.21 The striking alignment of PHD1 and 3 with HIF-2α in renal interstitial cells implicates a specific role of either or both of these enzymes in EPO regulation.
Within glomeruli we found that the expression of PHD1 and PHD3 is confined to podocytes. Details of the regulation of the HIF system and its pathophysiological relevance in glomeruli are yet poorly understood. Recent evidence suggests however, that HIF induction in podocytes can be detrimental. HIF stabilization by conditional deletion of VHL in podocytes was shown to induce the development of a pauci-immune, rapid progressive glomerulonephritis in the mouse, which was attributed to increased levels of the HIF target gene Chemokine Receptor 4 (Cxcr4).51 Thus, the constitutive PHD expression in podocytes may have a protective role in the homeostasis of HIF levels.
To further test the regulation of PHDs under pathophysiological conditions, we studied three models of acute renal injury: ischemia/reperfusion and contrast media nephropathy, which have previously been shown to be associated with HIF induction29,41 and cisplatin nephropathy, in which we found no accumulation of HIF.30 In ischemia/reperfusion and in the cisplatin, but not in the contrast media nephropathy a reduction in PHD mRNA levels was found. In the cisplatin model we observed a partial recovery during the next 7 days, but not in the ischemia/reperfusion model. The reason for this down-regulation remains unclear and it may reflect unspecific tissue damage, including apoptotic and necrotic cell death rather than specific effects on the mechanisms of PHD regulation. The down-regulation of PHDs in the absence of HIF induction in the cisplatin model indicates that a reduced PHD level is either not sufficient to induce HIF or that additional effects associated with this type of injury inhibit HIF accumulation.
Our findings may have implications for therapeutic attempts to induce HIF through inhibition of PHDs. Oxoglutarate analogues are being tested and developed as competitive PHD inhibitors. Some of these compounds stimulate erythropoiesis in rodents and primates32,52 and can confer tissue protection. In different models of acute kidney injury, preconditional HIF activation with oxoglutarate analogues or cobalt resulted in preservation of kidney structure and function.27,29,53 While current inhibitors are substrate analogues that presumably affect all PHDs unselectively, future development could result in compounds with differential effects on different PHDs. Based on our observations, selective PHD2 inhibition could primarily induce HIF in tubular cells, and thereby confer tissue protection. In contrast PHD1 and 3 inhibitors are likely to have less restricted effects, including HIF-2α stabilization in interstitial cells producing EPO.
Acknowledgments
We thank Christopher Pugh and Peter Ratcliffe for the PHD3 antiserum 188b and Andrea Kosel, Brigitte Rogge, and Hans Fees for excellent technical assistance.
Footnotes
Address reprint requests to Carsten Willam, M.D., Dept. of Nephrology and Hypertension, Friedrich-Alexander-University, Krankenhausstrasse 12, D-91054 Erlangen. E-mail: carsten.willam@uk-erlangen.de.
Supported by grants from the Deutsche-Forschungsgemeinschaft (SFB 423).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Brezis M, Rosen S. Hypoxia of the renal medulla–its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- O'Connor PM. Renal oxygen delivery: matching delivery to metabolic demand. Clin Exp Pharmacol Physiol. 2006;33:961–967. doi: 10.1111/j.1440-1681.2006.04475.x. [DOI] [PubMed] [Google Scholar]
- Maxwell PH. Hypoxia-inducible factor as a physiological regulator. Exp Physiol. 2005;90:791–797. doi: 10.1113/expphysiol.2005.030924. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ. Signalling hypoxia by HIF hydroxylases. Biochem Biophys Res Commun. 2005;338:617–626. doi: 10.1016/j.bbrc.2005.08.111. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr The von Hippel-Lindau protein, HIF hydroxylation, and oxygen sensing. Biochem Biophys Res Commun. 2005;338:627–638. doi: 10.1016/j.bbrc.2005.08.165. [DOI] [PubMed] [Google Scholar]
- Lando D, Gorman JJ, Whitelaw ML, Peet DJ. Oxygen-dependent regulation of hypoxia-inducible factors by prolyl and asparaginyl hydroxylation. Eur J Biochem. 2003;270:781–790. doi: 10.1046/j.1432-1033.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Fong GH, Takeda K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 2008;15:635–641. doi: 10.1038/cdd.2008.10. [DOI] [PubMed] [Google Scholar]
- Nangaku M, Kojima I, Tanaka T, Ohse T, Kato H, Fujita T. Novel drugs and the response to hypoxia: hIF stabilizers and prolyl hydroxylase. Recent Patents Cardiovasc Drug Discov. 2006;1:129–139. doi: 10.2174/157489006777442522. [DOI] [PubMed] [Google Scholar]
- Lieb ME, Menzies K, Moschella MC, Ni R, Taubman MB. Mammalian EGLN genes have distinct patterns of mRNA expression and regulation. Biochem Cell Biol. 2002;80:421–426. doi: 10.1139/o02-115. [DOI] [PubMed] [Google Scholar]
- Willam C, Maxwell PH, Nichols L, Lygate C, Tian YM, Bernhardt W, Wiesener M, Ratcliffe PJ, Eckardt KU, Pugh CW. HIF prolyl hydroxylases in the rat; organ distribution and changes in expression following hypoxia and coronary artery ligation. J Mol Cell Cardiol. 2006;41:68–77. doi: 10.1016/j.yjmcc.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Oehme F, Ellinghaus P, Kolkhof P, Smith TJ, Ramakrishnan S, Hutter J, Schramm M, Flamme I. Overexpression of PH-4, a novel putative proline 4-hydroxylase, modulates activity of hypoxia-inducible transcription factors. Biochem Biophys Res Commun. 2002;296:343–349. doi: 10.1016/s0006-291x(02)00862-8. [DOI] [PubMed] [Google Scholar]
- Willam C, Nicholls LG, Ratcliffe PJ, Pugh CW, Maxwell PH. The prolyl hydroxylase enzymes that act as oxygen sensors regulating destruction of hypoxia-inducible factor alpha. Adv Enzyme Regul. 2004;44:75–92. doi: 10.1016/j.advenzreg.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Nytko KJ, Spielmann P, Camenisch G, Wenger RH, Stiehl DP. Regulated function of the prolyl-4-hydroxylase domain (PHD) oxygen sensor proteins. Antioxid Redox Signal. 2007;9:1329–1338. doi: 10.1089/ars.2007.1683. [DOI] [PubMed] [Google Scholar]
- Koivunen P, Hirsila M, Gunzler V, Kivirikko KI, Myllyharju J. Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem. 2004;279:9899–9904. doi: 10.1074/jbc.M312254200. [DOI] [PubMed] [Google Scholar]
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1. PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26:8336–8346. doi: 10.1128/MCB.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Yi F, Sundy CM, Chen L, Hilliker ML, Donley DK, Muldoon DB, Li PL. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. Am J Physiol Renal Physiol. 2007;292:F207–F216. doi: 10.1152/ajprenal.00457.2005. [DOI] [PubMed] [Google Scholar]
- Soilleux EJ, Turley H, Tian YM, Pugh CW, Gatter KC, Harris AL. Use of novel monoclonal antibodies to determine the expression and distribution of the hypoxia regulatory factors PHD-1, PHD-2, PHD-3 and FIH in normal and neoplastic human tissues. Histopathology. 2005;47:602–610. doi: 10.1111/j.1365-2559.2005.02280.x. [DOI] [PubMed] [Google Scholar]
- Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002;13:1721–1732. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Kojima I, Ohse T, Inagi R, Miyata T, Ingelfinger JR, Fujita T, Nangaku M. Hypoxia-inducible factor modulates tubular cell survival in cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2005;289:F1123–F1133. doi: 10.1152/ajprenal.00081.2005. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Matsumoto M, Inagi R, Miyata T, Kojima I, Ohse T, Fujita T, Nangaku M. Induction of protective genes by cobalt ameliorates tubulointerstitial injury in the progressive Thy1 nephritis. Kidney Int. 2005;68:2714–2725. doi: 10.1111/j.1523-1755.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- Bernhardt WM, Campean V, Kany S, Jurgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Gunzler V, Amann K, Willam C, Wiesener MS, Eckardt KU. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol. 2006;17:1970–1978. doi: 10.1681/ASN.2005121302. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Bernhardt WM, Klanke B, Daniel C, Buchholz B, Campean V, Amann K, Warnecke C, Wiesener MS, Eckardt KU, Willam C. HIF activation protects from acute kidney injury. J Am Soc Nephrol. 2008;19:486–494. doi: 10.1681/ASN.2007040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt WM, Warnecke C, Willam C, Tanaka T, Wiesener MS, Eckardt KU. Organ protection by hypoxia and hypoxia-inducible factors. Methods Enzymol. 2007;435:221–245. doi: 10.1016/S0076-6879(07)35012-X. [DOI] [PubMed] [Google Scholar]
- Macdougall IC, Eckardt KU. Novel strategies for stimulating erythropoiesis and potential new treatments for anaemia. Lancet. 2006;368:947–953. doi: 10.1016/S0140-6736(06)69120-4. [DOI] [PubMed] [Google Scholar]
- Stoos BA, Naray-Fejes-Toth A, Carretero OA, Ito S, Fejes-Toth G. Characterization of a mouse cortical collecting duct cell line. Kidney Int. 1991;39:1168–1175. doi: 10.1038/ki.1991.148. [DOI] [PubMed] [Google Scholar]
- Letz B, Ackermann A, Canessa CM, Rossier BC, Korbmacher C. Amiloride-sensitive sodium channels in confluent M-1 mouse cortical collecting duct cells. J Membr Biol. 1995;148:127–141. doi: 10.1007/BF00207269. [DOI] [PubMed] [Google Scholar]
- Korbmacher C, Barnstable CJ. Renal epithelial cells show nonselective cation channel activity and express a gene related to the cGMP-gated photoreceptor channel. EXS. 1993;66:147–164. doi: 10.1007/978-3-0348-7327-7_11. [DOI] [PubMed] [Google Scholar]
- Taub M, Sato G. Growth of functional primary cultures of kidney epithelial cells in defined medium. J Cell Physiol. 1980;105:369–378. doi: 10.1002/jcp.1041050220. [DOI] [PubMed] [Google Scholar]
- Willam C, Masson N, Tian YM, Mahmood SA, Wilson MI, Bicknell R, Eckardt KU, Maxwell PH, Ratcliffe PJ, Pugh CW. Peptide blockade of HIFalpha degradation modulates cellular metabolism and angiogenesis. Proc Natl Acad Sci USA. 2002;99:10423–10428. doi: 10.1073/pnas.162119399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold SW, Vitzthum H, Filbeck T, Wolf K, Lattas C, Riegger GA, Kurtz A, Kramer BK. Gene expression of 5-, 12-, and 15-lipoxygenases and leukotriene receptors along the rat nephron. Am J Physiol Renal Physiol. 2006;290:F864–F872. doi: 10.1152/ajprenal.00169.2005. [DOI] [PubMed] [Google Scholar]
- Vitzthum H, Castrop H, Meier-Meitinger M, Riegger GA, Kurtz A, Kramer BK, Wolf K. Nephron specific regulation of chloride channel CLC-K2 mRNA in the rat. Kidney Int. 2002;61:547–554. doi: 10.1046/j.1523-1755.2002.00165.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-Step method of RNA isolation by acid guanidinium thiocyanate-phenol.chloroform extraction. Analytical Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Rosenberger C, Heyman SN, Rosen S, Shina A, Goldfarb M, Griethe W, Frei U, Reinke P, Bachmann S, Eckardt KU. Up-regulation of HIF in experimental acute renal failure: evidence for a protective transcriptional response to hypoxia. Kidney Int. 2005;67:531–542. doi: 10.1111/j.1523-1755.2005.67110.x. [DOI] [PubMed] [Google Scholar]
- Metzen E, Berchner-Pfannschmidt U, Stengel P, Marxsen JH, Stolze I, Klinger M, Huang WQ, Wotzlaw C, Hellwig-Burgel T, Jelkmann W, Acker H, Fandrey J. Intracellular localisation of human HIF-1 alpha hydroxylases: implications for oxygen sensing. J Cell Sci. 2003;116:1319–1326. doi: 10.1242/jcs.00318. [DOI] [PubMed] [Google Scholar]
- Stiehl DP, Wirthner R, Koditz J, Spielmann P, Camenisch G, Wenger RH. Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem. 2006;281:23482–23491. doi: 10.1074/jbc.M601719200. [DOI] [PubMed] [Google Scholar]
- Marxsen JH, Stengel P, Doege K, Heikkinen P, Jokilehto T, Wagner T, Jelkmann W, Jaakkola P, Metzen E. Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases. Biochem J. 2004;381:761–767. doi: 10.1042/BJ20040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprelikova O, Chandramouli GV, Wood M, Vasselli JR, Riss J, Maranchie JK, Linehan WM, Barrett JC. Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J Cell Biochem. 2004;92:491–501. doi: 10.1002/jcb.20067. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Duplan E, Boyer N, Vigne P, Frelin C. Hypoxia up-regulates prolyl hydroxylase activity: a feedback mechanism that limits HIF-1 responses during reoxygenation. J Biol Chem. 2003;278:38183–38187. doi: 10.1074/jbc.M302244200. [DOI] [PubMed] [Google Scholar]
- Eckardt KU, Koury ST, Tan CC, Schuster SJ, Kaissling B, Ratcliffe PJ, Kurtz A. Distribution of erythropoietin producing cells in rat kidneys during hypoxic hypoxia. Kidney Int. 1993;43:815–823. doi: 10.1038/ki.1993.115. [DOI] [PubMed] [Google Scholar]
- Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci USA. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, Marsden PA, Pippin J, Shankland S, Rastaldi MP, Cohen CD, Kretzler M, Quaggin SE. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med. 2006;12:1081–1087. doi: 10.1038/nm1460. [DOI] [PubMed] [Google Scholar]
- Hsieh MM, Linde NS, Wynter A, Metzger M, Wong C, Langsetmo I, Lin A, Smith R, Rodgers GP, Donahue RE, Klaus SJ, Tisdale JF. HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood. 2007;110:2140–2147. doi: 10.1182/blood-2007-02-073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safran M, Kim WY, O'Connell F, Flippin L, Gunzler V, Horner JW, Depinho RA, Kaelin WG., Jr Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci USA. 2006;103:105–110. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]