Abstract
This study explored the effect of acoustic trauma on cochlear pericytes. Transmission electron microscopy revealed that pericytes on capillaries of the stria vascularis were closely associated with the endothelium in both control guinea pigs and mice. Pericyte foot processes were tightly positioned adjacent to endothelial cells. Exposure to wide-band noise at a level of 120 dB for 3 hours per day for 2 consecutive days produced a significant hearing threshold shift and structurally damaged blood vessels in the stria vascularis. Additionally, the serum protein, IgG, was observed to leak from capillaries of the stria vascularis, and pericytes lost their tight association with endothelial cells. Levels of the pericyte structural protein, desmin, substantially increased after noise exposure in both guinea pigs and mice with a corresponding increase in pericyte coverage of vessels. Increased expression levels of desmin were associated with the induction of hypoxia inducible factor (HIF)-1α and the up-regulation of vascular endothelial growth factor (VEGF). Inhibition of HIF-1α activity caused a decrease in VEGF expression levels in stria vascularis vessels. Blockade of VEGF activity with SU1498, a VEGF receptor inhibitor, significantly attenuated the expression of desmin in pericytes. These data demonstrate that cochlear pericytes are markedly affected by acoustic trauma and display an abnormal morphology. HIF-1α activation and VEGF up-regulation are important factors for the alteration of the pericyte structural protein desmin.
Cochlear blood flow is critically important for maintaining the endocochlear potential, ion transport, and endolymphatic fluid balance.1,2,3,4,5 Dysfunction of the cochlear blood supply can cause serious hearing disorders. In particular, loud sound causes a dramatic change in cochlear blood flow, effects that include increased vascular permeability, capillary vasoconstriction, and blood stagnation in strial capillaries.6,7,8,9 However, little is known about the change of the vascular cells in the stria vascularis. In this study the focus is on pericytes under acoustic stress.
The cochlear microvasculature network has a high population of pericytes.10 Pericytes form a dense net over the capillaries of the stria vascularis. However, unlike pericytes in the capillaries of the cochlear spiral ligament or other organs, pericytes on the stria vascularis do not express contractile proteins such as α-SMA or tropomyosin but they are rich in the structural protein desmin.10 Pericytes, in general, are thought to give mechanical strength and enhance the general integrity of capillary networks, as a case in point, blood vessels deficient of pericytes are abnormally large and leaky.11
The pericytes are known to have important roles in the regulation of vascular development, stabilization, maturation, and remodeling.12,13,14,15,16,17,18,19,20 Pericytes also mediate physiological repair processes including adaptive responses that protect vulnerable tissues under stress.21,22,23,24,25 Proliferation and recruitment of pericytes have frequently been found in ischemic tissues damaged by stroke.25,26 They are also morphologically altered in ischemia and under other hypoxic conditions.26,27
The hypoxic environment induced in the cochlea by noise is immediate and the effects persist after the noise is terminated.6,28 Studies have shown that when cells encounter low oxygen tension, they adapt by promoting expression of genes associated with anaerobic cell metabolism, cell survival, and angiogenesis.29 This transcriptional response is mediated by a hypoxia-inducible factor (HIF),30,31 a principal transcription factor, involved in the regulation of transcriptional responses to hypoxia. HIF is a heterodimer comprised of α and β subunits, which are constitutively expressed.32,33 Expression of the β subunit is independent of oxygen, whereas the protein stability of the α subunit is regulated in accordance with cellular O2 levels.33 HIF-1α subunits are degraded in normoxia, but are stabilized and activated under hypoxic conditions, forming a complex with the constitutively expressed transcription factor ARNT/HIF1β, which increases the transcription of target genes. Target genes of Hif-1α including the gene of vascular endothelial growth factor (Vegf) are related to angiogenesis, cell proliferation, and survival.30,34 The biological activity of vascular endothelial growth factor (VEGF) protein has been studied extensively in vascular remodeling under the ischemia condition.35,36 In particular, emerging evidence shows that VEGF has important roles in pericyte proliferation and in turn, pericytes are critical in vascular remodeling.35,36,37
In this study, we investigated cochlear pericyte change in response to noise-induced hypoxia and involvement of HIF-1α and VEGF in the change. Our results show that high-level noise exposure increased the expression of pericyte structural protein desmin and that excess numbers of pericytes decorate vessels resulting in increased coverage over the vessels. HIF-1α induction and VEGF up-regulation are found to be influenced by loud sound and they are important components for altering pericyte structure in response to noise stimuli.
Materials and Methods
Animals
Experiments were performed on albino guinea pigs (both sexes; weight, 300 to 450 g) and 129S2/C57BL/6 mice (8 to 10 weeks old). All procedures in this study were reviewed and approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University.
Noise Exposure
The animals (guinea pigs and mice) with positive Preyer reflex were divided into control and noise-exposed groups. For noise exposure, the animals were placed in wire mesh cages and exposed to broadband noise at 120 dB SPL in a sound exposure booth for 30 minutes, 1 hour, 3 hours, or for an additional 3 hours the next day. This noise exposure regime is routinely used in our laboratory and produces a permanent cochlear sensitivity loss.38
Auditory Testing
Auditory brain-stem response audiometry to pure tones was used to evaluate hearing function before noise exposure and after noise exposure in five mice and five guinea pigs. For the auditory brain-stem response test, each animal was anesthetized with xylazine (10 mg/kg, i.m., IVX; Animal Health Inc., St. Joseph, MO) and ketamine (40 mg/kg, i.m.; Hospira, Inc., Lake Forest, IL), and placed on a heating pad in a sound-isolated chamber. The external ear canal and tympanic membrane were inspected using an operating microscope to ensure the ear canal was free of wax and that there was no canal deformity, no inflammation of the tympanic membrane, and no effusion in the middle ear. Needle electrodes were placed subcutaneously near the test ear, at the vertex and at the contralateral ear. Each ear was stimulated separately with a closed tube sound delivery system sealed into the ear canal. The auditory brain-stem response to a 1-ms rise-time tone burst at 4, 8, 12, 16, 24, and 32 kHz was recorded and thresholds obtained for each ear. Threshold was defined as an evoked response of 0.2 μV. This method was used to assess auditory brain-stem response both before noise exposure and immediately after noise exposure.
Immunohistochemistry
Primary antibodies used in the experiments included monoclonal rabbit anti-desmin (catalog no. ab32362; Abcam, Cambridge, MA), polyclonal rabbit anti-VEGF (catalog no. sc-507; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), polyclonal rabbit anti-HIF-1α (catalog no. sc-10790, Santa Cruz Biotechnology, Inc.), Alexa Fluor 568-conjugated goat anti-mouse IgG (H+L) (catalog no. A11001; Invitrogen, Eugene, OR), Alexa Fluor 568-conjugated goat anti-guinea pig IgG (H+L) (catalog no. A11073, Invitrogen). Secondary antibodies were Alexa Fluor 488-conjugated goat anti-rabbit (catalog no.11008, Invitrogen, Eugene, OR). Immunohistochemistry was performed as described previously.38 Tissue sections were briefly permeabilized in 0.5% Triton X-100 (Sigma, St. Louis, MO) for 1 hour, and immunoblocked with a solution of 10% goat serum and 1% bovine albumin in 0.02 mol/L phosphate-buffered saline (PBS) for 1 hour. The specimens were incubated overnight at 4°C with the primary antibody diluted in PBS-bovine serum albumin. After several washes in PBS, sections were incubated in secondary antibody for 1 hour at room temperature. Tissues were mounted in mounting medium (H-1000; Vector Laboratories, Inc., Burlingame, CA) and visualized under an Eclipse TE 300 inverted microscope (Nikon, Tokyo, Japan) fitted with a Bio-Rad (Richmond, CA) MRC 1024 confocal laser microscope system. Controls were prepared by replacing primary antibodies with 0.2% Triton X-100 in PBS.
Western Blot Analyses
Desmin expression under control and noise-stimulated conditions was compared. Guinea pigs and mice from control and on the second day of noise-exposed groups were anesthetized with an overdose of ketamine hydrochloride (100 mg/kg, i.m.) and 2% xylazine hydrochloride (10 mg/kg) (Abbott Laboratories, N. Chicago, IL). The cochleae were taken after cardiovascular perfusion with PBS (pH 7.4). Each guinea pig group was comprised of six animals; each mouse group was comprised of 10 animals. The guinea pig and mouse cochleae were removed and the whole cochlear lateral walls of the guinea pigs were immediately dissected in ice-cold PBS. For the mouse cochlea, the cochlear stria vascularis was isolated from the cochlear lateral wall in cold PBS. Tissue samples were washed twice with cold PBS and immediately frozen at −80°C in 1.5-ml Eppendorf tubes. Total protein was extracted following the manufacturer’s instructions (catalog no. 20-188; Upstate, Lake Placid, NY). The protein was removed and stored at −20°C. The protein concentrations were calculated using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Equal amounts of protein were loaded on 12% sodium dodecyl sulfate-polyacrylamide gels (90 V, room temperature for 80 minutes), electrophoresed, and transferred to nitrocellulose by electroblotting (30 V, overnight at 4°C) in 1× transfer buffer (Bio-Rad). Nitrocellulose membranes were blocked in 5% w/v nonfat dry milk and 0.1% v/v Tween 20 in PBS (pH 7.4, 0.12 mol/L) for 1 hour at 25°C before being incubated overnight at 4°C with primary antibodies [monoclonal rabbit anti-desmin 1:500 (catalog no. ab32362, Abcam, Cambridge, MA), polyclonal rabbit anti-VEGF 1:500 (catalog no. sc-507, Santa Cruz Biotechnology, Inc.)] in blocking buffer. Membranes were then incubated with a 1:3000 v/v secondary antibody (Bio-Rad) for 1 hour at room temperature. Protein bands were visualized on Kodak XAR-5 film (Eastman-Kodak, Rochester, NY) with Super Signal West Femto chemiluminescent substrate (Pierce, Rockford, IL). Equal protein loading was examined by stripping the blots and reprobing them with a 1:5000 v/v of monoclonal mouse antibody for GAPDH (Chemicon International, Temecula, CA) or monoclonal mouse antibody for α-actin (1:3000, catalog no. A1978; Sigma) followed by incubation with secondary antibody (catalog no. 170-6516, goat anti-mouse antibody; Bio-Rad) and visualization as described above.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total RNA from the cochlear lateral wall was separately extracted, with RNeasy (Qiagen, Valencia, CA), from the control, noise-exposed, and drug-pretreated noise-exposed groups. Each group was comprised of 10 mice for analysis of mRNA levels of Desmin and Vegf with quantitative real-time PCR. Two μg of total RNA and 100 ng of random hexamer were used to make 40 μl of cDNA by SuperScript II (Invitrogen) following the manufacturer’s instructions. Transcript quantities were assayed by corresponding TaqMan gene expression assay: Desmin (catalog no. Mm00802455_m1; Applied Biosystems, Foster City, CA) and Vegf (catalog no. Rh02621759_m, Applied Biosystems) with a model 7300 real-time PCR system (Applied Biosystems, Foster City, CA). Thermal cycle conditions were an initial hold of 50°C for 2 minutes, 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. For assessment of mRNA level of Hif-1α, total RNA from the cochlear lateral wall was separately extracted from the control, noise exposure (30 minutes), noise exposure (1 hour), and noise exposure 3 hours in each day for 2-day groups. Each group was comprised of three mice. Two μg of total RNA sample was reverse-transcribed by using RETROscript kit (Ambion, Austin, TX). The cDNA synthesized from total RNA was diluted 10-fold with DNase-free water. Transcript quantities were assayed by corresponding TaqMan gene expression assay Hif-1α (catalog no. Mm01283758_g1, Applied Biosystems) with a StepOnePlus real-time PCR system (Applied Biosystems). Cycling conditions of real-time PCR were 95°C for 20 seconds, 40 cycles of 95°C for 1 second, 60°C for 20 seconds. Mouse Gapdh (catalog no. 4352339E, Applied Biosystems) expression was used as an endogenous control. Samples were run in triplicate for Desmin and Vegf. Samples were run six times for Hif-1α. Quantitative PCR was performed according to the guidelines provided by Applied Biosystems. The comparative cycle threshold (CT) method (ΔΔCT quantitation) was used to calculate the difference between samples. Quantitative data analysis followed the suggestions of the manufacturer.
Transmission Electron Microscopy
Cochlear lateral wall tissues were dissected from the control and noise-exposed animals. Segments of the cochlear lateral wall from the basal turns were fixed overnight in phosphate-buffered 3% glutaraldehyde-1.5% paraformaldehyde and postfixed in 1% osmium. Tissues were dehydrated, embedded in Araldite plastic, sectioned, stained with lead citrate and uranyl acetate, and viewed in a Phillips, CM, 100 transmission electron microscope (Eindhoven, the Netherlands).
Double and Triple Labeling
To visualize pericytes on the endothelium of the cochlear lateral wall, we double labeled lateral wall tissues with a combination of antibody for desmin (to identify pericytes) and isolectin GS-IB4 Alexa Fluor 488 (to identify vessels) (catalog no. 121411, Invitrogen). The procedure after immunohistochemical labeling for desmin was the same as described above except that 1:400 isolectin GS-IB4 was added to the medium along with primary antibody for desmin.
To visualize HIF-1α translocation, we triple labeled lateral wall tissues with a combination of antibody for HIF-1α, propidium iodide (catalog no. P-3566, Molecular Probes, Eugene, OR) (to identify cell nuclei) and isolection GS-IB4 Alexa Fluor 647 (to identify vessels, catalog no. I32450, Invitrogen). The procedure after immunohistochemical labeling for HIF-1α was the same as described above except that 1:100 propidium iodide was added to the medium along with secondary antibody for HIF-1α for 1 hour.
Desmin Coverage on Vessels
Images were analyzed using Image J (V1.38X; National Institutes of Health, West Chester, PA). The cochlear lateral wall from the basal turn was used for the study. Images were acquired with a ×40 objective. A total of 60 images were recorded from five normal guinea pigs and a total of 80 images from seven noise-exposed guinea pigs were recorded. A total of 171 images were recorded from five normal mice and 65 images were recorded from three SU1498-treated normal mice. Seventy-five images were recorded from three sodium butyrate-treated animals. One hundred seventy-three images were recorded from seven noise-exposed mice. A total of 73 images were recorded from four noise-exposed mice treated with SU1498. Seventy-nine images were recorded from four noise-exposed mice treated with sodium butyrate and a total of ninety-one images were recorded from dimethyl sulfoxide (DMSO)-treated noise-exposed mice.
To determine pericyte coverage of endothelial cells (ECs), we labeled the pericytes with an antibody for desmin and labeled the ECs with isolectin GS-IB4 conjugated to Alexa Fluor 488 as described above. Images were analyzed for overlap of blood vessels and pericytes. For analysis, endothelium (red) and pericytes (green) were displayed and both sources of images were thresholded. A region tool in the Image J software was used to define and select the outer margin of the blood vessel under consideration. A delineated area in pixels and the percentage overlap (co-localization) of endothelium and pericytes was calculated. Pericyte coverage was quantified as a ratio of desmin-labeled area to isolectin-labeled area, in concurrence with a calculation method used by others.20
Evaluation of Immunostaining for VEGF
Immunostaining for VEGF was assessed and measured objectively by two independent observers (a senior researcher and a research assistant). VEGF labeling was measured with Image J (V1.38X) from a series of images obtained from the tissue segment. For each recorded image, the areas of the entire positive-labeled vessels were selected with a drawing tool, and the fluorescence intensity of the selected area was measured with the histogram function, obtaining a mean value. A background intensity was determined in a small window located away from the fluorescence of the vessels and was subtracted from the fluorescence intensity value from the vessels.9 Fluorescence was analyzed in 43 optical section images from control animals (n = 3), 87 optical section images from noise-exposed animals (n = 6), and 48 optical section images (n = 4) from sodium butyrate-treated noise-exposed animals.
SU1498 and Sodium Butyrate Treatments
To inhibit HIF-1α activity, mice were pretreated with an HIF-1α inhibitor, sodium butyrate (catalog no. 303410; Sigma-Aldrich, St. Louis, MO). Treatments were administered as acute, single-dose (intraperitoneal) injections (given in a 20-μl volume, 200 mg/kg, drugs were dissolved in saline as vehicle, Schroeder and colleagues,39) 30 minutes before the animal received the noise exposure. For inhibition of VEGF activity, the mice were pretreated with SU1498 (T4192, Sigma-Aldrich) by one periocular injection (given in a 10-μl volume, SU1498 50 mg/kg) 30 minutes before noise exposure. DMSO vehicle was the control. Dosage of SU1498 was based on studies by Saishin and colleagues,40 and Cebulla and colleagues,41 who demonstrated effects on vessel function from the injection of VEGF in C57BL/6J mice.40,41 In addition, to determine whether either sodium butyrate or SU1498 has side-effects on auditory function, auditory brain-stem response was used to measure hearing threshold. We found that neither sodium butyrate nor SU1498 had ototoxicities in all six tested mice in the two treated groups (n = 3). To determine whether dosage of SU1498 was sufficient to inhibit VEGF activity, the activity of focal adhesion kinase, a widely expressed cytoplasmic protein tyrosine kinase involved in VEGF-mediated signal transduction42 has been examined in the cochlear lateral wall tissues. We found that immunoreactivity for focal adhesion kinase in the noise-exposed mice was high compared with the nonnoise exposed mice. A pretreatment with SU1489 to the noise-exposed mice significantly attenuated focal adhesion kinase immunoreactivity (data not shown).
Statistics and Analysis
Data are presented as means ± SD. Differences between data sets were assessed with Student’s t-test. n refers to the number of animals. Differences were considered significant at P < 0.05.
Results
Noise Trauma Caused Cochlear Sensitivity Loss in Both Mice and Guinea Pigs
Both mice and guinea pigs had significant sensitivity loss after being exposed to intense noise at 120 dB SPL for 3 hours. Auditory brain-stem response thresholds at all test frequencies were increased (Figure 1; nmouse = 5; nGP = 5; P < 0.001 at all test frequencies).
Figure 1.
Noise-induced hearing loss, estimated using auditory brain-stem response thresholds before and immediately after noise at 4, 8, 16, and 32 kHz. Noise exposure caused significant hearing threshold shift at measured frequencies (nMS = 5, nGP = 5; P < 0.001 at all test frequencies).
Noise Trauma Caused Structural Damage and Functional Disruption of the Cochlear Blood Vessels in Both Mice and Guinea Pigs
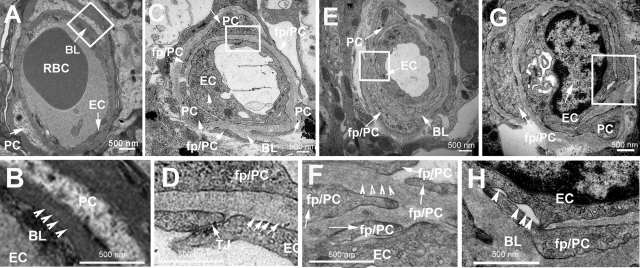
The cochlea of the guinea pig has ∼4 turns of cochlear lateral wall (Figure 2A) and 1.5 turns in the mouse (Figure 2B). The locations of the rectangles in the right panels of Figure 2, A and B, represent areas that we examined in the control and noise-exposed animals in this study. The stria vascularis, attached to the lateral wall fibrocyte (FC) layer, comprises marginal cells (MCs), intermediate cells (ICs), basal cells (BCs), and a dense capillary network. The capillary network in the stria vascularis (V/SV) lies between two cell layers connected by tight junctions (TJs); one is the epithelial MC layer, and the other is the mesodermal BC layer. Numerous basal infoldings of MCs and dendrite-like projections of ICs and BCs made close contact with the capillaries (Figure 2C).
Figure 2.
Cochlear anatomical structures from mice and guinea pigs, as well as electron transmission micrographs and immunofluorescence staining for serum protein IgG. Mouse (A) and guinea pig (B) cochleae before the cochlear bony wall were removed (left). Mouse and guinea pig cochleae after the cochlear bony wall were removed (right). The rectangular areas in A and B represent the locations we examined. C: Schematic diagram illustrating the location of capillary networks in the stria vascularis. It shows that the cochlear lateral wall is composed of two structures, the stria vascularis and the spiral ligament. The capillary network in the stria vascularis lies between two cell layers connected by TJs; one is the epithelial MC layer and the other is the mesodermal BC layer. The basal and IC layers face spiral ligament connective tissue composed of FCs, and the MC layer faces the cochlear duct. A dense capillary network (red circles) is limited in the interstitial space between the MCs and ICs. Low-magnification transmission electron micrographs of the stria vascularis in normal mouse (D) and guinea pig (E) show the stria vascularis composed of vessels (V/arrow), MCs (MC/arrow), ICs (IC/arrow), and BCs (BC/arrow). Under high magnification, the microvascular wall is composed of ECs surrounded by a pericyte in mice (F) and guinea pigs (G). Serum protein IgG confined to blood plasma (IgG/arrow) in the vessels of the stria vascularis in normal mice (H) and guinea pigs (I). The vascular wall is distorted and exhibited abnormal features in the noise-exposed mice (J) and guinea pigs (K). Arrowheads indicate interstitial volume in the stria vascularis between capillaries and surrounding cells in noise-exposed mice (J) and guinea pigs (K). Vacuolization is seen in an EC (L, arrow). Disruption of an EC is also seen in a vessel of a guinea pig (M, arrowheads). Serum protein IgG leaks from vessels (arrow/IgG) in noise-exposed mice (N) and guinea pigs (O). GP, guinea pig; MS, mouse; RBCs, red blood cells; PC, pericyte; V/SV, vessel of the stria vascularis; V/SL, vessel of the spiral ligament.
With low-magnification transmission electron microscopy, various cochlear lateral wall cells, including MCs, ICs, BCs, and capillaries (V), were seen in the normal mouse (MS) and guinea pig (GP) ear (Figure 2, D and E; MC/arrow, BC/arrow, IC/arrow, and V/arrow). Under high magnification, the microvascular wall comprises ECs surrounded by a pericyte (PC)/basal lamina (BL) in normal mice (Figure 2F) and normal guinea pigs (Figure 2G). PCs were closely associated with ECs and their foot processes (fp/PC) tightly positioned next to the endothelium in normal mice (Figure 2F, fp/PC/arrowheads) and guinea pigs (Figure 2G, fp/PC/arrowheads). The permeability of the endothelium was normal because no immunofluorescence signals of serum protein IgG H+L were found outside of the capillaries of the stria vascularis in normal mice (Figure 2H, IgG/arrow) and normal guinea pigs (Figure 2I, IgG/arrow). In contrast, mice (Figure 2J) and guinea pigs (Figure 2K) exposed to wide-band noise at the level of 120 dB for 3 hours per day for 2 consecutive days had edema and swelling of the stria vascularis. Intercellular spaces between ECs, PCs, and ICs were enlarged in the stria vascularis in the mice (Figure 2J, arrowheads) and guinea pig (Figure 2K, arrowheads). Endothelial cell vacuolization was seen in a capillary of the stria vascularis in a noise-exposed mouse (Figure 2L, arrow). A disrupted capillary wall was seen in a noise-exposed guinea pig (Figure 2M, arrowheads). Serum protein IgG was found outside of vessels in the stria vascularis of the mice (Figure 2N, arrowheads) and guinea pigs (Figure 2O, arrowheads).
Pericyte Coverage Was Significantly Increased in Both Mice and Guinea Pigs
Immunohistochemically labeled desmin enables pericytes to be visualized under fluorescence confocal microscopy. In a normal guinea pig and mouse ear, pericytes on the capillaries were oriented longitudinally along the vessels, with long thin foot processes, positioned next to the endothelium. We found pericyte coverage, as defined in the Materials and Methods, to be ∼12% in the guinea pig (Figure 3A, examples of pericyte distributions on two vessels of stria vascularis) and ∼19% (Figure 3B, an example of desmin distributions on vessels of the stria vascularis) in the mice. In contrast, when animals were exposed to wide-band noise at the level of 120 dB for 3 hours per day for 2 consecutive days, pericytes irregularly positioned along the vessel walls of the stria vascularis in both the guinea pigs (Figure 3D) and mice (Figure 3E). Some pericyte foot processes were detached from the capillaries of the guinea pigs (Figure 3D, arrow) and mice (Figure 3E, arrows). Interestingly, pericyte coverage was substantially increased to ∼40% in the capillaries of the stria vascularis of the guinea pig (Figure 3G; ncontrol = 5, nnoise = 7, **P < 0.01) and to 39% in the mice (Figure 3E; ncontrol = 5, nnoise = 7, **P < 0.01).
Figure 3.
Desmin up-regulation after noise exposure. A and B: Confocal fluorescent images of immunolabeled vessels from normal mice and guinea pigs. Pericytes containing desmin filaments are evenly distributed on the vessel walls of the stria vascularis in both guinea pig (A) and mice (B). Pericytes are labeled with an antibody for desmin (green), and vessels are labeled by isolectin IB4 (red). D and E: Confocal fluorescent images from noise-exposed guinea pigs and mice show abnormal pericyte morphology and increased pericyte coverage. Arrows point to irregular pericyte foot processes turning away from the vessel wall (D) and detached from it (E). C and F: Drawings illustrating the pattern of pericyte distribution on vessel walls in normal and noised-exposed animals. G: Histogram shows that pericyte coverage of vessel walls was significantly increased in noise-exposed animals compared with normal animals (GP: ncontrol = 5, nnoise = 7, **P < 0.01; mice: ncontrol = 5, nnoise = 7, **P < 0.01). V/SV, vessel of the stria vascularis; NE, noise exposure; GP, guinea pig; MS, mouse.
Desmin Expression Was Significantly Increased in Both Mice and Guinea Pigs
With Western blot analysis, we also found that the amount of desmin expression was increased in noise-exposed guinea pig whole cochlear lateral wall (Figure 4A) and the stria vascularis of noise-exposed mice (Figure 4B) after 2 consecutive days of noise exposure. The change of desmin protein expression measured by Western blot in guinea pigs appears to be greater in comparison with mice (Figure 4, A and B). This may be explained by the combined cell population of FCs, which also express desmin (unpublished data) and pericytes in the guinea pig lateral wall, whereas the mouse stria vascularis has only a single desmin-expressing cell population of pericytes. In contrast, desmin protein was not significantly up-regulated in both guinea pig retina and mouse retina (Figure 4, A and B). These findings indicate that pericyte desmin expression is a regional response to noise injury. With quantitative real-time PCR technique, the mRNA levels for Desmin in the cochlear lateral wall of the mice were also significantly increased after noise exposure to wide-band noise at the level of 120 dB for 30 minutes, 1 hour, and 3 hours per day for 2 consecutive days (Figure 4C; n = 10, **P30 minutes < 0.01, **P60 minutes < 0.01, **P2 days < 0.01).
Figure 4.
Western blot and quantitative real-time PCR analysis show the amount of desmin protein in different tissues from normal and noise-exposed animals. A: Desmin protein expression in whole cochlear lateral wall and retina in guinea pig. B: Desmin protein expression in cochlear stria vascularis and retina in mice. C: Quantitative real-time PCR analysis shows mRNA levels for Desmin at different time points after noise exposure. The mRNA level of Desmin is significantly increased at 30 minutes of noise exposure and 1 hour of noise exposure as well as on the 2nd day after an additional 3 hours of noise exposure (n = 10, **P30 minutes < 0.01, **P60 minutes < 0.01, **P2 days < 0.01). GP, guinea pig; MS, mouse.
Abnormalities of Pericyte Morphology Produced by Noise in Mice
Pericytes in the stria vascularis of normal mice, examined by electron microscopy, were tightly associated with ECs, with foot processes positioned next to the endothelium, as shown in Figure 2. Under high magnification, a dense and continuous BL is seen between pericytes and ECs (Figure 5, A and B). In contrast, pericytes in noise-exposed mice had an abnormally loose association with ECs, with wide spaces separating some regions of the two types of cells (Figure 5, C and D). Basal lamina appeared less electron-dense (Figure 5D). Also the numbers of small pericytes found on the capillaries suggest they were proliferating (Figure 5C, with arrows). Pericyte foot processes were migrating into the BL (Figure 5, E and F), and the size of pericyte foot processes was larger than in a normal animal (Figure 5G). The BL between pericytes and ECs was discontinuous in places. (Figure 5H). A similar abnormal morphological character was found in the guinea pigs (data not shown).
Figure 5.
Transmission electron micrographs of cochlear vessels from normal and noise-exposed mice. A: Endothelial cells (EC/arrow) were surrounded by pericytes (PC/arrow) and sheathed in continuous BL (BL/arrow). B: The BL (arrowheads) between normal PCs and ECs is homogenous with relatively high electron density at high magnification. C: In noise-exposed animals, greater numbers of pericytes were present on vessels in the stria vascularis (PC/arrow) and the cytoplasmic matrix of the ECs protruded into the lumen of the microvessel (EC/arrow). D: Basal lamina has less electron density at high magnification (arrows). The junction between ECs is loose (D, TJ/arrow). Pericyte foot processes migrate into loose BL (E, fp/PC) and form a multilayered BL seen at high magnification (F, arrows). Arrowheads represent abnormal basal lamina. G: An enlarged pericyte foot process surrounds a nearly closed capillary (fp/PC/arrow) and an EC protrudes to the vessel lumen (EC/arrow). H: The BL between PCs and ECs is discontinuous (arrowheads). Rectangular areas in A, C, E, and G indicate the locations of magnified micrographs B, D, F, and H. PC, pericyte; fp/PC, foot process of pericyte.
Noise Induced HIF-1α Translocation in Mice
Insufficient blood supply induced by noise causes pO2 to drop and the cochlear environment to turn hypoxic.7,43 The transcriptional complex hypoxia-inducible factor 1 (HIF-1) plays a key role in the cellular adaptations to the lack of oxygen supply.44 In this study, using an immunohistochemical technique, the immunoreactivity of HIF-1α protein was examined under fluorescence confocal microscopy. We found no or little immunoreactivity to HIF-1α in various cells of the cochlear lateral wall in the control mice (Figure 6A). This finding was consistent with the notion that HIF-1α is degraded under normoxia.34 However, within as little as 30 minutes of noise exposure, HIF-1α immunoreactivity was increased in various cells including vascular and nonvascular cells in the stria vascularis and remained active for 2 days of noise exposure. With double labeling for HIF-1α and cell nuclei with propidium iodide, HIF-1α induction and translocation can be seen in the pericytes (Figure 6, B and C; PC/arrow) and the ECs (Figure 6, B and C; EC/arrow) as well as in nonvascular MCs (Figure 6, B and C; MC/arrow) at as early as 30 minutes noise exposure and a prolonged noise exposure (Figure 6C). This can be better visualized from the high-magnification inset images from the location of the rectangle in Figure 6, B and C. With quantitative real-time PCR, the mRNA levels for Hif-1α in the cochlear lateral wall were also significantly increased after noise exposure to wide-band noise at the level of 120 dB for 30 minutes, 1 hour, and 3 hours per day for 2 consecutive days (Figure 6D; n = 3, **P30 minutes < 0.01; **P60 minutes < 0.01; *P2 days < 0.05).
Figure 6.
HIF-1α translocation and quantitative real-time PCR analyses. HIF-1α expression in normal (A) and noise-exposed (B) mice is shown with an antibody against HIF-1α (green), nuclei stained with propidium iodide (red), and vessels stained with isolectin (blue). A: No or little immunoreactivity is detected in the vascular cells of the stria vascularis in normal mice. B and C: Increased immunoreactivity for HIF-1α is found in ECs, pericytes, and MCs in noise-exposed mice. HIF-1α activation and translocation to the nucleus of ECs (B, EC/arrow) and nuclei of pericytes (B, EC/arrow) as well as nonvascular MCs (B, MC/arrow) as early as 30 minutes after noise exposure and remains active after 2 days of noise exposure (C). D: Quantitative real-time PCR analysis shows mRNA levels for Hif-1α at different time points after noise exposure (n = 3, **P30 minutes < 0.01; **P60 minutes < 0.01; *P2 days < 0.05). V, vessel; PCs, pericytes; NE, noise exposure.
Noise Induced Up-Regulation of VEGF Associated with HIF-1α Activation in Mice
VEGF production can be induced in cells that are not receiving enough oxygen and then it can bind to VEGF receptors on vascular cells, triggering a tyrosine kinase pathway leading to vascular remodeling.42 We hypothesize that the alteration in pericyte coverage may involve VEGF up-regulation and HIF-1α regulatory mechanisms. In this experiment, with immunohistochemical technique, we found VEGF constitutively expressed in various cells in the stria vascularis, including both vascular cells (pericytes and ECs) and nonvascular cells such as MCs in the control mice (Figure 7A; V/arrow, MC/arrow). In contrast, increased expression of VEGF was found in those cells in noise-exposed mice, (Figure 7B; V/arrow, MC/arrow). However, in animals pretreated with a HIF-1α inhibitor, sodium butyrate (SB), the expression of VEGF was significantly reduced (Figure 7C; V/arrow, MC/arrow). A significant statistical difference in expression of VEGF in the different groups was found for the VEGF expression shown in Figure 7D (ncontrol = 3; nNE = 6; nNE+SB = 4; **P < 0.01). Consistent with the results from immunofluorescence label for VEGF, the amount of VEGF protein in the stria vascularis analyzed by Western blot in the control, noise-exposed (NE) and SB-treated noise-exposed (NE+SB) groups also had a significant difference (Figure 7E). With quantitative real-time PCR, the mRNA level for Vegf was found to be increased as early as 30 minutes of noise exposure and remained at an elevated level after prolonged noise exposures (Figure 7F; n = 10, **P30 minutes < 0.01; **P60 minutes < 0.01; **P2 days < 0.01). In addition, as expected, mRNA for Vegf was significantly down-regulated when the noise-exposed mice were pretreated with a HIF-1α.inhibitor, sodium butyrate (SB) (Figure 7F; n = 10, *P2 days+SB < 0.05), suggesting that the mRNA for Vegf was regulated by HIF-1α.
Figure 7.
VEGF immunoreactivity, Western blot, and quantitative real-time PCR analysis. A: Expression of VEGF is seen in normal mice. Increased expression of VEGF is seen in the noise-exposed mice (B, ncontrol = 3; nNE = 6; nNE+SB = 4; **P < 0.01). C: Reduced expression of VEGF in sodium butyrate-pretreated noise-exposed mice. E: Western blot analysis shows the amounts of VEGF protein in the different groups. Quantitative real-time PCR (D and F) shows mRNA level for Vegf is significantly increased after noise exposure, but suppressed with the inhibition of HIF-1α activity (n = 10, **P30 minutes < 0.01; **P60 minutes < 0.01; **P2 days < 0.01). NE, noise exposure; SB, sodium butyrate. Arrows in A, B, and C indicate marginal cells (MC) and vessels (V).
Noise Induced Increased Pericyte Coverage Associated with Increased HIF-1α and VEGF Activities in Mice
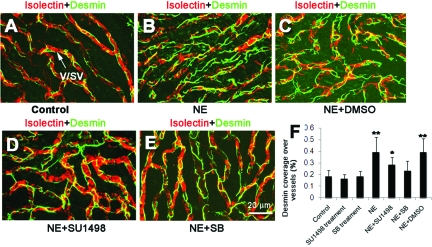
In this study, we determined whether increased pericyte coverage was related to HIF-1α induction and up-regulation of VEGF. The desmin coverage was assessed in normal mice (Figure 8A), noise-exposed mice (NE, Figure 8B), DMSO vehicle-pretreated noise-exposed mice (NE+DMSO, Figure 8C), SU1498-pretreated noise-exposed mice (NE+SU1498, Figure 8D), and sodium butyrate (SB)-pretreated animals (NE+SB, Figure 8E). We also assessed pericyte coverage in the stria vascularis of SU1498- and SB-treated normal (no noise exposure) mice as control groups (data not shown). We found that inhibition of either HIF-1α or VEGF activity could significantly suppress noise-induced increased pericytes coverage. Although SU1498 treatment did not completely reduce increased pericyte coverage, there was a significant difference between the nontreated noise-exposed animals and the SU1498-treated noise-exposed animals. SU1498 and SB treatment alone had no significant effect on pericyte coverage in nonnoise-exposed normal mice (Figure 8F; ncontrol = 5; nnoise = 7; nNE+SU1498 = 4; nNE+SB = 4; **P < 0.01; *P < 0.05).
Figure 8.
Quantification of pericyte coverage over vessels in different groups. A to E were stained with an antibody against desmin (green) to label pericytes and isolectin IB4 (red) to label vessels. Desmin coverage in normal mice (A), noise-exposed mice (B), DMSO vehicle-pretreated noise-exposed mice (C). SU1498-pretreated noise-exposed mice (D) and sodium butyrate (SB)-pretreated noise-exposed mice (E). Statistical analysis on pericyte coverage in the different groups (F) (ncontrol = 5; nnoise = 7; nNE+SU1498 = 4; nNE+SB = 4; **P < 0.01; *P < 0.05). NE, noise exposure; SB, sodium butyrate). Arrow in A indicates the stria vascularis (v/sv).
Discussion
This is the first report demonstrating noise-induced changes in cochlear pericytes in vivo through HIF-1α induction and up-regulation of VEGF.
Noise Damages the Blood-Labyrinth Barrier and Induces Vascular Leakage
Sound trauma significantly impairs the cochlear blood–labyrinth barrier (CLB). Noise causes significant hearing loss, and associated effects include disruption of the CLB and increased vascular permeability.38,45 Changes in the ultrastructure can be seen with transmission electron microscopy. In normal animals, pericytes are closely associated with ECs, with their processes tightly positioned adjacent to the endothelium (Figure 2, F and G). The pericytes and ECs share BL and are tightly coupled (Figure 2, H and I). The endothelium is functionally intact, indicated by absence of immunofluorescence for serum protein IgG leaked from capillaries (Figure 2, J and K). By contrast, animals exposed to wide-band noise at the level of 120 dB 3 hours/day for 2 consecutive days showed edema in the stria vascularis and swelling of ECs. The intercellular spaces between the ECs and pericytes were enlarged. The cytoplasm of ECs had vacuoles. Pericytes were irregularly positioned along the vessel walls of the stria vascularis, with foot processes that split the BL into several sheets and appeared as electron-transparent zones (Figure 5F). Serum proteins IgG and albumin (data not shown for albumin) were found leaking from vessels in the stria vascularis (Figure 2, R and S).
Complex physical and biochemical sequelae are likely responsible for the disruption of the CBL. Cochlear pericytes are a major component of CLB structure, and abnormal interaction between the ECs and pericytes may be significantly contributing to the noise induced vascular leakage. In addition to the physical deterioration of the CLB, up-regulation of VEGF may also be increasing vascular permeability. VEGF is known to be a potent angiogenic and vascular permeability factor.46,47 Increased vascular permeability, induced by VEGF, is reported in different microvasculatures, including the lung.48,49 Our data are consistent with those of Picciotti and colleagues50 and Selivanova and colleagues,51 who found that noise stimulation significantly increases VEGF expression in the stria vascularis.50
Noise Increases Expression of Desmin in Pericytes
The major finding of this study is that noise trauma increases the expression of desmin, a structural protein, in pericytes. In addition, the numbers of pericytes on vessel walls is increased by noise. The results are robust, found in both guinea pig and mice.
Desmin is a muscle- and pericyte-specific protein. It is a key subunit of intermediate filaments in cardiac, skeletal, and smooth muscle, critical to the structural and mechanical integrity of the contractile apparatus in muscle tissues.52,53 Results from mice deficient in desmin reveal the fundamental role desmin filaments play in cell architecture and force transmission. Mice lacking desmin develop cardiomyopathy with increased heart weight.54
The cochlear microvasculature is a vascular network dense with pericytes.10 However, unlike pericytes on the capillaries of the cochlear spiral ligament and other organs, pericytes on the vessels of the stria vascularis do not express contractile proteins such as α-SMA or tropomyosin. Instead, they are rich in desmin.10 Because pericytes are a major component of the vessel wall, desmin is thought to play a role integral to the mechanical strength of strial vessels.
Change in vascular architecture is known to be correlated with blood flow and vascular stability.55 Increased expression of desmin protein may be an adaptive strategy for coping with the physiological challenges imposed by loud sound. Higher internal pressure in capillaries may require more pericyte coverage to maintain vascular integrity.21 Blood flow in the stria vascularis is known to be slower than in the straight vessels of the spiral ligament,5 a flow configuration providing for effective exchange of oxygen and metabolites in cells such as MCs and ICs. High metabolic rates are needed to maintain the high-voltage endolymph potential.
Noise significantly impairs cochlear blood flow by vasoconstriction and blood stagnation in capillaries. Although it is unknown how this impairment occurs, mechanical strain, metabolic stress, or both are thought to be involved.7,56,57,58 Perturbation of blood flow caused by irregular vasomotion, such as overcontraction59 or dilation by a locally increased substrate of prostacyclin, NO, hemokines, and adhesion molecules,9,38 could change hydrostatic pressure and generate a pressure overload on the capillary networks. Shear forces on cells of the lateral wall during loud sound may also be important. Increased pericyte structural proteins may provide the physical strength that prevents the capillary network from deformation and loss of integrity under the forces of sound and osmotic pressure.
Increased pericyte coverage may also increase intercellular communication and material exchange between cells. Pericytes contact ICs through gap junctions. Pericytes have been shown to be dye-coupled to surrounding intermediate, basal, and capillary ECs.60 These coupled cells exchange ions, metabolites, and messengers through the gap junctions. Increased coverage may also be increasing signal transmission and interaction between ECs and ICs.
Disruption of normal endothelial-pericyte interactions may profoundly affect subsequent remodeling. The increased desmin may have a functional role in remodeling the blood-labyrinth barrier, particularly given the central role pericytes play in vascular stability, structural integrity, and angiogenesis in general. Pericytes prevent disruption of the endothelial barrier under hypoxic stress by contributing to cell-to-cell contacts and gap junctions between ECs.61
Involvement of HIF-1α and VEGF
Insufficient blood supply after noise exposure results in a drop in pO2 leaving a highly hypoxic environment in the cochlea.7,43,62 Induction of HIF-1α plays an essential role in triggering protective metabolic changes in response to oxygen deprivation. Up-regulation of VEGF expression in hypoxia is signaled through HIF-1α.30,34 We found that HIF-1α translocation and VEGF up-regulation also play a mediary role in noise-induced damage. Suppressing HIF-1α activity with sodium butyrate, a histone deacetylase inhibitor,19,63 down-regulated VEGF at both the transcription and protein levels (Figure 7). Pretreatment with an inhibitor for the VEGF receptor significantly reduced desmin expression (Figure 8D). Direct inhibition of HIF-1α also significantly suppressed desmin expression (Figure 8E). Our data suggest that alteration in pericyte coverage involves HIF-1α-induced VEGF regulatory mechanisms. Nonetheless, the specific mechanism involved in VEGF-related up-regulation of desmin remains unknown.
Noise increased the level of Hif-1α, Vegf, and Desmin transcripts in the cochlear lateral wall. Transcripts for Vegf and Desmin were found as early as 30 minutes after exposure. This is consistent with the view that a hypoxic environment is induced in the cochlea by noise immediate with the sound trauma.6,32 However, the increased mRNA level measured may not be exclusively of vascular origin. Nonvascular cells in the cochlear lateral wall such as MCs, ICs, BCs, and FCs may also be contributing. A further study will be required to determine changes in mRNA to loud sound localized to specific cells. Nevertheless, real-time PCR data from whole lateral wall showing a significant increase in the three gene products serves as additional evidence of increased HIF-1α and VEGF activity.
These findings support a hypothesis that noise provokes a homeostatic reaction to a hypoxic perturbation in the stria vascularis. In the hypoxia condition, HIF-1α is less degraded and stabilized. Accumulated stabilized HIF-1α translocates to the nucleus and then binds to hypoxia-response elements in the promoters of many genes including the Vegf gene.64 Specially, HIF-1α binds to the 5′-flanking region of the Vegf gene and not only stabilizes mRNA Vegf, but also increases its transcription rate, in which VEGF can be significantly increased.64,65 Consequentially, increased VEGF mediates pericyte remodeling such as structural protein desmin up-regulation through unknown mechanisms. These concepts are summarized diagrammatically in the model shown in Figure 9. The cellular pathways linking VEGF and the remodeling of pericytes dependent on vascular integrity, and specifically desmin expression, are yet to be explored.
Figure 9.
A model for the mechanism of noise-induced desmin up-regulation. Noise causes a perturbation (mechanical and metabolic stress), which promotes HIF-1α accumulation and translocation to the nucleus, leading to altered Vegf gene transcription and increased VEGF protein expression. Consequentially, increased VEGF mediates pericyte remodeling such as structural protein desmin up-regulation. V/SV, vessel of the stria vascularis.
In conclusion, pericytes are local regulatory cells, important for homeostasis and the maintenance of vascular integrity. Noise exposure increases the coverage of pericytes on vessels. We speculate about the importance of pericyte adaptive functions in remodeling cochlear vessels and in mediating regional microvessel structural stability under acoustic trauma. Ischemia from HIF-1α expression on noise exposure may be altering pericyte structure through VEGF up-regulation.
Acknowledgments
I thank Jackie DeGagne (Oregon Hearing Research Center, Oregon Health and Science University) for her assistance with transmission electron microscopy; Dr. Wen Xue Tang (Department of Otolaryngology, Yerkes Microarray Core, Emory University School of Medicine, Atlanta, GA) for his assistance with Western blot; Irina Omelchenko (Oregon Hearing Research Center, Oregon Health and Science University) for the auditory function tests (auditory brain-stem response); Dr. Jiaqing Pang (Oregon Health and Science University School of Medicine) for his contribution on real-time PCR analysis; Dr. Alfred Nuttall (Oregon Hearing Research Center, Oregon Health and Science University) for his critical review and valuable discussion of the manuscript; and Ms. Janice Moore for her edits on this manuscript.
Footnotes
Address reprint requests to Xiaorui Shi, M.D., Ph.D., Oregon Hearing Research Center, Oregon Health and Science University, 3181 SW Sam Jackson Park Rd., NRC04, Portland OR 97239-3098. E-mail: shix@ohsu.edu.
Supported by the National Institute of Deafness and Other Communications Disorders (grants R01 DC000141, R03 DC008888-02, and P30 DC005983).
References
- Kellerhals B. Perilymph production and cochlear blood flow. Acta Otolaryngol. 1979;87:370–374. doi: 10.3109/00016487909126435. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res. 1996;100:80–100. doi: 10.1016/0378-5955(96)00106-2. [DOI] [PubMed] [Google Scholar]
- Wangemann P. Potassium ion secretion and generation of the endocochlear potential in the stria vascularis. HNO. 1997;45:205–209. doi: 10.1007/s001060050105. [DOI] [PubMed] [Google Scholar]
- Salt AN, Melichar I, Thalmann R. Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope. 1987;97:984–991. [PubMed] [Google Scholar]
- Nuttall AL. Velocity of red blood cell flow in capillaries of the guinea pig cochlea. Hear Res. 1987;27:121–128. doi: 10.1016/0378-5955(87)90013-x. [DOI] [PubMed] [Google Scholar]
- Lamm K, Arnold W. Noise-induced cochlear hypoxia is intensity dependent, correlates with hearing loss and precedes reduction of cochlear blood flow. Audiol Neurootol. 1996;1:148–160. doi: 10.1159/000259195. [DOI] [PubMed] [Google Scholar]
- Nuttall AL. Sound-induced cochlear ischemia/hypoxia as a mechanism of hearing loss. Noise Health. 1999;2:17–31. [PubMed] [Google Scholar]
- Axelsson A, Dengerink H. The effects of noise on histological measures of the cochlear vasculature and red blood cells: a review. Hear Res. 1987;31:183–191. doi: 10.1016/0378-5955(87)90125-0. [DOI] [PubMed] [Google Scholar]
- Shi X, Nuttall AL. Expression of adhesion molecular proteins in the cochlear lateral wall of normal and PARP-1 mutant mice. Hear Res. 2007;224:1–14. doi: 10.1016/j.heares.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Shi X, Han W, Yamamoto H, Tang W, Lin X, Xiu R, Trune DR, Nuttall AL. The cochlear pericytes. Microcirculation. 2008;15:515–529. doi: 10.1080/10739680802047445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström M, Gerhardt H, Kalén M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Flores L, Guitierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 1991;6:269–286. [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson J. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53:637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005;94:115–125. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, Damore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ. Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab. 2006;26:209–217. doi: 10.1038/sj.jcbfm.9600181. [DOI] [PubMed] [Google Scholar]
- Braun A, Xu H, Hu F, Kocherlakota P, Siegel D, Chander P, Ungvari Z, Csiszar A, Nedergaard M, Ballabh P. Paucity of pericytes in germinal matrix vasculature of premature infants. J Neurosci. 2007;27:12012–12024. doi: 10.1523/JNEUROSCI.3281-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Imaizumi T. Pericyte biology and diseases. Int J Tissue React. 2005;27:125–135. [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Zhang X, Adamson RH, Curry FE, Weinbaum S. Transient regulation of transport by pericytes in venular microvessels via trapped microdomains. Proc Natl Acad Sci USA. 2008;105:1374–1379. doi: 10.1073/pnas.0710986105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner O, Tsimpas A, Kostin S, Valable S, Petit E, Schaper W, Marti HH. Time- and cell type-specific induction of platelet-derived growth factor receptor-beta during cerebral ischemia. Brain Res Mol Brain Res. 2003;113:44–51. doi: 10.1016/s0169-328x(03)00085-8. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Li L, Cunningham LA. Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab. 2006;26:545–555. doi: 10.1038/sj.jcbfm.9600214. [DOI] [PubMed] [Google Scholar]
- Duz B, Oztas E, Erginay T, Erdogan E, Gonul E. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: an ultrastructural study. Cryobiology. 2007;55:279–284. doi: 10.1016/j.cryobiol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Lamm K, Arnold W. The effect of blood flow promoting drugs on cochlear blood flow, perilymphatic pO(2) and auditory function in the normal and noise-damaged hypoxic and ischemic guinea pig inner ear. Hear Res. 2000;141:199–210. doi: 10.1016/s0378-5955(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18:2183–2194. doi: 10.1101/gad.1243304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268:21513–21518. [PubMed] [Google Scholar]
- Löfstedt T, Fredlund E, Holmquist-Mengelbier L, Pietras A, Ovenberger M, Poellinger L, Påhlman S. Hypoxia inducible factor-2alpha in cancer. Cell Cycle. 2007;6:919–926. doi: 10.4161/cc.6.8.4133. [DOI] [PubMed] [Google Scholar]
- van Weel V, van Tongeren RB, van Hinsbergh VWM, van Bockel JH, Quax PHA. Vascular growth in ischemic limbs: a review of mechanisms and possible therapeutic stimulation. Ann Vasc Surg. 2008;22:582–597. doi: 10.1016/j.avsg.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Birk D, Barbato J, Mureebe L, Chaer RA. Current insights on the biology and clinical aspects of VEGF regulation. Vasc Endovascular Surg. 2008;42:517–530. doi: 10.1177/1538574408322755. [DOI] [PubMed] [Google Scholar]
- Rissanen TT, Korpisalo P, Markkanen JE, Liimatainen T, Orden M-R, Kholova I, de Goede A, Heikura T, Grohn OH, Yla-Herttuala S. Blood flow remodels growing vasculature during vascular endothelial growth factor gene therapy and determines between capillary arterialization and sprouting angiogenesis. Circulation. 2005;112:3937–3946. doi: 10.1161/CIRCULATIONAHA.105.543124. [DOI] [PubMed] [Google Scholar]
- Shi XR, Nuttall AL. Upregulated iNOS and oxidative damage to cochlear stria vascularis due to noise stress. Brain Res. 2003;967:1–10. doi: 10.1016/s0006-8993(02)04090-8. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Saishin Y, Saishin Y, Takahashi K, Melia M, Vinores SA, Campochiaro PA. Inhibition of protein kinase C decreases prostaglandin-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:210–219. doi: 10.1002/jcp.10238. [DOI] [PubMed] [Google Scholar]
- Cebulla C, Jockovich M, Boutrid H, Pina Y, Ruggeri M, Jiao S, Bhattacharya S, Feuer W, Murray T. Lack of effect of SU1498, an inhibitor of vascular endothelial growth factor receptor-2, in a transgenic murine model of retinoblastoma. Open Ophthalmol J. 2008;2:62–67. doi: 10.2174/1874364100802010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Kanno S, Oda N, Abe M, Ito M, Shitara K, Shibuya M. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann NY Acad Sci. 2000;902:201–205. doi: 10.1111/j.1749-6632.2000.tb06314.x. [DOI] [PubMed] [Google Scholar]
- Lamm K, Arnold W. The effect of prednisolone and non-steroidal anti-inflammatory agents on the normal and noise-damaged guinea pig inner ear. Hear Res. 1998;115:149–161. doi: 10.1016/s0378-5955(97)00186-x. [DOI] [PubMed] [Google Scholar]
- Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- Goldwyn BG, Quirk WS. Calcium channel blockade reduces noise-induced vascular permeability in cochlear stria vascularis. Laryngoscope. 1997;107:1112–1116. doi: 10.1097/00005537-199708000-00019. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Nakao M, Kaga K. Interfield differences in intensity and frequency representation of evoked potentials in rat auditory cortex. Hear Res. 2005;210:9–23. doi: 10.1016/j.heares.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue L, Tyburski JG, Steffes CP, Wilson RF. Vascular endothelial growth factor modulates contractile response in microvascular lung pericytes. Am J Surg. 2006;191:349–352. doi: 10.1016/j.amjsurg.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Kim C, Huh A, Kim M, Chin B, Han S, Choi S, Jeong S, Choi H, Choi J, Song Y, Kim J. LPS-induced vascular endothelial growth factor expression in rat lung pericytes. Shock. 2008;30:92–97. doi: 10.1097/SHK.0b013e31815d19ad. [DOI] [PubMed] [Google Scholar]
- Picciotti PM, Fetoni AR, Paludetti G, Wolf FI, Torsello A, Troiani D, Ferraresi A, Pola R, Sergi B. Vascular endothelial growth factor (VEGF) expression in noise-induced hearing loss. Hear Res. 2006;214:76–83. doi: 10.1016/j.heares.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Selivanova O, Heinrich U-R, Brieger J, Feltens R, Mann W. Fast alterations of vascular endothelial growth factor (VEGF) expression and that of its receptors (Flt-1, Flk-1 and Neuropilin) in the cochlea of guinea pigs after moderate noise exposure. Eur Arch Otorhinolaryngol. 2007;264:121–128. doi: 10.1007/s00405-006-0154-3. [DOI] [PubMed] [Google Scholar]
- Stewart M. Intermediate filament structure and assembly. Curr Opin Cell Biol. 1993;5:3–11. doi: 10.1016/s0955-0674(05)80002-x. [DOI] [PubMed] [Google Scholar]
- Goldfarb LG, Park KY, Cerevenakova L, Gorokhova S, Lee HS, Vasconcelos O, Nagle JW, Semino-Mora C, Sivakumar K, Dalakas MC. Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nat Genet. 1998;19:402–403. doi: 10.1038/1300. [DOI] [PubMed] [Google Scholar]
- Balogh J, Merisckay M, Li Z, Paulin D, Arner A. Hearts from mice lacking desmin have a myopathy with impaired active force generation and unaltered wall compliance. Cardiovasc Res. 2002;53:439–450. doi: 10.1016/s0008-6363(01)00500-4. [DOI] [PubMed] [Google Scholar]
- Duvall AJD, Robinson KS. Local vs systemic effects of acoustic trauma on cochlear structure and transport. Arch Otolaryngol Head Neck Surg. 1987;113:1066–1071. doi: 10.1001/archotol.1987.01860100044019. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Axelsson A, Myers R, Woolf NK. Changes in cochlear blood flow during acoustic stimulation as determined by 14C-iodoantipyrine autoradiography. Acta Otolaryngol. 1988;105:232–241. doi: 10.3109/00016488809097003. [DOI] [PubMed] [Google Scholar]
- Quirk WS, Avinash G, Nuttall AL, Miller JM. The influence of loud sound on red blood cell velocity and blood vessel diameter in the cochlea. Hear Res. 1992;63:102–107. doi: 10.1016/0378-5955(92)90079-3. [DOI] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Konishi K, Sakamoto H, Matsuda Y, Iguchi H. Strial circulation impairment due to acoustic trauma. Acta Otolaryngol. 1991;111:85–93. doi: 10.3109/00016489109137358. [DOI] [PubMed] [Google Scholar]
- Hawkins JE., Jr The role of vasoconstriction in noise-induced hearing loss. Ann Otol Rhinol Laryngol. 1971;80:903–913. doi: 10.1177/000348947108000617. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Ando M. Dye-coupling of melanocytes with endothelial cells and pericytes in the cochlea of gerbils. Cell Tissue Res. 1998;293:271–275. doi: 10.1007/s004410051118. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Christer B. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Maass B, Baumgartl H, Lubbers DW. Local pO2- and pH2-measurements with needle electrodes for the examination of the hydrogen maintenance and microcirculation of the cochleae (author’s transl). Arch Otorhinolaryngol. 1976;214:109–124. doi: 10.1007/BF00453607. [DOI] [PubMed] [Google Scholar]
- Miki K, Unno N, Nagata T, Uchijima M, Konno H, Koide Y, Nakamura S. Butyrate suppresses hypoxia-inducible factor-1 activity in intestinal epithelial cells under hypoxic conditions. Shock. 2004;22:446–452. doi: 10.1097/01.shk.0000140664.80530.bd. [DOI] [PubMed] [Google Scholar]
- Levy NS, Chung S, Furneaux H, Levy AP. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem. 1998;273:6417–6423. doi: 10.1074/jbc.273.11.6417. [DOI] [PubMed] [Google Scholar]
- Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]