Abstract
To examine the functional significance and morphological characteristics of starvation-induced autophagy in the adult heart, we made green fluorescent protein-microtubule-associated protein 1-light chain 3 (LC3) transgenic mice starve for up to 3 days. Electron microscopy revealed round, homogenous, electron-dense lipid droplet-like vacuoles that initially appeared in cardiomyocytes as early as 12 hours after starvation; these vacuoles were identified as lysosomes based on cathepsin D-immunopositive reactivity and acid phosphatase activity. The increase in the number of lysosomes depended on the starvation interval; typical autophagolysosomes with intracellular organelles also appeared, and their numbers increased at the later phases of starvation. Myocardial expression of autophagy-related proteins, LC3-II, cathepsin D, and ubiquitin, increased, whereas both myocardial ATP content and starvation integral decreased. Treatment with bafilomycin A1, an autophagy inhibitor, did not affect cardiac function in normally fed mice but significantly depressed cardiac function and caused significant left ventricular dilatation in mice starved for 3 days. The cardiomyocytes were occupied with markedly accumulated lysosomes in starved mice treated with bafilomycin A1, and both the myocardial amino acid content, which was increased during starvation, and the myocardial ATP content were severely decreased, potentially contributing to cardiac dysfunction. The present findings suggest a critical role of autophagy in the maintenance of cardiac function during starvation in the adult.
Autophagy is a type of programmed cell death that occurs during tissue and organ development to eliminate unnecessary cells,1,2,3 but it also has survival-oriented functions, occurring under both basal conditions and conditions of stress, such as starvation.4,5,6 During autophagic degeneration, degraded membrane lipids and proteins within autophagosomes are recruited to maintain needed levels of ATP production and protein synthesis, thereby promoting cell survival. Autophagic cell death is actually a morphological term derived from electron microscopic observations and denotes a form of cell death in which abundant autophagic vacuoles appear in the cytoplasm. But this descriptive account tells us nothing about the pathophysiological significance of autophagy. Consequently, although cardiomyocytes exhibiting autophagic characteristics have recently been reported in failing hearts, suggesting autophagic hyperfunction,7,8,9,10,11 it is still undetermined whether an abnormal increase in autophagic vacuoles is the primary cause of cardiomyocyte death in heart failure, or even whether it usually reflects hyperfunction or hypofunction of the autophagic process. Moreover, accumulation of autophagic vacuoles can be caused by an impairment of digestion of the vacuolar contents because of lysosomal dysfunction, eg, Danon disease.12,13 Abnormal accumulation of autophagic vacuoles is properly recognized as a hypofunction, but not a hyerfunction, of the autophagic process in such a case. Such complications make it challengingly difficult to evaluate the actual pathophysiological significance of autophagy.14
Kuma and colleagues15 revealed a critically important role of autophagy in the survival of neonatal mice during the starvation period just after birth; Atg5-knockout mice with disturbed autophagic functional activity resulted in a deficiency of amino acids and energy that shortened the survival period of the mice. According to their report, autophagy was strongly induced during the early neonatal period in various organs of wild-type mice and interestingly enough, the induction was particularly massive in the heart. In the adult heart, however, the actual significance of autophagy that takes place in the basal state or in response to starvation has not been well understood. The aim in the present study was to morphologically and molecular biologically characterize starvation-induced autophagy and to determine its functional significance in the adult heart. For that purpose, we observed hearts of transgenic mice in which microtubule-associated protein 1 light chain 3 (LC3), a constituent of the autophagosome membrane, is labeled with green fluorescent protein (GFP).16 These mice were normally fed or starved for various periods up to 3 days. We next used bafilomycin A1 to inhibit the later digestion process of autophagy,17,18 and assessed the morphological and functional changes in the heart.
Materials and Methods
Animals and Experimental Protocols
This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Publication No. 85-23, revised 1996) and was approved by our institutional animal research committee. Pathogen-free heterozygous GFP-LC3 transgenic mice (strain GFP-LC3#53) (Riken BioResource Center, Tsukuba, Japan) containing a rat LC3-eGFP fusion construct under control of the chicken β-actin promoter16 were housed in a temperature-controlled environment with 12-hour light/dark cycles, and received food and water ad libitum. Genotyping of GFP-LC3 offspring was performed by polymerase chain reaction (PCR) analysis using the GFP-LC3 primers (5′-TCCTGCTGGAGTTCGTGACCG-3′ and 5′-TTGCGAATTCTCAGCCGTCTTCATCTCTCTCGC-3′; yielding a 400-bp PCR product) and the mouse LC3 internal control primers (5′-TGAGCGAGCTCATCAAGATAATCAGGT-3′ and 5′-GTTAGCATTGAGCTGCAAGCGCCGTCT-3′; yielding a 500-bp PCR product). For starvation studies, mice (8 to 10 weeks of age) were deprived of food for 12 hours, 1 day, 2 days, or 3 days (n = 6 to 9), but had free access to drinking water. Autophagy inhibition studies were performed by the intraperitoneal administration of BafA1 (Sigma, St. Louis, MO) at 0.3 mg/kg/day for 3 days to the normally fed (n = 6) or starved mice (n = 15). BafA1, one of the cell-permeable lysosomal inhibitors, is a vacuolar H+-ATPase inhibitor and inhibits autophagosome-lysosome fusion to prevent the final digestion step of autophagy.17,18 The same volume of saline was given to the normally fed (n = 6) or starved mice (n = 14) that served as the controls.
Physiological Studies
Physiological studies including echocardiography and cardiac catheterization were performed before sacrifice as described previously, with certain modifications.19 Animals were anesthetized with an intraperitoneal injection of pentobarbital (30 mg/kg). Echocardiograms were recorded using an echocardiographic system (Vevo770; Visualsonics, Toronto, Canada) equipped with a 45-MHz imaging transducer. After echocardiography, the right carotid artery was cannulated with a micromanometer-tipped catheter (SPR 671; Millar Instruments, Houston, TX) that was advanced into the aorta and then into the left ventricle for the recording of pressure and maximal and minimal dP/dt (±dP/dt).
Histology
Once the physiological measurements were complete, all mice were sacrificed, and the hearts were removed, weighed, and cut into two transverse slices through the middle of the ventricles between the atrioventricular groove and the apex. The basal specimens were fixed in 10% buffered formalin, embedded in paraffin or cryomold, cut into 4-μm-thick sections, and stained with hematoxylin and eosin, Masson’s trichrome, or Oil Red O. Cardiomyocyte size (expressed as the transverse diameter of myocytes cut at the level of the nucleus) was assessed in 20 randomly chosen high-power fields (×600) in each section.
Immunohistochemistry and Immunofluorescence
After deparaffinization, the 4-μm-thick sections were incubated with a primary antibody against GFP (Molecular Probes, Eugene, OR), cathepsin D (Santa Cruz Biotechnology, Santa Cruz, CA), or ubiquitin (DAKO Japan, Kyoto, Japan). A Vectastain Elite ABC system (Vector Laboratories, Burlingame, CA) was then used to immunostain the sections; diaminobenzidine served as the chromogen, and the nuclei were counterstained with hematoxylin. For immunofluorescence, the primary antibody against GFP was subsequently labeled with an Alexa 488-conjugated secondary antibody (Molecular Probes). Rhodamine-phalloidin (Invitrogen, Carlsbad, CA) was overlaid on the GFP-immunofluorescent section to document cardiomyocytes. Sections were then counterstained with Hoechst 33342 and observed under confocal microscopy (LSM510; Zeiss, Wetzlar, Germany). Quantitative assessments, including the number of immunopositive cells or immunopositive dots, were performed in 20 randomly chosen high-power fields using a multipurpose color image processor.
In situ terminal dUTP nick end-labeling (TUNEL) assays were performed with sections using an ApopTag kit (Chemicon, Temecula, CA) according to the manufacturer’s instructions. In addition, to separately evaluate apoptosis of cardiomyocytes and that of noncardiomyocytes in the heart, we performed double immunofluorescence for TUNEL and myoglobin. Tissue sections were first stained with Fluorescein-FragEL (Calbiochem, La Jolla, CA) and then labeled with anti-myoglobin antibody (DAKO) followed by Alexa Fluor 568. Nuclei were stained with Hoechst 33342. Mouse mammary tissue served as a positive control for the TUNEL assay.
Electron Microscopy
Cardiac tissue was quickly cut into 1-mm cubes, immersion-fixed with 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4) overnight at 4°C, and postfixed in 1% buffered osmium tetroxide. The specimens were then dehydrated through graded ethanol and embedded in epoxy resin. Ultrathin sections (90 nm), double-stained with uranyl acetate and lead citrate, were examined under electron microscopy (H-800; Hitachi, Tokyo, Japan).
Immunoelectron Microscopy for Cathepsin D
Immunoelectron microscopy was performed by the pre-embedding method for cathepsin D. In brief, after fixation in 4% paraformaldehyde in phosphate-buffered saline overnight, cardiac specimens were washed with 0.1 mol/L phosphate buffer containing 5% sucrose for 24 hours, and subsequently with the buffer containing 10%, 20%, and 40% sucrose for 4 hours each. Cryosections at the thickness of 20 μm were attached on the slide glasses and incubated with the primary antibody against cathepsin D (Santa Cruz Biotechnology) at a dilution of 1:100 overnight at 4°C. Next, they were incubated with horseradish peroxidase-conjugated F(ab′) 2 fragments of the secondary antibody for 1 hour. They were then postfixed with 1% osmium tetroxide for 30 minutes to make electron-dense osmium black precipitates, dehydrated with graded series of ethanol, and embedded with epoxy resin. Ultrathin sections (90 nm) were counterstained with uranyl acetate and lead citrate and examined under electron microscopy (H-700, Hitachi).
Enzyme Cytochemistry for Acid Phosphatase
Cytochemical study was performed to demonstrate acid phosphatase activity by the method of Robinson and Karrnovsky.20 Immediately after sampling, tissues were fixed for 1 hour at room temperature with 4% paraformaldehyde and 0.1% glutaraldehyde in 30 mmol/L PIPES buffer containing 7% sucrose (pH 7.2). After fixation, tissues were rinsed, embedded in cryomold, and cut into 40-μm-thick sections. The tissues were immersed in 10% dimethyl sulfoxide-containing buffer before frozen to avoid ice formation. Incubation was for 1 hour at 37°C to demonstrate acid phosphatase activity. The assay medium contained 0.1 mol/L acetate buffer (pH 5.0) with 1 mmol/L β-glycerophosphate (Sigma-Aldrich) as substrate, and 2 mmol/L cerium chloride (Wako Chemicals, Osaka, Japan) as tracer. Tissues were rinsed at 4°C in acetate buffer and serial dehydration in graded ethanols was followed by embedding in epoxy resin. Ultrathin sections (90 nm) were counterstained with uranyl acetate for 1 minute and examined under electron microscopy (H-700, Hitachi).
Western Blotting
Proteins (50 μg) extracted from hearts (n = 4 to 6 from each group) were subjected to 10 or 15% polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes. The membranes were then probed using primary antibodies against LC3 (MBL International, Woburn, MA), cathepsin D (Santa Cruz Biotechnology), and ubiquitin (DAKO Japan), after which the blots were visualized using enhanced chemiluminescence (Amersham, Arlington Heights, IL). α-Tubulin (analyzed using an antibody from Santa Cruz Biotechnology) served as the loading control.
Measurements of Blood Glucose and Serum-Free Fatty Acid Levels
Levels of blood glucose and serum-free fatty acid were measured by the standardized methods.
Measurement of Amino Acid Concentration in the Plasma and Myocardium
Plasma was deproteinized with 3% trichloroacetate. Hearts were weighed while frozen and homogenized in glass microhomogenizers with cold 5% sulfosalicylic acid. Free amino acids in the supernatants from plasma and tissue samples were measured using an automated amino acid analyzer (L8500, Hitachi). Amino acid concentration was expressed as the sum of concentrations of Asp, Thr, Ser, Asn, Glu, Gln, Pro, Gly, Ala, Val, Cys, Met, Ile, Leu, Tyr, Phe, Lys, His, and Arg.
ATP Measurement
Myocardial tissue ATP content was measured using an ATP bioluminescent assay kit (Toyo Ink, Tokyo, Japan) according to the manufacturer’s instructions. Experiments were performed in triplicate for each group.
Statistical Analysis
Data are expressed as the means ± SEM. The significance of differences between groups was evaluated using t-test or one-way analysis of variance with a posthoc Newman-Keul’s multiple comparisons test. Values of P < 0.05 were considered significant.
Results
Influence of Starvation on the Function, Morphology, and Autophagy-Related Molecules
For starvation studies, GFP-LC3 transgenic mice were deprived of food for 12 hours, 1 day, 2 days, or 3 days, but had free access to drinking water. The mice exposed to starvation as well as those normally fed all survived. The body weight of the mice subjected to starvation was decreased day by day (Table 1). No significant difference was observed in the left ventricular (LV) end-diastolic diameter (LVDd) and LV fractional shortening (LVFS) between the normally fed and starved groups (Table 1). Although heart rate was not affected by starvation, blood pressure indicated by LV peak systolic pressure was significantly lowered. Neither maximal nor minimal dP/dt, an indicator of LV systolic and diastolic function, respectively, was affected by starvation.
Table 1.
Influence of Starvation on Body Weight, Heart Weight, and Cardiac Function in Mice
| Baseline | Starvation periods (days)
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| n | 6 | 6 | 6 | 9 |
| BW, g | 25 ± 0.60 | 20 ± 0.15* | 20 ± 0.47* | 18 ± 0.18* |
| HW, mg | 113 ± 6 | 97 ± 6* | 93 ± 6* | 87 ± 6* |
| HW/BW, mg/g | 4.5 ± 0.04 | 4.8 ± 0.20 | 4.8 ± 0.07 | 4.9 ± 0.15* |
| LVDd, mm | 2.9 ± 0.04 | 3.0 ± 0.03 | 3.0 ± 0.03 | 2.9 ± 0.07 |
| LVDs, mm | 1.7 ± 0.08 | 1.6 ± 0.20 | 1.8 ± 0.03 | 1.7 ± 0.07 |
| LVFS, % | 43 ± 2.2 | 40 ± 1.5 | 38 ± 0.16 | 41 ± 1.9 |
| HR, bpm | 444 ± 16 | 431 ± 11 | 426 ± 9 | 421 ± 17 |
| LVSP, mm Hg | 121 ± 1.1 | N.A. | N.A. | 95 ± 2.7* |
| +dP/dt, mm Hg/s | 15,818 ± 870 | N.A. | N.A. | 14,833 ± 1994 |
| −dP/dt, mm Hg/s | −6397 ± 316 | N.A. | N.A. | −6419 ± 359 |
BW, body weight; HW, heart weight; HW/BW, heart-to-body weight ratio; LVDd and LVDs, left ventricular end-diastolic and end-systolic diameter; LVFS, left ventricular fractional shortening; HR, heart rate; LVSP, left ventricular peak systolic pressure.
P < 0.05 compared with the baseline value.
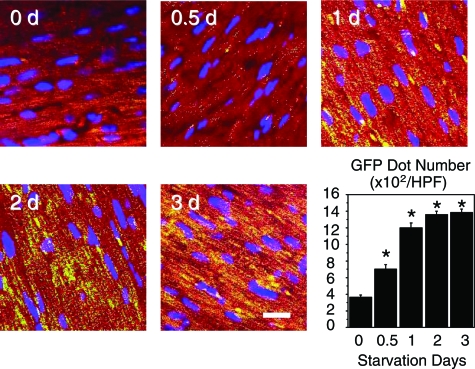
Although the heart weight decreased along with the starvation period, the heart-to-body weight ratio did not differ at 2 days but was significantly increased after starvation for 3 days (Table 1). The size of cardiomyocytes was not affected by starvation. The GFP puncta, which are developed in parallel to the LC3-II expression,16 significantly increased within cardiomyocytes depending on the starvation interval (Figure 1).
Figure 1.
GFP immunofluorescence (green) of the hearts of mice exposed to starvation for various intervals. Cardiomyocytes are stained red with rhodamine-phalloidin and the nuclei are stained blue with Hoechst 33342. Graph showing density (dots per cardiac tissue area) of GFP puncta. *P < 0.05 compared with the value at day 0 (one-way analysis of variance). Scale bar = 10 μm.
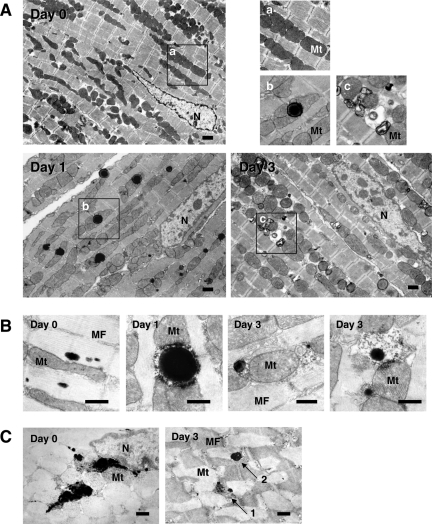
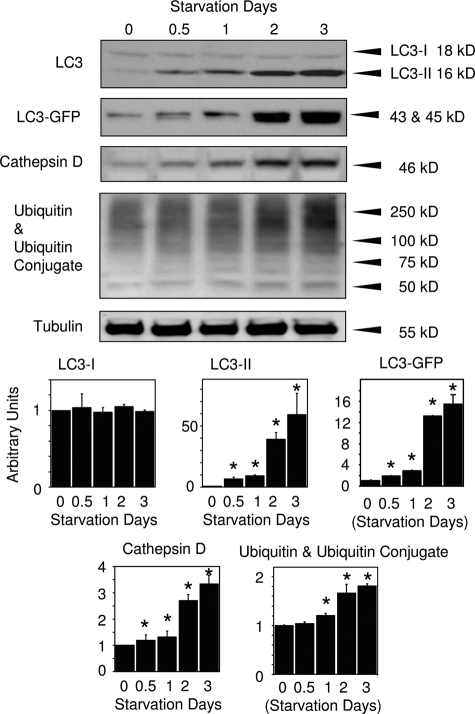
Electron microscopy revealed abundant vacuoles within cardiomyocytes of the starved mice as early as 12 hours after the initiation of starvation. Most vacuoles were homogenously electron-dense and appeared in the form of lipid droplets, and only a minority presented typical autophagosomes containing intracellular organelles, such as mitochondria and membrane-like structures, at the earlier starvation periods (up to 2 days) (Figure 2A). Although Oil Red O staining was performed on the cryosections because of presumed lipid droplets, the reaction was negative in any groups (data not shown), suggesting that those structures were not lipid droplets. Immunoelectron microscopy, on the other hand, revealed cathepsin D positivity (Figure 2B). Moreover, enzyme cytochemistry showed acid phosphatase activity on the vacuoles (Figure 2C). These indicate that the vacuoles presumed to be lipid droplets are in fact lysosomes. During the later stage of starvation (2 to 3 days), however, the ratio of the two types of the vacuoles turned out to be reversed; most were typical autophago(lyso)somes and some were lysosomes (Figure 2A). Another conspicuous finding was mitochondrial electron density; mitochondria in the cardiomyocyte appeared more electron-lucent in the starved mice compared with those in the normally fed mice (Figure 2A). According to the Western blot analyses, expression of LC3-II, LC3-GFP, cathepsin D, and ubiquitin, along with ubiquitinated proteins gradually increased along with the starvation interval, consistent with immunohistochemical findings (Figure 3).
Figure 2.
Electron microscopy, immunoelectron microscopy, and enzyme cytochemistry of cardiomyocytes from mice exposed to starvation for 0, 1, or 3 days. A: Electron microphotographs of cardiomyocytes from mice exposed to starvation for 0, 1, or 3 days. Most vacuoles were homogenously electron-dense at the earlier starvation periods (1 day later here), whereas during the later stage of starvation (3 days later), most of the vacuoles presented typical autophago(lyso)somes containing intracellular organelles such as mitochondria and membrane-like structures. As shown in the top right panels, mitochondria appeared more electron-lucent in the starved mice. N, nucleus; Mt, mitochondria. B and C: Immunoelectron microphotographs for cathepsin D (B) and enzyme cytochemistry for acid phosphatase (C). Cytoplasmic vacuoles were rarely seen in cardiomyocytes of the control animals (day 0), but immunopositive reaction of cathepsin D was observed as small dots among myofibrils and acid phosphatase activity was seen globally on lipofuscin granules as well as among myofibrils in the cardiomyocytes, ie, basal expressions. Cathepsin D expression and acid phosphatase activity showed similar distribution also during starvation; they localized on lipid droplet-like vacuoles at the early stage of starvation (day 1) and on autophagosomes at the late stage (day 3) in addition to the basal expressions. In the right panel of C, round vacuoles with (arrow 1) or without (arrow 2) degradation were found to have acid phosphatase activity (3 days after starvation). Scale bars: 1 μm (A); 500 nm (B, C).
Figure 3.
The effect of starvation on autophagy-related protein expression in hearts of mice. Western blots for LC3, LC3-GFP, cathepsin D, and ubiquitin along with ubiquitinated proteins in the hearts of mice exposed to starvation for various intervals. *P < 0.05 compared with the value at day 0 (one-way analysis of variance).
Influence of Autophagy Inhibition or Stimulation on Autophagy
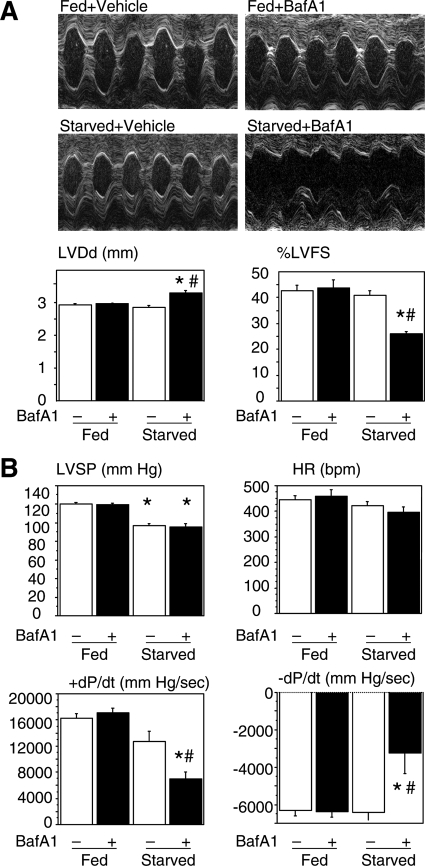
We treated mice with BafA1 at the dose of 0.3 mg/kg/day for 3 days to inhibit the final digestion step of the autophagy process and examined cardiac function by echocardiography and cardiac catheterization. Although BafA1 did not affect cardiac function in the normally fed mice, this reagent caused a significant cardiac dilatation (increase in left ventricular end-diastolic diameter) and hypofunction (decrease in %left ventricular fractional shortening and ±dP/dt) in the starved mice (Figure 4, A and B).
Figure 4.
Cardiac function assessed by echocardiography (A) and cardiac catheterization (B) of mice normally fed or starved for 3 days with or without BafA1. LVDd, LV end diastolic diameter; %FS, %fractional shortening; LVSP, LV peak systolic pressure; HR, heart rate. P < 0.05 compared with the fed groups (*) or with the starved group without BafA1 treatment (#) (one-way analysis of variance).
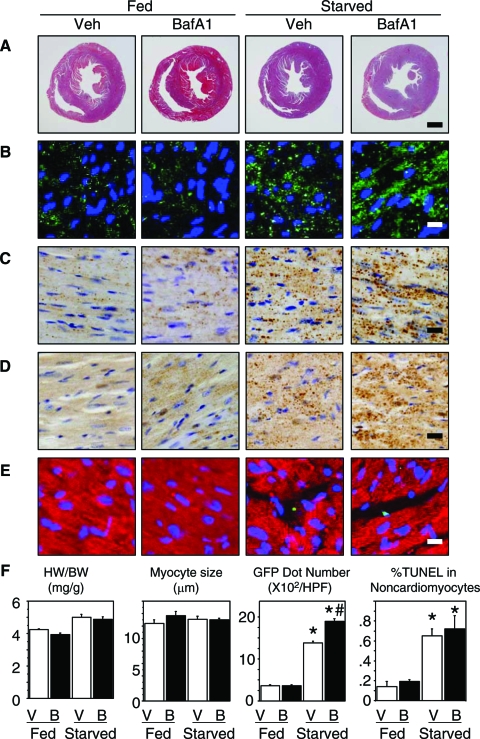
Treatment with BafA1 did not affect the heart-to-body weight ratio in either normally fed or starved mice. Necropsy revealed a biventricular dilatation accompanying biventricular wall thinning by the treatment with BafA1 in the starved, but not the normally fed mice (Figure 5A). Treatment with BafA1 did not affect the cardiomyocyte size in either normally fed or starved mice when assessed on H&E sections (Figure 5F). Treatment with BafA1 did not affect the immunohistochemical expression of GFP, cathepsin D, and ubiquitin in the heart of the normally fed mice but markedly augmented their expressions in the starved mice (Figure 5, B–D). TUNEL staining showed an increased level of apoptotic cells in the heart of the starved mice. The incidence of TUNEL-positive cardiomyocytes was extremely low in each group (0.048 ± 0.033% in the fed and untreated with BafA1; 0.049 ± 0.034% in the fed and treated with BafA1; 0.051 ± 0.037% in the starved and untreated with BafA1; 0.055 ± 0.038% in the starved and treated with BafA1) with no statistical difference between the groups and most of the TUNEL-positive cells in the heart were noncardiomyocytes such as interstitial and circulating cells (Figure 5E).
Figure 5.
Histological and immunohistochemical preparations of cardiac tissue from mice normally fed or starved for 3 days with or without BafA1. A: Transverse section of the hearts stained with Masson’s trichrome. B: GFP immunofluorescence (green dots) with nuclear stain by Hoechst 33342 (blue). C: Cathepsin D immunohistochemistry (brown dots). D: Ubiquitin immunohistochemistry (brown dots). E: Double immunofluorescence for TUNEL (green) and myoglobin (red). F: Graphs show the heart-to-body weight ratio, cardiomyocyte size, density of GFP puncta, and %TUNEL-positive noncardiomyocytes in each group. P < 0.05 compared with the fed groups (*) or with the starved group without BafA1 treatment (#) (one-way analysis of variance). Scale bars: 1 mm (A); 10 μm (B–E).
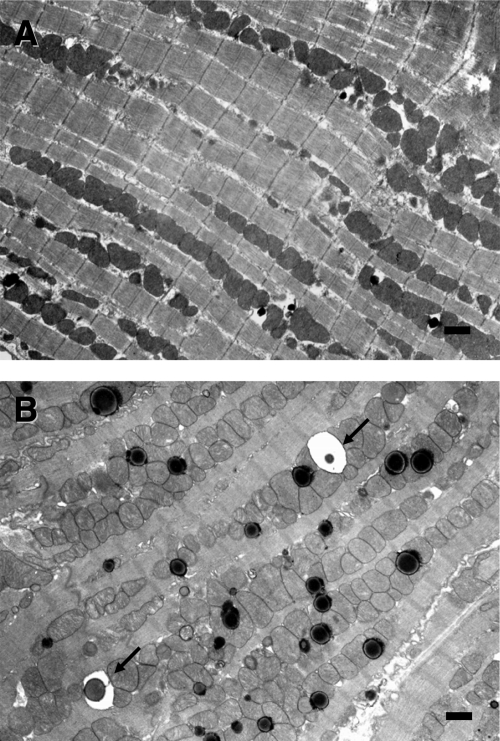
Electron microscopy revealed in cardiomyocytes of the 3-day starved mice treated with BafA1, a marked accumulation of vacuoles, most of which were electron-dense lysosomes whereas some of them contained almost intact or incompletely digested mitochondria and membrane structures (Figure 6, A and B). The ultrastructure of cardiomyocytes was not affected by the treatment with BafA1 in the normally fed mice.
Figure 6.
Electron microphotographs of cardiomyocytes from mice treated with BafA1: normally fed (A) and starved for 3 days (B). Arrows indicate autophagic vacuoles containing almost intact mitochondria. Scale bars = 1 μm.
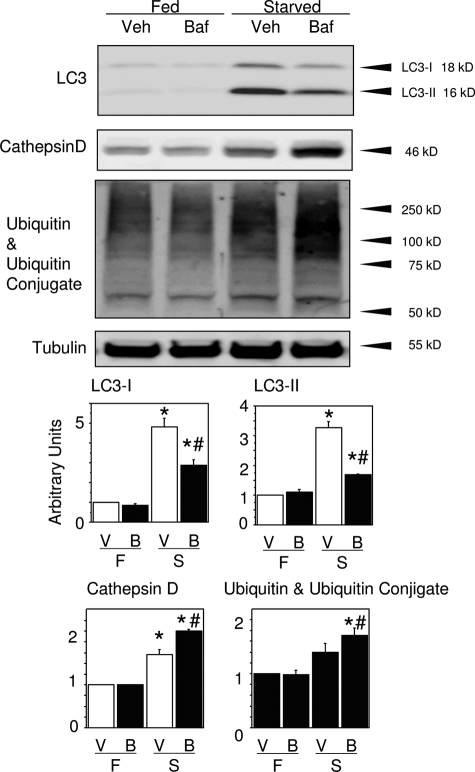
According to Western blotting, the myocardial expression of cathepsin D and ubiquitin was exaggerated in the starved mice treated with BafA1, consistent with the immunohistochemical findings (Figure 7). Inconsistent with GFP immunofluorescence, however, LC3 (both LC3-I and -II) expression was rather decreased in the later phase of starvation in the starved mice treated with BafA1.
Figure 7.
The effect of BafA1 on autophagy-related protein expression assessed by Western blots for LC3, cathepsin D, and ubiquitin with ubiquitinated proteins in the hearts of mice exposed to starvation for 3 days with or without BafA1 treatment. P < 0.05 compared with the fed groups (*) or with the starved group without BafA1 treatment (#) (one-way analysis of variance).
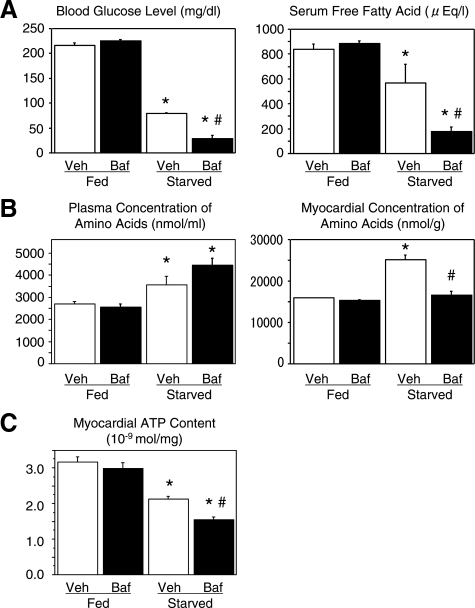
Both blood glucose and serum-free fatty acid levels were decreased after starvation for 3 days and the reductions were significantly augmented by treatment with BafA1 (Figure 8A). In contrast, the plasma amino acid concentration was increased under the starvation condition, which was not affected by BafA1. Content of amino acids in the heart was also increased after starvation. However, treatment with BafA1 inhibited this increase (Figure 8B). Myocardial tissue ATP content was significantly reduced to 68% of the baseline after starvation for 3 days. Treatment with BafA1 furthermore reduced myocardial ATP content, not in the fed mice, but in the starved mice (Figure 8C).
Figure 8.
The effect of BafA1 on blood glucose level, serum-free fatty acid, plasma and myocardial amino acid contents, and myocardial ATP content. A: Blood glucose and serum-free fatty acid levels in fed and starved mice for 3 days with or without BafA1 treatment. B: Amino acid concentrations in the plasma and myocardial tissues of fed and starved mice for 3 days with or without BafA1 treatment. C: Myocardial tissue ATP content in fed and starved mice for 3 days with or without BafA1 treatment. n = 5 for each group. P < 0.05 compared with the fed groups (*) or with the starved group without BafA1 treatment (#) (one-way analysis of variance).
Discussion
Functional Significance of Starvation-Induced Autophagy in the Adult Heart
Although autophagic vacuoles have been observed in the heart in response to various stresses such as starvation,15,21 ischemia,11,22,23 or pressure overload,24,25 the significance of autophagy on cardiac function has not been well established, even in the case of starvation. In general, starvation alone is unlikely to cause severe heart failure; this was confirmed in the present study. The present study demonstrates that autophagy inhibition by BafA1 caused severe cardiac dysfunction in the starved mice. Although autophagy is already known to be one of the most important mechanisms for survival under starvation conditions, our findings revealed that autophagy is critically important also for the maintenance of cardiac function of the adult heart under the starved condition. On the other hand, treatment with BafA1 did not affect cardiac function of the normally fed mice, indicating the critical importance of starvation-induced autophagy as a compensatory mechanism to maintain cardiac function under starvation.
Because the major role of autophagy is the degradation of proteins into amino acids, we measured the amino acid concentration in the plasma and myocardial tissue. Starvation significantly increased the concentrations. Although treatment with BafA1 did not affect the plasma amino acid concentration, it inhibited the starvation-induced increase of amino acid level in the myocardium. This suggests that the heart critically depends on degraded proteins via autophagy for the supply of amino acids. The myocardial tissue ATP content was found to have been decreased in parallel to the cardiac dysfunction. Thus, it is surmised that insufficient supply of ATP caused by inhibited autophagy might be one of the important contributors to cardiac dysfunction.
LC3 is a homologue of yeast Atg8, one of the autophagy-related genes (ATG genes), found in mammalian cells. The carboxyl terminal region of LC3 is cleaved, generating a soluble form known as LC3-I. LC3-I, in turn, is modified to a membrane-bound form, LC3-II (a LC3-phospholipid conjugate), by mammalian Atg7 and Atg3 homologues, which are E1- and E2-like enzymes, respectively, to then localize to autophagosomes and autolysosomes. Thus, the amount of LC3-II is a good early marker for the formation of autophagosomes.26 The present study revealed a starvation interval-dependent increase in expression of LC3-II in the adult heart. However, it is difficult to determine whether this increased expression of LC3-II reflects augmented autophagy or lowered turnover of autophagy during the later starvation period. We found that prolonged starvation when accompanied by treatment with BafA1 resulted in a reduction of LC3-II expression, which was inconsistent with GFP expression continuing to increase along with the starvation period. Although the degradation process of LC3-II in the autophagosomes is not well understood, a recent study revealed that LC3-II is degraded by lysosomal hydrolases after the formation of autophagosomes.14 Thus, the decreased expression of LC3-II at the later phase of starvation could reflect relatively rapid degradation of LC3-II compared with GFP.
The ubiquitin-proteasome system is another protein degradation pathway in addition to autophagy, in which the ubiquitination of proteins is the initial step.27 However, autophagy has been implicated in not only organelle turnover but also in the elimination of protein aggregates, which are often ubiquitinated.28 The present study showed that by Western blotting and immunohistochemistry, ubiquitin and polyubiquitinated substrates in the heart were increased by starvation and furthermore, augmented by the treatment with BafA1. The significance of such an increased expression remains to be clarified: the question is whether the ubiquitin-proteasome system might have been hyperfunctioning to help and/or compensate for autophagy, or undegraded ubiquitin and ubiquitinated substrates were not adequately processed so as to result in a simple accumulation.
We used BafA1 to demonstrate the physiological consequences of reduced autophagy during starvation. However, this approach used does not specifically target the heart, but rather affects metabolism in other vital organs. Thus, it is difficult to assess whether the profound loss of cardiac performance is the result of a local inhibition of autophagy, or because of the profound hypoglycemia and reduced free fatty acids in the circulation. This issue should be resolved in the future using some methods that inhibit autophagy specifically in the heart.
Characteristic Morphology of Autophagosomes in the Adult Heart
We observed a time-dependent transition of vacuolar ultrastructure within the adult mouse cardiomyocytes during starvation. During the early starvation period, the content of the majority of vacuoles was electron-dense and with homogenous lipid droplets whereas a relatively few vacuoles contained intracellular organelles, showing typical features of an autophagosome. Immunoelectron microscopy and enzyme cytochemistry have proven that such lipid-like homogenous vacuoles are lysosomes containing cathepsin D antigenicity and acid phosphatase activity. However, the ratio of the types of vacuoles became reversed during the later period; most of the vacuoles were typical autophagosomes, whereas a few were lysosomes. These findings suggest that lysosomal activation precedes the formation of morphologically typical autophagosomes, consistent with the earlier observation.29
Another conspicuous substructural change was seen in mitochondria. Mitochondria in the cardiomyocyte were more electron-lucent in the starved mice compared with those in the normally fed mice. Such a dramatic morphological change is presumed to reflect some important alterations of mitochondrial function that, however, were not elucidated in the present study.
Inhibition of autophagic digestion caused marked accumulation of lysosomes and autophagosomes containing a great many intact or undigested organelles. Such space-occupation in the cytoplasm as well as ATP reduction might have contributed to the functional disturbance of individual cardiomyocytes by interfering with cooperative movement activity of sarcomeres.
A previous study reported that the inactivation of autophagy triggers apoptosis in cell lines.30 We confirmed this in the cardiac interstitial cells and circulating cells but not in cardiomycytes. The specific absence of apoptosis in cardiomyocytes may be attributable to the relatively low sensitivity of cardiomyocytes to apoptotic stimuli such as Fas stimulation.31,32
Conclusions
The present study reveals a critical role for autophagy in the maintenance of cardiac function during starvation in the adult heart. This study also presents detailed ultrastructural changes in starvation-induced vacuoles within cardiomyocytes.
Acknowledgments
We thank the staff of Kyoto Women’s University (Chikako Koda, Aya Sakagami, Makiko Takeuchi, Megumi Tanigaki, Yuko Nakajima, Ayako Nozu) for technical assistance.
Footnotes
Address reprint requests to Genzou Takemura, M.D., Ph.D., Division of Cardiology, Gifu University Graduate School of Medicine, 1-1 Yanagido, Gifu 501-1194, Japan. E-mail: gt@gifu-u.ac.jp.
Supported in part by research grants from Gifu University.
References
- Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol. 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- Knaapen MW, Davies MJ, De Bie M, Haven AJ, Martinet W, Kockx MM. Apoptotic versus autophagic cell death in heart failure. Cardiovasc Res. 2001;51:304–312. doi: 10.1016/s0008-6363(01)00290-5. [DOI] [PubMed] [Google Scholar]
- Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn WP, Schaper J. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, Mora M, Riggs JE, Oh SJ, Koga Y, Sue CM, Yamamoto A, Murakami N, Shanske S, Byrne E, Bonilla E, Nonaka I, DiMauro S, Hirano M. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, Kanamori H, Khai NC, Maruyama R, Ogino A, Minatoguchi S, Fujiwara T, Fujiwara H. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113:535–543. doi: 10.1161/CIRCULATIONAHA.105.568402. [DOI] [PubMed] [Google Scholar]
- Robinson JM, Karnovsky MJ. Ultrastructural localization of several phosphatases with cerium. J Histochem Cytochem. 1983;31:1197–1208. doi: 10.1177/31.10.6309949. [DOI] [PubMed] [Google Scholar]
- Wildenthal K, Dees JH, Buja LM. Cardiac lysosomal derangements in mouse heart after long-term exposure to nonmetabolizable sugars. Circ Res. 1977;40:26–35. doi: 10.1161/01.res.40.1.26. [DOI] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000;10:524–530. doi: 10.1016/s0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- de Waal EJ, Vreeling-Sindelárová H, Schellens JP, James J. Starvation-induced microautophagic vacuoles in rat myocardial cells. Cell Biol Int Rep. 1986;10:527–533. doi: 10.1016/0309-1651(86)90027-5. [DOI] [PubMed] [Google Scholar]
- Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert KC, Heineke J, Westermann J, Ludde M, Fiedler B, Zierhut W, Laurent D, Bauer MK, Schulze-Osthoff K, Drexler H. The cardiac fas (APO-1/CD95) receptor/fas ligand system: relation to diastolic wall stress in volume-overload hypertrophy in vivo and activation of the transcription factor AP-1 in cardiac myocytes. Circulation. 2000;101:1172–1178. doi: 10.1161/01.cir.101.10.1172. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Takemura G, Koda M, Kawase Y, Maruyama R, Li Y, Minatoguchi S, Fujiwara T, Fujiwara H. Sensitivity to apoptosis signal, clearance rate, and ultrastructure of fas ligand-induced apoptosis in in vivo adult cardiac cells. Circulation. 2002;105:3039–3045. doi: 10.1161/01.cir.0000018651.89208.69. [DOI] [PubMed] [Google Scholar]