Abstract
Nuclear factor (NF)-κB participates in ischemia/reperfusion (I/R) hepatic signaling, stimulating both protective mechanisms and the generation of inflammatory cytokines. After analyzing NF-κB activation during increasing times of ischemia in murine I/R, we observed that the nuclear translocation of p65 paralleled Src and IκB tyrosine phosphorylation, which peaked after 60 minutes of ischemia. After extended ischemic periods (90 to 120 minutes) however, nuclear p65 levels were inversely correlated with the progressive induction of oxidative stress. Despite this profile of NF-κB activation, inflammatory genes, such as tumor necrosis factor (TNF) and interleukin (IL)-1β, predominantly induced by Kupffer cells, increased throughout time during ischemia (30 to 120 minutes), whereas protective NF-κB-dependent genes, such as manganese superoxide dismutase (Mn-SOD), expressed in parenchymal cells, decreased. Consistent with this behavior, gadolinium chloride pretreatment abolished TNF/IL-1β up-regulation during ischemia without affecting Mn-SOD levels. Interestingly, specific glutathione (GSH) up-regulation in hepatocytes by S-adenosylmethionine increased Mn-SOD expression and protected against I/R-mediated liver injury despite TNF/IL-1β induction. Similar protection was achieved by administration of the SOD mimetic MnTBAP. In contrast, indiscriminate hepatic GSH depletion by buthionine-sulfoximine before I/R potentiated oxidative stress and decreased both nuclear p65 and Mn-SOD expression levels, increasing TNF/IL-1β up-regulation and I/R-induced liver damage. Thus, the divergent role of NF-κB activation in selective liver cell populations underlies the dichotomy of NF-κB in hepatic I/R injury, illustrating the relevance of specifically maintaining NF-κB activation in parenchymal cells.
Liver damage induced by ischemia/reperfusion (I/R) is relevant in different clinical settings such as liver resection, transplantation, trauma, or hemorrhagic shock, in which nuclear factor (NF)-κB activation plays a controversial role because of its dual action in the induction of both protective and pro-inflammatory genes.1,2 For instance, hepatic NF-κB activation has been shown to diminish hepatic I/R injury and improve orthotopic liver transplantation, whereas NF-κB inactivation has been shown to protect against hepatic I/R.3,4,5,6 These diverse and seemingly discordant results have been explained on the basis of different degrees of residual NF-κB activation achieved depending on the mechanisms used to block NF-κB activation. Thus, although a total block of NF-κB may be detrimental as it prevents expression of survival genes, an incomplete NF-κB inhibition may suppress the up-regulation of proinflammatory mediators, while allowing the induction of protective genes. In addition, whether this dichotomy of NF-κB in promoting or protecting against hepatic I/R injury reflects the expression of NF-κB-dependent genes in different hepatic cell populations, such as parenchymal and Kupffer cells (KCs), remains primarily unknown.
In the liver, tumor necrosis factor (TNF), reactive oxygen species (ROS), and Toll-like receptors (TLR) are major players in NF-κB activation.7,8,9 TNF on binding to its membrane receptor TNF-R1 induces DISC recruitment, IKK activation, IκB serine phosphorylation, and subsequent proteasome degradation after ubiquitination.10 As a consequence, NF-κB is released from its inhibitory subunit allowing the transcription of target genes. This canonical pathway of NF-κB activation is also followed on engagement of Toll-like receptors (TLRs), a family of receptors that play a key role in innate immune responses as well as in inflammation.11 Moreover, TLRs, particularly TLR4, is drawing current attention as an important mediator of hepatic I/R injury.12,13
It has been shown that the generation of intracellular ROS activates Src tyrosine kinases leading to the phosphorylation of IκB at tyrosine residues, resulting in the dissociation of the p50-p65 heterodimer from its inhibitory IκB subunit followed by its nuclear translocation.14,15,16,17 In addition, the Src-mediated mechanism of NF-κB activation by IκB tyrosine phosphorylation has also been described during hypoxia in various cell types, including hepatocytes.18,19,20 Moreover, recent data have shown that NF-κB transactivation is diminished in hepatocytes after GSH depletion, involving IKK-dependent and -independent mechanisms21 and that GSSG generation, such as that caused by ROS overgeneration, inactivates NF-κB.22 Because ROS production could interfere with the synthesis of protective NF-κB-dependent genes in the liver, antioxidant therapies that increase NF-κB-dependent gene transcription in the hepatocyte, without promoting pro-inflammatory cytokines in nonparenchymal cells, particularly in KCs would be of interest to discriminate the role of NF-κB in hepatic I/R injury. In this regard, S-adenosylmethionine (SAM) is known to increase GSH levels in the hepatocyte through the transsulfuration pathway and shown to reduce ROS production in different liver pathologies, including I/R.23,24,25,26 Hence, the goal of this study was to examine the activation of NF-κB and the expression of protective and inflammatory target genes in relation with the length of ischemia during partial hepatic warm I/R and the influence of selective GSH loading in parenchymal cells.
Our work shows that SAM administration enhanced GSH stores in hepatocytes, decreasing ROS production, increasing the expression of NF-κB-dependent cytoprotective genes and preserving the liver against I/R exposure. In contrast, GSH depletion increased the up-regulation of proinflammatory genes and potentiated the hepatic damage induced by I/R. Therefore, our results suggest that, although indiscriminate NF-κB modulation may not produce the beneficial effects expected, therapies aimed to modulate the redox status of specific hepatic cells may increase selective κB-dependent proteins and be more effective in protecting the liver against I/R-induced injury.
Materials and Methods
Partial Hepatic Ischemia and Treatments
The experimental animal protocol was approved by the Institut d’Investigacions Biomèdiques August Pi i Sonyer (IDIBAPS) Animal Care and Use Committee. Hepatic partial warm ischemia was performed in male C57BL/6 mice using microvascular clamps (Biemer clip, 0.29 to 0.39 N) to prevent hepatic blood flow for different times (0 to 120 minutes) as described previously.27 Blood samples and liver biopsies were taken at different periods after reperfusion for further evaluation. Control animals were sham operated. Mice were pretreated 1 hour before surgery with SAM (5 mg/mouse; Sigma-Aldrich, St. Louis, MO), buthionine-sulfoximine (BSO) (4 mmol/kg, Sigma-Aldrich), MnTBAP (50 mg/kg; Calbiochem, San Diego, CA), or with an equal volume of the vehicle. In some cases, gadolinium chloride was administered 24 hours before surgery to antagonize KCs.28,29
Hepatocyte and KC Isolation
Primary hepatocytes were obtained from mouse liver by collagenase digestion and cultured on collagen-coated plates as described.23,25 KC isolation was performed by pooling nonparenchymal cells from three male mice (C57BL/6, 8 to 12 weeks old), using the method by Smedsrod and colleagues.30 In brief, after 0.05% collagenase perfusion of the liver, the cell suspension was centrifuged at 50 × g for 2 minutes to separate parenchymal from nonparenchymal cells. The supernatant, which contains nonparenchymal cells, was centrifuged (300 × g; 4 minutes; 4°C) to concentrate the cells, followed by centrifugation (800 × g; 10 minutes; 4°C) through a two-step Percoll gradient (25% + 50%), with the KCs and endothelial cells banding at the interface between the two density cushions. The pellet was suspended at 4 × 106 cells/ml in Dulbecco’s modified Eagle’s medium supplemented with 20% heat-inactivated fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Separation of pure KCs and endothelial cells was obtained from the selective adherence of KCs to plastic flasks after 60 minutes of incubation, yielding 2 to 3 million KCs per mouse liver, with 90 to 95% viability as determined by trypan blue exclusion. The cells showed typical macrophage morphological features and stained positively for F4/80. Macrophage cell line RAW 264.7, supplied by European Collection of Animal Cell Cultures (Sigma-Aldrich), was routinely grown at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum under normoxic atmosphere. Primary hepatocytes, Kupffer, and RAW 264.7 cells were exposed either to hypoxia (0.5% O2 and 5% CO2), TNF (Peprotech EC, London, UK) or lipopolysaccharide (LPS) (Sigma-Aldrich) for the indicated periods. In some cases, hepatocytes were treated with 10 μmol/L 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazole[3, 4-d]pyrimidine (PP2), and 100 μmol/L piceatannol (Calbiochem) before exposure to hypoxia as described19 and RAW 264.7 cells were pre-exposed to acetyl-leucyl-leucyl-norleucinal (Calbiochem) to inhibit the proteasome before exposure to LPS.
Western Blotting and Nuclear Extract Preparation
The phosphorylation of c-Src was analyzed in liver homogenates using phospho-Src (Tyr416) from Cell Signaling (Danvers, MA). To evaluate tyrosine phosphorylation of IκB-α after I/R, 1 mg of whole-liver lysate was immunoprecipitated using 2 μg of an IκB-α Ab (Santa Cruz Biotechnology, Santa Cruz, CA) and determined with anti-phosphotyrosine Ab (Santa Cruz Biotechnology). Manganese superoxide dismutase (Mn-SOD) and TNF protein levels were analyzed in total liver extracts using commercial antibodies (Santa Cruz Biotechnology), whereas NF-κB activation was analyzed in nuclear extracts using p65 Ab (Santa Cruz Biotechnology). Nuclear extracts from liver samples were prepared as previously described.19
Measurement of Plasma TNF Levels
Whole blood obtained by cardiac puncture was centrifuged and 50 μl of plasma aliquots were frozen for TNF assay. Plasma TNF levels were determined by an enzyme-linked immunosorbent assay using a commercial kit (Quantikine M Murine Tumor Necrosis Factor-α Assay; R&D Systems, Minneapolis, MN).
Liver Damage
After reperfusion, livers were fixed and sections (5 μm) were stained with hematoxylin and eosin (H&E) using standard methods, and slides were examined with a Zeiss (Göttingen, Germany) Axioplan microscope equipped with a Nikon (Tokyo, Japan) DXM1200F digital camera. Serum ALT levels were measured by the Centro de Diagnóstico Medico (Hospital Clinic, Barcelona, Spain).
Real-Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from mouse liver samples with the TRIzol reagent (Invitrogen, Carlsbad, CA). Real-time PCR was performed using the 2× SensiMix one-step kit with SYBR Green (Quantance, London, UK) following the manufacturer’s instructions. Threshold (CT) values for each mRNA were subtracted from that of 18S mRNA, averaged and converted from log-linear to linear term. The primer sequences used for 18s, cIAP-2, Mn-SOD, TNF, and interleukin (IL)-1β amplification were as follows: cIAP-2 forward 5′-GTGGAACATGCCAAGTGGTT-3′; cIAP-2 reverse 5′-GGAGGCAATACAGCATTGGT-3′; Mn-SOD forward 5′-AACTCAGGTCGCTCTTCAGC-3′; Mn-SOD reverse 5′-GAACCTTGGACTCCCACAGA-3′; TNF forward 5′-CTGAACTTCGGGGTGATCGGT-3′; TNF reverse 5′-ACGTGGGCTACAGGCTTGTCA-3′; IL-1β forward 5′-GAGCTGAAAGCTCTCCACCTC-3′; IL-1β reverse 5′-CTTTCCTTTGAGGCCCAAGGC-3′; 18S forward 5′-GTAACCCGTTGAACCCCATT-3′; 18S reverse 5′-CCATCCAATCGGTAGTAGCG-3′.
Lipid Peroxidation Assay
Lipid peroxidation in liver samples was determined after the production of malondialdehyde (MDA) using the thiobarbituric acid (TBA) method. Briefly, 0.4 ml of 0.6% TBA and 1.2 ml of 1% orthophosphoric acid were added to 100 μl of liver homogenate and boiled for 45 minutes. After cooling, 1.6 ml of 1-butanol were added; samples were mixed and centrifuged at 1200 rpm for 10 minutes. MDA concentrations were determined in the supernatants by measuring the optical density at 535 nm using a calibrated curve with TBA as standard.
Myeloperoxidase Assay
Myeloperoxidase, an enzyme predominantly found in the azurophilic granules of polymorphonuclear leukocytes, was used as an index of neutrophil accumulation in the liver. Tissue homogenates were assayed with o-dianisidine solution (43.2 mmol/L KH2PO4, 6.5 mmol/L Na2HPO4, 10 mmol/L ethylenediaminetetraacetic acid, 0.016% o-dianisidine, 0.001% H2O2; pH 6). Oxidized o-dianisidine forms a stable chromophore, which is detected by measuring the absorbance at 460 nm. In parallel, myeloperoxidase staining in liver samples was determined by immunohistochemistry as previously described.31
Assessment of Hepatic Reactive Oxygen Species (ROS) Content
Hepatic ROS content was measured using 2′-7′-dichlorofluorescein. Briefly, 10 μl of liver homogenates, diluted 100-fold with phosphate-buffered saline (pH 7.4), were loaded with 5 μmol/L 2′-7′-dichlorofluorescein and incubated at 37°C for 30 minutes. Fluorescence was measured at excitation wavelength of 485 nm and emission wavelength of 530 nm as previously described.19
Statistical Analysis
Results were expressed as mean ± SD with the number of individual experiments detailed in the figure legends. Statistical significance was established by the Student’s t-test.
Results
Time-Dependent Oxidative Stress and TNF Secretion during I/R
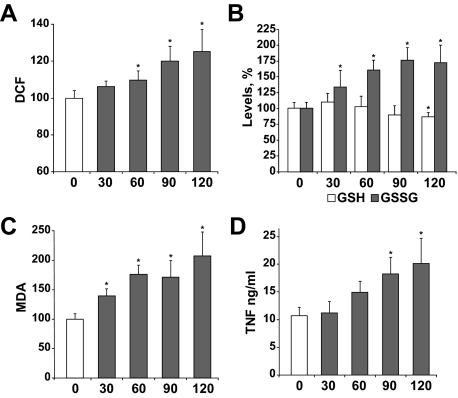
ROS and TNF are important players in different liver pathologies, such as hepatic I/R injury, and are known to modulate NF-κB activation. Therefore, we decided to measure parameters related to oxidative stress and TNF production as a function of the length of hepatic ischemia to ultimately examine their impact on NF-κB-dependent gene expression and liver injury. Hepatic ischemia was induced for various time periods (0 to 120 minutes), taking liver samples at the end of the reperfusion phase (1 hour). ROS production increased progressively as the time of ischemia was extended (Figure 1A). Even during short-time ischemia (30 minutes), substantial intracellular oxidative stress was detectable, as shown by the significant increase in the GSSG levels (Figure 1B), which affected the GSH/GSSG ratio (not shown) and the rise of lipid peroxidation (Figure 1C). In addition, as the time of ischemia increased, the levels of ROS, MDA, and GSSG were progressively elevated in samples from reperfused livers. Furthermore, in parallel with these changes, there was a significant rise in TNF levels detectable after 1 hour of ischemia that kept rising with extended ischemic periods (Figure 1D). These data underscore the induction of oxidative stress as a function of the time of ischemia after reperfusion.
Figure 1.
Variation of intracellular oxidative stress and TNF levels in liver after ischemia. Samples were taken from animals exposed to different times of ischemia (30, 60, 90, and 120 minutes or sham-operated and used as controls). After 1 hour of reperfusion, ROS production (A), levels of reduced and oxidized glutathione (B), and MDA (C) were measured in liver samples, whereas TNF levels (D) were analyzed in serum from I/R-treated mice (n ≥ 3). *P < 0.05 versus sham-operated mice.
Expression of NF-κB-Dependent Proinflammatory and Survival Genes during I/R
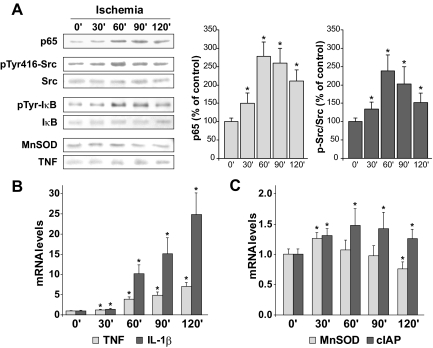
Next, we examined the time course of NF-κB activation assessed as nuclear p65 translocation. Interestingly, the nuclear p65 levels measured after 1 hour of reperfusion exhibited a dual pattern depending on the time of ischemia, increasing when ischemia was performed for up to 1 hour, but decreasing when the livers were clamped for longer periods of times (Figure 2A). Because tyrosine kinases, particularly Src, have been involved in NF-κB activation,18,19 we examined its activation reflected as Src phosphorylation at tyrosine 416. As seen, Src phosphorylation displayed a similar time-dependent pattern during ischemia as the nuclear translocation of p65 (Figure 2A). Indeed, a parallel increase in IκB-α tyrosine phosphorylation was detected by immunoprecipitation from liver extracts after different ischemia periods (Figure 2A).
Figure 2.
Time-dependent increase of nuclear p65, Src phosphorylation, and κB-dependent gene expression. A: Nuclear translocation of p65, phosphorylation of Src, and tyrosine phosphorylation of IκB were measured after 1 hour of reperfusion in liver samples from mice exposed to different times of ischemia. A representative blot is shown (n = 3). Quantification of the levels of p65 and ratio of p-Src/Src are included (n = 3). A: Protein levels of Mn-SOD and TNF were examined in total liver extracts after 6 hours of sham operation or reperfusion. mRNA levels of NF-κB-dependent proinflammatory (B) and antiapoptotic (C) genes were measured 6 hours after different times of ischemia and normalized by 18S mRNA levels. *P < 0.05 versus sham-operated mice.
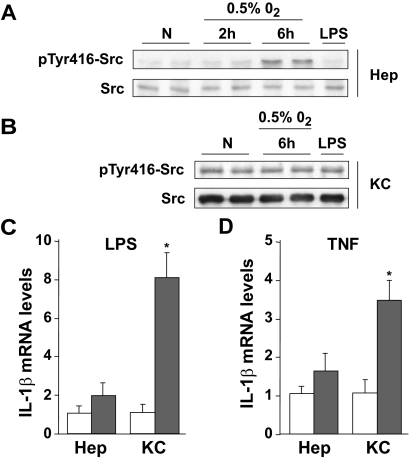
Because nuclear levels of p65 in hepatocytes do not necessarily reflect the transactivation of NF-κB in the face of substantial oxidative stress and GSH depletion,21 we next analyzed the pattern of activation of diverse κB target genes as a function of the time of ischemia. As seen, the mRNA levels of proinflammatory κB-dependent genes such as TNF and IL-1β were up-regulated with increasing ischemic periods (Figure 2B). In contrast, we observed that the mRNA levels of κB-dependent survival genes such as cIAP-2 increased moderately. Interestingly, the Mn-SOD mRNA levels increased for the first 30 minutes of ischemia, decreasing thereafter (Figure 2C). Moreover, we examined the protein expression of Mn-SOD and TNF in liver samples by Western blots to validate the results observed at the mRNA level. As seen (Figure 2A), Mn-SOD decreased as a function of the period of hepatic ischemia, whereas TNF levels increased thus matching the data obtained by mRNA analysis. The contrast in the time-dependent up-regulation of mRNA levels of proinflammatory versus survival genes may reflect the activation of NF-κB in specific hepatic cell types. To test this, we examined Src phosphorylation and IL-1β mRNA expression in primary mouse hepatocytes and KCs in response to hypoxia, LPS, or TNF exposure. As seen, hypoxia per se time-dependently phosphorylated Src at tyrosine 416 in primary mouse hepatocytes (Figure 3A), but not in KCs (Figure 3B). Indeed, the macrophage cell line RAW 264.7, a surrogate cell line for KCs, exhibited similar results (Supplemental Figure 1, see http://ajp.amjpathol.org). Consistent with our previous results in HepG2 cells,19 preincubation of primary isolated hepatocytes with PP2, a selective Src inhibitor, but not piceatannol, prevented hypoxia-induced Src phosphorylation at tyrosine 416 and p65 nuclear migration (data not shown). In addition, KCs failed to phosphorylate Src in response to LPS (Figure 3B) or TNF (not shown), despite strong induction of pro-inflammatory genes such as IL-1β in response to LPS and TNF exhibited (Figure 3, C and D). Moreover, RAW 264.7 macrophages, displayed increased IL-1β transcription after LPS or TNF exposure (Supplemental Figure 2, see http://ajp.amjpathol.org). Moreover, LPS-induced NF-κB activation in RAW 264.7 cells was accompanied by IκB degradation that was prevented by proteasomal inhibition with acetyl-leucyl-leucyl-norleucinal (not shown). In contrast, LPS and TNF failed to up-regulate IL-1β in primary hepatocytes (Figure 3, C and D). Thus, these data suggest that the activation of NF-κB via Src phosphorylation after I/R occurs in hepatocytes, and that KCs are the predominant source of proinflammatory cytokines.
Figure 3.
Primary hepatocytes and KCs display different responses in NF-κB activation after I/R-related stimuli. A: Increased Src phosphorylation was observed in hypoxic hepatocytes (0.5% O2), but not after LPS exposure (2 hours, 0.1 ng/ml), as shown in the top panel. B: The levels of pTyr416Src were not increased in KCs after exposure to hypoxia or LPS. Representative blots are shown (n = 2). C and D: Changes in IL-1β mRNA levels in hepatocytes and KCs after LPS (4 hours, 0.1 ng/ml) and TNF (4 hours, 250 ng/ml) treatment (full bars) with respect to nontreated cells (empty bars), normalized by 18S mRNA levels (n = 3). *P < 0.05 versus control cells.
KC Inactivation Down-Regulates TNF/IL-1β Production during I/R
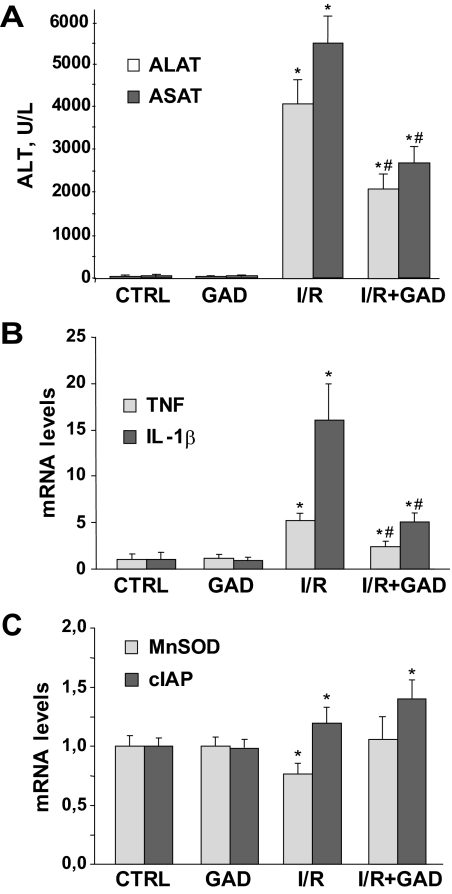
Because the preceding findings indicated that the up-regulation of proinflammatory cytokines were derived mainly from macrophages, we next examined the contribution of KCs in this process during I/R using gadolinium chloride to specifically abrogate their activity in vivo.9,28,29 Mice were injected intraperitoneally with gadolinium chloride (10 mg/kg) 24 hours before 90 minutes of ischemia and 6 hours after reperfusion. Confirming previous findings, KC inactivation protected against I/R-mediated liver injury, estimated by the reduction of serum ALT/AST (Figure 4A). Of note, this outcome was accompanied by the down-regulation of mRNA levels of TNF and IL-1β (Figure 4B). However, the Mn-SOD and cIAP-2 mRNA levels were either not affected or slightly increased during I/R after gadolinium chloride treatment (Figure 4C), indicating that the predominant source of TNF/IL-1β during I/R are KCs and that parenchymal cells are likely major contributors in the up-regulation of κB-dependent protective genes.
Figure 4.
Effect of KC inactivation by gadolinium chloride on hepatic I/R injury and κB-dependent gene expression. Mice pretreated with gadolinium chloride or vehicle for 24 hours were subjected to 90 minutes of ischemia and 6 hours of reperfusion. ALT levels in serum (A) and mRNA liver levels of inflammatory (B) and protective (C) genes were analyzed (n = 3). *P < 0.05 versus sham-operated mice; #P < 0.05 versus I/R-treated mice.
SAM Therapy Selectively Boosts GSH Stores in Parenchymal Cells Protecting Against I/R-Mediated Hepatic Injury
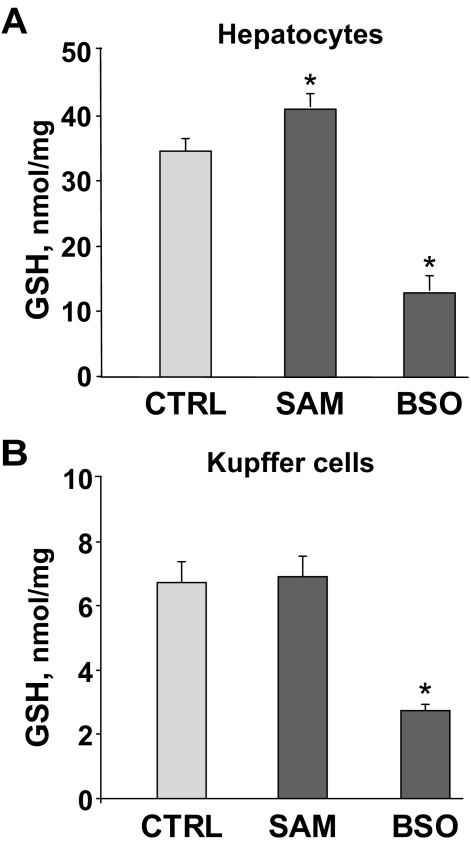
Because NF-κB activation and Src phosphorylation have been proved to depend on ROS production in hepatocytes and because GSH is a major antioxidant, we decided to specifically increase GSH levels in parenchymal cells by treating mice with SAM before I/R. SAM’s capacity to increase GSH levels is relevant in specific tissues such as liver or kidney in which the transsulfuration pathway allows the conversion of homocysteine to cysteine.32 Given that methionine adenosyltransferases (MATs) are suggested to be expressed in KCs and hepatic endothelial cells,33 we first assessed whether exogenous SAM boosts GSH levels in KCs and in isolated hepatocytes. Mouse hepatocytes displayed increased GSH content (Figure 5A) 4 hours after the administration of SAM (2 mmol/L). In contrast, the content of GSH in KCs did not change after SAM exposure (Figure 5B). However, the incubation with BSO, a specific inhibitor of γ-GCS, which catalyzes the rate-limiting step in GSH biosynthesis, significantly decreased GSH to a similar extent in both cell types (Figure 5, A and B). As expected from the data with KCs, BSO decreased GSH levels in RAW 264.7 cells whereas SAM did not exert any change in GSH content (Supplemental Figure 3, see http://ajp.amjpathol.org).
Figure 5.
GSH levels in hepatocytes and KCs. GSH concentration was measured in primary hepatocytes (A) and KCs (B) after 4 hours of exposure to SAM (2 mmol/L) or BSO (1 mmol/L) (n = 2 to 3). *P < 0.05 versus control cells.
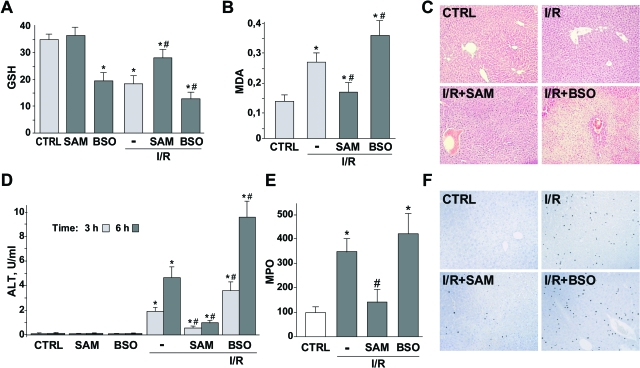
Next, we examined the effects of these strategies in vivo during I/R. Compared with I/R, SAM treatment was effective in replenishing hepatic GSH levels, which decreased in untreated samples after 90 minutes of ischemia and 6 hours after reperfusion (Figure 6A). In contrast, BSO pretreatment further decreased hepatic GSH levels during I/R, thus compromising the hepatic capacity to cope with oxidative stress during I/R. Consequently, SAM therapy efficiently prevented the increase in lipid peroxidation measured as MDA levels during I/R, whereas BSO treatment further enhanced this effect (Figure 6B). Consistent with these findings, ALT levels were reduced in SAM-treated animals after I/R (Figure 6D), indicative of lower liver injury as observed by H&E (Figure 6C) staining compared with untreated control mice subjected to I/R. In contrast, massive hepatic damage examined by H&E (Figure 6C) and ALT (Figure 6D) was observed in BSO-pretreated mice during I/R. In addition, and consistent with the above findings, we observed a differential effect of SAM versus BSO pretreatment in the extent of neutrophil infiltration during I/R as measured by myeloperoxidase enzymatic activity (Figure 6E) and immunohistochemistry (Figure 6F). Finally, to assess if the protective effect of SAM administration against I/R-mediated liver injury was dependent or not on GSH biosynthesis, we tested whether BSO abrogated the therapeutic effect of SAM pretreatment. Indeed, we observed that the serum ALT levels after I/R in mice treated with SAM plus BSO were similar to the group of mice pretreated with BSO alone (data not shown), indicating that the beneficial effect of SAM during I/R was dependent on the increase in parenchymal GSH stores.
Figure 6.
Differential effects of SAM and BSO in GSH levels and liver injury after I/R. Hepatic GSH levels (A), MDA generation (B), and liver damage measured by liver H&E staining (C) and ALT in serum (D) from vehicle-, SAM-, and BSO-treated animals exposed to 90 minutes of ischemia after 6 hours of reperfusion. Neutrophil infiltration by myeloperoxidase activity (E) and immunohistochemistry (F) were analyzed after 6 hours of reperfusion. No changes in liver damage or neutrophil infiltration were observed in animals treated with SAM or BSO alone (n ≥ 3). *P < 0.05 versus sham-operated mice; #P < 0.05 versus I/R-treated mice.
Differential Effects of SAM and BSO on TNF/IL-1β and Mn-SOD/cIAP-2 Expression during I/R
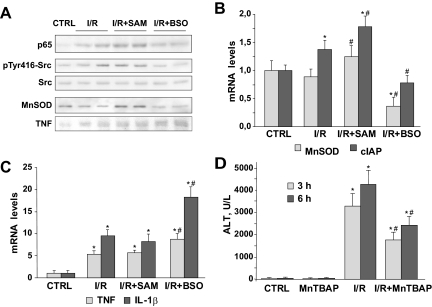
To evaluate if the changes in the liver redox status after SAM and BSO influence the degree of NF-κB activation during I/R, we analyzed the nuclear levels of p65, the transactivating subunit of the NF-κB heterodimer p50-p65, after 1 hour of reperfusion. As observed in Figure 7A, the nuclear translocation of p65 induced by I/R increased in SAM-treated mice, whereas GSH depletion in mice after BSO pretreatment diminished p65 nuclear levels during I/R. In addition, the levels of Src phosphorylation at tyrosine 416 after 90 minutes of ischemia were induced in SAM-treated animals, whereas BSO administration reduced phospho-Src levels, pointing again to Src tyrosine phosphorylation as the main mechanism involved in the observed NF-κB activation. SAM treatment alone in sham-operated mice did not affect the nuclear levels of p65 or Src phosphorylation at tyrosine 416 (data not shown). Moreover, total liver extracts after 90 minutes of ischemia followed by 6 hours of reperfusion displayed higher Mn-SOD protein levels in SAM-administered mice, suggesting an increased synthesis of this protective gene, whereas TNF was moderately enhanced in BSO-treated mice (Figure 7A).
Figure 7.
GSH restoration in liver after I/R increased p65 nuclear levels and the transcription of protective genes. SOD mimetic protected the liver against I/R-induced damage. A: Representative Western blots of nuclear p65, p-Src, and Src examined after 1 hour of reperfusion (n = 3). Mn-SOD and TNF protein levels were analyzed 6 hours after sham operation or reperfusion. mRNA quantification of protective (B) and proinflammatory (C) NF-κB-dependent genes in liver samples from mice exposed to 90 minutes of ischemia and 6 hours of reperfusion and pretreated with vehicle, SAM, or BSO. D: ALT levels in serum from vehicle- or MnTBAP-treated mice and exposed to I/R as above (n = 3). *P < 0.05 versus sham-operated mice; #P < 0.05 versus I/R-treated mice.
To analyze whether these changes in protein expression were reflected at the mRNA level, next we examined the effect of GSH modulation on NF-κB-dependent gene expression after I/R. We observed a significant increase in the expression of cIAP and Mn-SOD mRNA levels after SAM administration compared with the changes observed after GSH depletion after BSO injection (Figure 7B). Interestingly, TNF and IL-1β mRNA levels were unaffected by SAM administration, although these levels increased in BSO-pretreated mice before I/R (Figure 7C), suggesting a differential regulation of NF-κB-dependent gene expression in parenchymal and nonparenchymal cells. Besides, in RAW 264.7 macrophages incubated with LPS or TNF, pre-administration of SAM did not induce significant changes in the activation of these proinflammatory genes (data not shown).
Mn-SOD, the κB-dependent mitochondrial SOD isoform, has a relevant role in liver protection against diverse hepatotoxic stimuli that induce oxidative stress, such as I/R.7,34 In this regard, we have recently observed that Mn-SOD expression protects hepatoma cells under severe hypoxic conditions.18 Because Mn-SOD mRNA was increased in SAM-treated animals, we examined whether a Mn-SOD mimetic such as MnTBAP reproduces the protection observed in SAM-treated mice against I/R-induced liver damage. As seen, MnTBAP-treated animals were protected against I/R exposure examined by ALT serum levels (Figure 7D) as well as H&E and TUNEL staining (not shown). Collectively, these data suggest that strategies aimed to selectively activate NF-κB in parenchymal cells may be of relevance in I/R-mediated liver damage.
Discussion
As a master regulator of inflammation and survival pathways, NF-κB participates in both protective mechanisms and the generation of inflammatory cytokines whose balance ultimately controls the fate of the liver during I/R. As a result, although NF-κB signaling has become a potential therapeutic target to control and maintain hepatic function after I/R, conflicting results have been reported in which both NF-κB activation and inactivation protected against hepatic I/R injury.3,4,5,6 Our present work proposes that opposite effects of NF-κB after I/R are determined by distinctive mechanisms in parenchymal and nonparenchymal cells, which control autonomous NF-κB activation that affects separate sets of genes (Figure 8). Our results point to the ROS-mediated Src-dependent mechanism of NF-κB activation as a predominant pathway acting in hepatocytes, which is in charge for the induction of protective genes essential in the maintenance of liver function. In contrast, in nonparenchymal cells, particularly KCs the induction of κB-dependent pro-inflammatory genes most likely involves IκB serine-phosphorylation and proteasomal degradation. This canonical activation of NF-κB in KCs triggered by I/R through ligands such as TNF or LPS would be consistent with the liver protection observed in knockout models that block this receptor-mediated signaling5,6,8,35 or after KC inactivation.9,28,29
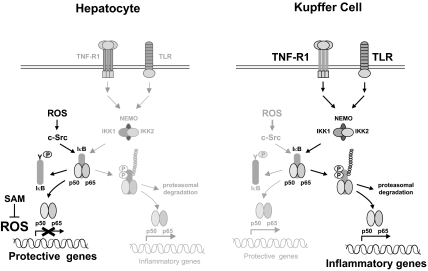
Figure 8.
Diagram showing different pathways of NF-κB signaling induced by I/R. In the hepatocyte, NF-κB controls protective genes after ischemia through a ROS-sensitive Src-mediated mechanism. However, high production of ROS by extended ischemia or by a deficient capacity to cope with oxidant production, as in GSH-depleted animals, reduces the transcription of NF-κB-responsive genes, and Src-mediated activation is ineffective. In contrast, KCs are mainly responsible for the production of NF-κB-dependent inflammatory proteins and show no evidence of reduction because of increased ROS production. In fact, levels of mRNAs from inflammatory genes rise as the time of ischemia was extended.
On the other hand, ROS generation after I/R is responsible for the induction of Src phosphorylation, NF-κB activation, and transcription of κB-dependent protective genes in the hepatocyte, such as Mn-SOD. This outcome is consistent with our previous findings indicating that hypoxia induced mitochondrial ROS, which played a signaling role in Src activation.19 However, extended ischemia induces a massive production of ROS and GSSG generation, which antagonizes NF-κB reflected by decreased nuclear p65 levels and down-regulation of κB-dependent survival genes such as Mn-SOD or cIAP-2. This is consistent with the notion that NF-κB activation requires a balanced level of GSSG.22 Paralleling these effects on nuclear p65 translocation, we observe the dual regulation of Src phosphorylation during I/R-mediated ROS generation. Although this activation during the early phase of ischemia fits with our previous finding in hypoxia,19 the decrease in Src phosphorylation at later times may reflect the activation of tyrosine phosphatases, although this remains to be established. This dual role of ROS in I/R signaling, depending on the duration of the ischemia, is consistent with the observation that the suppression of oxidative stress generated during ischemic preconditioning is sufficient to cancel the protective mechanisms triggered by preconditioning, including Mn-SOD overexpression.36,37
Mn-SOD is a critical enzyme in cellular protection acting as a first line of defense against superoxide anion generation, and consistent with this function Mn-SOD has been positioned as a decisive player against ischemic damage in different organs including the liver.34,38,39,40 Although we did not assess the source of ROS generation (mitochondrial versus extramitochondrial) during extended ischemia, consistent with the key protective role of Mn-SOD previous studies pointed to mitochondria as a major source for the burst of ROS during I/R or hypoxia.19,41 The scavenging of superoxide anion by Mn-SOD will be expected to yield hydrogen peroxide, which needs to be detoxified by the GSH redox cycle in the mitochondrial matrix. If the latter is compromised by impaired GSH peroxidase or GSH reductase or by limited GSH levels, it may result in higher hydrogen peroxide accumulation. Interestingly, SAM treatment will be expected to prevent GSH depletion not only in the cytosol via its conversion into cysteine in the transsulfuration pathway,32 but also to increase mitochondrial GSH levels from the transport of cytosol GSH.23,42 Consistent with the onset of oxidative stress during I/R, antioxidant strategies have been proved useful against I/R-mediated organ damage.31,38,41,43,44,45,46 Although MATs have been recently described in nonparenchymal cells,33 SAM conversion into GSH is only observed in parenchymal cells and this selectivity underscores the differential involvement of ROS in NF-κB-dependent gene regulation in parenchymal and nonparenchymal cells and their role in hepatic I/R injury. Thus, in nonparenchymal cells during extended times of ischemia, the increased transcription of κB-dependent inflammatory cytokines are not affected by SAM administration but are significantly reduced by KC inactivation. Interestingly, the liver protection observed after gadolinium treatment is accomplished almost exclusively by reducing the generation of inflammatory proteins, without inducing any decline in the levels of protective κB-dependent genes in parenchymal cells, such as Mn-SOD.
The above findings imply a careful regulation and/or modulation of NF-κB because of its complexity, diverse mechanisms of activation, and cellular heterogeneity. Several posttranslational modifications of p65 caused by different stimuli, particularly serine-phosphorylation and acetylation, may affect its affinity for specific domains located in the promoter of responsive genes.10,47 It is also proposed that NF-κB-regulated genes should be divided into groups depending on their requirement for chromatin modification for expression.48 Particularly, chromatin remodeling and histone acetylation have been shown to facilitate Mn-SOD transcription in response to TNF.48 Nevertheless, our results may help to appreciate that NF-κB signaling in the liver should be analyzed in particular cell types, and research approaches should take into account this autonomous behavior. Although in a different setting, Takahashi and colleagues49 described a dual activation profile of NF-κB during cold I/R-mediated liver injury, differentiating an early and a late phase of NF-κB activation. However, these investigators did not pursue whether selective liver cell populations contributed to the divergent role of NF-κB in cold ischemia.
In conclusion, because Src-mediated NF-κB activation in hepatocytes is induced by ROS generated after I/R, the NF-κB-dependent synthesis of protective proteins produced during short-time ischemia may be at risk by maneuvers aimed to diminish ROS production. In contrast, at prolonged time of ischemia the transcription of NF-κB-dependent protective genes may be compromised. In this scenario, the antagonism of oxidant species produced in hepatocytes during long ischemia by antioxidant-based strategies will preserve the levels of protective genes, such as Mn-SOD, to promote the integrity of the liver.
Footnotes
Address reprint requests to Albert Morales or José C. Fernandez-Checa, Instituto Investigaciones Biomédicas de Barcelona, C/Rosello, 161, 08036-Barcelona, Spain. Email: amorales@clinic.ub.es or checa229@yahoo.com.
Supported by the Centro de Investigación Biomédica en Red en el Área Temática de Enfermedades Hepáticas y Digestivas, the Instituto de Salud Carlos III (grants FIS06/0395, FIS07/0193, SAF2008-02199, and SAF2006-06780), the Ministry of Science and Innovation from Spain, and the US National Institute on Alcohol Abuse and Alcoholism (Research Center for Liver and Pancreatic Diseases grant P50 AA 11999).
A.M. and J.C.F.-C. share senior authorship.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Luedde T, Trautwein C. Intracellular survival pathways in the liver. Liver Int. 2006;26:1163–1174. doi: 10.1111/j.1478-3231.2006.01366.x. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- Kuboki S, Okaya T, Schuster R, Blanchard J, Denenberg A, Wong HR, Lentsch AB. Hepatocyte NF-kappaB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol. 2007;292:G201–G207. doi: 10.1152/ajpgi.00186.2006. [DOI] [PubMed] [Google Scholar]
- Suetsugu H, Iimuro Y, Uehara T, Nishio T, Harada N, Yoshida M, Hatano E, Son G, Fujimoto J, Yamaoka Y. Nuclear factor {kappa}B inactivation in the rat liver ameliorates short term total warm ischaemia/reperfusion injury. Gut. 2005;54:835–842. doi: 10.1136/gut.2004.043034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Assmus U, Wüstefeld T, Meyer zu Vilsendorf A, Roskams T, Schmidt-Supprian M, Rajewsky K, Brenner DA, Manns MP, Pasparakis M, Trautwein C. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury. J Clin Invest. 2005;115:849–859. doi: 10.1172/JCI23493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraza N, Lüdde T, Assmus U, Roskams T, Vander Borght S, Trautwein C. Hepatocyte-specific IKK gamma/NEMO expression determines the degree of liver injury. Gastroenterology. 2007;132:2504–2517. doi: 10.1053/j.gastro.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Urakami H, Abe Y, Grisham MB. Role of reactive metabolites of oxygen and nitrogen in partial liver transplantation: lessons learned from reduced-size liver ischaemia and reperfusion injury. Clin Exp Pharmacol Physiol. 2007;34:912–919. doi: 10.1111/j.1440-1681.2007.04640.x. [DOI] [PubMed] [Google Scholar]
- Teoh N, Field J, Sutton J, Farrell G. Dual role of tumor necrosis factor-alpha in hepatic ischemia-reperfusion injury: studies in tumor necrosis factor-alpha gene knockout mice. Hepatology. 2004;39:412–421. doi: 10.1002/hep.20035. [DOI] [PubMed] [Google Scholar]
- Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Qiao B, Shen XD, Gao F, Busuttil RW, Cheng G, Platt JL, Volk HD, Kupiec-Weglinski JW. Evidence for the pivotal role of endogenous toll-like receptor 4 ligands in liver ischemia and reperfusion injury. Transplantation. 2008;85:1016–1022. doi: 10.1097/TP.0b013e3181684248. [DOI] [PubMed] [Google Scholar]
- Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Li Q, Zhang Y, Liu X, Luo M, Abbott D, Zhou W, Engelhardt JF. IkappaBalpha and IkappaBbeta possess injury context-specific functions that uniquely influence hepatic NF-kappaB induction and inflammation. J Clin Invest. 2004;113:746–755. doi: 10.1172/JCI17337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koong AC, Chen EY, Giaccia AJ. Hypoxia causes the activation of nuclear factor kappa B through the phosphorylation of I kappa B alpha on tyrosine residues. Cancer Res. 1994;54:1425–1430. [PubMed] [Google Scholar]
- Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J Biol Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- Fan C, Li Q, Ross D, Engelhardt JF. Tyrosine phosphorylation of I kappa B alpha activates NF kappa B through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J Biol Chem. 2003;278:2072–2080. doi: 10.1074/jbc.M206718200. [DOI] [PubMed] [Google Scholar]
- Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Res. 2007;67:7368–7377. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- Sato H, Sato M, Kanai H, Uchiyama T, Iso T, Ohyama Y, Sakamoto H, Tamura J, Nagai R, Kurabayashi M. Mitochondrial reactive oxygen species and c-Src play a critical role in hypoxic response in vascular smooth muscle cells. Cardiovasc Res. 2005;67:714–722. doi: 10.1016/j.cardiores.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Lou H, Kaplowitz N. Glutathione depletion down-regulates tumor necrosis factor alpha-induced NF-kappaB activity via IkappaB kinase-dependent and -independent mechanisms. J Biol Chem. 2007;282:29470–29481. doi: 10.1074/jbc.M706145200. [DOI] [PubMed] [Google Scholar]
- Dröge W, Schulze-Osthoff K, Mihm S, Galter D, Schenk H, Eck HP, Roth S, Gmünder H. Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB J. 1994;8:1131–1138. [PubMed] [Google Scholar]
- Marí M, Colell A, Morales A, Pañeda C, Varela-Nieto I, García-Ruiz C, Fernández-Checa JC. Acidic sphingomyelinase downregulates the liver-specific methionine adenosyltransferase 1A, contributing to tumor necrosis factor-induced lethal hepatitis. J Clin Invest. 2004;113:895–904. doi: 10.1172/JCI19852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci USA. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ruiz C, Morales A, Colell A, Ballesta A, Rodés J, Kaplowitz N, Fernández-Checa JC. Feeding S-adenosyl-L-methionine attenuates both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology. 1995;21:207–214. doi: 10.1002/hep.1840210133. [DOI] [PubMed] [Google Scholar]
- Jeon BR, Lee SM. S-adenosylmethionine protects post-ischemic mitochondrial injury in rat liver. J Hepatol. 2001;34:395–401. doi: 10.1016/s0168-8278(00)00045-3. [DOI] [PubMed] [Google Scholar]
- Llacuna L, Marí M, Garcia-Ruiz C, Fernandez-Checa JC, Morales A. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology. 2006;44:561–572. doi: 10.1002/hep.21285. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Reinke LA. Antioxidants and gadolinium chloride attenuate hepatic parenchymal and endothelial cell injury induced by low flow ischemia and reperfusion in perfused rat livers. Free Radic Res. 2000;32:497–506. doi: 10.1080/10715760000300501. [DOI] [PubMed] [Google Scholar]
- Jang JH, Moritz W, Graf R, Clavien PA. Preconditioning with death ligands FasL and TNF-alpha protects the cirrhotic mouse liver against ischaemic injury. Gut. 2008;57:492–499. doi: 10.1136/gut.2007.137703. [DOI] [PubMed] [Google Scholar]
- Smedsrød B, Pertoft H, Eggertsen G, Sundstrom C. Functional and morphological characterization of cultures of Kupffer cells and liver endothelial cells prepared by means of density separation in Percoll, and selective substrate adherence. Cell Tissue Res. 1985;241:639–649. doi: 10.1007/BF00214586. [DOI] [PubMed] [Google Scholar]
- Marí M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, García-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin Chem Lab Med. 2007;45:1694–1699. doi: 10.1515/CCLM.2007.341. [DOI] [PubMed] [Google Scholar]
- Shimizu-Saito K, Horikawa S, Kojima N, Shiga J, Senoo H, Tsukada K. Differential expression of S-adenosylmethionine synthetase isozymes in different cell types of rat liver. Hepatology. 1997;26:424–431. doi: 10.1002/hep.510260224. [DOI] [PubMed] [Google Scholar]
- Wu TJ, Khoo NH, Zhou F, Day BJ, Parks DA. Decreased hepatic ischemia-reperfusion injury by manganese-porphyrin complexes. Free Radic Res. 2007;41:127–134. doi: 10.1080/10715760600801298. [DOI] [PubMed] [Google Scholar]
- Shen XD, Ke B, Zhai Y, Gao F, Tsuchihashi S, Lassman CR, Busuttil RW, Kupiec-Weglinski JW. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13:1435–1443. doi: 10.1002/lt.21251. [DOI] [PubMed] [Google Scholar]
- Rüdiger HA, Graf R, Clavien PA. Sub-lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver. J Hepatol. 2003;39:972–977. doi: 10.1016/s0168-8278(03)00415-x. [DOI] [PubMed] [Google Scholar]
- Rehman H, Connor HD, Ramshesh VK, Theruvath TP, Mason RP, Wright GL, Lemasters JJ, Zhong Z. Ischemic preconditioning prevents free radical production and mitochondrial depolarization in small-for-size rat liver grafts. Transplantation. 2008;85:1322–1331. doi: 10.1097/TP.0b013e31816de302. [DOI] [PubMed] [Google Scholar]
- Saba H, Batinic-Haberle I, Munusamy S, Mitchell T, Lichti C, Megyesi J, MacMillan-Crow LA. Manganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic Biol Med. 2007;42:1571–1578. doi: 10.1016/j.freeradbiomed.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GW, Kondo T, Noshita N, Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: implications for the production and role of superoxide radicals. Stroke. 2002;33:809–915. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Nakamura M, Teraoka S, Ota K. Ebselen, a novel anti-oxidant compound, protects the rat liver from ischemia-reperfusion injury. Transpl Int. 1997;10:96–102. doi: 10.1007/s001470050019. [DOI] [PubMed] [Google Scholar]
- Schauer RJ, Gerbes AL, Vonier D, Meissner H, Michl P, Leiderer R, Schildberg FW, Messmer K, Bilzer M. Glutathione protects the rat liver against reperfusion injury after prolonged warm ischemia. Ann Surg. 2004;239:220–231. doi: 10.1097/01.sla.0000110321.64275.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener G, Tosun O, Sehirli AO, Kaçmaz A, Arbak S, Ersoy Y, Ayanoğlu-Dülger G. Melatonin and N-acetylcysteine have beneficial effects during hepatic ischemia and reperfusion. Life Sci. 2003;72:2707–2718. doi: 10.1016/s0024-3205(03)00187-5. [DOI] [PubMed] [Google Scholar]
- Grattagliano I, Vendemiale G, Lauterburg BH. Reperfusion injury of the liver: role of mitochondria and protection by glutathione ester. J Surg Res. 1999;86:2–8. doi: 10.1006/jsre.1999.5620. [DOI] [PubMed] [Google Scholar]
- Natoli G, Saccani S, Bosisio D, Marazzi I. Interactions of NF-kappaB with chromatin: the art of being at the right place at the right time. Nat Immunol. 2005;6:439–445. doi: 10.1038/ni1196. [DOI] [PubMed] [Google Scholar]
- Guo Z, Boekhoudt GH, Boss JM. Role of the intronic enhancer in tumor necrosis factor-mediated induction of manganous superoxide dismutase. J Biol Chem. 2003;278:23570–23578. doi: 10.1074/jbc.M303431200. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Ganster RW, Gambotto A, Shao L, Kaizu T, Wu T, Yagnik GP, Nakao A, Tsoulfas G, Ishikawa T, Okuda T, Geller DA, Murase N. Role of NF-kappaB on liver cold ischemia-reperfusion injury. Am J Physiol. 2002;283:G1175–G1184. doi: 10.1152/ajpgi.00515.2001. [DOI] [PubMed] [Google Scholar]