Abstract
Studies in rodents have shown that brain perivascular macrophages are derived from bone marrow precursors. Less is known about the origin and turnover of perivascular cells in the human central nervous system. We took advantage of non-human primates reconstituted with autologous CD34+ hematopoietic stem cells that had been transduced with a lentiviral vector expressing the enhanced green fluorescent protein (EGFP) to study the ontogeny of brain macrophages of rhesus macaques. Flow cytometry and immunohistochemistry/fluorescence microscopy showed long-term reconstitution of monocytes/macrophages in the blood, lymphoid, and brain tissues 4 years post-transplant. In the brain, EGFP+ cells were detected in the choroid plexus, cerebellum, and cerebrum, where the percent engraftment between animals reflected the percentage of EGFP+ monocytes in the blood. Morphology and location of brain EGFP+ cells exclusively in the vicinity of blood vessels were consistent with perivascular macrophages. Up to 85% of brain EGFP+ cells expressed CD163, a marker of perivascular macrophages, and greater than 70% were CD68+ macrophages. These findings clearly demonstrate that a subpopulation of CD163+/CD68+ brain perivascular macrophages in rhesus macaques are renewed by CD34+ hematopoietic stem cell-derived precursors and exhibit a continuous long-lasting turnover. Because perivascular macrophages are significant targets of productive HIV/simian immunodeficiency virus infection in the brain, these observations point to hematopoietic stem cells as targets of both HIV/simian immunodeficiency virus infection and potential gene therapy.
Different populations of macrophages are found in the central nervous system (CNS). Microglia, the resident brain macrophages, are located in the parenchyma while other CNS macrophages are found in the perivascular spaces of Virchow-Robin at the interface between blood vessels and the surrounding brain parenchyma, in the meninges, and in the choroid plexus.1,2 Perivascular macrophages are immunophenotypically and functionally distinct from resident parenchymal microglia.1,3,4,5 Like peripheral macrophages and subpopulations of blood monocytes, they express molecules involved in antigen recognition (mannose receptor, DC-SIGN) and antigen presentation (MHC class II, CD40, B7-1, and B7-2).6,7,8,9,10 Perivascular macrophages in humans and non-human primates are a major target of productive infection by human immunodeficiency (HIV)11,12,13 and simian immunodeficiency (SIV)14,15 viruses. Therefore, precursors to perivascular macrophages in bone marrow and blood are likely targets that are either directly infected in bone marrow and/or blood or affected by HIV and SIV infection in these sites. Thus these cells are potential targets of infection as well as gene therapy approaches to make them resistant to infection.
The turnover of brain macrophages has been extensively studied in small animals and animal models of disease. Studies using chimeric rats,16,17 transplants of green fluorescent protein (GFP)-labeled unfractioned bone marrow cells,5,9,18 or dyes injected into the perivascular space19 have shown that perivascular macrophages are repopulated from bone marrow-derived cells and turnover within the CNS. Less is known, however, about the ontogeny of human perivascular macrophages although transplantation of human patients with bone marrow from sex-mismatched donors showed that they were of bone marrow origin.20 Whether long-term reconstitution of perivascular macrophages from hematopoietic stem cells (HSCs) occurs in primates is not known.
We took advantage of a non-human primate model of autologous HSC transplantation to study the ontogeny of perivascular macrophages of rhesus macaques. SIV vectors have been reported to successfully transduce non-human primate CD34+ HSCs capable of repopulating the hematopoietic system following transplantation.21,22 In these studies enhanced (E)GFP expression was examined long-term, within 1 year post-transplantation in multiple hematopoietic cell lineages. These data showed a stable repopulation by EGFP+ HSCs with 10% to 30% of cells in peripheral blood being EGFP+.21,22 Using four animals from this study and another animal (2RC003) whose CD34+ cells were transduced with a HIV-based vector constructed to express EGFP,23 we investigated the contribution of EGFP+ CD34+ HSCs in the repopulation of myeloid cells in blood, lymphoid tissues, and the CNS. We show that EGFP+ cells derived from rhesus macaque CD34+ HSCs give rise to monocytes and dendritic cells in blood and exclusively perivascular cells in the CNS 4 years post-transplantation. The majority of EGFP+ cells in the CNS are CD163+ perivascular macrophages, which are a major target of productive infection by HIV and SIV and point to important gene delivery in the CNS by HSCs/progenitor cells.
Materials and Methods
Animals
Five rhesus macaques (Macaca mulatta) were originally transplanted in 2002 (2RC003), 2003 (CJ5B and RQ3556), and 2005 (RQ5427 and RQ3570) with autologous CD34+ HSCs transduced by HIV-based (2RC003) or SIV-based vector particles constructed to express EGFP as described.21,22
Blood and Tissue Samples
Whole blood from all five adult rhesus macaques was collected in EDTA. Monkeys RQ3570, CJ5B, and RQ3556 were euthanized at days 1192, 1540, and 1624 post-transplant respectively. The remaining animals are still alive. Brains (RQ3570, CJ5B, and RQ3556), lymph nodes (RQ3570, CJ5B, and RQ3556), and spleen (RQ3556) were collected at time of necropsy. These tissues were collected in 10% neutral buffered formalin and embedded in paraffin. Formalin fixed, paraffin-embedded tissues were cut into 6 μm-thick sections.
Flow Cytometry
The following specific phycoerythrin-conjugated monoclonal antibodies were used: anti-CD16 (clone 3G8) and anti-CD123 (clone 7G3), both from BD Biosciences (San Jose, CA). The following specific PerCP-Cy5.5-conjugated monoclonal antibody was used: anti-HLA-DR (clone L243) from BD Biosciences. The following specific allophycocyanin-conjugated monoclonal antibodies were used: anti-CD14 (clone M5E2) and anti-CD11c (clone S-HCL-3), all from BD Biosciences. To analyze the expression level of EGFP in subsets of circulating cells, EDTA anti-coagulated fresh peripheral blood was tested by four-parameter flow cytometry. Briefly, erythrocyte lysis of 100 μl aliquots of whole blood was processed using Immunoprep reagent on a T-Q prep machine (Beckman-Coulter, Fullerton, CA). The remaining leukocytes were incubated with an antibody mix composed of phycoerythrin-, PerCP-Cy5.5- and allophycocyanin-conjugated antibodies and incubated for 20 minutes at room temperature in the dark. The samples were washed with PBS and resuspended in PBS with 2% formaldehyde, and analyzed on a FACS Calibur flow cytometer (BD Biosciences).
Immunohistochemistry
EGFP fluorescence in paraffin-embedded and frozen sections was too weak to be detected directly in tissues. Preliminary studies determined that low detection of EGFP in frozen sections is likely due to solubility of the antigen in frozen sections. Overfixation of tissues in 10% formalin likely affected immunogenicity in paraffin-embedded sections. For these reasons, we detected EGFP in tissues using primary antibodies against EGFP followed by routine immunohistochemistry and/or immunofluorescence.24 For immunohistochemistry, deparaffinized and rehydrated sections were incubated with peroxidase block reagent (Dako, Carpinteria, CA) for 5 minutes at room temperature, washed twice then incubated with nonserum protein block (Dako) for 30 minutes at room temperature. Sections were then incubated 1 hour with a polyclonal rabbit anti-GFP antibody (Abcam, Cambridge, MA). After incubation with a peroxidase-conjugated polymer reagent, the color reaction product was developed using diaminobenzidine (Dako) as the chromogenic substrate for horseradish peroxidase. Sections were counterstained with hematoxylin diluted 1:2 (Sigma), dehydrated, and mounted using Vectamount medium (Vector Laboratories, Burlingame, CA). Controls consisted of isotype specific immunoglobulins corresponding to the test antibody. Some sections were stained for EGFP and CD163 (clone EDHu-1, AbD serotec, Raleigh, NC) with the later marker used to define perivascular macrophages.25 Double-label immunohistochemistry was performed using EnVision Doublestain System (Dako), according to the manufacturers’ instructions. The colored reaction product was developed using diaminobenzidine and Vector blue (Vector Laboratories). Sections were dehydrated in ethanol and a final bath of Histoclear (National Diagnostics, Atlanta, GA) instead of xylenes to preserve blue the chromogen intensity, and mounted using Vectamount medium. Sections were visualized under a Zeiss Axio Imager M1 microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY). Cells expressing EGFP or CD163 or both were counted in 6 to 9 sections of cerebrum (frontal lobe), 5 to 8 sections of cerebellum, and 4 arbitrary fields from 6 to 10 sections of choroid plexus in monkeys CJ5B, RQ3556, and RQ3570. Data were expressed as the percentage of EGFP+ cells expressing CD163: (number of CD163+EGFP+ cells)/(total number of EGFP+ cells) × 100 and the percentage of CD163+ macrophages derived from EGFP+ CD34+ HSC: (number of CD163+EGFP+ cells)/(total number of CD163+ cells) × 100.
Immunofluorescence and Confocal Microscopy
Multilabel immunofluorescence was used to establish the location of EGFP+ cells using glucose transporter (Glut-1, CNS endothelial cells) and their phenotype using CD163 and CD68 (brain perivascular macrophages25), smooth muscle actin (SMA, pericytes26), glial fibrillary acidic protein (GFAP, astrocytes) and neuronal nuclei (NeuN, neurons). After deparaffinization and rehydration, sections were washed with PBS 1×, 0.2% fish skin gelatin (PBS/FSG), and permeabilized by treatment with PBS/FSG and 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) for 40 minutes. Sections were washed with PBS/FSG at room temperature and blocked with PBS/FSG, 10% normal goat serum (PBS/FSG/NGS). Primary antibodies were diluted in PBS/FSG/NSG and incubated overnight at 4°C or 1 hour at room temperature. Anti-CD163 antibody (10D6; Novocastra, Newcastle on Tyne, UK) was used followed by anti-mouse Alexa 568 (Molecular Probes, Eugene, OR); anti-GFAP (rabbit polyclonal; Dako) antibody was used followed by anti-rabbit Alexa 568; anti-SMA (1A4; Dako), anti-NeuN (A60; Millipore, Billerica, MA), and anti-CD68 (KP1; Dako) antibodies were used followed by anti-mouse Alexa 488 (Molecular Probes); and anti-Glut-1 antibody (rabbit polyclonal; Chemicon) was used followed by anti-rabbit Alexa 488. Biotinylated anti-EGFP antibody (rabbit polyclonal; Molecular Probes) was used followed by streptavidin-avidin Alexa 647 or Alexa 568 (Molecular Probes). Sections were washed with PBS/FSG before the addition of the next primary or secondary antibody. Secondary antibodies were diluted in PBS/FSG/NSG and incubated for 40 minutes at room temperature. After antibody treatment, sections were rinsed in PBS/FSG, and then washed in distilled water for 5 minutes. To quench endogenous autofluorescence, sections were treated with a 50 mmol/L CU3SO4 in ammonium acetate buffer for 45 minutes.27 The sections were rinsed in distilled water and coverslipped with Vectashield mounting medium with or without 4,6 diamino-2-phenylindole (DAPI; Vectashield; Vector Laboratories). Sections were visualized under a Zeiss Axio Imager.M1 microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) using Plan-Apochromat ×20/0.8 and ×40/0.95 Korr objectives. Individual channels were collected simultaneously using AxioVision (version 4.6.3; Carl Zeiss MicroImaging, Inc.). Immunohistochemistry and immunofluorescence staining results are representative of n = 3 animals using at least three different CNS regions and examining at least 10 slides per section. Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA). Optical slices were collected at 512 × 512 pixel resolution. Each individual slice represented 0.2 micrometers. National Institutes of Health Image (version 1.62; NIH, Bethesda, MD) and Acrobat Photoshop (version 7.0; Adobe, San Jose, CA) were used to assign colors to the four channels collected: Alexa 647 appears green; Alexa 568 appears red and Alexa 488 appears blue and the differential interference contrast (DIC) image is gray scale. The four channels were collected simultaneously.
Results
Long-Term Reconstitution of Blood Monocytes in Rhesus Macaques with Autologous, Genetically Modified CD34-Positive Hematopoietic Stem Cells
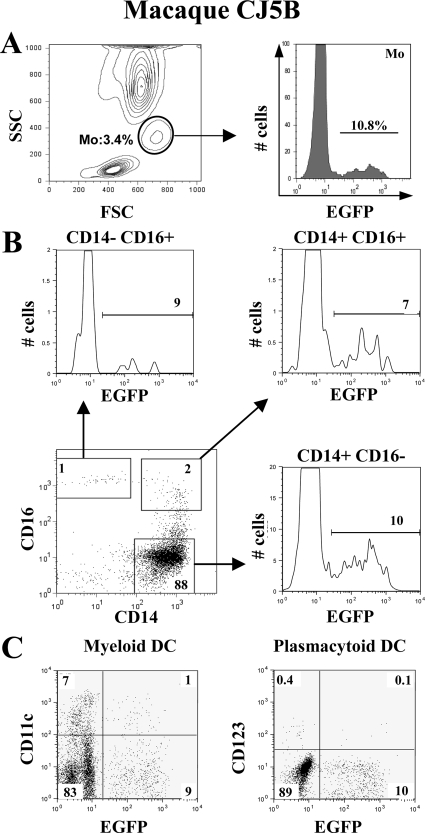
We assessed the expression of EGFP on whole blood from five rhesus macaques transplanted with autologous CD34+ HSCs that were transduced with either SIV-based or HIV-based (2RC003) vector particles constructed to express EGFP. We found that 8.1% ± 3.4% of monocytes still expressed EGFP as late as 4 years after transplantation (Figure 1A; mean ± SD, n = 5). Similar percentages of EGFP+ cells were detected within lymphocytes (6.2% ± 2.9%) and granulocytes (7.6% ± 2.7%) (mean ± SD, n = 5; Y. L. Kim et al, unpublished data). No significant differences were found between the percentages of EGFP-expressing cells within monocyte subsets: CD14+ CD16− (6.9% ± 2.7%), CD14+ CD16+ (4.8% ± 2.0%) and CD14− CD16+ (7.6% ± 2.3%) (Table 1, Figure 1B; mean ± SD, n = 5) suggesting that all subsets were equally repopulated from CD34+ progenitor/HSC-derived cells. Among lymphocytes, EGFP+ cells represented 7.1% ± 4.6% of CD4+ T lymphocytes, 5.6% ± 4.2% of CD8+ T lymphocytes and 6.1% ± 3.8% of CD20+ B lymphocytes (mean ± SD, n = 5; Y. L. Kim et al, unpublished data). The percentages of myeloid (HLA-DR+ CD11c+ CD123−) and plasmacytoid (HLA−DR+ CD11c− CD123+) dendritic cells expressing EGFP were 6% ± 3.6% and 6.5% ± 6.1% respectively (Figure 1C, Table 1; mean ± SD, n = 5). The overall percentages of circulating EGFP+ cells varied between animals (Table 1). Monkeys CJ5B and RQ3570 had the higher percentages of EGFP+ peripheral blood cells while monkey RQ3556 had a threefold lower percentage of EGFP+ cells (P < 0.02 according to non parametric Wilcoxon’s ranks test).
Figure 1.
Distribution of EGFP+ cells in peripheral blood of rhesus macaques transplanted with autologous CD34+ cells transduced with a lentivirus-based vector expressing EGFP. A: Expression of EGFP on monocytes (Mo). Monocytes were gated on the basis of their forward and side scatter properties. Representative histogram for the percentage of EGFP+ cells within this population is shown on the right. B: Expression of EGFP on CD14/CD16 monocyte subsets. Monocytes were first gated on HLA-DR+ cells. Monocyte subsets were then distinguished on the basis of CD14 and CD16 expression (dot plot). Histograms show the percentages of EGFP+ cells in each monocyte subset (CD14+CD16−, CD14+CD16+, CD14− CD16+). C: Expression of EGFP on DC subsets. DCs were first gated as HLA-DR+ cells. Non overlapping myeloid DC and plasmacytoid DC populations were then identified as CD11c+ CD123− and CD11c− CD123+ cells respectively. These data are from one animal (macaque CJ5B) out of five. Percentages of positive cells are indicated.
Table 1.
Percentages of EGFP+ Cells within Peripheral Blood Monocytes and Dendritic Cell Populations
| Cell population | CJ5B* | RQ3556* | RQ3570* | RQ5427 | 2RC003 | Mean ± SD |
|---|---|---|---|---|---|---|
| Total monocytes† | 10.8 | 3.3 | 10.6 | 5.7 | 9.8 | 8.1 ± 3.4 |
| CD14+ CD16− Mo | 10.0 | 3.6 | 9.4 | 5.8 | 5.9 | 6.9 ± 2.7 |
| CD14+ CD16+ Mo | 7.5 | 4.1 | 6.2 | 2.8 | 3.5 | 4.8 ± 2.0 |
| CD14− CD16+ Mo | 9.1 | 3.6 | 8.3 | 8.8 | 8.0 | 7.6 ± 2.3 |
| CD11c+ myeloid DC | 11.6 | 3.1 | 7.5 | 4.3 | 3.3 | 6.0 ± 3.6 |
| CD123+ plasmacytoid DC | 17.1 | 3.8 | 4.7 | 4.9 | 2.0 | 6.5 ± 6.1 |
Results presented as percentages of EGFP+ cells within each cell population.
Mo indicates monocytes and DC, dendritic cells.
Tissues collected from euthanized monkeys.
Total monocyte population was determined according to forward and side scatters properties. Monocyte and DC subsets were determined according to specific marker expression.
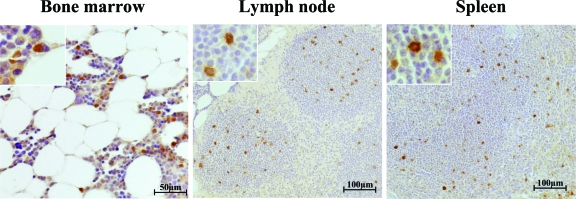
Macaques RQ3570, CJ5B, and RQ3556 were euthanized at day 1192, day 1540, and day 1624 post-transplant, respectively. To detect vector-expressed EGFP in tissue sections, we performed single label immunohistochemistry on lymphoid tissues. EGFP+ cells could be easily and specifically detected in the lymph node and the spleen as well as bone marrow at levels corresponding to the frequency of myeloid and lymphoid cells in the peripheral blood (Figure 2). As controls, lymph node, spleen, and bone marrow from non-transplanted animals were negative for EGFP. These results demonstrate that EGFP+ CD34+ HSCs repopulated peripheral blood and hematopoietic tissues for at least 4 years post-transplant.
Figure 2.
Distribution of EGFP+ cells in lymphoid tissues. Single staining immunohistochemistry reveals EGFP expression (brown, DAB chromogen) in bone marrow, lymph node, and spleen of monkey RQ3556. Insets show higher magnification of EGFP+ cells.
Partial Repopulation of CNS Macrophages in Rhesus Macaques by CD34+ HSC-Derived Cells Expressing EGFP
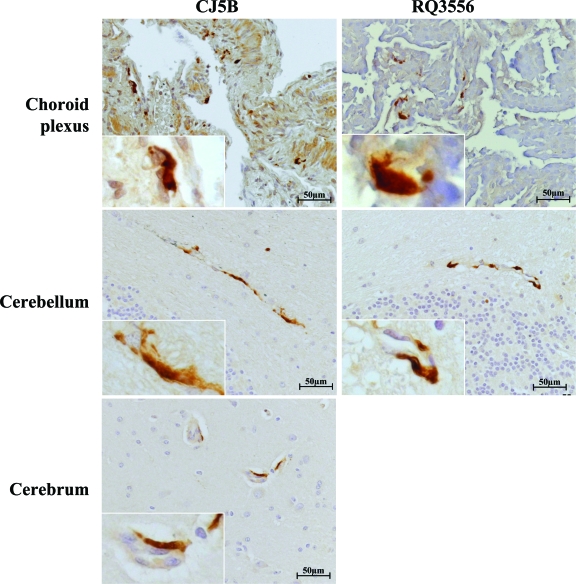
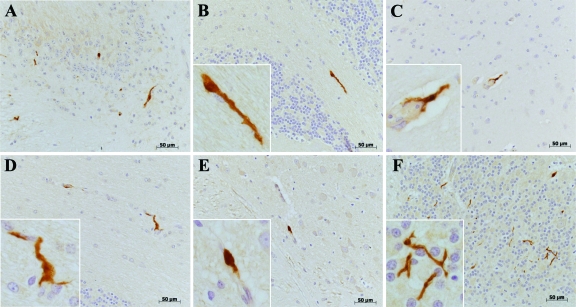
Little is known about the ontogeny of non-human primate perivascular macrophages in the CNS. We investigated whether EGFP+ CD34+ HSCs-derived cells could contribute to cell populations in the CNS of transplanted monkeys. In monkeys RQ3570, CJ5B, and RQ3556 we detected EGFP+ cells in different regions of the CNS. This staining seemed to be restricted to brain macrophages. EGFP+ cells were found in all brain sections examined including choroid plexus, cerebellum, and cerebrum (Figure 3). Interestingly we found fewer EGFP+ cells in brain tissues from macaque RQ3556 compared with macaques RQ3570 and CJ5B, consistent with the flow cytometric data showing lower percentages of circulating EGFP+ cells in RQ3556 than in RQ3570 or CJ5B (Table 1). Different morphologies of EGFP+ cells could be distinguished in the CNS (Figure 4). The majority of EGFP+ cells were elongated with small ramifications (Figure 4, A, C, D) or not (Figure 4B), while other cells had a round morphology (Figure 4E). In addition, we also detected stellate EGFP+ cells in the cerebellum (Figure 4F). All these morphologies are consistent with CNS perivascular macrophages. The distribution and percentage of EGFP+ cells within the CNS was not uniform. All EGFP+ cells were found exclusively in the vicinity of small capillaries and blood vessels, consistent with the hypothesis that they were CNS perivascular macrophages. We did not find EGFP+ cells within the CNS parenchyma representative of parenchymal microglia suggesting that EGFP+ CD34+ HSCs did not significantly contribute to parenchymal microglia repopulation.
Figure 3.
Distribution of EGFP+ cells in the CNS. Single staining immunohistochemistry of CNS tissues from monkeys CJ5B (left panels) and RQ3556 (right panels) reveals EGFP expression (brown, DAB chromogen) on cells within the choroid plexus, cerebellum, and cerebrum. Insets show higher magnification of EGFP+ cells.
Figure 4.
Different morphologies of EGFP+ cells detected in CNS tissues. Immunohistochemistry in different brain tissues reveals EGFP expression (brown, DAB chromogen). Different EGFP+ cells are detected on the basis of their morphology (A). Most of the EGFP+ cells were elongated without (B) or with some ramifications (C, D). Some EGFP+ cells were round (E). We also detected EGFP+ stellate cells in the cerebellum (F). Cerebellum: A, B, D, F; Cerebrum: C (mid brain) and E (frontal lobe). Insets show higher magnification of EGFP+ cells.
Brain EGFP+ Cells Express a Perivascular Macrophage Marker CD163
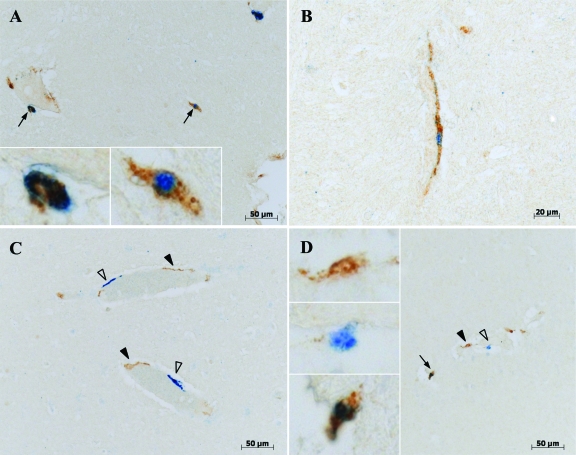
Based on the morphology and anatomical location of EGFP+ cells in the brain we hypothesized that EGFP+ cells detected in the CNS were perivascular cells. To further confirm this, we performed double-label immunohistochemistry for EGFP and CD163, a marker that selectively identifies perivascular macrophages in non-inflamed brains of monkeys and humans.28,29 We enumerated three populations of cells: EGFP+CD163+, EGFP+CD163−, and EGFP-CD163+ (Figure 5, A–D) in the choroid plexus, cerebellum, and cerebrum. We then calculated i) the proportion of EGFP+ cells that expressed CD163 (EGFP+CD163+ cells versus all EGFP+ cells) and ii) the percentage of CD163+ macrophages that were EGFP+ (EGFP+CD163+ cells versus all CD163+ cells) (Table 2). It was interesting to note that the percentage of CD163+ macrophages that were EGFP+ varied between different brain regions. There was a higher percentage of EGFP+ CD34+ HSC-derived CD163+ macrophages in the cerebellum as compared with the cerebrum and choroid plexus in all three animals. The CD163+ perivascular cells derived from the transplanted EGFP+ CD34+ progenitor/HSC represented 3% to 15%, 1% to 4%, and 3% to 6% of the total CD163+ macrophages in macaques CJ5B, RQ3556, and RQ3570 respectively (Table 2). These percentages were higher in CJ5B and RQ3570 in agreement with the higher percentage of EGFP+ cells in blood (Table 1) and the percentage of vector-modified hematopoiesis in macaques CJ5B and RQ3570 compared with RQ3556.22
Figure 5.
Phenotype of EGFP+ cells in the brain. Double-label immunohistochemistry of brain tissues (A–B: cortex; C–D: cerebellum) clearly shows three populations of cells. The open arrowheads point EGFP single-positive cells (blue, vector blue chromogen), black arrowheads point CD163 single-positive perivascular macrophages (brown, DAB chromogen), and black arrows point EGFP/CD163 double-positive cells (blue/brown). Insets show higher magnification of single or double positive cells.
Table 2.
Average Percentages of GFP+CD163+ Cells Within CNS Tissues
| Animal | Tissue | EGFP+CD163+ cells versus all EGFP+ cells (%)* | EGFP+CD163+ cells versus all CD163+ cells (%)† | n |
|---|---|---|---|---|
| CJ5B | Choroid plexus | 40 ± 8 | 3 ± 1 | 8 |
| Cerebellum | 37 ± 6 | 15 ± 3 | 5 | |
| Cerebrum (frontal lobe) | 60 ± 10 | 4 ± 1 | 9 | |
| RQ3556 | Choroid plexus | 27 ± 11 | 1 ± 0 | 10 |
| Cerebellum | 44 ± 10 | 4 ± 1 | 8 | |
| Cerebrum | ND | ND | ||
| RQ3570 | Choroid plexus | 72 ± 8 | 3 ± 1 | 6 |
| Cerebellum | 85 ± 7 | 6 ± 1 | 6 | |
| Cerebrum (frontal lobe) | 77 ± 7 | 2 ± 1 | 6 |
n is the number of tissue sections from which EGFP+CD163−, EGFP+CD163+ and EGFP−CD163+ cells were counted. For choroid plexus sections, four arbitrary microscope fields were counted per sections.
Mean ± SD of the percentage of EGFP+ cells expressing CD163 calculated as followed: (number of EGFP+CD163+ cells) / (total number of EGFP+ cells) ×100.
Mean ± SD of the percentage of CD163+ cells that derive from transplanted EGFP+ hematopoietic stem cells calculated as followed: (number of EGFP+CD163+ cells) / (total number of CD163+ cells) ×100.
ND denotes not done.
To clarify the phenotype of EGFP+ cells in the CNS that did not express CD163, we performed immunofluorescence staining for EGFP, CD163, and α-SMA, a marker for pericytes.26 We detected EGFP/CD163 double-positive cells but no EGFP+ cells expressing SMA (Figure 6A) suggesting that EGFP+CD163− cells were not pericytes. The staining for Glut-1 (Figure 6B), a marker for endothelial cells, confirmed that all EGFP+ cells were associated with blood vessels. Moreover, up to 71% of EGFP+ cells also expressed the macrophage marker CD68 (Figure 6C) suggesting that the majority of EGFP+ cells found in the CNS belong to the monocyte/macrophage lineage. In addition no EGFP+ cells were immunoreactive for GFAP and NeuN, markers for astrocytes and neurons respectively (Figure 6D). Altogether these results show that a significant proportion of EGFP+ cells detected in the CNS were perivascular macrophages and macrophages of choroid plexus, and that these cells were derived from CD34+ HSCs initially infused in rhesus macaques. These data further suggest that parenchymal microglia cells have a very low to non detectable turnover from HSCs in monkeys, as we did not detect EGFP+ cells that were morphologically and immunophenotypically consistent with microglial cells 4 years following transplant.
Figure 6.
EGFP+ cells located around blood vessels express CD163 but not SMA. Representative images of multilabel immunofluorescence staining of CNS tissue from a rhesus macaque are shown. A: Confocal microscopy image shows co-localization of EGFP and CD163 (yellow) indicating that EGFP+ cells (green, Alexa Fluor 647-streptavidin) express CD163 (red, Alexa Fluor 568-goat anti-mouse IgG1) but not SMA (blue, Alexa Fluor 488 goat anti-mouse IgG2a). The acquired EGFP, CD163 and SMA, the merge image and the differential interference contrast (DIC) are shown. B: Double-label immunofluorescence microscopy shows EGFP+ cells (green, Alexa Fluor 568-streptavidin) in the vicinity of a blood vessel stained with Glut-1 (red, Alexa Fluor 488-goat anti-rabbit). Co-localization of EGFP and DAPI (gray) results in a white. The acquired EGFP, Glut-1, and DAPI, the merge image and DIC are shown. C: Double-label immunofluorescence microscopy shows co-expression of EGFP (green, Alexa Fluor 647-streptavidin) and CD68 (red, Alexa Fluor 568-goat anti-mouse IgG1). Nuclei were stained with DAPI (gray). The acquired EGFP, CD68, and DAPI, the merge image, and DIC are shown. D: Multilabel immunofluorescence microscopy shows EGFP+ cells (green, Alexa Fluor 647-streptavidin) that are distinct from GFAP+ astrocytes (red, Alexa Fluor 568-goat anti-rabbit) and from NeuN+ neurons (blue, Alexa Fluor 488-goat anti-mouse IgG1). Nuclei were stained with DAPI (gray). The acquired EGFP, GFAP, NeuN, and DAPI, the merge image, and DIC are shown.
Discussion
We took advantage of rhesus macaques transplanted with autologous CD34+ HSCs expressing EGFP from replication-incompetent lentiviral vectors to investigate the origin and repopulation of perivascular macrophages in the CNS of non-human primates. These animals had long-lasting and stable expression of EGFP in peripheral blood cells, bone marrow, spleen, and lymph nodes 4 years post-transplant. This confirms the multilineage long-term reconstitution of rhesus transplanted with CD34+ cells transduced with an SIV-derived replication incompetent lentiviral vector21,22 (Y. L. Kim et al, unpublished data). No significant differences in the percentage of EGFP+ cells were observed between the different blood cell lineages, suggesting stable contribution from long-term repopulating multilineage CD34+ HSCs.
We detected significant numbers of EGFP+ macrophages in multiple regions of the CNS including the cerebellum, cerebrum, and choroid plexus. Interestingly, the percentage of EGFP+ circulating monocytes and dendritic cells as measured by flow cytometric analysis correlated well with the relative percentages of EGFP+ macrophages detected in the CNS of animals CJ5B, RQ3570, and RQ3556, suggesting that continuous seeding of this cell population from CD34+ HSC/progenitors engrafting post-transplantation had occurred and that by 4 years a steady-state had been reached, at least in the cerebellum, where the percentage of EGFP+ macrophages was almost identical to the percentage of EGFP+ monocytes in the peripheral blood. Together these observations support the notion of trafficking of bone marrow-derived progenitors to the CNS to populate brain macrophages, and that by 4 years the entire population of perivascular macrophages had likely turned over at least in the cerebellum, given that the percentage of EGFP+ cells in cerebellar brain macrophages was similar to the percentage in peripheral blood lineages. The somewhat lower levels of EGFP+ macrophages in the cerebrum and choroid plexus suggests that perhaps macrophages in these regions turn over more slowly.
Brain EGFP+ cells with heterogeneous morphologies were located exclusively in the vicinity of large and small blood vessels showing characteristics of perivascular cells and CNS macrophages. These observations are consistent with other studies on murine perivascular macrophages16 and bone marrow-derived cells in adult mouse brain.18 Depending on the animal and the region of the CNS analyzed, between 25% and 85% of these cells expressed CD163, a marker of perivascular macrophages in the CNS.6,28,29 This indicates that a significant proportion of the EGFP+ cells detected in the CNS were perivascular macrophages; greater than 70% of EGFP+ cells were CD68+, further confirming their lineage as macrophages.
Which cells in bone marrow and blood give rise to brain perivascular macrophages is not clearly established. Perivascular macrophages are located at the Virchow-Robin spaces that are the sites of infiltration of blood-derived cells in the CNS under normal and inflammatory conditions.19,30 Rodent studies have shown that perivascular macrophages are continuously renewed by bone marrow-derived blood monocytes.9,17,19,31 Different subsets of CD14+ monocytes can be distinguished on the basis of CD16 expression.32,33 The CD14+ CD16+ monocytes are described as “resident” cells migrating to tissues to renew macrophage populations while the CD14+ CD16− monocytes are “inflammatory” cells thought to play a role under pathological conditions.34 We and others have previously shown that a subset of “resident” CD14+ CD16+ blood monocytes express CD163 and could be precursors of brain CD163+ perivascular macrophages.13,28 Both circulating CD14+ CD16+ monocytes and brain perivascular macrophages of transplanted rhesus monkeys strongly expressed EGFP suggesting that the EGFP+ CD163+ perivascular macrophage population was replenished by monocytic precursors differentiated from genetically modified CD34+ progenitor/HSC.
In macaque brains, we found that a significant majority of EGFP+ cells, greater than 70%, expressed the macrophage marker CD68. EGFP immunofluorescence was not detected in populations of neurons, astrocytes, or pericytes. Studies in rodents using a similar GFP expression system in stem cells showed GFP+ parenchymal microglia and cerebellar neurons by direct fluorescence detection.35,36 Whether marked differences between primates and rodents account for different cells labeled with GFP is not known. In confirmation of our results Bechman and Vallieres have also demonstrated in rodents that EGFP-labeled bone marrow cells give rise selectively to CNS perivascular macrophages.18,31
Deciphering the turnover of brain perivascular macrophages is of importance because of their crucial role in the development of neuroAIDS and other neurological disorders.15,37 Monocyte/macrophages have been considered target cells that act as a “Trojan horse” carrying HIV/SIV into the brain.37 Our observations also underscore the importance of HSCs, bone marrow-derived progenitors, and perivascular macrophages as important targets of HIV/SIV. Tools allowing the modulation of their migration to CNS tissues or affecting their capacity to be infected by HIV/SIV have become major objectives in neuroAIDS therapeutic research, as well as therapy to selectively target such cells in bone marrow and blood using gene therapy approaches.38 An et al recently demonstrated the successful knockdown of CCR5 in circulating leukocytes from two rhesus macaques (RQ3570 and RQ5427) transplanted with autologous CD34+ HSCs transduced with a SIV vector containing short hairpin RNA against CCR5 and the EGFP reporter gene.21 EGFP+ lymphocytes have previously been isolated from these monkeys, infected by SIV mac239, and shown to have a threefold lower in vitro production of p27 SIV as compared with cells containing the mock vector.21 The detection of EGFP+ perivascular macrophages in the brain of animal RQ3570 suggests that the vector containing the short hairpin RNA against CCR5 has established residence in brain macrophage population via bone marrow-derived monocyte/macrophages. CCR5 expression by these perivascular macrophages is under investigation. Human macrophages containing lentiviral vector-expressed small interfering RNA against CCR5 are protected against HIV-1 infection.39 Our results suggest that not only can lymphocytes and monocyte/macrophages of the immune system be therapeutically targeted using CD34+ hematopoietic stem cell transplantation, but also the perivascular macrophages of the CNS. Finally the introduction of genes into the CNS via bone-marrow stem cells is also of great interest in the development of treatments of other degenerative diseases such as CNS storage disorders.40,41
In summary this study reports the detection of long-term EGFP-expressing cells following transplantation of rhesus macaques with autologous EGFP-expressing CD34+ HSCs. We show that brain CD163+ perivascular macrophages from rhesus macaques are derived from CD34+ HSCs. This autologous transplantation model will be of great use not only to decipher the ontogeny of perivascular macrophages in primates but also as a promising therapeutic tool to evaluate the ability to confer stable HIV/SIV resistance to monocyte/macrophages that are the target cells responsible for the development of AIDS-associated neuropathogenesis.
Acknowledgments
We thank the animal care staff and technicians for their excellent care and handling of the animals at 5 Research Court and Poolesville. We also especially thank Dr. Lauren Brinster and her staff in the Department of Pathology within the NIH Veterinary Resource Program for performing the necropsies. We thank Dr. Marcelo Kuroda and Dr. Woong-Ki Kim for critically reading the manuscript.
Footnotes
Address reprint requests to Kenneth C. Williams, Boston College, Department of Biology, Higgins Hall 445B, 140 Commonwealth Avenue, Chestnut Hill, MA 02467. E-mail: williauy@bc.edu.
Supported in part by the Intramural Research Program of the NIH (R.E.D. and C.E.D.), by National Heart Lung and Blood Institute Program Project PO1HL05374921765 and American Lebanese Associated Charities (D.A.P.), by Public Health Service Grants RR00164 (X.A.H.) and by grants from the NIH, including the National Institute of Neurologic Disease and Stroke NS040237 and NS037654 (to K.C.W.).
References
- Graeber MB, Streit WJ, Kreutzberg GW. Identity of ED2-positive perivascular cells in rat brain. J Neurosci Res. 1989;22:103–106. doi: 10.1002/jnr.490220114. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Gay D. Immunological and neuropathological significance of the Virchow-Robin space. J Neurol Sci. 1990;100:3–8. doi: 10.1016/0022-510x(90)90004-7. [DOI] [PubMed] [Google Scholar]
- Walker WS. Separate precursor cells for macrophages and microglia in mouse brain: immunophenotypic and immunoregulatory properties of the progeny. J Neuroimmunol. 1999;94:127–133. doi: 10.1016/s0165-5728(98)00237-9. [DOI] [PubMed] [Google Scholar]
- Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14:1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- Fabriek BO, Van Haastert ES, Galea I, Polfliet MM, Dopp ED, Van Den Heuvel MM, Van Den Berg TK, De Groot CJ, Van Der Valk P, Dijkstra CD. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia. 2005;51:297–305. doi: 10.1002/glia.20208. [DOI] [PubMed] [Google Scholar]
- Kida S, Steart PV, Zhang ET, Weller RO. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol. 1993;85:646–652. doi: 10.1007/BF00334675. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB. Heterogeneity of microglial and perivascular cell populations: insights gained from the facial nucleus paradigm. Glia. 1993;7:68–74. doi: 10.1002/glia.440070112. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Williams K, Ulvestad E, Antel JP. B7/BB-1 antigen expression on adult human microglia studied in vitro and in situ. Eur J Immunol. 1994;24:3031–3037. doi: 10.1002/eji.1830241217. [DOI] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Tedaldi EM, Rappaport J. CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses. 2008;24:417–421. doi: 10.1089/aid.2007.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner AA, Smith MO, Munn RJ, Martfeld DJ, Gardner MB, Marx PA, Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139:609–621. [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7:19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Vass K, Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992;51:246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T, Fraser PA, Cserr HF. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 1991;545:103–113. doi: 10.1016/0006-8993(91)91275-6. [DOI] [PubMed] [Google Scholar]
- Unger ER, Sung JH, Manivel JC, Chenggis ML, Blazar BR, Krivit W. Male donor-derived cells in the brains of female sex-mismatched bone marrow transplant recipients: a Y-chromosome specific in situ hybridization study. J Neuropathol Exp Neurol. 1993;52:460–470. doi: 10.1097/00005072-199309000-00004. [DOI] [PubMed] [Google Scholar]
- An DS, Donahue RE, Kamata M, Poon B, Metzger M, Mao SH, Bonifacino A, Krouse AE, Darlix JL, Baltimore D, Qin FX, Chen IS. Stable reduction of CCR5 by RNAi through hematopoietic stem cell transplant in non-human primates. Proc Natl Acad Sci USA. 2007;104:13110–13115. doi: 10.1073/pnas.0705474104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H, Hematti P, Keyvanfar K, Metzger ME, Krouse A, Donahue RE, Kepes S, Gray J, Dunbar CE, Persons DA, Nienhuis AW. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood. 2004;103:4062–4069. doi: 10.1182/blood-2004-01-0045. [DOI] [PubMed] [Google Scholar]
- Sander WE, Metzger ME, Morizono K, Bonifacino A, Penzak SR, Xie YM, Chen IS, Bacon J, Sestrich SG, Szajek LP, Donahue RE. Noninvasive molecular imaging to detect transgene expression of lentiviral vector in nonhuman primates. J Nucl Med. 2006;47:1212–1219. [PubMed] [Google Scholar]
- Swenson ES, Price JG, Brazelton T, Krause DS. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells. 2007;25:2593–2600. doi: 10.1634/stemcells.2007-0241. [DOI] [PubMed] [Google Scholar]
- Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, Bussolati G, Ravazzola M, Orci L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989;37:315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- Kim WK, Alvarez X, Fisher J, Bronfin B, Westmoreland S, McLaurin J, Williams K. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol. 2006;168:822–834. doi: 10.2353/ajpath.2006.050215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ES, Masliah E, Fox HS. CD163 identifies a unique population of ramified microglia in HIV encephalitis (HIVE). J Neuropathol Exp Neurol. 2004;63:1255–1264. doi: 10.1093/jnen/63.12.1255. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Kwidzinski E, Kovac AD, Simburger E, Horvath T, Gimsa U, Dirnagl U, Priller J, Nitsch R. Turnover of rat brain perivascular cells. Exp Neurol. 2001;168:242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW. Definition of human blood monocytes. J Leukoc Biol. 2000;67:603–606. doi: 10.1002/jlb.67.5.603. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, Fernandez-Klett F, Prass K, Bechmann I, de Boer BA, Frotscher M, Kreutzberg GW, Persons DA, Dirnagl U. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- Priller J, Persons DA, Klett FF, Kempermann G, Kreutzberg GW, Dirnagl U. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J Cell Biol. 2001;155:733–738. doi: 10.1083/jcb.200105103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Avarez X, Williams K. The role of monocytes and perivascular macrophages in HIV and SIV neuropathogenesis: information from non-human primate models. Neurotox Res. 2005;8:107–115. doi: 10.1007/BF03033823. [DOI] [PubMed] [Google Scholar]
- Hasson H, Mantelli B, Biswas P, Malnati M, Gianotti N, Vecchi A, Nozza S, Cernuschi M, Boeri E, Clerici M, Lazzarin A, Beretta A. Favorable outcome of ex-vivo purging of monocytes after the reintroduction of treatment after interruption in patients infected with multidrug resistant HIV-1. J Med Virol. 2007;79:1640–1649. doi: 10.1002/jmv.20977. [DOI] [PubMed] [Google Scholar]
- Anderson J, Akkina R. Complete knockdown of CCR5 by lentiviral vector-expressed siRNAs and protection of transgenic macrophages against HIV-1 infection. Gene Ther. 2007;14:1287–1297. doi: 10.1038/sj.gt.3302958. [DOI] [PubMed] [Google Scholar]
- Leimig T, Mann L, Martin Mdel P, Bonten E, Persons D, Knowles J, Allay JA, Cunningham J, Nienhuis AW, Smeyne R, d'Azzo A. Functional amelioration of murine galactosialidosis by genetically modified bone marrow hematopoietic progenitor cells. Blood. 2002;99:3169–3178. doi: 10.1182/blood.v99.9.3169. [DOI] [PubMed] [Google Scholar]
- Biffi A, De Palma M, Quattrini A, Del Carro U, Amadio S, Visigalli I, Sessa M, Fasano S, Brambilla R, Marchesini S, Bordignon C, Naldini L. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Invest. 2004;113:1118–1129. doi: 10.1172/JCI19205. [DOI] [PMC free article] [PubMed] [Google Scholar]