Abstract
Initiation of productive immune responses against Leishmania depends on the successful transition of dendritic cells (DC) from an immature to a mature phenotype. This process is characterized by high CD40 surface expression as well as interleukin-12 production, which are frequently seen in response to L. major infection. In vivo footpad infection of C3HeB/FeJ mice for 7 days with L. amazonensis promoted an immature CD11c+ DC phenotype characterized by both significantly low CD40 surface expression and significantly decreased interleukin-12p40 production compared with L. major infection of these same mice. In vitro infection of bone marrow-derived dendritic cells with L. amazonensis amastigotes resulted in rapid and significant phosphorylation of the mitogen activated protein kinase, extracellular signal-regulated kinase 1/2, observed within minutes of exposure to the parasite. Infection with L. amazonensis promastigotes led to increased 1/2 phosphorylation after 4 hours of infection compared with L. major infection, which correlated with promastigote transformation into amastigotes. Treatment of bone marrow-derived dendritic cells with a mitogen activated protein kinase kinase-specific inhibitor, PD98059, led to regained surface CD40 expression and interleukin-12p40 production following L. amazonensis amastigote infection compared with non-treated, infected DC. Treatment of L. amazonensis-infected mice with the highly-specific mitogen activated protein kinase kinase inhibitor, CI-1040, enhanced surface CD40 expression on CD11c+ DC obtained from the draining lymph node. L. amazonensis amastigotes, through activation of extracellular signal-regulated kinase 1/2, inhibit the ability of DC to undergo proper maturation both in vitro and in vivo.
Leishmaniasis is a vector-borne disease caused by intracellular protozoan parasites of the genus Leishmania. The intracellular amastigote stage of Leishmania predominates in the mammalian host while the vector stage exists as an extracellular, flagellated promastigote. Both promastigotes and amastigotes are capable of initiating mammalian infection. Leishmania amastigotes have been shown to interfere with host cell function, including modulation of signaling pathways, suppression of antimicrobial and pro-inflammatory mediators, and induction of cytokines that promote disease progression.1,2
Infection of C3HeB/FeJ mice with Leishmania major leads to the development of a healing TH1 immune response characterized by high levels of interleukin-12 and interferon (IFN)-γ-producing CD4+ T cells.3 In contrast, infection with L. amazonensis results in a non-healing immune response, leading to chronic disease and high parasite loads. Multiple studies have investigated and identified differences in the T cell response to these two parasites.4,5,6 Studies from our laboratory have revealed that antigen-responsive CD4+ T cells from C3HeB/FeJ mice chronically infected with L. amazonensis are impaired in their ability to transition from a naïve to an effector phenotype.6
Leishmania spp. infect phagocytic cells of the immune system, primarily macrophages and dendritic cells (DC). DC are antigen presenting cells that efficiently initiate antigen-specific immune responses by inducing the differentiation of naïve T cells.7 DC maturation can be characterized in vitro and ex vivo by the upregulation of co-stimulatory molecules including CD40 and the production of T cell-polarizing cytokines such as interleukin (IL)-12.7 Clearance and resistance to Leishmania infection is dependent on the development of a TH1 response3,8 and susceptibility to Leishmania infection has been associated with deficiencies in CD40, CD40 ligand,9,10,11 and IL-12 or IL-12 receptor subunits.12,13 Infection of C57BL/6 mice bone marrow-derived dendritic cells (BMDC) with L. amazonensis promastigotes indicated a significant reduction in CD40 surface expression and IL-12p40 production as compared with L. major promastigote-infected BMDC.14 Moreover, a recent publication indicated that L. amazonensis amastigote infection of BMDC significantly down-regulated CD40 expression and suppressed IL-12p40 and IL-12p70 production, leading to an impairment in antigen presenting cell function, characterized by an inability of L. amazonensis-amastigote infected BMDC to prime naïve CD4+ T cells or re-stimulate antigen-specific CD4+ T cells.15
Improper or insufficient activation of DC can promote a non-polarized, often tolerogenic, T cell response. DC maturation depends on the balance of particular molecular signaling cascades.7,16,17 One such pathway is mitogen activated protein kinase (MAPK) signaling cascades composed of three primary kinases, p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK). A recent report has linked ERK activation in macrophages with the induction of IL-10, a critical cytokine involved in host susceptibility to Leishmania infection.18 Work from our own laboratory has shown that inhibition of ERK in vitro increases the ability of L. amazonensis-infected macrophages to kill the intracellular parasites.19 Unlike p38 and JNK, ERK has been shown to play a role in preventing proper maturation of DC.16,20
As L. amazonensis-infected mice fail to develop an effective TH1 response,4,5,6 and L. amazonensis-infected BMDC in vitro have been shown to have an altered maturation phenotype,14,15 we hypothesized that L. amazonensis infection in vitro promotes an immature DC phenotype consistent with the observed lack in T cell polarization. In this study, we present novel ex vivo evidence that L. amazonensis infection promotes an immature CD11c+ DC phenotype characterized by significantly low CD40 surface expression and significantly decreased IL-12p40 production as compared with L. major infection. Furthermore, we explored the molecular mechanisms that may lead to impaired DC maturation and found that in vitro, BMDC infection with L. amazonensis amastigotes resulted in rapid and significant phosphorylation of the MAP kinase ERK1/2, observed within minutes of exposure to the parasite. Infection with L. amazonensis promastigotes led to increased ERK1/2 phosphorylation as compared with L. major infection; however, this phosphorylation was delayed several hours. This delay in phosphorylation correlated with promastigote transformation into amastigotes within infected DC, as confirmed by microscopic analysis of parasite stage before and after initiation of robust ERK phosphorylation. In vitro inhibition studies determined that treatment of DC with a mitogen activated protein kinase kinase (MEK)-specific inhibitor, PD98059, led to enhanced surface CD40 expression and IL-12p40 production following L. amazonensis-amastigote infection as compared with non-treated cells. Treatment of L. amazonensis-infected mice with the highly-specific MEK inhibitor, CI-1040, enhanced surface CD40 expression. Together, these data indicate that L. amazonensis amastigotes, through activation of the MAP kinase ERK1/2, inhibit the ability of DC to undergo proper maturation in vivo. This is the first report of use of a biochemical inhibitor, here targeted to the MAPK ERK, which restores the immune phenotype of dendritic cells after pathogen infection both in vitro and in vivo.
Materials and Methods
Mice
C3HeB/FeJ mice were bred in house or obtained from Jackson Laboratories (Bar Harbor, ME) and maintained in a specific-pathogen-free facility. The Institutional Animal Care and Use Committee at Iowa State University approved all protocols involving animals. Six- to eight-week old females were inoculated with 1 × 106 stationary-phase promastigotes in 50 μl of PBS in the left hind footpad.
C3H severe combined immunodeficiency mice (C3SnSmn. CB17-Prkdcscid/J) were inoculated with 1 to 2 × 107 stationary-phase promastigotes in 50 μl of PBS in the left hind footpad. These mice were later sacrificed for tissue-derived amastigotes.
For in vivo ERK inhibitor treatment, L. amazonensis- and L. major-infected C3HeB/FeJ mice were orally gavaged twice daily with 100 mg/kg of CI-1040 (kind gift from Pfizer Global Health, Groton, CT) in dimethyl sulfoxide supplemented with 0.5% hydroxypropyl methyl cellulose and 0.2% Tween 80, for a total of 7 days and sacrificed.
Isolation and Preparation of BMDC
BMDC were cultured in vitro in the presence of 10 ng/ml of murine granulocyte-macrophage colony-stimulating factor (PeproTech Inc., Rocky Hill, NJ) according to the method of Lutz et al.21 At day 10 of culture, approximately 90% of the BMDC were positive for the DC marker CD11c. For in vitro studies, 10-day-old BMDC were incubated for 24 hours at 37°C, 5% CO2 with fresh, tissue-derived amastigotes at a cell to parasite ratio of 1:3.
Parasites and Infection
Culture of L. amazonensis (MHOM/BR/00/LTB0016) and L. major (MHOM/IL/80/Friedlin) parasites was performed as previously described.5 For in vitro promastigote experiments, stationary phase L. amazonensis or L. major promastigotes were used. Where indicated, parasites were labeled using the dye carboxyfluorescein diacetate succinimidyl ester (Molecular Probes, Eugene, OR) as previously described22 with some modifications. Amastigotes were directly recovered from footpad lesions of infected C3H severe combined immunodeficiency mice. Infected feet were disinfected with 70% ethanol, and extraneous tissue dissected away. The remaining lesion was then homogenized with a Tenbrock tissue homogenizer. Cellular debris was removed by centrifuging at 180 × g for 15 minutes at 4°C. The resulting supernatant was centrifuged at 3000 × g for 15 minutes at 4°C. The number of viable amastigotes in the pellet was assessed by fluorescent microscopy with fluorescein diacetate (Acros Organics, Morris Plains, NJ) and propidium iodide (Sigma, St. Louis, MO). For in vitro infection of BMDC, cells were infected with either L. amazonensis or L. major at a multiplicity of infection of 3. Where indicated, as a positive control for maturation, some BMDC were stimulated with 500 ng of lipopolysaccharide (Sigma, St. Louis, MO) and 200iU of IFN-γ (Pharmigen, San Diego, CA), or treated with the MEK inhibitor PD98059 (Sigma, St. Louis, MO).
Flow Cytometry
For flow cytometry analysis of surface molecule expression, 1 × 106 BMDC were washed in 2 ml of fluorescence-activated cell sorting buffer (FACS, 0.1% sodium azide and 0.1% bovine serum albumin in phosphate buffer saline). Fcγ receptors were blocked with 10% purified rat anti-mouse CD16/CD32 antibody (BD Pharmingen, San Diego, CA) in 1 mg/ml rat IgG for 20 minutes at 4°C to prevent nonspecific binding. BMDC were then incubated with the appropriate antibody or isotype control for 30 minutes on ice. The antibodies used include fluorescein isothiocyanate-labeled CD11c (HL3), PerCP-Cy5.5-labeled CD11b (M1/70), phycoerythrin-labeled CD40 (3/23), allophycocyanin-labeled CD80 (16-10A1), phycoerythrin-Cy7-labeled CD86 (GL-1), allophycocyanin-Cy7-labeled CD19 (1D3), and allophycocyanin-Cy7-labeled CD3e (145-2C11). CD11c, CD11b, CD40, CD19, and CD3 were purchased from BD Pharmigen (San Diego, CA). CD80 and CD86 were purchased from Biolegend (San Diego, CA). Following staining, cells were washed in 2 ml of FACS buffer and fixed in 200 μl of 1% paraformaldehyde and stored at 4°C until analysis. Analysis was performed on a BD FACScanto flow cytometer (Becton Dickinson, San Jose, CA), and data analyzed using FlowJo software V8.5.2 (Tree Star, Inc., Ashland, OR).
Isolation of Cell Extracts
To make whole cell lysates, 3 × 106 BMDC were resuspended in 400 μl of 1× cell lysis buffer (Cell Signaling Technologies, Beverly, MA), supplemented with 1 mmol/L phenylmethylsulphonyl fluoride and a protease inhibitor cocktail (Roche, Indianapolis, IN) immediately before use. Samples were incubated on ice for 15 minutes and then centrifuged at 16,000 × g for 5 minutes at 4°C. Supernantants were collected as whole cell lysates and stored at −80°C.
Immunoblot Analysis
Protein content of all cell extracts was determined via BCA protein assay (Pierce, Rockford, IL) according to manufacturer’s recommendations, and all samples were normalized to 1 mg/ml using distilled water. Samples (20 to 30 μg of protein) were heated for 4 minutes at 95°C in 1× loading buffer and electrophoresis was performed on a 12% SDS-polyacrylamide electrophoresis gel. Gels were electroblotted onto polyvinylidene fluoride membranes, blocked with 5% bovine serum albumin, and probed with antibodies specific for phospho-ERK, p38 and JNK, total-ERK1/2, p38, JNK (1:1000) (Cell Signaling, Beverly, MA), and β-actin (1:5000) (Sigma, St. Louis, MO). Signals were detected with horseradish-peroxidase-conjugated goat anti-rabbit antibodies (1:20,000) (Jackson ImmunoResearch, West Grove, PA) using the SuperSignal West chemiluminescent substrate (Pierce, Rockford, IL) and expressed to autoradiography film (Midsci, St. Louis, MO).
Indirect Immunofluorescence and H&E Stain
BMDC (5 × 105) were plated onto 24-well plates containing tissue coverslips. BMDC were infected with L. amazonensis promastigotes at a multiplicity of infection of 3:1 and incubated at 34°C with 5% CO2. Tissue coverslips were then harvested and fixed with 4% paraformaldehyde in PBS for 20 minutes at room temperature and washed three times with PBS. BMDC were permeabilized with 0.1% saponin in PBS for 10 minutes at room temperature. Cells were incubated for 1 hour at room temperature with mouse anti-LPG CA7AE monoclonal antibody (kind gift from Dr. Jeffery Beetham, Iowa State University) at a 1:100 dilution in 0.1% saponin. After incubation, coverslips were washed three times with PBS and incubated for 1 hour at room temperature with rabbit anti-mouse Cy2-conjugated antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, kind gift from Dr. Bryan Bellaire, Iowa State University) at a 1:200 dilution in 0.1% saponin. BMDC were then counterstained with propidium iodide according to manufacturer’s instructions (Molecular Probes, Eugene, OR). Coverslips were then mounted onto slides using MOWIOL (Calbiochem, La Jolla, CA) and viewed using an Olympus IX71 inverted epifluorescence scope (Olympus America Inc., Center Valley, PA); 300 infected cells were counted on each coverslip.
For morphological analysis, coverslips were harvested at the indicated time points, fixed in 100% methanol for 5 minutes and stained with H&E according to manufacturer’s instructions (Fisher Diagnostics, Middletown, VA). Coverslips were analyzed using a Nikon Eclipse 50i light microscope (Nikon Instruments Inc., Melville, NY).
IL-12p40 Enzyme-Linked Immunosorbent Assay and ELIspot
Supernatants from BMDC cultures were harvested at indicated time points following amastigote or promastigote infection, and IL12-p40 enzyme-linked immunosorbent assay was performed using commercially available antibodies (BD Pharmigen, San Diego, CA), peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA) and ABTS microwell peroxidase substrate (Roche, Indianapolis, IN). IL-12p40 ELIspots were performed on total lymph node cells using commercially available purified IL-12p40 and biotinylated anti-IL12p40 antibodies (BD Pharmigen, San Diego, CA), and developed using 2-amino-2-methyl-1-propanol (ICN Biomedicals Inc., Aurora, OH) and 5-bromo-4-chloro-3-indoly-phosphate (Fisher, Fair Lawn, NJ).
Statistics
Statistical analysis between two values was determined via Student’s t-tests. P values of <0.05 were considered as statistically significant.
Results
DC in the Draining Lymph Node of L. amazonensis-Infected Mice Have an Immature Phenotype
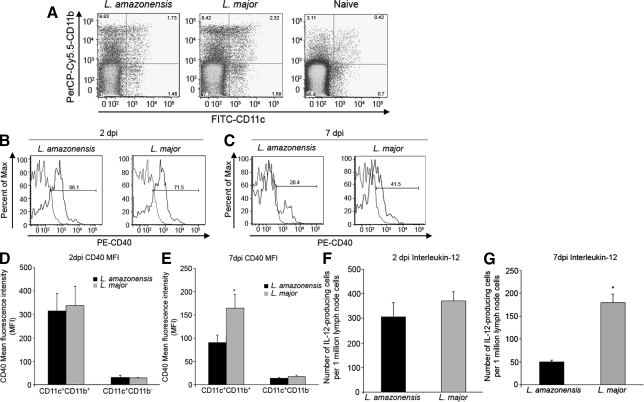
Recent studies demonstrated that BMDC infected with L. amazonensis amastigotes have an immature phenotype, as determined by CD40 expression and IL-12p40 and IL-12p70 production, and are impaired in their ability to prime naïve CD4+ T cells as compared with L. amazonensis promastigote-infected BMDC.15 Our laboratory has shown that CD4+ T cells from mice chronically infected with L. amazonensis are impaired in their ability to transition into an effector phenotype.6 We propose that these differences in BMDC phenotype previously observed to be elicited by L. amazonensis promastigotes and amastigotes in vitro, would be reflected during in vivo infection as the infection progresses from peracute (2 days post-infection [dpi]) to acute (7 dpi). Mice were infected subcutaneously in the left footpad with L. amazonensis or L. major promastigotes and sacrificed at 2 and 7 dpi. DC, as designated by CD11c+ cells23 from the draining lymph node (DLN) of infected mice, collected based on CD19 and CD3 double negative events (Figure 1A), were analyzed for DC maturation markers. As shown previously, infection with Leishmania not only increases the number of CD11c+-events in the DLN, but also increases CD40 expression in these cells.23 CD11c+ DC surface expression of CD40 (Figure 1, B and D), CD80 and CD86 (data not shown) was similar between L. amazonensis-infected and L. major-infected mice at 2 dpi. By 7 dpi, CD11c+ DC from L. amazonensis-infected mice had significantly lower CD40 surface expression as measured by FACS analysis as compared with DC from L. major-infected mice (Figure 1, C and E). No differences were observed in the surface expression of CD80 and CD86 (data not shown) between L. amazonensis-infected and L. major-infected mice at 7dpi, suggesting that CD40 expression is specifically targeted for down-regulation.
Figure 1.
Decreased CD40 expression and IL-12p40-producing cells from DC subsets of total draining lymph node cells from L. amazonensis-infected mice at 7 days postinfection. Mice were infected with L. amazonensis or L. major promastigotes and sacrificed at 2 and 7 days post-infection. CD11c+ DC subsets from total draining lymph node cells, collected on CD19 and CD3 double negative events (A) from L. amazonensis-infected, L. major-infected, and naïve mice. Cells were analyzed for CD40 surface expression via FACS scan. Histograms (B and C) indicate percent CD40 positive CD11c+CD11b+ cells (gray, isotype control; black, CD40) and bar graphs (D and E) indicate CD40 mean fluorescence intensity of cells at 2dpi (B and D) and 7 dpi (C and E). F and G: Number of IL-12-producing cells from DLN of mice infected with L. amazonensis or L. major at 2dpi (F) or 7 dpi (G) measured via ELispot. All data are from three separate experiments; error bars indicate ±SEM; *P ≤ 0.05.
The number of DLN cells producing IL-12p40 ex vivo was analyzed at 2 and 7 dpi via ELIspot. At 2 dpi, no significant differences were observed in IL-12p40 production from the DLN of L. amazonensis- or L. major-infected mice (Figure 1F). However, by 7 dpi the number of IL-12p40-producing cells was significantly decreased in response to L. amazonensis infection compared with L. major-infected mice (Figure 1G). These data suggest that early during infection, L. amazonensis and L. major promastigotes elicit similar DC phenotypes in the DLN, as measured by CD40 surface expression and IL-12p40 production. However, once the in vivo infection transitions to the acute stage (7 dpi) when the amastigote form of the parasite predominates,24 we observe a DC phenotype with significantly decreased CD40 surface expression and IL-12p40 production suggesting that L. amazonensis amastigotes can modulate the maturation phenotype of DC in vivo as it has been observed in vitro.
Increased ERK Phosphorylation Following Infection with L. amazonensis Amastigotes
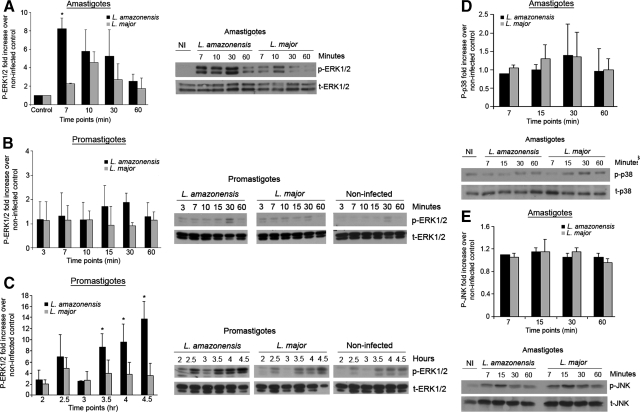
To determine a potential mechanism by which L. amazonensis could inhibit CD40 surface expression and IL-12 production during DC maturation, we determined which of several signaling pathways are differentially up-regulated in DC following infection with L. amazonensis and not with L. major infection. Several reports indicate that activation of mitogen-activated protein kinase ERK can prevent proper DC maturation.16,20 Recent published work from our laboratory and others has shown that activation of ERK1/2 following infection with L. amazonensis amastigotes leads to decreased ability of mouse macrophages to kill these intracellular parasites in vitro,19 and promotes a non-healing response in infected BALB/c mice.18 Based on these findings, we were interested in determining whether ERK phosphorylation occurs in C3HeB/FeJ-derived BMDC after infection with either L. amazonensis amastigotes or promastigotes. BMDC infected with L. amazonensis amastigotes (Figure 2A) or promastigotes (Figure 2, B and C) at a 3:1 parasite-cell ratio were lysed at the indicated time points and analyzed via Western blot for phosphorylated ERK1/2. Following L. amazonensis amastigote infection, a significant increase in ERK1/2 phosphorylation was observed within minutes of exposure to the parasite (Figure 2A), compared with L. major amastigote infection. In contrast, L. amazonensis promastigote infection did not result in significantly increased phosphorylated ERK1/2 until at least 3 hours post-infection (Figure 2, B and C) as compared with L. major promastigote-infected BMDC. Phosphorylation of ERK1/2 after L. amazonensis amastigote infection was unique to ERK1/2 as other MAP kinases, p38 and JNK, were not up-regulated following infection (Figure 2, D and E).
Figure 2.
Robust ERK1/2 phosphorylation in L. amazonensis amastigote-infected BMDC and delayed ERK1/2 activation following L. amazonensis promastigote infection. BMDC were infected with L. amazonensis or L. major amastigotes (A) or promastigotes (B and C). Whole cell lysates were collected at the indicated time points. Samples were analyzed via Western blot for phosphorylated ERK1/2 (A–C), p38 (D), JNK (E) and total ERK1/2, p38 and JNK. Densitometry values were normalized to t-ERK, t-p38, or t-JNK and then to noninfected controls. Densitometry analysis for at least three different experiments and representative blots are shown; error bars denote ±SD; *P ≤ 0.05.
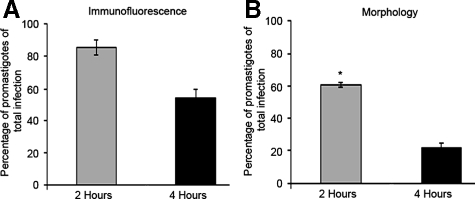
We hypothesized that the observed delay in ERK1/2 phosphorylation following L. amazonensis promastigote infection may correlate with intracellular transformation of the parasite into the amastigote stage. We analyzed L. amazonensis-infected BMDC at 2 and 4 hours postinfection to determine which parasite form, promastigote or amastigote, predominated at these time points in infected cells. Using both immunofluorescence via an anti-LPG antibody (Figure 3A), which selectively labels promastigotes and morphology analysis via H&E stain (Figure 3B), we show that while at 2 hours postinfection parasite form is dominated by promastigotes, the percentage of promastigotes present intracellularly is significantly decreased by 4 hours postinfection, indicating a growing predominance of amastigote infection. Four hours postinfection correlates with the rise of increased ERK1/2 phosphorylation observed after initial promastigote infection. These data suggest that L. amazonensis amastigotes may specifically induce ERK1/2 phosphorylation.
Figure 3.
L. amazonensis amastigotes predominate within infected cells at 4 hours post-infection. BMDC in 24 well plates containing coverslips were infected with L. amazonensis promastigotes. Coverslips were recovered at the indicated time points, fixed, and stained. A: Epifluorescent microscopy analysis of BMDC, (×100, oil). Infected cells were determined by propidium iodide (PI) nuclear staining, and promastigotes were identified via a CA7AE anti-LPG antibody and a secondary Cy2-conjugated antibody. Graph indicates mean and SD of 3 coverslips of one representative from two experiments. B: Coverslips were analyzed via light microscopy, (×100, oil). Parasite stage was identified by presence or absence of flagellum and by size. Data from at least three separate experiments; error bars denote ±SEM; *P ≤ 0.05.
ERK Inhibition Restores CD40 Surface Expression Both in Vitro and in Vivo Following L. amazonensis Infection
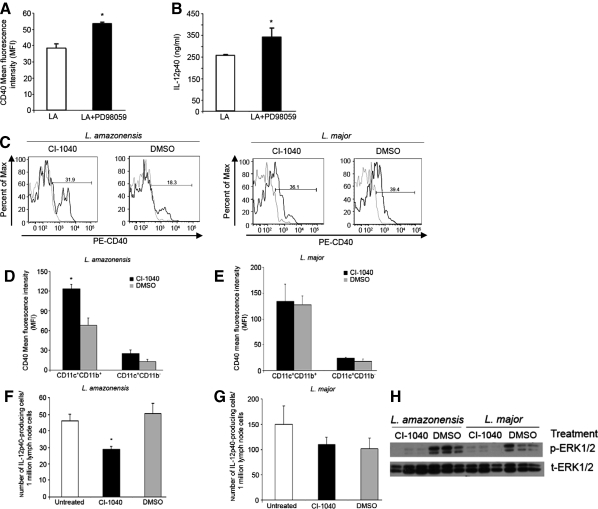
We have shown that MAPK ERK1/2 is selectively and rapidly phosphorylated following L. amazonensis amastigote infection of BMDC. To examine if increased ERK1/2 phosphorylation affects BMDC maturation in response to activating stimuli following Leishmania infection, BMDC were pre-treated with the specific MEK inhibitor PD98059 and then infected with either L. amazonensis or L. major amastigotes and activated with lipopolysaccharide and IFN-γ 2 hours postinfection. Following overnight culture, BMDC were analyzed for CD40 surface expression via FACS and resultant culture supernatants were analyzed to determine IL-12p40 production via enzyme-linked immunosorbent assay. ERK inhibition of L. amazonensis amastigote-infected BMDC significantly increased CD40 surface expression MFI (Figure 4A) and IL-12p40 production (Figure 4B) as compared with non-treated L. amazonensis amastigote-infected BMDC. Treatment with PD98059 had no effect on the ability of L. major-infected BMDC to express surface CD40 as measured by FACS analysis or to produce IL-12p40 (data not shown).
Figure 4.
ERK inhibition enhances DC maturation phenotype following L. amazonensis infection (A–B) BMDC were pretreated with the ERK inhibitor PD98059 (20 mmol/L) for 30 minutes and then infected with L. amazonensis amastigotes, and activated with LPS and IFN-γ. 24 hours post-infection cells were harvested and processed for (A) surface CD40 expression and analyzed via FACS scan and (B) supernatants were collected to determine IL-12p40 production via enzyme-linked immunosorbent assay. C–H: Mice were infected with L. amazonensis or L. major promastigotes and then treated with 100 mg/kg of CI-1040 via oral gavage started on day 0 post-infection and for the next 7 days, twice daily. On day 7 mice were sacrificed; draining lymph nodes were harvested to assess CD40 surface expression on DC populations. C: Representative histograms based on CD11c+CD11b+ population, (gray line, isotype control; black CD40). Mean fluorescence intensity of CD40 surface expression on CD11c+ cells in L. amazonensis-infected (D) or L. major-infected mice (E) treated with CI-1040 or DMSO-mock control. Number of IL-12p40-producing cells (F and G) as determined by Elispot analysis of total draining lymph node cells. Western blot of splenic lysates probed with anti-phosphorylated ERK1/2 and anti-total ERK1/2 (H). Data from at least three different experiments; error bars denote ±SEM; *P ≤ 0.05.
Based on these in vitro findings, where inhibition of ERK before L. amazonensis amastigote infection of BMDC enhanced CD40 expression and IL-12p40 production, we sought to determine the effect of ERK inhibition in vivo using a different ERK inhibitor, CI-1040. Mice were inoculated in the left footpad with stationary phase L. amazonensis or L. major promastigotes. Starting day 0 mice were treated with CI-1040 via oral gavage. Twice daily treatment continued for a total of 7 days. Mice were sacrificed 1 week after infection, DLNs were harvested, and CD11c+ DC phenotypes were analyzed via FACS as described previously (Figure 1). ERK inhibitor treatment of L. amazonensis-infected mice resulted in increased CD11c+ DC surface expression of CD40 (Figure 4C [left panel] and D) as determined by FACS analysis. ERK inhibitor treatment significantly decreased the number of IL-12p40-producing cells as measured by ELIspot (Figure 4F). CI-1040 treatment of L. major-infected mice had no effect on CD40 surface expression of CD11c+ DC (Figure 4C [right panel] and E) or on the number of IL-12p40 producing cells from the DLNs of infected mice (Figure 4G). Western blot analysis of splenic lysates showed that ERK1/2 phosphorylation was inhibited in CI-1040-treated animals (Figure 4H). These data indicate that in vitro L. amazonensis infection modulates DC maturation via ERK-mediated down-regulation of CD40 surface expression and decreased IL-12p40 production, and that in vivo, ERK activation down-regulates CD40 surface expression of CD11c+ DC.
Discussion
Here we describe a novel L. amazonensis amastigote-dependent mechanism modulating DC maturation via activation of the MAP kinase ERK and for the first time recover DC phenotype by inhibiting ERK phosphorylation. Alteration of surface marker expression, cytokine production, maturation, and function of DC following L. amazonensis infection has been previously characterized in vitro.15,25 Defects in DC maturation may, in part, contribute to the unpolarized T cell phenotype observed during L. amazonensis infection,6 and lead to a non-healing immune response. As early as 7 dpi CD11c+ DLN cells from L. amazonensis-infected C3HeB/FeJ mice have significantly reduced CD40 surface expression and a decreased number of IL-12p40-producing cells, as compared with L. major-infected mice. Consistent with another report indicating the activation of the MAP kinase ERK during L. amazonensis infection,18 we found that L. amazonensis amastigote infection of DC leads to phosphorylation of the MAP kinase ERK1/2. When ERK phosphorylation was inhibited, DC surface expression of CD40 increased both in vitro and in vivo, and production of IL-12p40 was enhanced in vitro.
Several pathways that alter DC maturation have been characterized, including MAP kinase pathways.16,20 p38 and JNK phosphorylation were not different between L. amazonensis-infected and L. major-infected BMDC (Figure 2, D and E). ERK1/2 phosphorylation in BMDC increased fourfold within minutes of contact with L. amazonensis amastigotes compared with L. major amastigotes (Figure 2A). ERK1/2 phosphorylation was also observed after L. amazonensis promastigote infection of BMDC but not until 3 to 4 hours post-infection (Figure 2C). Early ERK1/2 phosphorylation observed with in vitro following L. amazonensis amastigote infection of BMDC (Figure 2A) and microscopic analysis revealing that the amastigote form of L. amazonensis predominates within infected BMDC by 4 hours post-infection (Figure 3), suggest a correlation between ERK1/2 phosphorylation and amastigote predominance. We postulate that the amastigote form of L. amazonensis activates the ERK1/2 pathway.
ERK1/2 has multiple cytosolic and nuclear targets.26,27 The role of ERK1/2 in cellular function ranges from cell survival and cell cycle regulation, to modulation of additional signaling pathways and regulation of transcription. Both CD40 and IL-12p40 transcription can be negatively regulated by activation of ERK1/2.20 ERK1/2 has also been demonstrated to play a role in the DC survival rather than maturation.16 Moreover, in tumor cells, aberrant activation of ERK1/2 is implicated in inhibition of differentiation and apoptosis.28 Activation of ERK1/2 in infected DC by L. amazonensis amastigotes may provide two critical mechanisms to ensure parasite success within the mammalian host. First, by targeting specific genes for regulation, namely CD40 and IL-12p40, L. amazonensis could interfere with proper DC maturation, thereby preventing immune detection and induction of a proper adaptive immune response. Second, increased activation of host ERK1/2 would promote survival of the host cell therefore maintaining a viable host cell for an extended period of time.
ERK1/2 activation as an immunomodulatory mechanism for leishmaniasis has been previously described in other cells systems. Leishmania phosphoglycan has been shown to inhibit IL-12 production by macrophages via ERK1/2 activation.29 Recent work has demonstrated that antibody-opsonized L. amazonensis amastigotes induce ERK1/2 activation in BALB/c macrophages. Our work using a C3HeB/FeJ mouse model complement these findings, providing further support for ERK in the pathogenesis of L. amazonensis infection. We demonstrate that L. amazonensis amastigote infection of BMDC promotes increased ERK1/2 activation in the absence of additional activating stimuli. In contrast to these findings, Xin et al observed that both L. amazonensis promastigotes and amastigotes both reduce ERK1/2 phosphorylation in the presence or absence of activating stimuli.15 These differences could be explained by the time points chosen for analysis. Based on our data, the 6.5-hour time point occurs after the observed peak in ERK1/2 phosphorylation by both L. amazonensis amastigotes (7 minutes) and promastigotes (4.5 hours).
We report here that inhibition of ERK phosphorylation with the ERK inhibitor PD98059 before BMDC infection with L. amazonensis amastigotes leads to an enhanced CD40 surface expression and IL-12p40 production (Figure 4, A and B). In vivo, treatment of mice with the orally available ERK inhibitor CI-1040 enhanced CD40 surface expression of CD11c+ DC (Figure 4, C and D), but did not result in an increased number of IL-12p40-producing cells collected from the DLNs of L. amazonensis-infected mice (Figure 4F). We hypothesize that the observed decrease in the number of IL-12p40-producing cells from L. amazonensis-infected mice, in the presence of ERK inhibition, may be a result of the lower number of CD11c+ cells found in the DLN (CD11c+ events with no treatment, 2818 vs. CD11+ events with CI-1040, 1225). CI-1040-treatment of Raf-transformed hematopoietic cells leads to increased sensitivity to apoptosis.30 We suggest that systemic ERK inhibition via treatment with this inhibitor may adversely affect the survival of monocytes migrating into the site of infection and later into the DLN. A closer analysis of IL-12p40 production of individual CD11c+ events from the DLNs of infected mice may be required to determine the effects of ERK inhibition on the production of this cytokine.
Our observation of a more mature DC phenotype following treatment of L. amazonensis-infected C3HeB/FeJ mice with the ERK inhibitor CI-1040 complements work from our laboratory showing that ERK inhibition can promote parasite killing in L. amazonensis-infected macrophages in vitro.19 The data are also consistent with work indicating that inhibition of ERK slows disease progression of L. amazonensis-infected BALB/c mice.18 Although we did not observe a restoration in IL-12p40 production during in vivo L. amazonensis infection, ERK inhibition did enhance CD40 surface expression. Previously, it has been shown that CD40-CD40L interactions are important during L. amazonensis infection, as deficiencies in this interaction lead to increased susceptibility to infection.10 The work presented here indicates that L. amazonensis specifically targets CD40 expression in vivo, and that we can restore CD40 surface expression by inhibiting phosphorylation of ERK. We suggest that the mature phenotype restored to CD11c+ DC via ERK inhibition would promote the development of a productive CD4+ T cell response during L. amazonensis infection; however, further studies are necessary. The work presented here furthers our understanding of host-parasite amastigote-specific interactions and provides evidence for the use of ERK inhibitors as immunomodulators to directly enhance the host immune response after Leishmania infection.
Acknowledgments
We thank Pfizer Incorporated, Global Research & Development, for providing the ERK inhibitor CI-1040, Mr. Kyle Metz for his technical assistance, Dr. Marian Kohut for her critical review of this manuscript, Dr. Bryan Bellaire for technical advice with immunofluorescence procedures and microscope use, and Dr. Jeffery Beetham for kindly proving the anti-LPG CA7AE antibody.
Footnotes
Address reprint requests to Christine Petersen, Department of Veterinary Pathology, Iowa State University, 1600 University Boulevard, Ames, IA 50011. E-mail: kalicat@iastate.edu.
Supported by National Institutes of Health grants AI48357, AI50803.
References
- Gregory DJ, Oliver M. Subversion of host cell signaling by the protozoan parasite Leishmania. Parasitology. 2005;130:27–35. doi: 10.1017/S0031182005008139. [DOI] [PubMed] [Google Scholar]
- Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Ji J, Sun J, Qi H, Soong L. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am J Trop Med Hyg. 2002;66:338–345. doi: 10.4269/ajtmh.2002.66.338. [DOI] [PubMed] [Google Scholar]
- Jones DE, Ackermann MR, Wille U, Hunter CA, Scott P. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect Immun. 2002;70:2151–2158. doi: 10.1128/IAI.70.4.2151-2158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer AE, Vanloubbeeck YF, Jones DE. Antigen-responsive CD4+ T cells from C3H mice chronically Infected with Leishmania amazonensis are impaired in the transition to an effector phenotype. Infect Immun. 2006;74:1547–1554. doi: 10.1128/IAI.74.3.1547-1554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Scott P. Experimental cutaneous leishmaniasis: induction and regulation of T cells following infection of mice with Leishmania major. Chem Immunol. 1998;70:60–80. doi: 10.1159/000058698. [DOI] [PubMed] [Google Scholar]
- Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- Soong L, Xu JC, Grewal IS, Kima P, Sun J, Longley BJ, Jr, Ruddle NH, McMahon-Pratt D, Flavell RA. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- Mattner F, Magram J, Ferrante J, Launois P, Di Padova K, Behin R, Gately MK, Louis JA, Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur J Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- Chakir H, Campos-Neto A, Mojibian M, Webb JR. IL-12Rbeta2-deficient mice of a genetically resistant background are susceptible to Leishmania major infection and develop a parasite-specific Th2 immune response. Microbes Infect. 2003;5:241–249. doi: 10.1016/s1286-4579(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Xin L, Li Y, Soong L. Role of interleukin-1beta in activating the CD11c(high) CD45RB- dendritic cell subset and priming Leishmania amazonensis-specific CD4+ T cells in vitro and in vivo. Infect Immun. 2007;75:5018–5026. doi: 10.1128/IAI.00499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Li K, Soong L. Down-regulation of dendritic cell signaling pathways by Leishmania amazonensis amastigotes. Mol Immunol. 2008;45:3371–3382. doi: 10.1016/j.molimm.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Kröger A, Relloso M, Fernández-Capetillo O, Zubiaga A, Silva A, Bernabéu C, Corbí AL. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Immunobiology. 2001;98:2175–2182. doi: 10.1182/blood.v98.7.2175. [DOI] [PubMed] [Google Scholar]
- Yang Z, Mosser DM, Zhang X. Activation of the MAPK. ERK, following Leishmania amazonensis infection of macrophages. J Immunol. 2006;178:1077–1085. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukbel RM, Patten C, Gibson KN, Ghosh M, Petersen C, Jones DE. Macrophage killing of L. amazonensis amastigotes requires both nitric oxide and superoxide. Am Soc Trop Med Hyg. 2007;74:660–675. [PubMed] [Google Scholar]
- Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci. 2006;42:1–11. doi: 10.1016/j.jdermsci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Jones DE, Buxbaum LU, Scott P. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J Immunol. 2000;165:364–372. doi: 10.4049/jimmunol.165.1.364. [DOI] [PubMed] [Google Scholar]
- Iezzi G, Frohlich A, Ernst B, Ampenberger F, Saeland S, Glaichenhaus N, Kopf M. Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection. J Immunol. 2006;177:1250–1256. doi: 10.4049/jimmunol.177.2.1250. [DOI] [PubMed] [Google Scholar]
- Kima P. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int J Parasitol. 2007;37:1087–1096. doi: 10.1016/j.ijpara.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favali C, Tavares N, Clarêncio J, Barral A, Barral-Netto M, Brodskyn C. Leishmania amazonensis infection impairs differentiation and function of human dendritic cells. J Leuk Biol. 2007;82:1401–1406. doi: 10.1189/jlb.0307187. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dong C. MAP kinases in immune responses. Cell Mol Immunol. 2005;2:20–27. [PubMed] [Google Scholar]
- Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunology. 1999;163:6403–6412. [PubMed] [Google Scholar]
- Konopleva M, Shi Y, Steelman LS, Shelton JG, Munsell M, Marini F, McQueen T, Contractor R, McCubrey JA, Andreeff M. Development of a conditional In vivo model to evaluate the efficacy of small molecule inhibitors for the treatment of Raf-transformed hematopoietic cells. Cancer Res. 2005;65:9962–9970. doi: 10.1158/0008-5472.CAN-05-1068. [DOI] [PubMed] [Google Scholar]