Abstract
Gangliosides may be involved in the pathogenesis of Parkinson’s disease and related disorders, although the precise mechanisms governing this involvement remain unknown. In this study, we determined whether changes in endogenous ganglioside levels affect lysosomal pathology in a cellular model of synucleinopathy. For this purpose, dementia with Lewy body-linked P123H β-synuclein (β-syn) neuroblastoma cells transfected with α-synuclein were used as a model system because these cells were characterized as having extensive formation of lysosomal inclusions bodies. Treatment of these cells with d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP), an inhibitor of glycosyl ceramide synthase, resulted in various features of lysosomal pathology, including compromised lysosomal activity, enhanced lysosomal membrane permeabilization, and increased cytotoxicity. Consistent with these findings, expression levels of lysosomal membrane proteins, ATP13A2 and LAMP-2, were significantly decreased, and electron microscopy demonstrated alterations in the lysosomal membrane structures. Furthermore, the accumulation of both P123H β-syn and α-synuclein proteins was significant in PDMP-treated cells because of the suppressive effect of PDMP on the autophagy pathway. Finally, the detrimental effects of PDMP on lysosomal pathology were significantly ameliorated by the addition of gangliosides to the cultured cells. These data suggest that endogenous gangliosides may play protective roles against the lysosomal pathology of synucleinopathies.
Synucleinopathies, including Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy, are characterized by intraneuronal Lewy bodies and glial inclusions whose main constituent is mature fibrils of α-synuclein (α-syn).1,2 Because it is generally believed that oligomers and protofibrils of α-syn may play a causative role for neurotoxicity in synucleinopathies,3 inhibiting the aggregation of α-syn may constitute a rational approach for a therapeutic strategy. Reports show that β-synuclein (β-syn), a homologue of α-syn, may be the endogenous molecule that negatively regulates α-syn aggregation.4,5,6 Supporting this, overexpression of β-syn in α-syn transgenic mice by either crossing with β-syn transgenic mice or using lentivirus-mediated transfer results in amelioration of neuropathology.4,6,7 Thus, these results suggest endogenous molecules that inhibit α-syn aggregation may have a therapeutic potential against synucleinopathies.
GM1 was recently shown to interact with α-syn and inhibit aggregation of α-syn in vitro,8 and this raises the possibility that endogenous gangliosides may inhibit α-syn aggregation in vivo. Consistent with this notion, numerous studies have documented the beneficial effects of GM1 on MPTP-treated animal models of PD.9,10 Because 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induces mitochondrial dysfunction and oxidative stress,11 it is predicted that gangliosides may act to suppress the oxidative stress-induced α-syn aggregation. An alternative possibility is that gangliosides may be protective against the α-syn-induced deregulation of the signal transduction pathways12 because α-syn exists in caveolae and lipid rafts where gangliosides are abundantly expressed and many signal transduction events occur.13,14 Supporting the role of gangliosides in the pathogenesis of synucleinopathies, anti-GM1 antibodies have been shown to be increased in PD, principally in the tremor-dominant form of PD.15 In addition to PD, beneficial effects of GM1 have been documented for peripheral nerve damage, stroke, and spinal cord injuries, particularly during the acute phase of the diseases.16,17 In Alzheimer’s disease (AD), it was shown that intraventricular infusion of GM1 had a beneficial effect on early onset AD presumably through the sequestration of β-amyloid (Aβ) by GM1.17 However, this is still controversial because GM1-bound Aβ may act as a seed for Aβ, stimulating fibrillogenesis of Aβ, and leading to neurodegeneration.18 Therefore, the precise mechanism through which gangliosides act to protect against neurodegeneration remains poorly understood.
The pathological linkage of gangliosides to α-syn is further supported because α-syn pathology is frequently associated with various types of lysosomal storage diseases (LSDs), which are characterized by lysosomal accumulation of glycolipids attributable to defective lysosomal enzymes. In this regard, an increased incidence of synucleinopathies is noted in the relatives of Gaucher probands, whereas screening of patients with Parkinsonism is identified with a high frequency of glucocerebrosidase mutations.19 In relation to these epidemiology findings, Lewy body pathology was identified in the hippocampus regions of the Gaucher brains.19 Subsequently, accumulation of both α- and β-syn were detected in a mouse model of Sandhoff disease.20 Further, Lewy body-like structures were found in a wide range of LSDs and related disorders, including Niemann-Pick C1 disease, Tay-Sachs disease, metachromatic leukodystrophy, β-galactosialidosis, and adrenoleukodystrophy.21,22 Despite accumulating histological data, the role of increased syn proteins in the pathogenesis of the LSDs is unclear.
Recently, two missense mutations (P123H and V70M) in β-syn were identified in a DLB family in Seattle and in another solitary DLB case in Japan, suggesting alteration of β-syn may cause the pathogenesis of DLB.23 Curiously, overexpression of the DLB-linked mutant (P123H and V70M) β-syn in neuroblastoma cells resulted in formation of lysosomal inclusion bodies, which were similar to those formed in LSDs. Further, the number of inclusions were increased when these cells were further transfected with α-syn, thus providing a cellular model to investigate the relationship between gangliosides and synuclein pathology.24 Using this cellular model of synucleinopathies, here we show that treatment of these cells with d-Threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) resulted in various features of lysosomal pathology. PDMP is a ceramide analogue that efficiently inhibits the cell’s glucosylceramide synthase activities and has been widely used as a tool for studying various effects of endogenous glycosphingolipids, including gangliosides biosynthesized from glucosylceramide.25 Because the lysosomal pathology caused by PDMP treatment was reversed by addition of gangliosides, the result suggests that endogenous gangliosides may play a protective role for the lysosomal pathology in synucleinopathies.
Materials and Methods
Reagents
The reagents, including PDMP (D-PDMP) and L-PDMP (both were from Matreya, LLC, Pleasant Gap, PA), cathepsin B inhibitor (CA-074Me) (Calbiochem, San Diego, CA) and cathepsin D inhibitor (pepstatin A methyl ester) (Calbiochem), bovine ganglioside mixtures composed of various gangliosides, including GM1 (13.0%), GM2 (1.5%), GM3 (0.4%), GD1a (38.0%), GD1b (9.0%), GD3 (1.0%), GT1b (16.0%), GQ1b (1.0%), and other gangliosides (20.0%) (Funakoshi, Tokyo, Japan), MG132 (Sigma, St. Louis, MO), 3-methyadenine (3-MA) (Sigma) and rapamycin (Sigma), and etoposide (Calbiochem) were applied to cell cultures at the indicated concentrations. Unless otherwise described, the other reagents were purchased from Sigma.
The following antibodies were used: monoclonal anti-α-syn (syn-1) and anti-β-syn antibodies were from BD Biosciences (Franklin Lakes, NJ); rabbit polyclonal anti-Atg5 and anti-beclin-1 antibodies and monoclonal anti-β-actin (AC-15) antibody were from Sigma; monoclonal anti-ubiquitin antibody was from Chemicon (Temecula, CA); rabbit polyclonal anti-cathepsin B was from EMD Biosciences (San Diego, CA); rabbit polyclonal anti-cathepsin D (H-75) antibody and goat polyclonal anti-lysosome-associated membrane protein-2 (LAMP-2) antibody (C-20) were from Santa Cruz Biotechnology (Santa Cruz, CA); anti-V5 was from Bethyl Laboratories (Montgomery, TX); rabbit polyclonal anti-LAMP-2 (Igp96) antibody was from Invitrogen (Carlsbad, CA); rabbit polyclonal anti-ATP13A2 antibody was from ABR (Golden, CO); rabbit polyclonal anti-LC3B serum was from Novus (Littleton, CO); monoclonal anti-α-syn LB509 antibody was from Wako Pure Chemical Industries (Saitama, Japan); rabbit polyclonal anti-phospho-mammalian target of RAP (mTOR)(Ser2448), anti-mTOR, anti-phospho-p70 S6 kinase (Ser371), anti-p70 S6 kinase, anti-phospho-4E-BP1 (Thr37/46), anti-4E-BP1, anti-HSP70, and anti-cleaved caspase-3 antibodies (Asp175) were from Cell Signaling (Beverly, MA); guinea pig anti-P62 was from Progen (Heidelberg, Germany); rabbit anti-GM2 antibody was previously described24; cholera toxin subunit B conjugated with Alexa Fluor 488, Alexa Fluor 488-conjugated anti-goat and anti-rabbit antibodies, Alexa Fluor 555-conjugated anti-mouse antibody, and Alexa Fluor 647-conjugated anti-guinea pig antibody were from Molecular Probes (Eugene, OR).
Cell Cultures, Expression Vectors, and Transfection
The transfected rat B103 neuroblastoma cells, including vector-transfected cells, wild-type β-syn-overexpressing cells, and P123H β-syn-overexpressing cells with or without transfection of control vectors, wild-type α-syn, or A53T α-syn, were used as previously described.24 These cells were cultured in Dulbecco’s modified Eagle’s medium (high glucose) containing 10% fetal calf serum (BioWest, Nuaille, France) and 1% v/v penicillin/streptomycin (Invitrogen) in a 5% CO2/95% air atmosphere. For the transfection of P123H β-syn-overexpressing B103 neuroblastoma cells, cells were transfected with control vectors, wild-type α-syn, or familial PD-linked A53T α-syn. Briefly, P123H β-syn-overexpressing cells were inoculated into 6- (or 24- or 96-) well plates at a density of 5 × 105 (or 1 × 105 or 3 × 103) cells/well. The next day, the medium was changed with fresh growth medium, and the cells were transfected with pTarget (Invitrogen) or pTarget inserted with either wild-type α-syn or A53T α-syn cDNA24 using Lipofectamine 2000 (Invitrogen) for 36 hours. HEK293 cells were purchased from American Type Culture Collection (Manassas, VA). For the transfection of HEK293 cells, the cells were transfected with pcDNA3 (Invitrogen) or pcDNA3 containing V5-human ATP13A2 cDNA (a kind gift from Dr. Kubisch at University of Cologne in Germany).26 In some experiments, the transfection was performed for cells grown on the poly-l-lysine-coated coverslips in 24-well plates.
Immunofluorescence/Laser-Scanning Confocal Microscopy (LSCM)
An immunofluorescence study was performed as previously described.24 Briefly, cells were inoculated on poly-l-lysine-coated glass coverslips, grown to 70% confluence, fixed in 4% paraformaldehyde for 30 minutes, and pretreated with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 20 minutes. Fixed cells were blocked with PBS containing 3% goat serum and 5% bovine serum albumin at room temperature. For staining, the cells were incubated overnight at 4°C with the primary antibody (or antibodies for double/triple staining). After washing, the cells were incubated with Alexa Fluor-conjugated secondary antibody (or antibodies for double/triple staining) (Invitrogen) for 1 hour at room temperature. In some experiments, cells were stained with 6-diamino-2-phenylindole dihydrochloride (DAPI) to stain the nucleus. Coverslips were mounted on the slides with Gel/Mount (Biomeda Corp., Foster City, CA) and imaged using a LSCM (FV1000; Olympus, Tokyo, Japan).
Immunoblot Analysis
The cell extracts for immunoblot analysis was prepared as previously described.27 Briefly, cells were harvested and dissolved in lysis buffer: 1.0% Triton X-100, 50 mmol/L N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid), 150 mmol/L NaCl, 10% glycerol, 1.5 mmol/L MgCl2, 1 mmol/L ethylene glycol bis-(2-aminoethylether)-N,N,N′,N″-tetraacetic acid, 100 mmol/L sodium fluoride, and a protease inhibitor cocktail (Nacalai Tesque, Tokyo, Japan). The cells extracts were centrifuged for 10 minutes at 15,000 rpm and the supernatants were collected to measure the protein concentrations using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). The insoluble fractions were resuspended in the lysis buffer and solubilized with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The supernatants (detergent-soluble fractions) (10 μg) and the corresponding volume of the detergent-insoluble fractions were subjected to analysis.
For some analyses using anti-GM2, delipidated cell extracts were prepared as previously described.28 Briefly, cells were scraped into hypotonic buffer (10 mmol/L Tris, pH 7.3, 10 mmol/L MgCl2, 1 mmol/L EDTA, and 1 mmol/L ethylene glyod bis-(2-aminoethylether)-N,N,N′,N″-tetraacetic acid) for 10 minutes on ice. The cells were then homogenized by passing 15 times through a 21-gauge needle and centrifuged for 10 minutes at 1000 rpm to pellet the nuclei. The resulting supernatant was centrifuged for 30 minutes at 16,000 × g. Pelleted membranes were separated using SDS-PAGE, and the anti-GM2-immunoreactive bands were detected by immunoblotting. For delipidation a 20-μl membrane sample was transferred back to a fresh Pyrex tube, capped, and incubated at 60°C for 15 minutes in 800 μl of solvent [propanol/hexane/water, 55:20:25 (v/v/v)] with occasional shaking. While still warm, the sample was centrifuged at 500 × g for 3 minutes, and the supernatants were discarded. The pellets were extracted twice more with the solvent, and the pellet was subjected to SDS-PAGE.
In some experiments, lysosome-enriched fractions were harvested using a lysosome enrichment kit for tissue and cultured cells according to the manufacturer’s instruction (Pierce, Rockford, IL) with some modifications. Briefly, cells with or without treatment of PDMP for 48 hours were harvested and cell pellets (200 mg) were incubated with Lysosome Enrichment Reagent A (800 μl). After homogenization with a Dounce homogenizer, the cell lysates were incubated with Reagent B (800 μl) and were centrifuged at 500 × g for 10 minutes to remove nuclei and cell debris. At this point, ∼200 μl of the supernatant were subjected to ultracentrifuge at 100,000 × g for 1 hour to give rise to the supernatants as cytosolic fractions. The rest of the supernatants (∼1400 μl) were added with OptiPrep Media (15% of final concentrations), and the samples were overlaid to the top of the discontinuous density gradients of OptiPrep Gradients (30%, 27%, 23%, 20%, and 17%) in an ultracentrifuge tube (10 ml), after ultracentrifuge at 145,000 × g for 2 hours at 4°C. The top 2 ml of the gradients were then recovered, added with 2 vol of PBS, and were subjected to ultracentrifuge at 18,000 × g for 30 minutes at 4°C. Finally, the pellets were harvested as lysosome-enriched fractions and kept at −80°C until use for SDS-PAGE.
Immunoblot analysis was performed as previously described.27 Briefly, cell extracts were resolved using SDS-PAGE (7.5 ∼ 16%) and electroblotted onto nitrocellulose membranes (GE Healthcare, Piscataway, NJ) with 3-(cyclohexylamino)-1-propanesulfonic acid buffer (pH 11.0). In some experiments, recombinant α- and β-syn proteins were used as positive controls. The membranes were blocked with 3% bovine serum albumin in Tris-buffered saline (25 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl) plus 0.2% Tween 20, and followed by incubation with the primary antibodies in TBS containing 3% bovine serum albumin. After washing, the membranes were incubated with a secondary antibody conjugated with horseradish peroxidase (GE Healthcare) in Tris-buffered saline (1:10,000). The bands of target proteins were observed using an ECL or ECL advance kit (GE Healthcare) and detected on X-ray film. Quantitative analysis of immunoblot band intensities was performed using the image processing software ImageJ 1.38v (National Institutes of Health, Bethesda, MD) for the Macintosh.
Evaluation of Lysosomal (Cathepsin B) Activity
The cathepsin B activity assays were performed as previously described.27 Briefly, the transfected and untransfected cells growing in six-well plates were harvested in buffer containing 50 mmol/L N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) (pH 7.4), 10 mmol/L EDTA, and 10 mmol/L NaCl, subjected to freezing and thawing to rupture cell membranous structures, and centrifuged at 15,000 rpm for 10 minutes. The supernatants (10 μg) were then incubated with benzyloxycarbonyl-Arg-Arg-Glu-amidomethylcoumarin fluorogenic cathepsin B substrate (40 mmol/L). The enzymatic activities were assayed by continuous recording of the fluorescence activity released from the fluorogenic substrate using a Berthold Mithras LB940 microplate reader (Berthold, Bad Wildbad, Germany) for 1 hour at 37°C (excitation, 380 nm; emission, 460 nm), and the reaction rates were analyzed. The activities were expressed as arbitrary U/minute/mg of protein.
LysoSensor Dye Labeling and Its Fluorescence Measurement
The transfected cells (and/or untransfected cells) growing on the coverslips in 24-well plates were incubated with 500 μl of growth medium with or without treatment of PDMP (25 μmol/L). After addition of LysoSensor Green DND-153 (1 μmol/L) (Invitrogen) into the medium during the last 1-hour period of incubation, the cells on the coverslips were rinsed with PBS and inverted for viewing on a glass microscope slide, followed by observation using a LSCM. The intensity of LysoSensor green fluorescence was measured on a square window (640 pixels by 640 pixels) placed on the cells in the fields of 160 μm by 160 μm, and the mean fluorescence intensity was calculated by software FV10-ASW 1.7 (Olympus, Tokyo, Japan).
Lucifer Yellow Analysis
For Lucifer Yellow staining, the cells were preincubated with the dye Lucifer Yellow (CH, 100 μg/ml) during the last 16-hour period of transfection. The Lucifer Yellow-labeled cells were washed three times in PBS and then treated with or without PDMP (25 μmol/L) until observation. After various times of PDMP treatment, the cells on the coverslips were rinsed and observed using a LSCM. In some cases, the coverslips were fixed with 4% paraformaldehyde, stained with DAPI, and imaged using a LSCM.
Cell Death Assays
The effects of PDMP treatment on the cell death/survival of the transfected cells were evaluated by various methods, including terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, lactate dehydrogenase (LDH) assay, and 4-[3-{4-lodophenyl}-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) assay.
TUNEL assay was performed as previously described using In Situ Cell Death Detection kit, TMR red (Roche, Vienna, Austria).24 Briefly, the transfected cells growing on the coverslips in the 24-well plates were treated with PDMP at the indicated times. Cells were then fixed in 4% paraformaldehyde, rinsed in PBS, left overnight in 70% ethanol, and processed for TUNEL labeling as recommended by the manufacturer. The staining was evaluated using a LSCM. All cells were stained with DAPI to quantify the percentage of cell death.
An LDH release assay was performed using a cytotoxicity detection kit (LDH) from Roche. After transfection, cells were treated with or without PDMP for 24 hours. The supernatants of cell culture were collected and analyzed according to the manufacturer’s protocol, as low control cells were incubated with medium, and the high control was prepared with medium plus 2% Triton X-100. The LDH reagent mixture, 100 μl, was added and incubated for 30 minutes under light protection. Immediately after addition of stop solution, the absorbance was measured using a microplate reader (Bio-Rad model 450) with a test wave length of 490 nm and a reference wavelength of 690 nm. Cytotoxicity was calculated by the following equation: cytotoxicity [%] = 100 × (experimental value − low control)/(high control − low control).
WST-1 assay was performed using the Premix WST-1 cell proliferation assay system (Takara Bio Inc., Shiga, Japan). After transfection, cells were refed with 100 μl of fresh media containing PDMP for 24 hours. WST-1 reagent (10 μl) was then added to each well and the plates were incubated for 2 hours at 37°C. The absorbance was measured using a microplate reader with a test wave length of 450 nm and a reference wavelength of 690 nm. Negative controls consisted of the addition of 0.1% Triton X-100, which lyses cells and abolishes WST-1 reduction. The experimental value obtained from vector-transfected cells without PDMP treatment was taken as positive control (100%). Raw data were then scaled as follows: cell survival [%] = 100 × (experimental value − negative control)/(positive control − negative control). It was confirmed that both LDH release assay and WST-1 assay were not interfered by the tested chemicals, including PDMP, cathepsin B and D inhibitors, 3-MA, and rapamycin under the cell-free conditions.
Electron Microscopy and Morphometric Analysis
Electron microscopic analysis was performed as previously described.24 To investigate the effect of PDMP treatment on the characteristics of the lysosomal structures, both vector-transfected cells and P123H β-syn-overexpressing cells transfected with A53T α-syn were treated with or without PDMP. Cells were then harvested using trypsin-EDTA and fixed using 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer at 4°C for 2 hours. After centrifugation, the cells were washed with 0.1 mol/L sodium cacodylate buffer three times. Cell pellets were obtained by centrifugation, postfixed in 1% osmium tetroxide and 1% potassium ferrocyanide at room temperature for 2 hours, and processed for embedding in Quetol 812 (Nisshin EM, Tokyo, Japan). Ultrathin sections were stained with uranyl acetate and lead nitrate and observed using a Hitachi H-7500 electron microscope (Hitachi, Tokyo, Japan) equipped with a CanoScan 9900F digital imaging system (Canon, Tokyo, Japan). The cells on ultrathin sections were examined and photographed at 80 kV using a calibrated magnification of both ×5000 and ×25,000. To avoid counting the same cell twice, only one section from each block was used to prepare the photographs. Morphometric analysis of EM photomicrographs was performed according to the point counting method. Briefly, a lattice with points spaced 0.5 cm apart was superimposed on the printed and enlarged photographs. Lysosome and other organelles were tabulated. The number of lysosomal structures was counted and their diameters were measured. The inclusions were categorized as bigger (more than 1 μm) and smaller (400 nm ∼ 1 μm) lysosomal structures. Fifty cells with clear nuclear diameters more than 8 μm were randomly picked up from each group and surveyed in the quantitative studies.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
The total RNA was isolated from cultured cells using the RNA extraction buffer Isogen (Nippon Gene, Tokyo, Japan). cDNA was synthesized from 2 μg of total RNA using the Superscript III first-strand synthesis system (Invitrogen) according to the manufacturer’s instructions. PCR amplification was performed using TaqPCR polymerase (ABgene, Tokyo, Japan). The following primers were used for the PCR: rat ATP13A2 (XM-342962): the sense primer 5′-ATGGTGACAGGGGACAACCT-3′ [oligonucleotide position (op) 2643 to 2663], antisense 5′-ACTTGAAGACGCTGAATGAAG-3′ (op 3175 to 3195), rat LAMP-2 (NM-017068): the sense primer 5′-CAACTTAGGCTGAACAACAGC-3′ (op 922 to 942), antisense 5′-GCTCATATCCAGTATGATGGC-3′ (op 1316 to 1336), rat mTOR (NM-019906): the sense primer 5′-GACAGTGTCATGGCTGTGCTA-3′ (op 7297 to 7317) and antisense 5′-GCAGTTCCACTTGGGTTGGAA-3′ (op 7625 to 7645), rat Beclin-1 (AY033824): the sense primer 5′-GCAGTGGACAAAGGCGCTCAAGTTC-3′ (op 1263 to 1287) and antisense 5′-ATGGGGTTGAGCCAAACTACGGCAG-3′ (op 1529 to 1553), rat ATG5 (AM087012): the sense primer 5′-TGAACGGCCTTTCATTCAGAAGCTG-3′ (op 809 to 833) and antisense 5′-GGAAGTTGTCTGGATAGCTCAGATG-3′ (op 996 to 1020), human β-syn (BT006627): the sense primer 5′-ATGGACGTGTTCATGAAGGGC-3′ (op 1 to 21) and antisense 5′-CTACGCCTCTGGCTCATACTC-3′ (op 385 to 405), human α-syn (NM-000345): the sense primer 5′-ATGGATGTATTCATGAAAGGACTTTC-3′ (op 47 to 72) and antisense 5′-GGCTTCAGGTTCGTAGTCTTGATAC-3′ (op 442 to 466), rat cyclophilin A (M19533): sense 5′-TCCATGGCAAATGCTGGAC-3′ (op 337 to 355) and antisense 5′-GTCTTGCCATTCCTGGACCC-3′ (op 478 to 497), rat P62 (BC061575): the sense primer 5′-CCATCCACAGGTGAACTCCAG-3′ (op 1077 to 1097) and antisense 5′-GTCTGTAGGAGCCTGGTGAGC-3′ (op 1271 to 1291).
Statistical Analysis
All values in the figures are expressed as means ± SD. To determine statistical significance, the values were compared using two-group t-tests with differences considered significant for P values less than 0.05.
Results
Down-Regulation of Lysosomal Activity in PDMP-Treated Syn-Overexpressing Cells
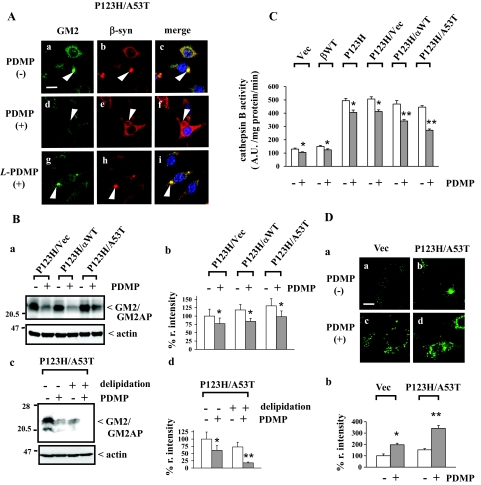
To determine whether lysosomal activity is affected by depletion of endogenous glycosphingolipids, PDMP was added to vector-transfected cells, wild-type β-syn-overexpressing cells and P123H β-syn-overexpressing cells with or without transfection of vectors, wild-type α-syn, or A53T α-syn. In the majority of the experiments, the cells were treated with 25 μmol/L of PDMP for up to 48 hours under 10% serum conditions. Similar experimental conditions were used in a previous report investigating the effects of PDMP on APP metabolism in SH-SY5Y neuronal cells.25 To confirm glycosphingolipids were depleted under these experimental conditions, the expression level of GM2, one of the typical gangliosides was analyzed using immunofluorescence/LSCM. The immunoreactivity of GM2 was significantly reduced by PDMP treatment in P123H β-syn-overexpressing cells transfected with A53T α-syn and also in other types of cells (Figure 1A, a–f; and unpublished data). Similar results were obtained for the immunoreactivity of GM1 (data not shown). We also confirmed that the inactive enantiomer L-PDMP not inhibiting glucosylceramide synthase had little effect on the immunoreactivities of GM1 and GM2 (Figure 1A, g–i; and data not shown). Immunoblot analysis using anti-GM2 antibody showed that the immunoreactivity of the 21-kDa band was significantly reduced by PDMP treatment (Figure 1B, a and b). Because the molecular weight of this band corresponded to the GM2 activating protein that is known to tightly bind with GM229 and because this band disappeared in the delipidated samples (Figure 1B, c and d), the 21-kDa band may be composed of a complex of GM2 with the GM2 activating protein.
Figure 1.
Compromised lysosomal activities in PDMP-treated syn-overexpressing cells. P123H β-syn-overexpressing cells were transfected with vector, wild-type α-syn, or A53T α-syn. Cells were then treated with or without PDMP (25 μmol/L) for 24 hours, followed by various evaluations. In some experiments, vector-transfected cells, wild-type β-syn-overexpressing cells, and P123H β-syn-overexpressing cells were also analyzed. A: Double immunofluorescence/LSCM of GM2 and β-syn for P123H β-syn-overexpressing cells transfected with A53T α-syn.. All figures are shown at the same magnification. Arrowheads indicate the inclusion formation. Note that the anti-GM2 immunoreactivity is remarkably diminished by treatment with PDMP (d--f) but not with L-PDMP (g--i). Similar results were observed in three independent experiments. B: Immunoblot analysis of the anti-GM2 immunoreactivities. In a, cell extracts (10 μg) were analyzed by immunoblot using anti-GM2. Equivalent protein loading in each lane was confirmed by immunoblotting with anti-actin antibody. In b, the band intensities corresponding to GM2/GM2AP were quantified and corrected with those of actin. The data are shown as a percentage of the relative intensity (% r. intensity) obtained from P123H β-syn-overexpressing cells transfected with vector and are expressed as the mean value ± SD (n = 4). *P < 0.05 versus PDMP-untreated cells. In c, cell extracts of P123H β-syn-overexpressing cells transfected with A53T α-syn were delipidated as described in Materials and Methods. In d, the band intensities corresponding to GM2/GM2AP were quantified and corrected with the intensities of actin bands. The data are shown as % r. intensity of the value obtained from PDMP-untreated/nondelipidated cells and are expressed as the mean value ± SD (n = 4). *P < 0.05, **P < 0.01 versus PDMP-untreated cells. C: Measurement of lysosomal activities. Cell extracts (10 μg) were incubated at 37°C with fluorogenic cathepsin B substrate to measure the activity of cathepsin B. Released fluorescence (excitation, 380 nm; emission, 460 nm) was monitored every 5 minutes up to 60 minutes. Fluorogenic intensity of each time point was plotted and the slope was calculated. The data are expressed as arbitrary units (A.U.) and shown as the mean value ± SD (n = 4). *P < 0.05, **P < 0.01 versus PDMP-untreated cells. D: LysoSensor staining to evaluate lysosomal activities. In a, P123H β-syn-overexpressing cells transfected with A53T α-syn and vector-transfected cells treated without (a, b) or with (c, d) PDMP were stained with LysoSensor Green DND-153. In b, the intensities of LysoSensor green fluorescence corresponding to a were measured by software FV10-ASW 1.7 (Olympus, Tokyo, Japan). The data are shown as % r. intensity of the fluorescence of vector-transfected cells without PDMP treatment and are expressed as the mean value ± SD (n = 4). *P < 0.05, **P < 0.01 versus PDMP-untreated cells. Scale bars: 20 μm (A); 10 μm (D).
Under these experimental conditions, the activity of cathepsin B, a lysosomal cysteine protease, was determined (Figure 1C). This showed that cathepsin B activities were significantly decreased by PDMP treatment in all types of cells. Further, the decrease in cathepsin B activities was greater in P123H β-syn-overexpressing cells transfected with α-syn compared with those in other cell types. To further confirm the lysosomal activities were compromised by PDMP treatment, cells with or without a treatment of PDMP were analyzed using staining with LysoSensor Green DND-153 (Figure 1D). Because this dye exhibits a pH-dependent increase in fluorescence intensity (pKa = 7.5), it can generate a brighter fluorescence at a neutral pH than at an acidic pH. We observed that the granular pattern of staining by this dye was increased in P123H β-syn-overexpressing cells transfected with A53T α-syn and vector-transfected cells by PDMP treatment (Figure 1Da). The intensities of fluorescence were significantly increased by PDMP treatment in the former cells and to a lesser extent in the latter cells (Figure 1Db). Together, the data shows that lysosomal activities were decreased by depletion of glycosphingolipids by PDMP treatment in our cellular model of synucleinopathies.
Lysosomal Membrane Permeabilization (LMP) Is Associated with PDMP-Induced Lysosomal Dysfunction in Syn-Overexpressing Cells
To determine whether LMP was induced by PDMP treatment, the Lucifer Yellow staining assay was performed for vector-transfected cells, wild-type β-syn-overexpressing cells, and P123H β-syn-overexpressing cells with or without the transfection of vector, wild-type α-syn, or A53T α-syn. Lucifer Yellow is a well-known marker of fluid-phase pinocytosis that accumulates in secondary lysosomes30 and is impermeable to cellular membranes.31 Notably, this staining has been successfully used for Aβ-induced LMP in neuronal cells.32,33 Here, Lucifer Yellow was visualized using immunofluorescence/LSCM (Figure 2A, a–h). The leakage of the dye in the cytosols 48 hours after PDMP treatment increased in P123H β-syn-overexpressing cells transfected with A53T α-syn (from 13.8 ± 0.7% to 46.2 ± 2.9%), and to a lesser extent in the cells transfected with wild-type α-syn (from 12.5 ± 0.4% to 27.2 ± 3.4%) (Figure 2Ba). In contrast, a modest leakage of the dye was induced by PDMP treatment in the cells transfected with control vector (from 1.2 ± 0.3% to 11.2 ± 1.7%), whereas little leakage of the fluorescence was observed in vector-transfected cells (from 0.2 ± 0.2% to 5.6 ± 0.9%) and in wild-type β-syn-overexpressing cells (from 0.3 ± 0.2% to 6.2 ± 0.6%). The leakage of Lucifer Yellow was then assessed during various intervals after PDMP treatment (Figure 2Bb). We observed that the leakage was significant as early as 6 hours after treatment with PDMP in P123H β-syn-overexpressing cells transfected with A53T α-syn, whereas it became significant at 12 hours in P123H β-syn-overexpressing cells transfected with wild-type α-syn or vector.
Figure 2.
Characterization of LMP in PDMP-treated syn-overexpressing cells. P123H β-syn-overexpressing cells were transfected with vector, wild-type α-syn, or A53T α-syn. Cells were then treated with or without PDMP (25 μmol/L) for the indicated times, followed by assessment of lysosomal leakage. In some experiments, vector-transfected cells, wild-type β-syn-overexpressing cells, and P123H β-syn-overexpressing cells were also analyzed. A: Lucifer Yellow staining at 24 hours. The fluorescence was increased by PDMP treatment in P123H β-syn-overexpressing cells transfected with A53T α-syn (c--h) and to a lesser extent in vector-transfected cells (a and b). Boxes in c and d are equivalent to e and f, respectively. In g and h, cells were fixed in 4% paraformaldehyde and nuclei were labeled with DAPI. Arrowheads indicate lysosomal inclusion bodies (e--h). Note that Lucifer Yellow is accumulated in inclusions before PDMP treatment (e and g), whereas it is leaked into cytoplasm after PDMP treatment (f and h). Ba: Quantification of Lucifer Yellow redistribution. The number of Lucifer Yellow-positive cells in their cytosols at 48 hours of treatment with PDMP was calculated as a percentage of the total number of cells. Data are shown as means ± SD (n = 4). *P < 0.05, **P < 0.01 versus PDMP-untreated cells. In b, time course quantification of Lucifer Yellow redistribution was performed. For P123H β-syn-overexpressing cells transfected with vector, wild-type α-syn, or A53T α-syn, the number of Lucifer Yellow-positive cells in their cytosols was evaluated at 6, 12, 24, and 48 hours. Data are shown as means ± SD (n = 4). *P < 0.05, **P < 0.01 versus PDMP-untreated cells. C: Immunoblot analyses of cathepsins B and D, LAMP-2, and HSP70 for cytosolic and lysosome-enriched fractions (each 10 μg) derived from P123H β-syn cells transfected with either control vector or A53T α-syn. A typical blot for each molecule is presented in a. Equivalent protein loading in each lane was confirmed by immunoblotting with anti-actin antibody. In b, the band intensity corresponding to each molecule was corrected with that of actin. The data are shown as % r. intensity of P123H β-syn-overexpressing cells transfected with vector (PDMP-treated cells for the cytosolic fractions and PDMP-untreated cells for the lysosome-enriched fractions) and are expressed as the mean value ± SD (n = 4). *P < 0.05, **P < 0.01 versus PDMP-untreated cells. D: Double immunofluorescence/LSCM of cathepsin B and β-syn (a), cathepsin D and β-syn (b), and cathepsin D and LAMP-2 (c) for P123H β-syn-overexpressing cells transfected with A53T α-syn. Cells were treated with (d–f, j–l, p–r) or without (a–c, g–i, m–o) PDMP for 24 hours. Arrowheads indicate inclusion bodies. Immunoreactivities of cathepsins B and D were co-localized with those of P123H β-syn (a and b) and LAMP-2 (c) in the lysosomal inclusion bodies before PDMP treatment, but were redistributed into the cytosols after PDMP treatment (a–c). In c, the immunoreactivity of LAMP-2 was retained in the lysosomal inclusions even after PDMP treatment (q). Nuclei are simultaneously stained with DAPI in the merged figures (c, f, i, l, o, and r). Similar results were observed in three independent experiments. Scale bars: 20 μm (Aa–Ad); 5 μm (Ae and Af); 10 μm (Ag, Ah, D).
To confirm that LMP is induced by PDMP treatment in our cellular model of synucleinopathies, the cell extracts were fractionated to isolate cytosolic and lysosome-enriched fractions, followed by immunoblot analysis of two typical lysosomal enzymes, cathepsins D and B, and a lysosomal membrane protein, LAMP-2 (Figure 2Ca). PDMP treatment resulted in the increase of cathepsins D and B in the cytosolic fractions in P123H β-syn cells transfected with A53T α-syn and to a lesser extent in the P123H β-syn cells transfected with control vector. Notably, oligomer formation of cathepsin D was observed in the cytosol fractions of PDMP-treated cells. It is possible that cathepsin D may be unstable in the cytoplasm and was sequestered by syn proteins, leading to aggregation of this enzyme. Such a mechanism was previously described for green fluorescence protein-α-syn transgenic mice.34 Concomitantly, expression levels of both cathepsins D and B in the lysosome-enriched fractions were decreased, indicating a redistribution of these enzymes from the lysosomes to the cytoplasm. Although the expression level of LAMP-2 in the lysosome-enriched fractions was significantly decreased by PDMP treatment, the expression level of LAMP-2 in the cytosolic fractions was little changed by PDMP treatment (Figure 2Cb).
Because previous studies have shown that heat shock chaperone HSP70 acts as a negative regulator of LMP in cancer cells,35 the expression of HSP70 was also investigated. In both cytosolic and lysosome-enriched fractions, the expression level of HSP70 was higher in P123H β-syn cells transfected with A53T α-syn compared with P123H β-syn cells transfected with vector. Furthermore, the expression level of HSP70 in both fractions was slightly but significantly up-regulated by PDMP treatment (Figure 2C and unpublished data). The immunoreactivity of HSP70 was also localized in lysosomal inclusion bodies (data not shown), suggesting a protective role of HSP70 against LMP caused by PDMP treatment.
To further characterize the effects of PDMP treatment on cellular distribution of lysosomal enzymes in P123H β-syn-overexpressing cells transfected with A53T α-syn, double-immunofluorescence/LSCM studies were performed (Figure 2D). The immunoreactivities of cathepsins B and D were co-localized with that of P123H β-syn in the lysosomal inclusion before treatment with PDMP, but was redistributed into the cytosols after treatment with PDMP (Figure 2D, a and b). Further study revealed that co-localization of cathepsin D with a lysosomal membrane protein LAMP-2 was observed in the lysosomal inclusions in PDMP nontreated cells. After PDMP treatment, the immunoreactivity of cathepsin D was detected in the cytoplasm, whereas that of LAMP-2 was relatively retained in the inclusions (Figure 2Dc). The immunoreactivity of cathepsin D exhibited granular patterns, being consistent with the result of oligomer formations of cathepsin D in immunoblotting (Figure 2Ca). Taken together, these results augment the notion that enhanced LMP is associated with PDMP-induced lysosomal pathology in these cells.
Enhanced LMP Stimulates Cytotoxicity in PDMP-Treated Syn-Overexpressing Cells
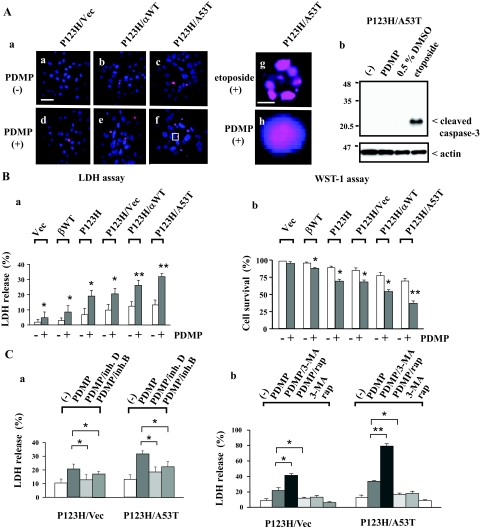
To determine whether PDMP-induced cellular toxicity is observed in our cellular model of synucleinopathies, the TUNEL assay was performed for vector-transfected cells, wild-type β-syn-overexpressing cells, and P123H β-syn-overexpressing cells with or without the transfection of vectors, wild-type α-syn, or A53T α-syn (Figure 3Aa, a–f). After 24 hours of PDMP treatment, the TUNEL-positive nuclei were small and round, and were also stained diffuse and even with DAPI (Figure 3Aa, h). In contrast, addition of etoposide, a topoisomerase II inhibitor, resulted in nuclear fragmentation reminiscent of apoptosis (Figure 3Aa, g). It was also shown that caspase-3 was activated by etoposide but not by PDMP in P123H β-syn-overexpressing cells transfected with A53T α-syn (Figure 3Ab).
Figure 3.
Increased cell death in PDMP-treated syn-overexpressing cells. P123H β-syn-overexpressing cells were transfected with vector, wild-type α-syn, or A53T α-syn. Cells were then treated with or without PDMP (25 μmol/L) for 24 hours, followed by various cell death assays. In some experiments, vector-transfected cells, wild-type β-syn-overexpressing cells, and P123H β-syn-overexpressing cells were also analyzed. Cells were also treated with etoposide (40 μmol/L) or 0.5% DMSO for 24 hours as a positive control for apoptosis. A: TUNEL staining at 24 hours. In a, P123H β-syn-overexpressing cells transfected with vector (a, d), wild-type α-syn (b, e), or A53T α-syn (c, f–h), were treated with either PDMP (d–f, h) or etoposide (40 μmol/L) (g) for 24 hours, followed by TUNEL staining. Areas in f are equivalent to h. In all of the figures, nuclei were simultaneously stained with DAPI. In b, P123H β-syn-overexpressing cells transfected with A53T α-syn were treated without or with PDMP, 0.5% DMSO, or etoposide for 24 hours. Cell extracts (10 μg) were analyzed by immunoblot analysis using anti-cleaved caspase-3. The filter was reprobed with anti-actin antibody. B: Cell death assay at 24 hours. a: LDH release was calculated as a percentage of the total cellular LDH. Data are shown as means ± SD (n = 4). *P < 0.05, **P < 0.01 versus PDMP-untreated cells. b: WST-1 assay was performed by measurement of the absorbance values at 450 nm. Cell survival was calculated as described in the Materials and Methods. Data are shown as means ± SD (n = 4). *P < 0.05, **P < 0.01 versus PDMP-untreated cells. C: Effects of cathepsin inhibitors and autophagy modulators on LDH release at 24 hours. In a, P123H β-syn-overexpressing cells transfected with either vector or A53T α-syn were treated without or with PDMP in the absence or presence of either cathepsin B inhibitor (0.1 μmol/L) or cathepsin D inhibitor (0.5 μmol/L). In b, cells were treated with or without PDMP in the absence or presence of either 3-MA (10 mmol/L) or rapamycin (rap) (0.2 μmol/L). Data are shown as means ± SD (n = 4). *P < 0.05, **P < 0.01 versus PDMP-treated cells. Scale bars: 100 μm (Aa–Af); 5 μm (Ag and Ah).
Next, because some cells were detached by the cytotoxicity caused by PDMP treatment, LDH release assay was performed to precisely characterize the number of cell death (Figure 3Ba). LDH release was significantly up-regulated after 24 hours of PDMP treatment in P123H β-syn-overexpressing cells transfected with A53T α-syn (from 13.3 ± 3.2% to 31.8 ± 2.1%), and to a lesser extent in the cells transfected with wild-type α-syn (from 12.5 ± 2.8% to 26.0 ± 3.4%). In contrast, a small induction of LDH release was observed for P123H β-syn-overexpressing cells transfected with vectors (from 9.9 ± 3.7% to 20.5 ± 3.7%), whereas little cell death was observed in other cell types. These results were consistent with the evaluation by WST-1 assay (Figure 3Bb). The cell survival rates, which were assessed based on the values of the absorbance at 450 nm in various types of syn-transfected cells, were inversely correlated with the results obtained in LDH release assay.
Based on the detection of cathepsins B and D in the cytosols of PDMP-treated P123H β-syn-overexpressing cells transfected with A53T α-syn, it was predicted that these lysosomal enzymes might contribute to increased cytotoxicity. To test this hypothesis, P123H β-syn-overexpressing cells transfected with either vector or A53T α-syn were treated with PDMP in the absence or presence of cathepsin inhibitors, followed by LDH release assay (Figure 3Ca). The LDH release was significantly suppressed by the cathepsin D inhibitor and to a lesser extent by the cathepsin B inhibitor in P123H β-syn-overexpressing cells transfected with A53T α-syn (from 32.5 ± 3.8% to 18.5 ± 3.6% by cathepsin D inhibitor and 22.4 ± 3.7% by cathepsin B inhibitor). Smaller but essentially similar effects were observed for P123H β-syn-overexpressing cells transfected with vector. Thus, these results suggested that the cell death caused by PDMP treatment was at least in some parts attributable to the leakage of lysosomal cathepsins B and D into the cytosols.
The effects of chemical autophagy modulators on PDMP-induced cell death was also investigated (Figure 3Cb). It was shown that addition of 3-MA, an inhibitor of autophagy, significantly enhanced the LDH release caused by PDMP treatment, whereas rapamycin, a typical autophagy inducer, suppressed it in P123H β-syn-overexpressing cells transfected with A53T α-syn (from 33.6 ± 2.3% to 79.8 ± 3.5% by 3-MA and 18.8 ± 3.0% by rapamycin). 3-MA by itself caused no significant cell death. Smaller but essentially similar effects were observed for P123H β-syn-overexpressing cells transfected with vector. These results suggest that autophagy may act protectively on the cell death caused by PDMP treatment.
Altered Expression of Lysosomal Membrane Proteins in PDMP-Treated Syn-Overexpressing Cells
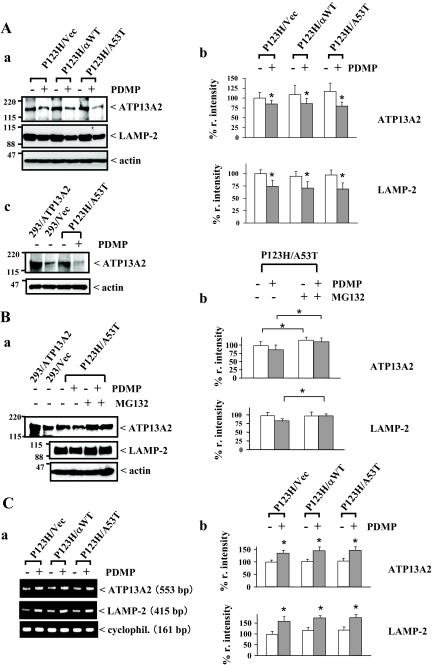
To determine whether PDMP-induced lysosomal dysfunction and enhanced LMP are reflected in altered expression of lysosomal membrane proteins in our cellular model of synucleinopathies, the effects of PDMP on the expression of two molecules, LAMP-2 and ATP13A2, were investigated for P123H β-syn-overexpressing cells transfected with vector, wild-type α-syn, or A53T α-syn. It was recently shown that missense mutation of ATP13A2 is linked to autosomal recessive hereditary parkinsonism and Kufor Rakeb disease (PARK9).26,36 On the other hand, it is known that LAMP-2 acts as a receptor for the selective import and degradation of cytosolic proteins in the lysosomes, or for chaperone-mediated autophagy.37,38 More recently, it was shown that LAMP-2 was indispensable to the maturation of the autophagosome.39 Thus, these molecules may be important in protection against the lysosomal pathology of synucleinopathies. The data show that expression levels of both ATP13A2 and LAMP-2 proteins were significantly decreased by PDMP treatment in all cell types (Figure 4A, a–c).
Figure 4.
Decreased expression of lysosomal membrane proteins in PDMP-treated syn-overexpressing cells. P123H β-syn-overexpressing cells were transfected with vector, wild-type α-syn, or A53T α-syn. Cells were then treated with or without PDMP (25 μmol/L) for 24 hours, followed by various analyses. A: Immunoblot analysis of lysosomal membrane proteins. a: Cell extracts (10 μg) were analyzed by immunoblot using anti-ATP13A2 (top), anti-LAMP-2 (middle), and anti-actin (bottom). In b, the band intensities corresponding to ATP13A2 and LAMP-2 were quantified and corrected with the intensities of actin bands. The data are shown as % r. intensity of the value obtained from P123H β-syn-overexpressing cells transfected with vector and are expressed as the mean value ± SD (n = 4). *P < 0.05 versus PDMP-untreated cells. In c, HEK293 cells transfected with either vector or ATP13A2 were used as controls. The blots are representative of three experiments. B: Effects of proteasome inhibitors on PDMP-induced down-regulation of lysosomal membrane proteins. a: P123H β-syn-overexpressing cells transfected with A53T α-syn were treated with or without PDMP in the absence or presence of MG132 (10 μmol/L). Cell extracts (10 μg) were analyzed by immunoblot using anti-ATP13A2 (top), anti-LAMP-2 (middle), and anti-actin (bottom). In b, quantification was performed. The data are shown as % r. intensity of the value obtained from P123H β-syn-overexpressing cells transfected with A53T α-syn and are expressed as the mean value ± SD (n = 4). *P < 0.05, versus MG132-untreated cells. C: RT-PCR analysis of ATP13A2 and LAMP-2 mRNAs. Representative figures are shown in a. Cyclophilin mRNA was used as an internal control. In b, the band intensities corresponding to ATP13A2 and LAMP-2 were quantified and corrected with the intensities of cyclophilin (cyclophil.) bands. The data are shown as % r. intensity of the value obtained from P123H β-syn-overexpressing cells transfected with vector without PDMP treatment and are expressed as the mean value ± SD (n = 4). *P < 0.05 versus PDMP-untreated cells.
Because the PARK9-linked mutant of ATP13A2 was shown to be susceptible to proteasomal degradation,26 it was speculated that wild-type ATP13A2 became unstable because of the alteration of lysosomal membranes by PDMP treatment, being subjected to degradation in the proteasome. Supporting this possibility, co-incubation with the proteasome inhibitor MG132 clearly up-regulated the immunoreactivity of ATP13A2 irrespective of the presence or absence of PDMP (Figure 4B, a and b). Similar to ATP13A2, PDMP-induced down-regulation of LAMP-2 expression was abrogated by co-incubation of MG132. Furthermore, RT-PCR analysis showed that both ATP13A2 and LAMP-2 mRNAs were significantly increased by PDMP treatment (Figure 4C, a and b), suggesting that the regulation of mRNAs of these molecules by PDMP treatment was not reflected in their decreased protein expression. Taken together, PDMP treatment resulted in altered expressions of lysosomal proteins, which might be potentially linked to stimulation of lysosomal pathology.
Ultrastructural Analysis of Lysosomal Pathology in PDMP-Treated Syn-Overexpressing Cells
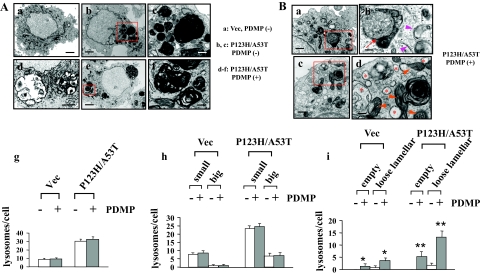
To determine whether PDMP treatment results in alteration of lysosomal morphology in a cellular model of synucleinopathies, electron microscopic analysis was performed for P123H β-syn-overexpressing cells transfected with A53T α-syn, and vector-transfected cells (Figure 5A). P123H β-syn-overexpressing cells transfected with A53T α-syn had electron-high dense myelinosome-like inclusions composed of concentric and/or multilamellar periodic membranes that were reminiscent of the lysosomal inclusion bodies formed in various types of LSDs (Figure 5A, a–f). Morphometric analysis revealed that total numbers of the lysosomal structures, including both small and big inclusions, were significantly higher in P123H β-syn-overexpressing cells transfected with A53T α-syn than in vector-transfected cells. However, the numbers of these lysosomal structures were little affected by PDMP treatment in both cells (Figure 5A, g and h). Notably, tightly packed lamellar structures of inclusions became fairly loose to give a gap between each membrane after treatment with PDMP (Figure 5A, e and f). Empty giant inclusion bodies were also observed (Figure 5Ad). Such changes were predominant in P123H β-syn-overexpressing cells transfected with A53T α-syn compared with vector cells (Figure 5Ai). These morphological alterations in the lysosomal inclusion bodies might be relevant to the dysfunction of lysosomes, such as decreased cathepsin B activity and enhanced LMP, in PDMP-treated cells, although no direct evidences were obtained to demonstrate the destructive profile of lysosomal membranes.
Figure 5.
Ultrastructural analyses of PDMP-treated syn-overexpressing cells. Electron microscopic analyses were performed for P123H β-syn-overexpressing cells transfected with A53T α-syn, followed by treatment with (Ad–Af, Ba–Bd) or without (Ab and Ac) PDMP treatment for 24 hours. Vector-transfected cells were also analyzed as a control (Aa). A: At a low magnification, P123H β-syn-overexpressing cells transfected with A53T α-syn exhibited various sizes of electron-dense inclusion clusters (b and e), whereas fewer lysosomal structures were found in vector-transfected cells (a). c: At a higher magnification, some large electron-dense inclusions were composed of multilamellar myelinosomes whose membranes were tightly packed with each other, whereas other small electron-dense inclusions made a fusion with relatively light dense structures of unknown origins. f: After treatment with PDMP, the tightly packed structures of multilamellar myelinosomes became so loose as to give rise to a gap between each membrane. d: Further, empty inclusion bodies, the contents of which were probably discharged into the cytoplasm, were frequently encountered. Small boxes in b and e are equivalent to c and f, respectively. In g–i, morphometric analysis was performed as described in the Materials and Methods. g: The numbers of lysosomal inclusions were higher in both P123H β-syn-overexpressing cells transfected with A53T α-syn compared with vector-transfected cells. However, there were no significant changes of the numbers of lysosomes before and after PDMP treatment. h: Similar results were obtained when the lysosomal structures were categorized into two groups according to their diameters; small: 400 nm to ∼1 μm, and big: more than 1 μm. In i, the numbers of empty giant inclusion and loose lamellar structures of inclusions were significantly increased after PDMP treatment in P123H β-syn-overexpressing cells transfected with A53T α-syn and to a lesser extent in vector-transfected cells. Data are shown as means ± SD (n = 50 cells). *P < 0.05, **P < 0.01 versus PDMP-untreated cells. B: In PDMP-treated P123H β-syn-overexpressing cells transfected with A53T α-syn, there was extensive formation of autophagic vacuoles (b, arrow) and unclosed double-membrane structures (b, arrowheads). Furthermore, mitochondria (d, arrowheads) and ER (d, asterisks) were frequently swollen and distorted. Small boxes in a and c are equivalent to b and d, respectively. In both A and B, the electron micrograph is representative of at least three independent experiments. Scale bars: 4 μm (Aa, Ab, Ad, Ae, Ba, Bc); 1 μm (Ac, Af, Bb, Bd).
Another pathological feature for PDMP-treated P123H β-syn-overexpressing cells transfected with A53T α-syn was the accumulation of autophagic vacuoles. Although typical autophagosomes were little encountered, there were many atypical double-membrane structures; some were flat and open-circular, whereas others were associated with multivesicular bodies, suggesting that the process of normal autophagy flux was deregulated by PDMP treatment (Figure 5B, a and b). Furthermore, swollen and distorted morphology of cytoplasmic organelles, such as mitochondria and ER, were frequently encountered (Figure 5B, c and d). These pathological features have been well characterized in both autophagic cell death and necrosis.40 Despite such dramatic changes in the cytoplasm, the morphology of the nucleus was invariably preserved (Figure 5, A and B). This finding is in line with the involvement of autophagy because it is known that autophagic cell death exhibits nuclear morphology of either apoptosis or necrosis.40 Taken together, it was suggested that autophagy might play a central role in PDMP-induced cell death in our cellular model of synucleinopathies.
Suppression of the Autophagy Pathway in PDMP-Treated Syn-Overexpressing Cells
To determine whether the process of autophagy was modified by PDMP treatment in P123H β-syn-overexpressing cells transfected with A53T α-syn, expression of LC3B was analyzed using immunoblotting for P123H β-syn-overexpressing cells transfected with A53T α-syn, and vector-transfected cells. Because the autophagosome membrane-associated form of LC3 (LC3-II) exhibits differential mobility compared with the cytoplasmic LC3 (LC3-I) on immunoblot analysis, the presence of LC3-II can be used as a marker protein for the autophagosome formation.41 Consistent with the result of electron microscopy, the expression of LC3B-II was increased after 24 hours of PDMP treatment in P123H β-syn-overexpressing cells transfected with A53T α-syn, and to a lesser extent in the cells transfected with wild-type α-syn (Figure 6A, a and b). In contrast, a modest increase of LC3B-II by PDMP treatment was observed for other cell types.
Figure 6.
Altered expression of the autophagy regulatory molecules in PDMP-treated syn-overexpressing cells. P123H β-syn-overexpressing cells were transfected with vector, wild-type α-syn, or A53T α-syn. Cells were then treated with or without PDMP (25 μmol/L) for 24 hours, followed by various analyses. Vector-transfected cells were also analyzed. A: Immunoblot analyses of the autophagy regulatory molecules. Cell extracts (10 μg) were analyzed using immunoblot for LC3B, Beclin-1, Atg5-Atg12, phospho (p)-mTOR, total (t)-mTOR, phospho (p)-p70 S6 kinase, total (t)-p70 S6 kinase, p-4E-BP1, t-4E-BP1, and actin. Representative figures are shown in a. In b, the band intensities corresponding to LC3B-I, LC3B-II, p62, Beclin-1, p-p70 S6 kinase, and t-p70 S6 kinase were quantified and corrected with the intensities of actin bands. The ratio of LC3B-II relative to LC3B-I and that of p-p70 S6 kinase relative to t-p70 S6 kinase were calculated, respectively, and are expressed as the mean value ± SD (n = 4). For p62 and Beclin-1, the data are shown as % r. intensity of the value obtained from vector-transfected cells without PDMP treatment and are expressed as the mean value ± SD (n = 4). *P < 0.05 versus PDMP-untreated cells. B: RT-PCR analysis of P62, Beclin-1, Atg5, and mTOR mRNAs. Representative figures are shown in a. Cyclophilin mRNA was used as an internal control. In b, the band intensities corresponding to p62 and Beclin-1 were quantified and corrected with the intensities of cyclophilin (cyclophil.) bands. The data are shown as % r. intensity of the value obtained from P123H β-syn-overexpressing cells transfected with vector without PDMP treatment and are expressed as the mean value ± SD (n = 4). *P < 0.05 versus PDMP-untreated cells. C: Double immunofluorescence/LSCM of LC3B and β-syn. Vector-transfected cells (a--c) and P123H β-syn-overexpressing cells transfected with A53T α-syn were treated without (d--f) or with (g--i) PDMP, followed by immunostaining for LC3B (green; a, d, and g) and β-syn (red; b, e, and h). Nuclei are simultaneously stained with DAPI in the merged figures (c, f, and i). Small boxes in d and g are equivalent to j and k, respectively. Arrowheads indicate lysosomal inclusion bodies. Similar results were observed in three independent experiments. D: Triple immunofluorescence/LSCM of LC3B, P62, and β-syn. P123H β-syn-overexpressing cells transfected with A53T α-syn were treated with PDMP, followed by immunostaining for LC3B (green; a), β-syn (red; b), and P62 (blue; c). d: Three molecules were co-localized in some inclusion bodies after PDMP treatment. Arrowheads indicate lysosomal inclusion bodies. Similar results were observed in three independent experiments. Scale bars: 20 μm (Ca--Ci); 10 μm (Cj, Ck, D).
It is known that the number of the autophagosomes is increased not only by stimulation of autophagy flux but also by compromised clearance of the autophagosomes. The latter is frequently caused by the failure of the fusion process between the autophagosomes and the lysosomes, which precedes the autolysosome formation.42 Because the present study showed that PDMP treatment resulted in compromised lysosomal activity, it was suspected that the autophagosomes may accumulate because of the latter mechanism. However, other mechanisms might be at play. To determine whether expression levels of other autophagy regulatory molecules are affected by PDMP treatment, immunoblot analysis was performed. The data shows that expressions of Atg5 and Beclin-1, which are both essential players during the initial stage of autophagy,42 were clearly down-regulated by PDMP treatment, whereas expression of mTOR, a negative regulator of autophagy,42 was up-regulated (Figure 6A, a and b). Furthermore, it was shown that phosphorylation of mTOR substrates, such as p70 S6 kinase and 4E-BP, was significantly stimulated by PDMP treatment. Similar to the immunoblot data, RT-PCR analysis showed that expression of both Atg5 and Beclin-1 mRNAs were decreased (Figure 6B, a and b; and unpublished data), whereas mTOR mRNA was increased by PDMP treatment (data not shown). Thus, these data show that several distinct mechanisms may contribute to the suppression of the autophagy pathway by PDMP treatment.
To further characterize the immunoreactivity of LC3B, immunofluorescence/LSCM was performed (Figure 6C). The LC3B dots were detected in the cytoplasm of P123H β-syn-overexpressing cells transfected with A53T α-syn (Figure 6Cd) and to a lesser extent in vector-transfected cells (Figure 6Ca). In the former cells, the immunoreactivity of LC3B was co-localized with that of P123H β-syn in the lysosomal inclusion bodies (Figure 6C, d–j). Such a co-localization of LC3B and P123H β-syn was also observed after PDMP treatment (Figure 6C, g–k). Furthermore, it was shown that the immunoreactivity of P62 was well merged with those of LC3B and P123H β-syn in some inclusion bodies before and after PDMP treatment (Figure 6D, l–o). The expression of p62 protein was increased by PDMP treatment (Figure 6A, a and b), whereas mRNA expression was little affected. These results are consistent with a recent report describing that LC3 is an aggregate-prone protein and is frequently incorporated in protein aggregates, such as polyQ proteins.43 Furthermore, it was recently shown that LC3 is associated with P62 in inclusion bodies formed in hepatocytes and neurons in the autophagy-deficient mice.44
Accumulation of Syn Proteins in PDMP-Treated Syn-Overexpressing Cells
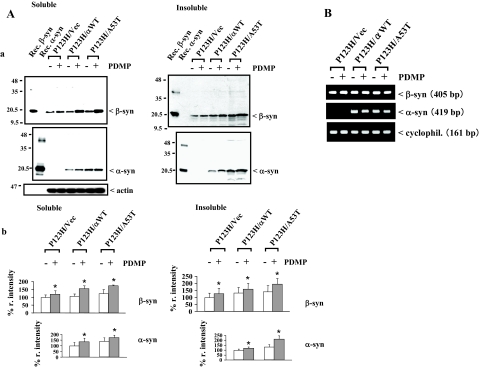
A recent study shows that the autophagy-lysosome pathway may play a central role in the degradation of α-syn.45 Because PDMP treatment resulted in suppression of autophagy, it was predicted that accumulation of syn proteins may be stimulated in our cellular model of synucleinopathies. To test this possibility, immunoblot analysis was performed to evaluate the expression of syn proteins in P123H β-syn-overexpressing cells transfected with vector, wild-type α-syn, or A53T α-syn. The data show that the expression levels of P123H β-syn in both detergent (1.0% Triton X-100)-soluble and -insoluble fractions were significantly increased by PDMP treatment in P123H β-syn-overexpressing cells transfected with A53T α-syn and to a lesser extent in the cells transfected with wild-type α-syn (Figure 7A, a and b). In contrast, a weak induction of the P123H β-syn expression by PDMP was observed for P123H β-syn-overexpressing cells transfected with vector. Essentially similar results were obtained for the expression of α-syn (Figure 7A, a and b). RT-PCR analysis showed that expression levels of both α- and β-syn mRNAs were little changed by PDMP treatment (Figure 7B). Together, these data support the notion that the suppression of autophagy by PDMP treatment might lead to accumulation of the syn proteins.
Figure 7.
Accumulation of syn proteins in PDMP-treated syn-overexpressing cells. P123H β-syn-overexpressing cells were transfected with vector, wild-type α-syn, or A53T α-syn. Cells were then treated with or without PDMP (25 μmol/L) for 24 hours, followed by immunoblot analysis and RT-PCR. A: Immunoblot analysis of syn proteins. The soluble and insoluble fractions of cell extracts (10 μg) were analyzed by immunoblotting using anti-β-syn, anti-α-syn, and anti-actin antibodies. Recombinant α- and β-syn proteins are used as positive controls. a: The blots are representative of at least three experiments. In b, the band intensities corresponding to α- and β-syn were quantified and corrected with the intensities of actin bands. The data are shown as % r. intensity of the value obtained from P123H β-syn-overexpressing cells transfected with either vector or wild-type α-syn without PDMP treatment and are expressed as the mean value ± SD (n = 3). *P < 0.05 versus PDMP-untreated cells. B: RT-PCR analysis of β- and α-syn mRNAs. Cyclophilin mRNA was used as an internal control. Similar results were obtained in three independent experiments and there were no significant effects of PDMP treatment (not shown).
Gangliosides Reverse the Lysosomal Pathology in PDMP-Treated Syn-Overexpressing Cells
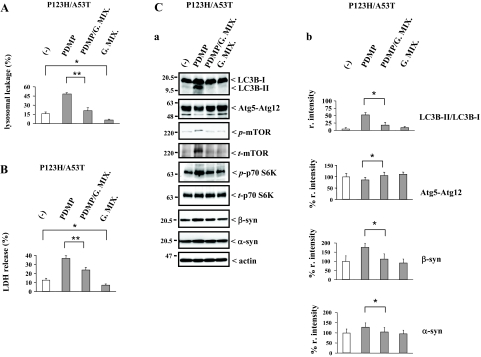
To determine whether enhanced lysosomal pathology by PDMP treatment is attributable to the depletion of gangliosides, in our cellular model of synucleinopathies, P123H β-syn-overexpressing cells transfected with A53T α-syn were co-incubated with the brain ganglioside mixtures during PDMP treatment, followed by several evaluations, including cell toxicity, LMP, and expression levels of autophagy regulatory molecules and syn proteins (Figure 8). As expected, the Lucifer Yellow-staining experiment showed that the stimulating effects of PDMP on the leakage of this dye were significantly abrogated by treatment with ganglioside mixtures, indicating that the PDMP-induced up-regulation of LMP was abrogated by an excess of gangliosides (Figure 8A). Furthermore, the leakage of Lucifer Yellow was significantly suppressed by gangliosides alone. Consistent with the result of Lucifer Yellow-staining experiment, the release of LDH induced by PDMP treatment in P123H β-syn-overexpressing cells transfected with A53T α-syn was significantly decreased by addition of ganglioside mixtures, suggesting that the cell toxicity caused by PDMP treatment was abrogated by an excess of gangliosides (Figure 8B). Furthermore, it was shown that the release of LDH was significantly suppressed by gangliosides alone.
Figure 8.
PDMP-induced lysosomal pathology is ameliorated by addition of gangliosides. P123H β-syn-overexpressing cells were transfected with A53T α-syn. Cells were then treated with or without PDMP (25 μmol/L) in the presence of either ganglioside mixtures (G. Mix.) (50 μg/ml) or vehicle (0.1% methanol) for 24 hours, followed by various analyses. A: Quantification of Lucifer Yellow redistribution. The number of Lucifer Yellow-positive cells in their cytosols was calculated as a percentage of the total number of cells. Data are shown as means ± SD (n = 4). *P < 0.05, **P < 0.01 versus G. Mix.-untreated cells. B: Quantification of LDH release. LDH release was calculated as a percentage of the total cellular LDH. Data are shown as means ± SD (n = 4). *P < 0.05, **P < 0.01 versus G. Mix.-untreated cells. C: Immunoblot analysis of the autophagy regulatory molecules and syn proteins. a: The soluble fractions of cell extracts (10 μg) were analyzed using immunoblot for LC3B, Atg5-Atg12, p-mTOR, t-mTOR, p-p70 S6 kinase, t-p70 S6 kinase, β-syn, α-syn, and actin. In b, the intensities of the bands of these molecules were quantified and corrected with that of actin. For LC3B, the ratio of LC3B-II relative to LC3B-I was calculated and is expressed as the mean value ± SD (n = 4). For Atg5-Atg12, β-syn, and α-syn, the data are shown as % r. intensity of the value obtained from the cells without PDMP treatment and are expressed as the mean value ± SD (n = 4). *P < 0.05 versus G. Mix.-untreated cells.
Under the same experimental conditions, expression levels of the autophagy regulatory proteins were evaluated by immunoblot analysis (Figure 8C). PDMP treatment-induced down-regulation of Atg5 and Beclin-1 expressions were mitigated in the presence of gangliosides, whereas up-regulation of mTOR and phosphorylated p70 S6 kinase expressions by PDMP treatment were abrogated by ganglioside treatment (Figure 8C, a and b; and unpublished data), suggesting that suppressive effects of PDMP treatment on the autophagy were reversed by addition of gangliosides. However, the effects of gangliosides alone on the expression of the autophagy regulatory molecules were unclear. Consistent with these data, it was further shown that PDMP-induced increase of syn proteins in both detergent-soluble and -insoluble fractions was significantly abrogated by addition of gangliosides (Figure 8C, a and b). Collectively, these results indicate that enhanced lysosomal pathology in PDMP-treated syn-overexpressing cells may be the result of the ganglioside depletion caused by PDMP treatment.
Discussion
Using a cellular model of synucleinopathies, we demonstrated here that glycosphingolipid depletion by PDMP treatment resulted in various features of lysosomal pathology. These include compromised lysosomal activity, enhanced LMP, decreased expression of lysosomal membranous proteins, alteration of lysosomal ultrastructures, and suppression of the autophagy-lysosome pathway. It was also shown that increased cytotoxicity was caused by enhanced LMP while both P123H β-syn and α-syn proteins accumulated through the suppression of the autophagy-lysosomal pathway. Finally, these detrimental effects by PDMP treatment on lysosomal pathology were significantly ameliorated by addition of gangliosides. These results suggested that endogenous gangliosides may play a protective role against the lysosomal pathology of synucleinopathies. Although the neuroprotective effects of gangliosides have been described for a variety of neurodegenerative and neurological conditions, the underlying molecular mechanisms are elusive. Our results provide a mechanism that may account for the neuroprotective effects of gangliosides on the pathogenesis of PD.
The lysosomal membrane has multiple important functions. It is responsible for acidification of the interior, sequestration of the active lysosomal enzymes,46 and transport of degradation products from the lysosomal lumen to the cytoplasm.47 Our data suggests that PDMP treatment resulted in lysosomal dysfunction, leading to enhanced LMP in our cellular model of synucleinopathies. First, PDMP treatment interfered with lysosomal functions as demonstrated by decreased activity of cathepsin B and a rise in pH in lysosomal inclusions. Second, increased LMP by PDMP treatment was demonstrated by cytoplasmic leakage of Lucifer Yellow fluorescence as well as the redistribution of lysosomal hydrolases, such as cathepsins B and D, to the cytosols. Third, lysosomal membranes were altered by PDMP treatment as shown by decreased expression of lysosomal membrane proteins including ATP13A2 and LAMP-2. Considering that missense mutations of ATP13A2 is linked to familial autosomal recessive PD (Park9)26,36 whereas LAMP-2 plays critical roles for chaperone-mediated autophagy38 and also for macroautophagy,48 we speculate that the decreased expression of these molecules may be prerequisite for neurodegeneration. Fourth, electron microscopy showed that the membranous structures of the inclusion bodies were loose and irregular in PDMP-treated cells compared with its tight and regular membranous structures in untreated cells. Because increased LMP by PDMP treatment was mitigated by addition of gangliosides, it was suggested but not demonstrated that endogenous gangliosides might be indispensable for the maintenance of the integrity of the lysosomal membrane, or otherwise the result is enhanced LMP.
Considering that the leakage of lysosomal enzymes may exert lethal effects, neurons might be equipped with certain mechanisms by which LMP by various stimuli is negatively regulated. In this context, it is worth noting that HSP70 has been characterized as a negative regulator of LMP in cancer cells.35 Because tumors are confronted with a variety of cytotoxic stimuli such as γ-irradiation and anti-cancer drugs, which are stimulators of LMP, increased expression of lysosomal HSP70 may confer resistance against increased LMP.35 The present study suggests that HSP70 may also play a protective role against LMP in neuronal cells. Supporting this notion, HSP70 was immunopositive in lysosomal inclusions in the P123H β-syn-overexpressing cells transfected with α-syn and was significantly up-regulated by PDMP treatment. Similar to HSP70, the gangliosides may be regarded as a negative regulator of LMP because enhanced LMP induced by PDMP treatment was causative for cell death, which was significantly reversed by the addition of gangliosides. Further supporting this notion, the gangliosides were shown to be protective against oxidative stress, a potent stimulator of LMP in various cells and tissues, including neuronal cells and mouse brain.35,49,50 Thus, we predict that endogenous gangliosides may play an important role as a negative regulator of LMP in neurons and possibly nonneuronal cells.
Because amyloid-fibrils are critical as stimulators of LMP,35 and because the gangliosides interact with amyloidogenic proteins and modulate the aggregation of these proteins,8,18 it is speculated that the effects of gangliosides on LMP may depend on each amyloidogenic protein accumulating in the lysosomes under the neurodegenerative conditions. In our cellular model of synucleinopathies, we previously observed that both P123H β-syn and α-syn were accumulated in the lysosomal inclusion bodies, forming thiofavin-S-positive amyloid like-fibrils.24 These aggregate-prone syn proteins were targeted by the autophagy-lysosome pathway and the fibril (or protofibril) form of these proteins might be refractory to lysosomal degradation, resulting in accumulation in the lysosomes. Because both GM1 and GM2 were immunopositive in the inclusions,24 these gangliosides may act inhibitory on the aggregation of α-syn,8 contributing to the protection against the lysosomal pathology in these cells. However, the situation may be complicated when Aβ is involved because gangliosides may stimulate aggregation of Aβ.18 Considering that the endosomal-lysosomal pathway is one major site where Aβ is produced,51 it is possible that aggregate-prone Aβ1-42 may be accumulated in the lysosomes, forming amyloid fibrils (or protofibrils) thereby. These fibrils may translocate into the cytoplasm through the impaired lysosomal membranes and interact with tau protein, leading to tangle formation. It is also possible that Aβ1-42 translocated into the cytosols may stimulate aggregation of α-syn, leading to Lewy body formation in AD. Such a mechanism could be applied to the overlapping pathology between AD and PD, including Lewy body variants of AD and early onset of familial AD associated with the formation of Lewy bodies.52 Under these conditions, it is possible that gangliosides may stimulate Aβ aggregation, leading to enhanced LMP. Thus, further investigations are required to determine whether the gangliosides may play differential effects on the lysosomal pathology of PD depending on the involvement of AD pathology.
The outcome of LMP has been characterized by multiple cell-destructive mechanisms.53 LMP may cause the proteolytic activation of Bid, cytochrome c release, and caspase activation, leading to apoptosis. On the other hand, massive LMP often results in cell death without caspase activation; this type of cell death may adopt a subapoptotic or necrotic appearance. Our present study may represent the latter case. Although TUNEL staining was positive by PDMP treatment, the nuclear staining with DAPI exhibited a weak and diffuse pattern, suggesting that pyknosis occurred without extensive DNA fragmentation and caspase-3 activation. Consistent with this notion, a relatively preserved nucleus was confirmed by electron microscopy. In contrast to pronounced pyknosis in apoptosis, such a mild pyknosis has been characterized in autophagic cell death.40 Further supporting the role of autophagy in PDMP-induced cell death, dynamic change of the cytoplasm was observed. In addition to extensive formation of autophagic vacuoles, the membranes of ER and mitochondria were frequently dilated by PDMP treatment. Such a swelling of intracellular organelles has been well characterized not only in necrosis, but also in autophagic cell death.40 Together, the results strongly suggest that autophagy may play a central role in PDMP-induced cell death. Then, what is the role of autophagy in the PDMP-induced cell death? In this regard, a further study showed that induction of autophagy by rapamycin rescued cell death by PDMP treatment, whereas suppression of autophagy by a chemical inhibitor 3-MA stimulated it, indicating that autophagy by itself may be protective. Because several lines of evidences in our study showed that autophagic flux was inhibited by PDMP treatment, one possible explanation is that decreased activities of autophagy failed to protect cell death. This notion is in line with a recent view regarding the role of autophagy in cell death. Autophagy has been a cytoprotective response, which can facilitate either apoptosis or necrosis if autophagy fails in pathological states.54
Regarding the role of gangliosides for protein aggregation in neurodegenerative disorders, previous studies have almost exclusively focused on the direct interaction of gangliosides with amyloidogenic proteins, including Aβ and α-syn. The present study may provide an alternative mechanism. In our cellular model of synucleinopathies, it was shown that suppression of the autophagic flux by depletion of the gangliosides might lead to accumulation of both P123H β-syn and α-syn, which was reversed by the addition of gangliosides. It was further shown that both LC3B and P62 were co-localized with P123H β-syn in the lysosomal inclusions, raising a possibility that these autophagy regulatory molecules were sequestered by P123H β-syn in the inclusions. Thus, these results suggest that alteration of the expression of gangliosides may be situated at the upstream of deregulation of autophagy and protein aggregation in the cascade of neurodegeneration. In neurodegenerative diseases, such as AD and PD, massive accumulations of autophagic vacuoles are observed within large swellings along with dystrophic and degenerating neuritis in which protein aggregations are prominent, suggesting that deregulation of autophagy and protein aggregation are associated with axonal degeneration.51,55 Based on our present result, one may speculate that deregulation of the expression of gangliosides may underlie the process of axonal degeneration. If this is the case, it is naturally expected that restoration of the protective action of gangliosides might be a useful therapeutic strategy. Thus, although previous clinical trials of gangliosides for the treatment of PD was suspended because of the occasional development of an acute motor neuropathy clinically presented as Guillain-Barré syndrome,56 it is worth re-evaluating the effects of gangliosides on the pathogenesis of PD, especially in terms of axonal pathology. Further, because previous studies have used drug-induced Parkinsonian models, such as those for 1-methyl-4-pheny1-1,2,3,6-tetrahydropyridine-treated animal and 6-hydroxydopamine-lesioned rat striatum, to assess the neuroprotective effects of gangliosides,9,10 an ideal investigation would consider using some syn transgenic mice, which may exhibit neuroaxonal degeneration in the striatum of their brains.
Acknowledgments
We thank Drs. Kazuhiko Watabe and Kiyomitsu Oyanagi (Tokyo Metropolitan Institute for Neuroscience) for their advice and continuous encouragement.
Footnotes
Address reprint requests to Jianshe Wei, Ph.D., or Makoto Hashimoto, M.D., Ph.D., Laboratory for Chemistry and Metabolism, Tokyo Metropolitan Institute for Neuroscience, Fuchu, Tokyo 183-8526, Japan. E-mail: jswei@tmin.ac.jp or mhashimoto@tmin.ac.jp.
Supported by a grant-in-aid for Science Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and Takeda Science Foundation.
References
- Hashimoto M, Masliah E. Alpha-synuclein in Lewy body disease and Alzheimer’s disease. Brain Pathol. 1999;9:707–720. doi: 10.1111/j.1750-3639.1999.tb00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Goedert M, Iwatsubo T, Lee VM. Fatal attractions: abnormal protein aggregation and neuron death in Parkinson’s disease and Lewy body dementia. Cell Death Differ. 1998;5:832–837. doi: 10.1038/sj.cdd.4400432. [DOI] [PubMed] [Google Scholar]
- Lansbury PT., Jr Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proc Natl Acad Sci USA. 1999;96:3342–3344. doi: 10.1073/pnas.96.7.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Mante M, Mallory M, Masliah E. Beta-synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron. 2001;32:213–223. doi: 10.1016/s0896-6273(01)00462-7. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Li J, Souillac P, Millett IS, Doniach S, Jakes R, Goedert M, Fink AL. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J Biol Chem. 2002;277:11970–11978. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- Fan Y, Limprasert P, Murray IV, Smith AC, Lee VM, Trojanowski JQ, Sopher BL, La Spada AR. Beta-synuclein modulates alpha-synuclein neurotoxicity by reducing alpha-synuclein protein expression. Hum Mol Genet. 2006;15:3002–3011. doi: 10.1093/hmg/ddl242. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Mante M, Crews L, Bar-On P, Gage FH, Marr R, Masliah E. An antiaggregation gene therapy strategy for Lewy body disease utilizing beta-synuclein lentivirus in a transgenic model. Gene Ther. 2004;11:1713–1723. doi: 10.1038/sj.gt.3302349. [DOI] [PubMed] [Google Scholar]
- Martinez Z, Zhu M, Han S, Fink AL. GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry. 2007;46:1868–1877. doi: 10.1021/bi061749a. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Mariani AP, Neff NH. GM1 ganglioside-induced recovery of nigrostriatal dopaminergic neurons after MPTP: an immunohistochemical study. Brain Res. 1989;484:297–303. doi: 10.1016/0006-8993(89)90373-9. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Pope A, Simpson K, Taggart J, Smith MG, DiStefano L. Recovery from experimental parkinsonism in primates with GM1 ganglioside treatment. Science. 1992;256:843–846. doi: 10.1126/science.1350379. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Ikebe S, Hattori N, Nakagawa-Hattori Y, Mochizuki H, Tanaka M, Ozawa T. Role of mitochondria in the etiology and pathogenesis of Parkinson’s disease. Biochim Biophys Acta. 1995;1271:265–274. doi: 10.1016/0925-4439(95)00038-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Takenouchi T, Rockenstein E, Masliah E. Alpha-synuclein up-regulates expression of caveolin-1 and down-regulates extracellular signal-regulated kinase activity in B103 neuroblastoma cells: role in the pathogenesis of Parkinson’s disease. J Neurochem. 2003;85:1468–1479. doi: 10.1046/j.1471-4159.2003.01791.x. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo S, Nemani VM, Chalkley RJ, Anthony MD, Hattori N, Mizuno Y, Edwards RH, Fortin DL. A combinatorial code for the interaction of alpha-synuclein with membranes. J Biol Chem. 2005;280:31664–31672. doi: 10.1074/jbc.M504894200. [DOI] [PubMed] [Google Scholar]
- Zappia M, Crescibene L, Bosco D, Arabia G, Nicoletti G, Bagala A, Bastone L, Napoli ID, Caracciolo M, Bonavita S, Di Costanzo A, Gambardella A, Quattrone A. Anti-GM1 ganglioside antibodies in Parkinson’s disease. Acta Neurol Scand. 2002;106:54–57. doi: 10.1034/j.1600-0404.2002.01240.x. [DOI] [PubMed] [Google Scholar]
- Nockels R, Young W. Pharmacologic strategies in the treatment of experimental spinal cord injury. J Neurotrauma. 1992;9(Suppl 1):S211–S217. [PubMed] [Google Scholar]
- Svennerholm L. Gangliosides—a new therapeutic agent against stroke and Alzheimer’s disease. Life Sci. 1994;55:2125–2134. doi: 10.1016/0024-3205(94)00393-9. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K. Role of gangliosides in Alzheimer’s disease. Biochim Biophys Acta. 2007;1768:1943–1951. doi: 10.1016/j.bbamem.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Hruska KS, Goker-Alpan O, Sidransky E. Gaucher disease and the synucleinopathies. J Biomed Biotechnol. 2006;2006:78549. doi: 10.1155/JBB/2006/78549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Iseki E, Katsuse O, Yamaguchi A, Katsuyama K, Aoki I, Yamanaka S, Kosaka K. Neuronal accumulation of alpha- and beta-synucleins in the brain of a GM2 gangliosidosis mouse model. Neuroreport. 2003;14:551–554. doi: 10.1097/00001756-200303240-00004. [DOI] [PubMed] [Google Scholar]
- Saito Y, Suzuki K, Hulette CM, Murayama S. Aberrant phosphorylation of alpha-synuclein in human Niemann-Pick type C1 disease. J Neuropathol Exp Neurol. 2004;63:323–328. doi: 10.1093/jnen/63.4.323. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Iseki E, Togo T, Yamaguchi A, Katsuse O, Katsuyama K, Kanzaki S, Shiozaki K, Kawanishi C, Yamashita S, Tanaka Y, Yamanaka S, Hirayasu Y. Neuronal and glial accumulation of alpha- and beta-synucleins in human lipidoses. Acta Neuropathol. 2007;114:481–489. doi: 10.1007/s00401-007-0264-z. [DOI] [PubMed] [Google Scholar]
- Ohtake H, Limprasert P, Fan Y, Onodera O, Kakita A, Takahashi H, Bonner LT, Tsuang DW, Murray IV, Lee VM, Trojanowski JQ, Ishikawa A, Idezuka J, Murata M, Toda T, Bird TD, Leverenz JB, Tsuji S, La Spada AR. Beta-synuclein gene alterations in dementia with Lewy bodies. Neurology. 2004;63:805–811. doi: 10.1212/01.wnl.0000139870.14385.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Fujita M, Nakai M, Waragai M, Watabe K, Akatsu H, Rockenstein E, Masliah E, Hashimoto M. Enhanced lysosomal pathology caused by beta-synuclein mutants linked to dementia with Lewy bodies. J Biol Chem. 2007;282:28904–28914. doi: 10.1074/jbc.M703711200. [DOI] [PubMed] [Google Scholar]
- Tamboli IY, Prager K, Barth E, Heneka M, Sandhoff K, Walter J. Inhibition of glycosphingolipid biosynthesis reduces secretion of the beta-amyloid precursor protein and amyloid beta-peptide. J Biol Chem. 2005;280:28110–28117. doi: 10.1074/jbc.M414525200. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Fujita M, Sugama S, Nakai M, Takenouchi T, Wei J, Urano T, Inoue S, Hashimoto M. {alpha}-Synuclein stimulates differentiation of osteosarcoma cells: relevance to down-regulation of proteasome activity. J Biol Chem. 2007;282:5736–5748. doi: 10.1074/jbc.M606175200. [DOI] [PubMed] [Google Scholar]
- Markham JE, Li J, Cahoon EB, Jaworski JG. Separation and identification of major plant sphingolipid classes from leaves. J Biol Chem. 2006;281:22684–22694. doi: 10.1074/jbc.M604050200. [DOI] [PubMed] [Google Scholar]
- Mahuran DJ. The GM2 activator protein, its roles as a co-factor in GM2 hydrolysis and as a general glycolipid transport protein. Biochim Biophys Acta. 1998;1393:1–18. doi: 10.1016/s0005-2760(98)00057-5. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Yirinec BD, Silverstein SC. Phorbol esters and horseradish peroxidase stimulate pinocytosis and redirect the flow of pinocytosed fluid in macrophages. J Cell Biol. 1985;100:851–859. doi: 10.1083/jcb.100.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko PV, Dahlstrom A. Studies on the 3-dimensional architecture of dendritic spines and varicosities in human cortex by confocal laser scanning microscopy and Lucifer yellow microinjections. J Neurosci Methods. 1995;57:55–61. doi: 10.1016/0165-0270(94)00125-z. [DOI] [PubMed] [Google Scholar]
- Yang AJ, Chandswangbhuvana D, Margol L, Glabe CG. Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Abeta1-42 pathogenesis. J Neurosci Res. 1998;52:691–698. doi: 10.1002/(SICI)1097-4547(19980615)52:6<691::AID-JNR8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ji ZS, Miranda RD, Newhouse YM, Weisgraber KH, Huang Y, Mahley RW. Apolipoprotein E4 potentiates amyloid beta peptide-induced lysosomal leakage and apoptosis in neuronal cells. J Biol Chem. 2002;277:21821–21828. doi: 10.1074/jbc.M112109200. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Schwach G, Ingolic E, Adame A, Crews L, Mante M, Pfragner R, Schreiner E, Windisch M, Masliah E. Lysosomal pathology associated with alpha-synuclein accumulation in transgenic models using an eGFP fusion protein. J Neurosci Res. 2005;80:247–259. doi: 10.1002/jnr.20446. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- Di Fonzo A, Chien HF, Socal M, Giraudo S, Tassorelli C, Iliceto G, Fabbrini G, Marconi R, Fincati E, Abbruzzese G, Marini P, Squitieri F, Horstink MW, Montagna P, Libera AD, Stocchi F, Goldwurm S, Ferreira JJ, Meco G, Martignoni E, Lopiano L, Jardim LB, Oostra BA, Barbosa ER, Bonifati V. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007;26:313–324. doi: 10.1038/sj.emboj.7601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Kuma A, Matsui M, Mizushima N. LC3, an autophagosome marker, can be incorporated into protein aggregates independent of autophagy: caution in the interpretation of LC3 localization. Autophagy. 2007;3:323–328. doi: 10.4161/auto.4012. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991;266:21327–21330. [PubMed] [Google Scholar]
- Saftig P, Beertsen W, Eskelinen EL. LAMP-2: a control step for phagosome and autophagosome maturation. Autophagy. 2008;4:510–512. doi: 10.4161/auto.5724. [DOI] [PubMed] [Google Scholar]
- Sokolova TV, Zakharova IO, Furaev VV, Rychkova MP, Avrova NF. Neuroprotective effect of ganglioside GM1 on the cytotoxic action of hydrogen peroxide and amyloid beta-peptide in PC12 cells. Neurochem Res. 2007;32:1302–1313. doi: 10.1007/s11064-007-9304-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto HA, Mohanan PV. Ganglioside GT1B and melatonin inhibit brain mitochondrial DNA damage and seizures induced by kainic acid in mice. Brain Res. 2003;964:100–106. doi: 10.1016/s0006-8993(02)04083-0. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120:4081–4091. doi: 10.1242/jcs.019265. [DOI] [PubMed] [Google Scholar]
- Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, Schmidt ML, Nee LE, O'Connell B, Pollen DA, St George-Hyslop P, Ghetti B, Nochlin D, Bird TD, Cairns NJ, Lee VM, Iwatsubo T, Trojanowski JQ. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153:1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lach B, Grimes D, Benoit B, Minkiewicz-Janda A. Caudate nucleus pathology in Parkinson’s disease: ultrastructural and biochemical findings in biopsy material. Acta Neuropathol. 1992;83:352–360. doi: 10.1007/BF00713525. [DOI] [PubMed] [Google Scholar]
- Nobile-Orazio E, Carpo M, Scarlato G. Gangliosides. Their role in clinical neurology. Drugs. 1994;47:576–585. doi: 10.2165/00003495-199447040-00002. [DOI] [PubMed] [Google Scholar]