Abstract
The (HER2/Neu) ErbB2 oncogene is commonly overexpressed in human breast cancer and is sufficient for mammary tumorigenesis in transgenic mice. Nuclear factor (NF)-κB activity is increased in both human and murine breast tumors. The immune response to mammary tumorigenesis may regulate tumor progression. The role of endogenous mammary epithelial cell NF-κB had not previously been determined in immune-competent animals. Furthermore, the role of the NF-κB components, p50 and p65, in tumor growth was not known. Herein, the expression of a stabilized form of the NF-κB-inhibiting IκBα protein (IκBαSR) in breast tumor cell lines that express oncogenic ErbB2 inhibited DNA synthesis and growth in both two- and three-dimensional cultures. Either NF-κB inhibition or selective silencing of p50 or p65 led to a loss of contact-independent tumor growth in vitro. IκBαSR reversed the features of the oncogene-induced phenotype under three-dimensional growth conditions. The NF-κB blockade inhibited ErbB2-induced mammary tumor growth in both immune-competent and immune-deficient mice. These findings were associated with both reduced tumor microvascular density and a reduction in the amount of vascular endothelial growth factor. The expression of IκBαSR in breast cancer tumors inhibited angiogenesis. Thus, mammary epithelial cell NF-κB activity enhances ErbB2-mediated mammary tumorigenesis in vivo by promoting both growth and survival signaling via the promotion of tumor vasculogenesis.
Nuclear factor (NF)-κB transcription factor complexes are composed of related protein dimers with similar DNA binding specificity.1 The canonical NF-κB pathway consists of dimers including RelA, c-Rel, and NF-κB1 (p50). The dimers are held in inactive cytoplasmic complexes with proteins of the IκB (inhibitor of NF-κB) family. IκB kinases (IKK) phosphorylate IκB bound to NF-κB, directing the IκB to ubiquitin-dependent degradation2 and releasing NF-κB dimers to enter the nucleus. Transcriptional targets of NF-κB include genes for cell cycle and cell survival proteins. The alternate pathway utilizes the RelB/NF-κB2 (p52) dimer, which is regulated primarily by IKKα and regulates a mostly distinct subset of target genes.3
Analysis with drug inhibitors of NF-κB signaling has provided inconsistent information on the role of NF-κB in cancers. Anti-inflammatory drugs that are known to inhibit IKK-dependent NF-κB activity reduced the cumulative incidence of colitis-associated cancer in patients with ulcerative colitis, suggesting a role for NF-κB in colonic tumor onset and/or progression.4 In contrast, inhibition of NF-κB in vivo promoted squamous cell tumors,5,6,7 indicating the possibility of cell-type-specific roles for NF-κB in tumorigenesis. Inactivation of IΚKβ in intestinal epithelial cells reduced colonic tumor formation in response to chemical procarcinogens,8 implying that IΚKβ plays a key role in promoting enterocyte tumorigenesis. NF-κB/Rel signaling is activated in many primary breast cancers and lymphoid malignancies.9,10,11 Increased nuclear translocation and overexpression of p50, p52, c-Rel, and Bcl-3 have been described in breast cancer cell lines and human tumors.10 Inhibition of NF-κB signaling in tumor cell lines enhances sensitivity to chemotherapy and radiation, suggesting a role for NF-κB in tumor maintenance.12 Collectively these studies provide evidence for NF-κB in breast tumor onset or progression.11,13
The receptor tyrosine kinase, ErbB2 (HER2/Neu) is overexpressed in more than 30% of human breast cancers. ErbB2 functions in heteromeric complexes with ErbB1 (epidermal growth factor receptor), ErbB3, and ErbB4). ErbB2 signaling is activated by ligands that include the heregulins (HRG), and ligands that bind the heteromeric receptor, such as growth factor.14 The use of humanized monoclonal antibodies directed to the extracellular domain of ErbB2 in patients with metastatic breast cancer resistant to standard chemotherapy, produces remissions in 10 to 15% of cases.15,16 The identification of additional molecular targets governing tumor progression is fundamental to tailoring patient therapy. Prior studies of NF-κB in breast cancer cellular growth have included studies of the IκB kinases. The NF-κB essential modulator (NEMO)-binding domain (NBD) peptide, an inhibitor of IκB kinase (IKK), reduced HRG-mediated proliferation of SKBr3 cells in culture.17 A dominant-negative kinase inhibitor blocked growth of MDA-MB-231 cell growth.18 The general proteosome inhibitor PS-341, which also inhibits IκB degradation and PS1145, an inhibitor of IKK, both inhibit SKBr3 cell proliferation.18
Several unanswered questions remain in understanding the role of NF-κB in breast cancer. Firstly, the immune response is of importance in mammary tumorigenesis.19 Infiltrating immune cells in the tumor microenvironment can either promote tumor development and tumor cell survival or exert anti-tumor effects.20 The interferons secreted by immune cells have antitumorigenic effects.21 In contrast tumor necrosis factor (TNF)-α is known to stimulate the production of genotoxic molecules (NO and reactive oxygen species).22 The interpretation of the role of mammary tumor NF-κB in prior studies conducted using xenografts in immune-deficient mice may therefore have been confounded by the abnormal immune status of the animal. It therefore remained important to discern the role of mammary epithelial cell NF-κB in mammary tumor growth using immune-competent mice. To this end, herein, we examined the role of NF-κB in ErbB2-induced tumors, derived in FVB mice, reimplanted into FVB hosts.
Secondly, although IKK kinase inhibition had been used to indirectly assess the role of NF-κB in breast cancer growth, the IKK complex has several additional functions, phosphorylating other substrates including Akt and β-catenin.23 Furthermore, a kinase-independent function of IKKα has been well characterized.24,25,26 Thus, prior studies inhibiting IκB kinase activity with dominant-negative kinase inhibitors or general proteosome inhibitors did not necessarily reflect only NF-κB inhibition. We therefore examined, herein, the effect of selectively inhibiting NF-κB with a dominant inhibitor of NF-κB (IκΒαSR). Thirdly, the role of the individual p65 and p50 components of NF-κB in breast cancer cellular proliferation had not been explicitly determined; therefore selective siRNA was used herein. Finally, the mechanisms by which NF-κB govern tumor growth in vivo is not well understood. The mechanism by which NF-κB regulates breast tumorigenesis was not known. Using immune-competent mice, we demonstrated NF-κB inhibition reduced ErbB2-induced breast tumor angiogenesis. Herein, we demonstrate the role of secreted factors regulated by NF-κB in neoangiogenesis assays.
Materials and Methods
Cell Culture, Retroviral Infections, Reagents, and Reporter Assays
The 293T, MCF-7 (ERα+), MCF10A (ERα+), SKBr3 (ERα−), and the NAFA murine mammary cell lines derived from a mammary tumor of MMTV-ErbB2 transgenic mice27 were cultured as described.27,28 The retroviral expression vector for IκBαSR was subcloned as an EcoRI fragment into MSCV IRES-GFP vector29 and viral supernatant was prepared as previously described.29 The NF-κB-responsive reporter, 3xRel-luc, and the expression vectors encoding activated Neu were previously described.30 Transfections were performed using Superfect transfection reagent (Qiagen, Valencia, CA) or GeneJuice transfection reagent (Novagen, Madison, WI) according to the manufacturers’ protocols. The relative firefly luciferase activities were calculated by normalizing transfection efficiency according to the Renilla luciferase activities. Statistical analyses were performed using the Student’s t-test. Reagents TNF-α (Sigma, Saint Louis, MO), HRG (R&D Systems, Minneapolis, MN), and Bay11-7082 (Sigma) were used as described in the text.
MTT, Flow Cytometric Analysis, DNA Synthesis Analysis, and Apoptosis Assays
Cell growth was determined by 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyl tetrazoliumbromide (MTT) assay. The cells were plated at 1 × 104 cells per well in 96-well microtiter plates. After incubation for the specified time, MTT (5 mg/ml) was added to each well and incubated for 4 hours. The absorbance was recorded on a microplate reader at a wavelength of 570 nm. DNA synthesis was analyzed by [3H] thymidine (TdR) incorporation as previously described.31,32 S phase was analyzed by flow cytometry. The Annexin V-PE apoptosis detection kit (BD Biosciences, Franklin, NJ) was used according to the manufacturer’s protocol, followed by flow cytometry.
Colony Formation in Soft Agar, 3D Cell Culture, and Western Blot
Cells (n = 4000) were seeded into 0.3% soft agar (Sigma) in a suspension dish (Nalge Nunc International, Rochester, NY). Colonies were stained by 0.04% crystal violet acetate and counted under a vertical microscope after 2 weeks of incubation. For 3D culture, MCF10A/NeuT (ErbB2*) and NAFA cells infected with IκBαSR or GFP control vector were resuspended in Dulbecco’s modified Eagle’s medium to 1 × 104 cells/ml. Equal volumes of cellular solution and growth-reduced Matrigel (BD Biosciences, Franklin Lakes, NJ) were mixed and 2000 cells in this mixture were then applied to precooled four-well chambers. After the gel solidified, medium was added, and the cells were allowed to grow in a 5% CO2 humidified incubator at 37°C. Medium was changed every 3 to 4 days. The colony size and morphology were monitored and photodocumented as previously described.33
Western blot analysis was conducted using antibodies to p65 (Rockland Immunochemicals Inc., Gilbertsville, PA), p50 (Upstate, Lake Placid, NY), phosphorylated p65 (93H1; Cell Signaling, Danvers, MA), Akt1 (2H10, Cell Signaling), phosphorylated Akt1 (S473, Cell Signaling), phosphorylated Erk (E-4, Santa Cruz Biotechnology, Santa Cruz, CA), Erk1 (C-16, Santa Cruz Biotechnology), Erk2 (C-14, Santa Cruz Biotechnology), FLAG (C-21, Santa Cruz Biotechnology), IκBα (C19, Santa Cruz Biotechnology), and the loading control guanine dissociation inhibitor (GDI), or β-actin (Upstate).
Nuclear Extracts and NF-κB Transcription Factor Assay
Nuclear extracts were isolated using the CelLytic NuCLEAR extraction kit, according to the manufacturer’s instructions (Sigma). To evaluate the DNA binding of the p50 and p65 subunits of NF-κB, we used a transcription factor assay kit combining the principles of an electrophoretic mobility shift assay with an enzyme-linked immunosorbent assay, according to the manufacturer’s instructions (Chemicon International, Temecula, CA).
Small Interfering RNA Transfection
Small interfering RNAs (siRNAs) against NF-κB p105 (J-003520-09-0010, NM-003998), NF-κB p65 (J-003533-08-0010, NM-021975), and nontargeting siRNA (D-001810-03) were acquired from Dharmacon RNA Technology (Lafayette, CO). SKBr3 cells (60% confluent) were incubated with oligofectamine transfection reagent (Invitrogen, Carlsbad, CA) and siRNA (100 nmol/L) according to the manufacturer’s protocol. MCF10A/ErbB2* cells were transfected by the Nucleofector technology using the Nucleofector Kit V and program T-024 (Amaxa Biosystems, Gaithersburg, MD). Transfection efficiency was monitored by nonsilencing fluorescein-labeled siRNA from Qiagen.
Immunohistochemistry
Immunohistochemical staining was performed using the EnVision Kit (DAKO, Carpinteria, CA) according to the manufacture’s manual. The primary antibodies used were CD31 (CM303A, 1/50; Biocare Medical, Concord, CA), vWF (A0082, 1/500; DAKO), vascular endothelial growth factor (VEGF) (SC-507, 1/250; Santa Cruz Biotechnology), VCAM-1 (SC-8304, 1/250; Santa Cruz Biotechnology). The secondary antibodies used were anti-mouse or -rabbit horseradish peroxidase-labeled polymer. The signal was visualized using a cyanine 3 (Cy-3)-labeled tyramide amplification system (Perkin Elmer Life Sciences, Inc., Boston, MA). Sections were mounted in aqueous medium containing 4,6-diamidino-2-phenylindole as a nuclear counterstain (Vector Laboratories, Burlingame, CA). A negative control was performed to ensure the specificity of fluorescent immunostaining by replacing primary antibody with a nonimmune rabbit IgG.
Microvascular density of the tumors was determined as previously described.31 Images were captured using an Olympus (Tokyo, Japan) BX61 confocal microscope. Vessels were quantified at ×200 using a linear encoded motorized Dr. John Ojelfo (Georgetown University, Washington, DC) provided the XY stage (Prior, NJ). At least 20 fields were quantified in five different tumor sections. Single endothelial cells or clusters of endothelial cells, positive for CD31 were considered as individual vessels. The number of capillary vessels per square millimeter of tumor tissue was quantified using a ×10 eyepiece equipped with 10 × 1 mm by 10 × 1 mm graticule and was expressed as a percentage of area of the grid. For controls, 30 fields were analyzed in five different tumor samples. At least 100 fields were counted for each experimental group.
Angiogenesis and Secreted Protein Assays
Human umbilical vein endothelial cells were obtained from LifeLine Cell Technology (Walkersville, MD). An In vitro angiogenesis kit (catalog no. ECM625) was obtained from Chemicon International. Briefly, a 96-well tissue culture plate was coated with ECMatrix gel and allowed to solidify at 37°C for 1 hour. Human umbilical vein endothelial cells were counted and seeded at 1 × 104 cells/well on the surface of the polymerized ECMatrix. One hundred μl of serum-free conditioned media from NAFA-GFP or NAFA-IκBαSR was added to the cells. Tube formation was monitored and phase contrast pictures were taken at ×10 magnification after 6 hours of incubation. Tube length and tube number were quantitated using the angiogenesis tube formation procedure in Metamorph software (Molecular Devices, Sunnyvale, CA). Each experiment was done in triplicate and repeated three times. The relative abundance of proteins secreted by NAFA and NAFA-IκBαSR cell lines was determined by cytokine/chemokine arrays as previously described.34
FVB and Nude Mice Study
NAFA cells infected stably expressing IκBαSR protein or its vector control were grown and harvested by trypsinization, washed, and resuspended with PBS at 2 × 107 cells/ml. Aliquots (0.1 ml) were injected into mammary gland of 4-week-old FVB mice, or injected subcutaneously into 4- to 6-week-old BALB/c athymic female nude mice purchased from the National Cancer Institute (Rockville, MD). Tumor development in mice was measured weekly by digital caliper and photographs were taken at 4 weeks after injection.
Results
ErbB2 Induction of NF-κB Activity
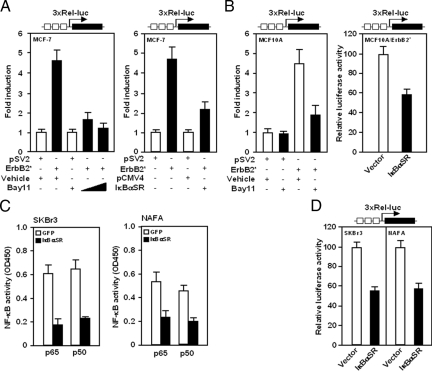
To systematically examine the role of NF-κB in oncogene-induced signaling in breast epithelial cells, analyses were conducted using an activating mutant of ErbB2. To determine whether ErbB2 directly activated NF-κB transcriptional activity, a luciferase reporter gene fused to three tandem repeats of the NF-κB sites from the MHC class I enhancer was assayed in MCF-7 and MCF10A cells. An activated mutant of ErbB2* (NeuT) induced NF-κB reporter activity 4.5-fold in MCF-7 and MCF10A cells (Figure 1, A and B). The human cyclin D1 promoter encodes a NF-κB-responsive reporter element30,35 and the cyclin D1 promoter was similarly activated sixfold by ErbB2* (data not shown). To determine the role of endogenous NF-κB in regulating the activity of the multimeric NF-κB response element, a dominant inhibitor (IκBαSR) of NF-κB signaling and chemical inhibitors of NF-κB signaling were used. IκBαSR, a mutant form of the IκBα inhibitor, in which serines at positions 32 and 36 have been changed to alanines, is resistant to phosphorylation and proteosomal degradation and thereby functions as a potent and specific inhibitor of NF-κB activity. We have previously shown by EMSA that IκBαSR inhibits NF-κB binding in nuclear extracts. The specific chemical inhibitors of NF-κB, Bay 11-7082,36 inhibited NF-κB reporter activity induced by ErbB2* (Figure 1, A and B). IκBαSR inhibited NF-κB activity, assayed using the multimeric NF-κB reporter construct in MCF-7 cells transiently transduced with ErbB2* from fourfold to fivefold to twofold (Figure 1A) and MCF10A cells transformed by oncogenic ErbB2* by ∼45% (Figure 1B). IκBαSR expression inhibited ErbB2 induction of p50 and p65 DNA-binding activity in SKBr3 and in NAFA cells by ∼60 to 50% (Figure 1C). IκBαSR inhibited 3xRel-luc reporter activity ∼50% in SKBr3 and NAFA cell lines (Figure 1D). Collectively these studies corroborate prior studies that ErbB2 induces NF-κB reporter activity in other cell lines and demonstrate that IκBαSR expression inhibits p50 and p65 binding activity.
Figure 1.
ErbB2 induces NF-κB activity. NF-κB activity was assessed using a multimeric NF-κB binding site (3xRel-luc) luciferase reporter and a NF-κB binding assay. A: MCF-7 cells were transfected with either an expression vector for an activating ErbB2* mutant (NeuT) or a control plasmid. 3xRel-luc reporter activity was reduced by the NF-κB inhibitor Bay 11-7082 (left) or transfection with an expression vector encoding IκBαSR (right). B: MCF10A cells, transiently transfected with an expression vector for ErbB2*, were treated with the NF-κB inhibitor Bay 11-7082 (left) or stably transduced with an expression vector for ErbB2* and an expression vector for IκBαSR, relative luciferase activity was determined (right). C and D: NF-κB binding activity (C) or luciferase reporter activity (D) was determined in SKBr3 and NAFA cells stably transduced with an IκBαSR expression vector. Data are relative luciferase activities (mean ± SEM of n > five separate transfections).
NF-κB Is Required for Oncogene-Induced Contact-Independent Growth
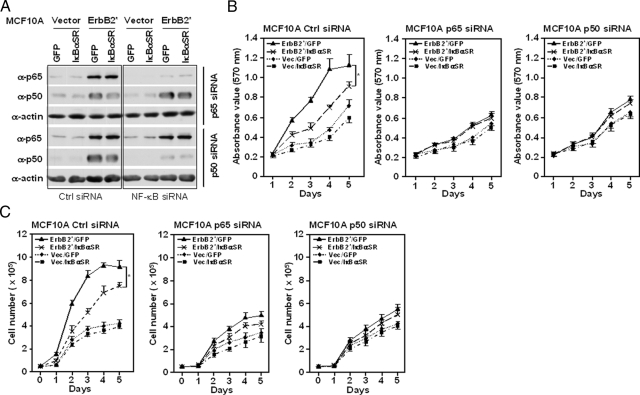
To determine the role of the individual p50 and p65 components of NF-κB in breast cancer cellular proliferation we used specific NF-κB siRNA. siRNA to p65 selectively reduced p65 abundance in MCF10A/ErbB2* (Figure 2A) or SKBr3 (data not shown). p50 siRNA selectively reduced abundance of p50 (Figure 2A and data not shown). Cellular proliferation, assayed by either MTT assay (Figure 2B) or cellular counting (Figure 2C), was reduced by p50 or p65 siRNA in MCF10A/ErbB2* cells and in SKBr3 cells (data not shown). IκBαSR reduced MCF10A/ErbB2* proliferation determined by MTT assay (Figure 2B, left) and cell growth curves (Figure 2C, left) by ∼40 to 50% at day 4, similar to the effect of p65 siRNA (Figure 2, B and C; middle) or p50 siRNA (Figure 2, B and C; right). These studies demonstrate for the first time the importance of the individual p50 and p65 components of the NF-κB complex in ErbB2*-induced human breast tumor cell line cellular proliferation.
Figure 2.
The NF-κB components p50 and p65 govern ErbB2-induced cellular proliferation. A: Western blot of MCF10A/ErbB2* (NeuT) cells transduced with p65 or p50 siRNA. MCF10A control or MCF10A cells stably expressing ErbB2* were transduced with siRNA and Western blot analysis was conducted 48 hours after transduction. Western blot analysis was conducted with antibodies as indicated in the figure. B and C: Cellular proliferation assays of p50 or p65 siRNA-transduced MCF10A cell lines were conducted using either MTT assay (B) or cell counting (C). The MCF10A cell lines were stably transfected with expression vectors encoding ErbB2* (NeuT) or control vector (Vec) and either PCMV-IκBαSR or the parental control vector (GFP). The cells were plated in equal number and daily MTT assays conducted as described in the Materials and Methods. The data are shown as mean ± SEM of three separate experiments. *P < 0.05; **P < 0.01.
To extend these studies, breast cancer cell lines (NAFA, MCF10A/ErbB2*, SKBr3) were examined (Figure 3 and data not shown). To determine the role of NF-κB in maintaining cellular DNA synthesis or contact-independent growth induced by ErbB2, a retroviral expression plasmid (MSCV-FLAG-IκBαSR-IRES-GFP) was used. The cells were transduced with an expression vector encoding IκBαSR to determine the role of endogenous NF-κB activity in maintaining cell-cycle distribution and contact-independent growth. The IκBαSR transgene expression was confirmed by Western blot (Figure 3, A and C). Inhibition of NF-κB activity with the chemical inhibitor Bay 11-7082 abrogated colony growth of SKBr3, NAFA, and MCF10A/ErbB2* cells (data not shown). Thus NF-κB activity is required for contact-independent growth of ErbB2-expressing breast cancer cells in culture. No inhibition of phospho-ERK or phospho-Akt was observed, two other pathways known to promote proliferation of breast epithelial cells (Supplemental Figure S1 available at http://ajp.amjpathol.org). Transduction of these cell lines with an expression vector encoding IκBαSR significantly reduced cellular proliferation (Figure 3, B and D; left), inhibited contact-independent growth as assessed by colony formation (Figure 3, B and D; right), and the proportion of cells in the DNA synthetic phase of the cell-cycle was reduced through inhibition of NF-κB activity (Figure 3, B and D; middle). Together, these studies demonstrate an important role for the NF-κB signaling pathway in maintaining DNA synthesis and contact-independent growth of breast cancer cell lines.
Figure 3.
Endogenous NF-κB maintains breast cancer epithelial cell contact-independent growth and DNA synthesis. Cell cycle analyses by flow cytometry and colony formation assays were conducted in three breast cancer cell lines stably transduced with a retroviral expression vector encoding IκBαSR or a control vector. The data were shown as mean ± SEM for n > three separate experiments. A and C: Western blot for IκBα in NAFA (A) MCF10A, and MCF10A-ErbB2* (C). B and D: Cell proliferation determined by MTT assay (left), cell-cycle distribution assessed by FACS (middle), and colony formation assayed by counting the number of colonies (right). *P < 0.05; **P < 0.01.
NF-κB Is Required for ErbB2-Induced Growth in 3D Matrix
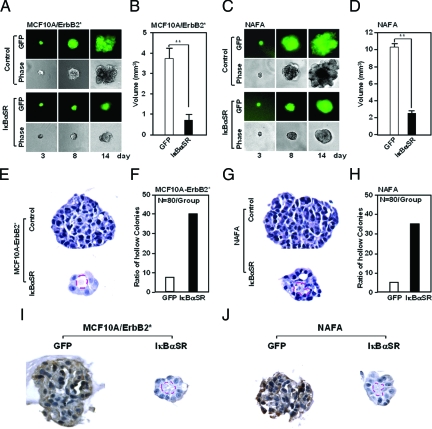
Mammary epithelial cells, when grown on basement membrane, form three-dimensional (3D) structures. The spontaneously immortalized human luminal epithelial cell line MCF10A, forms spherical structures when grown in 3D Matrigel.37 To examine the mechanism by which ErbB2* regulates mammary cell growth through NF-κB, the human immortalized MCF10A cell was used. ErbB2* transduction of MCF10A cells resulted in altered cellular morphology, characterized by a spindle-like appearance (Figure 4A, top). IκBαSR-transduced ErbB2*-expressing cells gave an intermediate morphology and were significantly less elongated and spindle shaped (Figure 4A, bottom). In contrast with MCF10A cells, which do not grow in soft agar, ErbB2*-transduced MCF10A cells formed colonies (Figure 3D, right). Direct comparison by Western blotting of either MCF10A- or ErbB2*-transduced MCF10A cells revealed increased expression of both p50 and RelA (p65) in ErbB2*-transduced cells (data not shown).
Figure 4.
NF-κB is required for ErbB2-induced disruption of mammary acinar growth in 3D culture. A and C: MCF10A/ErbB2* (NeuT) cells (A) or NAFA (C) cells transduced with IκBαSR-IRES-GFP (A and C, bottom) or control GFP vector (A and C, top) were grown in 3D Matrigel culture. Immunofluorescence and phase contrast microscopy shows the multilobular structure. Inhibition of NF-κB activity with IκBαSR reverts the transformed morphology to the spherical morphology observed with nontransformed mammary epithelial cells.33 The volume of MCF10A/ErbB2* (NeuT) (B) and NAFA (D) colonies grown in 3D is reduced 60 to 80% by expression of IκBαSR. E and G: H&E staining of MCF10A/ErbB2* (E) and NAFA (G) cell lines grown in 3D Matrigel demonstrate the solid ball of GFP control, and hollow ball of IκBαSR-expressing cells with (F and H) the relative number shown as mean data. I and J: Immunohistochemical staining for p50 of MCF10A/ErbB2* and NAFA cells. *P < 0.05; **P < 0.01.
Murine mammary epithelial cells also form spheroid-like structures in 3D Matrigel.38 We therefore examined the role of NF-κB in the morphology of NAFA cells in 3D Matrigel. NAFA cells are mammary epithelial cells derived from MMTV-ErbB2 transgenic mice. NAFA cells grew as complex multiacinar structures with a disorganized architecture (Figure 4C, top), resembling the morphology of MCF10A cells transduced with ErbB2.33 NAFA cells transduced with IκBαSR, however, grew as spheroids in 3D Matrigel resembling nontransformed murine mammary epithelium (Figure 4C, bottom).38
The mean volume of MCF10A/ErbB2* and NAFA cell acini grown in 3D Matrigel for 12 days was reduced by ∼60 to 80% through inhibition of NF-κB activity (Figure 4, B and D). These studies demonstrate that IκBαSR inhibits ErbB2*-induced growth of mammary epithelial cells in two dimensional (2D) and 3D culture, associated with a reduction in cyclin D1 abundance and pRb phosphorylation, without affecting cyclin A or cyclin E abundance (data not shown). Histological analysis of the 3D culture demonstrated that IκBαSR-expressing cells had increased prevalence of hollow spheres with a tendency toward polarized epithelium surrounding the central lumen. This trend was observed in both MCF10-ErbB2* and NAFA cell lines (Figure 4, E–H). Immunohistochemical analysis demonstrated a reduction in p50 immunostaining in the IκBαSR-expressing cells (Figure 4, I and J). Together, these studies demonstrated an essential role for NF-κB in maintaining contact-independent growth in the context of ErbB2*.
NF-κB Is Required for Growth Factor-Induced Breast Cancer Cellular DNA Synthesis and Survival
Having observed that NF-κB mediated ErbB2*-induced contact-independent growth, we investigated the contribution of endogenous NF-κB to ErbB2* on cell proliferation, DNA synthesis, cell cycle, and survival. The human mammary adenocarcinoma cell lines SKBr3 (which overexpresses ErbB2) and NAFA (murine mammary cell line) were exposed to HRG, after transduction with either the IκBαSR-expressing retrovirus or control. HRG increased 3H-thymidine incorporation by ∼30% (SKBr3) or ∼40% (NAFA) (Supplemental Figure S2, A and B, left; available at http://ajp.amjpathol.org). HRG increased the proportion of cells in the DNA synthetic (S) phase and reduced the proportion in the G1 phase. Expression of IκBαSR abolished the HRG-induced increase in the proportion of S phase cells (Supplemental Figure S2, A and B, right; available at http://ajp.amjpathol.org). IκBαSR expression abrogated the HRG-induced cell growth by MTT assay (Supplemental Figure S2, A and B, middle; available at http://ajp.amjpathol.org).
To determine the role of endogenous NF-κB in cellular apoptosis, PE-labeled annexin V binding was used to identify apoptotic and necrotic cells in cultures of SKBr3 and NAFA cells, transduced with either the IκBαSR-expressing retrovirus or control. Basal apoptosis rates were not altered by NF-κB inhibition, but NF-κB inhibition increased apoptosis rates in TNF-α-exposed cells from 3.1 to 6.4% in SKBr3 cultures and from 2.9 to 9.0% in NAFA cultures (Supplemental Figure S2, C and D, available at http://ajp.amjpathol.org). These findings are consistent with a prior study that showed that NF-κB inhibition increased TNF-α-induced apoptosis of a mammary tumor cell line grown in 3D Matrigel.39
NF-κB Is Required for ErbB2-Induced Tumor Growth in Immune-Competent and Immune-Deficient Mice
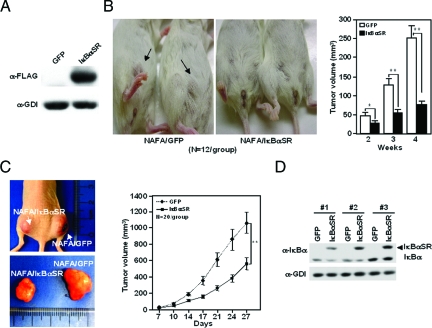
The growth of mammary tumors is modulated by heterotypic signals from local inflammatory cells.40 We therefore examined the role of IκBαSR in regulating the growth of ErbB2*-induced tumors in immune-competent mice. The MMTV-ErbB2 transgenic mice, from which the NAFA line was derived, are of FVB strain, allowing analysis in FVB mice. The presence of the IκBαSR expression vector was confirmed by Western blot analysis (Figure 5A). Comparison was made between the NAFA cells transduced with either the IκBαSR vector, or a control vector (Figure 5B). The growth of the NAFA cells was assessed in immune-competent mice. Each group consisting of 12 separate animals developed tumors. The volume of tumors at 4 weeks was reduced 80% by IκBαSR expression (Figure 5B). These studies indicate that NF-κB participates in the maintenance of implanted tumor epithelial cell growth in immune-competent mice in vivo.
Figure 5.
NF-κB is required for ErbB2-induced mammary tumor growth in immune-competent and immunodeficient mice. A: FLAG epitope of the IκBαSR protein. B: Tumor volume throughout 4 weeks after implantation of IκBαSR-expressing and control NAFA cells in the FVB strain of mice (n = 12 per group). The data are shown as mean ± SEM. Arrows represent mammary tumor. C: NAFA cells transduced with IκBαSR or control vector were implanted into nude mice. Representative examples of tumor size are shown (left), and tumor growth curves conducted of NAFA cells expressing IκBαSR or control vector in nude mice (n = 20 per group) (right). D: Representative Western blot analysis of NAFA cell-derived tumors transduced with the IκBαSR expression vector or control vector in three separate nude mice. *P < 0.05; **P < 0.01.
To determine whether the inhibitory effect of mammary epithelial cells IκBαSR involved the immune system, implantation experiments were conducted in female BALB/c nude mice (Figure 5C). These mice are athymic and have a defective cell-mediated immune response. IκBαSR expression reduced mean tumor volume by ∼50% at 27 days after implantation (Figure 5C). The presence of endogenous IκBα was detected postmortem in the extirpated vector control cell tumors (GFP) by Western blot analysis (Figure 5D). The transduced IκBαSR was detected as a higher molecular weight band, as a consequence of the 3xFLAG epitopes (Figure 5D). These studies indicate that mammary epithelial tumor cell NF-κB also participates in the maintenance of implanted mammary tumor epithelial cell growth in immune-deficient mice in vivo.
NF-κB Regulates Tumor Angiogenesis via VEGF in Mammary Epithelial Cells
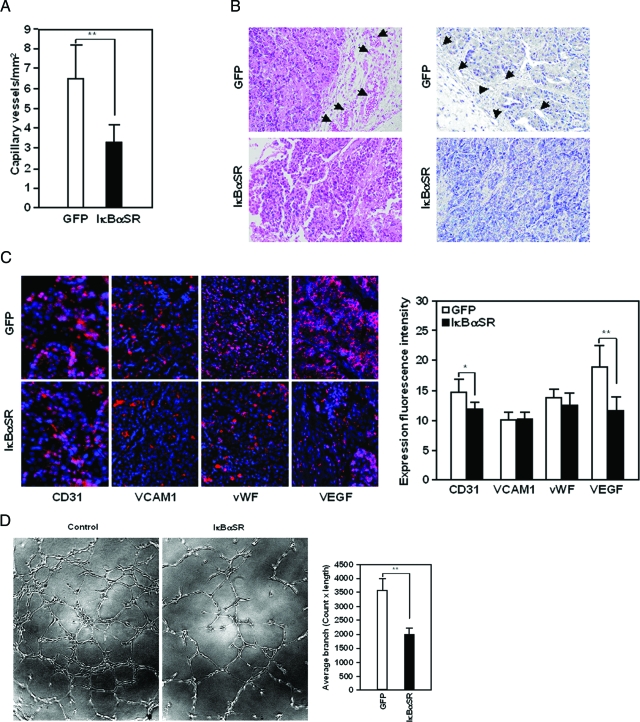
Direct inspection of the mammary tumor expression of IκBαSR suggested a reduction on vascularity when comparing similar sized tumors. Quantitative analysis was therefore conducted of neoangiogenesis by counting blood vessels as previously described (Figure 6A).31 IκBαSR expression reduced microvessel density by >50%, compared with size-matched tumors expressing the control vector (Figure 6A). Reduced vascularity in ErbB2 tumors expressing IκBαSR was also apparent on histomorphometric and immunohistochemical analyses [hematoxylin and eosin (H&E) staining and CD31 immunohistochemical staining shown (Figure 6B)]. We then examined candidate proangiogenic extracellular matrix (ECM) integrin-binding proteins, to further explore the mechanism by which NF-κB promoted mammary tumor angiogenesis. Neither the β1α4 integrin binding protein VCAM1 nor the αvβ3 binding ECM protein vWF, changed in abundance (Figure 6C). In contrast, VEGF abundance was significantly reduced on inhibition of mammary epithelial cell NF-κB activity in vivo (Figure 6C). To determine the mechanism by which mammary epithelial cell NF-κB regulates angiogenesis, we considered the possibility that NF-κB may regulate secreted factors. Angiogenesis assays were conducted using human umbilical vein endothelial cells, the supernatant of IκBαSR-transduced NAFA cells reduced neoangiogenesis assessed by the number and length of vascular branches (Figure 6D). Thus NF-κB induced the secretion of factors that stimulate angiogenesis and inhibiting NF-κB activity diminishes the secretion of these factors.
Figure 6.
Vasculogenesis in ErbB2-induced mammary tumors is NF-κB-dependent. A: Analysis of ErbB2*-induced mammary tumor (NAFA) expressing control vector (GFP) or IκBαSR (n = 5 per group) in nude mice to determine microvessel density. B: Histopathology by H&E staining (left) or immunostaining with CD31 antibody to demarcate tumor blood vessels (right) to which (arrows) point. C: Immunohistochemical staining for candidate pro-angiogenic factors (left) and quantitation of immunohistochemical staining for pro-angiogenic factors, shown as mean ± SEM (right). D: Angiogenesis assay conducted using human umbilical vein endothelial cells treated with media from either control vector or IκBαSR-transduced NAFA cells (left) and quantitation of vessel formation by branch number and length (right). *P < 0.05; **P < 0.01. Original magnifications, ×40.
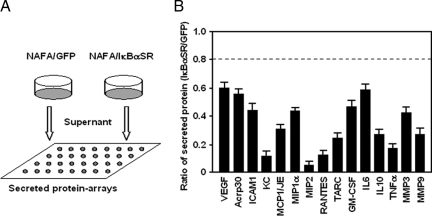
Our recent studies had suggested an increased abundance of VEGF in the circulation of MMTV-ErbB2 transgenic mice (180 mg/ml versus 43 mg/ml).41 To determine the NF-κB-regulated proteins secreted by NAFA cells, the supernatant of the NAFA and NAFA-IκBαSR were analyzed for secreted chemokines and cytokines using cytokine arrays (Figure 7A).34 Subtractive analysis was then conducted to identify NF-κB-regulated secreted proteins (Figure 7B). VEGF was identified as a protein secreted by NAFA cells and VEGF secretion was NF-κB-dependent. Several other secreted factors were identified as NF-κB-regulated, including GM-CSF and ACRP-30.
Figure 7.
NF-κB-regulated protein secretion. A: The relative abundance of secreted proteins was determined in the supernatant of NAFA versus NAFA-IκBαSR cells using cytokine arrays.34 B: Supernatant was analyzed from an equal number of cells after 24 hours of culture. Reduced abundance indicates suppressed by IκBαSR. The data are mean ± SEM of three separate experiments.
Discussion
The immune response, which is mediated by NF-κB, participates in promoting mammary tumorigenesis.19,40 Bone marrow macrophages, recruited to mammary tumors in mice, promote the progression of mammary tumor growth. F4/80-staining macrophages recruited via CSF1 are associated with increased tumor angiogenesis. TNF-α secreted by immune cells in contrast has substantial anti-tumor effects. Prior studies had examined the role of mammary epithelial cell NF-κB in cellular growth using immune-deficient mice.17 The current studies extend prior observations in several ways. First, these studies demonstrated that mammary epithelial cell NF-κB is required for mammary tumor growth in immune-competent mice in vivo. Second, these studies demonstrated the functional significance of mammary epithelial cell NF-κB in promoting vasculogenesis in vivo. Third, these studies demonstrated mammary tumor NF-κB governs vasculogenesis via a heterotypic secreted factor. Fourth, ErbB2 induced both p50 and p65 binding activity and using specific siRNA, the current studies demonstrated the specific subcomponents of NF-κB, p50, and p65, contribute to ErbB2-induced breast tumor cellular proliferation. Increased abundance of p50 and Rel-A is consistent with ErbB2 primarily activating the canonical pathway of NF-κB signaling.
The current studies demonstrate that ErbB2 induction of tumorigenesis in immune-competent mice in vivo involves NF-κB signaling. NF-κB enhanced angiogenesis and VEGF expression in vivo. Our studies extend prior studies conducted in cell culture. NF-κB signaling was required for DNA synthesis and contact-independent growth in both ERα+ (MCF-7, MCF10A) and ERα− (SKBr3, NAFA) breast cancer cell lines in culture. Breast cancer cells expressing low levels of ERα express high NF-κB activity and ERα− cells display constitutively active NF-κB.11 Loss of estrogen signaling through estradiol removal likewise allows NF-κB activity, which may contribute to hormone-independence of human breast cancer cells in culture.42
ErbB2 induced cyclin D1 expression in tissue culture27 and herein ErbB2 induced cyclin D1 in vivo via an NF-κB-dependent mechanism. This was accompanied by an increase in the proportion of pRb in the phosphorylated form that is permissive for cell-cycle entry. Cyclin D1 is required for ErbB2-induced tumorigenesis, as cyclin D1 anti-sense blocks ErbB2-induced tumor growth27 and cyclin D1 knockout mice are resistant to ErbB2-induced tumorigenesis. Cyclin D1 is a known target of NF-κB activation, by virtue of an NF-κB binding sequence at −30 to −39 bp in the promoter.30,35 In the studies reported here, increased abundance of both p50 and RelA accompanied the increase in NF-κB signaling induced by ErbB2 activity. In 3T3 cells, activated with serum or expressing activating mutations of Rac1, the cyclin D1 promoter NF-κB binding sequence bound increased abundance of p50/RelA heterodimers and p50 homodimers. A dominant-negative mutant of Rac (N17Rac) inhibited ErbB2-induced signaling to the cyclin D1 promoter and reduced binding of p50/RelA heterodimers to the cyclin D1 promoter.30,35 Thus Rac is also involved in the pathway by which ErbB2 activates NF-κB. Together these findings imply that cyclin D1 serves as a downstream target of ErbB2-NF-κB signaling.
The current studies, which reduced NF-κB activity in vivo with a dominant inhibitor of NF-κB are consistent with a prior study of the IκB kinase. A peptide inhibitor of the IκB kinase, NEMO, blocked breast cancer cellular proliferation in tissue culture.17 A dominant-negative mutant of IKKβ also inhibited breast xenografts in nude mice. The IκB kinase, however, activates additional substrates to NF-κB including Akt and β-catenin,23 both of which promote breast tumor growth.31,43 The current findings are consistent with the mammary tumor resistance of mice transgenic for mutations of the IκB kinase. However IKKα mutant mice have a substantial defect in mammary gland development.44 IKKαA/A mice, which have inhibitory mutations in the activation loop of IKKα, have failed terminal alveolar breast bud development resembling MMTV-IκBαSR mice or cyclin D1−/− mice.44 There was less cyclin D1 in the mammary glands of both the IKKαA/A and MMTV-IκBαSR transgenic animals. Cyclin D1 is induced by IKKα and the cyclin D1 promoter is a direct transcriptional target of IKKα.23
In the current studies, mammary epithelial cell NF-κB enhanced vasculogenesis in vivo. In vitro cultures indicated mammary epithelial cell NF-κB regulated the production of a secreted factor that enhanced vasculogenesis in tissue culture. Cytokine array analysis demonstrated NF-κB induced VEGF secretion in NAFA cells. Growth of solid tumors in vivo beyond 1 to 2 mm in diameter requires the induction and maintenance of tumor angiogenesis.45 Tumor angiogenesis is a consequence of increased production of proangiogenic factors (including fibroblast growth factor, platelet-derived growth factor, and VEGF) and reduced expression of antiangiogenic factors such as thrombospondin 1.46 VEGF abundance is increased in human tumors in response to hypoxia and cytokines. Oncogenes, including Ras and Src activate the Raf/MAPK pathway inducing VEGF transcription in mammary epithelial cells.47 VEGF binds the heterodimeric integrin, αvβ5.48 Integrins are expressed in newly synthesized blood vessels and allow interaction between endothelial cells and extracellular matrix (ECM). The αV integrins bind to ECM components that contain a RGD sequence, such as vWF, whereas the β1α4 integrin binds VCAM.48 NF-κB did not regulate the abundance of the integrin ligands vWF and VCAM. PECAM-1/CD31, an adhesion receptor member of the immunoglobulin superfamily that is expressed on endothelium to regulate tumor vasculogenesis49 was diminished by mammary epithelial cell NF-κB inhibition in vivo.
ErbB2, which is overexpressed in ∼30% of human breast cancers, is an important target for breast cancer therapy, but resistance to anti-ErbB2 monoclonal antibody treatment develops in 85% of patients. Inhibition of NF-κB improves chemotherapeutic sensitivity.50 The suppression of ErbB2 oncogenesis by NF-κB inhibition, observed in these experiments, provides further rationale for targeting NF-κΒ in human breast cancer.
Acknowledgments
We thank Grace Chung for the preparation of this manuscript.
Footnotes
Address reprint requests to Richard G. Pestell, Kimmel Cancer Center, Department of Cancer Biology, Thomas Jefferson University, 233 South 10th St., Philadelphia, PA 19107. E-mail: richard.pestell@jefferson.edu.
Supported in part by the National Institutes of Health (grants R01CA70896, R01CA75503, and R01CA86072 to R.G.P., and Cancer Center Core grant P30CA56036 to the Kimmel Cancer Center to R.G.P.), the Breast Cancer Research Foundation (to A.A.Q.), the Dr. Ralph and Marian C. Falk Medical Research Trust (to R.G.P.), and the Pennsylvania Department of Health (to R.G.P.). The Pennyslvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
A guest editor acted as editor-in-chief for this manuscript. No person at Thomas Jefferson University was involved in the peer review process or final disposition for this article.
References
- Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145–153. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, Khavari PA. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- Seitz CS, Lin Q, Deng H, Khavari PA. Alterations in NF-kappaB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-kappaB. Proc Natl Acad Sci USA. 1998;95:2307–2312. doi: 10.1073/pnas.95.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hogerlinden M, Auer G, Toftgard R. Inhibition of Rel/nuclear factor-kappaB signaling in skin results in defective DNA damage-induced cell cycle arrest and Ha-ras- and p53-independent tumor development. Oncogene. 2002;21:4969–4977. doi: 10.1038/sj.onc.1205620. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Epinat J-C, Barkett M. Ehrlich M, editor. Natick: Bio Techniques Books; Misregulation of a Signal Transduction PathwayThe Role of Rel/NF-κB Transcription Factors in Oncogenesis. 1999:pp 121–136. [Google Scholar]
- Dejardin E, Bonizzi G, Bellahcene A, Castronovo V, Merville MP, Bours V. Highly-expressed p100/p52 (NFKB2) sequesters other NF-kappa B-related proteins in the cytoplasm of human breast cancer cells. Oncogene. 1995;11:1835–1841. [PubMed] [Google Scholar]
- Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE. Aberrant nuclear factor-kB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit S, Ben-Neriah Y. NF-kappaB activation in cancer: a challenge for ubiquitination- and proteasome-based therapeutic approach. Semin Cancer Biol. 2003;13:15–28. doi: 10.1016/s1044-579x(02)00096-2. [DOI] [PubMed] [Google Scholar]
- Newton TR, Patel NM, Bhat-Nakshatri P, Stauss CR, Goulet RJ, Jr, Nakshatri H. Negative regulation of transactivation function but not DNA binding of NF-kappaB and AP-1 by IkappaBbeta1 in breast cancer cells. J Biol Chem. 1999;274:18827–18835. doi: 10.1074/jbc.274.26.18827. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA. From the molecule to the clinic—inhibiting HER2 to treat breast cancer. N Engl J Med. 2001;344:841–842. doi: 10.1056/NEJM200103153441110. [DOI] [PubMed] [Google Scholar]
- Ligibel JA, Winer EP. Trastuzumab/chemotherapy combinations in metastatic breast cancer. Semin Oncol. 2002;29:38–43. doi: 10.1053/sonc.2002.34054. [DOI] [PubMed] [Google Scholar]
- Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, Iglehart JD. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA. 2004;101:10137–10142. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Murphy RF, Ding XZ, Roginsky AB, Bell RH, Jr, Adrian TE. On the role of transforming growth factor-beta in the growth inhibitory effects of retinoic acid in human pancreatic cancer cells. Mol Cancer. 2007;6:82. doi: 10.1186/1476-4598-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- Albanese C, Wu K, D'Amico M, Jarrett C, Joyce D, Hughes J, Hulit J, Sakamaki T, Fu M, Ben-Ze'ev A, Bromberg JF, Lamberti C, Verma U, Gaynor RB, Byers SW, Pestell RG. IKKalpha regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Mol Biol Cell. 2003;14:585–599. doi: 10.1091/mbc.02-06-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descargues P, Sil AK, Sano Y, Korchynskyi O, Han G, Owens P, Wang XJ, Karin M. IKKalpha is a critical coregulator of a Smad4-independent TGFbeta-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc Natl Acad Sci USA. 2008;105:2487–2492. doi: 10.1073/pnas.0712044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. IKKα controls formation of the epidermis independently of NF-kB. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Albanese C, Fu M, D'Amico M, Lin B, Watanabe G, Haines GK, III, Siegel PM, Hung MC, Yarden Y, Horowitz JM, Muller WJ, Pestell RG. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol Cell Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese C, Reutens AT, Bouzahzah B, Fu M, D'Amico M, Link T, Nicholson R, Depinho RA, Pestell RG. Sustained mammary gland-directed, ponasterone A-inducible expression in transgenic mice. FASEB J. 2000;14:877–884. doi: 10.1096/fasebj.14.7.877. [DOI] [PubMed] [Google Scholar]
- Neumeister P, Pixley FJ, Xiong Y, Xie H, Wu K, Ashton A, Cammer M, Chan A, Symons M, Stanley ER, Pestell RG. Cyclin D1 governs adhesion and motility of macrophages. Mol Biol Cell. 2003;14:2005–2015. doi: 10.1091/mbc.02-07-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce D, Bouzahzah B, Fu M, Albanese C, D'Amico M, Steer J, Klein JU, Lee RJ, Segall JE, Westwick JK, Der CJ, Pestell RG. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-kappaB-dependent pathway. J Biol Chem. 1999;274:25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- Ju X, Katiyar S, Wang C, Liu M, Jiao X, Li S, Zhou J, Turner J, Lisanti MP, Russell RG, Mueller SC, Ojeifo J, Chen WS, Hay N, Pestell RG. Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci USA. 2007;104:7438–7443. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Liu M, Li A, Donninger H, Rao M, Jiao X, Lisanti MP, Cvekl A, Birrer M, Pestell RG. The cell fate determination factor DACH1 inhibits c-Jun induced contact-independent growth. Mol Biol Cell. 2007;18:755–767. doi: 10.1091/mbc.E06-09-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Li A, Rao M, Liu M, Dailey V, Yang Y, Di Vizio D, Wang C, Lisanti MP, Sauter G, Russell RG, Cvekl A, Pestell RG. DACH1 is a cell fate determination factor that inhibits cyclin D1 and breast tumor growth. Mol Cell Biol. 2006;26:7116–7129. doi: 10.1128/MCB.00268-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar S, Jiao X, Wagner E, Lisanti MP, Pestell RG. Somatic excision demonstrates c-Jun induces cellular migration and invasion through induction of stem cell factor. Mol Cell Biol. 2007;27:1356–1369. doi: 10.1128/MCB.01061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Majada V, Aguilera C, Villanueva A, Vilardell F, Robert-Moreno A, Aytes A, Real FX, Capella G, Mayo MW, Espinosa L, Bigas A. Nuclear IKK activity leads to dysregulated notch-dependent gene expression in colorectal cancer. Proc Natl Acad Sci USA. 2007;104:276–281. doi: 10.1073/pnas.0606476104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seton-Rogers SE LY, Hines LM, Koundinya M, LaBaer J, Muthuswamy SK, Brugge JS. Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci USA. 2004;101:1257–1262. doi: 10.1073/pnas.0308090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgia F, Williams TM, Schubert W, Medina F, Minetti C, Pestell RG, Lisanti MP. Caveolin-1 deficiency (−/−) conveys premalignant alterations in mammary epithelia, with abnormal lumen formation, growth factor independence, and cell invasiveness. Am J Pathol. 2006;168:292–309. doi: 10.2353/ajpath.2006.050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. Beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Chizea J, Dailey VK, Williams E, Johnson MD, Pestell RG, Ojeifo JO. Endothelial progenitor cells significantly contribute to vasculatures in human and mouse breast tumors. Open Hematol J. 2008;2:30–62. [Google Scholar]
- Pratt MA, Bishop TE, White D, Yasvinski G, Menard M, Niu MY, Clarke R. Estrogen withdrawal-induced NF-kappaB activity and bcl-3 expression in breast cancer cells: roles in growth and hormone independence. Mol Cell Biol. 2003;23:6887–6900. doi: 10.1128/MCB.23.19.6887-6900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands TM, Pechenkina IV, Hatsell SJ, Pestell RG, Cowin P. Dissecting the roles of beta-catenin and cyclin D1 during mammary development and neoplasia. Proc Natl Acad Sci. 2003;100:11400–11405. doi: 10.1073/pnas.1534601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Grill C, Gheyas F, Dayananth P, Jin W, Ding W, Qiu P, Wang L, Doll RJ, English JM. Analysis of the ERK1,2 transcriptome in mammary epithelial cells. Biochem J. 2004;381:635–644. doi: 10.1042/BJ20031688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang R, Varner J. The role of integrins in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:991–1006. doi: 10.1016/j.hoc.2004.09.010. vii. [DOI] [PubMed] [Google Scholar]
- Kim CS, Wang T, Madri JA. Platelet endothelial cell adhesion molecule-1 expression modulates endothelial cell migration in vitro. Lab Invest. 1998;78:583–590. [PubMed] [Google Scholar]
- Mayo MW, Wang CY, Cogswell PC, Rogers-Graham KS, Lowe SW, Der CJ, Baldwin AS. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]