Abstract
Background
Selection of antihypertensive therapy is often empiric and use of genetic information to guide drug therapy selection holds future promise.
Trial design
The objective of this trial is to identify the genetic determinants of the antihypertensive and adverse metabolic responses to a thiazide diuretic (hydrochlorothiazide, HCTZ), a β-blocker (atenolol) and their combination. This will be accomplished through candidate gene and genome wide association approaches. Individuals with uncomplicated hypertension (n=800), ages 17 and 65 years, are being enrolled. Current antihypertensive therapy is discontinued and hypertension is confirmed, along with collection of other baseline data. Subjects are then randomized to either HCTZ or atenolol, with one dose titration step, followed by assessment of response to therapy after at least 6 weeks on the target dose. Those with blood pressure > 120/70 mmHg have the second drug added, with similar dose titration and response assessment procedures. Data collected include home, office and 24 hour ambulatory blood pressure. Biological samples collected in the fasting state include plasma, serum, DNA (buffy coat), and urine. Epstein Barr virus transformed lymphocyte cell lines are also being created.
Conclusions
Pharmacogenetic-guided therapy holds clinical potential for hypertension, but the literature in the field is limited. This trial will add substantially to our understanding of the genetic determinants of antihypertensive and adverse metabolic responses to two commonly used antihypertensive drug classes.
Introduction
Hypertension is common, affecting approximately 73 million Americans, with an additional 70 million considered to have prehypertension.1 Serious sequelae of hypertension include stroke, heart failure, ischemic heart disease, and chronic renal failure.
Thiazide diuretics, β-blockers, ACE inhibitors, angiotensin receptor blockers (ARBs) and calcium channel blockers (CCBs) are considered appropriate first line treatment for hypertension in the US,2 although there is recent debate about the first line role of diuretics and β-blockers due to their adverse metabolic effects.3, 4 Despite availability of many effective agents, only about 40 percent of treated hypertensives have their blood pressure (BP) controlled.1, 5 Variable drug efficacy may contribute, in part, to poor BP control, as numerous studies have shown that any given drug is effective in only 40-60% of patients.6 Initial therapy is often selected empirically, and blood pressure responses to monotherapy vary widely within ethnic and gender subgroups. The low response rates to any particular antihypertensive drug suggest the current approach to therapy selection and hypertension management is not optimal. The Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) study seeks to address whether genetic predictors of blood pressure lowering in response to a thiazide diuretic, a beta-blocker, or their combination can be identified. A secondary objective is to determine whether genetic predictors of adverse metabolic effects (AME) to each monotherapy or combination therapy can be identified. The variable nature of these responses, previous studies suggesting genetic associations with blood pressure responses, and the heritability of high blood pressure, glucose and lipids all lend evidence to the hypothesis that antihypertensive and adverse metabolic responses to thiazides and beta-blockers may be under some genetic control. Information gained from this study may help individualize selection of antihypertensives in patients with uncomplicated essential hypertension.
Rationale for PEAR Study Design
PEAR is funded as part of the National Institute of Health’s (NIH) Pharmacogenetics Research Network (http://www.pharmgkb.org/network/pharmacogenetics_research_network.jsp) to investigate potential genetic contributors to antihypertensive and AME responses to thiazide diuretics and β-blockers, using both a candidate gene and genome-wide association approach. This broad approach will allow investigation of the contributions from multiple different genes to drug response variability.
Many previous hypertension pharmacogenetic studies have limited analyses to office (i.e., clinic) measures of BP response, but PEAR will incorporate both home and 24 hour ambulatory measures as well as office measures of BP response. Evidence suggests office BP is not the optimal BP phenotype for assessing genetic predictors of drug response. Specifically both ambulatory and home BP have been shown to be superior to office BP in predicting long-term outcomes.7, 8 Reproducibility for ambulatory and home BP is also better than for office BP.9 Additionally, we and others have shown a relatively poor correlation between office BP and either ambulatory BP or home BP, but good correlations between ambulatory BP and home BP.10-12 Finally, office BP is associated with a placebo effect, whereas ambulatory and home BP are not.13, 14 Therefore, the data suggest the most appropriate BP phenotypes are home and ambulatory BP rather than office BP, and these will be the primary focus in PEAR.
PEAR will also address genetic associations with monotherapy and combination therapy in hypertension. While information on the genetic predictors of BP response in the untreated patient is important, it is increasingly clear that a large percentage of patients will require more than one drug for BP control. It is therefore important to understand whether associations documented between genetic polymorphisms and response to monotherapy are preserved when the drug of interest is added to existing antihypertensive therapy, and the PEAR design will allow for such assessments.
Despite their widespread use for hypertension, thiazides and β-blockers are associated with AMEs that are the cause of increasing concern in the clinical community. Specifically, these AMEs have led some to suggest neither drug class should be considered first line therapy for uncomplicated hypertension.3, 4 However, only a relatively small portion of the population experiences these AMEs. If genetic contributors to AMEs could be identified a priori, clinicians could choose to avoid these drugs in at risk individuals. Despite the increasing focus on the AMEs of these drugs, data on genetic contributions to these effects is limited. In PEAR, these adverse metabolic response phenotypes will be assessed at the same time as BP responses, through determination of specific laboratory measures collected under fasting conditions. The primary endpoint for AMEs on glucose is the homeostatic model assessment (HOMA), which incorporates fasting glucose and insulin levels to arrive at a measure of insulin sensitivity and beta-cell function. Estimates of insulin resistance/sensitivity and pancreatic β-cell function, in diabetics and non-diabetics, by HOMA have been well correlated with invasive “gold standard” methods like euglycemic or hyperglycemic clamping, minimal modeling, and others.15 Change in triglycerides is our primary endpoint for lipid AMEs since sustained effects on triglycerides have been noted for beta-blockers and thiazides, and elevated triglycerides are a determinant of metabolic syndrome, and a modifiable risk factor for coronary disease.
Atenolol and HCTZ were specifically selected for several reasons. They are the most commonly used antihypertensive drugs within their respective classes, with each drug prescribed over 40 million times annually in the US. Given their widespread use, resulting data would potentially be of high clinical value. Both drugs are also dosed once daily, which is documented to enhance adherence to therapy.16 Finally, these drugs were chosen because of the relative lack of genetic influence on their pharmacokinetics, thus reducing confounding effects of pharmacokinetic variability. Specifically, both drugs are primarily eliminated by the kidneys and thus not influenced by genetic variation in drug metabolizing enzymes, as are other drugs, including other β-blockers. This trial was proposed and initiated prior to the publication of the ASCOT trial data,17 which has called into question the role of atenolol in hypertension management. Nonetheless, atenolol remains a widely used drug, and even if its use falls out of favor over the next decade, pharmacogenetic data on a variety of β-blockers suggest findings are consistent across the drug class. Thus pharmacogenetic findings with atenolol from this study are likely to be applicable to other β-blockers used in hypertension.
PEAR Study Design
Study population
Males or females (n=800) with mild to moderate essential hypertension, of any race or ethnicity, between the ages of 17 and 65 are being recruited to participate. Subjects are being enrolled at the University of Florida (Gainesville, FL), Emory University (Atlanta, GA) and Mayo Clinic (Rochester, MN). In Gainesville, subjects are recruited from Department of Community Health and Family Medicine clinics, which are primary care clinics. In Atlanta, subjects are recruited through outpatient medical clinics at Grady Memorial Hospital, the Hypertension and Renal Diseases Research Center at Emory University, advertisements in public media, and through mailings to registered voters. In Rochester, subjects are recruited from a list of all residents of Olmsted County seen by a health care provider in the previous three years who have a diagnosis of hypertension. Thus, study participants at all three sites are drawn almost exclusively from the primary care setting. The study has been approved the Institutional Review Boards at each institution, and all subjects provide informed, written consent prior to being screened for participation. Information collected on participants’ race and ethnicity are self-defined and collected according to the guidelines set forth by the NIH.
Potential subjects are those with newly diagnosed, untreated or known hypertension currently treated with one or two antihypertensive drugs. Inclusion and exclusion criteria are shown in Table 1. Subjects not meeting any exclusion criteria are further screened for BP inclusion, based on untreated home and office BP. Subjects undergo training by research personnel on the use of the home BP monitor, and are provided a monitor and appropriately sized cuff to take home. Those currently treated have their antihypertensive drug therapy tapered (as necessary) and discontinued, with a minimum antihypertensive-free period of 18 days, and a preferred washout period of 4-6 weeks.
Table 1.
PEAR Inclusion and Exclusion Criteria
Inclusion
|
Exclusion
|
Average home BP in week prior to visit
BP Inclusion
During the entire study, participants are requested to take their home BP twice daily, on rising from bed and prior to retiring. After at least 18 antihypertensive drug-free days, they are screened for inclusion based on both home and office BP data. BP inclusion requires an average (previous week) seated home DBP > 85 mmHg and an average seated (> 5 minutes) office DBP > 90 mmHg. Subjects are excluded if by either method DBP is > 110 mmHg or SBP is > 180 mmHg.
Study protocol
The study protocol (Figure 1) is initiated in subjects who meet all eligibility criteria for the study.
Figure 1.
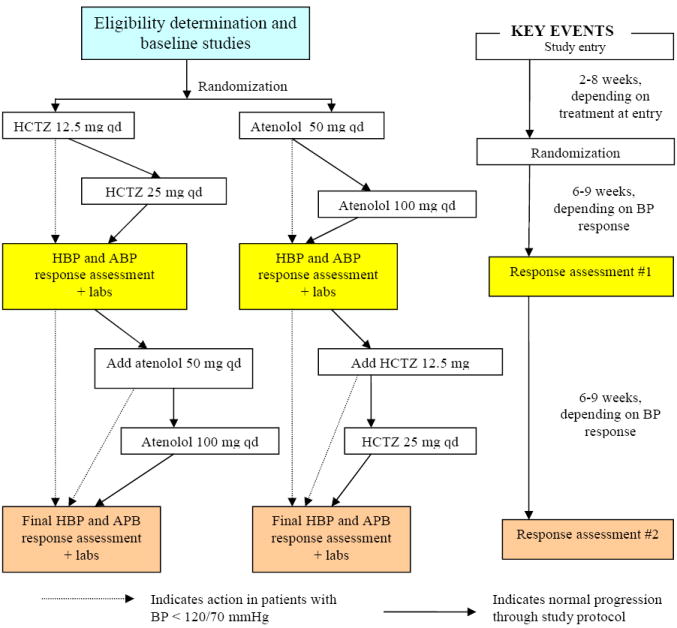
PEAR Study Protocol
Abbreviations: HCTZ – hydrochlorothiazide, BP – blood pressure, HPB – home BP, ABP – 24 hour ambulatory BP
Baseline studies
Data collected at various study visits are shown in Table 2. Subjects meeting eligibility criteria undergo baseline collection of home and 24 hour ambulatory BP (ABP) data. For ABP monitoring, subjects report to clinic for ABP monitor placement then return to usual daily activities. They return to clinic 24 hours after ABP placement. Biological samples are collected in the fasting state and include plasma, serum, buffy coat, and a spot urine (unpreserved and preserved with ascorbic acid) in sufficient quantities to meet the study aims and to support future research. Laboratory parameters determined in all subjects (from plasma or serum, as appropriate) for primary study analyses include glucose, insulin, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, sodium, potassium, magnesium, creatinine, and uric acid. There are no primary planned analyses for urine samples; they are collected to facilitate future research.
Table II.
Data collection summary by study visit
| Visit # | Visit Name | Clinical Labsa | Home BP & Office BP | Ambulatory BP | Research Labsb |
|---|---|---|---|---|---|
| 1 | Screening | X | |||
| 2 | BP Inclusion screening | X | |||
| 3 | Baseline studies | X | X | X | |
| 4 | Response & safety check | X | X | X | |
| 5 | Response assessment #1 | X | X | X | X |
| 6 | Response & safety check | X | X | X | |
| 7 | Response assessment #2 | X | X | X |
Clinical labs - for inclusion screening or clinical safety monitoring; measured at local clinical laboratory
Research labs - collected for research purposes, measured centrally.
Treatment phase
After completion of baseline studies, subjects are randomized to hydrochlorothiazide (HCTZ) 12.5 mg daily or atenolol 50 mg daily in an unblinded fashion. Given that all study phenotypes are objective in nature, and efficacy of the study drugs is well-established, it was not felt that blinding was needed. Throughout the protocol, those with an average home or office SBP > 120 mmHg or DBP > 70 mmHg continue to move through the titration protocol, while those with BPs ≤ 120/70 mmHg hold at their current treatment step. Subjects return with their home BP data after 3 weeks on the initial dose, and based on BP noted above, they undergo dose titration. They continue on this dose for a minimum of 6 additional weeks, after which they undergo studies to assess their response to the first study drug (Response assessment #1). Those with BP ≤ 120/70 mmHg proceed directly to response assessment #1 after at least 6 weeks on the initial dose (Figure 1). Response assessment studies are identical to baseline studies (Table 2). Additionally, samples are collected at this visit for creation of Epstein Barr virus transformed lymphocytes, to create a permanent source of DNA and support future tissue based studies.
After completion of Response Assessment #1, the majority of subjects (i.e. those with BP > 120/70 mmHg) have the alternate drug added, and continue through the protocol as in the first phase, after which they repeat response assessment studies.
Safety procedures
Data on adverse effects are collected at every study visit, and monitored to detect unexpected adverse events. Numerous safety procedures are in place to insure patient safety, particularly during the antihypertensive drug washout period. First, many of the exclusion criteria are designed to exclude those at moderate to high risk. Additionally, subjects are encouraged to conduct daily home BP monitoring, and to report immediately any readings with SBP > 180 mmHg or DBP > 110 mmHg. Subjects are withdrawn for any average home BP from the previous week, or an office BP of SBP > 180 mmHg or DBP > 110 mmHg, and restarted on their previous antihypertensive regimen or referred for care. The protocol also requires moving backward by one treatment step for symptomatic hypotension (regardless of BP), or for any SBP < 100 mmHg (regardless of symptoms). Any subject with HR < 55 bpm is precluded from receiving a higher atenolol dose, and in the case of symptomatic bradycardia, the atenolol dose is decreased or the drug is stopped.
Electrolytes are also carefully monitored, with clinical determination at a local laboratory of serum potassium at each visit while a subject is taking HCTZ. Study physicians can elect to replace potassium at any value, with protocol mandated prescription of oral potassium chloride (KCl) 40 mEq daily for any potassium below 3.2 mEq/L. Serum potassium is rechecked every 3-4 weeks and KCl doses increased as needed until potassium is normalized.
Finally, PEAR has an external Data Safety and Monitoring Board, charged with monitoring safety of the study protocol, along with data quality.
Methods for BP assessment
Home BP assessment
Home BP is determined using the Microlife model 3AC1-PC home BP monitor (Minneapolis, MN), a device that has met the standards of the British Society of Hypertension, and the Association for the Advancement of Medical Instrumentation.18 All BP measurements are taken in triplicate mode, and the monitor averages the values. SBP, DBP, HR, and a date/time stamp are recorded. The BP monitor stores up to 99 measurements, from which data are downloaded via a computer interface. Subjects bring their home BP monitor to each clinic visit, and the study coordinator downloads the data to the computer, using these data to make protocol-driven decisions. In order for the home BP data to be accepted, there must be at least 5 morning and 5 evening readings during the previous 7 days. If insufficient home BP data are recorded, study participants are asked to return when they have sufficient home BP data. Prior to giving the participant the home BP monitor on their first visit, study coordinators document the accuracy of the home BP monitor against manual measurement, with 6 measurements by home monitor and manual methods. If the 2 methods differ by more than 8 mmHg a different home BP monitor is used.
Office BP assessment
All office BPs are taken using the home BP monitor assigned to the subject, such that any differences in home and office BP can be attributed to the setting and not the device used to take the BP. Office BP is taken in triplicate, after the subject has been seated for at least 5 minutes.
Ambulatory BP assessment
ABP monitoring is performed using Spacelabs (Redmond, WA) model 90207, which has also met the standards for accuracy of the British Society of Hypertension, and the Association for the Advancement of Medical Instrumentation.18 The ABP monitor is preprogrammed to randomly record BPs four times hourly during the daytime or active hours (e.g., 0600 to 2200) and twice hourly during the nighttime or inactive hours (e.g., 2200 to 0600). Times are adjusted appropriately for night workers. A validation procedure similar to the one described for the home BP monitor is applied to the ABP monitor.
Assessing adherence to therapy
To aid adherence and its monitoring, study medication is provided in blister packs, labeled with the day of the week. This serves as a “pill-box” equivalent and aids the patient in remembering whether they took their dose. Participants are instructed to bring their blister packs to study visits, from which a pill count is made. This includes assessment of total number of doses missed, and the specific day on which doses are missed in the week prior to the study visit. Those with poor adherence are provided counseling on improved adherence strategies. Availability of detailed adherence data in the week prior to the study visit (during which home BP data are being captured) allows for exclusion of subjects with poor adherence (e.g. <70%), and/or inclusion of adherence data in analysis models.
Planned genetic analyses
Both candidate gene and genome-wide association analyses will be undertaken. Candidate gene genotyping will be accomplished using the IBC chip,19 a cardiovascular gene custom array that includes approximately 2,200 cardiovascular and metabolic-related genes, covered through assay of approximately 50,000 single nucleotide polymorphisms (SNPs) using a tag SNP approach. Genome-wide association genotyping will be accomplished using the Affymetrix 6.0 array. At study inception we proposed to study 70 biological candidate genes (Table 3), along with study of 20,000 genome-spanning putative functional (pf) SNPs. While the arrays described above will provide substantially more data than originally envisioned, our initial analyses will still focus on the original 70 candidate genes and the 20,000 pfSNPs.
Table III.
Candidate Genes for Antihypertensive & Adverse Responses to Diuretics & β-blockers
| Name | HUGO Gene Symbol | Chromosomal Location |
|---|---|---|
| Sympathetic Nervous System | ||
| β1-adrenergic receptor | ADRB1 | 10q24-q26 |
| β2-adrenergic receptor | ADRB2 | 5q32-q34 |
| α1A-adrenergic receptor | ADRA1A | 8p21 |
| α1B-adrenergic receptor | ADRA1B | 5q33 |
| α 2A-adrenergic receptor | ADRA2A | 10g24.q26 |
| α 2B-adrenertic receptor | ADRA2B | 2 |
| α 2C-adrenergic receptor | ADRA2C | 4p16.1 |
| Dopamine receptor, D1 | DRD1 | 5q35.1 |
| G protein αs | GNAS1 | 20q13.2 |
| Phosphodiesterase III | PDE3A | 12p12 |
| Adenylate cyclase 5 | ADCY5 | 3q13.2-g21 |
| Adenylate cyclase 6 | ADCY6 | 12q12-g13 |
| β-adrenergic receptor kinase 1 (GRK2) | ADRBK1 | 11cen-g13 |
| G protein coupled receptor kinase 5 (GRK5) | GPRK5 | 10q24-gter |
| G protein coupled receptor kinase 4 (GRK4) | GPR2L | 4p16.3 |
| β-arrestin 1 | ARRB1 | 11q13 |
| Dopamine β-hydroxylase | DBH | 9q34 |
| Catechol-O-methyltransferase | COMT | 22q11.2 |
| Monoamine oxidase A | MAOA | Xp11.23 |
| Renin-Angiotensin-Aldosterone System | ||
| Angiotensinogen | AGT | 1q42-43 |
| Renin | REN | 1q32 |
| Angiotensin converting enzyme | ACE | 17q23 |
| Angiotensin II receptor, type 1 | AGTR1 | 3q21-q25 |
| Aldosterone synthase | CYP11B2 | 8q21 |
| Aldosterone receptor | NR3C2 | 4g31.1 |
| 11β-hydroxysteriod dehydrogenase | HSD11B2 | 16q22 |
| Natriuretic peptides/receptors | ||
| Atrial natriuretic peptide | NPPA | 1p36.2 |
| Natriuretic peptide receptor A | NPR1 | 1q21-q22 |
| Brain natriuretic peptide | NPPB | 1p36.2 |
| Endothelial Systems | ||
| Nitric oxide synthase, endothelial | NOS3 | 7q36 |
| Endothelin-1 | EDN1 | 6p24-p23 |
| Endothelin-2 | EDN2 | 1p34 |
| Endothelin receptor A | EDNRA | 4 |
| Endothelin receptor B | EDNRB | 13q22 |
| Sodium Transport Systems | ||
| Na+-H+ antiporter, amiloride-sensitive | SLC9A3 | 5p15.3 |
| Na+-K+-2Cl- cotransporter, bumetanide-sensitive | SLC12A1 | 15q15-q21.1 |
| Na+-Cl- cotransporter, thiazide-sensitive | SLC12A3 | 16q13 |
| Epithelial sodium channel: | ||
| α-subunit | SCNN1A | 12p13 |
| β-subunit | SCNN1B | 16p13-p12 |
| γ-subunit | SCNN1G | 16p13-p12 |
| NEDD4 | NEDD4L | 15q |
| α-adducin | ADD1 | 4p16.3 |
| β-adducin | ADD2 | 2p14-p13 |
| γ1 –adducin | ADD3 | 1-q24.2-q24.3 |
| G protein β3 –subunit | GNB3 | 12p13 |
| Ion regulation systems | ||
| Sarcoendoplasmic reticulum calcium ATPase | ATP2A2 | 12q23-q24.1 |
| Ryanodine receptor 2, cardiac | RYR2 | 1q42.1-q43 |
| L type Calcium channel α1c subunit | CACNA1C | 12p13.3 |
| Calcium channel β1 subunit | CACNB2 | 10p12 |
| Ca-activated K channel β1 | KCNMB1 | 5q34 |
| Potassium inwardly-rectifying channel | KCNJ1 | 11q24 |
| Chloride channel, kidney, B | CLCNKB | 1p36 |
| Protein kinase, lysine deficient 1 | PRKWNK1 | 12p13 |
| Protein kinase, lysine deficient 4 | PRKWNK4 | 17q21-g22 |
| Glucose and lipid regulation | ||
| Peroxisome proliferative activated receptor | PPARG | 3p25 |
| ATP-binding cassette, C8 | ABCC8 | 11p15.1 |
| Transcription factor 1, hepatic; albumin proximal factor | TCF1 | 12q24.2 |
| Hepatocyte nuclear factor 4, alpha | HNF4A | 20q12-g13.1 |
| Uncoupling protein 2 | UCP2 | 11q13 |
| Calpain 10 | CAPN10 | 2q37.3 |
| Hepatic lipase | LIPC | 15 q21-q23 |
| B3-adrenergic receptor | ADRB3 | 8p12-p11.2 |
| Glucagon receptor | GCGR | 17q25 |
| Cholesteryl ester transfer protein | CETP | 16q21 |
| Apolipoprotein E | APOE | 19q13.2 |
| Lipoprotein lipase | LPL | 8p22 |
| Lecithin-cholesterol acyltransferase | LCAT | 16g22.1 |
| Paraoxonase 1 | PON1 | 7q21.3 |
| Paraoxonase 2 | PON2 | 7q21.3 |
| LDL receptor | LDLR | 19p13.2 |
Given that we have a large number of European Americans and African Americans, this presents certain potential challenges in the analyses. We will deal with these in several ways, including initial analyses that consider the two groups separately. If the associations are similar, then the groups will be combined, and the analyses will control for the self-defined race, along with estimates of ancestry, which will be determined based on ancestry informative markers that are contained on both arrays being utilized. More detailed information on this issue, and all other analysis issues will be contained in the individual manuscripts reporting genetic associations.
Data and sample sharing
Based on conditions of the NIH award, genotype and phenotype data will be deposited upon publication of the data in a database accessible to scientists. Primary phenotype and genotype data deposits deposits will be made to PharmGKB (www.pharmgkb.org). High-throughput genotype datasets will be deposited in dbGaP (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gap) with appropriate links and meta-data in PharmGKB. Additionally there is a sample sharing plan for biological samples, including DNA, cell lines, plasma, serum and urine, under which investigators may submit an ancillary proposal to the PEAR steering committee to conduct analyses in collaboration with PEAR investigators.
Funding and trial registration
PEAR is funded by the National Institutes of Health (U01 GM074492). It is registered at ClinicalTrials.gov, #NCT00246519; URL: http://clinicaltrials.gov/ct2/show/NCT00246519. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Results
Baseline demographic characteristics of the first 418 subjects to complete the PEAR protocol are shown in Table 4. These data reveal the subjects are, on average, middle aged, and obese (BMI = 31). Figure 2 displays the progression of the first 1,000 subjects enrolled in PEAR. It shows that a high percentage of subjects (over 40%) cannot be randomized into the trial, with failure to meet inclusion/exclusion criteria as the major reason. Prior to randomization, 3 subjects (0.3%) were excluded for an adverse event or safety concern and in no case was the adverse event definitely attributed to study participation. Following randomization 7 subjects (1.3%) were excluded from further participation either because their BP exceeded protocol safety limits or the patient was uncomfortable about their BP level. These data highlight the safety of the protocol.
Table IV.
Baseline demographics* for initial 418 completed PEAR participants
| Patients N = 418 | |
|---|---|
| Age | 50.1 (8.8) |
| Sex (% female) | 56.5% |
| Race (n, %) | |
| White - European American | 56.7% |
| Black - African American | 40.0% |
| Asian | 1.2% |
| Other/multiracial | 2.1% |
| Duration of hypertension (years) | 8.1 (7.7) |
| Family history of hypertensionˆ | 78.7% |
| Never taken an antihypertensive drug | 10.5% |
| Taking antihypertensive drug at entry | 84.8% |
| Smoking status | |
| Current smoker (%) | 10.9% |
| Number of cigarettes per day | 13.6 (9.6) |
| Ex-smoker (%) | 23.1% |
| BMI (kg/m2) | 31.0 (5.7) |
| Waist circumference (cm) | 98.2 (13.0) |
| Hip circumference (cm) | 111.3 (12.3) |
Mean ± SD unless otherwise noted
Family history of hypertension defined as hypertension in a parent or sibling Abbreviation: BMI: body mass index
Figure 2.
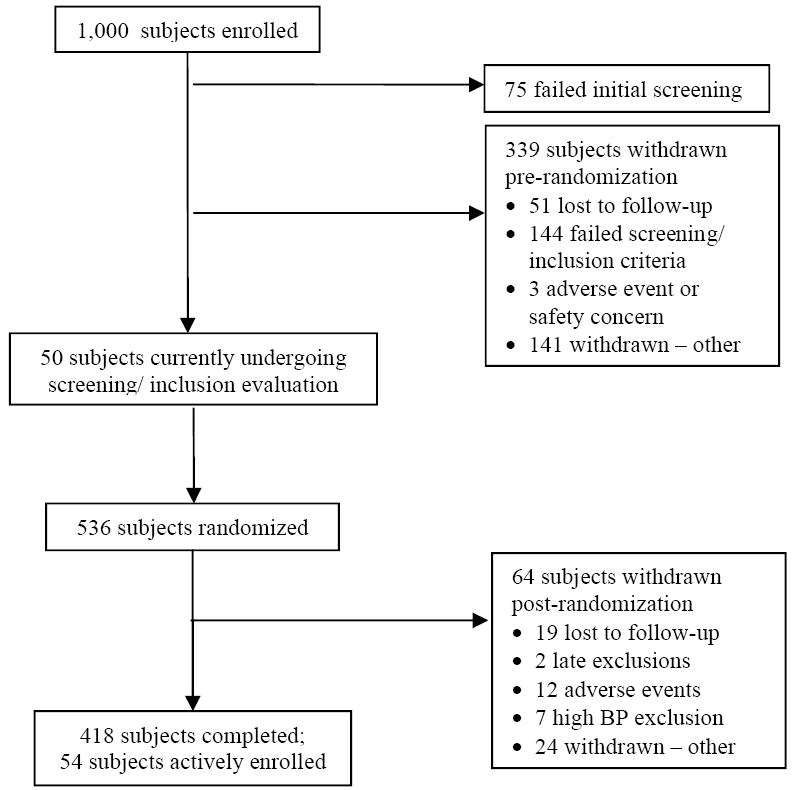
Progression of first 1,000 subjects enrolled in PEAR
Based on pill counts, 83.4% and 86.6% of subjects were 100% adherent at the time of response assessment to monotherapy and combination therapy, respectively. Focusing on adherence in the seven days prior to response assessments, 93.3% of subjects missed no doses, 4.8% missed one dose, and 1.9% missed two or more doses. The dose immediately prior to the response assessment visit was missed by 2.2% of subjects.
Discussion
Herein we describe the study design for the PEAR study, which is funded as part of the NIH Pharmacogenetics Research Network. The primary objectives of PEAR are to evaluate the genetic determinants of the blood pressure and adverse metabolic responses to β-blockers and thiazide diuretics, with the long-term goal of potentially being able to utilize such information to help guide selection of the most appropriate antihypertensive drug for an individual patient.
The study population consists of uncomplicated hypertensive subjects, where uncomplicated means they have no concomitant diseases that influence their initial antihypertensive therapy. Empiric therapy of hypertension is primarily with the uncomplicated hypertensive patient, thus this is the setting where pharmacogenetics may be of greatest clinical value. Other reasons for the focus on a sample of uncomplicated hypertensives is that it is a group with relatively fewer confounding variables (e.g. concomitant disease states), and the group for whom temporary washout of antihypertensive therapy is most likely to be safe. The data to date support this is a group in whom antihypertensive drugs can be safely withdrawn for several weeks.
In summery, PEAR will be unique as a hypertension pharmacogenetics study due to its focus not only on genetic associations of BP response, but also on adverse metabolic responses to these drugs.
Acknowledgments
This work is funded by the National Institutes of Health (Bethesda, MD) grant number U01-GM074492, as part of the Pharmacogenetics Research Network.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.AHA. Heart Disease and Stroke Statistics - 2008 Update. Dallas, Texas: American Heart Assocation; 2008. [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Cutler JA, Davis BR. Thiazide-type diuretics and beta-adrenergic blockers as first-line drug treatments for hypertension. Circulation. 2008;117(20):2691–704. doi: 10.1161/CIRCULATIONAHA.107.709931. discussion 2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messerli FH, Bangalore S, Julius S. Risk/benefit assessment of beta-blockers and diuretics precludes their use for first-line therapy in hypertension. Circulation. 2008;117(20):2706–15. doi: 10.1161/CIRCULATIONAHA.107.695007. discussion 2715. [DOI] [PubMed] [Google Scholar]
- 5.Ma J, Stafford RS. Screening, treatment, and control of hypertension in US private physician offices, 2003-2004. Hypertension. 2008;51(5):1275–81. doi: 10.1161/HYPERTENSIONAHA.107.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Materson BJ. Variability in Response to Antihypertensive Drug Treatment. Hypertension. 2004;43(6):1166–1167. doi: 10.1161/01.HYP.0000127916.65346.5c. [DOI] [PubMed] [Google Scholar]
- 7.Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348(24):2407–15. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 8.Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16(7):971–5. doi: 10.1097/00004872-199816070-00010. [DOI] [PubMed] [Google Scholar]
- 9.Stergiou GS, Baibas NM, Gantzarou AP, et al. Reproducibility of home, ambulatory, and clinic blood pressure: implications for the design of trials for the assessment of antihypertensive drug efficacy. Am J Hypertens. 2002;15(2 Pt 1):101–4. doi: 10.1016/s0895-7061(01)02324-x. [DOI] [PubMed] [Google Scholar]
- 10.Beitelshees AL, Zineh I, Yarandi HN, et al. Discordant beta-blocker effects on clinic, ambulatory, resting, and exercise hemodynamics in patients with hypertension. Pharmacotherapy. 2006;26(9):1247–54. doi: 10.1592/phco.26.9.1247. [DOI] [PubMed] [Google Scholar]
- 11.Finkielman JD, Schwartz GL, Chapman AB, et al. Lack of agreement between office and ambulatory blood pressure responses to hydrochlorothiazide. Am J Hypertens. 2005;18(3):398–402. doi: 10.1016/j.amjhyper.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Stergiou GS, Efstathiou SP, Skeva II et al. Assessment of drug effects on blood pressure and pulse pressure using clinic, home and ambulatory measurements. J Hum Hypertens. 2002;16(10):729–35. doi: 10.1038/sj.jhh.1001477. [DOI] [PubMed] [Google Scholar]
- 13.Mancia G, Omboni S, Parati G, et al. Lack of placebo effect on ambulatory blood pressure. Am J Hypertens. 1995;8(3):311–5. doi: 10.1016/0895-7061(94)00250-F. [DOI] [PubMed] [Google Scholar]
- 14.Imai Y, Ohkubo T, Hozawa A, et al. Usefulness of home blood pressure measurements in assessing the effect of treatment in a single-blind placebo-controlled open trial. J Hypertens. 2001;19(2):179–85. doi: 10.1097/00004872-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Estevez DA, Araujo-Vilar D, Fiestras-Janeiro G, et al. Comparison of several insulin sensitivity indices derived from basal plasma insulin and glucose levels with minimal model indices. Horm Metab Res. 2003;35(1):13–7. doi: 10.1055/s-2003-38385. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med. 2004;164(7):722–32. doi: 10.1001/archinte.164.7.722. [DOI] [PubMed] [Google Scholar]
- 17.Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien E, Waeber B, Parati G, et al. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322(7285):531–536. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keating BJ, Tischfield S, Murray SS, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS ONE. 2008;3(10):e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]


