Abstract
Exercise expends energy, but without dietary intervention, exercise does not appear to produce substantial weight loss. The present study examined whether overweight, sedentary individuals increase their energy intake after moderate intensity exercise, particularly in the presence of negative mood. A repeated measures design was used where overweight, sedentary individuals (N=65) completed, in counterbalanced order, two conditions: 3 minutes of exercise (Active) and 3 minutes of sedentary activity (Sedentary) during one session. Snack foods were presented 10 minutes after each activity. Mixed-effects regression modeling revealed no significant effect of Active versus Sedentary condition on energy intake. However, moderational analyses revealed that change in negative mood interacted with condition to predict energy intake, such that participants who reported increased negative mood during exercise consumed more calories in the Active compared to the Sedentary condition. That a short bout of exercise resulted in mood deterioration and increased energy intake for some overweight, sedentary individuals is concerning. Further research examining behavioral and physiological mechanisms of mood deterioration and caloric overcompensation following exercise in overweight, sedentary individuals is warranted.
Keywords: energy intake, exercise, obesity, mood
1.1 Introduction
Obesity is characterized by positive energy balance, a condition in which energy intake exceeds expenditure. Although heredity contributes to positive energy balance (Ravussin & Bogardus, 2000), the recent steep increase in U.S. obesity prevalence in the absence of a change in gene pool suggests that environmental and behavioral variables play a strong role (Hill & Melanson, 1999). Although associated with improved weight loss maintenance (Cowburn, Hillsdon, & Hankey, 1997; Foreyt & Goodrick, 1994; Pavlou, Krey, & Steffee, 1989; Wing, 1999), exercise without a dietary prescription is generally not effective at generating clinically meaningful weight loss (Garrow & Summerbell, 1995; Prentice & Jebb, 2004; Saris, 1995; Votruba, Horvitz, & Schoeller, 2000). The energy deficit created by exercise might not lead to appreciable weight loss if people increase their calorie intake following exercise, however the extent to which such energy compensation occurs is unclear (Titchenal, 1988).
Studies finding no increases in energy intake following exercise examined regular exercisers (Dionne, Johnson, White, St-Pierre, & Tremblay, 1997; Hubert, King, & Blundell, 1998; King, Lluch, Stubbs, & Blundell, 1997; Lluch, King, & Blundell, 1998), used small sample sizes (Durrant, Royston, & Wloch, 1982; Keim, Canty, Barbieri, & Wu, 1996; Kissileff, Pi-Sunyer, Segal, Meltzer, & Foelsch, 1990; Thompson, Wolfe, & Eikelboom, 1988; Woo, Garrow, & Pi-Sunyer, 1982), or used modestly palatable foods during the post-exercise period (Dionne et al., 1997; Kissileff et al., 1990; Lluch et al., 1998). Conversely, studies that examined intake of highly palatable, high fat foods by sedentary, overweight individuals suggest a link between exercise and increased energy intake (George & Morganstein, 2003; Pi-Sunyer, 1985; Stubbs et al., 2002). Whether such exercise-induced energy imbalance occurs and why remains unclear, but the impact of exercise on mood might play a role (Blundell & King, 1998). Palatable, high fat foods may heighten temptation to eat following exercise, particularly if negative mood is generated by the exercise bout (Stice, Akutagawa, Gaggar, & Agras, 2000).
While numerous studies substantiate the mood-enhancing effects of exercise (Arent, Lander, & Etnier, 2000; Byrne & Byrne, 1993; Penedo & Dahn, 2005; Yeung, 1996), sedentary, overweight samples have not been well studied, and these individuals may be more likely to experience exercise as fatiguing, strenuous, and difficult (Salmon, 2001). In sedentary individuals, acute exercise bouts have been found to both increase (Petruzzello, Jones, & Tate, 1997) and decrease negative mood (Dunn & McAuley, 2000), as well as both increase (Dunn & McAuley, 2000) and decrease positive mood (Boutcher, McAuley, & Courneya, 1997; Gauvin, Rejeski, Norris, & Lutes, 1997; Lochbaum, Karoly, & Landers, 2004; Petruzzello et al., 1997). Variation in the intensity and length of activity, relative to an individual’s level of fitness, may predict whether a person experiences negative or positive mood in response to exercise. For example, lean, active individuals reported worsened mood when exercise intensity exceeded their ventilatory threshold (Ekkekakis, Hall, & Petruzzello, 2004; Hall, Ekkekakis, & Petruzzello, 2002). Plausibly, overweight, sedentary individuals may experience post-exercise mood deterioration at a lower exercise intensity and shorter duration compared to sedentary, lean individuals, because they report more problems with exercise (Ball, Crawford, & Owen, 2000; Donnelly et al., 1992; Evers Larsson & Mattsson, 2001; Hulens, Vansant, Lysens, Claessens, & Muls, 2001; Mattsson, Larsson, & Rossner, 1997; Wilfley & Brownell, 1994). Some evidence supports the premise that exercise initiation can prompt mood deterioration in overweight, sedentary individuals (Ekkekakis & Lind, 2006). Those who find exercise unpleasant may be especially prone to increasing food intake following exercise in an attempt to restore more positive mood states.
Two primary hypotheses were examined in the present study. The Activity hypothesis posits that energy intake will be greater following moderate intensity exercise than following sedentary activity. The Mood hypothesis proposes that mood deterioration during moderate intensity exercise moderates the relationship between Condition (exercise or sedentary pastime) and energy intake, such that participants who experience mood deterioration from exercise will consume disproportionately more calories than participants who do not report mood deterioration.
2.1 Method
2.1.1 Recruitment and Screening
Data for the present study were collected during the baseline session of a parent study that compared different diet and physical activity prescriptions (e.g., increasing fruit/vegetables, increasing physical activity, decreasing saturated fat and decreasing sedentary activity). Both study protocols were approved by the human subjects institutional review board at the University of Illinois at Chicago and Northwestern University.
Online Screening
The primary mode of recruitment was bulk emails sent to students and staff. Interested candidates accessed a website that provided information and screening materials for the parent study. If interested, they were asked to complete a questionnaire about their activity habits. To be eligible for telephone screening, potential participants were required to be sedentary (operationalized as engaging in less than 20 minutes of moderate or vigorous intensity activity per day and at least 3 hours per day of the following sedentary leisure time activities: watching television, watching movies, playing video games and recreational computer use).
Telephone Screening
After online screening, potential participants completed a telephone screening. Interested participants were interviewed regarding study inclusion and exclusion criteria. Inclusion criteria required participants to be ages 18 to 60 and overweight or obese [body mass index (BMI) ≥ 25 and < 50 kg/m2]. Eligible participants completed an online medical history form, which was reviewed by the study physician.
Exclusion Criteria
Those with unstable medical conditions (e.g., recent myocardial infarction) or more than 3 signs/symptoms of heart disease were excluded. Participants with current or past medical conditions, muscular-skeletal problems or 1-3 signs/symptoms of heart disease were required to obtain permission from their physician to participate. Participants were ineligible if they met DSM-IV criteria for current or past anorexia or bulimia, current substance abuse or dependence other than nicotine or if they reported active suicidal ideation or behavior. To increase generalizability, individuals who met criteria for binge eating disorder were included. Participants with allergies to the food ingredients or who were dieting or using appetite suppressants were excluded. Participants who could not read the questionnaires and who were pregnant or lactating were ineligible. Peri-menopausal women were excluded because of the inability to control for changes in mood due to irregular menstrual cycles.
Participants
The sample of 65 participants was 66.2% female, had a mean age of 34.43 (SD=10.76) and an average BMI of 33.54 (SD=5.53). The sample was diverse with 38.5% African-American, 35.4% Caucasian, 12.3% Hispanic, 6.2% Asian-American and 3.1% Multi-ethnic (4.5% were missing ethnicity data). Participants were well-educated: 10.8% completed some graduate school and 23.0% had a graduate degree. The majority (30.8%) had a bachelor’s degree, 27.7% completed some college, 4.6% had an associate’s degree, and 3.1% had a high school diploma only.
Experimental Session
Eligible participants were scheduled for the experimental session. Menstruating women were scheduled between days 7-21 of their menstrual cycle to minimize hormonal effects on mood and appetite. Sessions were scheduled between meals (i.e., either between breakfast and lunch or between lunch and dinner). Participants were instructed not to eat two hours prior to the session, but not to fast for more than four hours prior to the session so that participants would not exercise on a full or empty stomach and limited the possibility that participants would become satiated during the first food exposure.
Written consent was obtained. Height and weight were assessed without shoes on a balance beam scale. Participants completed baseline measurements of mood, blood pressure, and heart rate and then began their first randomly assigned activity (Active or Sedentary). After the 3-minute activity, the experimenter administered the mood questionnaire, asking participants to rate how they felt during the activity and measured blood pressure, heart rate and perceived exertion. Ten minutes after the activity, participants were asked to complete a taste test. Although some research suggests that exercise produces brief appetite suppression, such effects have been shown to dissipate within 15 minutes (King, Burley, & Blundell, 1994). Participants were presented with two 800 kilocalorie portions of cookies and chips (1600 kcal total) with instructions to eat as much as desired because the excess will be thrown away. Prior to eating, participants rated their hunger. Participants were left alone during the food presentation and had 15 minutes to eat and then rate the cookies and chips on palatability. Two different brands of cookies and chips, all with similar nutritional content, were equally distributed across the two conditions. Cookies and chips were used because they have been identified as highly craved foods (Rodin, Mancuso, Granger, & Nelbach, 1991; Weingarten & Elston, 1991). Bottled water was also provided.
Participants were offered different explanations for the activities and the food presentation to prevent them from guessing the study hypotheses. For the parent study, participants spend two four-hour sessions in a recreational laboratory, during which they engage in exercise and sedentary activity and consume different foods. Participants were informed that their participation in exercise and sedentary activity was for the purpose of familiarizing them with the recreational laboratory equipment for the parent study. Participants were also informed that their ratings of chips and cookies were to help determine the foods they would be offered in the dietary component of the parent study. To further increase the participants’ belief that the activities and the food presentations were unrelated, participants completed visual analogue scales of palatability for the food.
At least 2 hours separated the first condition from the second condition. After completing the first condition, participants underwent an interview to gather information and provide training for the parent study. Participants completed the Marlowe-Crown Social Desirability Scale at the end of the experimental session to avoid the possibility that completing this questionnaire might bias food consumption or mood ratings.
Sedentary activity condition (Sedentary)
During the Sedentary condition, participants sat and watched a 3-minute clip from a nature video. To mirror the Active condition, participants had their heart rate assessed halfway through the sedentary activity. They completed the Borg scale after the sedentary activity so that they were not distracted from the video clip.
Exercise condition (Active)
During the Active condition participants completed the Dundee Step Test (P. Lim, Shiels, Anderson, & MacDonald, 1999), a 3-minute task during which participants step up and down on a 17.5 cm. bench (roughly 7 inches) for 3 minutes at a rate of 92 steps per minute. Step tests have been used in previous studies of exercise and mood (Hayakawa, Miki, Takada, & Tanaka, 2000; Turnbull & Wolfson, 2002), including those involving sedentary, overweight individuals (P. O. Lim, Shiels, & MacDonald, 1998). A metronome was used to pace participants and a nature video was also shown to ensure that the only difference between the Active and Sedentary conditions was the engagement in moderate intensity exercise.
To ensure that participants stepped at a moderate pace, they wore a heart rate monitor to establish that they were not exercising at a level greater than 55% to 69% of their estimated maximum heart rate. Estimated maximum heart rate was calculated using the formula 220 – age (Fox, Naughton, & Haskell, 1971). Although all participants began stepping at the same pace, halfway through the step test, heart rate and perceived exertion (Borg scale) were assessed and participants were instructed to adjust their stepping speed to maintain a moderate pace. Those with a heart rate greater than 69% maximum heart rate were asked to decrease their stepping pace to 88 steps per minute. Participants with a maximum heart rate less than 55% maximum heart rate were asked to increase their stepping pace to 98 steps per minute. If participants reported a Borg rating greater than moderate intensity, they decreased their stepping pace to 88 steps per minute, regardless of their heart rate. If the Borg rating was lower than moderate intensity, participants only increased their step pace if their heart rate was also lower than moderate intensity. In both conditions, participants retrospectively rated their perceived exertion, yielding parallel measurement for the two conditions.
2.1.2 Measures
Physical and Sedentary Activity
The online screening included a revised version of the Physical Activity Recall Questionnaire (PAR-Q) to assess usual activity output (Blair et al., 1985). The PAR-Q is an 8-item questionnaire with adequate validity to assess moderate and vigorous activity for the past 3 months (Blair et al., 1985). The revised questionnaire inquired about time spent in leisure time sedentary activity (i.e., watching television or movies, playing videogames and leisure time computer use).
Body Mass Index
Body mass index (BMI) was calculated using the formula: (weight in pounds/(height in inches)2) × 704.5. At the experimental session, a standard balance beam scale was used to record the participants’ weight and height without shoes.
Social Desirability
The Marlowe-Crowne Social Desirability Scale was used to control for the possibility that participants might respond in a manner that reflects their desire to give socially desirable answers (Crowne & Marlowe, 1960). Developed as a vehicle to assess social desirability independent of psychopathology, the Marlowe-Crowne Social Desirability Scale consists of 33 true/false questions. High internal reliability and construct validity have been reported (Crowne & Marlowe, 1960). Participants completed this scale at the end of the session. In the present study, α=.65.
Craving Questionnaire
Participants completed a craving questionnaire that asked participants to rate the extent to which they have the urge to participate in a variety of physical and sedentary activities (e.g., stair climbing, eating). In this study, α=.89.
Heart Rate Monitoring
During each activity, participants wore a heart rate monitoring belt (Ecko E10) on their chest. Heart rate measurements were taken before each activity, half-way through the activity and after the activity was completed.
Perceived Exertion
Half-way through the step test, participants completed the Borg Rating of Perceived Exertion Scale (Borg, 1998). Ratings between 12 and 14 are indicative of moderate intensity activity (Borg, 1998). After each activity, participants retrospectively rated their perceived exertion for comparability across conditions.
Hunger
Participants rated their hunger using a visual analogue scale (VAS) where 0 represented not at all hungry and 100 represented extremely hungry. VAS measures have demonstrated satisfactory reliability (Flint, Raben, Blundell, & Astrup, 2000).
Mood Measure
To assess the degree to which positive and negative mood influence energy intake, participants completed the Profile of Mood States (POMS) before (pre-activity) and after engaging in the sedentary and physical activities (post-activity). For the post-activity rating, participants were asked to retrospectively recall how they felt during the activity, to avoid distracting participants during the activity. The POMS is a 65-item self-report questionnaire containing adjectives rated on 5-point scales ranging from 1 (not at all) to 5 (extremely) (McNair, Lorr, & Droppleman, 1971). The POMS consists of 6 factor-analytically derived subscales reflecting vigor, anger, depression, confusion, fatigue and tension. Internal consistencies for the subscales are in the range of .90 and the subscales demonstrate adequate construct and predictive validity (McNair et al., 1971). The vigor subscale represented positive mood (PM) and a composite distress subscale consisting of the anger, depression, confusion, fatigue and tension subscales served as a measure of negative mood (NM). Difference scores were calculated by subtracting the pre-activity mood scale from the post-activity mood scale. Thus, positive scores represent an increase in mood (positive or negative), while negative scores represent a decrease in mood. For the current study, the alphas ranged from .89 to .92 for PM and from .73 to .82 for NM.
Calorie Intake
The primary outcome is calorie intake. Before presenting the food and after the 15 minute consumption period, food was weighed using a digital scale to calculate energy intake. The weight of the food container was subtracted from the total weight, and the pre-consumption food weight and post-consumption weight were converted from grams to kilocalories. Energy intake was calculated by subtracting the post-consumption food weight from the pre-consumption weight.
2.1.3 Preliminary Analyses
The possible covariates, moderator variables and dependent variable were examined to determine whether they violated the assumption of normality. With the exception of energy intake, all primary study variables demonstrated acceptable skew and kurtosis. Energy intake in the Sedentary and Active conditions showed elevated kurtosis values, which improved with a square root transformation (kurtosis range: 0.63-0.86). Thus, the square root energy intake variable was used in all analyses.
To ensure that the Active condition induced greater moderate intensity activity compared to the Sedentary condition, paired samples t-tests were conducted on Borg scores and heart rate. Paired samples t-tests revealed significant differences in Borg scores, t(63)=24.01, p<.001, and heart rate, t(57)=4.63, p<.0012, such that participants reported higher Borg scores during the Active condition (M=11.61, SD=1.72) and experienced higher heart rates (M=91.25, SD=25.44), compared to during the Sedentary condition (M=6.19, SD=0.69 and M=75.29, SD=11.78, respectively).
Since the participant’s liking for the presented foods and hunger could influence their energy intake, paired samples t-tests were conducted to determine whether the two conditions significantly differed on palatability and hunger ratings. Prior to analyzing the palatability data, the chip and cookie palatability ratings were summed to create one rating for palatability. Paired samples t-tests revealed that palatability, t(62)=0.11, p=.92 and hunger, t(62)=0.54, p=.59, were equal between the two conditions.
2.1.4 Statistical Analyses
The Activity Hypothesis posited that participants would consume more calories following the Active condition compared to the Sedentary condition. Mixed-effects regression modeling, implemented via SAS PROC MIXED, was used to examine the effect of Condition (Active vs. Sedentary) on energy intake. The model included a random intercept trend. Gender, activity order, hunger (time varying) and BMI were included as covariates.
The Mood Hypothesis proposed that mood change from pre- to post-activity would interact with Condition to predict energy intake. Mixed-effects regression was used to examine whether the Condition by Mood Change interaction predicted energy intake, with separate analyses conducted for positive mood (PM) and negative mood (NM) change. The main effects of Condition and Mood Change were included in the model, along with the interaction term (Condition*Mood Change). Gender, activity order, BMI, social desirability and hunger (time varying) were included as covariates. For significant effects, means and standard deviations were used to calculate an effect size, such that 0.2 indicates a small effect, 0.5 a medium effect and 0.8 a large effect (Cohen, 1992).
3.1 Results
Contrary to the Activity Hypothesis, Condition did not predict energy intake, t(61)=-0.49, p=.63. No differences were observed in energy intake between the Active and Sedentary conditions. Hunger was the only covariate that significantly predicted energy intake, t(61)=3.64, p<.001, with greater hunger ratings predicting greater energy intake.
The test of the Mood Hypothesis revealed a non-significant Condition × PM Change interaction, t(57)=0.99, p=.33, and a significant Condition × NM Change interaction, t(57)=-2.49, p=.02 [Table 1]. To examine the simple effects, two levels were created for NM, such that participants whose NM increased by 2 or more during the Active condition were categorized as “increased NM” (n=24) and remaining participants were categorized as “no change/decreased NM” (n=41). Mixed-effects regression was conducted for each level of NM, comparing mean caloric consumption across the two conditions, and including the same covariates as the overall model. As hypothesized, increased NM participants consumed more calories in the Active condition compared to the Sedentary condition, t(20)=-2.44, p=.02 [Table 2]. Cohen’s d for the differences in energy intake equaled 0.43. The increased NM group consumed an average of 68.75 more kilocalories (0.29 mj) during the Active condition compared to the no change/decreased NM group [Figure 1]. The consumption of no change/decreased NM participants did not differ significantly between the two conditions, t(38)=1.22, p=.23.
Table 1.
Condition × Negative Mood Change effect on energy intake (N=65).
| Estimate | Standard error | t | p | |
|---|---|---|---|---|
| Intercept | 17.51 | 4.03 | 4.35 | <.001 |
| Gender | 0.11 | 1.27 | 0.09 | .93 |
| Body mass index | 0.02 | 0.12 | 0.18 | .86 |
| Hunger | 0.06 | 0.02 | 3.41 | .001 |
| Condition order | -1.07 | 1.20 | -0.89 | .38 |
| Social desirability | -0.24 | 0.14 | -1.65 | .10 |
| Condition | -0.84 | 0.70 | -1.20 | .24 |
| Negative Mood | 0.20 | 0.08 | 2.50 | .02 |
| Condition × Negative Mood | -0.27 | 0.11 | -2.49 | .02 |
Table 2.
Simple effect analysis of Condition on energy intake for participants who experience increased negative mood during the Active condition (n=24).
| Estimate | Standard error | t | p | |
|---|---|---|---|---|
| Intercept | 20.46 | 7.57 | 2.70 | .01 |
| Gender | -0.86 | 2.40 | -0.36 | .72 |
| Body mass index | -0.04 | 0.22 | -0.16 | .88 |
| Hunger | 0.07 | 0.03 | 2.23 | .04 |
| Condition order | -0.90 | 2.36 | -0.38 | .71 |
| Social desirability | -0.19 | 0.28 | -0.69 | .50 |
| Condition | -2.74 | 1.12 | -2.44 | .02 |
Figure 1.
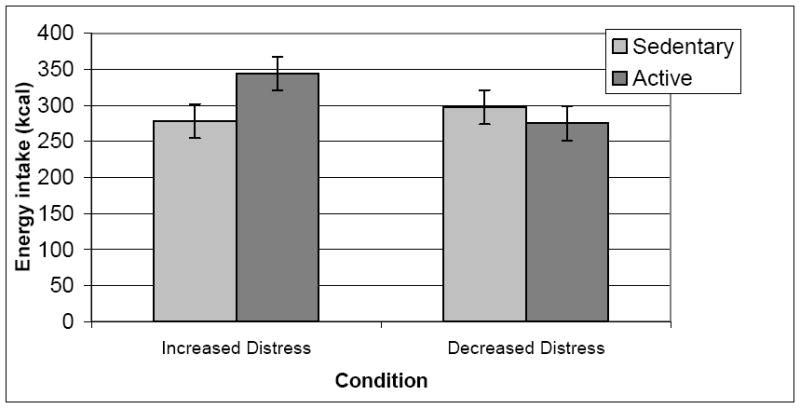
Condition × Negative Mood Change interaction predicting energy intake (N=65).
Note. Actual kilocalorie values were used instead of the square root transformed variableto ease interpretation.
Exploratory analyses were conducted to understand whether the increased NM group differed from the no change/decreased NM mood on variables that could explain the greater energy intake during the Active condition in the increased NM group. Independent samples t-tests examined whether participants who experienced increased NM differed from participants who did not on consumption-related variables (hunger, palatability, BMI, eating craving) and activity-related variables (perceived exertion, heart rate). T-tests revealed no differences between those who did and did not experience increased NM on ratings of perceived exertion for the Active condition, t(63)=0.89, p=.38, heart rate after the Active condition, t(57)=0.34, p=.74, hunger ratings after the Active condition, t(62)=0.56, p=.58, or palatability ratings after the Active condition, t(62)=-0.15, p=.88. However, the increased NM group had greater BMIs, t(63)=2.49, p=.02 (M=34.69, SD=5.70) and endorsed greater craving for eating, t(62)=2.09, p=.04; (M=7.00, SD=1.48) compared to participants who did not report increased NM (M=31.29, SD=5.08 and M=6.05, SD=2.16, respectively). Cohen’s d for the differences in BMI equaled 0.63 and Cohen’s d for differences in craving for eating equaled 0.51.
4.1 Discussion
Contrary to the Activity Hypothesis, participants did not consume more calories in the Active condition compared to the Sedentary condition. However, consistent with the Mood Hypothesis, change in NM interacted with Condition to predict energy intake. Participants who experienced increased NM in the Active condition consumed significantly more calories in the Active condition versus the Sedentary condition, but no differences in consumption were found in participants who did not experience increased NM. Exploratory analyses suggested that while participants who experienced increased NM weighed more, they were not working harder as evidenced by no differences in their subjective (perceived exertion) and objective (heart rate) measures of exertion, compared to participants who did not experience increased NM. Participants who reported increased NM also endorsed greater food craving.
Results may help to explain contradictory findings regarding the occurrence of increased energy intake after exercise in sedentary, overweight individuals. Present findings suggest that there is an individual difference factor, mood change during exercise, which moderates the effect of exercise on energy intake. Results also lend support to the hypothesis that some sedentary, overweight individuals experience mood deterioration while exercising (Van Landuyt, Ekkekakis, Hall, & Petruzzello, 2000), and such individuals are prone to overeat after exercising.
For participants who reported mood deterioration in the Active condition, the difference in energy intake between the two conditions was modest. However, the average calories consumed more than negated the amount of calories expended during the step test, indicating a pattern of overcompensation that would, if sustained, result in weight gain. Recent guidelines recommend 60 minutes of moderate intensity activity per day to achieve weight loss (Department of Health and Human Services (HHS) & Department of Agriculture (USDA), 2005). If participants who report mood deterioration during exercise increased their activity to recommended levels, and their energy intake increased at a rate proportional to the current study, weight gain would be more likely than weight loss. However, mood deterioration might prevent the exercise habit from developing.
That mood deterioration occurred for a proportion of participants during a 3 minute step exercise is quite striking. Research suggests that mood enhancement from exercise is an important contributor to exercise adherence (Courneya et al., 2004; McAuley, Jerome, Elavsky, Marquez, & Ramsey, 2003). Overweight, sedentary individuals who experience mood deterioration during exercise may be less likely to adopt and adhere to an exercise routine. Although exercise may initially result in mood deterioration, mood benefits from exercise have been found to accrue over time (Matsouka, Kabitsis, Harahousou, & Trigonis, 2005), although this has not been consistently demonstrated in the overweight (Nieman, Custer, Butterworth, Utter, & Henson, 2000). Understanding what factors predict enhanced mood effects from exercise over time may be critical to increasing exercise adherence in overweight, sedentary individuals. Kiviniemi and colleagues (2007) suggest that interventions targeting mood responses to exercise might improve exercise engagement.
As in all research, this study contains limitations. Like a number of studies examining relationships between exercise and energy intake (Durrant et al., 1982; Verger, Lanteaume, & Louis-Sylvestre, 1992), this research was conducted in a laboratory and thus may not reflect actual behavior occurring outside the laboratory. However, the laboratory setting allowed direct observation of exercise and intake, eliminating the need to rely on less valid self-report measures. That participants were offered only high fat, high calorie foods could be a limitation. Patterns of intake may have been different if other types of foods or a wider variety were offered. However, greater food variety has been linked to greater energy intake (Raynor & Epstein, 2001). That participants completed both conditions in relatively close temporal proximity may have resulted in carry over satiation from the first to the second food presentation. To offset this effect, participants were asked to fast 2 hours prior to the first condition, at least 2 hours separated the conditions, condition order was counterbalanced, condition order did not significantly predict energy intake, and hunger ratings, comparable between conditions, were used to control for any possible influence of satiety on intake. Offering participants bottled water and not controlling for consumption of water may also have affected the participant’s energy intake. Although, water consumption was not measured or controlled for statistically, the effect of water consumption on energy intake is likely minimal as consumption 30 minutes prior to a meal (Davy, Dennis, Dengo, Wilson & Davy, 2008; Van Walleghen, Orr, Gentile & Davy, 2007), not during a meal (Rolls, Bell & Thorwart, 1999) has been found to influence energy intake.
The short length of the activity might explain the null finding for the Activity hypothesis. Other studies that have reported increased energy intake following exercise typically used bouts of 20 minutes or more. Furthermore, the use of a single type of exercise limits the generalizability of findings to other types of exercise. However, research has observed increased energy intake after walking (George & Morganstein, 2003; Visona & George, 2002), cycling (Almeras, Lavallee, Despres, Bouchard, & Tremblay, 1995) aerobic exercise (Keim et al., 1996; Verger et al., 1992) and strength training (Keim et al., 1996). Since the calculation of maximum heart rate was merely an estimate, and the usefulness of this equation has been questioned (Rohbergs & Landwehr, 2002), it is possible that participants were stepping at an intensity less than or greater than moderate intensity. To minimize this possibility, perceived exertion was also used to monitor the intensity level.
Participants may have inadvertently misreported their mood during the activities since they retrospectively rated how they felt during each activity. However, at the conclusion of exercise, participants may be more likely to retrospectively report enhanced mood, rather than mood deterioration because the activity ended. For example, Durrant, Royston & Wloch (1982) found no significant negative effects of exercise on post-exercise retrospectively reported mood, despite the fact that participants complained of pain and fatigue during exercise. Thus, the current study’s finding that 37% of participants reported mood deterioration during exercise might be conservative.
That some individuals increase energy intake following exercise may help to explain why exercise without dietary change fails to successfully generate weight loss (Garrow & Summerbell, 1995; Prentice & Jebb, 2004; Saris, 1995). A recent examination of a supervised exercise intervention for weight loss found that there was variability in caloric compensation that predicted differences in the degree of weight loss (King, Hopkins, Caudwell, Stubbs, & Blundell, 2008). Although, on average, the exercise intervention generated significant weight loss, participants who lost less weight than expected, given their energy expenditure, consumed more calories compared to participants who lost the expected amount of weight (King et al., 2008). However, such findings in no way support the removal of exercise from weight loss treatment because of evidence that exercise produces greater loss of fat mass compared to dieting alone (Abdel-Hamid, 2003), improves weight loss maintenance (Cowburn et al., 1997; Foreyt & Goodrick, 1994; Pavlou et al., 1989; Wing, 1999) and prevents disease (Blair et al., 1996). Instead, results suggest that dietary monitoring may be necessary when exercise prescriptions are made in overweight, sedentary individuals who dislike exercise and are at risk for increased energy intake. A thorough understanding of the mechanisms responsible for increased energy intake after exercise in these individuals might inform approaches to limiting intake after exercise. Overweight individuals may benefit from working toward diet goals first and exercise goals second to facilitate greater dietary control when exercise is adopted. Ebbeling and Rodriguez (1999) introduced an exercise program after a diet plan in children and found weight loss and increased protein synthesis. Further research on the timing of exercise and dietary interventions is needed.
Present findings highlight the importance of monitoring mood during exercise. Initial notions about the ability of exercise to universally enhance mood are being questioned as more detailed theories about individual variability in mood responses to exercise arise (Ekkekakis et al., 2004). Ekkekakis (2003) described a model where both cognitive and physiological processes influence mood responses during exercise. For overweight, sedentary individuals, cognitive factors such as self-efficacy and social physique anxiety, might predict mood responses to exercise during exercise initiation. Future research should examine whether cognitive variables influence mood during exercise and food consumption in overweight, sedentary individuals.
The relationship between energy expenditure and intake is a complex one, affected by both behavioral and biological factors. Theories integrating psychological and physiological variables, genetics, activity characteristics, and food properties are required to better understand and explain the interplay between energy expenditure and intake. Theory testing must include overweight, sedentary individuals given the limited research with this population and emerging evidence that relationships between energy intake and expenditure differ from that of their normal weight, more active counterparts.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health to Dr. Schneider (1 F31 Mh070107-01) and from the National Heart, Lung and Blood Institute to Dr. Spring (5 R01HL075451-04).
Footnotes
One participant was missing Borg ratings and 7 participants were missing heart rate ratings, which accounts for the different degrees of freedom. Similarly, 2 participants were missing palatability and hunger ratings.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Hamid TK. Exercise and diet in obesity treatment: An integrative system dynamics perspective. Medicine and Science in Sports and Exercise. 2003;35(3):400–413. doi: 10.1249/01.MSS.0000053659.32126.2D. [DOI] [PubMed] [Google Scholar]
- Almeras N, Lavallee N, Despres JP, Bouchard C, Tremblay A. Exercise and energy intake: Effect of substrate oxidation. Physiology & Behavior. 1995;57(5):995–1000. doi: 10.1016/0031-9384(94)00360-h. [DOI] [PubMed] [Google Scholar]
- Arent SM, Lander DM, Etnier JL. The effects of exercise on mood in older adults: A meta-analytic review. Journal of Aging and Physical Activity. 2000;8:407–430. [Google Scholar]
- Ball K, Crawford D, Owen N. Too fat to exercise? Obesity as a barrier to physical activity. Australian & New Zealand Journal of Public Health. 2000;24(3):331–333. doi: 10.1111/j.1467-842x.2000.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. American Journal of Epidemiology. 1985;122(5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- Blair SN, Horton E, Leon AS, Lee IM, Drinkwater BL, Dishman RK, et al. Physical activity, nutrition, and chronic disease. Medicine and Science in Sports and Exercise. 1996;28(3):335–349. doi: 10.1097/00005768-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Blundell JE, King NA. Effects of exercise on appetite control: Loose coupling between energy expenditure and energy intake. International Journal of Obesity and Related Metabolic Disorders. 1998;22(Suppl 2):S22–29. [PubMed] [Google Scholar]
- Borg G. Perceived exertion and pain scales. Champaign, IL: Human Kinetics Publishers, Inc.; 1998. [Google Scholar]
- Boutcher SH, McAuley E, Courneya KS. Positive and negative affective response of trained and untrained subjects during and after aerobic exercise. Australian Journal of Psychology. 1997;49:28–32. [Google Scholar]
- Byrne A, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. Journal of Psychosomatic Research. 1993;37(6):565–574. doi: 10.1016/0022-3999(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Friedenreich CM, Sela RA, Quinney HA, Rhodes RE, Jones LW. Exercise motivation and adherence in cancer survivors after participation in a randomized controlled trial: An attribution theory perspective. International Journal of Behavioral Medicine. 2004;11(1):8–17. doi: 10.1207/s15327558ijbm1101_2. [DOI] [PubMed] [Google Scholar]
- Cowburn G, Hillsdon M, Hankey CR. Obesity management by life-style strategies. British Medical Bulletin. 1997;53(2):389–408. doi: 10.1093/oxfordjournals.bmb.a011619. [DOI] [PubMed] [Google Scholar]
- Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. Journal of Consulting Psychology. 1960;24:349–354. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- Davy BM, Dennis EA, Dengo AL, Wilson KL, Davy KP. Water consumption reduces energy intake at a breakfast meal in obese older adults. Journal of the American Dietetics Association. 2008;108:1236–1239. doi: 10.1016/j.jada.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services (HHS), & Department of Agriculture (USDA) Dietary guidelines for Americans. 2005 Retrieved September 19 2007, from http://www.healthierus.gov/dietaryguidelines/
- Dionne I, Johnson M, White MD, St-Pierre S, Tremblay A. Acute effect of exercise and low-fat diet on energy balance in heavy men. International Journal of Obesity and Related Metabolic Disorders. 1997;21(5):413–416. doi: 10.1038/sj.ijo.0800426. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Jacobsen DJ, Jakicic JM, Whatley J, Gunderson S, Gillespie WJ, et al. Estimation of peak oxygen consumption from a sub-maximal half mile walk in obese females. International Journal of Obesity and Related Metabolic Disorders. 1992;16(8):585–589. [PubMed] [Google Scholar]
- Dunn EC, McAuley E. Affective responses to exercise bouts of varying intensities. Journal of Social Behavior and Personality. 2000;15:201–214. [Google Scholar]
- Durrant ML, Royston JP, Wloch RT. Effect of exercise on energy intake and eating patterns in lean and obese humans. Physiology & Behavior. 1982;29(3):449–454. doi: 10.1016/0031-9384(82)90265-7. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Hall EE, Petruzzello SJ. Practical markers of the transition from aerobic to anaerobic metabolism during exercise: rationale and a case for affect-based exercise prescription. Preventive Medicine. 2004;38(2):149–159. doi: 10.1016/j.ypmed.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Ekkekakis P, Lind E. Exercise does not feel the same when you are overweight: The impact of self-selected and imposed intensity on affect and exertion. International Journal of Obesity. 2006;30(4):652–660. doi: 10.1038/sj.ijo.0803052. [DOI] [PubMed] [Google Scholar]
- Evers Larsson U, Mattsson E. Functional limitations linked to high body mass index, age and current pain in obese women. International Journal of Obesity and Related Metabolic Disorders. 2001;25(6):893–899. doi: 10.1038/sj.ijo.0801553. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. International Journal of Obesity and Related Metabolic Disorders. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- Foreyt JP, Goodrick GK. Impact on behavior therapy on weight loss. American Journal of Health Promotion. 1994;8(6):466–468. doi: 10.4278/0890-1171-8.6.466. [DOI] [PubMed] [Google Scholar]
- Fox SM, 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Annals of Clinical Research. 1971;3(6):404–432. [PubMed] [Google Scholar]
- Garrow JS, Summerbell CD. Meta-analysis: Effect of exercise, with or without dieting, on the body composition of overweight subjects. European Journal of Clinical Nutrition. 1995;49(1):1–10. [PubMed] [Google Scholar]
- Gauvin L, Rejeski JW, Norris JL, Lutes L. The curse of inactivity: Failure of acute exercise to enhance feeling states in a community sample of sedentary adults. Journal of Health Psychology. 1997;2:509–523. doi: 10.1177/135910539700200408. [DOI] [PubMed] [Google Scholar]
- George VA, Morganstein A. Effect of moderate intensity exercise on acute energy intake in normal and overweight females. Appetite. 2003;40(1):43–46. doi: 10.1016/s0195-6663(02)00146-0. [DOI] [PubMed] [Google Scholar]
- Hall EE, Ekkekakis P, Petruzzello SJ. The affective beneficence of vigorous exercise revisited. British Journal of Health Psychology. 2002;7(Pt 1):47–66. doi: 10.1348/135910702169358. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Miki H, Takada K, Tanaka K. Effects of music on mood during bench stepping exercise. Perceptual and Motor Skills. 2000;90(1):307–314. doi: 10.2466/pms.2000.90.1.307. [DOI] [PubMed] [Google Scholar]
- Hill JO, Melanson EL. Overview of the determinants of overweight and obesity: Current evidence and research issues. Medicine and Science in Sports and Exercise. 1999;31(11 Suppl):S515–521. doi: 10.1097/00005768-199911001-00005. [DOI] [PubMed] [Google Scholar]
- Hubert P, King NA, Blundell JE. Uncoupling the effects of energy expenditure and energy intake: Appetite response to short-term energy deficit induced by meal omission and physical activity. Appetite. 1998;31(1):9–19. doi: 10.1006/appe.1997.0148. [DOI] [PubMed] [Google Scholar]
- Hulens M, Vansant G, Lysens R, Claessens AL, Muls E. Exercise capacity in lean versus obese women. Scandinavian Journal of Medicine & Science in Sports. 2001;11(5):305–309. doi: 10.1034/j.1600-0838.2001.110509.x. [DOI] [PubMed] [Google Scholar]
- Keim NL, Canty DJ, Barbieri TF, Wu MM. Effect of exercise and dietary restraint on energy intake of reduced-obese women. Appetite. 1996;26(1):55–70. doi: 10.1006/appe.1996.0005. [DOI] [PubMed] [Google Scholar]
- King NA, Burley VJ, Blundell JE. Exercise-induced suppression of appetite: Effects on food intake and implications for energy balance. European Journal of Clinical Nutrition. 1994;48(10):715–724. [PubMed] [Google Scholar]
- King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Individual variability following 12 weeks of supervised exercise: Identification and characterization of compensation for exercise-induced weight loss. International Journal of Obesity. 2008;32(1):177–184. doi: 10.1038/sj.ijo.0803712. [DOI] [PubMed] [Google Scholar]
- King NA, Lluch A, Stubbs RJ, Blundell JE. High dose exercise does not increase hunger or energy intake in free living males. European Journal of Clinical Nutrition. 1997;51(7):478–483. doi: 10.1038/sj.ejcn.1600432. [DOI] [PubMed] [Google Scholar]
- Kissileff HR, Pi-Sunyer FX, Segal K, Meltzer S, Foelsch PA. Acute effects of exercise on food intake in obese and nonobese women. The American Journal of Clinical Nutrition. 1990;52(2):240–245. doi: 10.1093/ajcn/52.2.240. [DOI] [PubMed] [Google Scholar]
- Lim P, Shiels P, Anderson J, MacDonald T. Dundee step test: A simple method of measuring the blood pressure response to exercise. Journal of Human Hypertension. 1999;13(8):521–526. doi: 10.1038/sj.jhh.1000869. [DOI] [PubMed] [Google Scholar]
- Lim PO, Shiels P, MacDonald TM. The Dundee Step Test: A novel exercise test suitable for the outpatient management of hypertension. Journal of Hypertension. 1998;16(11):1701. [PubMed] [Google Scholar]
- Lluch A, King NA, Blundell JE. Exercise in dietary restrained women: No effect on energy intake but change in hedonic ratings. European Journal of Clinical Nutrition. 1998;52(4):300–307. doi: 10.1038/sj.ejcn.1600555. [DOI] [PubMed] [Google Scholar]
- Lochbaum MR, Karoly P, Landers DM. Affect responses to acute bouts of aerobic exercise: A test of opponent-process theory. Journal of Sport Behavior. 2004;27(4):330–348. [Google Scholar]
- Matsouka O, Kabitsis C, Harahousou Y, Trigonis I. Mood alterations following an indoor and outdoor exercise program in healthy elderly women. Perceptual and Motor Skills. 2005;100(3 Pt 1):707–715. doi: 10.2466/pms.100.3.707-715. [DOI] [PubMed] [Google Scholar]
- Mattsson E, Larsson UE, Rossner S. Is walking for exercise too exhausting for obese women? International Journal of Obesity and Related Metabolic Disorders. 1997;21(5):380–386. doi: 10.1038/sj.ijo.0800417. [DOI] [PubMed] [Google Scholar]
- McAuley E, Jerome GJ, Elavsky S, Marquez DX, Ramsey SN. Predicting long-term maintenance of physical activity in older adults. Preventive Medicine. 2003;37(2):110–118. doi: 10.1016/s0091-7435(03)00089-6. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of mood states manual. San Diego, CA: Education and Industrial Testing Service; 1971. [Google Scholar]
- Nieman DC, Custer WF, Butterworth DE, Utter AC, Henson DA. Psychological response to exercise training and/or energy restriction in obese women. Journal of Psychosomatic Research. 2000;48(1):23–29. doi: 10.1016/s0022-3999(99)00066-5. [DOI] [PubMed] [Google Scholar]
- Pavlou KN, Krey S, Steffee WP. Exercise as an adjunct to weight loss and maintenance in moderately obese subjects. American Journal of Clinical Nutrition. 1989;49(5 Suppl):1115–1123. doi: 10.1093/ajcn/49.5.1115. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry. 2005;18(2):189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Petruzzello SJ, Jones AC, Tate AK. Affective responses to acute exercise: A test of opponent-process theory. Journal of Sports Medicine and Physical Fitness. 1997;37(3):205–212. [PubMed] [Google Scholar]
- Pi-Sunyer FX. Effect of exercise on food intake. In: Hirsch J, Van Itallie TB, editors. Recent Advances in Obesity Research: Proceedings of the 4th International Congress on Obesity. London: John Libbey & Company Limited; 1985. [Google Scholar]
- Prentice A, Jebb S. Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nutrition Reviews. 2004;62(7 Pt 2):S98–104. doi: 10.1111/j.1753-4887.2004.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Bogardus C. Energy balance and weight regulation: Genetics versus environment. The British Journal of Nutrition. 2000;83(Suppl 1):S17–20. doi: 10.1017/s0007114500000908. [DOI] [PubMed] [Google Scholar]
- Raynor HA, Epstein LH. Dietary variety, energy regulation, and obesity. Psychological Bulletin. 2001;127(3):325–341. doi: 10.1037/0033-2909.127.3.325. [DOI] [PubMed] [Google Scholar]
- Rodin J, Mancuso J, Granger J, Nelbach E. Food cravings in relation to body mass index, restraint and estradiol levels: A repeated measures study in healthy women. Appetite. 1991;17(3):177–185. doi: 10.1016/0195-6663(91)90020-s. [DOI] [PubMed] [Google Scholar]
- Rohbergs RA, Landwehr R. The surprising history of the “HRmax=220-age” equation. Journal of Exercise Physiology. 2002;5:1–10. [Google Scholar]
- Rolls BJ, Bell EA, Thorwart ML. Water incorporated into a food but not served with a food decreases energy intake in lean women. American Journal of Clinical Nutrition. 1999;70:448–55. doi: 10.1093/ajcn/70.4.448. [DOI] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clinical Psychology Review. 2001;21(1):33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Saris WH. Exercise with or without dietary restriction and obesity treatment. International Journal of Obesity and Related Metabolic Disorders. 1995;19(Suppl 4):S113–116. [PubMed] [Google Scholar]
- Stice E, Akutagawa D, Gaggar A, Agras WS. Negative affect moderates the relation between dieting and binge eating. The International Journal of Eating Disorders. 2000;27(2):218–229. doi: 10.1002/(sici)1098-108x(200003)27:2<218::aid-eat10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Stubbs RJ, Sepp A, Hughes DA, Johnstone AM, King N, Horgan G, et al. The effect of graded levels of exercise on energy intake and balance in free-living women. International Journal of Obesity and Related Metabolic Disorders. 2002;26(6):866–869. doi: 10.1038/sj.ijo.0801874. [DOI] [PubMed] [Google Scholar]
- Thompson DA, Wolfe LA, Eikelboom R. Acute effects of exercise intensity on appetite in young men. Medicine and Science in Sports and Exercise. 1988;20(3):222–227. doi: 10.1249/00005768-198806000-00002. [DOI] [PubMed] [Google Scholar]
- Titchenal CA. Exercise and food intake. What is the relationship? Sports Medicine. 1988;6(3):135–145. doi: 10.2165/00007256-198806030-00002. [DOI] [PubMed] [Google Scholar]
- Turnbull M, Wolfson S. Effects of exercise and outcome feedback on mood evidence for misattribution. Journal of Sport Behavior. 2002;25:394–406. [Google Scholar]
- Van Landuyt LM, Ekkekakis P, Hall EE, Petruzzello SJ. Throwing the mountains into the lake: On the perils of nomothetic conceptions of the exercise-affect relationship. Journal of Sport & Exercise Psychology. 2000;22(3):208–234. [Google Scholar]
- Van Walleghen E, Orr JS, Gentile CL, Davy BM. Energy intake in older but not younger subjects. Obesity. 2007;15:93–99. doi: 10.1038/oby.2007.506. [DOI] [PubMed] [Google Scholar]
- Verger P, Lanteaume MT, Louis-Sylvestre J. Human intake and choice of foods at intervals after exercise. Appetite. 1992;18(2):93–99. doi: 10.1016/0195-6663(92)90186-a. [DOI] [PubMed] [Google Scholar]
- Visona C, George VA. Impact of dieting status and dietary restraint on postexercise energy intake in overweight women. Obesity Research. 2002;10(12):1251–1258. doi: 10.1038/oby.2002.170. [DOI] [PubMed] [Google Scholar]
- Votruba SB, Horvitz MA, Schoeller DA. The role of exercise in the treatment of obesity. Nutrition. 2000;16(3):179–188. doi: 10.1016/s0899-9007(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Weingarten HP, Elston D. Food cravings in a college population. Appetite. 1991;17(3):167–175. doi: 10.1016/0195-6663(91)90019-o. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Brownell KD. Physical activity and diet in weight loss. In: Dishman RK, editor. Advances in Exercise Adherence. Champaign, IL: Human Kinetics Publishers, Inc.; 1994. pp. 351–383. [Google Scholar]
- Wing RR. Physical activity in the treatment of the adulthood overweight and obesity: Current evidence and research issues. Medicine and Science in Sports and Exercise. 1999;31(11 Suppl):S547–552. doi: 10.1097/00005768-199911001-00010. [DOI] [PubMed] [Google Scholar]
- Woo R, Garrow JS, Pi-Sunyer FX. Effect of exercise on spontaneous calorie intake in obesity. The American Journal of Clinical Nutrition. 1982;36(3):470–477. doi: 10.1093/ajcn/36.3.470. [DOI] [PubMed] [Google Scholar]
- Yeung RR. The acute effects of exercise on mood state. Journal of Psychosomatic Research. 1996;40(2):123–141. doi: 10.1016/0022-3999(95)00554-4. [DOI] [PubMed] [Google Scholar]


