ABSTRACT
We report three cases of hydatid disease of the skull base and the treatment thereof. The first involved the anterior cranial fossa and paranasal sinuses. The second was located in the infratemporal fossa. The last involved the temporal bone and posterior cranial fossa. Hydatid disease is endemic in many parts of the world where livestock farming is practiced and is highly endemic in sub-Saharan Africa. Although hydatid disease of the head and neck is rare, it should be considered in the differential diagnosis of cystic disease in the head and neck region. Of the three forms of hydatid disease, Echinococcus granulosis is most common and gives rise to cystic hydatid disease. Most hydatid cysts are “silent,” but become clinically apparent because of their mass effects, when they rupture, or if they become superinfected. Computed tomography scanning and magnetic resonance imaging are the best diagnostic tools. Hydatid disease can be successfully treated by a combination of surgery and chemotherapy.
Keywords: Hydatid disease, skull base, extradural, sinusitis, infratemporal fossa
Hydatid disease of the head and neck is rare.1,2,3 It accounts for only two out of every thousand cases of hydatid disease.4 Most hydatid cysts are “silent,” but become clinically apparent because of their mass effects, when they rupture, or if they become superinfected.5 Hydatid disease is a worldwide parasitic disease, particularly in areas where livestock and subsistence farming are practiced.6,7 Echinococcus infection may cause serious morbidity and even death in the intermediate host.8 It is an endemic disease in many parts of the world including the Mediterranean, the Middle East, South America, Australasia, and is highly endemic in sub-Saharan Africa.9
Of the three forms of hydatid disease, Echinococcus granulosis is most common and gives rise to cystic hydatid disease. Echinococcus granulosis is a small tapeworm, 3 to 5 mm in length, comprising three to four segments. It lives in the small intestine of the definitive host, the dog. The oldest segments at the end of the worms produce eggs, which are excreted in feces. Intermediate hosts ingest contaminated feces. Humans are accidental intermediate hosts. The eggs hatch in the intestine of the intermediate host and the embryo migrates through the intestinal wall into the portal system. Most embryos (65%) lodge in the liver. Some (25%) escape this first filter and lodge in the lung. A small percentage escape to the systemic circulation where they may lodge in any organ, most commonly the spleen, kidneys, heart, bone, muscle, or central nervous system. The life cycle is completed when the definitive canid host eats infested offal.
We report three cases of cystic hydatid disease in the skull base. One case presented with complicated sinusitis, which to our knowledge has not previously been reported in the literature. One occurred in a patient infected with human immunodeficiency virus (HIV), which to our knowledge has been reported only once in the literature, also from South Africa.10
CASE 1
An 8-year-old boy was referred to our otolaryngology service with a 4-week history of swelling of the right eye with associated headaches and a purulent nasal discharge. He had no other significant medical history. Initially he had sought treatment at the local clinic and was treated with oral amoxicillin 250 mg three times daily for 1 week. He relapsed after 10 days and was admitted to a rural secondary hospital with a swinging fever, proptosis, and nasal discharge, and was treated with intravenous cloxacillin, gentamicin, and metronidazole. During this time he had a single seizure, which was treated with phenobarbitone. There was a poor clinical response after 1 week's treatment and he was referred to our hospital. On examination, the child was lethargic, pyrexial (38°C), and had right-sided periorbital hyperpigmentation, edema, and mild proptosis. Vision, eye movements, and pupillary reflexes were normal. There was a 1 × 1-cm firm, tender swelling arising from the superomedial orbital rim. A small amount of purulent material was visible in the right middle meatus of the nose. The clinical picture was consistent with complicated sinusitis with periorbital involvement.
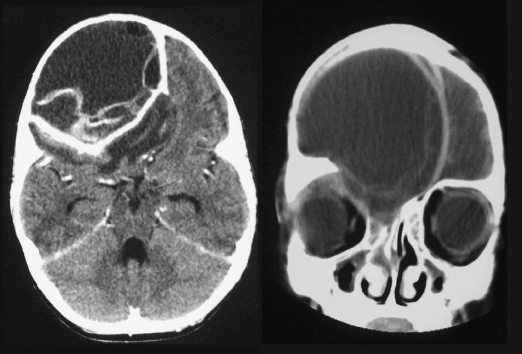
Computed tomography (CT) scans revealed a large, cystic mass in the anterior cranial fossa with surrounding cerebral edema. Bony remodeling of the cranium was evident, suggesting this was an acute-on-chronic event. Based on the CT findings, a presumptive diagnosis of a superinfected hydatid cyst was made (Fig. 1).
Figure 1.
Case 1: Cystic hydatid disease of anterior cranial fossa.
The patient was begun on albendazole 250 mg twice a day and the phenobarbitone dosage was increased to 30 mg twice a day. A small craniectomy was done via a brow incision, and free drainage of pus was encountered on entering an extradural cyst. The cyst and its contents were removed completely. Following surgery, the patient made a rapid and uneventful recovery. The patient was discharged on day 10 on albendazole. He remains well after 18 months with no obvious sequelae.
CASE 2
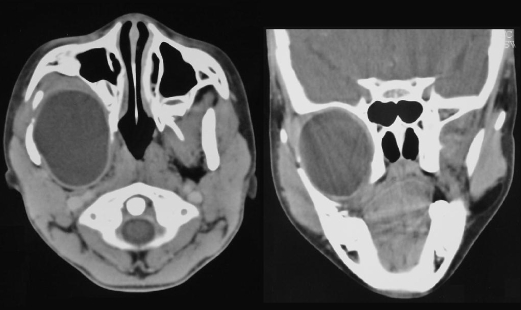
An 8-year-old boy presented with a 4-month history of painless right facial swelling. There was no other medical history of note. The child appeared generally well. The right mandibular ramus was displaced laterally, but there was normal dental occlusion and no trismus. The right tonsil was slightly displaced toward the midline, and sensation was reduced in the distributions of the maxillary and mandibular divisions of the trigeminal nerve. Computed tomography scans revealed a cystic mass in the infratemporal fossa, consistent with a diagnosis of cystic hydatid disease (Fig. 2).
Figure 2.
Case 2: Hydatid cyst in infratemporal fossa.
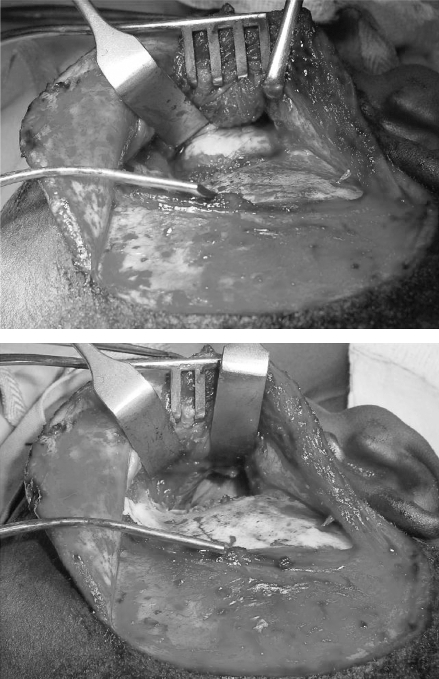
The patient was begun on albendazole 200 mg twice a day, and the cyst was completely enucleated via a preauricular infratemporal fossa approach (Fig. 3). The postoperative course was unremarkable and he was discharged on the sixth postoperative day on albendazole treatment for 6 months.
Figure 3.
Case 2: Infratemporal exposure (top) and resection (bottom) of cyst.
CASE 3
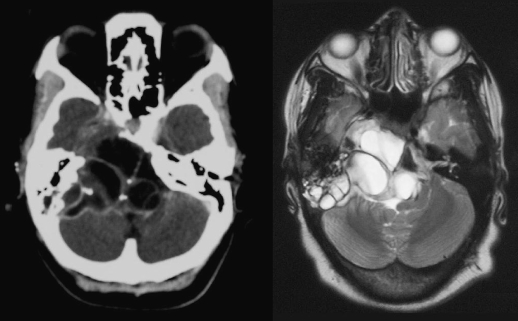
A 25-year-old HIV-positive female, not on antiretroviral therapy, presented to the neurosurgery department with a 3-month history of progressive weakness of the legs, the left arm, and the right face. She did not complain of headache. She had previously been treated for pulmonary tuberculosis. Her Glasgow Coma Scale was 15/15. She had complete right facial nerve paralysis. Power was reduced in the left arm and the lower limbs bilaterally. Plantar reflexes were up going bilaterally. A mass was visible in the inferior quadrant of the middle ear on the left side, medial to an intact tympanic membrane. She had mixed sensorineural and conductive hearing loss in the same ear. Computed tomography scan of the head revealed a multicystic lesion involving the left temporal bone and the posterior cranial fossa, causing obstructive hydrocephalus (Fig. 4). A diagnosis of cystic hydatid disease was made on the basis of the CT scan findings.
Figure 4.
Case 3: Cystic hydatid disease of temporal bone and posterior cranial fossa.
The patient was started on albendazole. Mastoidectomy was performed via a postauricular approach and a huge cystic, multiloculated mass filling the mastoid air-cell system was found, which was consistent with cystic hydatid disease. The hydatid cysts were partially resected, owing to difficulty freeing the cysts from the dura. The remaining cysts were irrigated with 40 mL of hypertonic saline. The patient made a full neurological recovery.
DISCUSSION
Hydatid cysts have been reported in a variety of sites in the head and neck region including orbit, thyroid gland, parotid gland, superficial temporal muscles, paraspinal muscles, cervical soft tissues, sternocleidomastoid muscle, retropharyngeal tissues, submandibular gland, tongue, mastoid, infratemporal fossa, pterygopalatine fossa, and posterior cranial fossa.4 They do not appear to have a predilection for any particular site. Only 2% of the hydatid cysts are located in the skeleton and of these only 3 to 4% are in the skull.11 Because they are so rare in the head and neck region, hydatid cysts are usually not considered in the differential diagnosis of cystic masses in the head and neck, especially in the absence of cysts elsewhere.3
Intracranial extracerebral hydatid disease may occur in three forms: cranial, cranial-extradural, and combined.11 In the cranial form, the bony spongiosa is usually the first to be involved. With the extradural form, the extradural space may be infected by embolization of scolices or embryos via blood vessels, by extrusion of intracerebral cysts via healthy dura mater, or erosion of osseus hydatid into the extradural space. Extradural hydatid disease occurs extremely rarely.2,12 With the combined form, there may be simultaneous intracerebral, extradural, and bony cysts.
Diagnosis of hydatid disease in the head and neck region can be challenging due to the complex anatomy. Serological tests have limited value in osseus hydatid disease as false-positive and false-negative tests are common,3,4 but can provide a useful confirmation of clinical infection. Computed tomography and MRI scanning are usually sufficient for making a diagnosis of hydatid disease.1 Cysts containing membranes and/or daughter cysts and debris are highly suggestive of cystic hydatid disease.13 Ultrasound is generally unsuitable in the head and neck due to the complex anatomy and surrounding bony structures. Aspiration of cyst contents may reveal crystal clear or straw-colored fluid containing scolices, membranes, and hooklets.4 Full blood count shows eosinophilia only in cases where cysts have leaked or ruptured.5 Indirect immunofluorescence assays have 95% sensitivity but are not readily available.5
Conservative management, simply by careful surveillance, is recommended for small densely calcified cysts that can be presumed to be dead.5
In hepatic hydatid disease, chemotherapy is used for cysts < 4 cm in diameter, for cysts with insignificant mass effect, and for cases that may involve destructive surgery.5 Benzimidazole carbamates, albendazole, and mebendazole are commonly used. Because of its increased absorption and bioavailability, albendazole is recommended by the World Health Organization at a dosage of 10 to 15 mg/kg/day for at least 3 months.5
Surgery remains the most effective treatment,2,14,15 and many authors combine this with long-term chemotherapy.11,12,14 Preoperative chemotherapy or intraoperative scolicides (alcohol or hypertonic saline) are recommended.2 It is crucial to avoid spillage of cyst contents, as fatal anaphylaxis has been reported.2,15 Chemotherapy should be continued postoperatively for 3 to 6 months.5
CONCLUSIONS
Hydatid disease should be included in the differential diagnosis of cystic disease in the head and neck region, especially in regions where livestock farming is practiced and hydatid disease is endemic. Computed tomography and MRI scanning are the best means to diagnose hydatid disease in the head and neck preoperatively. Complex hydatid disease of the skull base can be successfully treated by a combination of surgery and chemotherapy.
REFERENCES
- Adaletli I, Yigiter R, Selcuk D, Sirikci A, Senyuz O F. Primary hydatid cyst of the head and neck diagnosed with ultrasound and computed tomography: a report of two cases. South Med J. 2005;98:830–832. doi: 10.1097/01.smj.0000170732.24324.ea. [DOI] [PubMed] [Google Scholar]
- Sennaroglu L, Onercij M, Turan E, Sungur A. Infratemporal hydatid cyst—unusual location of echinococcosis. J Laryngol Otol. 1994;108:601–603. doi: 10.1017/s0022215100127562. [DOI] [PubMed] [Google Scholar]
- Gangopadhyay K, Abuzeid M O, Kfoury H. Hydatid cyst of the pterygopalatine-infratemporal fossa. J Laryngol Otol. 1996;110:978–980. doi: 10.1017/s0022215100135509. [DOI] [PubMed] [Google Scholar]
- El Kohen A, Benjelloun A, El Quessar A, et al. Multiple hydatid cysts of the neck, the nasopharynx and the skull base revealing cervical vertebral hydatid disease. Int J Pediatr Otorhinolaryngol. 2003;67:655–662. doi: 10.1016/s0165-5876(03)00059-4. [DOI] [PubMed] [Google Scholar]
- Shaw J M, Bornman P C, Krige J E. Hydatid disease of the liver. S Afr J Surg. 2006;44:70–72, 74–77. [PubMed] [Google Scholar]
- Moro P L, Schantz P M. Echinococcosis: historical landmarks and progress in research and control. Ann Trop Med Parasitol. 2006;100:703–714. doi: 10.1179/136485906X112257. [DOI] [PubMed] [Google Scholar]
- Craig P S, McManus D P, Lightowlers M W, et al. Prevention and control of cystic echinococcosis. Lancet Infect Dis. 2007;7:385–394. doi: 10.1016/S1473-3099(07)70134-2. [DOI] [PubMed] [Google Scholar]
- Jenkins D J, Romig T, Thompson R C. Emergence/re-emergence of Echinococcus spp.–a global update. Int J Parasitol. 2005;35(11–12):1205–1219. doi: 10.1016/j.ijpara.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Magambo J, Njoroge E, Zeyhle E. Epidemiology and control of echinococcosis in sub-Saharan Africa. Parasitol Int. 2006;55(Suppl):S193–S195. doi: 10.1016/j.parint.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Smedema E, Mayosi B M, Smedema J P. Hydatid disease—the “water lily” sign. S Afr Med J. 2006;96:1042. [PubMed] [Google Scholar]
- Behari S, Banerji D, Phadke R V, et al. Multiple infected extradural parasellar hydatid cysts. Surg Neurol. 1997;48:53–57. doi: 10.1016/s0090-3019(97)85702-3. [DOI] [PubMed] [Google Scholar]
- Beskonakli E, Cayli S, Yalcinlar Y. Primary intracranial extradural hydatid cyst extending above and below the tentorium. Br J Neurosurg. 1996;10:315–316. doi: 10.1080/02688699650040223. [DOI] [PubMed] [Google Scholar]
- Kovoor J M, Thomas R D, Chandrashekhar H S, et al. Neurohydatidosis. Australas Radiol. 2007;51:406–411. doi: 10.1111/j.1440-1673.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- Pasaoglu E, Damgaci L, Tokoglu F, et al. CT findings of hydatid cyst with unusual location: infratemporal fossa. Eur Radiol. 1998;8:1570–1572. doi: 10.1007/s003300050588. [DOI] [PubMed] [Google Scholar]
- Lath R, Ratnam B G, Ranjan A. Diagnosis and treatment of multiple hydatid cysts at the craniovertebral junction. Case report. J Neurosurg Spine. 2007;6:174–177. doi: 10.3171/spi.2007.6.2.174. [DOI] [PubMed] [Google Scholar]