Abstract
Myostatin, a member of the TGF-β family, has been identified as a powerful inhibitor of muscle growth. Absence or blockade of myostatin induces massive skeletal muscle hypertrophy that is widely attributed to proliferation of the population of muscle fiber-associated satellite cells that have been identified as the principle source of new muscle tissue during growth and regeneration. Postnatal blockade of myostatin has been proposed as a basis for therapeutic strategies to combat muscle loss in genetic and acquired myopathies. But this approach, according to the accepted mechanism, would raise the threat of premature exhaustion of the pool of satellite cells and eventual failure of muscle regeneration. Here, we show that hypertrophy in the absence of myostatin involves little or no input from satellite cells. Hypertrophic fibers contain no more myonuclei or satellite cells and myostatin had no significant effect on satellite cell proliferation in vitro, while expression of myostatin receptors dropped to the limits of detectability in postnatal satellite cells. Moreover, hypertrophy of dystrophic muscle arising from myostatin blockade was achieved without any apparent enhancement of contribution of myonuclei from satellite cells. These findings contradict the accepted model of myostatin-based control of size of postnatal muscle and reorient fundamental investigations away from the mechanisms that control satellite cell proliferation and toward those that increase myonuclear domain, by modulating synthesis and turnover of structural muscle fiber proteins. It predicts too that any benefits of myostatin blockade in chronic myopathies are unlikely to impose any extra stress on the satellite cells.
Keywords: muscle growth, muscular dystrophy, TGF-beta, muscle stem cells, myonuclear domain
Loss of muscle mass and strength is a major clinical feature of inherited myopathies such as Duchenne muscular dystrophy (DMD) and also of more common acquired atrophies associated with disuse, aging, and cancer. This loss has fostered widespread interest in the powerful inhibitory effect of myostatin, a member of the TGF-β family of signaling molecules, on muscle growth (1) with specific focus on the prospect of modulating this system to counteract atrophic processes. Indeed, muscle fiber hypertrophy arising from absence or blockade of myostatin has been reported to be associated with therapeutic benefits in the mdx mouse model of DMD (2, 3). This hypertrophy has been attributed to proliferation of satellite cells (4, 5), the principal cellular source for growing and regenerating skeletal muscle (6–10), consequent upon their release from myostatin inhibition (5, 11, 12).
Here, we have investigated the contribution of satellite cells in 2 myostatin-null mouse models, constitutive (mstn−/−) and compact (BEHc/c), and following myostatin blockade by AAV-mediated overexpression of myostatin propeptide. These data, together with our results from in vitro studies on the effect of presence or absence of myostatin on satellite cells contradict comprehensively the widely held view that postnatal muscle hypertrophy elicited by the blockade or absence of myostatin is the result of increased satellite cell activity.
Results
Lack of Myostatin Results Predominantly in Hypertrophy of Individual Muscle Fibers.
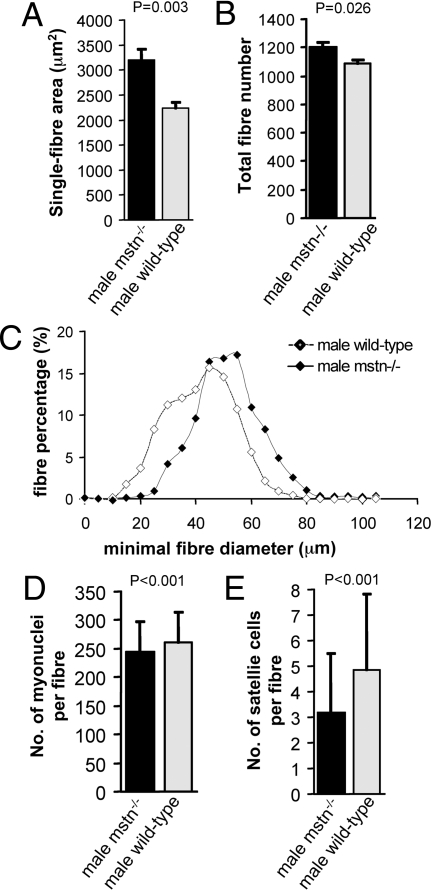
Having shown previously that mstn−/− extensor digitorum longus (EDL) muscles were 66% heavier than wild type (13), we analyzed their cellular composition. They contained only 11% more myofibers (P = 0.026) than controls but these myofibers were of 43% greater cross-sectional area (P = 0.003) and 6% longer (P = 0.001; Table 1; Fig. 1). Similarly, increased muscle size in the Berlin High mouse line compact (BEHc/c), which harbors a naturally occurring mutation in the myostatin gene (14, 15), reflects in part 37% more myofibers (P = 0.023) but, more importantly, a 93% larger mean fiber cross-sectional area (P < 0.001) and a significantly greater fiber length (P < 0.001) than BEH+/+ controls (supporting information (SI) Table S1). Thus, the large size of myostatin-deficient muscle is attributable chiefly to myofiber hypertrophy.
Table 1.
Cellular morphometric properties of male Mstn−/− and C57BL/6 wild-type EDL muscles
| Variable | Age (months) | Mstn−/− | C57BL/6 wild type | P* |
|---|---|---|---|---|
| Number of myofibers | 7 | 1,200 ± 71 (5) | 1,083 ± 65 (5) | 0.026* |
| Fiber area (μm2) | 7 | 3,192 ± 507 (5 mice, 974 fibers) | 2,235 ± 293 (6 mice, 1,251 fibers) | 0.003* |
| Satellite cells per fiber | 2 | 3.2 ± 2.3 (212 fibers, 4 mice) | 4.8 ± 3.0 (272 fibers, 6 mice) | <0.001*,** |
| Myonuclei per fiber | 2 | 244.5 ± 52 (212 fibers, 4 mice) | 261.3 ± 53 (244 fibers, 5 mice) | <0.001*,** |
| Fiber length (μm) | 2 | 4,047 ± 529 (89 fibers, 3 mice) | 3,820 ± 457 (117 fibers, 4 mice) | 0.001* |
Values are given as means together with standard deviation; number of muscles examined is given in parentheses.
*, for statistical analysis unpaired t-test and
**, Kolmogorov-Smirnov 2-sample test were used and P below 0.05 considered as significant.
Fig. 1.
Morphometric and cellular properties of EDL muscles from adult male mstn−/− mice compared to age-matched C57BL/6 wild types. (A) Total fiber number of whole EDL muscles from mstn−/− mice (black column) compared to wild types (gray column) (P = 0.026). (B) EDL fiber area from Mstn−/− mice (black column) compared to wild types (gray column) (P = 0.003). (C) Fiber size distribution in the EDL from mstn−/− mice (black diamonds) and wild-type mice (white diamonds). (D) Number of myonuclei per isolated muscle fiber from EDL muscles from mstn−/− mice (black column) compared to wild types (gray column) (P < 0.001). (E) Number of Pax-7 positive satellite cells per isolated muscle fiber from EDL muscles from mstn−/− mice (black column) compared to wild types (gray column) (P < 0.001).
Muscle Fiber Hypertrophy Is Not Dependent on Satellite Cell Activity.
The number of myonuclei per myofiber reflects the cumulative history of muscle precursor recruitment during fiber growth. Surprisingly, EDL fibers of both mstn−/− and BEHc/c mice contained fewer myonuclei per fiber (P < 0.001) than their respective wild-type controls (Table 1; Table S1; Fig. 1). This combination of larger size and fewer myonuclei must entail a markedly higher cytoplasmic-to-nuclear ratio.
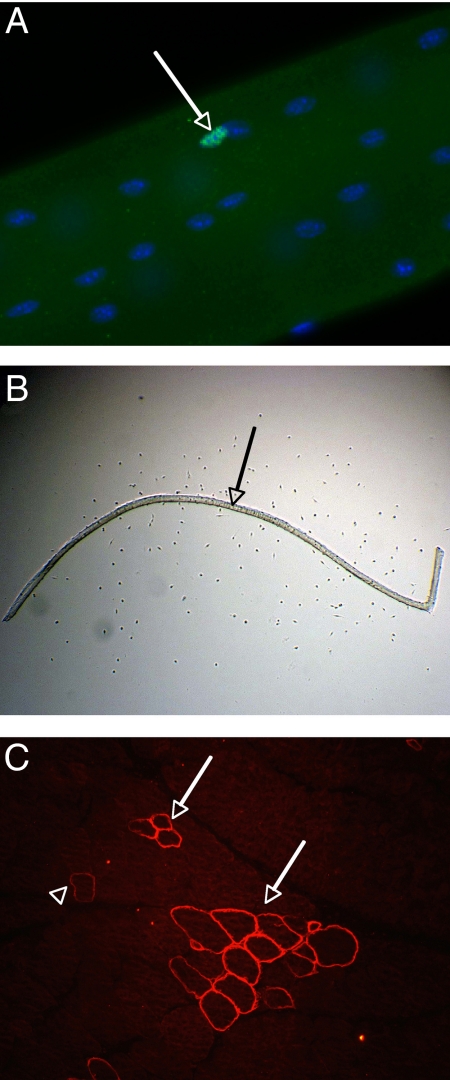
Such a pattern of myostatin-null-induced hypertrophy suggests a lack of satellite cell recruitment. Immunostaining for Pax7, which is expressed as a marker of both quiescent and activated satellite cells (10), (Fig. 2A), we found fewer (P < 0.001) satellite cells per myofiber from mstn−/− EDLs (3.2 ± 2.3 SD) than on equivalent wild-type myofibers (4.8 ± 3.0 SD) (Table 1; Figs. 1 and 2), with similar observations in BEHc/c (P < 0.001; Table S1). Thus, the sustained muscle hypertrophy arising from lack of myostatin is not associated with increase in either the number or recruitment of satellite cells. (Counts in 2 other muscles confirmed the lack of satellite cell increase in mstn−/− mice: soleus, wild type, 9.6 ± 3.5, n = 84; mstn−/−, 10.4 ± 4.0, n = 90; biceps brachii, wild type, 3.9 ± 2.1, n = 64; mstn−/−, 3.1 ± 1.9, n = 65.)
Fig. 2.
Analysis of muscle fibers. (A) Part of an isolated fiber from wild-type EDL, combined immunostaining against Pax-7 (green), and nuclear stain with DAPI (blue). The image depicts 1 satellite cell (arrow) and numerous myonuclei. (B) Example of a culture of an isolated muscle fiber (arrow) from wild-type EDL muscle after 3 days of in vitro incubation. Numerous myoblasts are present in the proximity of the muscle fiber, which are visible as little dots at this magnification in phase microscopy. (C) Immunostaining against revertant fibers on a cross-section of EDL muscle from mstn+/+mdx mouse depicts 2 clusters of revertant fibers (3 and 13 revertant fibers, respectively, arrows) and 1 paler isolated revertant fiber (arrowhead).
Satellite Cell Proliferation Is Independent of Myostatin.
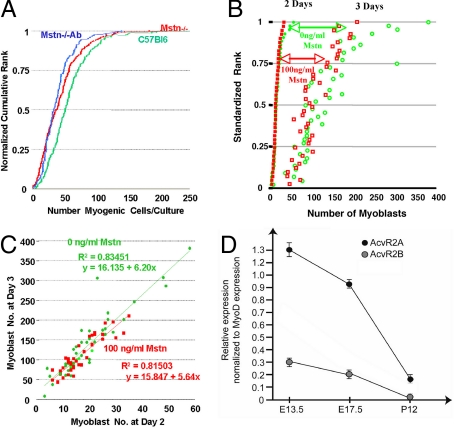
To examine the direct effect of myostatin on myoblast proliferation we monitored the rate of increase in satellite cell numbers around isolated myofibers in tissue culture growth medium. This constitutes a biologically relevant model of the basic self-replacing muscle unit, standardizing myoblast proliferation to the variability both in number and behavior of fiber-associated satellite cells (16, 17), (Fig. 2B). After 72 h in culture, fewer myoblasts (43 ± 32 SD) had accumulated in cultures from 2-month-old mstn−/− mice than in those from age-matched wild-type mice (58 ± 35 SD; P < 0.001; Fig. 3A). Although starting numbers of cells per fiber were smaller in the former, the gradients, indicating rates of increase in cell number, are indistinguishable. Moreover, blockade of any potential contaminant myostatin in the serum and chicken embryo extract with J16-mouse anti-myostatin monoclonal antibody did not affect the number of satellite cell progeny accumulating around mstn−/− myofibers (39 ± 25 SD; P = 0.228; Fig. 3A). Similarly, no difference in myoblast accumulation was found between 1-year-old mstn−/− and wild-type mice, with or without J16-antibody treatment (data not shown).
Fig. 3.
Analysis of responsiveness of primary myoblasts to myostatin. (A) Influence of myostatin on myoblast proliferation. Data are shown as cumulative rank ogives of the numbers of myoblasts accumulating around isolated fiber cultures from wild-type mice (green), mstn−/− mice (red), and J16-antibody-treated cultures of isolated fibers from mstn−/− mice (blue). The number of cells that had accumulated around each single myofiber during 72 h in tissue culture is plotted on the horizontal axis. The vertical axis is the individual ranks, normalized to the rank total for each experiment to permit comparison of data sets of different sample size. (B) Effect of myostatin on growth of primary cultures of satellite cells from single myofibers isolated from wild-type mice. After 2 days in culture, 100 ng/mL myostatin was added to half of the cultures (full red squares) but not to the control group (full green circles). The numbers of cells present 1 day later in each individual culture are displayed as empty red squares for the myostatin-treated and open green circles for the nontreated cultures, pairing the data for each culture between days 2 and 3. (C) Plot of the data from B of the cell number present at day 2 against that in the same culture on day 3 in cultures treated with myostatin (red squares) and in controls (green circles). The regression lines for myostatin-treated cultures (red) and controls (green) are shown with mean square error of prediction (R2) and regression equation that indicates the rate of cell increase before and after treatment with myostatin. (D) Expression profiling of activin receptors during muscle progenitor maturation. Real-time PCR comparing of activin receptors 2 a and b expression in Pax3GFP/+ muscle progenitors during embryonic (E13.5), fetal (E17.5), and postnatal (P12) stages. The relative levels of expression of activin receptors AcvR2A and AcvR2B, shown normalized to MyoD expression, decrease dramatically between early development and the neonatal growth phase.
For the complementary experiment, we exposed satellite cells to recombinant myostatin, bioactivity of which was verified on high-density cultures of limb bud mesenchyme (see SI Text). Addition of 100 ng/mL recombinant myostatin to 2-day isolated fiber cultures from wild-type EDL muscles had no effect on myoblast proliferation over the subsequent 24 h (Fig. 3B). Regression analysis of day 2 against day 3 values revealed a 5.5- to 6-fold increase in number of myoblasts, requiring >2 cell divisions over this 24-h period. So high a rate of proliferation, lying toward the upper limit for mammalian cells, forms a sensitive test for any inhibitory activities, rendering the lack of effect of myostatin particularly impressive (Fig. 3C). We conclude that neither the presence nor the absence of myostatin has any influence on satellite cell proliferation in vitro.
Myostatin Blockade in Adult Muscle Results in Satellite Cell Independent Muscle Hypertrophy.
Myostatin propeptide binds noncovalently to myostatin and inhibits its activity (18, 19). Induction of this type of blockade in adult muscle, by injection of recombinant AAV-2/1 encoding the myostatin propeptide (AAV-prop) yielded essentially the same results as mutations in the myostatin gene. Injection of AAV-prop into the tibialis anterior (TA) muscles of 2-month-old C57Bl6 mice induced a marked weight increase compared with contralateral control TA muscles injected with AAV-2/1 encoding murine-secreted alkaline phosphatase (AAV-muSeAP). By 14 days the difference was 15% (P = 0.038), increasing by 28 days to 28% (P < 0.001; Table 2); thereafter, the weight did not increase significantly. Morphometric analysis at 1 month revealed a 12% greater fiber area in AAV-prop- than in AAV-muSeAP-treated controls, rising by 2 months to 16% (P = 0.004; Table 2), reflecting a general shift toward larger diameters but with no change in number of fiber profiles (Table 2; Fig. S1). Myonuclear number per muscle fiber profile in cross-sections was unchanged from controls by treatment with AAV-prop vector (P = 0.77); this was concordant with a diminution of 24% in myonuclei per muscle total surface area (P = 0.031; Table 2). To examine the effect of myostatin blockade on satellite cell number we injected AAV-prop into TA muscles of Myf5nlacZ/+ mice (20), in which nlacZ marks satellite cells (21). One month later, no significant difference was observed between AAV-prop and AAV-muSeAP-injected muscles in the number of satellite cells per myofiber profile (P = 0.31; Table 2).
Table 2.
Morphometric properties after injection of AAV myostatin propeptide into tibialis anterior muscles compared to AAV-MSeap injection
| Variable | Days postinjection | AAV-prop | AAV-MSeap | P* |
|---|---|---|---|---|
| Muscle weight (mg) | 14* | 44.72 ± 2.92 (6) | 38.76 ± 2.38 (6) | 0.038 |
| 28* | 50.10 ± 2.77 (6) | 39.05 ± 0.55 (6) | 0.0003 | |
| 42* | 51.55 ± 2.40 (6) | 40.12 ± 1.49 (6) | 0.00033 | |
| 56* | 49.98 ± 4.32 (6) | 39.05 ± 0.97 (6) | 0.002 | |
| 70* | 48.63 ± 2.3 (6) | 41.20 ± 1.23 (6) | 0.0021 | |
| Number of myofibers | 28* | 3,245.8 ± 59.5 (5) | 3,184.6 ± 71.5 (5) | 0.729 |
| 56* | 3,328 ± 131 (5) | 3,420 ± 72 (5) | 0.47 | |
| Fiber area (μm2) | 28* | 2,242 ± 163 (16,035 fibers, 5 mice) | 1,996 ± 91 (16,238 fibers, 5 mice) | 0.089 |
| 56* | 2,156 ± 41 (16,763 fibers, 5 mice) | 1,865 ± 116 (16,200 fibers, 5 mice) | 0.004 | |
| Myonuclei per fiber | 31** | 0.65 ± 0.06 (475 fibers, 4 mice) | 0.63 ± 0.07 (816 fibers, 4 mice) | 0.774 |
| Myonuclei/surface area (nE−05/pixel) | 31** | 3.4 ± 0.48 (4 mice) | 4.5 ± 0.38 (4 mice) | 0.031 |
| Myf5 + ve cells/100 fibers | 31* | 3.2 ± 2.5 (6 mice) | 2.4 ± 0.8 (6 mice) | 0.31 |
AAV injections were performed into TA muscles of C57Bl6 mice at 8 weeks of age* or at 6 weeks of age**. Values are given as means together with standard deviation; number of muscles examined is given in parentheses; for statistical analysis paired t-test was used and P < 0.05 considered as significant.
Postnatal Satellite Cells Downregulate Activin Receptors.
Whereas our data confirmed a recent demonstration that postnatal myostatin blockade results in myofiber hypertrophy (22), we found no evidence of accompanying satellite cell hyperactivity. Seeking an explanatory mechanism for this finding, we assessed the expression of the activin receptors of myostatin signaling, AcvR2A and 2B, in muscle progenitors. Using a gfp reporter gene targeted into the Pax3 locus (21) as an identifier, we were able by FACS to obtain pure preparations of muscle progenitor cells from embryonic (E13.5) and fetal (E17.5) (23) stages and the predominant subset of satellite cells in postnatal (P12) hypaxial trunk muscles (24). Real-time PCR analysis demonstrated that while activin receptors are clearly expressed in muscle progenitor cells from developmental stages, they were barely detectable in postnatal satellite cells, in accord with the latter's obliviousness to myostatin blockade (Fig. 3D).
Myostatin Blockade Does Not Augment Muscle Regeneration in mdx Mice.
Next we reexamined the dystrophin-null mdx mouse model of DMD (25, 26) in which beneficial effects of lack or blockade of myostatin have been reported (2, 3). EDL muscles from constitutive mstn−/−mdx mice weighed 72% more (33 mg ± 1.7 SD) than those from mstn+/+mdx (19.2 mg ± 2.1 SD; P < 0.001; Table S2). They did contain 30% more fiber profiles/section, although the large intersample variation rendered this nonsignificant (P = 0.078; Table S2). Fiber profile numbers are difficult to interpret in mstn−/−mdx and mstn+/+mdx mice, being dependent on the lengths and branching frequency of regenerated fibers in addition to any putative increase in fiber number that might be associated with the constitutive lack of myostatin.
Counts of Pax7 expressing satellite cells on EDL myofibers of 15-month-old mice revealed no significant difference between mstn−/−mdx (11.8 ± 5.67 SD) and mstn+/+mdx fibers (12.2 ± 6.18 SD) (P = 0.44; Table S2), indicating no difference in the satellite cell resource in the mstn−/− environment.
In mouse muscle, persistence of centrally located myonuclei is an indicator of previous regenerative activity. Muscle from mstn−/−mdx mice and mstn+/+mdx mice showed no significant difference in the frequency of such central nucleation of fibers (P = 0.25; Table S2), again showing no effect of lack of myostatin on mdx muscle regeneration.
As a further indicator of regenerative activity, we analyzed the frequency of dystrophin-positive “revertant” fibers found sporadically in both mdx and DMD muscles (Fig. 2C). Progressive expansion of revertant fiber clusters has been shown to be a cumulative indicator of satellite cell-dependent muscle regeneration (27). Comparison of revertant fiber frequency in EDL muscles from mstn−/−mdx mice and mstn+/+mdx mice revealed no significant difference in total numbers, in numbers per cluster, or the numbers of isolated revertant fibers (Table S2; Fig. S2): further argument against any significant boost of net regenerative activity of dystrophic muscle in the absence of myostatin.
Discussion
Together, the above data comprehensively challenge the generally accepted mechanism of action of myostatin on satellite cells in postnatal muscle. On the contrary, it attributes postnatal muscle hypertrophy generated by lack of myostatin largely to hypertrophy of individual fibers that owes little or nothing to satellite cell activity, being generated mainly by increase in size of the myonuclear domain. True, the larger number of fibers in the genetically myostatin-null mice, especially in the BEHc/c strain, does imply some excess proliferation of muscle progenitors during early muscle development. This accords with our detection of expression of activin receptors at prenatal stages and aligns with recent demonstrations that, during early myogenesis, myostatin acts to limit myogenic cell proliferation by favoring their entry into terminal diffentiation (28). Even then, the larger-than-normal size of muscle fibers in the myostatin-null adults involved no increase in numbers of myonuclei per fiber, implying some limiting influence other than myostatin on the numbers of myogenic cells fusing into individual fibers over their period of formation and growth. Moreover, blockade of myostatin in postnatal muscle produced no evidence for either de novo generation of muscle fibers or for any increase in myonuclei per myofiber profile. Furthermore, in the dystrophic mstn−/−mdx mice, enhanced muscle growth was not attributable to an increase above normal levels of muscle regeneration. This lack of responsiveness of postnatal satellite cells to myostatin signaling is explicable by their perinatal downregulation of activin receptors.
Our in vitro data supporting this view are in agreement with previous studies employing similar concentrations of myostatin (29) but conflict with a previous publication employing 10- to 20-fold higher concentrations (4). The essentially artifactual nature of tissue culture leaves open the debate as to what the biologically appropriate doses and individual tissue culture conditions are for evaluation of myostatin activity. However our in vivo findings firmly contradict the notion that myostatin-mediated control of satellite cell proliferation has any significant influence on muscle fiber hypertrophy during normal postnatal growth or during regeneration of dystrophic muscle. This revelation points us away from the signaling pathways of satellite cell activation and proliferation that are linked to the accepted view of myostatin action (11), in both normal postnatal and regenerating myopathic muscle. It implicates, instead, mechanisms whereby myostatin regulates protein balance within the muscle fibers themselves. This accords well with recent biochemical studies of muscle hypertrophy in myostatin null conditions (30, 31), finding little or no concordant increase in DNA content and thus attributing much of the effect to enlargement of the myonuclear domain, with associated increased transcription of myofibrillar RNAs. A recent debate concluded that the relationship between muscle fiber hypertrophy and myonuclear number was variable between models and situations (32). Our data places the fiber hypertrophy associated with lack of myostatin activity at the extreme in which large size of muscle fibers mainly reflects enlargement of myonuclear domain. It implies, too, that this mechanism, rather than augmented satellite cell activation, operates even in regenerating dystrophic muscle in which satellite cells are actively proliferating in response to the stimuli that mediate muscle repair.
From a practical viewpoint, we need to revise current views that therapeutic strategies based on myostatin blockade counteract muscle defects by stimulating satellite cell activity. Such an adjustment does not conflict with a recent report on the effect of myostatin blockade in adult muscular dystrophy patients, which showed a tendency toward bigger muscle fibers with no evidence of increased muscle regeneration (33). But the change in rationale would usefully inform the design of future human clinical trials of myostatin blockade such as those in DMD. The implication that beneficial amounts of muscle hypertrophy are independent of satellite cell activity raises the prospect of combating forms of muscle weakness such as disuse atrophy that are not directly associated with satellite cell function. It is important, however, before further clinical trials, to explore the consequences of an increased myonuclear domain on homeostasis and physiological function of muscle tissue: specifically its relationship to the severe decrease in specific force and the mitochondrial depletion observed in myostatin-deficient muscle (13).
Methods
Animals.
Mstn−/+ founder breeding pairs on a C57BL/6 background were a gift of Se-Jin Lee (Johns Hopkins University, Baltimore) (1). Myostatin knockout and wild-type mice (C57BL/6) were bred and kept in the animal facilities of The Royal Veterinary College, London, under guidelines of the Home Office (UK) under license PPL/70/5218.
Muscles from a subline of the Berlin High mouse line (BEH), which is homozygous for the compact mutation (13–15, 34), were a kind gift from Lutz Bunger. Muscles from 1.5-year-old male mstn−/−mdx mice and mstn+/+mdx littermates were a kind gift from Kathryn Wagner (Johns Hopkins University, Baltimore) (3). Myf5nlacZ/+ mice (20) and Pax3GFP/+ mice (21) were bred in the animal facilities of the UMR-S 787, Medical Faculty Pitié-Salpétrière, under institutional guidelines.
Production and Injection of AAV Vector.
The myostatin propeptide construct was prepared by PCR amplification of C57Bl6 cDNA and introduced into an AAV-2-based vector under the control of the CMV promoter. The AAV-muSeAP was described elsewhere (35). (Production details appear in supporting information.)
Single Fiber Isolation and Culture.
EDL muscles were carefully dissected from mice killed by cervical dislocation. Age and number of animals are given in Tables 1 and 2; Tables S1 and S2. Myofibers isolated as described previously (36, 37) were either fixed in 4% PFA/PBS or cultured as detailed in SI Text (36, 37).
Immunohistochemistry of Single Muscle Fibers.
Fixed myofibers were permeabilized with 0.5% (vol/vol) Triton X-100/PBS and blocked with 20% (vol/vol) goat serum/PBS, as described previously (36). Satellite cells were visualized with anti-Pax-7 antibody (DSHB) and Alexa Fluor 488 goat anti-mouse IgG (Molecular Probes) before mounting in Faramount fluorescent mounting medium (DakoCytomation) containing 100 ng/mL DAPI.
Morphometric Analysis of mstn−/− and Compact Mice.
EDL muscles were weighed and then mounted in OCT (BDH) and frozen in melting isopentane cooled in liquid nitrogen. Ten-micrometer transverse sections from the midbelly were cut on a cryostat and total number was counted in H&E-stained transverse sections using Leica QWin software. Minimal and maximal fiber diameters and fiber area of all fibers were determined using KS 300 software (Carl Zeiss). Number and age of mice are given in Table 1 and Table S1.
Histological Analysis of AAV-Injected Muscles.
After killing of AAV-injected mice, both tibialis anterior muscles were removed, weighed, mounted in OCT, and frozen in isopentane cooled in liquid nitrogen. Transverse cryostat sections (8 μm) were fixed in 2% paraformaldehyde for 30 min, blocked for 1 hour in 1% BSA, 1% sheep serum, 0.1% triton X-100, and 0.001% sodium azide before incubation with a rabbit anti-laminin antibody (Dako, Z0097, 1/300) followed by Cy3-conjugated goat anti-rabbit IgG secondary antibody (Jackson ImmunoResearch, 111–165-144, 1/200). After immunostaining for laminin, fiber number, and CSA, and myonuclear number and position were analyzed by Ellix software (Microvision).
LacZ-expressing nuclei were visualized in Myf5nlacZ/+ mice and counted, together with myonuclei, on 2 complete transverse cryostat sections (10 μm) of each AAV-injected TA muscle using standard protocols for X-Gal staining. Most X-Gal positive nuclei were found adjacent to the sarcolemma and designated satellite cells; the occasional X-Gal positive nucleus found within muscle fibers, was not included in the satellite cell counts.
Real-Time Quantitative PCR.
Real-Time PCR was performed according to standard protocols on a DNA Engine Opticon 2 System (Bio-Rad) as detailed in supporting information.
FACS Sorting of Pax3 Positive Cells.
Isolation and cell sorting of GFP+ cells were performed as previously described (24). Expression of the receptors was normalized to MyoD expression to make conservative allowance for the lower frequency of activation of postnatal satellite cells.
Statistical Analysis.
All values are expressed as mean ± standard deviation. To determine significance between 2 groups, we made comparisons using the unpaired Student's t-tests and Kolmogorov-Smirnov 2-sample test for comparison of different mouse genotypes and paired Student's t-tests for comparison of ipsilateral and contralateral muscles. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank Elaine Shervill, Helen Hunt, and Daniela Kittel for excellent technical assistance. We are indebted to Professor Se-Jin Lee for providing founder mice for our mstn−/− colony, Professor Kathryn Wagner for providing mstn−/−mdx muscles, and Dr. Lisa-Anne Whittemore and Dr. Simon Hughes for providing antibodies. The work was funded by The Wellcome Trust (066195 and 078649 to R.M. and K.P.) and the Biotechnology and Biological Sciences Research Council (11020 to K.P. and A.O.). H.A. is supported by the Muscular Dystrophy Association (3870), the Bundesministerium für Bildung und Forschung (as member of the MD-NET, 01 GM0302, Project R24), and by the Association Monégasque contre les Myopathies. C.H.'s postdoctoral salary is supported by the Association Monégasque contre les Myopathies. F.R. is supported by the Inserm Avenir program, AFM strategic plan to UMR-S 787 and Decrypthon program. A.R.'s postdoctoral salary is supported by the Decrypthon program to F.R. The laboratory of P.S.Z. is supported by The Medical Research Council, The Muscular Dystrophy Campaign, the Association of International Cancer Research, and the Association Française contre les Myopathies. T.A.P. is supported by funding from the Wellstone Center (U54HD053177), and the United States Department of Defense Congressionally Directed Medical Research Program (W81XWH-05-1-0616).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811129106/DCSupplemental.
References
- 1.McPherron AC, Lawler AM, Lee S-J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 2.Bogdanovich S, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 3.Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol. 2002;52:832–836. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- 4.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCroskery S, et al. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci. 2005;118:3531–3541. doi: 10.1242/jcs.02482. [DOI] [PubMed] [Google Scholar]
- 6.Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- 7.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins CA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Sherwood RI, et al. Isolation of adult mouse myogenic progenitors: Functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Zammit PS, et al. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFarlane C, et al. Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp Cell Res. 2008;314:317–329. doi: 10.1016/j.yexcr.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Wagner KR. Muscle regeneration through myostatin inhibition. Curr Opin Rheumatol. 2005;17:720–724. doi: 10.1097/01.bor.0000184163.61558.ca. [DOI] [PubMed] [Google Scholar]
- 13.Amthor H, et al. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci USA. 2007;104:1835–1840. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabo G, et al. A deletion in the myostatin gene causes the compact (Cmpt) hypermuscular mutation in mice. Mamm Genome. 1998;9:671–672. doi: 10.1007/s003359900843. [DOI] [PubMed] [Google Scholar]
- 15.Varga L, et al. Inheritance and mapping of Compact (Cmpt), a new mutation causing hypermuscularity in mice. Genetics. 1997;147:755–764. doi: 10.1093/genetics/147.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bockhold K, Rosenblatt J, Partridge T. Aging normal and dystrophic mouse muscle: Analysis of myogenicity in cultures of living single fibers. Muscle Nerve. 1998;21:173–183. doi: 10.1002/(sici)1097-4598(199802)21:2<173::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Heslop L, Morgan JE, Partridge TA. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J Cell Sci. 2000;113:2299–2308. doi: 10.1242/jcs.113.12.2299. [DOI] [PubMed] [Google Scholar]
- 18.Hill JJ, et al. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem. 2002;277:40735–40741. doi: 10.1074/jbc.M206379200. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tajbakhsh S, et al. Gene targeting the myf-5 locus with nlacZ reveals expression of this myogenic factor in mature skeletal muscle fibers and early embryonic muscle. Dev Dyn. 1996;206:291–300. doi: 10.1002/(SICI)1097-0177(199607)206:3<291::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 22.Qiao C, et al. Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum Gene Ther. 2008;19:241–254. doi: 10.1089/hum.2007.159. [DOI] [PubMed] [Google Scholar]
- 23.Relaix F, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montarras D, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 25.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sicinski P, et al. The molecular basis of muscular dystrophy in the mdx mouse: A point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 27.Yokota T, et al. Expansion of revertant fibers in dystrophic mdx muscles reflects activity of muscle precursor cells and serves as an index of muscle regeneration. J Cell Sci. 2006;119:2679–2687. doi: 10.1242/jcs.03000. [DOI] [PubMed] [Google Scholar]
- 28.Manceau M, et al. Myostatin promotes the terminal differentiation of embryonic muscle progenitors. Genes Dev. 2008;22:668–681. doi: 10.1101/gad.454408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner KR, Liu X, Chang X, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA. 2005;102:2519–2524. doi: 10.1073/pnas.0408729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welle S, Bhatt K, Pinkert CA. Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab. 2006;290:E409–415. doi: 10.1152/ajpendo.00433.2005. [DOI] [PubMed] [Google Scholar]
- 31.Welle S, Bhatt K, Pinkert CA, Tawil R, Thornton CA. Muscle growth after postdevelopmental myostatin gene knockout. Am J Physiol Endocrinol Metab. 2007;292:E985–991. doi: 10.1152/ajpendo.00531.2006. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor RS, Pavlath GK, McCarthy JJ, Esser K. A last word on point:counterpoint: Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol. 2007;103:1107. doi: 10.1152/japplphysiol.00502.2007. [DOI] [PubMed] [Google Scholar]
- 33.Wagner KR, et al. A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 34.Bunger L, et al. Inbred lines of mice derived from long-term growth selected lines: Unique resources for mapping growth genes. Mamm Genome. 2001;12:678–686. doi: 10.1007/s00335001-3018-6. [DOI] [PubMed] [Google Scholar]
- 35.Bartoli M, et al. AAV-mediated delivery of a mutated myostatin propeptide ameliorates calpain 3 but not alpha-sarcoglycan deficiency. Gene Ther. 2007;14:733–740. doi: 10.1038/sj.gt.3302928. [DOI] [PubMed] [Google Scholar]
- 36.Beauchamp JR, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim. 1995;31:773–779. doi: 10.1007/BF02634119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.