Abstract
Inclusions of TAR DNA-binding protein-43 (TDP-43), a nuclear protein that regulates transcription and RNA splicing, are the defining histopathological feature of frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-Us) and sporadic and familial forms of amyotrophic lateral sclerosis (ALS). In ALS and FTLD-U, aggregated, ubiquitinated, and N-terminally truncated TDP-43 can be isolated from brain tissue rich in neuronal and glial cytoplasmic inclusions. The loss of TDP-43 function resulting from inappropriate cleavage, translocation from the nucleus, or its sequestration into inclusions could play important roles in neurodegeneration. However, it is not known whether TDP-43 fragments directly mediate toxicity and, more specifically, whether their abnormal aggregation is a cause or consequence of pathogenesis. We report that the ectopic expression of a ≈25-kDa TDP-43 fragment corresponding to the C-terminal truncation product of caspase-cleaved TDP-43 leads to the formation of toxic, insoluble, and ubiquitin- and phospho-positive cytoplasmic inclusions within cells. The 25-kDa C-terminal fragment is more prone to phosphorylation at S409/S410 than full-length TDP-43, but phosphorylation at these sites is not required for inclusion formation or toxicity. Although this fragment shows no biological activity, its exogenous expression neither inhibits the function nor causes the sequestration of full-length nuclear TDP-43, suggesting that the 25-kDa fragment can induce cell death through a toxic gain-of-function. Finally, by generating a conformation-dependent antibody that detects C-terminal fragments, we show that this toxic cleavage product is specific for pathologic inclusions in human TDP-43 proteinopathies.
Keywords: caspase, inclusion, amyotrophic lateral sclerosis, cell death, frontotemporal lobar degeneration with ubiquitin-positive inclusions
The aggregation and deposition of ubiquitinated proteins in neuronal and glial cells is a characteristic feature of a number of neurodegenerative diseases, like Alzheimer's disease, Parkinson's disease, frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS). Recently, TAR DNA-binding protein of 43-kDa molecular mass (TDP-43) was identified as the major component of the predominately cytoplasmic inclusions observed in ALS and FTLD with ubiquitin-positive inclusions (FTLD-U), the most common form of FTLD (1, 2). Normally, TDP-43 is a nuclear protein but neurons with cytoplasmic inclusions have a substantial loss of nuclear TDP-43, suggesting it redistributes to the cytoplasm (1). In addition to the abnormal distribution and aggregation of TDP-43 in disease, the molecular signature of pathologic TDP-43 includes ubiquitination, phosphorylation and proteolytic cleavage generating C-terminal fragments (1).
TDP-43 is a 414-amino acid protein and the primary amino acid structure has strong homology in domain composition to members of the heterogeneous ribonucleoprotein (hnRNP) family (3, 4). Like other members of this family, TDP-43 has 2 highly conserved RNA recognition motifs (RRM1 and RRM2) flanked by the N-terminal and the C-terminal tail. The C-terminal tail contains a glycine-rich region often found to mediate protein–protein interactions. This region is reported to bind members of the hnRNP protein family, such as hnRNP A1, A2/B1 and A3 (5). The N-terminal region contains 2 nuclear localization signals (NLS), and 3 potential caspase-3 cleavage consensus sites span the TDP-43 molecule (6).
Rare mutations in genes encoding the major protein component of inclusions in familial neurodegenerative diseases have provided unequivocal evidence that the proteins in the inclusions are causally related to neurodegeneration. For example, mutations in MAPT cause tau-positive FTLD (7) and mutations in the amyloid precursor protein (APP) lead to Alzheimer's disease, which is characterized by amyloid deposits (8). Extensive mutation analysis of TARDBP, the gene encoding TDP-43, lead to the discovery of missense mutations in sporadic and familial ALS (9–14). All but 1 of the 14 reported mutations cluster in exon 6 of TARDBP, which encodes the highly-conserved glycine-rich domain of TDP-43. Mutant forms of TDP-43 form fragments more readily than wild-type TDP-43 in Chinese hamster ovary cells and cause neural apoptosis and developmental delay in chick embryos (12). In lymphoblastoid cell lines derived from patients carrying TARDBP mutations, the accumulation of detergent-insoluble TDP-43 cleavage products of ≈25 and 35-kDa is observed (10, 11), providing additional indication for a pathological role of TDP-43 fragments in disease.
We have shown that the proteolytic cleavage of TDP-43 by caspases generates C-terminal fragments (25 and 35 kDa) similar to those observed in FTLD-U brains (6). Caspase-cleavage of TDP-43 appears to be a prerequisite for its redistribution from the nucleus to the cytosol in H4 neuroglioma cells (6), and C-terminal TDP-43 fragments are associated with enhanced aggregation potential and toxicity in yeast (15). Whether TDP-43 C-terminal fragments are prone to aggregate in mammalian cells has not yet been determined, nor has the mechanism of toxicity of these C-terminal fragments been examined. Thus, this study compared the aggregation and toxicity of C-terminal TDP-43 fragments and full-length TDP-43 in human cell lines and investigated the mechanisms of toxicity induced by the 25-kDa C-terminal TDP-43 fragment.
Results
C-Terminal Fragments of TDP-43 Corresponding to Caspase-Cleavage Products Form Cytoplasmic, Ubiquitin-Positive Inclusions.
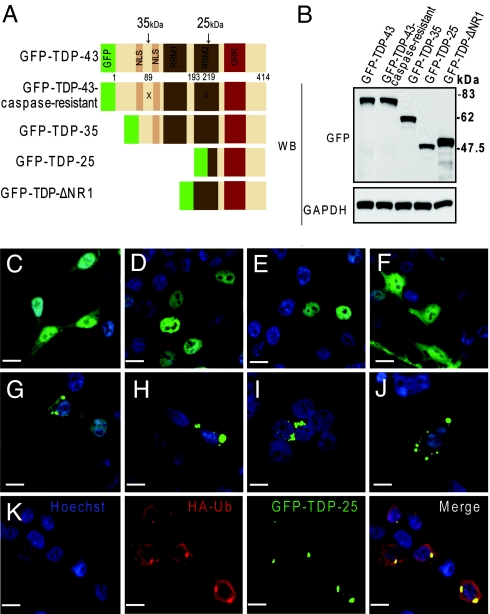
The pathological inclusions in ALS and FTLD-U are composed of TDP-43, which is abnormally ubiquitinated, phosphorylated and truncated in affected brain regions (1). To elucidate the involvement of the C-terminal fragments of TDP-43 in inclusion formation, HEK293 cells were made to express a 35-kDa (amino acid 90–414) or 25-kDa (amino acid 220–414) TDP-43 fragment, corresponding to the C-terminal truncation products generated when TDP-43 is cleaved by caspases at residues 89 and 219, respectively (Fig. 1A). To this end, expression vectors were generated encoding the 35- or 25-kDa C-terminal fragments tagged at the amino terminal with enhanced green fluorescence protein (GFP-TDP-35, GFP-TDP-25). Also made were expression vectors for a third C-terminal fragment (amino acid 193–414) lacking the N terminus and the RRMI (GFP-TDP-ΔNR1), full-length wild-type TDP-43 (GFP-TDP-43) and caspase-resistant TDP-43 in which the aspartic acid at positions 89 and 219 was substituted to glutamic acid (GFP-TDP-43-caspase-resistant) (6) (Fig. 1A). The transient expression of all fusion proteins in HEK293 cells resulted in a similar level of protein expression (Fig. 1B). The cellular distribution of the GFP control, examined by confocal fluorescence microscopy, showed diffuse cytoplasmic and nuclear distribution (Fig. 1C), while wild-type GFP-TDP-43 (Fig. 1D) and the caspase-resistant mutant (Fig. 1E) localized almost exclusively to the nucleus. In contrast, both GFP-TDP-35 (Fig. 1G) and GFP-TDP-25 (Fig. 1H) showed cytoplasmic and nuclear distribution. Moreover, both of these fragments formed compact cytoplasmic inclusions (Fig. 1 G and H) with more inclusions observed in cells expressing GFP-TDP-25 (≈75% of cells) compared with GFP-TDP-35 (≈25% of cells). Inclusion formation was specific to caspase-cleavage TDP-43 products, since the GFP-TDP-ΔNR1 C-terminal fragment exhibited diffuse cytoplasmic and nuclear immunoreactivity with no detectable inclusions (Fig. 1F). Of interest, the inclusions formed by GFP-TDP-25 and GFP-TDP-35 were not disrupted by Triton X-100 (Fig. 1I) nor by the microtubule-destabilization agent, nocodazole (Fig. 1J). Additionally, the inclusions were positive for endogenous ubiquitin and the coexpression of GFP-TDP-25 and HA-tagged ubiquitin demonstrated colocalization of the HA-ubiquitin and GFP within cytoplasmic inclusions (Fig. 1K). Similar results were observed in primary hippocampal neurons and 7 cell lines, including differentiated M17 neuroblastoma cells (Fig. 2), and also when Flag-tagged-TDP-43, -TDP-35, and -caspase-resistant constructs were expressed. Since the 25-kDa fragment is believed to be the pathologic species in FTLD-U and ALS (1), we focused our studies on this fragment.
Fig. 1.
C-terminal, caspase-dependent fragments form cytoplasmic, ubiquitin-positive inclusions. (A) Schematic representation of the TDP-43 molecule and various TDP-43 constructs. The “X” symbol indicates 2 of the caspase-cleavage sites that result in either 35- or 25-kDa C-terminal TDP-43 fragments. NLS, nuclear localization signal; RRM, RNA recognition motif; GRR, glycine-rich region. (B) Expression of GFP fusion proteins in vitro. HEK293 cells were transfected with one of the constructs for 48 h, and cell lysates were subjected to Western blot analysis using an anti-GFP antibody to compare expression levels of the fusion proteins. The membrane was stripped and reprobed with an anti-GAPDH antibody to verify protein loading. (C–K) Fluorescent confocal microscopy demonstrates the diffuse cytoplasmic and nuclear distribution of GFP (C) and GFP-TDP-ΔNR1 (F), as well as the predominantly nuclear localization of wild-type GFP-TDP-43 (D) and the caspase-resistant mutant (E). Note the cytoplasmic inclusions formed in cells transfected with GFP-TDP-35 (G) or GFP-TDP-25 (H). Inclusions formed from the 25-kDa fragment persist after treatment with 0.2% Triton X-100 (I) or nocodazole (J). (K) Ubiquitin immunostaining shows that cytoplasmic inclusions formed from GFP-TDP-25 are ubiquitin-positive. (Scale bar: 10 μm.)
Fig. 2.
The 25-kDa C-terminal fragment of TDP-43 is prone to phosphorylation, but its phosphorylation is not required for inclusion formation. (A) Cells were transfected for GFP-TDP-43 or GFP-TDP-25, then immunostained with anti-pTDP-43, which detects TDP-43 when phosphorylated at S409/S410. Fluorescent confocal microscopy demonstrates enhanced staining of GFP-TDP-25 compared with GFP-TDP-43. (B) Lysates from HEK293 cells transfected with GFP-TDP-43, GFP-TDP-43NLSmut, GFP-TDP-25, or GFP-TDP-25S409A/S410A were subjected to Western blot analysis and probed with anti-pTDP-43. The 25-kDa C-terminal fragment showed marked anti-pTDP-43 immunoreactivity compared with GFP-TDP-43 or GFP-TDP-43NLSmut. GFP-TDP-25S409A/S410A did not exhibit any immunoreactivity. The membrane was reprobed with anti-GAPDH to verify protein loading. (C) Fluorescent confocal microscopy demonstrates that GFP-TDP-25S409A/S410A, which is not phosphorylated at S409/S410, can form cytoplasmic inclusions.
The 25-kDa C-Terminal Fragment of TDP-43 Is Prone to Phosphorylation at S409/S410, but Its Phosphorylation Is Not Required for Inclusion Formation.
It has been reported that the TDP-43 C-terminal fragments in the brain of patients with FTLD-U and ALS are phosphorylated at serine 409 and serine 410 (16). To determine if phosphorylation at these sites is required for inclusion formation, HEK293 cells were transfected with GFP-TDP-43 or GFP-TDP-25 and immunostained with a polyclonal antibody specific to TDP-43 when phosphorylated at S409/S410 (anti-pTDP-43). Fluorescent confocal microscopy revealed that the inclusions in cells expressing GFP-TDP-25 were intensely stained by anti-pTDP-43, whereas GFP-TDP-43 was not (Fig. 2A). In a similar fashion, Western blot analysis using extracts from transfected cells showed that, compared with GFP-TDP-43, GFP-TDP-25 was strongly immunoreactive for anti-pTDP-43 (Fig. 2B). In contrast, anti-pTDP-43 failed to detect a mutant TDP-25 incapable of being phosphorylated (GFP-TDP-25S409A/S410A), confirming the specificity of the antibody (Fig. 2B). One possible reason for the enhanced phosphorylation of GFP-TDP-25 is that its cytoplasmic localization renders it readily available to kinases. To establish if full-length TDP-43 would be comparably phosphorylated if it too were present in the cytoplasm instead of the nucleus, an expression vector encoding full-length TDP-43 containing mutations (K82A, R83A, K84A) in the NLS was generated (GFP-TDP-43NLSmut). Substituting the charged amino acid (lysine or arginine) to alanine results in cytoplasmic expression (17). Cells transfected with GFP-TDP-43NLSmut had the same modest anti-pTDP-43 immunoreactivity as GFP-TDP-43 (Fig. 2B), suggesting that cytoplasmic distribution does not account for the enhanced phosphorylation of GFP-TDP-25. Finally, since GFP-TDP-25 expression causes inclusion formation and since it is heavily phosphorylated, we sought to determine if preventing phosphorylation of GFP-TDP-25 at S409/S410 would prevent inclusion formation. Thus, cells expressing GFP-TDP-25S409A/S410A were examined by fluorescent confocal microscopy. As shown in Fig. 2C, despite the inability to be phosphorylated, GFP-TDP-25S409A/S410A formed cytoplasmic inclusions similar to those formed by GFP-TDP-25 indicating that phosphorylation at S409/S410 is not required for TDP-25 aggregation. Together, these results suggest that the 25-kDa C-terminal fragment of TDP-43 is more prone to phosphorylation at S409/S410 than full-length TDP-43, but enhanced phosphorylation is not required for its aggregation into inclusions.
The 25-kDa C-Terminal Fragment of TDP-43 Causes Cellular Toxicity.
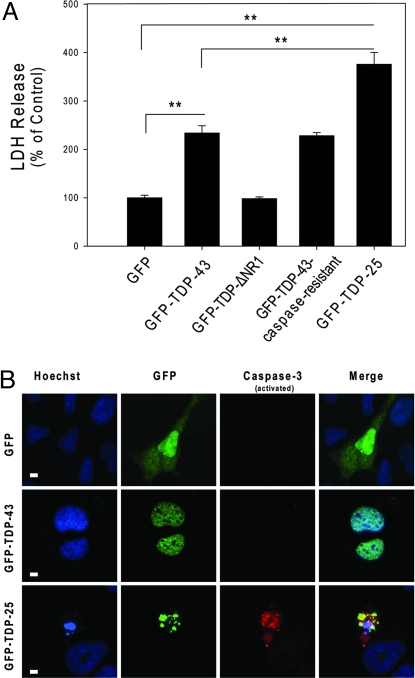
Next, we examined if GFP-TDP-25 cytoplasmic aggregates increase cellular toxicity. Differentiated M17 neuroblastoma cells were transiently transfected with the GFP, GFP-TDP-43, GFP-TDP-43-caspase-resistant, GFP-TDP-ΔNR1, or GFP-TDP-25 vector. The medium was collected 72 h later and lactate dehydrogenase (LDH) levels were measured. Compared with LDH levels in cells expressing only GFP, LDH levels were no different in GFP-TDP-ΔNR1-expressing cells but were comparably increased in GFP-TDP-43 and GFP- TDP-43-caspase-resistant-expressing cells. Of particular interest, LDH release was significantly higher in cells expressing the 25-kDa C-terminal fragment (Fig. 3A) and GFP-TDP-25S409A/S410A compared with cells transfected with full-length or caspase-resistant TDP-43.
Fig. 3.
The 25-kDa C-terminal fragment enhances cellular toxicity. (A) The release of LDH into the media was used as an indicator of cell toxicity. LDH levels were measured 72 h after cells were transfected with the indicated constructs. Data from 3 separate experiments were analyzed by 1-way ANOVA, followed by Tukey's posthoc analysis (**, P < 0.001). (B) Increased apoptosis in differentiated M17 neuroblastoma cells expressing GFP-TDP-25 compared with cells expressing GFP alone or GFP-TDP-43. Cultures were fixed and stained with Hoechst to label nuclei (blue) and activated caspase-3 antibody (red). (Scale bar: 10 μM.)
To examine transfection efficiency and the extent of apoptosis, fixed cells were stained with bisbenzimide (henceforth referred to as Hoechst) and an antibody against activated caspase-3, and then viewed by confocal microscopy. Hoechst labeling of nuclei revealed numerous fragmented nuclei, indicative of apoptotic cell death, only in cells expressing GFP-TDP-25-positive inclusions (Fig. 3B). These inclusions were also immunopositive for activated caspase-3 (Fig. 3B). Based on quantitative analysis, ≈50% of the inclusion-positive cells were positive for fragmented nuclei and activated caspase-3. In contrast, cells expressing GFP-alone, GFP-TDP-43 (Fig. 3B) or GFP-TDP-43-caspase-resistant did not stain for activated caspase-3. These results suggest that GFP-TDP-25 expression and inclusion formation is deleterious to differentiated neuroblastoma cells.
The 25-kDa C-Terminal Fragment of TDP-43 Does Not Sequester Full-Length TDP-43.
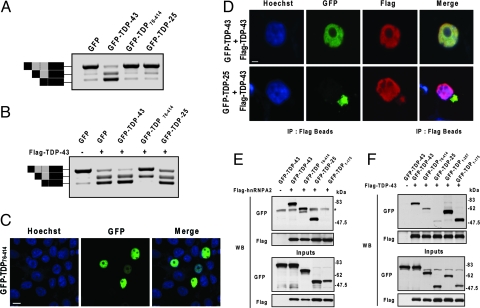
The toxicity associated with the expression of the 25-kDa C-terminal fragment may result from a toxic gain-of-function due to its abnormal presence in the cytoplasm, or from the sequestration of endogenous wild-type TDP-43. If the 25-kDa fragments lead to depletion of endogenous nuclear TDP-43, a decrease in the activity of TDP-43 is anticipated. One of the biological activities of TDP-43 is promoting the skipping of cystic fibrosis transmembrane conductance regulator (CFTR) exon 9 (18). To examine if the 25-kDa fragment would reduce this activity, HeLa cells were cotransfected with an expression vector for GFP-TDP-25, GFP-TDP-43 or GFP, and a CFTR minigene construct, previously shown to mimic the splicing pattern of endogenous exon 9 CFTR (19) (Fig. 4 A and B). Additionally, since little is known about the normal biological function of the N-terminal of TDP-43, except that it contains the nuclear localization sequence, the effect of a second C-terminal fragment was examined: GFP-TDP76–414. Unlike GFP-TDP-25, this fragment retains nuclear distribution (Fig. 4C), contains all of the critical functional domains (Fig. 1A) and does not increase toxicity as assessed by LDH release. After cotransfection, the relative mRNA levels of exon inclusion and exclusion products were monitored by RT-PCR. In cells cotransfected with GFP, little exon exclusion activity was present, suggesting that endogenous TDP-43 activity is low, especially when compared with the marked increase in exon exclusion observed with GFP-TDP-43 expression (Fig. 4A). If GFP-TDP-25 causes toxicity by depleting endogenous nuclear TDP-43, a decrease in TDP-43 activity is expected. However, the exon inclusion/exclusion profiles did not differ between cells expressing only GFP and those expressing GFP-TDP-25 (Fig. 4A). This suggests that GFP-TDP-25 has no effect on endogenous TDP-43 activity, perhaps because it lacks an N-terminal region required for TDP-43 sequestration. Interestingly, despite its nuclear distribution (Fig. 4C), GFP-TDP76–414 did not promote exon 9 exclusion indicating the importance of the N-terminal domain for normal TDP-43 activity (Fig. 4A).
Fig. 4.
The 25-kDa C-terminal fragment does not affect full-length TDP-43 function or cellular localization. (A and B) To examine the effect of C-terminal fragments on endogenous TDP-43 function, cells were cotransfected with a CFTR minigene construct and a vector encoding the GFP-fusion proteins, as indicated (A). To examine the effect of C-terminal fragments on exogenous TDP-43, cells were also transfected with a vector for FLAG-TDP-43 (B). Two days after transfection, the exon inclusion and exclusion products were examined by RT-PCR. The schematic shown to the left of the gels in A and B depicts transcripts containing or lacking exon 9 with or without a cryptic splice variant (gray box). Black boxes depict non-CFTR exons. Top and lower bands correspond to transcripts that include or exclude exon 9, respectively. The middle band represents the cryptic splice varient. Note that TDP-25 inhibited neither endogenous nor exogenous TDP-43 exon exclusion activity. Yet, GFP-TDP76–414 did markedly attenuate exogenous TDP-43 activity, despite its nuclear localization (C), which was examined by staining cells with the nuclear marker, Hoechst (blue), after transfection. (Scale bar: 10 μM.) (D) Fluorescent confocal microscopy reveals that GFP-TDP-25 inclusions fail to sequester wild-type FLAG-TDP-43. HEK293 cells were cotransfected with FLAG-TDP-43 and GFP-TDP-43 or GFP-TDP-25 and stained with Hoescht (blue) and anti-FLAG antibody (red). (Scale bar: 10 μM.) (E and F) Coimmunoprecipitation experiments reveal that GFP-TDP-25 and GFP-TDP76–414 bind strongly to hnRNPA2 (E) but only weakly to wild-type FLAG-TDP-43 (F). Note that GFP-TDP-43 and the C-terminal deletion products, GFP-TDP1–257 and GFP-TDP1–175, do bind FLAG-TDP-43, indicating that TDP-43 molecules interact and that their binding to one another is enhanced by the presence of the N terminal. (* represents a nonspecific band.)
The results above suggest that the 25-kDa C-terminal fragment has no effect on endogenous TDP-43 activity. Because endogenous TDP-43 exon skipping activity was low (Fig. 4A), any reduction caused by GFP-TDP-25 could be difficult to accurately detect. Therefore, we next examined the effect of the 25-kDa C-terminal fragment on the exon skipping activity in cells overexpressing full-length TDP-43. To this end, cells were transfected with the CFTR minigene construct and Flag-TDP-43, and either the GFP-TDP-25, GFP-TDP-43, GFP-TDP76–414 or GFP vector. Consistent with our previous result, the coexpression of Flag-TDP-43 and GFP-TDP-43 promoted exon 9 exclusion (Fig. 4B). In contrast, the expression of GFP-TDP-25 with Flag-TDP-43 did not reduce Flag-TDP-43 activity (Fig. 4B). Of interest, the expression of GFP-TDP76–414 markedly reduced the exon skipping activity of Flag-TDP-43 (Fig. 4B).
Based on these results, it seems unlikely that the 25-kDa C-terminal fragment causes toxicity by sequestering full-length TDP-43 in the cytoplasm and preventing its nuclear function. This is further supported by confocal fluorescent microscopy experiments in which HEK293 cells were cotransfected with Flag-TDP-43 and GFP-TDP-25 or GFP-TDP-43. As expected, anti-Flag immunoreactivity and GFP fluorescence colocalized in the nucleus of cells cotransfected with Flag-TDP-43 and GFP-TDP-43 (Fig. 4D). In cells expressing both Flag-TDP-43 and GFP-TDP-25, immunostaining with an anti-Flag antibody strongly stained nuclear Flag-TDP-43 but showed only weak cytoplasmic staining. Thus, the nuclear localization of Flag-TDP-43 was not appreciably altered by GFP-TDP-25, further indicating that this fragment does not sequester nuclear TDP-43 to the cytoplasm.
Although the 25-kDa C-terminal fragment did not affect TDP-43 activity, the nuclear C-terminal fragment, GFP-TDP76–414, inhibited the activity of TDP-43 (Fig. 4B). This result was surprising since it was presumed that GFP-TDP76–414 would enhance activity because it contains all of the functional domains similar to wild-type TDP-43 and is present in the nucleus. In an effort to determine why GFP-TDP76–414 had little activity compared with GFP-TDP-43, we examined its ability to bind hnRNPA2, an interaction that is likely essential for the splicing inhibitory activity of TDP-43 (5). Thus, coimmunoprecipitation experiments were performed in which HeLa cells were cotransfected with Flag-tagged hnRNPA2 and GFP-TDP76–414, GFP-TDP-43, GFP-TDP-25 or a C-terminal deletion mutant (GFP-TDP1–175), used as a negative control. Coimmunoprecipitation of Flag-tagged hnRNPA2 followed by probing for GFP indicated that GFP-TDP76–414 did indeed interact with hnRNPA2, as did GFP-TDP-43 and GFP-TDP-25 (Fig. 4E). GFP-TDP1–175, which lacks the critical glycine-rich region necessary for interaction, failed to bind hnRNPA2 (Fig. 4E). Thus, the loss of GFP-TDP76–414 activity is not due to an inability to bind hnRNPA2.
Next, to determine if the inhibitory effect of GFP-TDP76–414 on TDP-43 activity resulted from an interaction between GFP-TDP76–414 and wild-type TDP-43, coimmunoprecipitation experiments were performed. HeLa cells were cotransfected with Flag-TDP-43 and the vector for GFP-TDP76–414,GFP-TDP-43, GFP-TDP-25, GFP, or the C-terminal deletion products (GFP-TDP1–175 and GFP-TDP1–257). Coimmunoprecipitation of Flag-tagged TDP-43 followed by probing for GFP revealed that Flag-TDP-43 bound strongly to GFP-TDP-43 and to the C-terminal deletion products, GFP-TDP1–175 and GFP-TDP1–257 (Fig. 4F). In contrast, GFP-TDP76–414 and GFP-TDP-25 only weakly interacted with Flag-TDP-43, despite comparable expression levels (Fig. 4F). Thus, the ability of GFP-TDP76–414 to inhibit Flag-TDP-43 activity does not occur through the interaction of these 2 proteins. It is noteworthy that these results indicate that the interaction of TDP-43 with other TDP-43 molecules appears enhanced by the presence of an intact N-terminal.
A Conformation-Dependent TDP-43 Antibody Detects C-Terminal TDP-43 Fragments and Specifically Labels the Pathologic Inclusions in Human TDP-43 Proteinopathies.
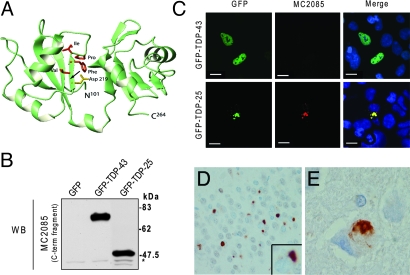
Since the caspase-derived C-terminal fragments aggregate into cytoplasmic inclusions, we hypothesized that they may be the major pathological species in FTLD-U and ALS. Previous studies have shown that C-terminal specific TDP-43 antibodies detect TDP-43 pathology in FTLD-U and ALS (20); however, all current commercially available TDP-43 antibodies detect both normal nuclear TDP-43 and TDP-43-positive pathological structures, like neuronal cytoplasmic inclusions (NCI), dystrophic neurites (DN) and neuronal intranuclear inclusions (NII). In some cases, extensive nuclear labeling makes it difficult to detect mild TDP-43 pathology. Hasegawa et al. (16) have shown that antibodies to TDP-43 phospho-peptides appear to be lesion-specific. Hence, we reasoned that an antibody that would detect the neoepitope formed upon caspase cleavage of TDP-43 might also be lesion specific. We thus generated a polyclonal TDP-43 antibody (MC2085) to a peptide sequence in the 25-kDa C-terminal fragment. Based on composite analysis of the TDP-43 aa sequence and 3-dimensional modeling (10) (Fig. 5A), we chose the amino acid residues, VFIPKPFR, C-terminal to the putative caspase cleavage site, as the peptide antigen. This region is buried within the TDP-43 molecule and so is predicted to be detected only when exposed by proteolytic cleavage or under denaturing conditions.
Fig. 5.
A conformation-dependent TDP-43 antibody that detects C-terminal fragments is specific for pathologic inclusions in human TDP-43 proteinopathies. (A) Three-dimensional modeling of wild-type TDP-43 (amino acids 101–264). The positions of the 8 amino acid residues following the caspase cleavage motif, selected to generate a polyclonal antibody, are indicated. Note that aspartate 219, which is part of the caspase cleavage motif, is buried within the molecule (arrow). (B) Western blot analysis of lysates from HEK293 cells transfected with GFP only, GFP-TDP-43, or GFP-TDP-25 and probed with MC2085. *, Endogenous TDP-43. (C) MC2085 immunostaining of HEK293 cells transfected with GFP-TDP-43 or GFP-TDP-25. Note the absence of staining in cells expressing wild-type TDP-43 compared with those expressing the 25-kDa C-terminal fragment. (Scale bars: 10 μm.) (D and E) Immunostaining of tissue from FTLD-U brain (D) and ALS brain (E) with MC2085 shows cytoplasmic inclusions with little or no nuclear TDP-43. (Magnification: 40×.)
To determine if MC2085 detects the 25-kDa C-terminal fragment, wild-type TDP-43 or both, we transfected cells with GFP, GFP-TDP-43, or GFP-TDP-25 for Western blot analysis and immunofluorescent confocal microscopy. By Western blot analysis, both GFP-TDP-43 and GFP-TDP-25 were immunoreactive for MC2085 (Fig. 2B). In contrast, immunostaining with MC2085 showed it to react only with the inclusions in GFP-TDP-25 transfected cells and not with nuclear TDP-43 in GFP-TDP-43 transfected cells (Fig. 2C). Of interest, the inclusions were not detected by a TDP-43 N-terminal-specific antibody (MC2079), suggesting they are composed mainly of C-terminal fragments (Fig. S1). Next, immunohistochemistry of FTLD-U and ALS tissue was conducted using MC2085. Neuronal cytoplasmic inclusions in FTLD-U (Fig. 5D) and ALS (Fig. 5E) cases stained positively for MC2085 but minimal or no staining of normal neuronal TDP-43 and glial nuclei was observed (Fig. 5 D and E). These findings suggest that some inclusions in both FTLD-U and ALS contain caspase-derived C-terminal fragments of TDP-43.
Discussion
Our data highlight the pathologic relevance of caspase-derived C-terminal TDP-43 fragments. Using molecular and biochemical assays in various cell culture systems, we demonstrate that: (i) the 25-kDa fragment forms cytoplasmic inclusions that are ubiquitinated and phosphorylated at S409/S410; (ii) phosphorylation of these fragments at S409/S410 is not required for their aggregation; and (iii) inclusion formation is associated with enhanced cellular toxicity, likely through a toxic gain-of-function. Moreover, using an antibody that selectively detects caspase-derived C-terminal fragments in human tissue, we provide evidence that these fragments are diagnostic characteristics of TDP-43 proteinopathies.
Herein, we show that the expression of the 25-kDa C-terminal fragment, corresponding to a caspase-cleavage product of TDP-43, causes inclusion formation and cellular toxicity. Interestingly, just as the inclusions in FTLD-U and ALS are immunoreactive to phosphorylation-specific antibodies toward pS409/S410 (16, 21), so are the inclusions formed by the 25-kDa fragments (Fig. 2). In fact, GFP-TDP-25 was much more prone to phosphorylation at S409/S410 than full-length TDP-43. This is consistent with the findings of Hasegawa et al., who show by Western blot that a ≈25-kDa fragment in sarkosyl-insoluble, urea soluble fractions of FTLD-U and ALS cases is more intensely stained with an antibody to pS409/S410 than full-length TDP-43 (16). This group also reports that treating recombinant full-length TDP-43 with casein kinase I leads to TDP-43 phosphorylation and facilitates filament formation in vitro (16). While phosphorylation may expedite the aggregation of full-length TDP-43 in vitro, we show that S409/S410 phosphorylation is not required for inclusion formation nor toxicity resulting from GFP-TDP-25 expression. These results strongly suggest that cleavage is a prerequisite for TDP-43 hyperphosphorylation, aggregation, and toxicity, and highlight the importance of the 25-kDa C-terminal fragment as a pathological hallmark of disease. Indeed, the antibody generated to detect C-terminal fragments, MC2085, strongly stained inclusions in histological sections from FTLD-U and ALS brains, making it a powerful tool for the detection of TDP-43 pathology by IHC, especially since it does not stain normal neuronal or glial nuclei.
Although our results establish the toxicity of the 25-kDa fragment, the exact mechanism by which this occurs is not known. Toxicity could result from the aggregation of the fragments and their interference with normal cellular activity through a toxic gain-of-function. Alternatively, it may result from the sequestration of normal TDP-43 into inclusions, thus depriving the cell of the normal biological functions of TDP-43. For example, Winton and colleagues have shown that the exogenous expression of full-length TDP-43 with NLS mutations leads to the accumulation of cytoplasmic aggregates and the sequestration of endogenous TDP-43, thereby depleting normal nuclear TDP-43 (22). Yet, this seems not to be the case with caspase-cleaved TDP-43 since the expression of GFP-TDP-25, while toxic, did not reduce the exon skipping activity of either endogenous or exogenous TDP-43, nor did it alter the nuclear distribution of Flag-TDP-43 (Fig. 4). Also, the inclusions were not detected by the N-terminal specific antibody, MC2079, further suggesting that full-length TDP-43 is not sequestered into the inclusions (Fig. S1). Rather, the toxicity associated with C-terminal fragment inclusions appears to occur independently of their effect on normal TDP-43 function. This is consistent with the work by Johnson and colleagues who examined the molecular determinants of TDP-43 aggregation and its cellular consequences in yeast models. They concluded that aggregation is critical for toxicity, with the most toxic fragments containing both the C terminus and RRM2, similar to the 25-kDa fragment of the present study (15). As observed in yeast cells (15), TDP-43 overexpression was toxic to M17 cells, albeit to a lesser extent than GFP-TDP-25. While the mechanism of cell death is not yet clear, it may result from the burden of high TDP-43 levels in the nucleus and the dysregulation of TDP-43 activity. Indeed, TDP-43-caspase-resistant, which also localized predominately to the nucleus, was comparably toxic, whereas GFP-TDP-ΔNR1, which exhibited both diffuse cytoplasmic and nuclear staining, was not and neither was GFP-TDP76–414 which did localize to the nucleus but had no biological activity. Just as depletion of TDP-43 results in apoptosis in human cells lines (23), so may elevated TDP-43 levels. Until more is known about TDP-43 function, it will be difficult to ascertain how changes in nuclear TDP-43 levels will affect cell survival.
It is important to note that, while inclusions formed from the 25-kDa fragment are toxic, it does not rule out the potential for TDP-43 loss-of-function to cause toxicity in TDP-43 proteinopathies. In fact, loss of TDP-43 in cells by RNAi causes cell death and aberrant nuclear morphology through the misregulation of the retinoblastoma protein (pRb) (23). We have previously shown that caspase-cleavage of endogenous TDP-43 results in the redistribution of TDP-43 from the nucleus to the cytoplasm (6), perhaps due to the loss of the NLS. This would result in the depletion of nuclear TDP-43 and, thus, loss of function. Indeed, the 25-kDa fragment has no biological activity (Fig. 4A). In the present study, the effect of C-terminal TDP-43 fragments on toxicity was examined by exogenously expressing the 25-kDa fragment and not by inducing the cleavage of endogenous TDP-43, so it is expected (based on the findings shown in Fig. 4) that endogenous remaining nuclear TDP-43 remains functional.
Our findings provide previously undescribed insights on TDP-43 function. We show that the N-terminal region of TDP-43 is required for normal TDP-43 exon 9 exclusion activity (Fig. 4A). The lack of activity of N-terminal deletion mutants, like GFP-TDP-25 and GFP-TDP76–414, does not result from impaired hnRNPA2 binding, an interaction thought critical for the splicing inhibitory activity of TDP-43 (5). Although it could be assumed that loss of activity for GFP-TDP-25 results from its cytosolic localization, this cannot be said for GFP-TDP76–414 which is present in the nucleus. Indeed, when GFP-TDP76–414 is coexpressed with full-length TDP-43, it inhibits full-length TDP-43 function (Fig. 4B). This effect is not caused by an interaction between wild-type TDP-43 and GFP-TDP76–414 since they seem to bind poorly to one another (Fig. 4F). Instead, it is possible that the functionally inert GFP-TDP76–414 competes with wild-type TDP-43 for binding the substrate and partners necessary for activity, like hnRNPA2. Finally, we show that the binding of full-length TDP-43 to other TDP-43 molecules is enhanced by the presence of an intact N-terminal.
Overall, these findings, along with previous biochemical, pathological and genetic studies, indicate that cleavage of TDP-43 results in the abnormal cytosolic localization of C-terminal TDP-43 fragments and the formation of toxic aggregates. Further study of the pathways that generate these toxic C-terminal fragments and their mechanisms of toxicity may lead to the identification of new therapeutic strategies to treat or prevent ALS, FTLD-U and other TDP-43 proteinopathies.
Materials and Methods
Plasmids, Antibodies, and Reagents.
For information about the materials used in this study please refer to SI Materials and Methods.
Cell Culture and Treatments.
Cell culture studies are detailed in SI Materials and Methods.
Immunofluorescence and Western Blot Analysis.
Immunofluroscence and Western blot analysis are detailed in SI Materials and Methods.
Toxicity Assay.
Toxicity assay is detailed in SI Materials and Methods.
RNA Extraction and Semiquantitative RT-PCR.
CFTR splicing assay was followed according to a protocol adapted from ref. 18 and detailed in SI Materials and Methods.
Generation of Antibodies and Immunohistochemistry.
Please refer to SI Materials and Methods for details regarding antibodies and immunohistochemistry.
Supplementary Material
Acknowledgments.
This work was supported by the Mayo Clinic Foundation, National Institutes of Health/National Institute on Aging Grants R01AG026251 and P01-AG17216-08, National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant R01 NS 063964-01, and the Amyotrophic Lateral Sclerosis Association Grant 1736.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900688106/DCSupplemental.
References
- 1.Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 2.Arai T, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfuss G, Kim VN, Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol. 2002;3:195–205. doi: 10.1038/nrm760. [DOI] [PubMed] [Google Scholar]
- 4.Krecic AM, Swanson MS. hnRNP complexes: Composition, structure, and function. Curr Opin Cell Biol. 1999;11:363–371. doi: 10.1016/S0955-0674(99)80051-9. [DOI] [PubMed] [Google Scholar]
- 5.Buratti E, et al. TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: An important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J Biol Chem. 2005;280:37572–37584. doi: 10.1074/jbc.M505557200. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YJ, et al. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27:10530–10534. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutton M, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 8.Goate A, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 9.Gitcho MA, et al. TDP-43 A315T mutation in familial motor neuron disease. Ann Neurol. 2008;63:535–538. doi: 10.1002/ana.21344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabashi E, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 11.Rutherford NJ, et al. Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet. 2008;4:e1000193. doi: 10.1371/journal.pgen.1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sreedharan J, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Deerlin VM, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: A genetic and histopathological analysis. Lancet Neurol. 2008;7:409–416. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoseki A, et al. TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol. 2008;63:538–542. doi: 10.1002/ana.21392. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BS, McCaffery JM, Lindquist S, Gitler AD. A yeast TDP-43 proteinopathy model: Exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc Natl Acad Sci USA. 2008;105:6439–6444. doi: 10.1073/pnas.0802082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa M, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayala YM, et al. Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci. 2008;121:3778–3785. doi: 10.1242/jcs.038950. [DOI] [PubMed] [Google Scholar]
- 18.Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- 19.Pagani F, et al. Splicing factors induce cystic fibrosis transmembrane regulator exon 9 skipping through a nonevolutionary conserved intronic element. J Biol Chem. 2000;275:21041–21047. doi: 10.1074/jbc.M910165199. [DOI] [PubMed] [Google Scholar]
- 20.Igaz LM, et al. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of fronto-temporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol. 2008;173:182–194. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inukai Y, et al. Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS. FEBS Lett. 2008;582:2899–2904. doi: 10.1016/j.febslet.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Winton MJ, et al. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J Biol Chem. 2008;283:13302–13309. doi: 10.1074/jbc.M800342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayala YM, Misteli T, Baralle FE. TDP-43 regulates retinoblastoma protein phosphorylation through the repression of cyclin-dependent kinase 6 expression. Proc Natl Acad Sci USA. 2008;105:3785–3789. doi: 10.1073/pnas.0800546105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.