Abstract
Electrogenic events due to the activity of wild-type lactose permease from Escherichia coli (LacY) were investigated with proteoliposomes containing purified LacY adsorbed on a solid-supported membrane electrode. Downhill sugar/H+ symport into the proteoliposomes generates transient currents. Studies at different lipid-to-protein ratios and at different pH values, as well as inactivation by N-ethylmaleimide, show that the currents are due specifically to the activity of LacY. From analysis of the currents under different conditions and comparison with biochemical data, it is suggested that the predominant electrogenic event in downhill sugar/H+ symport is H+ release. In contrast, LacY mutants Glu-325→Ala and Cys-154→Gly, which bind ligand normally, but are severely defective with respect to lactose/H+ symport, exhibit only a small electrogenic event on addition of LacY-specific substrates, representing 6% of the total charge displacement of the wild-type. This activity is due either to substrate binding per se or to a conformational transition after substrate binding, and is not due to sugar/H+ symport. We propose that turnover of LacY involves at least 2 electrogenic reactions: (i) a minor electrogenic step that occurs on sugar binding and is due to a conformational transition in LacY; and (ii) a major electrogenic step probably due to cytoplasmic release of H+ during downhill sugar/H+ symport, which is the limiting step for this mode of transport.
Keywords: bioenergetics, membrane proteins, permease, solid-supported membrane, transport
Secondary active transporters in the bacterial plasma membrane are of fundamental importance for the cell. To name only a few functions, they catalyze uptake of nutrients, export of toxic compounds, translocation of macromolecules, regulation of cell turgor, and creation of electrochemical ion gradients important for the function of other membrane proteins. Bacterial homologues of mammalian transporters have also become important, because they can be conveniently obtained, purified readily in large amounts, and used for crystallization and structure determination. One of the best-characterized systems is the lactose permease from Escherichia coli (LacY), structures of which have been solved recently at atomic resolution (1–3). Because of the wealth of biochemical and biophysical data available for LacY (4–8), it represents an ideal model system for the investigation of the basic principles and molecular details of secondary active transport.
Among the many methods for functional characterization, electrophysiology is arguably the most universal, because it does not require labeled substrate. Also, it is an extremely sensitive, highly time-resolved technique that allows direct measurement of charge movement. Although it has been known for many years that lactose/H+ symport catalyzed by LacY is an electrogenic reaction (9–11), despite numerous efforts, LacY has so far resisted all attempts at electrophysiological analysis. Although the lacY gene is expressed well in frog oocytes and other eukaryotic cells, LacY remains in the cis-Golgi and the perinuclear membrane, and does not target to the plasma membrane to any extent whatsoever. In this report, we present the successful electrophysiological study of LacY by using purified, reconstituted proteoliposomes with solid-supported membrane (SSM) based electrophysiology (12).
Results
Downhill Sugar/H+ Symport Generates Transient Currents.
Proteoliposomes containing reconstituted LacY were immobilized on an SSM-coated gold electrode (the sensor), and charge displacement induced by downhill sugar/H+ symport into the proteoliposomes was detected by capacitive coupling (13). Transport was initiated at 1.5 s by a sugar concentration jump using rapid solution exchange (Fig. 1). Approximately 40 ms later, sugar reaches the surface of the SSM, and a transient current starts abruptly. The time course of the signals is characterized by 2 distinct phases: a rapid rise to a maximum followed by a much slower decay toward the baseline. Because the decay is not exponential, it was quantified by the decay time from peak to half-maximal current, τ1/2, which is ≈50 ms for all LacY substrates tested.
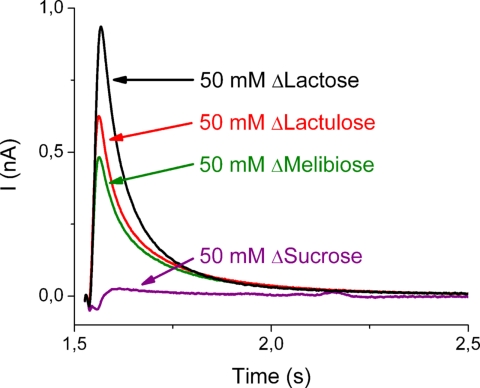
Fig. 1.
Transient currents obtained with wild-type LacY proteoliposomes after a 50 mM sugar concentration jump at t = 1.5 s. The traces in black, red, and green correspond to concentration jumps of lactose (50 mM; ΔLactose), lactulose (50 mM; ΔLactulose), melibiose (50 mM; ΔMelibiose), and sucrose (50 mM; ΔSucrose), respectively. The nonactivating solution (50 mM glucose) and all activating solutions were prepared in 100 mM potassium phosphate at pH 7.6 plus 1 mM DTT. All traces shown were recorded from 1 sensor.
Because the amount of proteoliposomes adsorbed to an individual sensor exhibits some variability, only current amplitudes obtained from the same sensor are compared directly. As shown, the maximal value of the peak current observed after a 50 mM concentration jump of lactose is 950 pA, and the peak currents generated after 50 mM concentration jumps of lactulose and melibiose are ≈65% and ≈50%, respectively, of that recorded with lactose. However, addition of 50 mM sucrose (Fig. 1), a sugar that is not a substrate of LacY, has no effect.
Sugar binding and transport catalyzed by LacY are inactivated by alkylation of Cys-148 primarily (14–16). Consistently, no electrical transient is observed after treatment with N-ethylmaleimide (NEM; Fig. S1).
Varying Lipid-to-Protein Ratio (LPR).
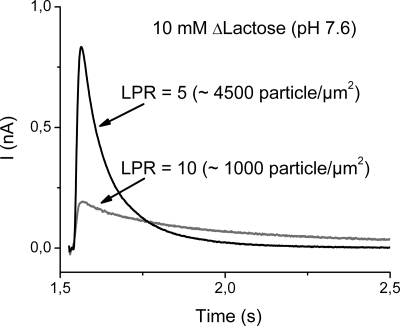
Electrogenic transport by a reconstituted protein leads to transient currents in the capacitively coupled system (13, 17). With wild-type LacY, downhill sugar/H+ symport into the proteoliposomes generates an inside-positive potential, which acts to decelerate the downhill symport reaction catalyzed by LacY, leading to transient currents. However, any conformational transition that displaces charged amino acyl side chains or reorients electrical dipoles also represents an electrogenic transition that may contribute to the transient nature of the currents. Indeed, it has been shown with the melibiose permease from E. coli (MelB) that melibiose binding triggers an electrogenic conformational transition that is a major component of the transient currents observed (18, 19). To discriminate between downhill sugar/H+ symport and electrogenic conformational transition, experiments were performed with proteoliposomes reconstituted at different LPR (wt/wt). As shown by freeze–fracture electron microscopy (Fig. S2 A and B), at LPRs of 10 or 5, liposomes with LacY particle densities of ≈1,000 and ≈4,500 particles per μm2, respectively, are observed. An electrogenic conformational transition is expected to yield identical time constants for decay of the transients at different particle densities. In contrast, charging of the liposomal membrane by downhill sugar/H+ symport should lead to decreasing decay time (lower τ1/2) at increasing protein density (i.e., lower LPR). As shown in Fig. 2, increased LacY particle density clearly leads to significantly faster decay. Thus, the transient currents observed for wild-type LacY represent mainly charging of the liposome membrane due to downhill sugar/H+ symport activity.
Fig. 2.
Transient currents obtained at 2 different LPR (10 and 5). The solution exchange protocol and the nonactivating solution were as described in Fig. 1, but the activating solution contained 40 mM glucose plus 10 mM lactose. Therefore, the difference between the test and nonactivating solutions represents a 10 mM lactose concentration jump (10 mM ΔLactose). Wild-type LacY proteoliposomes reconstituted at a LPR of 10 (≈1,000 particles per μm2; Fig. S2A) or at a LPR of 5 (≈4,500 particles per μm2; Fig. S2B) were activated with a 10 mM lactose concentration jump. The decay phase of the transient currents is decreased almost 5-fold from a τ1/2 = 53 ± 2 ms at an LPR of 5 (black trace) to a τ1/2 = 260 ± 2 ms at an LPR of 10 (gray trace).
Effect of pH.
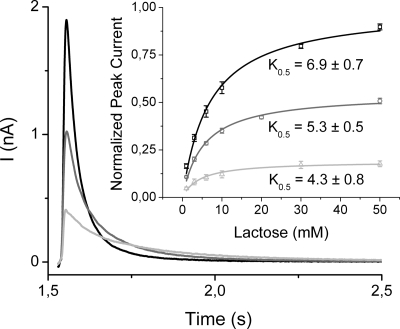
The shape and magnitude of the transients generated by downhill lactose/H+ symport strongly depends on pH (Fig. 3). An overall increase in pH from 6.6 to 8.5 causes a 5-fold increase in the magnitude of the peak current (Fig. 3; Table 1). The decay time also depends on pH, because τ1/2 decreases from ≈103 to 46 to 27 ms, respectively, at pH 6.6, 7.6, and 8.5 (Fig. 3; Table 1). This trend is anticipated, because higher electrogenic activity of the transporter leads to faster charging of the liposome membrane and a concomitant faster current decay (i.e., lower τ1/2) (17). Therefore, the effect of pH on the amplitude and time dependence of the transient currents is consistent with the proposed assignment of the peak currents to the symport activity of LacY.
Fig. 3.
Effect of pH on the transient currents generated after 50 mM lactose concentration jumps at different pH values. The traces were successively recorded on the same sensor after equilibration was reached and are, therefore, directly comparable. To equilibrate the pH across the proteoliposome membrane after changing the pH of the solutions, the immobilized proteoliposomes are incubated for ≈20 min at the new pH. Subsequent lactose concentration jumps produced constant currents indicating that the pH value had indeed equilibrated. The nonactivating solution contained 50 mM glucose and the activating solutions 50 mM lactose. Both solutions were prepared in 100 mM potassium phosphate buffer at pH 8.5 (black trace), 7.6 (gray trace), or 6.6 (light gray trace) plus 1 mM DTT. (Inset) Dependence of peak currents on lactose concentration at 3 pH values. The nonactivating solution contained 50 mM glucose, the activating solutions a given concentration of x mM lactose plus 50 − x mM glucose to maintain a constant sugar concentration. The solutions were prepared in 100 mM potassium phosphate buffer at a given pH value plus 1 mM DTT, and the pH was equilibrated across the proteoliposome membrane. The peak currents recorded at pH 8.5 for each lactose concentration jump were fitted with a hyperbolic function, and all data obtained with that sensor (every lactose concentration at the 3 pH values) were expressed as fraction of maximum value at pH 8.5 (Ipeakmax). This normalization procedure yields datasets that can be directly compared between sensors. For a statistical analysis, the complete dataset was recorded on 3 different sensors, and the averaged values and errors (SE) are shown. From the hyperbolic fits, apparent K0.5 values with SE were obtained at every pH (Table 1).
Table 1.
Kinetic parameters of the transient currents measured for wild-type LacY
| pH | Efflux, % | Peak current, % | τ1/2, ms | K0.5, mM |
|---|---|---|---|---|
| 8.5 | 100 | 100 | 27 ± 0.6 | 6.9 ± 0.7 |
| 7.6 | 35 | 55 | 46 ± 2 | 5.3 ± 0.5 |
| 6.6 | 6 | 19 | 103 ± 7 | 4.3 ± 0.8 |
Proteoliposomes adsorbed to an SSM surface are stable for hours without loss of activity, allowing investigation of the effect of pH on the kinetics of the transient currents induced at different lactose concentrations (Fig. 3 Inset; Table 1). An increase in pH from 6.6 to 8.5 generates a 5-fold increase in the saturating peak current (Fig. 3; Table 1). Indeed, rates of efflux (i.e., downhill lactose/H+ symport in the opposite direction) from right-side-out membrane vesicles (20), or proteoliposomes reconstituted with purified LacY (21, 22), exhibit a similar dependence on pH (Table 1). The half saturating concentration increases only slightly with the pH (Fig. 3 Inset; Table 1). At pH 7.6, a K0.5 of 5.3 ± 0.5 mM is obtained, which is close to that of 3.1 ± 0.7 mM determined for downhill lactose/H+ influx in proteoliposomes reconstituted with purified LacY (23).
Transient Currents in Mutants.
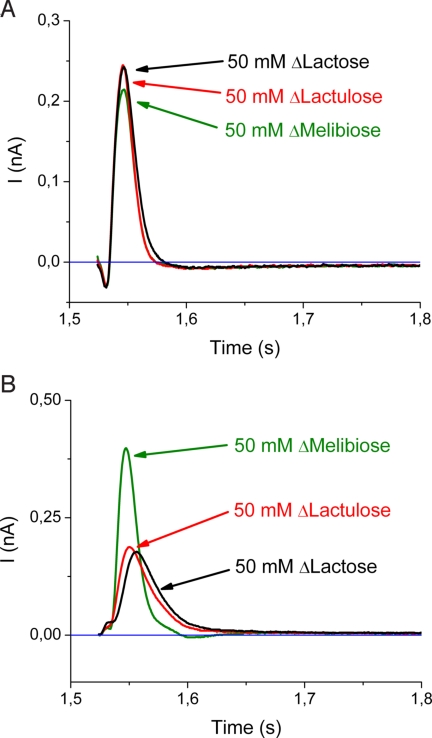
To dissect the overall electrogenic response, mutants of LacY that bind ligand but do little or no lactose/H+ symport were used. Mutant E325A is specifically defective in all steps involving H+ release from LacY but catalyzes exchange and counterflow at least as well as wild-type (24, 25). E325A LacY was reconstituted into proteoliposomes at an LPR of 5 and a particle density comparable with that of the wild-type preparation (≈3,500 particles per μm2; Fig. S3A). Concentration jumps of 50 mM lactose, lactulose, or melibiose produce transient currents with virtually identical kinetics and negligible differences in magnitude (Fig. 4A), which are abolished after treatment with NEM (Fig. S4A). However, the transient currents are ≈5-times smaller than those observed for lactose with wild-type LacY (Table 2), and exhibit mono-exponential decays (τ ≈ 10 ms) followed by a shallow negative phase (τ ≈ 300 ms). Notably, the nonexponential decay of the wild-type is characterized by τ1/2, whereas here the time constant τ is used. The negative component represents discharge of the liposome membrane after rapid charge translocation. This phenomenon is common for the capacitively coupled system (13), and indicates absence of significant steady-state charge transport across the liposome membrane (i.e., downhill sugar/H+ symport activity).
Fig. 4.
Transient currents obtained with LacY mutants. The solution exchange protocol and composition of the solutions was the same as described for Fig. 1. The baseline is represented in blue. (A) E325A LacY was reconstituted into liposomes, and activated with 50 mM concentration jumps of lactose (50 mM; ΔLactose), lactulose (50 mM; ΔLactulose), or melibiose (50 mM; ΔMelibiose) at pH 7.6. All traces exhibit virtually identical kinetics and only small differences in magnitude with an exponential decay toward the baseline (τ ≈ 10 ms) followed by a negative phase (τ ≈ 300 ms). (B) Transient currents obtained with C154G LacY proteoliposomes after 50 mM sugar concentration jumps at pH 7.6. The transient currents corresponding to 50 mM ΔLactose or 50 mM ΔLactulose decay mono-exponentially toward the baseline, with time constants of ≈20 ms, whereas the transients observed with 50 mM ΔMelibiose exhibit the largest peak current and a significantly faster exponential decay (τ ≈ 10 ms) followed by a small negative phase.
Table 2.
Characteristics of LacY wild-type and mutant preparations
| Characteristic | Wild-type LacY | C154G LacY | E325A LacY |
|---|---|---|---|
| Particles per μm2 | ≈4,500 | ≈3,500 | ≈3,500 |
| Peak current, pA | 960 ± 190 | 102 ± 67 | 211 ± 50 |
| Q, pC | 96 ± 19 | 5 ± 2 | 4.4 ± 0.4 |
The number of particles per μm2 was estimated from freeze–fracture images (Fig. S2 and Fig. S3). The peak currents refer to the transients after 50 mM lactose concentration jumps at pH 7.6. Conditions are as described in Figs. 1 and 4. The total translocated charge (Q) is obtained from numerical integration of the currents. Charge translocation per turnover of the wild-type was estimated by using the current of 960 pA and an estimated turnover rate of 10 s−1.
Mutant C154G binds sugar as well as wild-type and exhibits extremely low, but significant, transport activities (26–29). Proteoliposomes were prepared at an LPR of 5 and a particle density of ≈3,500 particles per μm2 (Fig. S3B). Concentration jumps with 50 mM lactose, lactulose, or melibiose generate transient currents of comparable magnitude as E325A LacY (compare Fig. 4 A and B), which are also abolished by NEM treatment (Fig. S4B). In contrast to E325A, the magnitude and kinetics of the peak currents recorded with C154G LacY depend on the sugar used (Fig. 4B). Concentration jumps of 50 mM lactose or lactulose trigger transient currents that decay mono-exponentially, with time constants of ≈20 ms. Interestingly, a 50 mM melibiose concentration jump generates the largest peak current with a significantly faster exponential decay (τ ≈ 10 ms) followed by a small negative phase.
From the transient currents measured, the kinetics of the true transport currents generated by the mutants can be reconstructed by using an iterative least-squares deconvolution algorithm (Fig. S5 A and B). This operation requires a transfer function for the specific measurements determined as described (30). The transfer function is the derivative of the substrate concentration rise at the surface of the SSM, and corresponds to the time resolution of the measurement (15 ms) (13, 30). However, significantly faster processes (k < 200 s−1; see Fig. S5) can be resolved with a least-square deconvolution algorithm (30). With this deconvolution procedure, the time constants for the underlying charge displacement are determined. For E325A LacY, regardless of the sugar used, the transient currents are indistinguishable from the transfer function, indicating that charge translocation is too fast to be resolved by the measurements. In this case, we can only estimate a lower limit for the rate constant (k) of the process as >200 s−1 from the iterative least-squares algorithm (Fig. S5A). For C154G LacY, the rate constants for 50 mM concentration jumps of lactose or lactulose are 53 ± 5 s−1 or 72 ± 5 s−1, respectively, but the k for a 50 mM concentration jump of melibiose is too rapid for accurate measurement (k > 200 s−1).
Discussion
Wild-Type LacY.
Reconstitution of LacY using the procedure described previously (21, 31, 32) results in proteoliposomes with ≈85% of the LacY molecules in the right-side-out orientation (i.e., with the periplasmic side facing the exterior of the proteoliposomes) (33). Therefore, application of a substrate concentration jump corresponds to substrate transport in the physiological direction. All transported sugars trigger positive transient currents, in agreement with the displacement of positive charge (H+) into the proteoliposomes, as a result of downhill sugar/H+ symport. Of all sugars tested, lactose generates the largest transient current, consistent with it being the most efficiently transported substrate. The currents depend on pH and substrate concentration, and are blocked by alkylation with NEM. Also, good correlation is observed between kinetic parameters determined in our electrophysiological measurements and the values previously obtained from downhill sugar/H+ symport in proteoliposomes (23). All these observations strongly support the contention that the transient currents reflect specifically the electrogenic activity of LacY.
Because the decay time constant of the currents strongly depends on the number of transporters incorporated into the proteoliposomes, and decreases at high transport activity, it is concluded that this phase reflects charging of the liposomes as a result of downhill lactose/H+ symport. A comparable dependence of the decay time constant on the electrogenic transport activity of bacteriorhodopsin was described for purple membrane adsorbed to a planar lipid bilayer (17). Likewise, increasing the amount of reconstituted Na+/H+ exchanger in proteoliposomes adsorbed to the SSM resulted in a faster decay of the transient currents (34). In both cases the measured peak currents have been attributed to the continuous transport activity of the corresponding transporter, and the same applies to LacY.
It is interesting to compare the LacY currents with those observed with MelB, at similar time resolutions. With MelB, biphasic current patterns are observed decaying with time constants of τ1 ≈ 17 ms and τ2 ≈ 380 ms (18). The fast phase τ1 is due to an electrogenic conformational transition triggered by melibiose binding, whereas the slow phase τ2 is related to downhill sugar/Na+ symport activity of MelB. With LacY, a fast initial current phase is not observed. This deficiency is especially apparent in the current recorded at low transporter density, which decays slowly with a τ1/2 = 260 ± 2 ms (Fig. 2). Such behavior can only be explained if the initial electrogenic reactions are slow or if charge translocation occurs late in the LacY reaction cycle. As discussed below, sugar binding occurs with a rate constant of >50 s−1, ruling out a slow initial step. Although this argument is strictly valid for the mutants only, the fact that the mutants are fully capable of sugar binding indicates that the same rate constants apply also to the wild-type. Together, the data indicate that the major electrogenic step in wild-type LacY occurs late in the reaction cycle.
LacY Mutants.
The transient currents of the mutants E325A and C154G differ drastically from the response of the wild-type. They are 5–10 times smaller (Table 2) and considerably faster than the transients observed with wild-type LacY. Smaller transient currents could be interpreted as residual downhill sugar/H+ symport with reduced turnover in E325A and C154G. However, this residual symport would lead not only to smaller current amplitude, but also to slower decay (17). A good example for this behavior is the transient currents observed with wild-type LacY at pH 6.6, which are ≈5 times smaller, and decay 5 times slower than at pH 8.5 (Table 1; Fig. 3). In contrast, the transient currents observed with both mutants at pH 7.5 decay even faster than the transients observed with the wild-type at maximal activity (pH 8.5). This observation indicates that the currents observed with E325A and C154G LacY are not generated by sugar/H+ symport, but rather represent sugar binding induced electrogenic conformational transitions.
A comparison between mutants and wild-type (Table 2) must take into account the amount of reconstituted protein in the membrane of the adsorbed proteoliposomes on the sensor. Freeze fracture electron microscopy shows a comparable transporter density for both mutants and the wild-type. The integrated charge of C154G and E325A LacY of ≈5 pC (Table 2) represents the charge translocated in a single turnover by all transporters on the SSM electrode. By comparison, the translocated charge per turnover of wild-type LacY is much larger; ≈96 pC (Table 2). By taking into account the somewhat different particle density of wild-type and mutant proteoliposomes, it is concluded that substrate binding to the mutants induces a charge displacement corresponding to the transport of the equivalent of only ≈6% of an elementary charge across the membrane.
Although smaller, these transient currents can be used to estimate binding rate constants for the different sugars and mutants. They range between 50 and >200 s−1, and depend on the nature of substrate and mutant. Recent stopped-flow experiments reveal that the binding of p-nitrophenyl α,d-galactopyranoside (α-NPG) to C154G/V331C LacY in detergent micelles is a 2-step process: a binding step followed by a slower conformational change with a rate constant of 238 s−1 detected with fluorescent-labeled protein (35). Clearly, sugar binding triggers a conformational change with a rate constant similar to that observed in the electrophysiological experiments, which may be responsible for the charge translocation observed. Unfortunately, a direct comparison of rate constants is not possible because of the strong interaction of α-NPG with the membrane that generates large electrical artifacts.
Electrogenic Steps in the LacY Reaction Cycle.
Combining the results obtained with wild-type LacY and the mutants, there is clear evidence for a major electrogenic step late in the reaction cycle, and a rapid electrogenic reaction during or immediately after sugar binding with relatively low electrogenicity (6% of an elementary charge). It seems unlikely that the latter is a unique property of the E325A and C154G mutants. Most probably, this electrogenic event also takes place in the wild-type, but is completely masked by the 20-fold greater charge transport activity of the wild-type, and is only observed under conditions where sugar/H+ symport is blocked, as in the mutant proteins.
Based on previous observations on the kinetics of LacY (20, 22, 36, 37), and on these electrophysiological findings, we propose that the main electrogenic step corresponds to deprotonation of wild-type LacY in the inward-facing conformation. In this context, it is notable that: (i) structural, biochemical, and biophysical data strongly support the contention that wild-type and C154G LacY are predominantly in an inward-facing conformation (1, 2, 5–8); (ii) the rate of efflux from proteoliposomes reconstituted with purified LacY is strongly influenced by the voltage across the membrane, whereas exchange is completely voltage independent (21); thus, the main electrogenic step is related to protonation/deprotonation of LacY in either the inward- or outward-facing conformations or the return of the empty carrier; and (iii) the shape of the transient currents indicates that the main electrogenic step in wild-type LacY occurs late in the reaction cycle (see above). Considering these points, it is likely that the inward-facing deprotonated transporter is the initial state, and that deprotonation corresponds to the main electrogenic step of the transport cycle. Following this argument, the main electrogenic step would take place at the end of the transport cycle, in agreement with the conclusions obtained from the analysis of the transient currents. Because downhill lactose/H+ symport is specifically inhibited 3- to 4-fold in deuterium oxide, deprotonation is probably not only the main electrogenic step, but is also rate limiting (22).
With C154G LacY, all transport reactions, including equilibrium lactose exchange, are almost abolished (16, 38), indicating that the substrate translocation step is blocked. In the absence of substrate, this mutant is in an inward-facing conformation, and paralyzed in an open conformation on the periplasmic side, but able to close on the cytoplasmic side on lactose binding (6–8, 39). E325A LacY exhibits no active transport whatsoever; however, in contrast to C154G LacY, mutant E325A catalyzes exchange and counterflow at least as well as wild-type LacY, indicating that this mutant is permanently protonated (i.e., H+ dissociation is blocked in E325A LacY but binding is normal) (24, 25, 28). In both mutants, a rapid but weakly electrogenic reaction with similar magnitude and kinetic properties is seen, which agrees with our notion that periplasmic H+ binding is not responsible for the charge translocation observed, and that the rapid initial charge displacement of minor electrogenicity is associated with sugar binding. Possibly, the rapid conformational transition after sugar binding (35) leads to rearrangement of charged residues within LacY (3, 40), which may account for this phenomenon.
Materials and Methods
Construction of Mutants and LacY Purification.
Construction of mutants and purification of the His-tagged proteins were carried out as described (7). Purified proteins (10–15 mg/mL) in 50 mM NaPi/0.02% n-dodecyl-beta-d-maltoside (pH 7.5) were frozen in liquid nitrogen and stored at −80 °C until use.
Reconstitution of Proteoliposomes.
Reconstitution of purified wild-type or the E325A and C154G mutants was carried out with E. coli phospholipids (Avanti Polar Lipids) by using dodecyl maltoside/octyl glucoside dilution, followed by 1 cycle of freeze–thaw/sonication (41, 42). Purified wild-type LacY and liposomes were mixed at an LPR of 10 or 5 (wt/wt), as indicated. Mutant E325A or C154G was reconstituted at an LPR of 5. Before use, the samples were thawed on ice and gently sonicated for 2–5 s. Reconstitution was verified in all cases by freeze–fracture electron microscopy.
SSM Measurements.
SSM measurements were performed as described previously (18, 30, 43). Briefly, 40 μL of proteoliposomes at a protein concentration of 1 mg/mL was allowed to adsorb for 1 h to an octadecanethiol/phosphatidylcholine hybrid bilayer on a gold surface (the sensor). The solution exchange protocol consisted in 3 phases of duration 1.5, 2, and 1.5 s, respectively. The nonactivating solution flows through the cuvette during the first and third phases (from t = 0 to 1.5 s, and from t = 3.5 to 5 s), whereas the activating solution flows during the second phase (from t = 1.5 to t = 3.5 s). A valveless diverted fluidic geometry was chosen to apply the different solutions (30) at a flow rate of 0.46 mL/s. The nonactivating solution always contained 50 mM glucose, and the activating solution contained a given sugar at a concentration of 50 mM, unless stated otherwise (Fig. 3). All solutions were buffered in 100 mM potassium phosphate buffer at a given pH value plus 1 mM DTT. Currents were recorded throughout the entire time, and amplified with a current amplifier set to a gain of 109-1010 V/A and low pass filtering set to 300–1,000 Hz.
Supplementary Material
Acknowledgments.
We thank Lina Hatahet and Andre Bazzone for excellent assistance in the laboratory, Ernst Bamberg for helpful discussions and support, and Winfred Haase for the excellent freeze–fracture micrographs of the reconstituted liposomes. This work was funded by Deutsch Forschungsgemeinshaft SFB 807 (to K.F.); National Institutes of Health Grants DK051131, DK069463, GM073210, and GM074929; and National Science Foundation Grant 0450970 (to H.R.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902471106/DCSupplemental.
References
- 1.Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 2.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci USA. 2007;104:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H+ symport in LacY. EMBO J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaback HR, et al. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci USA. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majumdar DS, et al. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Natl Acad Sci USA. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smirnova I, et al. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci USA. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc Natl Acad Sci USA. 2008;105:3774–3778. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West IC. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970;41:655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- 10.West IC, Mitchell P. Proton movements coupled to transport of beta-galactosides into Escherichia coli. Biochem J. 1972;127:P56. doi: 10.1042/bj1270056pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel L, Garcia ML, Kaback HR. Direct measurement of lactose/proton symport in Escherichia coli membrane vesicles: Further evidence for the involvement of histidine residue(s) Biochemistry. 1982;21:5805–5810. doi: 10.1021/bi00266a013. [DOI] [PubMed] [Google Scholar]
- 12.Seifert K, Fendler K, Bamberg E. Charge transport by ion translocating membrane proteins on solid supported membranes. Biophys J. 1993;64:384–391. doi: 10.1016/S0006-3495(93)81379-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz P, Garcia-Celma JJ, Fendler K. SSM-based electrophysiology. Methods. 2008;46:97–103. doi: 10.1016/j.ymeth.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Trumble WR, Viitanen PV, Sarkar HK, Poonian MS, Kaback HR. Site-directed mutagenesis of cys148 in the lac carrier protein of Escherichia coli. Biochem Biophys Res Commun. 1984;119:860–867. doi: 10.1016/0006-291x(84)90853-2. [DOI] [PubMed] [Google Scholar]
- 15.Bieseler B, Prinz H, Beyreuther K. Topological studies of lactose permease of Escherichia coli by protein sequence analysis. Ann N Y Acad Sci. 1985;456:309–325. doi: 10.1111/j.1749-6632.1985.tb14882.x. [DOI] [PubMed] [Google Scholar]
- 16.van Iwaarden PR, Driessen AJ, Lolkema JS, Kaback HR, Konings WN. Exchange, efflux, and substrate binding by cysteine mutants of the lactose permease of Escherichia coli. Biochemistry. 1993;32:5419–5424. doi: 10.1021/bi00071a017. [DOI] [PubMed] [Google Scholar]
- 17.Bamberg E, et al. Photocurrents generated by bacteriorhodopsin on planar bilayer membranes. Biophys Struct Mech. 1979;5:277–292. [Google Scholar]
- 18.Ganea C, Pourcher T, Leblanc G, Fendler K. Evidence for intraprotein charge transfer during the transport activity of the melibiose permease from Escherichia coli. Biochemistry. 2001;40:13744–13752. doi: 10.1021/bi011223k. [DOI] [PubMed] [Google Scholar]
- 19.Meyer-Lipp K, Ganea C, Pourcher T, Leblanc G, Fendler K. Sugar binding induced charge translocation in the melibiose permease from Escherichia coli. Biochemistry. 2004;43:12606–12613. doi: 10.1021/bi0489053. [DOI] [PubMed] [Google Scholar]
- 20.Kaczorowski GJ, Kaback HR. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 1. Effect of pH on efllux, exchange, and courterflow. Biochemistry. 1979;18:3691–3697. doi: 10.1021/bi00584a009. [DOI] [PubMed] [Google Scholar]
- 21.Garcia ML, Viitanen P, Foster DL, Kaback HR. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 1. Effect of pH and imposed membrane potential on efflux, exchange, and counterflow. Biochemistry. 1983;22:2524–2531. doi: 10.1021/bi00279a033. [DOI] [PubMed] [Google Scholar]
- 22.Viitanen P, Garcia ML, Foster DL, Kaczorowski GJ, Kaback HR. Mechanism of lactose translocation in proteoliposomes reconstituted with lac carrier protein purified from Escherichia coli. 2. Deuterium solvent isotope effects. Biochemistry. 1983;22:2531–2536. doi: 10.1021/bi00279a034. [DOI] [PubMed] [Google Scholar]
- 23.Viitanen P, Garcia ML, Kaback HR. Purified reconstituted lac carrier protein from Escherichia coli is fully functional. Proc Natl Acad Sci USA. 1984;81:1629–1633. doi: 10.1073/pnas.81.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrasco N, Antes LM, Poonian MS, Kaback HR. lac permease of Escherichia coli: Histidine-322 and glutamic acid-325 may be components of a charge-relay system. Biochemistry. 1986;25:4486–4488. doi: 10.1021/bi00364a004. [DOI] [PubMed] [Google Scholar]
- 25.Carrasco N, et al. Characterization of site-directed mutants in the lac permease of Escherichia coli. 2. Glutamate-325 replacements. Biochemistry. 1989;28:2533–2539. doi: 10.1021/bi00432a028. [DOI] [PubMed] [Google Scholar]
- 26.Menick DR, Sarkar HK, Poonian MS, Kaback HR. cys154 Is important for lac permease activity in Escherichia coli. Biochem Biophys Res Commun. 1985;132:162–170. doi: 10.1016/0006-291x(85)91002-2. [DOI] [PubMed] [Google Scholar]
- 27.Menick DR, Lee JA, Brooker RJ, Wilson TH, Kaback HR. Role of cysteine residues in the lac permease of Escherichia coli. Biochemistry. 1987;26:1132–1136. doi: 10.1021/bi00378a022. [DOI] [PubMed] [Google Scholar]
- 28.Sahin-Toth M, Kaback HR. Arg-302 facilitates deprotonation of Glu-325 in the transport mechanism of the lactose permease from Escherichia coli. Proc Natl Acad Sci USA. 2001;98:6068–6073. doi: 10.1073/pnas.111139698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smirnova IN, Kaback HR. A mutation in the lactose permease of Escherichia coli that decreases conformational flexibility and increases protein stability. Biochemistry. 2003;42:3025–3031. doi: 10.1021/bi027329c. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Celma JJ, et al. Rapid activation of the melibiose permease MelB immobilized on a solid-supported membrane. Langmuir. 2008;24:8119–8126. doi: 10.1021/la800428h. [DOI] [PubMed] [Google Scholar]
- 31.Foster DL, Garcia ML, Newman MJ, Patel L, Kaback HR. Lactose-proton symport by purified lac carrier protein. Biochemistry. 1982;21:5634–5638. doi: 10.1021/bi00265a038. [DOI] [PubMed] [Google Scholar]
- 32.Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981;256:11804–11808. [PubMed] [Google Scholar]
- 33.Herzlinger D, Viitanen P, Carrasco N, Kaback HR. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 2. Binding studies with membrane vesicles and proteoliposomes reconstituted with purified lac carrier protein. Biochemistry. 1984;23:3688–3693. doi: 10.1021/bi00311a018. [DOI] [PubMed] [Google Scholar]
- 34.Zuber D, et al. Kinetics of charge translocation in the passive downhill uptake mode of the Na+/H+ antiporter NhaA of Escherichia coli. Biochim Biophys Acta. 2005;1709:240–250. doi: 10.1016/j.bbabio.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Smirnova IN, Kasho VN, Kaback HR. Direct sugar binding to LacY measured by resonance energy transfer. Biochemistry. 2006;45:15279–15287. doi: 10.1021/bi061632m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaczorowski GJ, Robertson DE, Kaback HR. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 2. Effect of imposed delta psi, delta pH, and delta mu H+ Biochemistry. 1979;18:3697–3704. doi: 10.1021/bi00584a010. [DOI] [PubMed] [Google Scholar]
- 37.Robertson DE, Kaczorowski GJ, Garcia ML, Kaback HR. Active transport in membrane vesicles from Escherichia coli: The electrochemical proton gradient alters the distribution of the lac carrier between two different kinetic states. Biochemistry. 1980;19:5692–5702. doi: 10.1021/bi00566a005. [DOI] [PubMed] [Google Scholar]
- 38.van Iwaarden PR, Driessen AJ, Menick DR, Kaback HR, Konings WN. Characterization of purified, reconstituted site-directed cysteine mutants of the lactose permease of Escherichia coli. J Biol Chem. 1991;266:15688–15692. [PubMed] [Google Scholar]
- 39.Nie Y, Sabetfard FE, Kaback HR. The Cys154→Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379:695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinglass A, Whitelegge JP, Faull KF, Kaback HR. Monitoring conformational rearrangements in the substrate-binding site of a membrane transport protein by mass spectrometry. J Biol Chem. 2004;279:41858–41865. doi: 10.1074/jbc.M407555200. [DOI] [PubMed] [Google Scholar]
- 41.Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- 42.Costello MJ, et al. Purified lac permease and cytochrome o oxidase are functional as monomers. J Biol Chem. 1987;262:17072–17082. [PubMed] [Google Scholar]
- 43.Pintschovius J, Fendler K. Charge translocation by the Na+/K+-ATPase investigated on solid supported membranes: Rapid solution exchange with a new technique. Biophys J. 1999;76:814–826. doi: 10.1016/S0006-3495(99)77245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.