Abstract
Estrogen receptor α (ERα) and E-cadherin are primary markers of luminal epithelial breast cancer cells with E-cadherin being a main caretaker of the epithelial phenotype. E-cadherin repression is needed for cancer cells to acquire motile and invasive properties, and it is known that in ER-positive breast cancer cells, estrogen down-regulate E-cadherin gene transcription. We report here that ERα is bound to the E-cadherin promoter in both the presence and the complete absence of estrogen, suggesting an unexpected role for unliganded ERα in E-cadherin transcription. Indeed, our data reveal that activation by unliganded ERα and repression by estrogen-activated ERα require direct binding to a half-estrogen response element within the E-cadherin promoter and exchange from associated coactivators to corepressors. Therefore, these results suggest a pivotal role for unliganded ERα in controlling a fundamental caretaker of the epithelial phenotype in breast cancer cells. Here, we show that ERα-positive breast cancer T47D cells transduced with the sfRON kinase undergo a full epithelial–mesenchymal conversion and lose E-cadherin and ERα expression. Our data show that, although the E-cadherin gene becomes hypermethylated and heterochromatic, kinase inhibitors can restore E-cadherin expression, together with an epithelial morphology in an ERα-dependent fashion. Similarly, transfection of ERα, in the absence of ligands, was sufficient to restore E-cadherin transcription in both sfRON-T47D and other ERα-, E-cadherin-negative cells. Therefore, our results suggest a novel role for the ERα that plays the dual role of ligand-independent activator and ligand-dependent repressor of E-cadherin in breast cancer cells.
Keywords: E-cadherin, epithelial to mesenchymal transition, estrogen, invasion
Estrogen receptor α (ERα) plays a fundamental role in mammary gland development and function and in breast cancer development and progression. The picture emerging from extensive gene expression profiling of human breast tumors tissues and cell lines clearly links ERα to the genetic program specifying the epithelial phenotype of luminal type (1–4). ER+ breast carcinoma cells grow in vitro as organized, polygonal cells that maintain cell-to-cell contacts, whereas ER− cells more often show a mesenchymal morphology. This observation agrees with the older notion that ER+ tumors are generally more differentiated and less invasive.
In carcinoma, the loss of epithelial characteristics is a prerequisite for the acquisition by cancer cells of several properties, including cell motility, invasion, intravasation, and metastasis (5), in a process that mimics the developmental epithelial-to-mesenchymal transition (EMT). EMT is characterized by a wide genetic reprogramming that primarily involves the suppression of E-cadherin, a central caretaker of the epithelial phenotype (6), in addition to activation or repression of several other genes. Transcription of the E-cadherin encoding gene, CDH1, in epithelial cells is guaranteed by several positive transcription factors, primarily Sp1 (7) and AP-2 proteins (8, 9), whereas during EMT the concerted and cell-specific action of a number of repressors (Snail, Slug, Zeb1, Zeb2, Twist) binding to several E-boxes in the E-cadherin promoter is controlled by different signal transduction pathways and leads to hypermethylation and heterochromatization of the gene (8, 10). In development, a number of signaling pathways are involved in E-cadherin silencing, including those activated by FGF, PDGF, EGF, TGFβ, BMP, and Wnt. Other signals, including hypoxia, inflammation, and other microenvironmental conditions also play a relevant role in other contexts (6). The loss of E-cadherin expression in invasive cancer is accompanied in some cases by gene mutation, but more often E-cadherin is silenced with no structural alteration and shows promoter CpG island hypermethylation (11), suggesting that mechanisms linked to normal suppressive pathways may operate in cancer cells. Coherently, overexpression of repressors such as Snail, Slug, or Twist is often found in cancer (12, 13) and is possibly linked to the constitutive activation of the signaling pathways described above.
Several studies have reported down-regulation of E-cadherin mRNA and protein expression by estrogen in breast, ovarian, and endometrial cancer cells (14–16). E-cadherin was also found among down-regulated genes in microarray studies (17). Down-regulation of E-cadherin by estrogen is congruent with the common notion that the hormone stimulates progression of breast cancer and, consequently, with the mitogenic and motogenic activity of estrogen (18). However, estrogen induces an easily-reversible and only partial EMT-like response (18) that is very different from the complete EMT, accompanied by E-cadherin silencing, that is seen in ER-, mesenchymal-like breast cells.
Therefore, it is important to ascertain whether a direct link exists between the expression and activity of ERα and the transition to a mesenchymal phenotype mediated by regulation of E-cadherin transcription.
In this work, we set out to understand the functional relationship of ERα and E-cadherin in breast cancer cells. Our results show that unliganded ERα is necessary and sufficient to sustain basal expression of E-cadherin, also when reversal of a silenced heterochromatic status is required, through direct binding to the E-cadherin gene promoter.
Results
ERα Recruitment at the E-Cadherin Promoter Is Independent of Ligand.
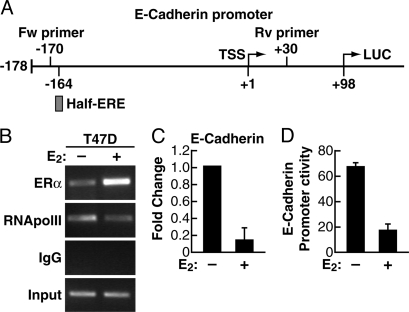
The concept that ERα can repress transcription of many genes by direct interaction has emerged clearly from ChIP on chip (ChIP-chip) studies (19, 20). Interestingly in one report E-cadherin transcriptional repression by estrogen in MCF7 cells was shown to be associated to ERα binding to a transfected, but not to the endogenous, E-cadherin promoter sequence (14). By screening the 5′ flanking sequence of E-cadherin at low stringency with a matrix obtained by examining several ERα promoter binding sites (20) we identified several putative estrogen response element (ERE) sequences, including a perfect half-ERE at −164 (Fig. 1A). Therefore, we investigated whether the proximal portion of the endogenous E-cadherin promoter may bind ERα during estrogen treatment. ChIP analysis of ER+ T47D breast carcinoma cells showed that indeed ERα is bound to the region containing this half-ERE (Fig. 1B) during estrogen down-regulation of E-cadherin transcription (Fig. 1C). In keeping with published results (14), 17β-estradiol (E2) also repressed the activity of a transfected reporter vector carrying the proximal E-cadherin promoter (−178/+92) and driving luciferase transcription (Fig. 1D), further supporting the notion that this fragment is necessary for mediating estrogen activity on E-cadherin transcription. Moreover, in these experiments, we noticed that ERα was also bound to E-cadherin in anaestrogenic conditions, though to a lesser extent (Fig. 1B), suggesting that unliganded ERα may play a role in basal E-cadherin transcription, in keeping with the fact that ERα and E-cadherin are consistently coexpressed in cells with epithelial phenotype and both are lost during EMT.
Fig. 1.
Association of unliganded and ligand-activated ERα with E-cadherin promoter and effect on E-cadherin expression. (A) Schematic representation of the Luc-reporter construction, showing location of the half-site ERE at position −164/−160 in the E-cadherin promoter. (B) The ER+ T47D cells were grown in estrogen-free medium for 72 h. Then, the cells were treated with either ethanol vehicle (−E2) or E2 for 90 min. ChIP assay was performed with anti-ERα and anti-RNApolII antibodies. Input DNA was used to normalize the results. (C) Quantitative real-time PCR was used to evaluate changes in E-cadherin mRNA level in T47D cells similarly grown and treated with either ethanol vehicle (−E2) or E2 for 90 min. (D) T47D cells were grown in estrogen-free medium for 24 h, then were transiently transfected with E-cadherin promoter-Luc vector. After 48 h, cells were treated with either ethanol vehicle (−E2) or E2 for 90 min, and luciferase activity was measured and normalized by using β-gal activity.
EMT Transition is Accompanied by Silencing of E-Cadherin Together with ERα in T47D Cells Transduced with sfRON.
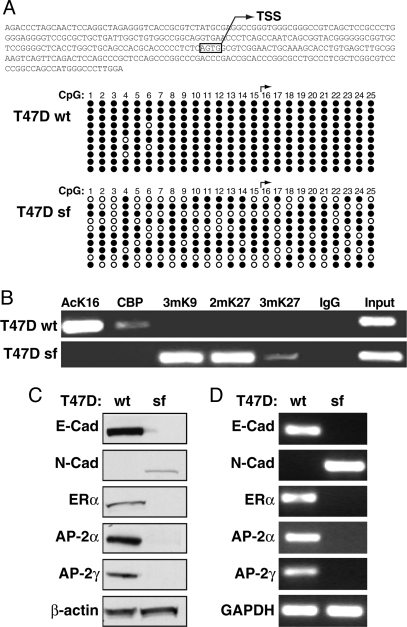
To examine this hypotesis, we looked for a cell model system where silencing of E-cadherin and ERα and consequent EMT were obtained starting from an ER+ epithelial background. We reported previously that transduction of a constitutively-active truncated form of the RON tyrosine kinase (MST1R) into breast carcinoma T47D cells led to morphological changes, increased growth, motility, and invasion, and suppression of E-cadherin expression, compatible with an EMT, apparently caused by increased expression of the zinc-finger repressor Slug (21). Thus, we first characterized sfRON-T47D cells to evaluate the extent of their mesenchymal conversion. The chromatic status of E-cadherin was addressed by CpG methylation analysis, revealing that the E-cadherin promoter is hypermethylated in sfRON-T47D cells, as compared with wild-type (wt) T47D cells (Fig. 2A). ChIP analysis of the same E-cadherin region also demonstrated that H3K9 is trimethylated, and H3K27 is dimethylated and trimethylated, while general histone acetylaton is lost (Fig. 2B), clearly showing that the E-cadherin gene is heterochromatic in sfRON-T47D cells. In addition to E-cadherin, these cells have lost other features of breast epithelial cells such as the expression of AP-2α and AP-2γ factors, which are positive regulators of E-cadherin (8, 9), while they express the nonepithelial N-cadherin gene. As expected, sfRON-T47D have also completely lost ERα expression at both the RNA and protein level (Fig. 2 C and D).
Fig. 2.
Effect of a constitutively-active tyrosine kinase (sfRON) on chromatin organization at the E-cadherin gene promoter and change of ERα status in sfRON-T47D cells. (A) CpG methylation analysis of the CDH-1 promoter. The scheme shows the region analyzed. For each CpG (numbered from 5′ to 3′) black dots are unmethylated and open dots are methylated CpG. (B) Heterochromatic markers are enriched at the E-cadherin promoter in sfRON-T47D. ChIP assay was performed by using antibodies against acetyl-lysine 16 histone H4 (AcK16), trimethyl-lysine 9 of histone H3 (3mK9), dimethyl-lysine 27 of histone H3 (2mK27), trimethyl-lysine 27 of histone H3 (3mK27), and CBP. (C) Whole-cell protein extracts were subjected to immunoblotting for E-cadherin (E-Cad), N-cadherin (N-Cad), ERα, AP-2α, AP-2γ, and β-actin, showing equal protein loading. (D) End-point PCR was used to evaluate changes in E-cadherin (E-Cad), N-cadherin (N-Cad), ERα, AP-2α, and AP-2γ mRNA level as compared with GAPDH control.
ERα Is Sufficient to Activate Basal Expression of E-Cadherin in sfRON-T47D.
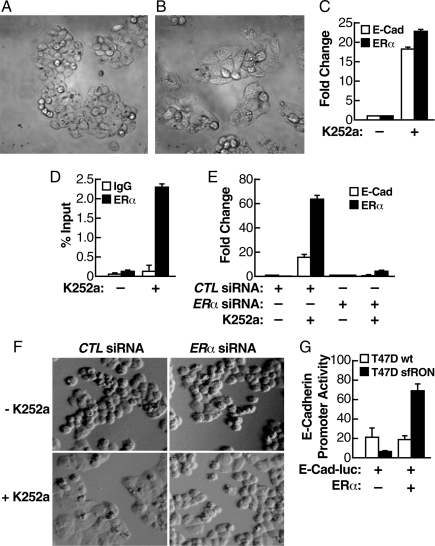
Next, we attempted to reverse the mesenchymal phenotype by interfering with the RON transduction pathway. We have used several reagents to extinguish the Ron-activated signaling pathway, and the most effective molecule turned out to be the kinase inhibitor K252a that inhibits several receptor and nonreceptor kinases in this pathway (22). All of the experiments were run in estrogen-free media to avoid potential repression of E-cadherin by estrogen-activated ERα, as shown before. As shown in Fig. 3C, treatment of sfRON-T47D cells with K252a led to reexpression of both E-cadherin and ERα mRNA, together with an evident morphological reversion to a polygonal cell type (Fig. 3B, cells treated with K252a, compared with Fig. 3A, cells treated with vehicle alone). Interestingly, as demonstrated by ChIP analysis, unliganded ERα was recruited to the E-cadherin promoter after K252a treatment (Fig. 3D).
Fig. 3.
Unliganded ERα is sufficient to activate the basal expression of E-cadherin in sfRON-T47D. (A and B) Reversion of the morphological penotype in sfRON-T47D cells by a kinase inhibitor. Cells were grown in estrogen-free medium for 72 h and then treated with K252a (B) or control vehicle (A) for 48 h. Images were taken at 40× magnification. (C) Quantitative real-time PCR was used to evaluate changes in E-cadherin and ERα mRNA level in sfRON-T47D cells in the presence of K252a or control vehicle. (D) K252a treatment induces ERα recruitment at the E-cadherin promoter. ChIP with quantitative real-time PCR was performed by using antibodies against ERα. (E) Induction of E-cadherin mRNA by K252a depends on ERα. sfRON-T47D cells were transfected with a control siRNA or a siRNA against ERα, grown in estrogen-free medium for 72 h, and treated with K252a or control vehicle. (F) The same experiment as in E demonstrates that ERα is required for morphological reversal in the presence of K252a. The images were taken at 40× magnification. (G) ERα expression induces E-cadherin-Luc reporter activity. Cells were grown in estrogen-free medium for 24 h then were transiently transfected with E-cadherin promoter-Luc vector, ERα-expressing vector, or empty vector. After 48 h luciferase activity was measured and normalized to β-gal activity.
These observations strongly suggest that unliganded ERα has a functional role in the reexpression of E-cadherin. To address this question directly, we transfected sfRON-T47D cells with a siRNA specific for ERα or a control siRNA, then we treated the cells with K252a for 48 h in estrogen-free medium. As shown in Fig. 3E, ERα is down-regulated by specific siRNA and its down-regulation prevents reexpression of E-cadherin mRNA after K252a treatment. In agreement, cells treated with control siRNA showed an evident morphological change in response to K252a treatment with most of the cells flattened and contiguous (Fig. 3F Left), whereas cells treated with ERα siRNA did not change morphology under K252a treatment (Fig. 3F Right). To obtain direct proof of the action of ERα on reactivation of E-cadherin transcription, we transfected ERα in sfRON-T47D cells, together with the E-cadherin-Luc reporter, in estrogen-free medium. ERα induced transcription from the E-cadherin promoter in transduced cells, but had no effect on ER+ wt T47D cells (Fig. 3G).
Unliganded ERα Restores E-Cadherin Expression in Other E-Cadherin Negative Cells.
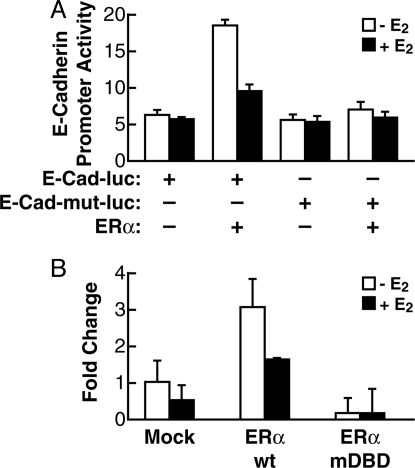
These data strongly suggest that ERα has a pivotal role for E-cadherin gene transcription in sfRON-T47D cells, where E-cadherin is completely silenced, heterochromatic, and hypermethylated. Therefore, we asked whether this action could be seen also in different cells, not of breast origin, where E-cadherin is silenced. In HeLa cells of cervical cancer origin it was demonstrated that E-cadherin is silenced by hypermethylation (23). HeLa cells also lack detectable ERα protein expression (24). Using HeLa cultured in estrogen-free medium, we performed a reporter assay using the wild-type E-cadherin-Luc vector and a mutated version disrupting the putative half-ERE at position −164. As illustrated in Fig. 4A, transfection of ERα increased the activity of the WT reporter, confirming the role of ERα on the basal activity of E-cadherin promoter in the absence of ligand, but not that of the ERE-dead mutant, indicating that this element is required for induction of transcription by unliganded ERα. Notably, also in this case, the treatment with E2 inhibited reporter transcription in the presence of ERα.
Fig. 4.
Unliganded ERα regulates E-cadherin expression in HeLa cells. (A) HeLa cells were grown in estrogen-free medium for 24 h then transiently transfected with E-cadherin promoter-Luc vector or E-cadherin promoter-Luc vector ERE-dead mutant, along with ERα-expressing vector or empty vector. After 48 h of transfection, the cells were treated with either ethanol vehicle (−E2) or E2 for 90 min, and luciferase activity was measured and normalized to β-gal activity. (B) HeLa cells were grown as above and transiently transfected with of ERα wt or ERα mutDBD-expressing vectors or empty vector. After 48 h the cells were treated with either ethanol vehicle (−E2) or E2 for 90 min. Quantitative real-time PCR was used to evaluate changes in E-cadherin mRNA.
To see whether in HeLa cells forced expression of ERα could reactivate endogenous E-cadherin transcription, we transfected wild-type ERα and an ERα mutant carrying a triple point mutation in the DNA binding domain (mutDBD), which greatly reduces the affinity of the receptor for the ERE (25), and measured E-cadherin mRNA levels. As predicted by our previous observation, ERα is sufficient to reactivate transcription of the endogenous E-cadherin gene in these cells (Fig. 4A). We further demonstrated that direct binding of ERα to DNA is necessary, because the DBD-dead mutant did not show any activity in this assay (Fig. 4B).
ERα Has a Dual Role in E-Cadherin Transcriptional Regulation.
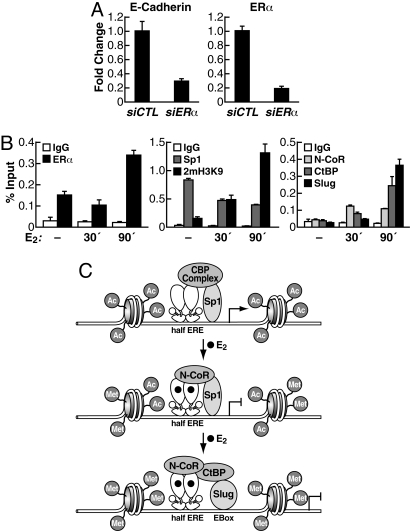
Taken together, our results support the idea that unliganded ERα is involved in the basal expression of E-cadherin and maintenance of the epithelial morphology. To further test this hypothesis we asked whether perturbation of ERα expression in a cell line that normally expresses ER would result in simultaneous alteration of E-cadherin expression. Indeed, transfection of siRNA against ERα in MCF-7 cells, which resulted in the evident down-regulation of ERα mRNA (Fig. 5A, Right), led to a marked reduction in E-cadherin mRNA level, whereas no decline in E-cadherin or ERα occurred with control siRNA (Fig. 5A).
Fig. 5.
Dual role of unliganded or estrogen-activated ERα on E-cadherin gene expression in ER+ cells. (A) ERα is needed for basal E-cadherin expression in epithelial cells. MCF7 cells were grown in estrogen-free medium for 24 h then were transiently transfected with siRNA against ERα or control siRNA. After 48 h quantitative real-time PCR was used to evaluate changes in E-cadherin and ERα mRNA. (B) Increased ERα binding accompanies cofactor exchange at E-cadherin promoter after estradiol treatment. MCF7 cells were grown in estrogen-free medium for 72 h; the cells were treated with either ethanol vehicle (−E2) or E2 for 30 and 90 min. ChIP with quantitative real-time PCR was performed by using antibodies against ERα, histone H3 dimethyl-lysine 9 (2mH3K9), Sp1, NCoR (rabbit serum), CtBP, and Slug. (C) Proposed model for regulation of E-cadherin gene expression mediated by unliganded and estrogen-activated ERα.
Our results in HeLa cells (Fig. 4A) support the idea that the half-ERE at the E-cadherin promoter mediates estrogen-dependent repression, in addition to ligand-independent activation by ERα. To confirm this idea, ChIP analysis was performed in MCF-7 cells by using antibodies against ERα and dimethyl-lysine 9 of histone H3 (2mH3K9), which is a marker of repressed promoters. As shown in Fig. 5B, treatment with E2 led to an increase of ERα bound to E-cadherin promoter, and H3 became more methylated on lysine 9, indicating that the chromatin in this region became a less permissive environment for transcription. The half-ERE is embedded in a CG-rich element, and Sp1 was shown to play important roles in promoting E-cadherin transcription (7). Therefore, we examined the recruitment of Sp1 at the E-cadherin promoter as a function of E2 treatment. As shown in Fig. 5B Center, Sp1 is bound to E-cadherin promoter in the absence of estrogen and is progressively dismissed after E2 treatment, indicating that when ligand-activated ERα is present the E-cadherin promoter loses an important transactivator. To examine possible corepressor complex recruitment by ligand-activated ERα at the E-cadherin promoter, we tested the presence of N-CoR, one of the major corepressor complexes for ERα, and the CtBP complex, whose function in E-cadherin silencing was associated to the action of zinc-finger repressors. As shown in Fig. 5B Right, N-CoR was recruited at the E-cadherin promoter 30 min after E2 treatment, whereas Slug and CtBP were recruited after 90 min.
Taken together, our results provide evidence of a novel role for ERα in allowing basal transcription of E-cadherin in epithelial cells, by direct binding, in conjunction with Sp1 factors, to the E-cadherin promoter. Ligand activation of ERα leads to Sp1 dismissal, recruitment of N-CoR, and consequent function of zinc-finger repressor to silence E-cadherin expression (Fig. 5C).
Discussion
Results presented here demonstrate that unliganded ERα is needed for basal transcription of the E-cadherin gene and that it is sufficient to induce reexpression of E-Cadherin, even when the E-cadherin gene is heterochromatic. This effect depends on ERα binding to a half-ERE present in the E-cadherin gene promoter; surprisingly, from the same site ERα directs E-cadherin transcriptional repression when E2 is present. We can exclude the possibility that the repression effect is caused by ERβ because our cell models do not express ERβ (Fig. S1). We have demonstrated a direct role of ERα in controlling E-cadherin expression, with elimination of ERα from an ER-positive cell line or its reintroduction in a ER-negative context, respectively triggering repression or transcription of E-cadherin. Thus, ERα may represent the prime factor controlling the expression of this gene in breast cancer cells, an idea previously suggested only by indirect evidence. In addition to a number of relational observations, the absence of ERα had been mechanistically linked to E-cadherin suppression and EMT by other studies (26, 27); indirect evidence that reexpression of endogenous ERα is linked to reversion of the invasive phenotype in breast cancer cells was recently reported (28). However, interpretation of these results was confounding, because the activation of ERα by estrogen would lead to results similar to those elicited by the absence of ERα, i.e., reversible EMT (18) and E-cadherin down-regulation (14). Results presented here reconcile these data, because they have revealed that unliganded and liganded ERα exert opposite effects on E-cadherin gene transcription.
The occupancy of the E-cadherin promoter by ERα in both estrogenic and anaestrogenic medium was suggested by Oesterreich et al. (14) who used a transfected E-cadherin promoter to show ERα binding. Here, we demonstrate ERα binding to the endogenous E-cadherin locus in T47D and MCF7 cells. In addition, we provide evidence of direct interaction of ERα with a half-ERE in E-cadherin promoter by using both an E-cadherin-luciferase reporter with deleted half-ERE and a DNA binding domain-dead ERα version. In a different context, represented by endometrial carcinoma cells, ERα binding to E-cadherin was also confirmed (16).
Ligand-independent activity of ERα has been reported by other studies (29, 30). Also, several papers reported activation of unliganded ERα by phosphorylation, phosphatases, or cAMP (31) as activated by growth factor pathways (32, 33). Moreover, ERα is known to interact with other transcription factors, such as Sp1, NF-κB, and AP-1, and may use the constitutive transactivation function of these factors in certain contexts.
One distinctive character of the present study is that, in our model system, ERα binds to, and reactivates, the silenced E-cadherin gene in sfRON-T47D cells, where it was shown to be clearly hypermethylated and heterochromatic. This result means that unliganded ERα finds access to the heterochromatic promoter and initiate events leading to chromatin remodeling and transcription initiation. We excluded the presence of very low doses of estrogen coming from cell metabolism because we observe the same effect on E-cadherin expression treating the cells with Letrozole, a common aromatase inhibitor (Fig. S2).
Other authors have attempted reexpression of ERα in a context of ER−, fibroblastoid cells such as the MDA.MB.231 cells, by adenoviral transduction. However, estrogen treatment of ERα reexpressing cells resulted in growth inhibition, rather than stimulation, either because of receptor overloading or these cells may have embryological derivation different from epithelium-like cells such as commonly used ER+ cell lines (34, 35). However, we see here reactivation of E-cadherin by ERα also in HeLa cells that are of cervical carcinoma origin.
Our results also confirm that estrogen inhibits transcription of E-cadherin. This was clearly shown by previous studies reporting down-regulation of E-cadherin protein, mRNA and reporter activity by estrogen in MCF7 cells (14) and in cells of other origins (15). While studying the activity of the Mi-Nurd component MTA3, Fujita et al. (26) reached a different conclusion, i.e., that estrogen may activate, rather than repress, E-cadherin expression. However, results reported in this study may be caused mostly by ERα per se, rather than by estrogen, because they were obtained by overexpressing ERα and evaluating the effects of tamoxifen, which is known to exert both agonistic and antagonistic context-dependent actions (17, 36). Indirect support to a suppressive effect of estrogen on E-cadherin expression is also given by several observations that estrogen treatment results in EMT-like phenotypic changes (18).
Repression by estrogen-activated ERα depends on direct interaction with the half-ERE in E-cadherin promoter. Our finding of a sequential recruitment of N-CoR and CtBP complexes after E2 treatment suggests a temporal checkpoint regulation of E-cadherin repression. Our proposed model is that E2–ERα interaction induces first the recruitment of N-CoR at the E-cadherin promoter. Formation of this first complex leads to hypoacetylation of histones (37) and, after that, the recruitment of Slug/CtBP complexes induces hypermethylation of histones (38), such as histone H3 lysine 9, which causes stabilization of the nucleosome structure, limiting accessibility to the basal transcriptional machinery and thus repressing E-cadherin gene expression. Assuming several possibilities, because Sp1 is required to promote E-cadherin expression (7), ERα might bind the half-ERE by interacting with Sp1 or ligand-activated ERα binding might lead to a conformational change that allows the increase of ERα recruitment and of corepressors assembling (Fig. 5C). This behavior in repression has been observed for other genes also containing ERE-like sequence in their promoters (39). Another possibility, not necessarily an alternative, is that nongenomic action of estrogen, perhaps acting on coactivator/corepressor distribution, may influence the response.
Factors like Slug and Snail are involved in the control of E-cadherin transcription and may also be implicated in the effects of unliganded and liganded ERα (27). Estrogen up-regulates Snail and Slug (15) that can mediate, in part, the repressive effect on E-cadherin transcription. However, half-ERE deletion experiments clearly show that ERα binding to the promoter is required for repression. Together, assembled data permit a model where ERα play a master function in a circuit that regulates transition from epithelial to mesenchymal phenotypes and reverse. Unliganded ERα binds to and activates the E-cadherin promoter and down-regulates Snail expression, further relieving repression on E-cadherin. Conversely, estrogen treatment directly represses E-cadherin transcription and stimulates Snail expression, resulting in more robust repression of E-cadherin. Overexpression of Snail is known to repress E-cadherin, but also represses the ERα encoding ESR1 gene (27).
In conclusion, our results unravel a role for the ERα that plays the dual role of ligand-independent activator and ligand-dependent repressor of E-cadherin in breast cancer cells. To understand the full genetic program that is similarly regulated by unliganded ERα and fully determine the identity of genomic locations that recruit ERα in the absence of estrogen in breast cancer cells will be of great interest.
Materials and Methods
Detailed protocols for cell culture, pharmacological treatment of cells, transient cell transfection, site-directed mutagenesis, RNA preparation, RT-PCR, and real-time quantitative PCR analysis are described in SI Text.
ChIP Assay.
Protein–DNA cross-linking was performed by adding 1% (wt/vol) formaldehyde and soluble chromatin extract, immunoprecipitation was performed as described (14), and the DNA was purified on Qiaquick spin columns (Qiagen) and eluted in 50 μL of water. Specific sequences from immunoprecipitated and input DNA were detected by end-point PCR (forward, 5′-tagagggtcaccgcgtctat-3′ and reverse, 5′-tcacaggtgctttgcagttc-3′) or quantitative real-time PCR and SYBR Green-detection (Stratagene) (forward, 5′-ccccatctccaaaacgaacaa-3′ and reverse, 5′-ccggtggctcactaagacctg-3′). Antibodies used were: anti-ERα (MC-20 and H-184); anti-CBP (C-20 and A22); anti hSlug (H-140 and D-19); anti-CtBP (C-1 and H-440); anti-Sp1 (H-225); and anti-RNA polymerase II (N-20) (Santa Cruz Biotechnology). Antibodies against histone 3 (H3) trimethyl-lysine 9, H3 dimethyl-lysine 27, H3 trimethyl-lysine 27, H3 acetyl-lysine 16 were from Upstate Biotech.
Immunoblot Analysis.
Total protein extracts were prepared with boiling 2.5% SDS and 0.125 M Tris·HCl, pH 6.8. Proteins were separated by SDS/PAGE and blotted to PVDF membranes (Bio-Rad). Blots were probed with primary antibodies [ERα (H-184), ERβ (H-150) E-cadherin (H-108), N-cadherin (H-163), AP-2α (C18), AP-2γ (6E4), β-actin (C-2) (Santa Cruz)] and visualized by enhanced chemiluminescence (ECL; Amersham Biosciences).
CpG Methylation Assay.
DNA was extracted by using the QIAmp DNAmini Kit (Qiagen), then 10 μg of DNA was digested with HindIII and denatured with 0.2 M NaOH for 10 min at 37 °C. Thirty microliters of freshly prepared 10 mM hydroquinone (Sigma) and 520 μL of 3 M sodium bisulfite (Sigma), pH 5.0 were added and mixed. The samples were overlaid with mineral oil to prevent evaporation and incubated at 50 °C for 16 h. The bisulfite-treated DNA was isolated by using the Wizard DNA Clean-Up System (Promega). The DNA was eluted by 50 μL of warm water, and 5.5 μL of 3 M NaOH was added for 5 min. The DNA was ethanol-precipitated with glycogen as a carrier and resuspended in 20 μL of water. A 50-μL PCR was carried by using specific PCR primer (forward 1, 5′-atttagtggaattagaatagtgtaggtttt-3′ and reverse 1, 5′-ctacaactccaaaaacccataactaac-3′) as described (40), A seminested PCR was then performed by using primers forward 2, 5′-gattttagtaattttaggttagaggg-3′ and reverse,1 5′-ctacaactccaaaaacccataactaac-3′ as described (40). The final PCR products were purified and cloned with the TOPO-TA cloning kit (Stratagene) per the manufacturer's protocol. Minipreps were prepared with a QIAprep Spin Miniprep Kit (Qiagen) and sequenced.
Supplementary Material
Acknowledgments.
We thank V. Perissi for critical reading of the manuscript and valuable comments and J. Hightower for figure preparation. M.D.C. was supported by a American-Italian Cancer Foundation Postdoctoral Research Fellowship Award, the PhD Program of the University of Turin, and the Cassa di Risparmio di Torino Foundation of Torino. M.G.R. is a Howard Hughes Medical Institute Investigator. This work was supported by Italian Ministry of Health, Programmi Speciali 2003, 2005, Regione Piemonte Progetti 2004-07, Ricerca Locale 2004-08 Università degli Studi di Torino, the Associazione Italiana per la Ricerca sul Cancro, and National Institutes of Health grants to M.G.R.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903033106/DCSupplemental.
References
- 1.Perou CM, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sotiriou C, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wirapati P, et al. Meta-analysis of gene expression profiles in breast cancer: Toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008;10:R65. doi: 10.1186/bcr2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 7.Liu YN, et al. Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene. 2005;24:8277–8290. doi: 10.1038/sj.onc.1208991. [DOI] [PubMed] [Google Scholar]
- 8.Batsche E, et al. RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol Cell Biol. 1998;18:3647–3658. doi: 10.1128/mcb.18.7.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hennig G, Lowrick O, Birchmeier W, Behrens J. Mechanisms identified in the transcriptional control of epithelial gene expression. J Biol Chem. 1996;271:595–602. doi: 10.1074/jbc.271.1.595. [DOI] [PubMed] [Google Scholar]
- 10.Peinado H, Olmeda D, Cano A. Snail, Zeb, and bHLH factors in tumor progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 11.Lombaerts M, et al. E-cadherin transcriptional down-regulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94:661–671. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Elloul S, et al. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–1643. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 14.Oesterreich S, et al. Estrogen-mediated down-regulation of E-cadherin in breast cancer cells. Cancer Res. 2003;63:5203–5208. [PubMed] [Google Scholar]
- 15.Park SH, Cheung LW, Wong AS, Leung PC. Estrogen regulates Snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor α. Mol Endocrinol. 2008;22:2085–2098. doi: 10.1210/me.2007-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng YG, et al. Induction of the LRP16 gene by estrogen promotes the invasive growth of Ishikawa human endometrial cancer cells through the down-regulation of E-cadherin. Cell Res. 2007;17:869–880. doi: 10.1038/cr.2007.79. [DOI] [PubMed] [Google Scholar]
- 17.Scafoglio C, et al. Comparative gene expression profiling reveals partially overlapping but distinct genomic actions of different antiestrogens in human breast cancer cells. J Cell Biochem. 2006;98:1163–1184. doi: 10.1002/jcb.20820. [DOI] [PubMed] [Google Scholar]
- 18.Planas-Silva MD, Waltz PK. Estrogen promotes reversible epithelial-to-mesenchymal-like transition and collective motility in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2007;104:11–21. doi: 10.1016/j.jsbmb.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 19.Carroll JS, et al. Genomewide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 20.Kwon YS, et al. Sensitive ChIP-DSL technology reveals an extensive estrogen receptor α-binding program on human gene promoters. Proc Natl Acad Sci USA. 2007;104:4852–4857. doi: 10.1073/pnas.0700715104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardella C, et al. Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell–cell adhesion through E-cadherin transcriptional repression. Cancer Res. 2004;64:5154–5161. doi: 10.1158/0008-5472.CAN-04-0600. [DOI] [PubMed] [Google Scholar]
- 22.Morotti A, et al. K252a inhibits the oncogenic properties of Met, the HGF receptor. Oncogene. 2002;21:4885–4893. doi: 10.1038/sj.onc.1205622. [DOI] [PubMed] [Google Scholar]
- 23.Chen CL, et al. E-cadherin expression is silenced by DNA methylation in cervical cancer cell lines and tumors. Eur J Cancer. 2003;39:517–523. doi: 10.1016/s0959-8049(02)00175-2. [DOI] [PubMed] [Google Scholar]
- 24.Bake S, Ma L, Sohrabji F. Estrogen receptor-α overexpression suppresses 17β-estradiol-mediated vascular endothelial growth factor expression and activation of survival kinases. Endocrinology. 2008;149:3881–3889. doi: 10.1210/en.2008-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stossi F, Likhite VS, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen-occupied estrogen receptor represses cyclin G2 gene expression and recruits a repressor complex at the cyclin G2 promoter. J Biol Chem. 2006;281:16272–16278. doi: 10.1074/jbc.M513405200. [DOI] [PubMed] [Google Scholar]
- 26.Fujita N, et al. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–219. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 27.Dhasarathy A, Kajita M, Wade PA. The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor α. Mol Endocrinol. 2007;21:2907–2918. doi: 10.1210/me.2007-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belguise K, Guo S, Sonenshein GE. Activation of FOXO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor α expression reversing invasive phenotype of breast cancer cells. Cancer Res. 2007;67:5763–5770. doi: 10.1158/0008-5472.CAN-06-4327. [DOI] [PubMed] [Google Scholar]
- 29.Cvoro A, et al. Distinct roles of unliganded and liganded estrogen receptors in transcriptional repression. Mol Cell. 2006;21:555–564. doi: 10.1016/j.molcel.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Alotaibi H, Yaman EC, Demirpence E, Tazebay UH. Unliganded estrogen receptor α activates transcription of the mammary gland Na+/I− symporter gene. Biochem Biophys Res Commun. 2006;345:1487–1496. doi: 10.1016/j.bbrc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 31.Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor α: Regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- 32.Hafner F, Holler E, von Angerer E. Effect of growth factors on estrogen receptor-mediated gene expression. J Steroid Biochem Mol Biol. 1996;58:385–393. doi: 10.1016/0960-0760(96)00054-4. [DOI] [PubMed] [Google Scholar]
- 33.Ciana P, et al. In vivo imaging of transcriptionally active estrogen receptors. Nat Med. 2003;9:82–886. doi: 10.1038/nm809. [DOI] [PubMed] [Google Scholar]
- 34.Moggs JG, et al. Antiproliferative effect of estrogen in breast cancer cells that reexpress ERα is mediated by aberrant regulation of cell cycle genes. J Mol Endocrinol. 2005;34:535–551. doi: 10.1677/jme.1.01677. [DOI] [PubMed] [Google Scholar]
- 35.Licznar A, et al. Identification of genes involved in growth inhibition of breast cancer cells transduced with estrogen receptor. FEBS Lett. 2003;553:445–450. doi: 10.1016/s0014-5793(03)01090-1. [DOI] [PubMed] [Google Scholar]
- 36.Frasor J, et al. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: Insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 37.Jepsen K, Rosenfeld MG. Biological roles and mechanistic actions of corepressor complexes. J Cell Sci. 2002;115:689–698. doi: 10.1242/jcs.115.4.689. [DOI] [PubMed] [Google Scholar]
- 38.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu P, et al. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124:615–629. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 40.Corn PG, et al. E-cadherin expression is silenced by 5′ CpG island methylation in acute leukemia. Clin Cancer Res. 2000;6:4243–4248. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.