Abstract
IDH1 encodes isocitrate dehydrogenase 1, which participates in the citric acid cycle and was recently reported to be mutated in 12% of glioblastomas. We assessed IDH1 mutations in 321 gliomas of various histological types and biological behaviors. A total of 130 IDH1 mutations was detected, and all were located at amino acid residue 132. Of these, 91% were G→A mutations (Arg→His). IDH1 mutations were frequent in low-grade diffuse astrocytomas (88%) and in secondary glioblastomas that developed through progression from low-grade diffuse or anaplastic astrocytoma (82%). Similarly, high frequencies of IDH1 mutations were found in oligodendrogliomas (79%) and oligoastrocytomas (94%). Analyses of multiple biopsies from the same patient (51 cases) showed that there were no cases in which an IDH1 mutation occurred after the acquisition of either a TP53 mutation or loss of 1p/19q, suggesting that IDH1 mutations are very early events in gliomagenesis and may affect a common glial precursor cell population. IDH1 mutations were co-present with TP53 mutations in 63% of low-grade diffuse astrocytomas and with loss of heterozygosity 1p/19q in 64% of oligodendrogliomas; they were rare in pilocytic astrocytomas (10%) and primary glioblastomas (5%) and absent in ependymomas. The frequent presence of IDH1 mutations in secondary glioblastomas and their near-complete absence in primary glioblastomas reinforce the concept that despite their histological similarities, these subtypes are genetically and clinically distinct entities.
Gliomas are the most common primary brain tumors and show wide diversity with respect to location, morphology, genetic status, and response to therapy. Glioblastoma (World Health Organization [WHO]grade IV), the most frequent and most malignant glioma, may develop rapidly after a short clinical history and without evidence of a less malignant precursor lesion (primary or de novo glioblastoma), or slowly through progression from low-grade diffuse or anaplastic astrocytoma (secondary glioblastoma).1,2,3 Both glioblastoma subtypes show frequent loss of heterozygosity (LOH) 10q (63% to 70%), but differ significantly with respect to the frequency of other genetic alterations: LOH 10p, EGFR amplification, MDM2 amplification, and PTEN mutations are typical of primary glioblastomas,3,4 whereas TP53 mutations, LOH 19q, and LOH 22q are more frequent in secondary glioblastomas.3,5
Low-grade diffuse astrocytomas (WHO grade II) are slowly growing, well-differentiated tumors, but they diffusely infiltrate into surrounding brain tissues and have an intrinsic tendency to progress to anaplastic astrocytoma (WHO grade III) and eventually to secondary glioblastoma.6 In the genetic pathway to secondary glioblastoma, TP53 mutations are considered an early event, since their frequency in low-grade diffuse astrocytomas is similar to that in secondary glioblastomas.2
Recently, Parsons et al7 analyzed 20,661 protein coding genes in 22 glioblastomas, and showed that 12% of cases contained mutations in the IDH1 gene. All were located at position 395 (amino acid residue 132) of the IDH1 transcript and most were a G→A change with amino acid substitution of Arg→His.7 Interestingly, many of the glioblastomas carrying IDH1 mutations were secondary glioblastomas and contained TP53 mutations.7
It remained uncertain whether IDH1 mutations are early events during astrocytoma progression and to what extent they are specific to the pathways leading to secondary glioblastomas. We screened a total of 321 gliomas for IDH1 mutations, with the aim of assessing their role in the development of a large range of human gliomas of different histological types and biological behavior.
Materials and Methods
Tumor Samples
We examined a total of 321 gliomas in 266 patients, diagnosed at the University Hospital Zurich, Switzerland.2,8,9,10,11 They include 68 low-grade diffuse astrocytomas (mean age 37.0 ± 10.0 years, 36 males, 32 females), 39 oligodendrogliomas (40.5 ± 13.9 years, 16 males, 23 females), 17 oligoastrocytomas (42.4 ± 11.4 years, 9 males, 8 females), 27 anaplastic astrocytomas (39.5 ± 13.5 years, 15 males, 12 females), 14 anaplastic oligoastrocytomas (45.4 ± 16.6 years, 7 males, 7 females), 8 anaplastic oligodendrogliomas (50.0 ± 18.4 years, 7 males, 1 female), 34 secondary glioblastomas (45.5 ± 14.5 years, 14 males, 20 females), 59 primary glioblastomas (56.8 ± 10.0 years, 40 males, 19 females), 31 pilocytic astrocytomas (15.0 ± 11.0 years, 18 males, 13 females), and 24 ependymomas (26.0 ± 20.2 years, 14 males, 10 females). Tumors were considered primary (de novo) glioblastomas if a glioblastoma diagnosis was made at the first biopsy, without clinical or histological evidence of presence of a less malignant precursor lesion and with a clinical history of less than three months. A diagnosis of secondary glioblastoma was made only in cases with histopathological evidence of a preceding low-grade or anaplastic glioma.1,2 In 51 patients (including 34 patients with secondary glioblastoma), we were able to assess two or more biopsies. DNA was extracted from formalin-fixed, paraffin-embedded tissue sections as previously described.2 Results on TP53 mutations and loss of chromosome 1p/19q have been reported previously.2,8,9,10,11
SSCP Analysis and Direct DNA Sequencing for IDH1 Mutations
SSCP analysis was performed to prescreen for mutations in exon 4 of the IDH1 gene. Primer sequences were 5′-CGGTCTTCAGAGAAGCCATT-3′ (sense) and 5′-CACATTATTGCCAACATGAC-3′ (antisense) to cover nucleotides 6617 to 6738, corresponding to codons 107 to 138. Briefly, PCR was performed in a total volume of 10 μl, consisting of 1 μl of DNA solution (approximately 100 ng/μl), 0.5 U of PLATINUM TaqDNA polymerase (Invitrogen, Cergy Pontoise), 0.1 mCi of [α-33P]dCTP (ICN Biomedicals, Inc., Costa Mesa, CA; specific activity, 3000 Ci/mmol/L), 2.5 mmol/L MgCl2, 0.1 mmol/L of each dNTP, 0.25 mmol/L of each primer, 10 mmol/L Tris-HCl, pH 8.3, and 50 mmol/L KCl in a thermal cycler (Biometra, Archamps, France) with an initial denaturing step at 95°C for 3 minutes, followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 56°C for 30 seconds, extension at 72°C for 40 seconds, and a final extension at 72°C for 10 minutes. PCR products (10 μl) were mixed with 20 μl loading buffer (0.02 N NaOH, 95% formamide, 20 mmol/L EDTA, 0.05% xylene cyanol, and bromophenol blue), denatured at 95°C for 10 minutes and quenched on ice. Then 5.5 μl of the above mixture was loaded onto a 12.5% polyacrylamide non-denaturing gel containing 10% glycerol. Electrophoresis was performed at 45 W for 2.5 hours at room temperature with cooling by fan. Gels were dried at 80°C and autoradiography was performed for 24 to 36 hours. Samples exhibiting mobility shifts in single-strand conformation polymorphism (SSCP) analyses were subsequently reamplified using the same primers as for SSCP and sequenced using the Big DyeTM Terminator cycle sequencing kit (ABI PRISM, Applied Biosystems, Foster City, CA) in an ABI 3100 PRISM DNA sequencer (Applied Biosystems, CA).
Statistical Analyses
The Fisher’s exact test was performed to analyze the significance of the associations between TP53 mutations and IDH1 mutations. The Student’s t-test was performed to compare the mean age of patients with and without IDH1 mutations. The χ2 test and Fisher’s exact test were performed to analyze the timing of IDH1 mutations and other genetic alterations.
Results
Frequency of IDH1 Mutations
A total of 130 IDH1 mutations was detected and all were located at amino acid residue 132. All except for 12 mutations were CGT→CAT with amino acid substitution of Arg→His; six mutations were CGT→TGT (Arg→Cys), five were CGT→GGT (Arg→Gly), and one was CGT →AGT (Arg→Ser). All IDH1 mutations were heterozygous.
IDH1 mutations were frequent in low-grade diffuse astrocytomas (88%), anaplastic astrocytomas (78%), and secondary glioblastomas (82%), as well as in oligodendrogliomas (79%), anaplasic oligodendrogliomas (75%), oligoastrocytomas (94%), and anaplastic oligoastrocytomas (71%). They were rare in pilocytic astrocytomas (10%) and primary glioblastomas (5%), and absent in ependymomas.
For 27 cases with IDH1 mutation (15 oligodendrogliomas, eight low-grade astrocytomas, three secondary glioblastomas, and one primary glioblastoma), we were able to obtain peritumoral normal brain tissue (20 cases) or peripheral blood samples (seven cases). There was no IDH1 mutation in these normal tissues, indicating that the IDH1 mutations are somatic.
Patient Age and IDH1 Mutations
Patients with low-grade diffuse astrocytomas carrying IDH1 mutations were significantly younger (36.2 ± 9.4 years) than those without IDH1 mutations (51.6 ± 19.6 years; P = 0.0004). The mean ages of oligodendroglioma patients with and without IDH1 mutations were not significantly different (42.6 ± 12.3 years vs. 37.1 ± 13.1 years; P = 0.307).
The mean age of all glioblastoma patients with IDH1 mutations was significantly lower than that of those without IDH1 mutations (44.2 ± 12.6 years vs. 56.4 ± 10.7 years; P = 0.0001). However, when primary and secondary glioblastomas were separately analyzed, there was no correlation between age of patients and IDH1 mutations (primary glioblastomas P = 0.202; secondary glioblastomas, P = 0.405).
IDH1 and Other Genetic Alterations in Low-Grade Diffuse Gliomas
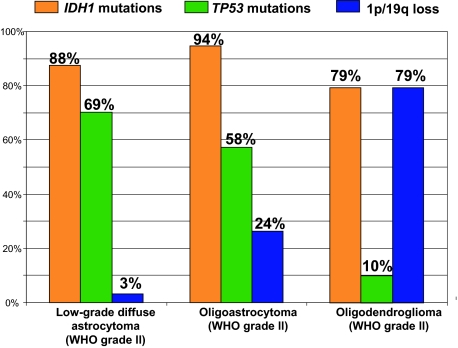
Data from our laboratory on other genetic alterations (TP53 mutations, loss of 1p/19q) in low-grade diffuse astrocytomas, oligoastrocytomas, and oligodendrogliomas have been reported previously (Figure 1).2,8,9,10,11
Figure 1.
Frequency of genetic alterations in low-grade diffuse astrocytomas, oligoastrocytomas, and oligodendrogliomas. Note that IDH1 mutations are frequent in all these low-grade diffuse gliomas.
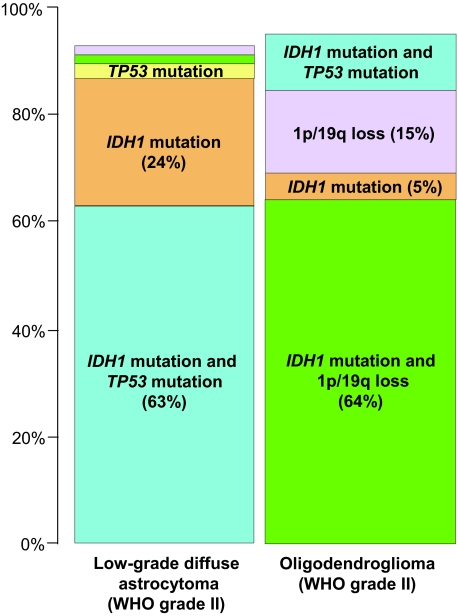
In low-grade astrocytomas, IDH1 mutations and TP53 mutations were co-present in 63% of cases (Figure 2). Sixteen low-grade astrocytomas (24%) carried IDH1 mutations alone (Figure 2). There was a significant association between TP53 mutations and IDH1 mutations in low-grade astrocytomas (P = 0.015).
Figure 2.
Frequency of genetic alterations in low-grade diffuse astrocytomas and oligodendrogliomas. Note that the majority of low-grade astrocytomas contain both IDH1 and TP53 mutations (63%), and that the majority of oligodendrogliomas show both IDH1 mutations and 1p/19q loss (64%).
In oligodendrogliomas, IDH1 mutations and 1p/19q loss were co-present in 64% of cases (Figure 2), but there was no significant association between 1p/19q loss and IDH1 mutations (P = 0.658).
Timing of IDH1 Mutations and Other Genetic Alterations
IDH1 mutations were assessed in relation to TP53 mutations and loss of 1p/19q in 51 patients who had two or more biopsies. IDH1 mutations were observed at the first biopsy in 42 of 51 cases (82%), whereas TP53 mutations or loss of 1p/19q were found at the first biopsy in 34 cases (67%; χ2 test; P = 0.1117). Of the nine patients who did not have IDH1 mutations at the first biopsy, two (22%) carried an IDH1 mutation at the last biopsy, whereas of the 17 cases who lacked TP53 mutations or loss of 1p/19q at the first biopsy, seven (41%) carried these alterations at the last biopsy (Fisher’s exact test; P = 0.3989).
Of 40 patients who had both IDH1 mutations and other genetic alterations at the last biopsy, 33 (83%) had both IDH1 mutations and other genetic alterations from the first biopsy. In four patients, the first biopsy (low-grade diffuse astrocytomas) had an IDH1 mutation alone, while the second biopsy (one low-grade astrocytoma and three anaplastic astrocytomas) showed both IDH1 and TP53 mutations. In three patients, the first biopsy (oligoastrocytoma) had an IDH1 mutation alone, while the second biopsy (two oligoastrocytomas and one secondary glioblastoma) showed both IDH1 mutations and 1p/19q loss. There was no case in which an IDH1 mutation occurred after the acquisition of a TP53 mutation or loss of 1p/19q.
Discussion
Low-grade diffuse astrocytomas (WHO grade II) and oligodendrogliomas (WHO grade II) are well-differentiated, slowly-growing tumors that typically affect adults and preferentially develop in cerebral hemispheres. Low-grade diffuse astrocytomas are histologically composed of well-differentiated fibrillary or gemistocytic neoplastic astrocytes, whereas oligodendrogliomas are composed of monomorphic cells with uniform round nuclei and perinuclear halos on paraffin sections (honeycomb appearance).6,10 However, in a substantial fraction of cases, the histological diagnosis is difficult, with marked interobserver variability. This is particularly true for oligoastrocytomas, ie, tumors composed of a conspicuous mixture of two distinct neoplastic cell types morphologically resembling astrocytes and oligodendrocytes.6
Low-grade diffuse astrocytomas typically carry frequent TP53 mutations (>65%) and infrequent loss of 1p/19q (<10%), whereas oligodendrogliomas contain frequent loss of 1p/19q (70%) and infrequent TP53 mutations (<15%).6,9,10,12 Oligoastrocytomas contain either TP53 mutations or loss of 1p/19q.9,10,13,14 Importantly, TP53 mutations and 1p/19q loss are mutually exclusive in low-grade diffuse gliomas.9,10,13,14
The present study provides the first evidence that low-grade diffuse astrocytomas, oligodendrogliomas and oligoastrocytomas share a frequent genetic alteration, ie, IDH1 mutations. The vast majority of low-grade diffuse astrocytomas contained both an IDH1 mutation and a TP53 mutation, and a similar majority of oligodendrogliomas showed both IDH1 mutations and 1p/19q loss. Furthermore, analyses of multiple biopsies from the same patients did not reveal a single case in which an IDH1 mutation occurred after the acquisition of a TP53 mutation or loss of 1p/19q. These findings suggest that IDH1 mutations are very early genetic events, before TP53 mutations or loss of 1p/19q occur, and suggest that low-grade diffuse astrocytomas, oligoastrocytomas, and oligodendrogliomas may originate from common glial precursor cells carrying IDH1 mutations. It is possible that the additional acquisition of TP53 mutations may lead to astrocytic differentiation, while subsequent loss of 1p/19q favors acquisition of the oligodendroglial phenotype.
Our results show that IDH1 mutations are frequent in diffuse astrocytic and oligodendroglial gliomas, but rare in other gliomas such as pilocytic astrocytomas (WHO grade I) and apparently absent in ependymomas. Pilocytic astrocytomas are relatively circumscribed, slowly growing WHO grade I tumors that typically manifest in children and young adults.6 In contrast to diffuse astrocytomas, they very rarely undergo malignant progression and the prognosis is very favorable after surgical resection.15 They are genetically distinct from low-grade diffuse astrocytomas, and do not usually contain TP53 mutations,6 but have frequent gains at chromosome 7q34 involving the BRAF gene.16 The present study provides further evidence that pilocytic astrocytomas differ significantly in genetic characteristics from diffuse low-grade astrocytomas and may be derived from a different type of glial progenitor.
Our observation that secondary glioblastomas but not primary glioblastomas share IDH1 mutations with oligodendrogliomas suggests that these glioblastoma subtypes have different origins. The present study also shows that IDH1 mutations are currently the most reliable genetic marker for secondary glioblastomas.
The data from Parsons et al7 and the present study show that the mean age of glioblastoma patients with IDH1 mutations is significantly lower than that of patients without IDH1 mutations. This difference is largely due to the fact that the majority of tumors with IDH1 mutations were secondary glioblastomas, which develop in younger patients.2 In the present study, we further show that patients with low-grade diffuse astrocytomas carrying IDH1 mutations were significantly younger (mean 36.2 years) than those without IDH1 mutations (51.6 years; P = 0.0004).
The IDH1 gene (at 2q33) encodes isocitrate dehydrogenase 1 (IDH1),17 one of the enzymes of the citric acid (Krebs) cycle.18 In aerobic organisms, the citric acid cycle is part of a metabolic pathway involved in the chemical conversion of carbohydrates, fats and proteins into carbon dioxide and water to generate a form of usable energy.19 Five distinct forms of isocitrate dehydrogenase have been identified.18 In contrast to other IDHs in the mitochondria, IDH1 is present in the cytosol, and its biochemical role is not yet fully understood. The biological function of mutated forms of IDH1 and their role in the development of low-grade diffuse gliomas and secondary glioblastomas remain to be elucidated.
Acknowledgments
We thank Mrs. Anne-Marie Camus and Ms. Christine Carreira for technical assistance.
Footnotes
Address reprint requests to Dr. Hiroko Ohgaki, Head, Pathology Group, International Agency for Research on Cancer, 150 cours Albert Thomas, 69372 Lyon, France. E-mail: ohgaki@iarc.fr.
Supported by a grant from the Foundation for Promotion of Cancer Research, Japan. T.W. was supported by the Naito Foundation.
References
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC, Baeza N, Pisani P, Yonekawa Y, Yasargil MG, Lutolf UM, Kleihues P. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H, Reis RM, Nakamura M, Colella S, Yonekawa Y, Kleihues P, Ohgaki H. Loss of heterozygosity on chromosome 10 is more extensive in primary (de novo) than in secondary glioblastomas. Lab Invest. 2000;80:65–72. doi: 10.1038/labinvest.3780009. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ishida E, Shimada K, Kishi M, Nakase H, Sakaki T, Konishi N. Frequent LOH on 22q12.3 and TIMP-3 inactivation occur in the progression to secondary glioblastomas. Lab Invest. 2005;85:165–175. doi: 10.1038/labinvest.3700223. [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. Lyon: IARC; WHO Classification of Tumours of the Central Nervous System. 2007 doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JCH, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SKN, Shinjo SMO, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Sato K, Biernat W, Tachibana O, von Ammon K, Ogata N, Yonekawa Y, Kleihues P, Ohgaki H. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin Cancer Res. 1997;3:523–530. [PubMed] [Google Scholar]
- Okamoto Y, Di Patre PL, Burkhard C, Horstmann S, Jourde B, Fahey M, Schuler D, Probst-Hensch NM, Yasargil MG, Yonekawa Y, Lutolf U, Kleihues P, Ohgaki H. Population-based study on incidence, survival rates, and genetic alterations of low-grade astrocytomas and oligodendrogliomas. Acta Neuropathol. 2004;108:49–56. doi: 10.1007/s00401-004-0861-z. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakamura M, Kros JM, Burkhard C, Yonekawa Y, Kleihues P, Ohgaki H. Phenotype versus genotype correlation in oligodendrogliomas and low-grade diffuse astrocytomas. Acta Neuropathol. 2001;103:267–275. doi: 10.1007/s004010100464. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Watanabe T, Yonekawa Y, Kleihues P, Ohgaki H. Promoter hypermethylation of the DNA repair gene MGMT in astrocytomas is frequently associated with G:C — A:T mutations of the TP53 tumor suppressor gene. Carcinogenesis. 2001;22:1715–1719. doi: 10.1093/carcin/22.10.1715. [DOI] [PubMed] [Google Scholar]
- Reifenberger G, Louis DN. Oligodendroglioma: toward molecular definitions in diagnostic neuro-oncology. J Neuropathol Exp Neurol. 2003;62:111–126. doi: 10.1093/jnen/62.2.111. [DOI] [PubMed] [Google Scholar]
- Maintz D, Fiedler K, Koopmann J, Rollbrocker B, Nechev S, Lenartz D, Stangl AP, Louis DN, Schramm J, Wiestler OD, von Deimling A. Molecular genetic evidence for subtypes of oligoastrocytomas. J Neuropathol Exp Neurol. 1997;56:1098–1104. doi: 10.1097/00005072-199710000-00003. [DOI] [PubMed] [Google Scholar]
- Mueller W, Hartmann C, Hoffmann A, Lanksch W, Kiwit J, Tonn J, Veelken J, Schramm J, Weller M, Wiestler OD, Louis DN, von Deimling A. Genetic signature of oligoastrocytomas correlates with tumor location and denotes distinct molecular subsets. Am J Pathol. 2002;161:313–319. doi: 10.1016/S0002-9440(10)64183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard C, Di Patre PL, Schüler D, Yasargil MG, Lütolf U, Kleihues P, Ohgaki H. A population-based study on the incidence and survival of patients with pilocytic astrocytoma. J Neurosurg. 2003;98:1170–1174. doi: 10.3171/jns.2003.98.6.1170. [DOI] [PubMed] [Google Scholar]
- Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67:878–887. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- Narahara K, Kimura S, Kikkawa K, Takahashi Y, Wakita Y, Kasai R, Nagai S, Nishibayashi Y, Kimoto H. Probable assignment of soluble isocitrate dehydrogenase (IDH1) to 2q33.3. Hum Genet. 1985;71:37–40. doi: 10.1007/BF00295665. [DOI] [PubMed] [Google Scholar]
- Geisbrecht BV, Gould SJ. The human PICD gene encodes a cytoplasmic and peroxisomal NADP(+)-dependent isocitrate dehydrogenase. J Biol Chem. 1999;274:30527–30533. doi: 10.1074/jbc.274.43.30527. [DOI] [PubMed] [Google Scholar]
- Devlin TM, editor. Wiley-Liss: Hoboken, N.J.; Textbook of biochemistry with clinical correlations. 2006 [Google Scholar]