Abstract
The present study aimed to evaluate whether levels of urinary L-type fatty acid-binding protein (L-FABP) could be used to monitor histological injury in acute kidney injury (AKI) induced by cis-platinum (CP) injection and ischemia reperfusion (IR). Different degrees of AKI severity were induced by several renal insults (CP dose and ischemia time) in human L-FABP transgenic mice. Renal histological injury scores increased with both CP dose and ischemic time. In CP-induced AKI, urinary L-FABP levels increased exponentially even in the lowest dose group as early as 2 hours, whereas blood urea nitrogen (BUN) levels increased at 48 hours. In IR-induced AKI, BUN levels increased only in the 30-minute ischemia group 24 hours after reperfusion; however, urinary L-FABP levels increased more than 100-fold, even in the 5-minute ischemia group after 1 hour. In both AKI models, urinary L-FABP levels showed a better correlation with final histological injury scores and glomerular filtration rates measured by fluorescein isothiocyanate-labeled inulin injection than with levels of BUN and urinary N-acetyl-d-glucosaminidase, especially at earlier time points. Receiver operating characteristic curve analysis demonstrated that urinary L-FABP was superior to other biomarkers for the detection of significant histological injuries and functional declines. In conclusion, urinary L-FABP levels are better suited to allow the accurate and earlier detection of both histological and functional insults in ischemic and nephrotoxin-induced AKI compared with conventional renal markers.
Acute kidney injury (AKI) is still a critical problem because the mortality rate of severely ill patients complicated with AKI is much higher than non-AKI patients.1 There is no effective treatment for human AKI except for supportive renal replacement therapy such as dialysis. Novel renal biomarkers are indispensable to develop a new therapeutic strategy for AKI because it will allow us to detect AKI and start treatment early.2 In addition to early detection, renal biomarkers should be able to estimate renal injury accurately. Because acute tubular necrosis (ATN) is the common feature of most AKI, predicting histological injuries in renal tubular cells would be one of the required characteristics of renal biomarkers in AKI.
We have recently demonstrated that L-type fatty acid-binding protein (L-FABP) is localized to proximal tubular cells in human kidney and projected to urinary space after ischemia reperfusion (IR) injury in living-related kidney transplantation.3 In human AKI after cardiac surgery, urinary L-FABP levels were increased at 4 hours after surgery in AKI patients at least, whereas serum creatinine started to increase 48 hours later.4 Mouse L-FABP in proximal tubules of C57BL/6 wild-type mice was virtually defective because of the silencing sequence localizing in the upstream of the promoter region. Therefore, we used human L-FABP transgenic mice, which are humanized by expressing higher protein levels of human L-FABP in proximal tubules that resemble the human kidney. This study is aimed to clarify the sensitivity and dynamics of urinary L-FABP in AKI. We induced nephrotoxic and ischemic AKI by cis-platinum (CP) injection and IR on human L-FABP transgenic mice. It has been reported that the mechanisms of CP-induced AKI include mitochondrial damage, activation of apoptosis pathway, reactive oxygen species production, and inflammatory responses in renal tubular epithelial cells mainly regulated by tumor necrosis factor-α.5 In IR renal injury, reactive oxygen species (ROS) play a crucial role.6,7 We further evaluated whether urinary L-FABP levels can detect renal insults before blood urea nitrogen (BUN) increases and monitor the histological severity of renal injury induced by different CP doses and ischemic times.
Materials and Methods
Experimental Protocols
Male heterozygous human L-FABP transgenic mice (C57BL/6 background) were used in this experiment. The engineering of human L-FABP chromosomal transgenic (Tg) mice is described previously.3 Male L-FABP Tg mice weighing 25 to 35 g were allowed food and water ad libitum. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (US Department of Health and Human Services Public Health Services, National Institutes of Health, NIH Publication No. 86-23, 1985).
For the CP-induced AKI experiment, animals were kept in glass-shielded metabolic cages (Metabolica; Sugiyamagen, Tokyo, Japan), and fed with a standard chow diet for several days before the CP injection. CP (generously provided by Nippon Kayaku Co. Ltd., Tokyo, Japan) at the dose of 5, 10, or 20 mg/kg or vehicle (normal saline) was administered by intraperitoneal injection. Animals were sacrificed at 72 hours after CP injection for collecting the kidneys.
For the IR-induced AKI experiment, animals had a similar adaptation process. IR surgery was performed as previously described.3 After 5, 15, or 30 minutes of ischemia, mice were kept in metabolic cages. Sham operation was performed for control. Animals were sacrificed at 24 hours after surgery for collecting the kidneys.
Renal biomarkers were compared with inulin clearance, the gold standard of glomerular filtration rate (GFR) as described below. After the same adaptation and basal blood and urine collection, animals were subjected to CP injection (5, 10, 20 mg/kg) or IR surgery (5, 15, 30 minutes), respectively. Blood and urine samples were also serially collected.
Renal Marker Measurement
BUN was measured using the urease-indophenol method with Urea N B (Wako Pure Chemical Industries Ltd., Osaka, Japan). The urinary levels of N-acetyl-d-glucosaminidase (NAG) and creatinine were measured using the NAG test (Shionogi and Co. Ltd., Osaka, Japan) and Nescoat VLII CRE (Alfresa Pharma Corp., Osaka, Japan), respectively. Urinary human L-FABP was measured using the sandwich ELISA kit (CMIC Co. Ltd., Tokyo, Japan). The measurable range of this kit is between 4 and 400 ng/ml. This assay system does not detect rodent L-FABP. Urinary h-L-FABP levels and NAG are expressed as the ratio to the creatinine concentration.
Measurement of GFR in Conscious Mice
GFR was measured following the previously described method.8,9 Briefly, mice were injected with fluorescein isothiocyanate-labeled inulin (Sigma-Aldrich, St. Louis, MO) into the retro-orbital plexus, and blood was serially collected from the tail vein at 3, 7, 10, 15, 35, 55, and 75 minutes after single bolus injection of fluorescein isothiocyanate-labeled inulin (3.7 μl/g body weight). Fluorescence intensity was determined by a fluorescence microplate reader (fMax; Molecular Devices, Sunnyvale, CA) with an excitation wavelength of 485 nm and an emission wavelength of 538 nm and analyzed using software (SoftMax Pro, Molecular Devices). Inulin clearance was calculated with JMP 7.0.1 software (SAS Institute Inc., Cary, NC) using a two-compartment model of two-phase exponential decay.
Morphological Evaluation of Kidneys
Formalin-fixed sections were stained with periodic acid-Schiff. Morphological evaluation was performed using well-established criteria as the ATN score in a blind manner.6,10,11 Five categories including tubular necrosis, tubular dilatation, interstitial edema, cast formation, and brush border loss were evaluated with the score of 0 to 2 and the total score ranged from 0 to 10.
Statistical Analysis
The results of statistical analyses are expressed as means ± SEM; P < 0.05 was considered significant. Differences from vehicle (CP) and sham (IR) at the same time point were examined by analysis of variance followed by the Dunnett multiple comparison test. Correlations between two indicators were evaluated using the Spearman rank test. Receiver operating characteristic (ROC) curve analysis was conducted; the value of the area under the curve can range from 0 to 1.0, where a value of 1.0 signifies a perfect biomarker and a value of 0.5 is no better than random chance. These calculations were performed with JMP 7.0.1 software.
Results
Responses of Renal Biomarkers in CP-Induced AKI
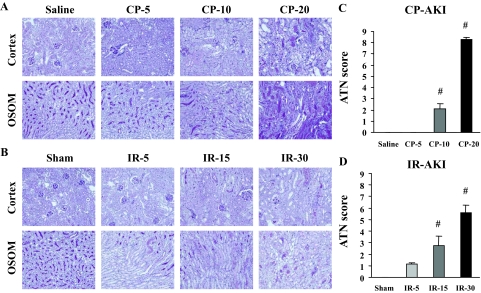
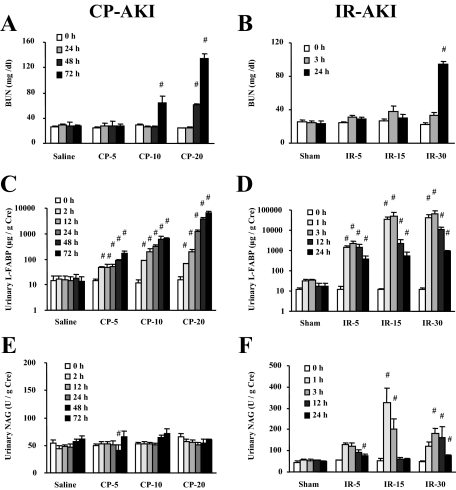
Human L-FABP transgenic mice were administered different doses of CP (5, 10, 20 mg/kg) to induce different degrees of AKI. Pathological analysis demonstrated that acute tubular injury evaluated by ATN scoring was found in the 10- and 20-mg/kg group and the degree of injury was dose-dependent (Figure 1, A and C). BUN started to increase at 72 hours and 48 hours after CP administration in the 10-mg/kg and the 20-mg/kg group, respectively, but did not increase at all in the 5-mg/kg group (Figure 2A). Urinary L-FABP showed a small but significant increase even 2 hours after administration in all of the groups. Urinary L-FABP could distinguish the difference of dose dependence in CP at all the time points except for 2 hours (Figure 2C). On the other hand, urinary NAG was completely insensitive for the detection of CP-AKI (Figure 2E).
Figure 1.
Histological evaluation of CP- and IR-induced AKI. CP with different dose injections (0, 5, 10, 20 mg/kg) and different ischemia times (0, 5, 15, 30 minutes) were conducted. Representative histology in CP (A)- and IR (B)-induced AKI are shown. Stepwise increases of total ATN score in CP (C)- and IR (D)-induced AKI along with CP dose and ischemia time were found. [n = 5 ∼ 10 per group, #P < 0.05 versus saline (CP) or sham (IR) group]. OSOM, outer stripe of outer medulla. Original magnifications, ×200.
Figure 2.
Renal biomarkers in CP- and IR-induced AKI. Renal biomarkers of BUN (A, B), urinary L-FABP (C, D), and urinary NAG (E, F) in response to CP with different dose injections (0, 5, 10, 20 mg/kg) and different ischemia times (0, 5, 15, 30 minutes) are shown. (n = 5 ∼ 10 per group, #P < 0.05 versus sham or saline group).
Responses of Renal Biomarkers in IR-Induced AKI
In IR-induced AKI, acute tubular damage was partly found even in the 5-minute ischemia group and the degree of ATN was dependent on the ischemia time (Figure 1, B and D). BUN level did not increase at all after IR in the 5-minute and 15-minute ischemia groups. BUN increase achieved to significant level 24 hours after reperfusion when animals were subjected to 30 minutes of ischemia (Figure 2B). Urinary L-FABP started to increase earlier than BUN (1 hour after reperfusion) in all of the ischemic time groups (Figure 2D). A significant increase was found even in the 5-minute ischemia group. The dynamic range of urinary L-FABP was sufficiently wide to detect the different level of injury induced by different ischemic time. Urinary L-FABP in all of the ischemia groups showed a rapid increase that peaked at 3 hours, and gradually decreased, but remained at significantly high levels (∼60-fold) even at 24 hours after reperfusion. Urinary NAG levels also increased at 1 hour after reperfusion even in the 5-minute ischemia group and decreased to the baseline at 12 to 24 hours after reperfusion (Figure 2F). Although urinary NAG responded early and sensitively, the dynamic range was not wide enough to detect the difference of ischemic level.
Prediction of Histological Injuries by Renal Biomarkers
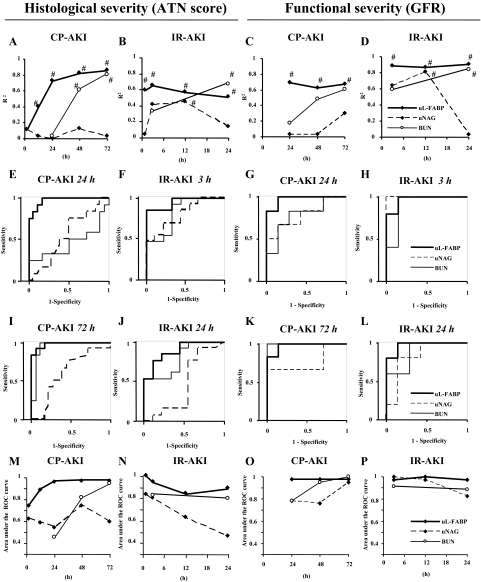
Correlations of renal biomarkers with the final histological injuries were examined. Urinary L-FABP levels showed the best correlations with ATN scores in CP- and IR-induced AKI (Figure 3, A and B). It is of note that the R2 values of urinary L-FABP at the early time points were much higher than BUN, although BUN in the later phase certainly showed good correlations with ATN scores. ROC curve analysis was performed whether each renal biomarker could distinguish mild to moderate histological injuries after reshuffling without considering dose difference in CP AKI or ischemia time in IR AKI. Here, mild injury or normal histology was defined as below the median values of ATN score. At earlier time points of 24 hours after CP injection and 3 hours after IR, urinary L-FABP showed the highest values of the area under the ROC curve (Figure 3, E and F) and these highly accurate responses were still observed at the time point when AKI had been histologically established (Figure 3, I and J). Urinary L-FABP had higher values of the area under the ROC curve than BUN and urinary NAG at any time point in both CP- and IR-induced AKI for detecting histologically mild to moderate severity of AKI (Figure 3, M and N).
Figure 3.
Correlation of renal biomarkers with histological injury and functional decline in AKI. R2 values were calculated with the renal biomarkers at each time point and the ATN score at 72 hours in CP-AKI (A, n = 30) or 24 hours in IR-AKI (B, n = 22). R2 values were also calculated with the renal biomarkers at each time point and measured GFR at 72 hours in CP-AKI (C, n = 13) or at 24 hours in IR-AKI (D, n = 12). #P < 0.001. ROC curve analysis for detecting moderate to severe histological injuries in CP and IR AKI was performed with renal biomarkers at 24 hours (E) or 72 hours (I) after CP injection (n = 30) and 3 hours (F) or 24 hours (J) after IR (n = 22). The areas under the ROC curve were calculated with the renal biomarkers at each time point in CP-AKI (M, n = 30) and IR-AKI (N, n = 22). ROC curve analysis for detecting the functional change of GFR >25% decrease in CP- and IR-induced AKI was performed with renal biomarkers at 24 hours (G) or 72 hours (K) after CP injection (n = 13) and 3 hours (H) or 24 hours (L) after IR (n = 12). The areas under the ROC curve were calculated with the renal biomarkers at each time point in CP-AKI (O, n = 13) and IR-AKI (P, n = 12).
Prediction of Functional Decline by Renal Biomarkers
To evaluate whether renal biomarkers can predict functional changes of the kidney, we measured GFR by fluorescein isothiocyanate-labeled insulin injection at 24 hours after ischemia and 72 hours after CP injection. Urinary L-FABP levels showed the best correlations with GFRs in CP- and IR-induced AKI. Although BUN clearly showed good correlations with GFRs at the time of GFR measurement (24 hours in IR and 72 hours in CP), urinary L-FABP at the early time points had higher R2 values than BUN (Figure 3, C and D). ROC curve analysis was performed as described above. AKI was defined as more than 25% decrease compared with sham-operated and saline-injected animals. For predicting functional declines, urinary L-FABP at the earlier time points showed high values of the area under the ROC curve (Figure 3, G and H). Urinary L-FABP also detected decreases of GFR at the time point of later stages (Figure 3, K and L). Urinary L-FABP had high values of the area under the ROC curve at any time point in both CP- and IR-induced AKI for detecting functional declines in CP and IR AKI models (Figure 3, O and P). This observation indicates urinary L-FABP can reflect and predict not only histological injury but functional change of glomerular filtration induced by IR and CP AKI.
Discussion
In the present study, we demonstrated that urinary L-FABP levels can predict acute renal histological injuries more sensitively than BUN and urinary NAG in two different animal AKI models of CP injection and IR injury. Moreover, urinary L-FABP responded earlier than BUN. Urinary L-FABP can also reflect functional decline of glomerular filtration. These data indicate that urinary L-FABP can serve as a sensitive and specific renal biomarker in AKI.
The requirements of an excellent biomarker are a quick and stress-dependent response.12 Urinary L-FABP increased earlier than BUN in all of the groups in CP model and a higher CP dose induced higher urinary excretion of L-FABP (∼400-fold). In the IR model, urinary L-FABP increased even in the 5- and 15-minute ischemia group, whereas no BUN increase was found in these groups. Longer ischemia induced higher excretion of L-FABP in the urine (∼1000-fold). Urinary L-FABP increased earlier than BUN and returned toward the baseline earlier. This may be because IR is a reversible renal insult in a week and therefore urinary L-FABP levels started to decrease 24 hours after IR. We previously reported that urinary L-FABP levels in pediatric cardiac surgery patients increased at 4 hours after the surgery (before serum creatinine increase) and started to decrease at 12 hours after.4 Two other urinary biomarkers have been studied for early diagnosis of human AKI: neutrophil gelatinase-associated lipocalin (NGAL) and interleukin-18 (IL-18). Although NGAL showed 100% accuracy at the early time point in pediatric cardiac surgery patients,13 the area under the ROC curve of NGAL in critically ill children and adult cardiac surgery patients was not so high.14,15,16 Several studies tried to clarify the role of urinary IL-18 for early diagnosis of AKI and found that urinary IL-18 had high specificity but low sensitivity.17,18 In the present study, we demonstrated that urinary L-FABP can diagnose two different types of AKI at the early time points. In addition to histological injury, urinary L-FABP level certainly reflected renal functional deterioration referencing to inulin clearance in this report and may have a great potential as an advance-warning reporter of AKI when used in point-of-care at bedside. Further clinical investigations with various AKI populations are necessary to confirm the role of urinary L-FABP as a novel AKI biomarker.
In addition to the advantage of urinary L-FABP on early detection and wide dynamic range compared with BUN and urinary NAG, urinary L-FABP could predict the histological injuries more accurately than the other renal markers in CP- and IR-induced AKI. Moreover, urinary L-FABP could detect modest but significant histological injury even in the animals without any BUN elevation. Most AKI diagnosis criteria including RIFLE use serum creatinine, GFR, and urine output for the definition of AKI.19 The severity of AKI is usually evaluated by these indicators that do not reflect tubular cell injury directly. In the present study, we evaluated whether urinary L-FABP could predict tubular cell injury examined by histological analysis because L-FABP is demonstrated to be localized in proximal tubules in human kidney3 and excreted into urine by hypoxic stress independently from serum L-FABP levels.11 Kidney injury molecule-1 (KIM-1) is another tubular protein that has been demonstrated to be elevated in AKI.20,21 However, it is still unclear whether urinary KIM-1 can predict histological injury accurately. In the present study, urinary L-FABP showed better correlations (higher R2 values) with ATN score than BUN and urinary NAG especially at the early time points. ROC analysis also demonstrated the superiority of urinary L-FABP for predicting the future histological injuries of ATN. This study is the first report that evaluates the significance of renal biomarker for predicting histological damages to our knowledge. Although renal biopsy is rarely performed in human AKI, the presence of ATN is one of the most crucial characteristics for distinguishing AKI from oliguria caused by dehydration or prerenal azotemia. Recently developing imaging techniques such as magnetic resonance (MR)22 might be applied in future study to evaluate the correlation of urinary L-FABP with morphological changes in the human kidney. We also demonstrate that urinary L-FABP can detect and predict functional decline in CP and IR AKI models. Considering that urinary L-FABP is derived from proximal tubular cells, the good predictions of urinary L-FABP on GFR may be indirect. However, reflecting both histological and functional insults of AKI is truly required biomarker characteristics in clinical use. Validation clinical studies should be needed to confirm the findings on our animal models.
In conclusion, urinary L-FABP can detect the histological and functional insults in mouse CP- and IR-induced AKI models earlier and more accurately than conventional renal biomarkers. Application for ROC curve analysis is unique but useful to compare renal biomarkers. Further evaluation on various human AKI populations should be necessary to confirm the significance of urinary L-FABP as a forecasting biomarker of pathological and functional damage.
Acknowledgments
We thank Mrs. Mami Haba and Mrs. Kaoru Amitani (University of Tokyo) for their technical support and Dr. Christoph Eisner (National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases) for his suggestion on GFR measurement.
Footnotes
Address reprint requests to Eisei Noiri, M.D., Ph.D., 107 Lab., Departments of Nephrology and Endocrinology, University of Tokyo, 7-3-1 Hongo, Bunkyo, Tokyo, Japan 113-8655. E-mail: noiri-tky@umin.ac.jp.
Supported by the BioBank Japan Project on the Implementation of Personalized Medicine (grant 3023168, MEXT, Japan to E.N.); KAKEN-HI (grant 19590935, MEXT, Japan to E.N. and T.S.), the Special Coordination Funds for Promoting Science and Technologies (grant 1200015, MEXT, Japan to E.N.), the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases grant RO1-DK075976 to S.P.), and the Veterans Administration (merit award to D.P.).
References
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73:1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T. Renal L-type fatty acid binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73:465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- Doi K, Suzuki Y, Nakao A, Fujita T, Noiri E. Radical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidney. Kidney Int. 2004;65:1714–1723. doi: 10.1111/j.1523-1755.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol. 2007;292:F27–F37. doi: 10.1152/ajprenal.00193.2006. [DOI] [PubMed] [Google Scholar]
- Doi K, Leelahavanichkul A, Hu X, Sidransky KL, Zhou H, Qin Y, Eisner C, Schnermann J, Yuen PS, Star RA. Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int. 2008;74:1017–1025. doi: 10.1038/ki.2008.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiri E, Peresleni T, Miller F, Goligorsky MS. In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clin Invest. 1996;97:2377–2383. doi: 10.1172/JCI118681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K, Noiri E, Maeda R, Portilla D, Sugaya T, Fujita T. Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int. 2008;73:1374–1384. doi: 10.1038/ki.2008.106. [DOI] [PubMed] [Google Scholar]
- Hewitt SM, Dear J, Star RA. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004;15:1677–1689. doi: 10.1097/01.asn.0000129114.92265.32. [DOI] [PubMed] [Google Scholar]
- Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Kasza KE, O'Connor MF, Konczal DJ, Trevino S, Devarajan P, Murray PT. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–1069. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- Dear JW, Kobayashi H, Brechbiel MW, Star RA. Imaging acute renal failure with polyamine dendrimer-based MRI contrast agents. Nephron Clin Pract. 2006;103:c45–c49. doi: 10.1159/000090608. [DOI] [PubMed] [Google Scholar]