Abstract
Alternative splicing of transcripts from many cancer-associated genes is believed to play a major role in carcinogenesis as well as in tumor progression. Alternative splicing of one such gene, the neural cell adhesion molecule CD56 (NCAM), impacts the progression, inadequate therapeutic response, and reduced total survival of patients who suffer from numerous malignant neoplasms. Although previous investigations have determined that CD56 exists in three major isoforms (CD56120kD, CD56140kD, and CD56180kD) with individual structural and functional properties, neither the expression profiles nor the functional relevance of these isoforms in malignant tumors have been consistently investigated. Using new quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) strategies and novel CD56 isoform-specific antibodies, CD56140kD was shown to be exclusively expressed in a number of highly malignant CD56+ neoplasms and was associated with the progression of CD56+ precursor lesions of unclear malignant potential. Moreover, only CD56140kD induced antiapoptotic/proliferative pathways and specifically phosphorylated calcium-dependent kinases that are relevant for tumorigenesis. We conclude, therefore, that the specific detection of CD56 isoforms will help to elucidate their individual functions in the pathogenesis and progression of malignant neoplasms and may have a positive impact on the development of CD56-based immunotherapeutic strategies.
The neural cell adhesion molecule CD56 (NCAM) is a founding member of a large family of cell surface glycoproteins that share structural motifs related to immunoglobulin and fibronectin type III domains.1,2 Human CD56 is encoded by a single-copy gene on chromosome 11 that spans more than 314 kb and contains 19 major exons as well as 6 additional smaller exons.2,3,4 Alternative splicing results in the expression of three major isoforms that differ in their membrane association and their intracellular domains: the isoform CD56120kD, which is linked to the plasma membrane by a glycosylphosphatidylinositol anchor, and the isoforms CD56140kD/CD56180kD, which both have a transmembrane domain and cytoplasmatic tails of different lengths.2
Originally, CD56 was characterized as a mediator of cell-cell adhesion, but now it is also considered to be a signaling receptor that impacts cellular adhesion, migration, proliferation, apoptosis, differentiation, survival, and synaptic plasticity.5,6,7,8,9,10 CD56-mediated signaling can be activated after homophilic interaction or via heterophilic dimerization to a broad range of other molecules including the closely related adhesion molecule L1, fibroblast growth factor 1 (FGFR 1), the glial cell line-derived neurotrophic factor, and sulfate proteoglycans (CSPG and HSPGs).11,12,13,14,15,16,17,18,19,20,21,22,23
Physiologically, CD56 is abundantly expressed in the developing as well as in the adult human brain and plays a pivotal role in neurogenesis, neuronal migration, and neurite outgrowth,19,24,25,26 on natural killer (NK) cells, a subset of T lymphocytes,27,28 as well as on neuroendocrine cells.29 In human diseases, CD56 is a specific histological immune marker for the diagnosis of malignant nervous tumors (eg, medulloblastoma and astrocytoma),29,30 malignant NK/T-cell lymphomas (NK/T-NHLs),31,32 and neuroendocrine carcinomas (NECs).33,34,35,36 Moreover, increased serum levels of CD56 are associated with the progression of dementia of Alzheimer’s type37 as well as multiple myeloma (MM).38,39,40,41,42 Its overexpression in malignant neoplasms is associated with an aggressive tumor type, inadequate therapeutic response, and a reduced total survival time in a broad range of malignancies including lymphoblastic and myeloid leukemias (ALLs/AMLs),43,44,45 malignant melanomas,46,47 and numerous carcinomas.48,49,50,51,52,53
Despite the correlation between CD56 expression and the progression of degenerative and neoplastic diseases, no reports of consistent investigations concerning the expression of different CD56 isoforms have been published. However, these data appear relevant as i) the different CD56 isoforms exhibit varying intramembrane localizations, mobility, and interaction partners2; ii) alternative splice products of many cancer genes that impact tumorigenesis are known to occur during tumor progression54,55; and iii) CD56 transfected cardiomyocytes with stable overexpression of CD56 isoforms revealed strongly different, isoform-specific, gene expression profiles (S.G., unpublished data). Finally, because it has been determined that CD56 induces increased proliferation and decreased apoptosis in acute myeloid leukemias (AMLs) via the nuclear factor (NF)-κB/bcl2 pathway,56 an effect that can be inhibited using the NF-κB inhibitor wedelolactone,56 the specific detection of CD56 isoforms may further elucidate their different functions in human malignant and degenerative diseases and therefore be the basis for novel CD56-related immunotherapeutic strategies.
Materials and Methods
Cell Lines and Human Tissue Samples
The human lymphoma/leukemia cell lines K562, U937, HL-60, Jurkat, Karpas, MEG01, Mo7e, SU-DH-L1, THP1, and MUTZ-2 were provided by the American Type Culture Collection (Manassas, VA) and by the DSMZ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany). Plasmacytoma cell lines INA6, AMO-1, MOLP-8, RPM-8226, U266, KMS-12-BM, and MMIS were made available by Dr. T. Stühmer (University of Würzburg, Würzburg, Germany). Normal human tissue samples and tumor specimens were obtained from biopsy and autopsy material as described.56,57
Specific Detection of CD56 Isoforms by Quantitative RT-PCR (qRT-PCR)
For qRT-PCR, RNA was extracted as previously described and 5 μg of total RNA was reverse-transcribed adding 1 μCi of 32P-dCTP. Adjustment to equal amount and quantification by radioactive RT-PCR were performed as described56 using 0.1 μCi 32P-dCTP per 25 μl of reaction mix and GAPDH control RT-PCR. The primers for amplification of CD56 isoforms were as follows: A: CD56com-UP, 5′-ATGCTGCAAACTAAGGATCTCA-3′; B: CD56120kD-LP, 5′-CTAACAGAGCAAAAGAAGAGTC-3′; C: CD56140kD/180kD-LP, 5′-TCATGCTTTGCTCTCGTTCTCC-3′; D: CD56180kD-UP, 5′-CGGACCCGGAGCCCACCCAGCC-3′. Amplifications were performed to detect CD56120kD/125kD [primers A + B, size of PCR products 2173 bp (120 kDa) and 2283 bp (125 kDa)], CD56140kD/180kD [primers A + C, size of PCR products 2544 bp (140 kDa) and 3354 bp (180 kDa)] and CD56180kD (primers C + D, size of PCR product 399 bp) at 60°C annealing temperature and for 35 cycles each.
Cloning and Sequencing of Human CD56 Isoforms
Amplificates from CD56 isoform-specific qRT-PCRs (CD56120kD, CD56140kD, and CD56180kD) were isolated from agarose gels and cloned into the pGEM T-vector (Promega, Heidelberg, Germany), transformed in DH5 α Escherichia coli competent cells (Invitrogen, Heidelberg, Germany) and DNA from recombinant colonies was isolated by minipreparation and sequenced by the cycle-sequencing method as previously described.56 Homology to known CD56 isoforms was confirmed by comparison with CD56 isoform-specific sequences using Blast search (National Center for Biotechnology Information, National Institutes of Health, Bethesda, MD).
Recombinant Protein Expression of CD56 Isoforms
CD56 isoforms (CD56120kD, CD56140kD, and CD56180kD) were subcloned into the pTNT vector (Promega) and the corresponding proteins were expressed in the eukaryotic TNT reticulocyte lysate system using SP6 RNA polymerase and methionine S35 (following protocols from Promega). For bacterial protein expression and epitope mapping, overlapping isoform-specific subclones were generated by cloning corresponding fragments from the common N-terminal regions and specific C-terminal regions into the pGEX4T2 vector. After transformation of E. coli BL21 competent cells (Stratagene, Heidelberg, Germany), recombinant GST-fusion proteins were expressed and purified using glutathione Sepharose 4B following the instructions of the manufacturer (Amersham Biosciences, Heidelberg, Germany) and detected by Western blot using an anti-GST monoclonal mouse antibody (Calbiochem, Heidelberg, Germany).
Production and Testing of Novel CD56 Antibodies
Unmodified peptides and N-terminally acetylated and/or C-terminally amidated derivatives, respectively, were coupled to keyhole limpet hemocyanin via an additional terminal cysteine residue using sulfosuccinimidyl-4-[-(N-maleimidomethyl)cyclohexane-1]-carboxylate (Pierce, Rockford, IL) or via primary amino groups using glutaraldehyde (Sigma-Aldrich, St. Louis, MO). Rabbits were immunized intradermally with peptide conjugates emulsified in complete Freund’s adjuvant (primary injections) and Montanide ISA 206 (boosts). All sera were routinely affinity purified on peptide affinity matrices (Immunoglobe, Himmelstadt, Germany). Matrices for coupling through cysteine and primary amino groups were UltraLink iodoacetyl supported (Pierce) and NHS-activated Sepharose 4 Fast Flow (GE Healthcare, Freiburg, Germany), respectively. The CD56 sequence information used for peptide design was as follows: anti-CD56120kD antibody IG-656: peptides specific for CD56120kD appeared not to be suitable as antigens. Therefore, a CD56120kD-derived sequence (SAQPTAIPATLGGNSAS) was chosen despite an extended region of sequence identity with the CD56140kD and CD56180kD isoforms. CD56120kD specificity was obtained in a second step by purification on a peptide matrix based on a CD56120kD-specific sequence (PATLGGNSAS). Anti-CD56180kD antibodies IG-640, IG-658: PSTADSSVSPAPA (CD56180kD-specific sequence both for immunization and purification). Extraction of whole protein and detection of CD56 isoforms/controls by Western blotting were performed as described.56
Immunohistochemistry and Clonality Analysis
Immunohistochemical and immunofluorescence stainings as well as molecular detection of clonal plasma cell populations by PCR amplification of the immunoglobulin heavy chain gene locus (FR3A and FR2A) with subsequent fragment analysis were performed as previously described.56,58
Cell Transfection and Microarray Analysis
For eukaryotic cell transfections, the CD56 isoforms (CD56120kD, CD56140kD, and CD56180kD) were cloned into the mammalian expression MIDGE vector pMCV1.2 (Mologen, Berlin, Germany). Cells (106) of the CD56-negative plasmacytoma cell line INA6 (provided by T. Stühmer, University of Würzburg), were transfected by electroporation with each 20 μg/ml of pMCV1.2 clones corresponding to individual CD56 isoforms, 4 μg/ml pEGFP (enhanced green fluorescent protein), and 4 μg/ml of the expression plasmid for human truncated CD4 (pCD4Δ) for subsequent enrichment of transfected cells after 6 days of cell culture as previously described.59 Purified cells highly enriched (∼98%) for GFP/pCD4Δ were collected and the overexpression of CD56 isoforms was confirmed by CD56 isoform-specific RT-PCR (see above). For microarray experiments, total RNA was extracted with TriPure isolation reagent (Roche, Mannheim, Germany). The samples were analyzed for gene expression using Affymetrix (Santa Clara, CA) human HG U133 plus 2.0 arrays. Expression data were sorted by difference in expression and visualized using the Chester and Treeview software from Michael Eisen (Berkeley, CA). Overexpression of up-regulated genes was confirmed by qRT-PCR using the following gene-specific primers: keratin 8, forward 5′-CTGGTGGAGGACTTCAAGAAC-3′; reverse 5′-GACCTCAGCAATGATGCTGTC-3′; HSP70, forward 5′-TTCCAGCCCCCAATCTCAGAGCCG-3′, reverse 5′-GTCTCCGTCGTTGATCACCTGGAA-3′; RhoA GTPase, forward 5′-TTCGGAATGACGAGCACACG-3′, reverse 5′-GTCTAGCTTGCAGAGCAGCT-3′; thrombospondin 1, forward 5′-ACCGCATTCCAGAGTCTG-3′, reverse 5′-GACGTCCAACTCAGCATT-3′; NF-YB, forward 5′-GCCATACCTCAAACGGGAAAG-3′, reverse 5′-TCTCCTTTCATAGCCTCTCTG-3′; Malat 1, forward 5′-TAGTTGGCAGTGGCGTGTTAC-3′, reverse 5′-GTTTCCCCTCCCTCATCACAA-3′; neuroligin 1, forward 5′-GAATAACGAGGGAGGGGGAGGTGG-3′, reverse 5′-CCGCTGGAGAAAGATGGAATGGTG-3′; NPPA, forward 5′-CAGTGAGCCGAATGAAGAAGC-3′, reverse 5′-CTCCAATCCTGTCCATCCTGC-3′; NFKBIB, forward 5′-CTTGTACCCCGATTCCGACTT-3′, reverse 5′-GCTTCTTGCCTGCCGGTCTAT-3′; tankyrase 2, forward 5′-GCTGAGCCAACCATCCGAAAT-3′, reverse 5′-GTAGGCGGCAGGTTTGTAGAT-3′; GAPDH, forward 5′-CAACAGCGACACCCACTCCTC-3′, reverse 5′-CATGTGGGCCATGAGGTCCAC-3′.
Detection of Apoptosis and Phospho-Kinases
Presence of phosphatidylserine in the outer leaflet of the plasma membrane was detected by flow cytometry using fluorescein isothiocyanate-coupled annexin V (annexin V; BD Biosciences, Heidelberg, Germany) as described.56 For further analysis of apoptotic cells, the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL) assay was used (Apo-BRDU kit; BD, Pharmingen, Heidelberg, Germany) following the instructions of the manufacturer. For the detection of Ca2+-dependent phosphorylated kinases, whole protein extracts from INA6 cells transfected with individual CD56 isoforms (see above) were tested by Western blotting56 using phospho-antibody sampler kits (Cell Signaling, Danvers, MA) specific for the Akt-pathway, the erk1/2 pathway, the p38 MAP kinase pathway, the SAPK/JNK pathway, as well as for CamKII, phospho-CamKII, and phospho-PKA C. The detection of intracellular calcium was performed by loading CD56-transfected INA-6 cells with the calcium indicator Fluo-4 following the instructions of the manufacturer (Molecular Probes, Invitrogen, Heidelberg, Germany).
Statistical Analysis
Data are expressed as means ± SD. Comparisons were made using the Mann-Whitney U-test and the SPSS for Windows software package (SPSS Inc., Chicago, IL). Differences were considered significant when P < 0.05.
Results
Detection of CD56 Isoforms in Normal Human Tissues, Cell Lines, and Malignant Human Tumors by Isoform-Specific qRT-PCR
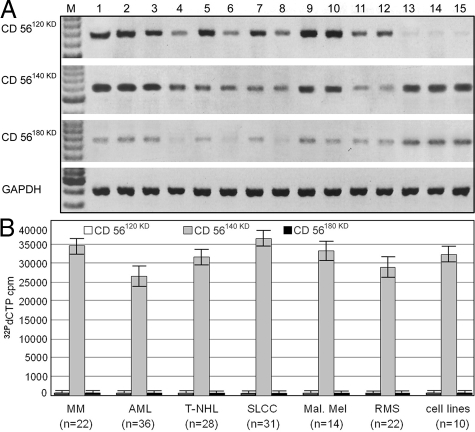
For the specific amplification of CD56 isoforms, an isoform-specific qRT-PCR strategy with one oligonucleotide primer from the homologous N-terminus of CD56 combined with isoform-specific C-terminal primers was used. As shown in Figure 1a, a consistently strong expression of the CD56120kD and CD56140kD isoforms (except the muscle-specific CD56125kD version that is exclusively expressed in skeletal muscle instead of the CD56120kD isoform) and only small amounts of CD56180kD mRNA were detected in all extraneural normal human tissues tested, whereas in samples from the central nervous system a predominant expression of CD56140kD and CD56180kD was found. In contrast, all human CD56+ tumor cell lines (n = 10) as well as all ex vivo samples from CD56+ human malignancies (n = 153), including multiple myelomas (n = 22), acute myeloid leukemias (n = 36), malignant T-NHLs and NK/T cell lymphomas (n = 28), small lung cell carcinomas (n = 31), malignant melanomas (n = 14), and rhabdomyosarcomas (n = 22), exhibited an exclusive expression of CD56140kD, the level of which is significantly higher than in normal tissues (Figure 1b).
Figure 1.
CD56 isoform-specific quantitative RT-PCRs56 using cDNAs from normal tissues and malignant neoplasms. A: Detection of CD56 isoform RNA in normal tissues (lane 1 = duodenum, lane 2 = salivary gland, lane 3 = thymus, lane 4 = lymph node, lane 5 = spleen, lane 6 = liver, lane 7 = testis, lane 8 = lung, lane 9 = kidney, lane 10 = pancreas, lane 11 = tonsil, lane 12 = placenta, lane 13 = cerebral cortex, lane 14 = basal ganglia, lane 15 = cerebellum) in five different samples from individual patients in comparison with GAPDH transcripts (loading control). In all extraneural normal human tissues (lanes 1 to 12) a ubiquitous expression of CD56120kD as well as CD56140kD and only few CD56180kD transcripts were detected, whereas CD56140kD and CD56180kD were the predominantly expressed isoforms in tissue samples from the central nervous system (lanes 13 to 15). B: Detection of CD56 isoform RNA in human ex vivo samples from CD56+ malignancies (n = 153) of different type [multiple myeloma (MM, n = 22), acute myeloid leukemia (AML, n = 36), malignant T-NHLs and NK/T cell lymphomas (T-NHL, n = 28), small lung cell carcinomas (SLCC, n = 31), malignant melanomas (Mal. Mel, n = 14), and rhabdomyosarcomas (RMS, n = 22)] as well as in CD56+ tumor cell lines (cell lines, n = 10). In contrast to normal tissue, CD56140kD was the only CD56 isoform found in human ex vivo samples from CD56+ malignancies and tumor cell lines.
Generation of CD56 Isoform-Specific Antibodies
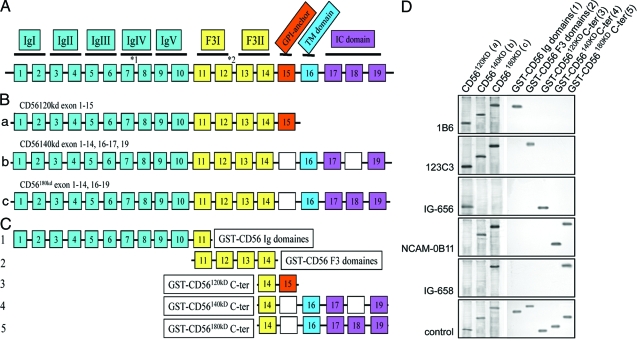
To test the specificity of commercial and novel CD56 antibodies, we cloned the human CD56 isoforms CD56120kD, CD56140kD, and CD56180kD (Figure 2a) and expressed them recombinantly either as full-length proteins in reticulocyte lysates (Figure 2b) or as glutathione S-transferase fusion proteins coding for the common N-termini as well as the isoform-specific C-terminal regions (Figure 2c). Among 12 commercial anti-CD56 antibodies, 7 (Table 1) revealed a strong detection of CD56 and its isoforms. In particular, clones 1B6 (Novocastra, Newcastle upon Tyne, UK) and H-300 (Santa Cruz Biotechnology, Santa Cruz, CA) bind within the common N-terminus of CD56 and recognize all isoforms (Figure 2d), clone 123C3 (Santa Cruz; Abcam, Cambridge, MA) and N-CAM 13 (BD; Fitzgerald Industries, Concord, MA) bind within exon 11/12 and also recognize CD56120kD, CD56140kD, and CD56180kD (Figure 2d). Interestingly, clone C-20 (Santa Cruz) was shown to bind in part at the end of C-terminus of exon 14 common to all CD56 isoforms and to the 5′-end of exon 15, which is specific for CD56120kD. Finally, clone 12F11 (BD) and NCAM-OB11 (Sigma) were shown to detect the C-terminus-specific for both CD56140kD and CD56180kD (Figure 2d). Because none of the antibodies tested showed specificity for CD56120kD or CD56180kD, we generated specific polyclonal rabbit antisera directed against the specific C-termini of CD56120kD (IG-656, Immunoglobe) and CD56180kD (IG-658, Immunoglobe), respectively. As shown in Figure 2d, all of these antisera bound specifically to their corresponding isoforms and their binding was shown to be directed against the corresponding isoform-specific C-terminal regions used for immunization (Figure 2d).
Figure 2.
The exon structure of CD56 isoforms and identification of CD56 isoform-specific antibodies. A: Schematic presentation of the CD56 gene locus showing exons 1 to 19 constituting CD56 isoforms (*1 indicates the insertion point of the small VASE- or p-exon located between exons 7 and 8; *2 indicates the locus of the SEC-exon as well as the locus of four additional small exons located between exon 12 and 13).83,84,85,86 IgI-V indicates immunoglobulin homology modules; F3-I and -II correspond to fibronectin type III homology modules. B: Exon composition of the predominant human CD56 isoforms CD56120kD, CD56140kD, and CD56180kD. Corresponding cDNAs were expressed as full-length proteins in reticulocyte lysates (see D). C: CD56-GST fusion proteins representing the N-terminus containing the immunoglobulin domains (GST-CD56 Ig domains) and fibronectin domains (GST-CD56 F3 domains) common to all CD56 isoforms as well as the isoform-specific C-terminal regions of individual CD56 isoforms (GST-CD56120kD C-ter, GST-CD56140kD C-ter, GST-CD56180kD C-ter). D: Identification of CD56 isoform-specific antibodies by Western blotting using recombinantly expressed full-length CD56 isoforms (left: a to c) and CD56-GST fusion proteins (right: 1 to 5). As indicated in Table 1, clone 1B6 and clone 123C3 recognize all CD56 isoforms by binding either within the N-terminal immunoglobulin domain region (clone 1B6) or the fibronectin domain region (clone 123C3). In contrast, clone NCAM-OB-11 binds to an epitope in the common C-terminus of the CD56140kD and CD56180kD isoforms, whereas the novel antibodies IG 656 and IG 658 specifically detect CD56120kD and CD56180kD, respectively. As control (ctr), full-length CD56 isoforms were expressed in the presence of methionine S35 (left) and GST-fusion proteins were detected by an anti-GST antibody.
Table 1.
Determination of the Isoform Specificity of CD56 Antibodies by Western Blot Epitope Mapping
| Clone number/company/source | CD56 isoform specificity | Binding region |
|---|---|---|
| 1B6, Novocastra (mouse mc) | 120 kDa, 140 kDa, 180 kDa | N-terminus |
| H-300, Santa Cruz (rabbit pc) | 120 kDa, 140 kDa, 180 kDa | N-terminus |
| 123C3, Santa Cruz, Abcam (mouse mc) | 120 kDa, 140 kDa, 180 kDa | Exon 11/12 (F3-1) |
| N-CAM 13, BD, Fitzgerald (mouse mc) | 120 kDa, 140 kDa, 180 kDa | Exon 11/12 (F3-1) |
| C-20,* Santa Cruz (goat pc) | 120 kDa, 140 kDa, 180 kDa | C-terminus |
| IG-656, immunoglobe (rabbit pc) | 120 kDa | C-terminus |
| 12F11, BD (rat mc) | 140 kDa, 180 kDa | C-terminus |
| NCAM-0B11, Sigma (mouse mc) | 140 kDa, 180 kDa | C-terminus |
| IG-658, immunoglobe (rabbit pc) | 180 kDa | C-terminus |
This antibody was mapped to react with the specific C-terminus of CD56120kD/125kD (exon 15) and the C-terminal region of exon 14 common to all CD56 isoforms (not shown).
No specific binding was achieved for the following antibodies (clone, company): UJ13A, Abcam; NKI-nbl-1, Abcam; MEM-188, Abcam; 12F8, BD; 56-C04, oncogene.
The bold indicated antibodies were produced and tested in cooperation with immunoglobe (see also Material and Methods). mc, monoclonal, pc, polyclonal.
Immunohistochemical Detection of CD56 Isoforms in Normal Human Tissues and Malignant Tumors Using Isoform-Specific Antibodies
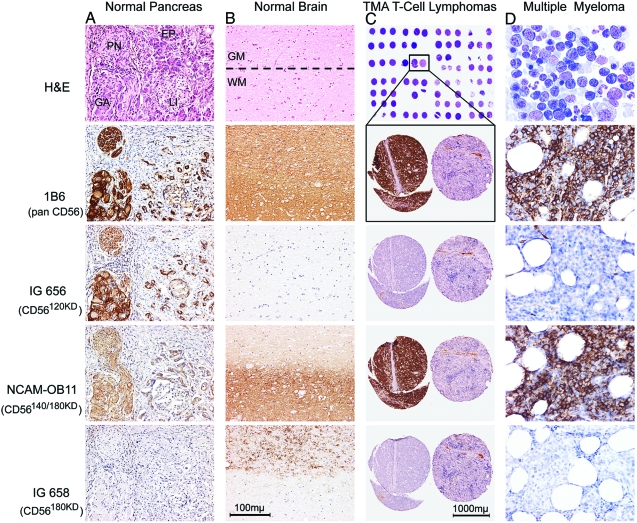
The specificity of the CD56 isoform-specific antibodies was tested by Western blot analysis (not shown) as well as immunostaining on normal human tissue samples with high mRNA levels of individual CD56 isoforms. As shown in Figure 3, a and b, all isoform-specific antibodies detected CD56 isoform protein levels that correlated to the mRNA amounts of each respective isoform detected in the corresponding human formalin-fixed and paraffin-embedded tissue [eg, pancreas (Figure 3a) and cerebral cortex (Figure 3b)]. As expected after the qRT-PCR experiments, significantly stronger immunostaining than in normal tissues was observed in CD56-positive aggressive malignant neoplasms [eg, NK/T cell lymphoma (Figure 3c) and multiple myeloma (Figure 3d)] using the CD56pan antibody (clone 1B6, Novocastra) as well as the CD56140/180kD-specific antibody (clone NCAM-OB11, Sigma), whereas the reactions with antibodies specific for CD56180kD (IG-658, Immunoglobe) and for CD56120kD (IG-656, Immunoglobe) revealed negative results. Furthermore, as was to be expected for CD56, which is a cell membrane protein with transmembrane and intracellular domains, staining was located on the cell membrane as well as within the cytoplasm.
Figure 3.
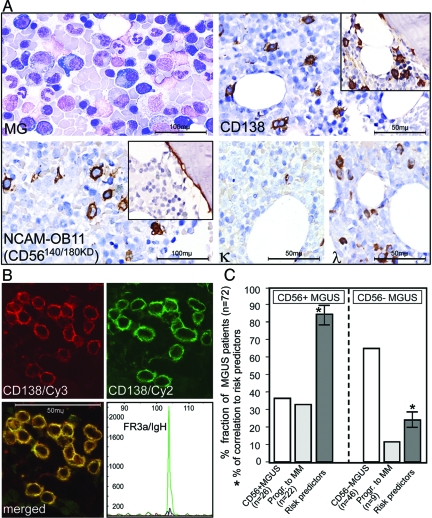
A and B: Detection of CD56 isoforms using immunohistochemistry in formalin-fixed, paraffin-embedded normal human pancreas (A: PN = peripheral nerve, GA = ganglion, EP = exocrine pancreatic gland, LI = Langerhans island) and brain (B: WM = white matter, GM = gray matter) from different individuals (n = 15) using anti-CD56 antibodies (top row: H&E; second row: pan CD56 antibody 1B6 immunostain; third row: CD56120kD-specific antibody IG656 immunostain; fourth row: CD56140kD/180kD-specific antibody NCAM-OB-11 immunostain; bottom row: CD56180kD-specific antibody IG658 immunostain). A: In normal pancreatic tissue, a co-expression of CD56120kD and CD56140kD was found in exocrine pancreatic glands, peripheral nerves, and ganglion cells, whereas a weak but exclusive expression of CD56120kD was demonstrated in Langerhans islands. In contrast, CD56180kD is neither expressed in exocrine/endocrine pancreatic tissue nor in peripheral nerves. No CD56 at all was detected in the perineural pancreatic stroma. B: In the normal cerebral cortex, CD56140kD was found in the gray matter as well as in the white matter, whereas CD56180kD is expressed exclusively in the gray matter and no CD56120kD was detectable in either of the regions. C: Tissue microarray (TMA) of samples from 83 individual CD56+ or CD56− T-cell lymphoma cases [top: excerpt of sample overview; bottom: enlargements from two representative cases (left: CD56+; right: CD56−)] using anti-CD56 antibodies [top enlargement: 1B6 (pan CD56); second enlargement: IG656 (specific for CD56120kD); third enlargement: NCAM-0B-11 (specific for CD56140kD/180kD); bottom enlargement: IG658 (specific for CD56180kD)]. In all CD56-positive peripheral T-cell lymphomas examined (n = 28), CD56140kD was the only detectable isoform (negative immunostaining using antibodies IG656 and IG658). D: Detection of CD56 isoforms in bone marrow trephines from 72 cases of multiple myeloma (MM) using conventional staining (May-Grünwald stain, top) as well as immunostaining with anti-CD56 antibodies [second panel: 1B6 (pan CD56); third panel: IG656 (specific for CD56120kD); fourth panel: NCAM-OB-11 (specific for CD56140kD/180kD); bottom panel: IG658 (specific for CD56180kD)]. CD56140kD (positive staining with 1B6 and NCAM-OB-11) was found in every sample examined, whereas none of the samples contained CD56120kD (negative staining using IG656) or CD56180kD (negative staining using IG658). One representative sample is shown.
CD56 Isoform-Specific Gene Expression Profiles and Phosphorylation of CamKII
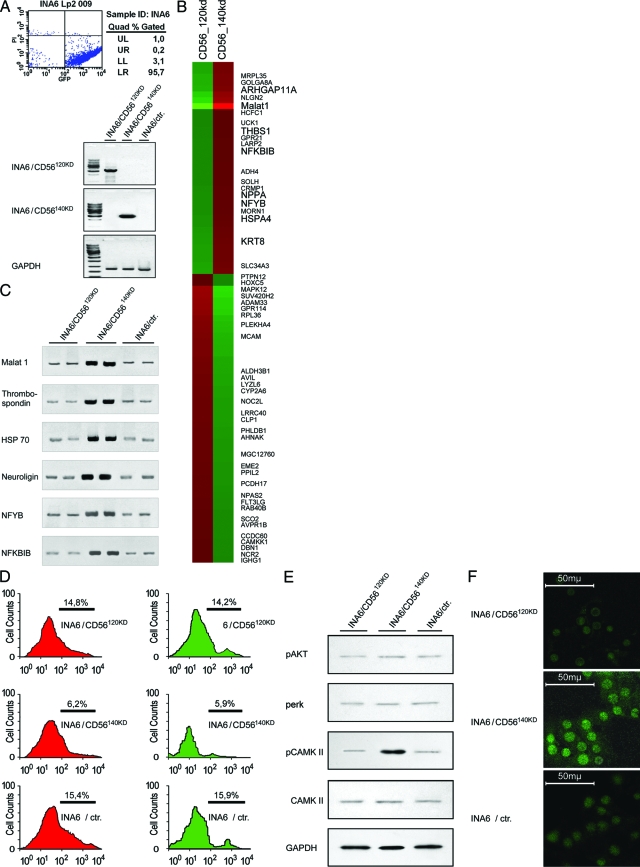
To investigate CD56 isoform-specific gene expression profiles in malignant neoplasms, the CD56-negative plasmacytoma cell line INA6 was transfected with the human CD56 isoforms CD56120kD and CD56140kD. Using co-transfection of pEGFP (enhanced green fluorescent protein) and truncated human CD4 (pCD4Δ), transfected cells were purified to >95% transfected cells using CD4 affinity chromatography (Figure 4a).59 CD56120kD and CD56140kD isoform-specific gene expression profiles were generated using the Affymetrix whole genome chip U133-2.0. In comparison with the mock control, 99 differentially expressed genes (55 up-regulated, 31 down-regulated; not shown) were detected in INA6 cells transfected with CD56120kD, whereas INA6 cells transfected with CD56140kD showed 82 differentially regulated genes (41 up-regulated, 41 down-regulated; not shown). In direct comparison between the expression profiles of CD56140kD and CD56120kD, 85 probesets showed at least a fourfold difference in gene expression (36 up-regulated and 49 down-regulated for CD56140kD; Figure 4b). Interestingly, most of the genes induced by CD56140kD in comparison with CD56120kD that have previously been functionally characterized exhibit an association to multiple myeloma (RhoGTPase,60 HSP70,61 thrombospondin,62 NPPA,63 keratin 8,64 tankyrase 265) or to procarcinogenic, antiapoptotic signaling pathways (NFYB,66 NF-κB,56 neuroligin,67 and Malat168,69). The validity of the gene expression data were verified by measuring the expression levels of selected genes by quantitative RT-PCR56 using gene-specific primers (Figure 4c). Moreover, the functional relevance of the up-regulation of antiapoptotic pathways was shown by annexin V staining as well as using a TUNEL assay. Here, it was determined that CD56140kD transfection in INA6 cells induces a 2.5-fold decrease in apoptosis in comparison with mock controls and CD56120kD-transfected cells (Figure 4d).
Figure 4.
CD56 isoform-specific gene expression profiles and induction of antiapoptotic/proproliferative pathways. A: After co-transfection of the CD56-negative MM cell line INA6 with ΔCD4, GFP, and either CD56120kD or CD56140kD, transfected cells were enriched by affinity chromatography. FACS analyses for GFP expression (top) and CD56 isoform-specific RT-PCRs (bottom) show highly enriched INA 6 cells with specific overexpression of individual CD56 isoforms. B: Eighty-five probesets representing genes with at least a fourfold difference in expression between the isoforms CD56140kD and CD56120kD. Red color depicts increased expression and green color decreased expression. C: Analysis of MM-associated procarcinogenic/antiapoptotic gene induction by CD56140kd in INA6 cells using quantitative RT-PCR.56 In comparison with INA6 cells transfected with CD56120kD and INA6 cells alone, INA6 cells transfected with CD56140kD had elevated transcript levels of Malat 1, thrombospondin, HSP70, neuroligin, NFYB, and NFKBIB. D: Annexin V FACS analysis (left) and TUNEL assay (right) demonstrate a 2.5-fold decrease in apoptotic rate in INA6 cells transfected with CD56140kD in comparison with CD56120kD and controls. E: Western blot analyses using antibodies specific for phosphorylated CD56-dependent kinases2 show an increased phosphorylation of CamKII in CD56140kD-overexpressing INA6 cells, whereas no changes were seen for other CD56-related kinases such as Akt and erk. The presence of similar amounts of total unphosphorylated CamKII in all samples was shown by Western blotting using a CamKII-specific antibody (Cell Signaling, Danvers, MA). F: Detection of intracellular calcium using the green fluorescent chromogen fluo-4 show increased calcium content in the cytoplasm of INA6 cells transfected with CD56140kD cells in comparison with INA6 cells transfected with CD56120kD cells or INA6 cells alone.
In addition to these gene expression analyses, we used the purified INA6 cells expressing individual CD56 isoforms to investigate their effect on the activation of CD56-dependent kinase pathways.2 To do this, we used phospho-specific anti kinase antibody sampler kits and were able to show an increase in the phosphorylation of the calcium-dependent calmodulin kinase II (CamKII, Figure 4e) for CD56140kD, but not for CD56120kD or controls. Using immunofluorescence with the fluo calcium indicator fluo 4, we found that this effect is accompanied by an increase in intracellular calcium (Figure 4f).
CD56 Expression and Isoform Pattern in the Differential Diagnosis of Neoplasms with Uncertain Malignant Potential
As the clinical course of CD56 neoplasms with low/uncertain malignant potential is often unclear, there is need for indicators to better predict the progression of these tumor types. Based on the evidence for the tumorigenic potential of CD56140kD we had found, we examined the predictive value of the expression of CD56 isoforms in monoclonal gammopathies of uncertain significance (MGUS), which is an example for such a precursor lesions of CD56-positive malignant neoplasms. To this end, we performed CD56 isoform-specific immunohistochemical stainings on bone marrow trephines that were infiltrated by MGUS, a plasma cell disorder that evolves to symptomatic multiple myeloma in 30 to 40% of cases.70 As shown in Figure 5a, ∼30% of the 72 MGUS patients examined (n = 72) exhibited CD56-positive clonal plasma cells in bone marrow trephines with exclusive expression of CD56140kD. Coexistence of CD138 and CD56 in clonal plasma cells was confirmed by immunofluorescence (Figure 5b). More interestingly however, in longitudinal retrospective analyses (8 to 12 years), ∼85% of CD56-positive MGUS cases showed progression to multiple myeloma characterized by clinical progression and nodular clonal plasma cell infiltrates in the bone marrow (>20%, Figure 5c). Not surprisingly, the attributes of these patients were in good correlation with the known risk factors for the progression of MGUS to MM.70,71 In contrast, 75% of the patients among the 70% MGUS cases with CD56-negative clonal plasma cells remained histologically and clinically stable and exhibited no increase of clonal plasma cell infiltrates during the time of the analysis.
Figure 5.
Identification of CD56140kD as a prognostic marker in MGUS, an example of a CD56-positive precursor neoplasia of uncertain malignant potential. A (top): Scattered (5 to 10%) atypical plasma cells (top left, May-Grünwald stain) with immunohistochemical staining for the plasma cell marker CD138 (top right) in conventional bone marrow smears and trephines from cases of CD56+ (main panel) and CD56− (inset) MGUS show positive results irrespective of CD56 expression. A (bottom): Trephines from CD56+ MGUS cases show specific CD56 staining (antibody NCAM-OB11, main panel), whereas no specific staining is visible in samples from CD56− MGUS cases (inset) using the same antibody. A (bottom right): Predominant staining of λ and weak staining of κ indicate immunoglobulin light chain restriction for λ in conventional bone marrow smears and trephines from patients with MGUS. B: Confirmation of the coexistence of CD138 (Cy3, red) and CD56 (Cy2, green) in clonal plasma cells in MGUS by immunofluorescence showing expression of both markers in identical plasma cell populations (merged yellow). The clonality was confirmed by the detection of a clonal rearrangement of the framework 3a (FR3a) region of the immunoglobulin heavy chain (bottom right). C: Of 26 CD56+ cases of MGUS (36% of total, first column), 22 progressed to morphologically and clinically manifest multiple myeloma within 1.5 years after initial diagnosis (31% of total, second column), whereas this was the case for a significantly smaller proportion of CD56− MGUS patients [9 (13% of total, fifth column) of 46 (64% of total, fourth column)]. Eighty-five percent of CD56+ MGUS cases were also diagnosed with other known risk factors70,71 for the progression of MGUS to multiple myeloma (third column), whereas this was only the case for 25% of CD56− MGUS cases (sixth column). Significant correlations (P < 0.001) between CD56 expression and the progression of MGUS to multiple myeloma as well as established risk predictors for this progression were confirmed using the Mann-Whitney U-test.
Discussion
In this study, we present the specific molecular genetic and immunohistochemical evidence of the predominant CD56 isoforms CD56120kD, CD56140kD, and CD56180kD present in the human using a novel qRT-PCR strategy as well as identification/generation of novel CD56 isoform-specific antibodies. Using these techniques, we were able to show that i) CD56140kD is the only CD56 isoform expressed in numerous aggressive CD56-positive malignant neoplasms (n = 155) and cell lines (n = 10); that ii) gene expression profiles specific for CD56140kD include the induction of tumor-specific genes as well as antiapoptotic signaling pathways and increased calcium influx with subsequent activation/phosphorylation of Ca2+-dependent kinases; and iii) in MGUS, an example of a CD56-positive precursor lesion with uncertain dignity and malignant potential, the expression of CD56140kD is a specific marker for progression of a clonal plasma cell population and for transition to symptomatic MM that is correlated with known risk factors for disease progression.
The production of several protein isoforms from one pre-mRNA by alternative splicing contributes to proteomic diversity and controlled switching of many cancer genes to specific splicing alternatives that occurs during tumor progression and plays a major role in carcinogenesis.54,55 Therefore, the detection of CD56 isoforms using the isoform-specific qRT-PCR strategy and antibodies described in this report may prove to be of significant relevance because the CD56 isoforms predominantly expressed in humans exhibit large structural and functional variations. In contrast to the membrane-associated CD56120kD isoform present in various healthy tissues, the predominant CD56140kD isoform in aggressive malignant neoplasms exhibits a transmembrane domain with a cytoplasmatic portion and dependent regulatory tyrosine and nonreceptor tyrosine kinases.2 Moreover, various studies have demonstrated that CD56 isoforms harbor different intramembrane microdomain localizations and mobility. These differences may be related to interactions with intracellular molecules and components of the cytoskeleton because individual CD56 isoforms have been demonstrated to have a number of direct or indirect differential interactions with various intracellular molecules such as Fyn (CD56120kD), GAP (CD56120kD and CD56140kD), FAK and spectrin (CD56140kD).72,76 According to our data, these structural variations also appear to be of major functional importance in tumorigenesis because we were able to show an overexpression of MM-associated and antiapoptotic/proproliferative antigens as well as a 2.5-fold decreased rate of apoptosis in annexin V stainings and TUNEL assays after transfection of the CD56-negative MM cell line INA-6 with CD56140kD, but not with CD56120kD or mock controls. Moreover, as inhibition of Cam kinases augments cell death77 and down-regulation of Cam kinase activator results in cell cycle arrest with impact for cancer therapy,78,79 the CD56140kD-dependent calcium influx and increased phosphorylation/activation of the calcium-dependent kinase CamKII could be a trigger of CD56140kD-dependent tumor growth and may therefore be the basis for further investigations addressing the therapeutical potential of CD56-dependent pathways. Finally, the CD56-dependent phosphorylation/activation of CamKII also appears functionally regulated by CD56140kD because CD56 is known to increase the Ca2+ influx via phosphatidylinositol 4,5-bisphosphate (PIP2), diacylglycerol (DAG), and arachidonic acid (AA),2 leading to a subsequent increase in the phosphorylation of Ca2+-dependent kinases such as CamKII.
Concerning the obvious tumorigenic potential of CD56140kD and its relation to the progression of malignant neoplasms, we finally investigated MGUS as an example for a CD56 neoplasm with low/uncertain malignant potential and could demonstrate the predictive value of CD56140kD in a CD56-positive precursor lesion of unknown dignity and malignant potential. Although the clinical relevance of CD56 in MM has been the subject of extensive investigations40,41,71 and CD56140kD was shown to be the exclusively expressed isoform in MM (see Figures 1b and 3d), the prognostic value of the expression of CD56 and its isoforms in MGUS remained to be clarified.70,80,81 In the retrospective analysis presented here, which is, to the best of our knowledge, the first of its kind concerning the expression of CD56 isoforms in a larger number of MGUS patients (n = 72, 8- to 12-year time span), a significant correlation between CD56140kD expression and clinical as well as morphological transition of MGUS to MM was found, whereby the attributes of the patients in the group showing progressive MGUS were in good correlation with the known risk factors for the progression of MGUS to MM.70,71 These results are in agreement with previous reports in which CD56 was already described as progression marker in other neoplasms of unknown dignity (eg, the transition of histiocytosis X to Langerhans cell sarcoma82), but further investigations will be needed to clarify the CD56 isoform specificity and its functional relevance in such neoplasms.
We therefore conclude that the specific detection of CD56 and its isoforms as well as the identification of CD56 isoform-dependent signaling cascades may contribute to the further elucidation of the progression and pathogenesis of CD56 malignant neoplasms. With regard to the predominantly inadequate response of CD56-positive aggressive neoplasms to conventional radio- and chemotherapy, our data may be the basis of alternative CD56-associated immune therapeutic approaches in analogy to the CD56/NF-κB/bcl2 signaling pathway in AMLs described by us previously.56
Acknowledgments
We thank Mrs. Margrit Bonengel, Mrs. Jaqueline Maar, Mrs. Ilona Pietrovski, and Mr. Erwin Schmitt for expert technical assistance.
Footnotes
Address reprint requests to Prof. Dr. Stefan Gattenlöhner, Institute of Pathology, University of Würzburg, Josef-Schneiderstr.2, D-97080 Würzburg, Germany. E-mail: stefan.gattenloehner@mail.uni-wuerzburg.de.
Supported by the Wilhelm-Sander-Stiftung (grant 2007.068.01) and the Deutsche Forschungs-Gesellschaft (DFG-SFB-Transregio 52, TPA8).
References
- Brümmendorf T, Lemmon V. Immunoglobulin superfamily receptors: cis-interactions, intracellular adapters and alternative splicing regulate adhesion. Curr Opin Cell Biol. 2001;13:611–618. doi: 10.1016/s0955-0674(00)00259-3. [DOI] [PubMed] [Google Scholar]
- Walmod PS, Kolkova K, Berezin V, Bock E. Zippers make signals: NCAM-mediated molecular interactions and signal transduction. Neurochem Res. 2004;29:2015–2035. doi: 10.1007/s11064-004-6875-z. [DOI] [PubMed] [Google Scholar]
- Walsh FS, Putt W, Dickson JG, Quinn CA, Cox RD, Webb M, Spurr N, Goodfellow PN. Human N-CAM gene: mapping to chromosome 11 by analysis of somatic cell hybrids with mouse and human cDNA probes. Brain Res. 1986;387:197–200. doi: 10.1016/0169-328x(86)90012-4. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Mattei MG, Mattei JF, Santoni MJ, Goridis C, Jordan BR. Localization of the human NCAM gene to band q23 of chromosome 11: the third gene coding for a cell interaction molecule mapped to the distal portion of the long arm of chromosome 11. J Cell Biol. 1986;102:711–715. doi: 10.1083/jcb.102.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoureux MC, Cunningham BA, Edelman GM, Crossin KL. N-CAM binding inhibits the proliferation of hippocampal progenitor cells and promotes their differentiation to a neuronal phenotype. J Neurosci. 2000;20:3631–3640. doi: 10.1523/JNEUROSCI.20-10-03631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambon K, Venero C, Berezin V, Bock E, Sandi C. Post-training administration of a synthetic peptide ligand of the neural cell adhesion molecule, C3d, attenuates long-term expression of contextual fear conditioning. Neuroscience. 2003;122:183–191. doi: 10.1016/s0306-4522(03)00597-9. [DOI] [PubMed] [Google Scholar]
- Ditlevsen DK, Kohler LB, Pedersen MV, Risell M, Kolkova K, Meyer M, Berezin V, Bock E. The role of phosphatidylinositol 3-kinase in neural cell adhesion molecule-mediated neuronal differentiation and survival. J Neurochem. 2003;84:546–556. doi: 10.1046/j.1471-4159.2003.01538.x. [DOI] [PubMed] [Google Scholar]
- Kiryushko D, Kofoed T, Skladchikova G, Holm A, Berezin V, Bock E. A synthetic peptide ligand of neural cell adhesion molecule (NCAM), C3d, promotes neuritogenesis and synaptogenesis and modulates presynaptic function in primary cultures of rat hippocampal neurons. J Biol Chem. 2003;278:12325–12334. doi: 10.1074/jbc.M211628200. [DOI] [PubMed] [Google Scholar]
- Prag S, Lepekhin EA, Kolkova K, Hartmann-Petersen R, Kawa A, Walmod PS, Belman V, Gallagher HC, Berezin V, Bock E, Pedersen N. NCAM regulates cell motility. J Cell Sci. 2002;115:283–292. doi: 10.1242/jcs.115.2.283. [DOI] [PubMed] [Google Scholar]
- Ronn LC, Berezin V, Bock E. The neural cell adhesion molecule in synaptic plasticity and ageing. Int J Dev Neurosci. 2000;18:193–199. doi: 10.1016/s0736-5748(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Ibanez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- Cole GJ, Schubert D, Glaser L. Cell-substratum adhesion in chick neural retina depends upon protein-heparan sulfate interactions. J Cell Biol. 1985;100:1192–1199. doi: 10.1083/jcb.100.4.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GJ, Glaser L. A heparin-binding domain from N-CAM is involved in neural cell-substratum adhesion. J Cell Biol. 1986;102:403–412. doi: 10.1083/jcb.102.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GJ, Akeson R. Identification of a heparin binding domain of the neural cell adhesion molecule N-CAM using synthetic peptides. Neuron. 1989;2:1157–1165. doi: 10.1016/0896-6273(89)90182-7. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- Frei T, von Bohlen und Halbach F, Wille W, Schachner M. Different extracellular domains of the neural cell adhesion molecule (N-CAM) are involved in different functions. J Cell Biol. 1992;118:177–194. doi: 10.1083/jcb.118.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MA, Halfter W, Cole GJ. Analysis of proteoglycan expression in developing chicken brain: characterization of a heparan sulfate proteoglycan that interacts with the neural cell adhesion molecule. J Neurosci Res. 1995;41:49–64. doi: 10.1002/jnr.490410107. [DOI] [PubMed] [Google Scholar]
- Grumet M, Rutishauser U, Edelman GM. Neural cell adhesion molecule is on embryonic muscle cells and mediates adhesion to nerve cells in vitro. Nature. 1982;295:693–695. doi: 10.1038/295693a0. [DOI] [PubMed] [Google Scholar]
- Grumet M, Flaccus A, Margolis RU. Functional characterization of chondroitin sulfate proteoglycans of brain: interactions with neurons and neural cell adhesion molecules. J Cell Biol. 1993;120:815–824. doi: 10.1083/jcb.120.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Meyer-Puttlitz B, Margolis RK, Margolis RU. Complex-type asparagine-linked oligosaccharides on phosphacan and protein-tyrosine phosphatase-zeta/beta mediate their binding to neural cell adhesion molecules and tenascin. J Biol Chem. 1995;270:24650–24653. doi: 10.1074/jbc.270.42.24650. [DOI] [PubMed] [Google Scholar]
- Storms SD, Kim AC, Tran BH, Cole GJ, Murray BA. NCAM-mediated adhesion of transfected cells to agrin. Cell Adhes Commun. 1996;3:497–509. doi: 10.3109/15419069609081026. [DOI] [PubMed] [Google Scholar]
- Storms SD, Anvekar VM, Adams LD, Murray BA. Heterophilic NCAM-mediated cell adhesion to proteoglycans from chick embryonic brain membranes. Exp Cell Res. 1996;223:385–394. doi: 10.1006/excr.1996.0093. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Grumet M, Edelman GM. Neural cell adhesion molecule mediates initial interactions between spinal cord neurons and muscle cells in culture. J Cell Biol. 1983;97:145–152. doi: 10.1083/jcb.97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM. Cell adhesion molecules in neural histogenesis. Annu Rev Physiol. 1986;48:417–430. doi: 10.1146/annurev.ph.48.030186.002221. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Jones FS. Gene regulation of cell adhesion: a key step in neural morphogenesis. Brain Res Brain Res Rev. 1998;26:337–352. doi: 10.1016/s0165-0173(97)00034-9. [DOI] [PubMed] [Google Scholar]
- Moretta A, Ciccone E, Tambussi G, Bottino C, Viale O, Pende D, Santoni A, Mingari MC. Surface molecules involved in CD3-negative NK cell function. A novel molecule which regulates the activation of a subset of human NK cells. Int J Cancer Suppl. 1989;4:48–52. doi: 10.1002/ijc.2910440713. [DOI] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Pende D, Tripodi G, Tambussi G, Viale O, Orengo A, Barbaresi M, Merli A, Ciccone E, Moretta L. Identification of four subsets of human CD3−CD16+ natural killer (NK) cells by the expression of clonally distributed functional surface molecules: correlation between subset assignment of NK clones and ability to mediate specific alloantigen recognition. J Exp Med. 1990;172:1589–1598. doi: 10.1084/jem.172.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeromski J, Szczepanski M, Mozer L. [Prevalence of CD56 /NCAM molecule in nervous system immune system and endocrine glands—accidental coincidence?]. Endokrynol Pol. 2005;56:78–82. [PubMed] [Google Scholar]
- Etzell JE, Keet C, McDonald W, Banerjee A. Medulloblastoma simulating acute myeloid leukemia: case report with a review of “myeloid antigen” expression in nonhematopoietic tissues and tumors. J Pediatr Hematol Oncol. 2006;28:703–710. doi: 10.1097/01.mph.0000243647.66734.0f. [DOI] [PubMed] [Google Scholar]
- Liang X, Graham DK. Natural killer cell neoplasms. Cancer. 2008;112:1425–1436. doi: 10.1002/cncr.23316. [DOI] [PubMed] [Google Scholar]
- Liang X, Greffe B, Garrington T, Graham DK. Precursor natural killer cell leukemia. Pediatr Blood Cancer. 2008;50:876–878. doi: 10.1002/pbc.21189. [DOI] [PubMed] [Google Scholar]
- Wick MR. Immunohistology of neuroendocrine and neuroectodermal tumors. Semin Diagn Pathol. 2000;17:194–203. [PubMed] [Google Scholar]
- Lechner O, Dietrich H, Oliveira dos Santos A, Wiegers GJ, Schwarz S, Harbutz M, Herold M, Wick G. Altered circadian rhythms of the stress hormone and melatonin response in lupus-prone MRL/MP-fas(Ipr) mice. J Autoimmun. 2000;14:325–333. doi: 10.1006/jaut.2000.0375. [DOI] [PubMed] [Google Scholar]
- Wick MR. Neuroendocrine neoplasia. Current concepts. Am J Clin Pathol. 2000;113:331–335. doi: 10.1309/ETJ3-QBUK-13QD-J8FP. [DOI] [PubMed] [Google Scholar]
- Wick MR, Ritter JH. Neuroendocrine neoplasia of the lung. Ann Thorac Surg. 2000;69:307–308. [PubMed] [Google Scholar]
- Strekalova H, Buhmann C, Kleene R, Eggers C, Saffell J, Hemperly J, Weiller C, Muller-Thomsen T, Schachner M. Elevated levels of neural recognition molecule L1 in the cerebrospinal fluid of patients with Alzheimer disease and other dementia syndromes. Neurobiol Aging. 2006;27:1–9. doi: 10.1016/j.neurobiolaging.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Kaiser U, Oldenburg M, Jaques G, Auerbach B, Havemann K. Soluble CD56 (NCAM): a new differential-diagnostic and prognostic marker in multiple myeloma. Ann Hematol. 1996;73:121–126. doi: 10.1007/s002770050212. [DOI] [PubMed] [Google Scholar]
- Kaiser U, Auerbach B, Oldenburg M. The neural cell adhesion molecule NCAM in multiple myeloma. Leuk Lymphoma. 1996;20:389–395. doi: 10.3109/10428199609052420. [DOI] [PubMed] [Google Scholar]
- Ely SA, Knowles DM. Expression of CD56/neural cell adhesion molecule correlates with the presence of lytic bone lesions in multiple myeloma and distinguishes myeloma from monoclonal gammopathy of undetermined significance and lymphomas with plasmacytoid differentiation. Am J Pathol. 2002;160:1293–1299. doi: 10.1016/S0002-9440(10)62556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Samiee S, Yi QL. Prognostic relevance of CD56 expression in multiple myeloma: a study including 107 cases treated with high-dose melphalan-based chemotherapy and autologous stem cell transplant. Leuk Lymphoma. 2006;47:43–47. doi: 10.1080/10428190500272549. [DOI] [PubMed] [Google Scholar]
- Kraj M, Sokolowska U, Kopec-Szlezak J, Poglod R, Kruk B, Wozniak J, Szpila T. Clinicopathological correlates of plasma cell CD56 (NCAM) expression in multiple myeloma. Leuk Lymphoma. 2008;49:298–305. doi: 10.1080/10428190701760532. [DOI] [PubMed] [Google Scholar]
- Raspadori D, Damiani D, Michieli M, Stocchi R, Gentili S, Gozzetti A, Masolini P, Michelutti A, Geromin A, Fanin R, Lauria F. CD56 and PGP expression in acute myeloid leukemia: impact on clinical outcome. Haematologica. 2002;87:1135–1140. [PubMed] [Google Scholar]
- Raspadori D, Damiani D, Lenoci M, Rondelli D, Testoni N, Nardi G, Sestigiani C, Mariotti C, Birtolo S, Tozzi M, Lauria F. CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia. 2001;15:1161–1164. doi: 10.1038/sj.leu.2402174. [DOI] [PubMed] [Google Scholar]
- Ravandi F, Cortes J, Estrov Z, Thomas D, Giles FJ, Huh YO, Pierce S, O’Brien S, Faderl S, Kantarjian HM. CD56 expression predicts occurrence of CNS disease in acute lymphoblastic leukemia. Leuk Res. 2002;26:643–649. doi: 10.1016/s0145-2126(01)00188-6. [DOI] [PubMed] [Google Scholar]
- Abbott JJ, Amirkhan RH, Hoang MP. Malignant melanoma with a rhabdoid phenotype: histologic, immunohistochemical, and ultrastructural study of a case and review of the literature. Arch Pathol Lab Med. 2004;128:686–688. doi: 10.5858/2004-128-686-MMWARP. [DOI] [PubMed] [Google Scholar]
- Johnson JP. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999;18:345–357. doi: 10.1023/a:1006304806799. [DOI] [PubMed] [Google Scholar]
- Pujol JL, Simony J, Demoly P, Charpentier R, Laurent JC, Daures JP, Lehmann M, Guyot V, Godard P, Michel FB. Neural cell adhesion molecule and prognosis of surgically resected lung cancer. Am Rev Respir Dis. 1993;148:1071–1075. doi: 10.1164/ajrccm/148.4_Pt_1.1071. [DOI] [PubMed] [Google Scholar]
- Cho EY, Choi Y, Chae SW, Sohn JH, Ahn GH. Immunohistochemical study of the expression of adhesion molecules in ovarian serous neoplasms. Pathol Int. 2006;56:62–70. doi: 10.1111/j.1440-1827.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- Choi YL, Xuan YH, Shin YK, Chae SW, Kook MC, Sung RH, Youn SJ, Choi JW, Kim SH. An immunohistochemical study of the expression of adhesion molecules in gallbladder lesions. J Histochem Cytochem. 2004;52:591–601. doi: 10.1177/002215540405200504. [DOI] [PubMed] [Google Scholar]
- Evans AJ, Humphrey PA, Belani J, van der Kwast TH, Srigley JR. Large cell neuroendocrine carcinoma of prostate: a clinicopathologic summary of 7 cases of a rare manifestation of advanced prostate cancer. Am J Surg Pathol. 2006;30:684–693. doi: 10.1097/00000478-200606000-00003. [DOI] [PubMed] [Google Scholar]
- Zoltowska A, Stepinski J, Lewko B, Serkies K, Zamorska B, Roszkiewicz A, Izycka-Swieszewska E, Kruszewski WJ. Neural cell adhesion molecule in breast, colon and lung carcinomas. Arch Immunol Ther Exp (Warsz) 2001;49:171–174. [PubMed] [Google Scholar]
- Daniel L, Bouvier C, Chetaille B, Gouvernet J, Luccioni A, Rossi D, Lechevallier E, Muracciole X, Coulange C, Figarella-Branger D. Neural cell adhesion molecule expression in renal cell carcinomas: relation to metastatic behavior. Hum Pathol. 2003;34:528–532. doi: 10.1016/s0046-8177(03)00178-3. [DOI] [PubMed] [Google Scholar]
- Brinkman BM. Splice variants as cancer biomarkers. Clin Biochem. 2004;37:584–594. doi: 10.1016/j.clinbiochem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Skotheim RI, Nees M. Alternative splicing in cancer: noise, functional, or systematic? Int J Biochem Cell Biol. 2007;39:1432–1449. doi: 10.1016/j.biocel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Gattenloehner S, Chuvpilo S, Langebrake C, Reinhardt D, Muller-Hermelink HK, Serfling E, Vincent A, Marx A. Novel RUNX1 isoforms determine the fate of acute myeloid leukemia cells by controlling CD56 expression. Blood. 2007;110:2027–2033. doi: 10.1182/blood-2007-02-074203. [DOI] [PubMed] [Google Scholar]
- Gattenlöhner S, Waller C, Ertl G, Bultmann BD, Muller-Hermelink HK, Marx A. NCAM(CD56) and RUNX1(AML1) are up-regulated in human ischemic cardiomyopathy and a rat model of chronic cardiac ischemia. Am J Pathol. 2003;163:1081–1090. doi: 10.1016/S0002-9440(10)63467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Quintanilla-Martinez L, Raffeld M, Richter M, Krugmann J, Burek C, Hartmann E, Rudiger T, Jaffe ES, Muller-Hermelink HK, Ott G, Fend F, Rosenwald A. IgVH mutational status and clonality analysis of Richter’s transformation: diffuse large B-cell lymphoma and Hodgkin lymphoma in association with B-cell chronic lymphocytic leukemia (B-CLL) represent 2 different pathways of disease evolution. Am J Surg Pathol. 2007;31:1605–1614. doi: 10.1097/PAS.0b013e31804bdaf8. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Jain S, Stuhmer T, Andrulis M, Ungethum U, Kuban RJ, Lorentz H, Bommert K, Topp M, Kramer D, Muller-Hermelink HK, Einsele H, Greiner A, Bargou RC. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90alpha and beta in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood. 2007;109:720–728. doi: 10.1182/blood-2006-05-024372. [DOI] [PubMed] [Google Scholar]
- Kobune M, Chiba H, Kato J, Kato K, Nakamura K, Kawano Y, Takada K, Takimoto R, Takayama T, Hamada H, Niitsu Y. Wnt3/RhoA/ROCK signaling pathway is involved in adhesion-mediated drug resistance of multiple myeloma in an autocrine mechanism. Mol Cancer Ther. 2007;6:1774–1784. doi: 10.1158/1535-7163.MCT-06-0684. [DOI] [PubMed] [Google Scholar]
- Nimmanapalli R, Gerbino E, Dalton WS, Gandhi V, Alsina M. HSP70 inhibition reverses cell adhesion mediated and acquired drug resistance in multiple myeloma. Br J Haematol. 2008;142:551–561. doi: 10.1111/j.1365-2141.2008.07217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendtlew Danielsen JM, Knudsen LM, Dahl IM, Lodahl M, Rasmussen T. Dysregulation of CD47 and the ligands thrombospondin 1 and 2 in multiple myeloma. Br J Haematol. 2007;138:756–760. doi: 10.1111/j.1365-2141.2007.06729.x. [DOI] [PubMed] [Google Scholar]
- Mock BA, Hartley J, Le Tissier P, Wax JS, Potter M. The plasmacytoma resistance gene, Pctr2, delays the onset of tumorigenesis and resides in the telomeric region of chromosome 4. Blood. 1997;90:4092–4098. [PubMed] [Google Scholar]
- Adams H, Schmid P, Dirnhofer S, Tzankov A. Cytokeratin expression in hematological neoplasms: a tissue microarray study on 866 lymphoma and leukemia cases. Pathol Res Pract. 2008;204:569–573. doi: 10.1016/j.prp.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Xu D, Zheng C, Bergenbrant S, Holm G, Bjorkholm M, Yi Q, Gruber A. Telomerase activity in plasma cell dyscrasias. Br J Cancer. 2001;84:621–625. doi: 10.1054/bjoc.2000.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatti P, Basile V, Merico D, Fantoni LI, Tagliafico E, Imbriano C. A balance between NF-Y and p53 governs the pro- and anti-apoptotic transcriptional response. Nucleic Acids Res. 2008;36:1415–1428. doi: 10.1093/nar/gkm1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariazi EA, Gould MN. Identifying differential gene expression in monoterpene-treated mammary carcinomas using subtractive display. J Biol Chem. 1996;271:29286–29294. doi: 10.1074/jbc.271.46.29286. [DOI] [PubMed] [Google Scholar]
- Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Muller-Tidow C. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- Perez DS, Hoage TR, Pritchett JR, Ducharme-Smith AL, Halling ML, Ganapathiraju SC, Streng PS, Smith DI. Long, abundantly expressed non-coding transcripts are altered in cancer. Hum Mol Genet. 2008;17:642–655. doi: 10.1093/hmg/ddm336. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smouldering multiple myeloma: emphasis on risk factors for progression. Br J Haematol. 2007;139:730–743. doi: 10.1111/j.1365-2141.2007.06873.x. [DOI] [PubMed] [Google Scholar]
- Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, Dalva K, Fuhler G, Gratama J, Hose D, Kovarova L, Lioznov M, Mateo G, Morilla R, Mylin AK, Omede P, Pellat-Deceunynck C, Perez Andres M, Petrucci M, Ruggeri M, Rymkiewicz G, Schmitz A, Schreder M, Seynaeve C, Spacek M, de Tute RM, Van Valckenborgh E, Weston-Bell N, Owen RG, San Miguel JF, Sonneveld P, Johnsen HE. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93:431–438. doi: 10.3324/haematol.11080. [DOI] [PubMed] [Google Scholar]
- Krämer EM, Klein C, Koch T, Boytinck M, Trotter J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J Biol Chem. 1999;274:29042–29049. doi: 10.1074/jbc.274.41.29042. [DOI] [PubMed] [Google Scholar]
- Pollerberg GE, Schachner M, Davoust J. Differentiation state-dependent surface mobilities of two forms of the neural cell adhesion molecule. Nature. 1986;324:462–465. doi: 10.1038/324462a0. [DOI] [PubMed] [Google Scholar]
- Pollerberg GE, Burridge K, Krebs KE, Goodman SR, Schachner M. The 180-kD component of the neural cell adhesion molecule N-CAM is involved in cell-cell contacts and cytoskeleton-membrane interactions. Cell Tissue Res. 1987;250:227–236. doi: 10.1007/BF00214676. [DOI] [PubMed] [Google Scholar]
- Leshchyns’ka I, Sytnyk V, Morrow JS, Schachner M. Neural cell adhesion molecule (NCAM) association with PKCbeta2 via betaI spectrin is implicated in NCAM-mediated neurite outgrowth. J Cell Biol. 2003;161:625–639. doi: 10.1083/jcb.200303020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner B, Kannicht C, Reutter W, Horstkorte R. The neural cell adhesion molecule is associated with major components of the cytoskeleton. Biochem Biophys Res Commun. 2003;310:967–971. doi: 10.1016/j.bbrc.2003.09.105. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mora OG, Lahair MM, Evans MJ, Kovacs CJ, Allison RR, Sibata CH, White KS, McCubrey JA, Franklin RA. Inhibition of the CaM-kinases augments cell death in response to oxygen radicals and oxygen radical inducing cancer therapies in MCF-7 human breast cancer cells. Cancer Biol Ther. 2006;5:1022–1030. doi: 10.4161/cbt.5.8.2910. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mora OG, LaHair MM, McCubrey JA, Franklin RA. Calcium/calmodulin-dependent kinase I and calcium/calmodulin-dependent kinase kinase participate in the control of cell cycle progression in MCF-7 human breast cancer cells. Cancer Res. 2005;65:5408–5416. doi: 10.1158/0008-5472.CAN-05-0271. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mora O, LaHair MM, Howe CJ, McCubrey JA, Franklin RA. Calcium/calmodulin-dependent protein kinases as potential targets in cancer therapy. Expert Opin Ther Targets. 2005;9:791–808. doi: 10.1517/14728222.9.4.791. [DOI] [PubMed] [Google Scholar]
- Dunphy CH, Nies MK, Gabriel DA. Correlation of plasma cell percentages by CD138 immunohistochemistry, cyclin D1 status, and CD56 expression with clinical parameters and overall survival in plasma cell myeloma. Appl Immunohistochem Mol Morphol. 2007;15:248–254. doi: 10.1097/01.pai.0000213136.93912.84. [DOI] [PubMed] [Google Scholar]
- Pérez-Andrés M, Almeida J, Martin-Ayuso M, Moro MJ, Martin-Nunez G, Galende J, Borrego D, Rodriguez MJ, Ortega F, Hernandez J, Moreno I, Dominguez M, Mateo G, San Miguel JF, Orfao A. Clonal plasma cells from monoclonal gammopathy of undetermined significance, multiple myeloma and plasma cell leukemia show different expression profiles of molecules involved in the interaction with the immunological bone marrow microenvironment. Leukemia. 2005;19:449–455. doi: 10.1038/sj.leu.2403647. [DOI] [PubMed] [Google Scholar]
- Kawase T, Hamazaki M, Ogura M, Kawase Y, Murayama T, Mori Y, Nagai H, Tateno M, Oyama T, Kamiya Y, Taji H, Kagami Y, Naoe T, Takahashi T, Morishima Y, Nakamura S. CD56/NCAM-positive Langerhans cell sarcoma: a clinicopathologic study of 4 cases. Int J Hematol. 2005;81:323–329. doi: 10.1532/IJH97.04142. [DOI] [PubMed] [Google Scholar]
- Barthels D, Vopper G, Boned A, Cremer H, Wille W. High degree of NCAM diversity generated by alternative RNA splicing in brain and muscle. Eur J Neurosci. 1992;4:327–337. doi: 10.1111/j.1460-9568.1992.tb00880.x. [DOI] [PubMed] [Google Scholar]
- Dickson G, Gower HJ, Barton CH, Prentice HM, Elsom VL, Moore SE, Cox RD, Quinn C, Putt W, Walsh FS. Human muscle neural cell adhesion molecule (N-CAM): identification of a muscle-specific sequence in the extracellular domain. Cell. 1987;50:1119–1130. doi: 10.1016/0092-8674(87)90178-4. [DOI] [PubMed] [Google Scholar]
- Reyes AA, Small SJ, Akeson R. At least 27 alternatively spliced forms of the neural cell adhesion molecule mRNA are expressed during rat heart development. Mol Cell Biol. 1991;11:1654–1661. doi: 10.1128/mcb.11.3.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni MJ, Barthels D, Vopper G, Boned A, Goridis C, Wille W. Differential exon usage involving an unusual splicing mechanism generates at least eight types of NCAM cDNA in mouse brain. EMBO J. 1989;8:385–392. doi: 10.1002/j.1460-2075.1989.tb03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]