Abstract
Adhesion of ovarian cancer cells to the peritoneal mesothelium is a key step in the malignant progression of the disease. In an in vitro study, we showed that the adherence of ovarian cancer cells (of the OVCAR-3, SKOV-3, and A2780 cell lines) to senescent human omentum-derived peritoneal mesothelial cells (HOMCs) was greater than to early passage cells. The process was mediated primarily by the increased interaction of the α5β1 integrin on cancer cells with HOMC-associated fibronectin (FN). In comparison with early passage HOMCs, senescent cells exhibited increased FN mRNA expression levels and produced significantly more FN. To assess the effect of senescence-associated oxidative stress on FN release, HOMCs were rendered senescent by exposure to an oxidant, tert-butyl hydroperoxide. Treatment with tert-butyl hydroperoxide resulted in a significant increase in HOMC FN mRNA and protein expression levels. The effect of oxidative stress on FN synthesis was found to be mediated by transforming growth factor-β1, whose signaling pathway was controlled at upstream and downstream levels by p38 MAPK. The activity of p38 MAPK increased markedly in senescent HOMCs. Treatment of HOMCs with antioxidants significantly attenuated senescence-associated increases in p38 MAPK activity, production of both transforming growth factor-β1 and FN, and ovarian cancer cell adhesion. These data indicate that oxidative stress that accompanies senescence may increase FN production by HOMCs and thus facilitate binding and dissemination of ovarian cancer cells.
Ovarian cancer is still associated with high mortality. The poor outcome is related to a large extent to metastatic spreading of cancer cells within the peritoneal cavity.1 Attachment of malignant cells to the peritoneal mesothelium is thought to be a critical step in peritoneal dissemination of the disease. Available data indicate that the process is mediated by interactions between extracellular matrix components produced by mesothelial cells and the corresponding adhesion molecules on ovarian cancer cells. A key role is thought to be played by CD44 and β1 integrin receptors and their ligands—hyaluronan, fibronectin (FN), and vitronectin.2,3,4,5,6
FN is a ubiquitous constituent of extracellular matrix. Secreted by cells as a soluble dimer, it is then processed and assembled into insoluble fibrils at the cell surface.7 FN is involved in many cellular functions including the maintenance of cell shape, tissue repair, cell differentiation, adhesion, and migration. These processes are primarily mediated by binding of the Arg-Gly-Asp (RGD) sequence of the FN molecule to integrin receptors (especially to α5β1 and αVβ3).8 It has been demonstrated that FN released by peritoneal mesothelial cells stimulates ovarian cancer cell motility in vitro.9 Moreover, competitive inhibition of FN by RGD-containing peptides has been reported to decrease peritoneal spreading of ovarian cancer cells in mice.10 More recently it has been demonstrated that the attachment of ovarian cancer cells to the peritoneum results in up-regulation of their matrix metalloproteinase-2, which cleaves mesothelial cell-derived FN into small fragments that further augment cancer cell binding.6
Interestingly, increased production of FN appears to be one of the most typical features of senescent cells.11,12 Increased levels of FN have also been detected in plasma and cells from aged individuals.13,14 Although a link between cancer progression and the age-dependent FN production and processing has been postulated to occur,14 the exact nature of such a relationship remains unclear.
Cellular senescence occurs in response to irreparable DNA damage with potentially oncogenic potential and such DNA injuries are caused primarily by oxidative stress.15,16 By inhibiting cell proliferation, cellular senescence may thus act as a powerful tumor-suppressing mechanism.17 It is believed to protect effectively young organisms against neoplasia, however, it may produce some unfavorable effects later in life. Paradoxically, they may include accelerated cancer progression. It appears that the accumulation of senescent cells with age may alter the tissue architecture to the extent that it provides a permissive environment for uncontrolled growth of adjacent premalignant cells.18 Indeed, it has been demonstrated that proliferation of premalignant breast epithelial cells in vitro and tumor growth in vivo increased in the presence of senescent rather than early-passage fibroblasts.19,20 Moreover, fibroblasts adjacent to the malignant tissue in ovarian cancer specimens, in contrast to those neighboring the normal epithelium, have been found to be senescent.21 It has also been demonstrated that senescent fibroblasts promoted tumor-associated angiogenesis,22 which could facilitate malignant progression.
We have recently analyzed mechanisms of senescence in human peritoneal mesothelial cells in vitro23,24 and detected senescent mesothelial cells in freshly explanted specimens of omentum.25 Because the omentum is the commonest location of ovarian cancer metastases,26 in the present study we have set out to examine whether and how senescence of human omentum-derived mesothelial cells (HOMCs) modulates the adhesion of ovarian cancer.
Materials and Methods
Materials
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich Corp. (St. Louis, MO). All tissue culture plastics were from Nunc (Roskilde, Denmark). Monoclonal antibodies against α5β1 integrin (clones HA5 and JBS5) and against αVβ3 integrin (clone LM609), as well as isotype control antibodies and fluorescein-conjugated anti-mouse IgG were obtained from Chemicon/Millipore (Bedford, MA). Monoclonal mouse anti-β1 integrin (clone P5D2), affinity-purified polyclonal anti-transforming growth factor (TGF)-β (AF-101-NA), appropriate control antibodies, and horseradish peroxidase-labeled polyclonal goat anti-rabbit IgG were purchased from R&D Systems (Wiesbaden, Germany). Polyclonal rabbit anti-FN antibody was obtained from DAKO (Hamburg, Germany). GRGDSP, a FN binding blocking peptide and GRADSP, an inactive control peptide, as well as SB202190 were obtained from Calbiochem/Merck (Warsaw, Poland).
Cell Culture
HOMCs were isolated from specimens of omentum obtained from consenting patients undergoing elective abdominal surgery. Cells were isolated, propagated, and characterized as described in detail elsewhere.27 The donors had no evidence of peritoneal inflammation and/or malignancy. The study was approved by the institutional ethics committee. Cells were cultured in medium M199 supplemented with l-glutamine (2 mmol/L), penicillin (100 U/ml), streptomycin (100 μg/ml), hydrocortisone (0.4 μg/ml), and 10% (v/v) fetal bovine serum.
The ovarian cancer cell line OVCAR-3 was purchased from the American Type Culture Collection (Rockville, MD) and maintained in RPMI 1640 medium supplemented with l-glutamine (2 mmol/L), HEPES (10 mmol/L), sodium pyruvate (1 mmol/L), glucose (4500 mg/L), and 10% fetal bovine serum. The ovarian cancer cell lines SKOV-3 and A2780 were obtained from the European Collection of Cell Cultures (Porton Down, UK) and propagated in McCoy’s 5a and RPMI 1640 media, respectively, both supplemented with l-glutamine (2 mmol/L), penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum.
Induction of HOMC Senescence
HOMCs were grown to senescence by serial passages performed at 4- to 5-day intervals with a fixed seeding density of 3 × 104 cells/cm2. Cultures were considered senescent when cells failed to increase in number during 4 weeks, showed enlarged morphology, and when >70% of cells stained positively for senescence-associated β-galactosidase (SA-β-Gal).28 SA-β-Gal was detected essentially as described by Dimri and colleagues.29 To prepare co-cultures of early-passage and senescent cells, an aliquot of early-passage cells was frozen in liquid nitrogen and stored until the remaining cells became senescent.25 Then, the early-passage cells were defrosted and mixed with senescent cells to produce co-cultures containing required fraction of senescent cells. In selected experiments HOMCs were cultured to senescence in the presence of reactive oxygen species (ROS) scavenger α-phenyl-N-tert-butyl-nitrone (PBN) at a dose of 800 μmol/L.30
In some tests HOMC senescence was induced by oxidative stress using tert-butyl hydroperoxide (t-BHP).31 Briefly, early-passage cells at 70% confluence were treated with 30 μmol/L t-BHP for 1 hour daily for 7 consecutive days. Between the exposures cells were maintained in standard medium supplemented with or without PBN (800 μmol/L). After 7 days of such treatment cells were allowed to recover in standard growth medium for 5 days, during which they developed the senescence phenotype, as evidenced by hypertrophic morphology and extensive positive staining for SA-β-Gal. When required, the incubation was performed in the presence of specific inhibitors or antibodies, as detailed in legends to figures.
Cell Adhesion Assay
Adhesion of ovarian cancer cells to HOMCs was determined as described by Alkhamesi and colleagues32 with minor modifications. Briefly, HOMCs were plated in flat-bottom 96-well plates (8 × 104 cells/well) and left to settle overnight. Ovarian cancer cells were detached by trypsinization, washed with phosphate-buffered saline (PBS), and probed with 5 μmol/L calcein-AM (Molecular Probes, Invitrogen, Eugene, OR) for 30 minutes at 37°C. Calcein-labeled cells were washed with M199 medium with 0.1% fetal bovine serum to remove the free dye and added (3 × 104 cells/well) on top of mesothelial cells. After 45 minutes of incubation at 37°C, total fluorescence in each well was recorded in a spectrofluorimeter (Perkin-Elmer, Turku, Finland) using 485-nm and 535-nm wavelengths for excitation and emission, respectively. Then, the nonadherent cells were removed by gentle washing and the measurement of fluorescence was repeated. To calculate the percentage of bound cells, the values recorded were compared with those representing total fluorescence.
In the inhibition studies, HOMCs and/or ovarian cancer cells were treated with appropriate antibodies or inhibitors for 30 minutes before and during the adhesion assay. To confirm the role of FN in ovarian cancer cell adhesion, a 96-well plate was coated overnight with increasing doses of human plasma FN in PBS. After the blockade of nonspecific binding with 1% bovine serum albumin for 2 hours, the adhesion assay was performed as described above.
Measurement of Cell-Associated FN
Cell-bound FN was measured with an immunoassay. Briefly, HOMCs in 96-well plates were incubated with 10% Roti-Block (Carl Roth, Karlsruhe, Germany) in PBS for 2 hours to block unspecific binding sites, then washed three times with PBS and incubated with anti-FN antibody (2.5 μg/ml) overnight. After extensive washing, bound antibodies were detected with secondary horseradish peroxidase-labeled anti-IgG antibody (1:2000 for 2 hours) and subsequent exposure to horseradish peroxidase substrate reagent (BD Pharmingen, Franklin Lakes, NJ). The color reaction was stopped with 2 N H2SO4 and OD was read at 450 nm. The results were normalized per 105 cells.
Flow Cytometry
Expression of α5β1 integrin on ovarian cancer cells was assessed by flow cytometry, as described.33 Briefly, harvested cells were washed twice in cold PBS, divided into 105 cell aliquots, and incubated for 45 minutes on ice with either the anti-human integrin α5β1 monoclonal antibody or the isotype control antibody. After washing with cold PBS, cells were incubated for 45 minutes on ice with the anti-mouse fluorescein-conjugated secondary antibody. Cells were then washed and analyzed with the FACSCanto flow cytometer (Becton Dickinson, Franklin Lakes, NJ). The data obtained were analyzed and graphically presented using PC-Lysis software (Becton Dickinson).
Reverse Transcription (RT) and Polymerase Chain Reaction (PCR)
Total RNA was extracted from HOMCs with the TRIzol reagent (Invitrogen, Carlsbad, CA), purified, and reverse-transcribed into cDNA with random hexamer primers, as described.34 PCR was performed with Platinum TaqDNA polymerase (Invitrogen) using oligonucleotide primers synthesized by TIB MolBiol SyntheseLabor (Berlin, Germany). Because alternative RNA splicing may produce several transcript variants of the FN gene, the assessment of FN mRNA expression was performed with a primer pair detecting the most typical variants 1 to 5. Detailed evaluation of FN transcript variants was performed using primer specific for each variant, as shown in Table 1.35,36,37,38,39
Table 1.
PCR Primer Sequences
| Sequence | Product size (bp) | Reference |
|---|---|---|
| α-Actin | 204 | 35 |
| F: 5′-GGAGCAATGATCTTGATCTT-3′ | ||
| R: 5′-TGCTCACAGGCAAGGTGTAG-3′ | ||
| FN | 439 | 36 |
| F: 5′-AGCCGCCACGTGCCAGGATTAC-3′ | ||
| R: 5′-CTTATGGGGGTGGCCGTTGTGG-3′ | ||
| FN ED-A | 151, 420 | 37 |
| F: 5′-GACTATTGAAGGCTTGCAGCC-3′ | ||
| R: 5′-CTTGTGGGTGTGCCTGAGTG-3′ | ||
| FN ED-B | 185, 458 | 37 |
| F: 5′-GGTCCATGCTGAATCAGAGCTC-3′ | ||
| R: 5′-CAGGTGACACGCATGGTGTCTG-3′ | ||
| FN IIICS | 124, 316, 391, 409, 484 | 37 |
| F: 5′-GGCTACTATTACTGGCCTGG-3′ | ||
| R: 5′-CTGAGAGAGAGCTTCTTGTCC-3′ | ||
| TGF-β1 | 510 | 38 |
| F: 5′-GGCAGTGGTTGAGCCGTGGA-3′ | ||
| R: 5′-TGTTGGACAGCTGCTCCACCT-3′ | ||
| Integrin α5 | 313 | 39 |
| F: 5′-ACCTGCAGCTGGAAGTGTTT-3′ | ||
| R: 5′-TTAGTGTCCCGGAGATGAGG-3′ | ||
| Integrin-β1 | 526 | 39 |
| F: 5′-AGACCTGCCTTGGTGTGTCT-3′ | ||
| R: 5′-CAGTGTTGTGGGATTTGCAC-3′ |
F, forward; R, reverse; bp, base pairs
Measurement of TGF-β1
TGF-β1 concentration in cell culture supernatants was measured with an immunoassay using the DuoSet ELISA development system (R&D Systems). The samples to be measured were activated with 1 N HCI for 15 minutes and subsequently neutralized with 1.2 N NaOH/0.5 mol/L HEPES. The assay was performed as per the manufacturer’s instructions. Its sensitivity was 15 pg/ml.
Measurement of p38 Mitogen-Activated Protein Kinase (MAPK) Activity
Activation by phosphorylation of p38 MAPK was quantified by in-cell Western technique using Fast Activated Cell-Based ELISA (FACE) p38 kit (Active Motif, Rixensart, Belgium) according to the manufacturer’s instructions. The results were expressed as a ratio of phosphorylated to total p38 activity.
Statistics
Statistical analysis was performed using GraphPad Prism 4.00 software (GraphPad Software, San Diego, CA). The data were analyzed with the t-test or repeated measures analysis of variance, as appropriate. Results were expressed as means ± SD. Differences with a P value <0.05 were considered significant. The number of experiments indicated (n) always refers to the number of independent experiments performed with HOMCs from different donors.
Results
Characterization of OVCAR-3 Cell Adhesion to HOMCs
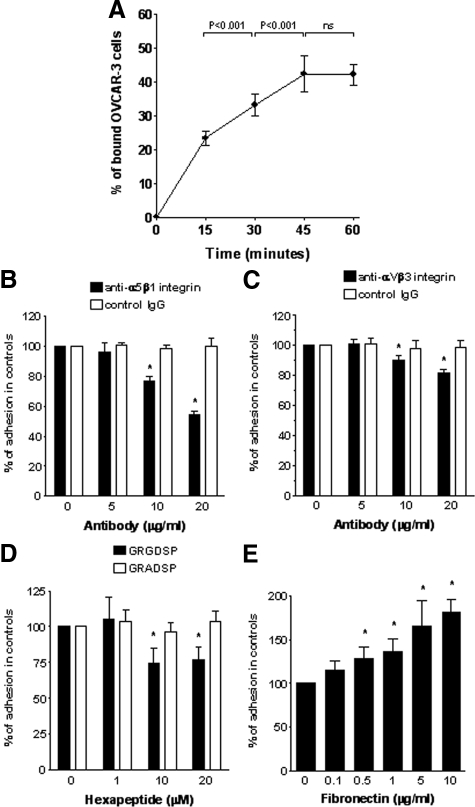
Adhesion of OVCAR-3 cells to freshly isolated HOMCs increased in a time-dependent manner (Figure 1A). The plateau was reached after 45 minutes. Therefore, this time point was chosen for all subsequent experiments. Because the attachment of ovarian cancer cells to the mesothelium is thought to be partly mediated by β1 integrins, and these are expressed on both OVCAR-3 cells2,40 and HOMCs,41 we began by performing the adhesion assay with cells treated separately with blocking antibodies. The pretreatment of OVCAR-3 cells with an anti-β1 integrin antibody, but not with control IgG, led to a significant dose-dependent inhibition of OVCAR-3 cell adhesion to early-passage HOMCs. The maximal inhibition observed was 55.4 ± 6.4% (n = 3, P < 0.05). In contrast, when the same antibody was applied to HOMCs, the inhibition of OVCAR-3 cell adhesion was of considerably lower magnitude and reached only 11.5 ± 10.6% (n = 3, P < 0.05). Simultaneous blockade of β1 integrins on OVCAR-3 cells and HOMCs did not produce any greater inhibition compared with neutralization of OVCAR-3 cell β1 integrins only.
Figure 1.
OVCAR-3 cell adhesion to HOMCs. A: Time effect of calcein-labeled OVCAR-3 cell binding to HOMCs. Results were expressed as a fraction of bound cells relative to the number of cells added, n = 4. B and C: Effect of the specific blockade of OVCAR-3 cell α5β1 integrin or αVβ3 integrin on cell adhesion to HOMCs. OVCAR-3 cells were treated with either integrin-blocking antibodies (black bars) or control antibodies (white bars) for 30 minutes before and during the experiment. Results were expressed as a percentage of binding in the absence of antibodies. Asterisks indicate significant differences compared with cells treated with control antibodies at equal doses, n = 4. D: Effect of competitive inhibition of the FN RGD sequence. HOMCs were pretreated with either GRGDSP or GRADSP peptides for 30 minutes before and during the adhesion assay. Results were expressed as a percentage of binding to untreated cells. Asterisk indicates a significant difference compared with cells treated with a control peptide (GRADSP) at the same dose, n = 5. E: Adhesion of OVCAR-3 cells to culture plates coated with increasing doses of FN. Asterisks indicate significant differences compared with the controls, n = 4.
The above data suggested that OVCAR-3 cell binding was mediated primarily through their β1 integrins. Because β1 integrin is one of the subunits of the main FN receptor, integrin α5β1, to characterize the involvement of the receptor more precisely, the adhesion assay was performed with OVCAR-3 cells treated with a specific integrin α5β1-neutralizing antibody (Figure 1B). These experiments showed that the α5β1 integrin blockade on OVCAR-3 cells reduced their binding to HOMCs by up to 46.4 ± 2.9%. Because FN may also bind to an additional receptor (integrin αVβ3), the adhesion of OVCAR-3 cells was assessed after its neutralization (Figure 1C). This led to a 18.5 ± 2.3% inhibition of OVCAR-3 adherence.
To determine the role of HOMC-derived FN as a ligand for FN receptors on OVCAR-3 cell adhesion, HOMCs were incubated with GRGDSP, a peptide with attachment sequence of FN that acts as a competitive inhibitor of integrin binding.33 The presence of GRGDSP, but not of a control peptide GRADSP, resulted in a significant inhibition of OVCAR-3 cell adhesion to HOMCs (Figure 1D). The most effective inhibition (by 26 ± 11%) was achieved with GRGDSP at 10 μmol/L. The assessment of adhesion of OVCAR-3 cells to FN-coated plates showed a significant dose-dependent effect of FN (Figure 1E). The adhesion-promoting effect of FN became significant at a dose of 0.5 μg/ml and at the highest dose tested (10 μg/ml) the OVCAR-3 adhesion was increased by 81 ± 15%.
Increased Adherence of OVCAR-3 Cells to Senescent HOMCs
The attachment of OVCAR-3 cells to senescent HOMCs was greater by up to 50% than to their young counterparts (Figure 2, A and B). To confirm the effect of senescent HOMCs, the adhesion assay was performed with HOMC cultures prepared by mixing young and senescent cells at different ratios.25 The adhesion of OVCAR-3 cells was found to increase in proportion to the fraction of senescent cells (Figure 2C). The effect became significant when senescent cells made up at least 50% of the population. Blocking of α5β1 and αVβ3 integrins on OVCAR-3 cells showed that the relative magnitude of OVCAR-3 cell adhesion mediated by these integrins was similar during binding to early-passage and senescent HOMCs (Figure 2D). On the other hand, experiments with GRGDSP peptide revealed that RGD sequence of FN mediated significantly more adherence of OVCAR-3 cells to senescent HOMCs than to early-passage cells (Figure 2E).
Figure 2.
Effect of HOMC senescence on OVCAR-3 cell binding. A: Representative microphotographs of early-passage and senescent HOMCs with bound OVCAR-3 cells. Insets show digitally enlarged fragments to better visualize a difference in size and shape of early-passage and senescent HOMCs. B: Quantification of OVCAR-3 cell binding to early-passage and senescent HOMCs. An asterisk represents a significant difference (P < 0.0001) between the groups, n = 6. C: Adhesion of OVCAR-3 cells to cultures of HOMCs prepared to contain early-passage and senescent cells from the same donor at different proportions. Asterisks indicate significant differences compared with a group containing early-passage HOMCs only, n = 4. D: Adhesion of OVACR-3 cells to early-passage and senescent HOMCs mediated by either α5β1 integrin or αVβ3 integrin. Results were expressed as a percentage of total binding, which could be inhibited by pretreatment of OVCAR-3 cells with antibodies (20 μg/ml) blocking either α5β1 integrin or αVβ3 integrin, n = 5. E: Adhesion of OVCAR-3 cells to early-passage and senescent HOMCs mediated by the RGD sequence of FN. HOMCs were pretreated with either GRGDSP or GRADSP (20 μmol/L) for 30 minutes before and during the adhesion assay. An asterisk represents a significant difference (P < 0.01) between early-passage and senescent HOMCs, n = 5. Original magnifications, ×100.
Mesothelial Adherence of Ovarian Cancer Cells with Different Expression of the α5β1 Integrin
To assess how the adhesion-promoting effect of senescent HOMCs affects ovarian cancer cells with various levels of the α5β1 integrin expression, the experiments were performed in parallel with three cancer cell lines. As assessed by flow cytometry, the α5β1 integrin expression was similar in SKOV-3 and A2780 cells, and higher than in OVCAR-3 cells (Figure 3A). Compared with the OVCAR-3 line, SKOV-3 and A2780 cells displayed also higher expression of the α5 integrin subunit mRNA (Figure 3B).
Figure 3.
Adhesion of ovarian cancer cell lines with different expression of α5β1 integrin to HOMCs. A: Expression of the α5β1 integrin on OVCAR-3, SKOV-3, and A2780 cells, as assessed by flow cytometry with either a specific anti-α5β1 integrin antibody (black areas) or an isotype control antibody (transparent areas). MFI, mean fluorescence intensity. B: Expression of mRNAs encoding α5 and β1 integrin subunits and α-actin (as a housekeeping gene) in OVCAR-3, SKOV-3, and A2780 cells, as analyzed by RT-PCR. C: Comparison of adhesion of OVCAR-3, SKOV-3, and A2780 cells to early-passage and senescent HOMCs. Results were expressed as a fraction of bound cells relative to the number of cells added. Asterisks represent significant differences between values recorded with early-passage and senescent HOMCs, separately for each ovarian cancer cell line. Δ represents a calculated relative difference in binding to early-passage and senescent HOMCs; these values were then statistically evaluated, n = 5. D: Comparison of adhesion of OVCAR-3, SKOV-3, and A2780 cells to early-passage and senescent HOMCs that was mediated through a α5β1-integrin-dependent mechanism. Adhesion of cancer cells was assessed in the presence of either a blocking anti-α5β1 integrin antibody or a control antibody. Results were expressed as a percentage of binding in the presence of control antibodies, n = 5.
The number of cancer cells binding to HOMCs under the assay conditions was greater for SKOV-3 and A2780 cells than for OVCAR-3 cells (Figure 3C). All cancer cell lines tested adhered better to the senescent mesothelium than to early-passage HOMCs. However, the proportional increase in binding to senescent HOMCs was greater for OVACR-3 cells than for SKOV-3 and A2780 cells. In all cell lines, the relative contribution of α5β1 integrin-mediated binding was similar in early-passage and senescent HOMCs (Figure 3D).
Increased Production of FN during HOMC Senescence
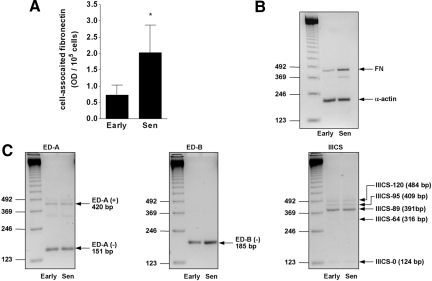
Senescence of HOMCs led to a 2.8-fold increase in cell-associated FN, as measured by cell-bound immunoassay (Figure 4A). The effect was accompanied by an increase in FN mRNA expression, as visualized by RT-PCR with primers amplifying at least five mRNA variants (Figure 4B). The appearance of an additional band (∼370 bp) might have suggested the presence of other variants of the primary transcript. Detailed analysis of three alternatively spliced regions revealed that early-passage HOMCs expressed both ED-A exon-included and ED-A exon-skipped FN mRNA isoforms (Figure 4C). Of five differentially spliced variants of the region IIICS, HOMCs clearly expressed three isoforms, whereas two variants were barely detectable. In contrast, only one (exon-skipped) isoform of the ED-B region was detected, and this isoform was found to be expressed more strongly in senescent cells. No apparent differences in the expression of other FN mRNA variants between early-passage and senescent cells were recorded.
Figure 4.
Expression of FN by senescent HOMCs. A: Production of cell-associated FN by early-passage and senescent HOMCs. An asterisk represents a significant difference (P < 0.01) between the groups, n = 5. B: Global FN mRNA expression by early- and late-passage HOMCs, as assessed by RT-PCR with primers amplifying the commonest FN mRNA variants. Results of a representative analysis of three performed. C: Effect of HOMC senescence on the expression of alternatively spliced FN mRNA variants, as assessed by RT-PCR with primers detecting transcriptional variations in the ED-A, ED-B, and IIICS regions of the FN gene. Results of a representative analysis of six performed.
Senescence-Associated Oxidative Stress as a Cause of Increased FN Production
To characterize the role of senescence-associated oxidative stress in FN production, the process of HOMC senescence was induced by exposure to t-BHP.42 The treatment resulted in a rise in the percentage of cells staining positively for SA-β-Gal from 14 ± 4% to 83 ± 10% (n = 4, P < 0.001). The exposure to t-BHP resulted also in a 2.7-fold increase in cell-associated FN (Figure 5A). In contrast, when the treatment with t-BHP was followed by an exposure to PBN, a ROS scavenger, the amount of cell-associated FN did not differ from that in control cells. Similar changes were reflected at the mRNA level (Figure 5B).
Figure 5.
Regulation of senescence-associated FN production in HOMCs. HOMC senescence was induced by exposure to 30 μmol/L t-BHP for 1 hour daily for 7 consecutive days. After each exposure, cells were allowed to recover in standard medium with supplements as indicated below. A: Effect of t-BHP on the production of cell-associated FN. After each exposure, cells were incubated in standard medium with or without PBN (800 μmol/L). An asterisk represents a significant difference (P < 0.001) compared with control cells, n = 6. B: FN mRNA expression by HOMCs treated with t-BHP in the presence or absence of PBN, as in A. A representative RT-PCR analysis of three performed. C: Effect of TGF-β1 neutralization on t-BHP-induced FN production. During each recovery period cells were incubated in the presence of either blocking anti-TGF-β1 antibody or control chicken IgY (both at 400 ng/ml). Asterisks represent a significant difference (P < 0.01) compared with cells not exposed to t-BHP, n = 5. D: FN mRNA expression by HOMCs treated with t-BHP in the presence or absence of antibodies, as in C. A representative RT-PCR analysis of three performed. E: Effect of p38 MAPK blockade on t-BHP-induced FN production. HOMCs were incubated with 10 μmol/L SB202190 for 1 hour before and during exposure to t-BHP. During each recovery period cells were incubated in plain standard medium. Asterisks represent a significant difference (P < 0.001) compared with cells not exposed to t-BHP, n = 3. F: FN mRNA expression by HOMCs treated with t-BHP in the presence or absence of SB202190, as in E. A representative RT-PCR analysis of three performed.
Because both TGF-β and p38 MAPK have been implicated in controlling oxidative stress-induced FN synthesis,43,44,45 we tested whether their neutralization affected senescence-associated FN release. These experiments showed that a t-BHP-induced increase in cell-associated FN could be reduced to control levels with a TGF-β1-blocking antibody (Figure 5C). The specificity of the effect was confirmed by the lack of inhibition in the presence of nonimmune control antibody. Changes in FN mRNA expression followed a similar pattern (Figure 5D). Moreover, t-BHP-stimulated FN production and expression was decreased to control levels with SB202190, a p38 MAPK inhibitor (Figure 5, E and F).
Signaling Pathways of Senescence-Induced FN Production
The above data suggested that both TGF-β and p38 MAPK are involved in senescence-associated FN release. To clarify the exact signaling pathway, we first examined induction of TGF-β1 and p38 MAPK in senescent HOMCs. Consistently with our previous report,46 we observed an increase in TGF-β1 release by HOMCs rendered senescent either by passages (a 4.5–fold increase; Figure 6A) or by exposure to t-BHP (a 3.2-fold increase; Figure 6C). However, when both experimental protocols were modified by adding PBN, the amount of TGF-β1 released was markedly reduced. In addition, we found an almost similar degree of inhibition with SB202190 (Figure 6E), which indicated that p38 MAPK mediates ROS-induced TGF-β1 production. In all cases, the effects observed at the protein level were accompanied by corresponding changes in TGF-β1 mRNA expression (Figure 6, B, D, and F).
Figure 6.
Regulation of senescence-associated TGF-β1 production in HOMCs. A: TGF-β1 release from HOMCs grown to senescence by serial passages in the presence or absence of PBN (800 μmol/L). At each stage TGF-β1 release was assessed throughout a period of 4 days. Asterisks indicate a significant difference compared with early-passage cells in each group, n = 6. B: TGF-β1 mRNA expression by HOMCs grown senescent, as in A. A representative RT-PCR analysis of three performed. C: Effect of PBN on t-BHP-induced TGF-β1 release. HOMC senescence was induced with t-BHP, as described in the Materials and Methods. After each exposure cells were incubated in standard medium with or without PBN (800 μmol/L). An asterisk represents a significant difference (P < 0.01) compared with control cells, n = 6. D: TGF-β1 mRNA expression by HOMCs treated with t-BHP in the presence or absence of PBN, as in C. A representative RT-PCR analysis of three performed. E: Effect of p38 MAPK blockade on t-BHP-induced TGF-β1 production. HOMCs were incubated with 10 μmol/L SB202190 for 1 hour before and during exposure to t-BHP. During each recovery period cells were incubated in plain standard medium. Asterisks represent a significant difference (P < 0.001) compared with cells not exposed to t-BHP, n = 6. F: TGF-β1 mRNA expression by HOMCs treated with t-BHP in the presence or absence of SB202190, as in E. A representative RT-PCR analysis of three performed.
Role of p38 MAPK in Senescence-Induced FN Production
We then went on to analyze directly p38 MAPK activity and found that the ratio of phosphorylated p38 MAPK to its total form was 23 ± 7-fold greater in senescent HOMCs than in early-passage cells (Figure 7A). An equally spectacular rise in p38 MAPK activity was seen in response to t-BHP (Figure 7B). These effects could be significantly reduced by PBN, which confirmed that senescence-associated oxidative stress led to the activation by p38 MAPK. Interestingly, significant inhibition of t-BHP-induced p38 MAPK activation was also caused by anti-TGF-β1 neutralizing antibody, but not by control IgY. The degree of inhibition by anti-TGF-β1 was of lower magnitude that by PBN. These data suggested that TGF-β activated by p38 MAPK might have subsequently contributed to further p38 MAPK activation. Indeed, when assessed directly in early-passage cells, TGF-β1 produced a clear dose-dependent increase in p38 MAPK activation (Figure 7C). To examine whether p38 MAPK may also act as a downstream mediator of TGF-β1 signaling, HOMCs were treated with TGF-β1 in the presence or absence of SB202190. Inhibition of p38 MAPK by SB202190 resulted in a significant reduction of TGF-β1-induced FN production and mRNA expression (Figure 7, D and E).
Figure 7.
Regulation of senescence-associated TGF-β1 and FN production by p38 MAPK. A: Activity of p38 MAPK in HOMCs rendered senescent by serial passages in the presence or absence of PBN (800 μmol/L). Asterisks indicate a significant difference compared with early-passage cells in each group, n = 4. B: Effect of PBN and TGF-β1 on t-BHP-induced p38 MAPK activity in HOMCs. HOMC senescence was induced with t-BHP, as described in the Materials and Methods. After each exposure cells were incubated in standard medium with either PBN (800 μmol/L), anti-TGF-β1 neutralizing antibody (400 ng/ml), or control chicken IgY (400 ng/ml). Asterisks represent a significant difference (P < 0.01) compared with control cells, n = 4. C: Effect of TGF-β1 on p38 MAPK activation in HOMCs. Cells were treated with TGF-β1 for 24 hours. Asterisks represent a significant difference compared with untreated cells, n = 3. D: Effect of p38 MAPK blockade on TGF-β1-induced production of cell-associated FN. HOMCs were incubated with TGF-β1 (1 ng/ml) for 5 days in the presence or absence of 10 μmol/L SB202190 added for 2 hours daily. Asterisks represent a significant difference (P < 0.001) compared with cells not exposed to TGF-β1, n = 6. E: FN mRNA expression by HOMCs treated with TGF-β1 in the presence or absence of SB202190, as in D. A representative RT-PCR analysis of three performed.
Antioxidants and Ovarian Cancer Cell Adhesion to Senescent HOMCs
Because antioxidants have recently been shown to delay the appearance of senescence features in cultured HOMCs,25 we went on to examine whether this treatment could also affect ovarian cancer cell adhesion. To this end, HOMCs were cultured until senescent in the presence or absence of PBN and then tested for the capacity to bind ovarian cancer cells. These experiments revealed that—for all ovarian cancer cell lines tested—an increase in adherence to senescent HOMCs could be significantly reduced if HOMCs were propagated with PBN (Figure 8A). The degree of reduction recorded was almost to the level seen in early-passage cells. A very similar effect was observed when HOMC senescence was induced by the exposure to t-BHP (Figure 8B). Albeit the magnitude of increase in cancer cell binding was less pronounced compared with when HOMC were rendered senescent by replication, this rise could still be alleviated by the treatment with PBN. Such an effect was evident for all ovarian cancer cell lines examined.
Figure 8.
Effect of antioxidants on ovarian cancer cell binding to the senescent mesothelium. HOMCs were rendered senescent either by replication (A) or by exposure to t-BHP (B), as described in the Materials and Methods. Both procedures were performed in the presence or absence of PBN (800 μmol/L). HOMCs were then assessed for the ability to bind ovarian cancer cells. The results were expressed as a percentage of binding to early-passage HOMCs, separately for each cell line. Asterisks indicate significant differences compared with values recorded in early-passage cells, n = 5.
Discussion
The major finding of the present study is that the adhesion of ovarian cancer cells to senescent HOMCs is greater than to early-passage cells and that the process is primarily related to a senescence-associated and ROS-mediated increase in FN production. Recent studies have strongly implicated FN in ovarian cancer cell adhesion.6 Because the main FN receptor is the β1 integrin-containing heterodimer α5β1, we have examined whether specific blockade of the integrin α5β1 on OVCAR-3 cells affects their binding to the mesothelium. These experiments showed that neutralization of the α5β1 integrin decreased OVCAR-3 cell adhesion almost by half. Neutralization of the additional FN receptor, integrin αVβ3, reduced OVCAR-3 cell binding by a further 20%. The latter effect cannot be entirely attributed to FN, however, because the integrin αVβ3 acts also as a receptor for vitronectin, which contributes significantly to ovarian cancer cell adhesion.6
Because FN is expressed in abundance by mesothelial cells,4 we blocked HOMC-derived FN with GRGDSP and observed a reduction in OVCAR-3 cell binding as a result. Accordingly, the adhesion of OVCAR-3 cells to FN-coated culture plates increased in a FN dose-dependent manner. Although some authors found only a minor increase in OVCAR-3 adhesion to FN-coated plates,2 the others observed that GRGDSP markedly decreased adhesion of OVCAR-3 cells either to FN-coated dishes or to peritoneal mesothelial cells of the LP-9 line.33
Having previously observed increased secretion of FN by senescent HOMCs,46,47 we went on to analyze whether this effect might impact on the adhesion of OVCAR-3 cells. We found that the attachment of OVCAR-3 to senescent HOMCs was greater than to early-passage cells. By preparing co-cultures with a different proportion of senescent cells we were able to demonstrate that this effect was clearly related to the number of senescent cells in a population. Importantly, increased adhesion of ovarian cancer cells to the senescent mesothelium was not restricted to OVCAR-3 cells, but was evident also in other ovarian cancer strains such as SKOV-3 and A2780. Although in comparison with OVCAR-3 cells, these cell lines display increased expression of the α5β1 integrin, the relative increases in their adhesion to senescent HOMCs were surprisingly smaller. One may hypothesize that fewer cells with higher α5β1 expression will suffice to saturate a given amount of FN available for binding. Furthermore, the comparison of inhibitory effects of FN receptor-blocking antibodies showed that the relative contribution of the integrin α5β1 in mediating adhesion to early-passage and senescent HOMCs was similar in all ovarian cancer cell lines examined.
In an elegant study, Kenny and colleagues6 have recently demonstrated the importance of early interactions between ovarian cancer cells and FN at the HOMC surface. Our experiments with GRGDSP suggested that FN availability rather than integrin α5β1 expression was instrumental in augmenting ovarian cancer cell adhesion to senescent HOMCs. Therefore, we focused on cell-bound FN and detected it at significantly higher levels in senescent HOMCs. We also found increased FN mRNA expression in senescent HOMCs. More detailed analysis of alternatively spliced mRNA variants revealed that this increase was predominantly related to the increased expression of the exon-spliced out transcript of the ED-B region. In this respect, increased expression of such a variant was also observed in various strains of late-passage fibroblasts.48,49
ROS have been linked to increased FN synthesis predominantly in the context of diabetes.50,51,52 We have observed that high-glucose-induced FN release by senescent HOMCs could be alleviated with antioxidants.47 In the present study we focused on how oxidative stress that is associated with senescence per se might regulate FN synthesis. To this end we used a model of t-BHP-induced HOMC senescence. Indeed, HOMCs rendered senescent by exposure to t-BHP produced more cell-associated FN. The causal involvement of ROS in FN production was confirmed by demonstrating the inhibitory effect of a ROS scavenger, PBN. Interestingly, the effect of oxidative stress was also blocked by the inhibition of TGF-β1. Given that TGF-β1 is a known inducer of FN in HOMCs,46 we hypothesized that TGF-β1 acted as a downstream mediator of ROS. Such a role for TGF-β1 has been demonstrated in mesangial cells.53 Indeed, we found that both replicative senescence and t-BHP-induced senescence resulted in an ROS-dependent increase in TGF-β1 production in HOMCs. These results are in line with those of Frippiat and colleagues,43 who demonstrated that TGF-β1 acted as a mediator of increased FN production in fibroblasts entering premature senescence as a result of exposure to either hydrogen peroxide or t-BHP. Furthermore, we found that the induction of TGF-β1 in response to t-BHP could be abrogated with SB202190, a p38 MAPK inhibitor. This observation positioned p38 MAPK upstream of TGF-β1. As expected, senescent HOMCs were found to have markedly increased p38 MAPK activity, which could be reduced with PBN. Moreover, inhibition of p38 MAPK led to a decrease in t-BHP-induced FN release. In this respect, it has been demonstrated that the inhibition of p38 MAPK in fibroblasts treated with hydrogen peroxide resulted in reduced FN expression.44,54
Interestingly, it has been reported that TGF-β1 could also phosphorylate p38 MAPK45 and that neutralization of TGF-β1 reduced the enhanced activity of p38 MAPK in cells under oxidative stress.44 We observed similar effects in HOMCs, which indicated that, on the one hand, TGF-β1 could be induced by a p38 MAPK-dependent mechanism, and on the other hand, it could activate p38 MAPK. Furthermore, we showed that p38 MAPK mediated TGF-β1-induced FN synthesis. These data show that p38 MAPK operates at many levels in mediating oxidative stress-induced FN synthesis in senescent HOMCs. They also support the concept of p38 MAPK being a key executory pathway of senescence triggered by different stimuli.54,55
We have previously demonstrated that the development of senescence in cultured HOMCs could be significantly delayed by the timely administration of the strong antioxidant PBN. Having documented that increased production of FN by senescent HOMCs is primarily related to oxidative stress, we hypothesized that the same procedure could also affect ovarian cancer cell binding. Indeed, we observed that increased adhesion of several ovarian cancer cell lines to HOMCs rendered senescent either by replication or by exposure to t-BHP could be significantly reduced by antioxidants.
Exact causes and the rate of HOMC senescence in vivo remains to be determined. Because inflammation may impose significant oxidative stress on cells, it may be viewed as a potential culprit. In this respect it has been demonstrated that HOMCs from older donors are more vulnerable to oxidative stress42 and show increased expression of pro-inflammatory mediators.56 Tumor growth-promoting properties of senescent cells could thus add to a known association between inflammation and cancer.57
In summary, our results indicate that some phenotypic features of senescent peritoneal mesothelial cells may facilitate malignant dissemination of ovarian cancer cells. They support the idea of senescent cells promoting late-life cancer.17 They also demonstrate that targeting cellular senescence may be a complementary approach to prevent age-related pathology.58
Footnotes
Address reprint requests to Prof. Janusz Witowski, Department of Pathophysiology, Poznan University of Medical Sciences, Swiecickiego 6, 60-781 Poznan, Poland. E-mail: jwitow@ump.edu.pl.
Supported by the Polish Ministry of Science and Higher Education (grant N401 094 31/2181).
References
- Amadori D, Sansoni E, Amadori A. Ovarian cancer: natural history and metastatic pattern. Front Biosci. 1997;2:g8–g10. [PubMed] [Google Scholar]
- Ahmed N, Riley C, Rice G, Quinn M. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin Exp Metastasis. 2005;22:391–402. doi: 10.1007/s10585-005-1262-y. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Catterall JB, Jones LM, Turner GA. Human ovarian tumour cells can bind hyaluronic acid via membrane CD44: a possible step in peritoneal metastasis. Clin Exp Metastasis. 1996;14:325–334. doi: 10.1007/BF00123391. [DOI] [PubMed] [Google Scholar]
- Lessan K, Aguiar DJ, Oegema T, Siebenson L, Skubitz AP. CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154:1525–1537. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel T, Cannistra SA. Beta1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol Oncol. 1999;73:362–367. doi: 10.1006/gyno.1999.5388. [DOI] [PubMed] [Google Scholar]
- Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118:1367–1379. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Schwarzbauer JE. The ins and outs of fibronectin matrix assembly. J Cell Sci. 2003;116:3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Rieppi M, Vergani V, Gatto C, Zanetta G, Allavena P, Taraboletti G, Giavazzi R. Mesothelial cells induce the motility of human ovarian carcinoma cells. Int J Cancer. 1999;80:303–307. doi: 10.1002/(sici)1097-0215(19990118)80:2<303::aid-ijc21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Murae M, Yasuda M. RGD-containing peptides inhibit experimental peritoneal seeding of human ovarian cancer cells. Nippon Sanka Fujinka Gakkai Zasshi. 1991;43:1687–1692. [PubMed] [Google Scholar]
- Fodil-Bourahla I, Drubaix I, Robert L, Labat-Robert J. Effect of in vitro aging on the modulation of protein and fibronectin biosynthesis by the elastin-laminin receptor in human skin fibroblasts. Gerontology. 1999;45:23–30. doi: 10.1159/000022051. [DOI] [PubMed] [Google Scholar]
- Grillari J, Hohenwarter O, Grabherr RM, Katinger H. Subtractive hybridization of mRNA from early passage and senescent endothelial cells. Exp Gerontol. 2000;35:187–197. doi: 10.1016/s0531-5565(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Kumazaki T, Kobayashi M, Mitsui Y. Enhanced expression of fibronectin during in vivo cellular aging of human vascular endothelial cells and skin fibroblasts. Exp Cell Res. 1993;205:396–402. doi: 10.1006/excr.1993.1103. [DOI] [PubMed] [Google Scholar]
- Labat-Robert J. Fibronectin in malignancy. Semin Cancer Biol. 2002;12:187–195. doi: 10.1016/S1044-579X(02)00022-6. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Passos JF, von Zglinicki T. Oxygen free radicals in cell senescence: are they signal transducers? Free Radic Res. 2006;40:1277–1283. doi: 10.1080/10715760600917151. [DOI] [PubMed] [Google Scholar]
- Campisi J, d'Adda di, Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Campisi J. Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int J Biochem Cell Biol. 2002;34:1401–1414. doi: 10.1016/s1357-2725(02)00053-5. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- Yang G, Rosen DG, Zhang Z, Bast RC, Jr, Mills GB, Colacino JA, Mercado-Uribe I, Liu J. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci USA. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- Ksiazek K, Piwocka K, Brzezinska A, Sikora E, Zabel M, Breborowicz A, Jörres A, Witowski J. Early loss of proliferative potential of human peritoneal mesothelial cells in culture: the role of p16INK4a-mediated premature senescence. J Appl Physiol. 2006;100:988–995. doi: 10.1152/japplphysiol.01086.2005. [DOI] [PubMed] [Google Scholar]
- Ksiazek K, Passos JF, Olijslagers S, Saretzki G, Martin-Ruiz C, von Zglinicki T. Premature senescence of mesothelial cells is associated with non-telomeric DNA damage. Biochem Biophys Res Commun. 2007;362:707–711. doi: 10.1016/j.bbrc.2007.08.047. [DOI] [PubMed] [Google Scholar]
- Ksiazek K, Mikula-Pietrasik J, Jörres A, Witowski J. Oxidative stress-mediated early senescence contributes to the short replicative life span of human peritoneal mesothelial cells. Free Radic Biol Med. 2008;45:460–467. doi: 10.1016/j.freeradbiomed.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Doig T, Monaghan H. Sampling the omentum in ovarian neoplasia: when one block is enough. Int J Gynecol Cancer. 2006;16:36–40. doi: 10.1111/j.1525-1438.2006.00273.x. [DOI] [PubMed] [Google Scholar]
- Yung S, Li FK, Chan TM. Peritoneal mesothelial cell culture and biology. Perit Dial Int. 2006;26:162–173. [PubMed] [Google Scholar]
- Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T, Pilger R, Sitte N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic Biol Med. 2000;28:64–74. doi: 10.1016/s0891-5849(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Dumont P, Burton M, Chen QM, Gonos ES, Frippiat C, Mazarati JB, Eliaers F, Remacle J, Toussaint O. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radic Biol Med. 2000;28:361–373. doi: 10.1016/s0891-5849(99)00249-x. [DOI] [PubMed] [Google Scholar]
- Alkhamesi NA, Ziprin P, Pfistermuller K, Peck DH, Darzi AW. ICAM-1 mediated peritoneal carcinomatosis, a target for therapeutic intervention. Clin Exp Metastasis. 2005;22:449–459. doi: 10.1007/s10585-005-2893-8. [DOI] [PubMed] [Google Scholar]
- Said N, Najwer I, Motamed K. Secreted protein acidic and rich in cysteine (SPARC) inhibits integrin-mediated adhesion and growth factor-dependent survival signaling in ovarian cancer. Am J Pathol. 2007;170:1054–1063. doi: 10.2353/ajpath.2007.060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörres A, Dinter H, Topley N, Gahl GM, Frei U, Scholz P. Inhibition of tumour necrosis factor production in endotoxin-stimulated human mononuclear leukocytes by the prostacyclin analogue iloprost: cellular mechanisms. Cytokine. 1997;9:119–125. doi: 10.1006/cyto.1996.0145. [DOI] [PubMed] [Google Scholar]
- O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R, III, Le Beau MM, Earp HS, Liu ET. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AO, Steadman R, Morrisey K, Martin J, Eynstone L, Williams JD. Exposure of human renal proximal tubular cells to glucose leads to accumulation of type IV collagen and fibronectin by decreased degradation. Kidney Int. 1997;52:973–984. doi: 10.1038/ki.1997.419. [DOI] [PubMed] [Google Scholar]
- Parkar MH, Bakalios P, Newman HN, Olsen I. Expression and splicing of the fibronectin gene in healthy and diseased periodontal tissue. Eur J Oral Sci. 1997;105:264–270. doi: 10.1111/j.1600-0722.1997.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Phillips AO, Steadman R, Topley N, Williams JD. Elevated D-glucose concentrations modulate TGF-beta 1 synthesis by human cultured renal proximal tubular cells. The permissive role of platelet-derived growth factor. Am J Pathol. 1995;147:362–374. [PMC free article] [PubMed] [Google Scholar]
- Cheuk BL, Cheng SW. Differential expression of integrin alpha5beta1 in human abdominal aortic aneurysm and healthy aortic tissues and its significance in pathogenesis. J Surg Res. 2004;118:176–182. doi: 10.1016/S0022-4804(03)00351-2. [DOI] [PubMed] [Google Scholar]
- Cannistra SA, DeFranzo B, Niloff J, Ottensmeir C. Functional heterogeneity of CD44 molecules in ovarian cancer cell lines. Clin Cancer Res. 1995;1:333–342. [PubMed] [Google Scholar]
- Gardner MJ, Jones LM, Catterall JB, Turner GA. Expression of cell adhesion molecules on ovarian tumour cell lines and mesothelial cells, in relation to ovarian cancer metastasis. Cancer Lett. 1995;91:229–234. doi: 10.1016/0304-3835(95)03743-g. [DOI] [PubMed] [Google Scholar]
- Ksiazek K, Piatek K, Witowski J. Impaired response to oxidative stress in senescent cells may lead to accumulation of DNA damage in mesothelial cells from aged donors. Biochem Biophys Res Commun. 2008;373:335–339. doi: 10.1016/j.bbrc.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276:2531–2537. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- Frippiat C, Dewelle J, Remacle J, Toussaint O. Signal transduction in H2O2-induced senescence-like phenotype in human diploid fibroblasts. Free Radic Biol Med. 2002;33:1334–1346. doi: 10.1016/s0891-5849(02)01044-4. [DOI] [PubMed] [Google Scholar]
- Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, Lee HB. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- Ksiazek K, Korybalska K, Jörres A, Witowski J. Accelerated senescence of human peritoneal mesothelial cells exposed to high glucose: the role of TGF-beta1. Lab Invest. 2007;87:345–356. doi: 10.1038/labinvest.3700519. [DOI] [PubMed] [Google Scholar]
- Ksiazek K, Breborowicz A, Jörres A, Witowski J. Oxidative stress contributes to accelerated development of the senescent phenotype in human peritoneal mesothelial cells exposed to high glucose. Free Radic Biol Med. 2007;42:636–641. doi: 10.1016/j.freeradbiomed.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Kumazaki T, Mitsui Y, Hamada K, Sumida H, Nishiyama M. Detection of alternative splicing of fibronectin mRNA in a single cell. J Cell Sci. 1999;112:1449–1453. doi: 10.1242/jcs.112.10.1449. [DOI] [PubMed] [Google Scholar]
- Magnuson VL, Young M, Schattenberg DG, Mancini MA, Chen DL, Steffensen B, Klebe RJ. The alternative splicing of fibronectin pre-mRNA is altered during aging and in response to growth factors. J Biol Chem. 1991;266:14654–14662. [PubMed] [Google Scholar]
- Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- Ihnat MA, Thorpe JE, Kamat CD, Szabo C, Green DE, Warnke LA, Lacza Z, Cselenyak A, Ross K, Shakir S, Piconi L, Kaltreider RC, Ceriello A. Reactive oxygen species mediate a cellular ‘memory’ of high glucose stress signalling. Diabetologia. 2007;50:1523–1531. doi: 10.1007/s00125-007-0684-2. [DOI] [PubMed] [Google Scholar]
- Lee HB, Yu MR, Song JS, Ha H. Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int. 2004;65:1170–1179. doi: 10.1111/j.1523-1755.2004.00491.x. [DOI] [PubMed] [Google Scholar]
- Iglesias-De La, Cruz MC, Ruiz-Torres P, Alcami J, Diez-Marques L, Ortega-Velazquez R, Chen S, Rodriguez-Puyol M, Ziyadeh FN, Rodriguez-Puyol D. Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells. Kidney Int. 2001;59:87–95. doi: 10.1046/j.1523-1755.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–144. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- Zdanov S, Debacq-Chainiaux F, Remacle J, Toussaint O. Identification of p38 MAPK-dependent genes with changed transcript abundance in H2O2-induced premature senescence of IMR-90 hTERT human fibroblasts. FEBS Lett. 2006;580:6455–6463. doi: 10.1016/j.febslet.2006.10.064. [DOI] [PubMed] [Google Scholar]
- Nevado J, Vallejo S, El Assar M, Peiro C, Sanchez-Ferrer CF, Rodriguez-Manas L. Changes in the human peritoneal mesothelial cells during aging. Kidney Int. 2006;69:313–322. doi: 10.1038/sj.ki.5000082. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Curtis R, Geesaman BJ, DiStefano PS. Ageing and metabolism: drug discovery opportunities. Nat Rev Drug Discov. 2005;4:569–580. doi: 10.1038/nrd1777. [DOI] [PubMed] [Google Scholar]