Abstract
Activation of latent transforming growth factor β (TGF-β) by αvβ6 integrin is critical in the pathogenesis of lung injury and fibrosis. We have previously demonstrated that the stimulation of protease activated receptor 1 promotes αvβ6 integrin-mediated TGF-β activation via RhoA, which is known to modulate cell contraction. However, whether other G protein-coupled receptors can also induce αvβ6 integrin-mediated TGF-β activation is unknown; in addition, the αvβ6 integrin signaling pathway has not yet been fully characterized. In this study, we show that lysophosphatidic acid (LPA) induces αvβ6-mediated TGF-β activation in human epithelial cells via both RhoA and Rho kinase. Furthermore, we demonstrate that LPA-induced αvβ6 integrin-mediated TGF-β activity is mediated via the LPA2 receptor, which signals via Gαq. Finally, we show that the expression levels of both the LPA2 receptor and αvβ6 integrin are up-regulated and are spatially and temporally associated following bleomycin-induced lung injury. Furthermore, both the LPA2 receptor and αvβ6 integrin are up-regulated in the overlying epithelial areas of fibrosis in patients with usual interstitial pneumonia. These studies demonstrate that LPA induces αvβ6 integrin-mediated TGF-β activation in epithelial cells via LPA2, Gαq, RhoA, and Rho kinase, and that this pathway might be clinically relevant to the development of lung injury and fibrosis.
Transforming growth factor (TGF)-β includes a pleiotropic group of cytokines that exist in three mammalian isoforms (TGF-β1, -β2, and -β3) that are all secreted as latent complexes. This latent complex needs to be activated for TGF-β family members to exert their biological effect. The small latent complex contains the latency associated peptide (LAP), which, in TGF-β1 and TGF-β3, contains an arginine-glycine-aspartate (RGD) motif. This RGD motif can bind integrins, facilitating TGF-β activation. The LAP of TGF-β2 does not contain an RGD motif and no role for integrin mediated TGF-β2 activation has been described. TGF-β1 exerts profound effects on matrix deposition and is a central mediator of lung injury and fibrosis. There are several mechanisms by which TGF-β1 may be activated, including extremes of heat, oxidation, proteolytic cleavage, deglycosylation, and activation by thrombospondin-1.1,2,3,4,5,6,7,8 In vivo, activation by integrins appears to play a major role in activating TGF-β1 during development9 and in various disease models.10,11,12,13,14
Integrins are heterodimeric transmembrane proteins made up of α and β subunits. Six, of the 24 currently described integrins are able to bind the RGD motif in the LAP of TGF-β. Four of these integrins (αvβ3, αvβ5, αvβ6, and αvβ8) are thought to be able to activate TGF-β1.13,14,15 The role of integrin-mediated TGF-β activation in vivo has only been confirmed for the αvβ6 and αvβ8 integrins.13,14 Mice in which the aspartic acid in the RGD site of TGF-β1 is replaced by glutamic acid, preventing integrin-mediated TGF-β1 activation, completely phenocopy TGF-β1 null mice, highlighting the importance of TGF-β1 interactions with integrins.9 Furthermore, activation of TGF-β1 by the epithelially restricted αvβ6 integrin is central to the pathogenesis of acute lung injury and pulmonary fibrosis.12,14
Further regulation of TGF-β bioavailability is afforded by interaction of the small latent complex with the latent TGF-β binding proteins (LTBPs). There are four LTBPs (1, 2, 3, and 4) that belong to the LTBP/fibrillin family of extracellular glycoproteins. Of these, three, LTBP-1, -3, and -4, associate with the small latent complex through covalent attachment with the LAP, forming the large latent complex.16 The LTBPs are required to ensure correct post-translational modification of the small latent complex,17 and they target storage of TGF-β in the extracellular matrix by crosslinking the large latent complex to the matrix via the actions of tissue transglutaminase.18,19 The LTBPs are also likely to determine, at least in part, the specificity of TGF-β activation. LTBPs-1 and -3 can bind all isoforms of TGF-β, whereas LTBP-4 can only bind TGF-β1.16,20 There is further evidence of the importance of LTBP modulating TGF-β activation from in vivo studies using mice null for various LTBPs. LTBP-1 null mice have reduced TGF-β activity and are protected from hepatic fibrosis.21 LTBP-3 null mice have phenotypic features consistent with reduced TGF-β activity in the bones.22 Mice with a gene trap disruption of LTBP-4 show reduced epithelial Smad2 phosphorylation and abnormal cardiopulmonary development and develop colonic tumors similar to those seen in Smad3 null mice.23
Overexpression of the αvβ6 integrin is not sufficient to promote fibrosis and the αvβ6 integrin itself must be activated during injury to promote TGF-β1 activation.12,24 αvβ6-dependent TGF-β activation also requires an intact actin cytoskeleton14 and is critically dependent on association of latent complexes with the specific LTBP family member, LTBP-1.25 In cells lacking LTBP-1, αvβ6 cannot activate TGF-β, but this response can be rescued by expression of a short fusion protein composed of the region of LTBP-1 that forms a disulfide bond to TGF-β1 LAP and the region required to cross-link LTBP-1 to the extracellular matrix protein fibronectin.25 These findings are consistent with a model by which the integrin-expressing cell activates latent TGF-β by exerting traction on the tethered latent complex. Such a mechanism received further support from a recent report that myofibroblasts can activate latent TGF-β using the αvβ5 integrin and cell contraction.26 It is thought that cellular injury may induce contractile forces through the cytoskeleton and integrins to the latent TGF-β1 molecule, which is itself tethered by the LTBP-1 to either the cell surface or the extracellular matrix.25,26 We identified that agonists of the seven-transmembrane domain, G protein-coupled receptor (GPCR), PAR1, can promote αvβ6 integrin-mediated TGF-β activation via RhoA, known to modulate cell contraction, and that this pathway is important in acute lung injury.12 However, whether other GPCRs are able to contribute to αvβ6 integrin-mediated, TGF-β activation is not known. Furthermore, the G protein involved in mediating injury-induced αvβ6 integrin-mediated, TGF-β activation has not yet been identified.
Lysophosphatidic acid (LPA), is a bioactive phospholipid known to mediate contraction in a number of cell types.27 It is released from activated platelets at sites of injury,28 contributing to wound repair. LPA is present in bronchoalveolar lavage fluid and increased during inflammation,29 and can mediate pro-inflammatory effects on several cells types within the lung.30,31,32 Furthermore, LPA is increased in patients with pulmonary fibrosis.33 LPA mediates its cellular effects via the LPA class of GPCRs. Currently five subtypes have been identified (LPA1-5), and like PAR1, these receptors couple to the small G proteins Gαi, Gαq, and Gα12/13.34,35 It has recently been shown that LPA can induce fibroblast chemotaxis through an LPA1-dependent mechanism, and this could be important in the pathogenesis of pulmonary fibrosis.33
The purpose of the present study was to investigate whether LPA can activate TGF-β in epithelial cells via the αvβ6 integrin and to dissect the proximal signaling pathway from the relevant LPA receptor to RhoA. We demonstrate that the LPA2 receptor is the predominant receptor for transducing LPA-induced, αvβ6 integrin-mediated, TGF-β activation and that this pathway is coupled to the G protein, Gαq. Finally we provide evidence that both the αvβ6 integrin and LPA2 receptor are induced in epithelial cells overlying areas of pulmonary fibrosis in the lungs of mice treated with intratracheal bleomycin and in samples from patients with idiopathic pulmonary fibrosis.
Materials and Methods
Cells and Reagents
The gαq−/− and gα12/13−/− murine embryonic fibroblasts (MEFs) were a gift of Stefan Offermanns (University of Heidelberg, Germany). The lpa1−/− and lpa2−/− were a gift of Jerold Chun (The Scripps Research Institute). MEFs were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. The transformed mink lung (TMLC) cells stably expressing firefly luciferase under the control of a TGF-β–sensitive portion of the plasminogen activator inhibitor-1 (PAI-1) promoter were a gift from Dan Rifkin (New York University, New York) and maintained in DMEM, 10% fetal calf serum (FCS) containing 250 μg/ml of Geneticin (G-418 sulfate; Sigma-Aldrich, Dorset, UK). All media were supplemented with l-glutamine (2 mmol/L), penicillin (100 U/ml), and streptomycin sulfate (100 μg/ml). All cells were maintained at 37°C and 5% CO2 in a humid environment. The anti-αvβ6 integrin antibodies 6.3G9 and 2A1 were generated as previously described.36 All other reagents were commercially obtained. The antibodies used were mouse monoclonal anti-TGF-β (clone 1D11, R&D systems, Abingdon, UK), rabbit polyclonal anti phospho-Smad2 and anti-total Smad2 (Cell Signaling, Hertfordshire, UK), and rabbit polyclonal anti-LPA1 and anti-LPA2 (Acris Antibodies GmbH, Herford, Germany). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse antibodies for Western blot were purchased from Dako (Cambridge, UK). The stimulants used were TGF-β1 (R&D Systems, Abingdon, UK) and LPA (Sigma-Aldrich, Dorset, UK). Inhibitors used were Ki16425, pertussis toxin (Sigma-Aldrich, Dorset, UK), botulinum toxin exoenzyme C3-transferase (Tebu-bio, Peterborough, UK), H-1152 (Calbiochem, Nottingham, UK), and GP-antagonist 2A (Calbiochem, Nottingham, UK). Normal Human Bronchial Epithelial (NHBE) cells and growth media were obtained from Lonza Walkersville Inc (Workingham, UK).
Generation of Stable Cell Lines
MEFs were infected with the wild-type human β6 subunit in the retroviral vector pWZL. Retroviruses were generated by lipofectamine-mediated (Invitrogen, Paisley, UK) transfection of the ϕ-ε replication-incompetent ectropic virus packaging cell line. The virus-containing supernatant was harvested and filtered through a 0.22-μm filter and then added to 50% confluent cultures in the presence of 5 μg/ml Polybrene (Sigma-Aldrich, Dorset, UK) and cultured for 24 hours. The virus-containing medium was removed, and cells were cultured in 5% DMEM-FCS containing 5 μg/ml blasticidin (InvivoGen, San Diego, CA). Cells expressing the αvβ6 integrin were identified by flow cytometry with the anti-αvβ6 antibody 6.3G9. All cell lines continuously expressed high surface levels of αvβ6 as determined by flow cytometry with 6.3G9.
Flow Cytometry
Cultured cells were harvested by trypsinization. Nonspecific binding was blocked with normal goat serum at 4°C for 20 minutes. Cells were then incubated with primary antibody for 20 minutes at 4°C, followed by secondary goat anti-mouse antibody conjugated with phycoerythrin (Chemicon International, Hertfordshire, UK). Between incubations, cells were washed twice in PBS with Ca2+ and Mg2+. Stained cells were resuspended in 100 μl of PBS with Ca2+ and Mg2+, and the fluorescence was quantified on 10,000 cells with FacsCalibur with Cellquest software (Becton-Dickinson, Cowley, UK).
TMLC Assay
The TMLC coculture assay was used to detect active TGF-β activity. Experimental cell lines were plated at 2.5 × 105 cells/ml in 10% DMEM-FCS for 16 hours to allow adhesion and cell spreading. This medium was then changed to serum-free culture medium for 2 hours. TMLC cells were harvested by trypsinization and resuspended in serum-free DMEM at 5 × 105 cells per ml. An aliquot of TMLC cells was removed and mixed with αvβ6 or TGF-β antibody. The medium was then removed from the experimental cells, and an equal volume (100 μl) of TMLC cells (with or without antibodies) and medium containing experimental stimulant or inhibitor was added. The TMLC cells were incubated at 37°C overnight, and cells were washed once in PBS before cell lysis in reporter lysis buffer (Promega, Hampshire, UK). The cell layer was agitated with a pipette and centrifuged at 1500 × g for 5 minutes at 4°C. The supernatant was added to luciferase assay buffer (Promega, Hampshire, UK) and the luminescence measured using a MicroLumatPlus microplate luminometer (EG&G, Berthold, Hertfordshire, UK). To determine total TGF-β, the experimental cell layer was acidified with 0.1 volume of 1N HCl for 10 minutes at room temperature before neutralizing in 0.1 volume 1.2 N NaOH, and TMLC coculture was performed as above. In both cases, TGF-β was quantified by comparing values obtained under experimental conditions to readings obtained from a standard curve derived from increasing concentrations of active TGF-β1 added to coculture under identical conditions.
Cell Viability Assay
The effect of Rho/Rho kinase inhibitor on cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay. NHBE cells were seeded in 96-well culture plates (1 × 104/well) with complete bronchial-epithelial growth medium (BEGM) for 16 hours to allow adhesion and cell spreading. This medium was then changed to supplement-free BEGM medium for 2 hours. NHBE cells were treated with various doses of inhibitors in supplement-free BEGM medium for 24 hours. After incubating the cells with inhibitors, the media was removed; then 100 μl of supplement-free BEGM medium containing 0.4 mg/ml MTT (Sigma-Aldrich, Dorset, UK) reagent were then added to each well. Followed incubation with MTT for 2 hours, the media was removed and then 200 μl of dimethyl sulfoxide was to each well and optical density measured at 550 nm using a plate reader (Tecan Genios, Theale, UK).
Measurement of Intracellular Calcium
NHBE cells (1 × 104/well) with complete BEGM medium were seeded in black walled, clear bottom 96 well plates for 16 hours to allow adhesion and cell spreading. This medium was then changed to supplement-free BEGM medium for 2 hours. Then cells were loaded with Fluo-4AM (Invitrogen, Paisley, UK) for 1 hour at room temperature in 100 μl loading buffer (Hanks’ balanced saline solution, containing 0.1% bovine serum albumen, 5 mmol/L glucose, 10 mmol/L HEPES, 2.5 mmol/L probenecid, 0.5 mmol/L brilliant black, 0.1 mmol/L CaCl2, and 1 mmol/L MgCl2). The fluorescence was continuously recorded at wavelengths of 485 nm excitation and 520 nm emissions using a Flexstation (Molecular Devices, Wokingham, UK). Data are presented as changes in fluorescence intensity, measured in arbitrary fluorescence units.
Western Blot Analysis
After washing the cells with PBS twice, they were incubated on ice with cold buffer containing 50 mmol/L Tris, 150 mmol/L NaCl, 20 mmol/L NaF, 1 mmol/L NaVaO3, 1 mmol/L EDTA, 1 μmol/L phenylmethane sulphonyl fluoride glycerol, 1% v/v Triton X-100, 0.1% v/v sodium dodecyl sulfate, and a protease inhibitor cocktail (Sigma-Aldrich, Dorset, UK) and harvested by all scraping. The cell extract was centrifuged (4000 × g, 4°C, 10 minutes), and protein concentration was measured using a bicinchoninic acid assay (Pierce Biotechnology, Northumberland, UK). One hundred micrograms of protein was fractionated by SDS-polyacrylamide gel electrophoresis, transferred onto polyvinylidene difluoride membranes, and incubated with blocking buffer containing 5% nonfat dry milk in Tris-buffered saline with Tween (50 mmol/L Tris, 150 mmol/L NaCl, 0.1% Tween-20, pH 7.4) for 1 hour. Membranes were incubated with primary antibody in 5% skimmed milk Tris-buffered saline with Tween overnight at 4°C before washing and incubating for 1 hour at room temperature with HRP-conjugated secondary antibody. Immunoreactive bands were visualized by enhanced chemiluminescence (GE Health care, Buckinghamshire, UK). Membranes were next stripped (100 mmol/L 2-mercaptoethanol, 2% SDS, 62.5 mmol/L Tris-HCl) at 50°C for 30 minutes at room temperature before immunoblotting with total Smad2 polyclonal rabbit anti-mouse antibody (Cell Signaling, Hertfordshire, UK) or glyceraldehyde-3-phosphate dehydrogenase monoclonal mouse anti-rabbit antibody (Upstate, Hertfordshire, UK) to assess total protein and loading controls.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated from 6-well plates for culture cells or frozen powdered lung tissue using the RNeasy mini kit (Qiagen, West Sussex, UK) with on-column DNase digestion. 1 μg of total RNA was reverse transcribed in 20 μl containing 200units of Moloney murine leukemia virus reverse transcriptase (Promega, Hampshire, UK), 25units of RNase inhibitor (Promega, Hampshire, UK), 0.5 μg of oligo(dT)15 primer, 0.5 mmol/L of each dNTPs, and 1×first-strand buffer provided by Promega. The reaction was incubated at 42°C for 90 minutes.
Aliquots of the reverse transcription (RT) products were used for PCR amplification. 2.5 μl of cDNA in 50 μl containing 1 mmol/L MgCl2, 0.12 mmol/L of each dTNPs, 1 unit of Taq polymerase (Promega, Hampshire, UK), and 0.5 μmol/L of both the upstream and downstream PCR primers (Shown in the Table 1), and 1× PCR buffer (Promega, Hampshire, UK).
Table 1.
Primers for RT-PCR and Real-Time PCR
| Gene | Strand | Primer |
|---|---|---|
| Human LPA1 | Sense | 5′-GGTGATGGGACTTGGAAT-3′ |
| Antisense | 5′-AAACCGTAATGTGCCTCT-3′ | |
| Human LPA2 | Sense | 5′-TGGCTCAACCCAACCAAC-3′ |
| Antisense | 5′-CCTCATTACCCAGTCATACCG-3′ | |
| hLPA2 for real time | Sense | 5′-CACCCTTTAGCTACCTTGAACT TCA-3′ |
| Antisense | 5′-CAGTCCTGTTGGTTGGGTTGA-3′ | |
| Human LPA3 | Sense | 5′-TGCTTCCCTCACCAACTT-3′ |
| Antisense | 5′-CCGCAGGTACACCACAAC-3′ | |
| Human LPA4 | Sense | 5′-AGTTGTTGGGTTTATCATTC-3′ |
| Antisense | 5′-AAACAGGGACTCCATTCT-3′ | |
| Mouse LPA1 | Sense | 5′-ATGAGTCTATCGCCTTCTTT-3′ |
| Antisense | 5′-CGGGTATTAGGTCCTGTATT-3′ | |
| Mouse LPA2 | Sense | 5′-CTCCAACCGACGCTTCCA-3′ |
| Antisense | 5′-ATGCGTGAGCAACTGTCCAA-3′ | |
| mLPA2 for real time | Sense | 5′-GTCACACTCATCGTGGGTGTGT-3′ |
| Antisense | 5′-GTCACACTCATCGTGGGTGTGT-3′ | |
| Mouse LPA3 | Sense | 5′-CGAGTGGACAGGGACAAA-3′ |
| Antisense | 5′-CCAGCAGGTAGTAGAAGGGA-3′ | |
| Mouse LPA4 | Sense | 5′-GAGAAGTGAGACGGCTAT-3′ |
| Antisense | 5′-GTGGTGGAGAACAAAGAA-3′ | |
| Mouse Gαq | Sense | 5′-CAGACAATGAGAACCGCA-3′ |
| Antisense | 5′-GGAATACATGATTTTCTCC TCT-3′ | |
| Mouse Gα12 | Sense | 5′-TGAACATCTTCGAGACCATCG-3′ |
| Antisense | 5′-ACAGACTTCACCTTCTCCA CCA-3′ | |
| Mouse Gα13 | Sense | 5′-TGATGGCATTTGATACCCGC-3′ |
| Antisense | 5′-ACCACTGTCCTCCCATAAG GCT-3′ | |
| Integrin β6 | Sense | 5′-AAACGGGAACCAATCCTCTGT-3′ |
| Antisense | 5′-GCTTCTCCCTGTGCTTGTAGGT-3′ | |
| GAPDH | Sense | 5′-CCACCCATGGCAAATTCCATGGCA-3′ |
| Antisense | 5′-TCTAGACGGCAGGTCAGGTCC ACC-3′ | |
| PAI-1 | Sense | 5′-TTCCAGTCACATTGCCATCAC-3′ |
| Antisense | 5′-TGGCCTTTGGCCTGTCA-3′ | |
| 18srRNA | Sense | 5′-AAACGGCTACCACATCCAAG-3′ |
| Antisense | 5′-CAATTACAGGGCCTCGAAAG-3′ |
RNA was amplified after initial denaturation at 94°C for 2 minutes. This was followed by 30 cycles of denaturation at 94°C for 1 minute, annealing at 58°C for 30 seconds, extension at 72°C for 1 minute, and a final extension of 72°C for 5 minutes (PTC-100, MJ Research Inc., Watertown, MA). The PCR products were visualized by electrophoresis on a 2% agarose gel in 0.5× Tris borate-EDTA buffer after staining with 0.5 μg/ml ethidium bromide.
Quantitative Real-Time RT-PCR
PAI-1, integrin β6 and LPA2 gene expression were determined using primer sequences listed in Table 1. GAPDH 18srRNA and β2m were used as the housekeeping gene. One nanogram of reverse-transcribed cDNA was subjected to real-time PCR using Excite Real-time Mastermix with SYBR green (Biogene, Cambridge, UK) and the ABI Prism 7700 detection system (Applied Biosystems, Warrington, Cheshire, UK). Each reaction consisted of 1× Excite mastermix, SYBR green (1:60,000 final concentration), 40 nmol/L of both sense and antisense primers, 1.6 μl of DNA (or dH2O), and H2O to a final volume of 20 μl. Thermal cycler conditions included incubation at 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Integration of the fluorescent SYBR green into the PCR product was monitored after each annealing step. Amplification of one specific product was confirmed by melting curve analysis, where a single melting peak eliminated the possibility of primer-dimer association. For melting curve, the products were heated from 60°C to 95°C over 20 minutes after the 40 cycles.
Negative controls consisting of no template were included, and all reactions were set up in triplicate. Gene expression was normalized to the housekeeping gene by dividing the mean of the sample triplicate value by the mean of the housekeeping gene triplicate value. This was then expressed as fold increase at each time point.
Small Interfering RNA for LPA2
RNA interference used small hairpin RNA targeted against LPA2 and scrambled control sequence using the SureSilencing shRNA Plasmids System (SuperArray, Frederick, MD) for MEF cells. Cells seeded at 35% confluence (1.5 × 105 cells per well) in 6-well plates were transfected with 2.5 μg of plasmid DNA using lipofectamine 2000 in the presence of 10% fetal bovine serum; 24 to 48 hours following transfection cells were split to 10% confluence and cultured in medium containing 1 mg/ml G418 for 1 week. Success of RNA interference was determined by the expression of LPA2 using real-time PCR and immunoblot.
For NHBE cells, small interfering RNA duplex (siRNA) directed against LPA2 and scrambled siRNA were obtained from Eurofins MWG Operon according to published sequences.37 NHBE cells cultured onto 6-well plates (40% to 50% confluence) were transiently transfected with siRNA using Transmessenger transfection reagent (Qiagen, West Sussex, UK). LPA siRNA or scrambled siRNA (50 nmol/L) was condensed with enhancer R and formulated with Transmessenger reagent according to the manufacturer’s instructions. The transfection complex was diluted into 900 μl of bronchial epithelial cell basal medium and added directly to the cells for 3 hours and then replaced with normal growth media until stimulation experiments were performed. Cells were used for experiments at 24 hours after transfection for Real-time PCR or at 72 hours after transfection for Western blotting.
Animal Model of Pulmonary Fibrosis
Lung fibrosis was induced by oropharyngeal aspiration of bleomycin (2 mg/kg body weight) using a previously described protocol.38 All animals were used in accordance with local and UK Government regulations. Lungs for immunohistochemical analyses were fixed after perfusion. The trachea was cannulated, and lungs were insufflated with 4% paraformaldehyde in PBS at a pressure of 25 cm H2O. The heart and inflated lungs were removed en bloc, immersed in 4% paraformaldehyde for 4 hours before transferring to 15% sucrose in PBS overnight at 4°C, and processed to paraffin wax. For real-time RT-PCR analysis, lungs were carefully removed and immediately snap-frozen in liquid nitrogen.
Human Lung Tissue Samples
Samples of lung tissue were obtained from patients with idiopathic pulmonary fibrosis (IPF) undergoing surgical lung biopsy or lung transplantation. All patients fulfilled a consensus clinical, histological and radiological diagnosis of usual interstitial pneumonia (UIP)/IPF in accordance with the criteria proposed by the American Thoracic Society and European Respiratory Society.39 Control lung sections, from histologically normal regions of lung, were obtained from archived tissue. All tissue was obtained with appropriate consent and its use approved by the local research ethics committee.
Immunohistochemistry
Histological sections of lung tissue were cut (5 μm), dewaxed in xylene, rehydrated through decreasing concentrations of ethanol, and washed in Tris-buffered saline. Antigens were unmasked by microwaving of sections in 10 mmol/L citrate buffer, pH 6.0 (twice for 10 minutes) for assessment of LPA receptors and by pepsin (0.5% in 5 mmol/L HCl) for assessment of αvβ6 integrin expression. Sections were incubated with 3% H202 in methanol for 30 minutes, and then washed twice with Tris-buffered saline before blocking of nonspecific binding (using normal goat serum for mouse tissues and using normal horse serum for human tissues) for 1 hour at room temperature. After washing twice in Tris-buffered saline, immunostaining was undertaken using the avidin-biotinylated enzyme complex method (Vector Laboratories, Peterborough, UK) with antibodies against LPA receptor and β6 integrin. After incubation with biotin-conjugated secondary antibody and subsequently with ABC complex solution, color development was performed using 3,3′-diaminobenzidine tetrahydrochloride (Vector Laboratories, Peterborough, UK). Sections were counterstained using Mayer’s Hematoxylin (Raymond A Lamb Limited, East Sussex, UK).
Masson’s Trichrome Staining
Sections were dewaxed in xylene and rehydrated through decreasing concentrations of ethanol, then were mordant in Bouin’s solution (Sigma-Aldrich, Dorset, UK) at room temperature overnight. Following washing in running tap water for 5 minutes, sections were stained for 5 minutes in Weigert’s Iron Hematoxylin Solution (1% hematoxylin in 95% ethanol, 1.2% ferric chloride, and 1% acetic acid in distilled water). After washing in running tap water for 5 minutes, sections were then stained in Biebrich scarlet-acid fuchsin (Sigma-Aldrich, Dorset, UK) for 5 minutes. Followed by rinsing in distilled water, sections were incubated in phosphomolybdic/phosphotungstic acid solution (Sigma-Aldrich, Dorset, UK) for 5 to 10 minutes to allow uptake of aniline blue solution (Sigma-Aldrich, Dorset, UK). Then sections were incubated in 1% (v/v) acetic acid solution for 3 to 5 minutes to differentiate. After staining, sections were dehydrated through increasing concentrations of ethanol and xylene before they were mounted in DPX (BDH/Merck, Nottingham, UK).
Immunofluorescence
Cells were plated on 8-well chamber glass-slides for 24 hours. Cells were fixed in 4% paraformaldehyde for 10 minutes at room temperature after LPA stimulation for 4 hours. Once fixed, the cells were permeabilized (0.2% Triton X-100), blocked (10% goat serum in PBS), and incubated with anti αvβ6 monoclonal primary antibody (human/mouse chimeric form of the anti αvβ6 mAb 2A1 for mouse origin and murine 2A1 for human origin) for 1 hour at room temperature following three washes with PBS. Alexafluor labeled goat anti-human IgG (Invitrogen, Paisley, UK) for MEF cells, or Alexafluor labeled horse anti-mouse IgG (Invitrogen, Paisley, UK) for NHBE cells was incubated in the dark for 1 hour at room temperature and subsequently slides were washed three times with PBS. For stress fiber staining, fluorescein isothiocyanate-conjugated phalloidin (Sigma-Aldrich, Dorset, UK) was used. An additional 4,6-diamidino-2-phenylindole stain (1 μg/ml for 1 minute, room temperature) was added to visualize the nucleus immediately before mounting. Images were acquired by Leica TCS SP2 confocal microscope (Milton Keynes, UK).
Statistical Analysis
Data were analyzed by two-tailed Student’s t-test for single and by one-way analysis of variance for multiple group comparisons. Differences were considered significant at P < 0.05.
Results
LPA Induces αvβ6-Mediated TGF-β Activation in Bronchial Epithelial Cells
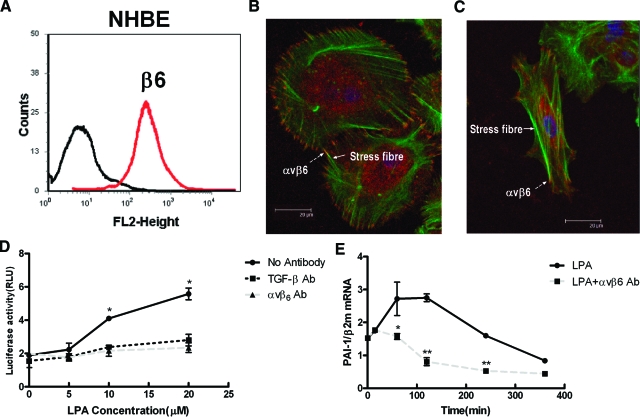
To investigate whether LPA can induce αvβ6-dependent TGF-β activation in epithelial cells, NHBE were used. These cells are primary lung epithelial cells that express high levels of αvβ6 integrin on the cell surface (Figure 1A) that is stable for up to four passages (data not shown). The αvβ6 integrin was associated with cortical actin and the ends of stress fibers (Figure 1B). Stimulation of NHBE cells with LPA lead to increased stress fiber formation and reorganization of αvβ6 integrin at the ends of stress fibers (Figure 1C).
Figure 1.
Stimulation of αvβ6-expressing human lung epithelial cells with LPA leads to αvβ6-dependent TGF-β activation. A: NHBE cells express high levels of αvβ6 integrins on the cell surface. B: Confocal overlay micrographs of NHBE cells stained with phalloidin (green) and a β6 antibody (red) demonstrates widespread colocalization of the αvβ6 integrin (dashed arrow) with cortical actin and the end of stress fibers (solid arrow). C: Following stimulation with LPA, there is cell contraction and reorganization of the cytoskeleton with αvβ6 integrin prominent at the end of actin stress fibers. D: NHBE cells were cocultured with TMLC cells and stimulated with increasing concentrations of LPA in the absence (bold line) or presence of a pan-TGF-β blocking antibody (dotted line) or an αvβ6-blocking antibody (dashed line), and luciferase activity from the TGF-β responsive PAI-1 promoter was measured. E: NHBE cells were stimulated with 10 μmol/L LPA in the absence (bold line) or presence of αvβ6-blocking antibody (dashed bars) and the time course of TGF-β activity was determined by real-time PCR assessment of endogenous PAI-1 mRNA levels. All experiments were performed in triplicate and a representative experiment is shown. Data presented as mean + SEM. *P < 0.05, **P < 0.01 in comparison with no antibody control.
LPA was also used to stimulate NHBE cells in coculture with TMLC containing the TGF-β responsive PAI-1 promoter driving luciferase expression. LPA induced a concentration-dependent increase in luciferase activity that was completely abolished by αvβ6 blocking antibody (Figure 1D). To determine the proportion of LPA-induced TGF-β activity that was mediated by αvβ6 integrin, a pan-TGF-β blocking antibody was also used. The pan-TGF-β blocking antibody inhibited luciferase activity to the same extent as the αvβ6 integrin-blocking antibody, demonstrating that there was no αvβ6-independent TGF-β activation induced by LPA in these cells (Figure 1D).
Because the coculture bioassay depends on the induction of luciferase protein, it cannot be used for short-term time course experiments. To determine the time course of LPA-induced, αvβ6-mediated TGF-β activity, the expression level of endogenous PAI-1 mRNA levels in NHBE was measured by real-time PCR in the absence of reporter cells. LPA caused a time-dependent increase in PAI-1 expression that was maximal 2 hours following stimulation (Figure 1E). LPA-induced PAI-1 expression was blocked by αvβ6 antibody, again confirming that LPA-mediated TGF-β activation in NHBE is mediated by αvβ6.
LPA Signals to the αvβ6 Integrin in Epithelial Cells via RhoA and Rho Kinase
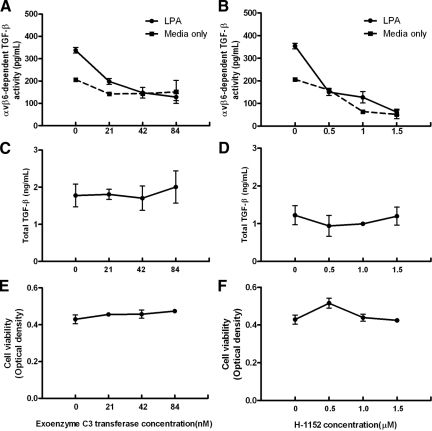
We have previously shown that RhoA and Rho kinase are involved in PAR1-induced, αvβ6-mediated, TGF-β activation in MEFs engineered to express the β6 integrin.12 We therefore hypothesized that LPA induced αvβ6 integrin-mediated TGF-β activity in NHBE cells would also signal via RhoA and Rho kinase. We stimulated NHBE cells with 10 μmol/L LPA in the presence of increasing concentrations of botulinum toxin exoenzyme C3-transferase (a specific inhibitor of RhoA40) and H-1152 (a specific inhibitor of Rho kinase41). Specific αvβ6 integrin mediated TGF-β activity was calculated by subtracting the relative light unit values from cocultures treated with an αvβ6-integrin blocking antibody from values obtained from cocultures simultaneously performed without blocking antibody treatment. αvβ6 integrin-mediated TGF-β activity was quantified by generating a standard curve of reporter cells in coculture with NHBE cells treated with increasing concentrations of active TGF-β1, or with a TGF-β blocking antibody to allow subtraction of baseline TGF-β-independent luminescence. Both botulinum toxin exoenzyme C3-transferase (Figure 2A) and H-1152 (Figure 2B) caused a concentration-dependent inhibition of αvβ6 integrin-mediated TGF-β activation. H-1152 also reduced basal (unstimulated) αvβ6 integrin-mediated TGF-β activation, whereas botulinum toxin exoenzyme C3-transferase had little effect. This difference could reflect differences in cellular uptake or potency of the two inhibitors, but we cannot exclude a contribution of activation of ROCK by RhoA-independent pathways. To ensure these effects were not due to non-specific effects of these inhibitors on total TGF-β levels or cell viability, total TGF-β and cell death were measured by TMLC coculture and MTT assay, respectively. At the concentrations of botulinum toxin exoenzyme C3-transferase used to inhibit αvβ6 integrin-dependent TGF-β activity, there was no significant effect on total TGF-β levels (Figure 2C) or cell viability (Figure 2E). Likewise there were no concentration-dependent effects on total TGF-β levels (Figure 2D), and only a very slight effect on cell viability (Figure 2F) at the highest concentration of H-1152 used. These results confirm that LPA-induced αvβ6-mediated TGF-β activation in epithelial cells is mediated via RhoA and Rho kinase.
Figure 2.
LPA signals via RhoA and Rho kinase to induce αvβ6-mediated TGF-β activation. NHBE cells were stimulated with 10 μmol/L LPA (solid lines) in the presence of increasing concentrations (A, C, E) of the RhoA inhibitor botulinum toxin exoenzyme C3-transferase and (B, D, F) Rho kinase inhibitor H-1152. A and B: αvβ6 integrin-mediated TGF-β activity was compared with unstimulated NHBE cells (dashed lines). C and D: To ensure the effects of the inhibitors were not due to non-specific effects on TGF-β synthesis, total TGF-β levels were determined. E and F: To determine whether there were toxic effects of these compounds, an MTT cell viability assay was performed. All experiments were performed in triplicate and a representative experiment is shown. Data presented as mean + SEM.
LPA-Induced, αvβ6-Mediated TGF-β Activity Requires Gαq
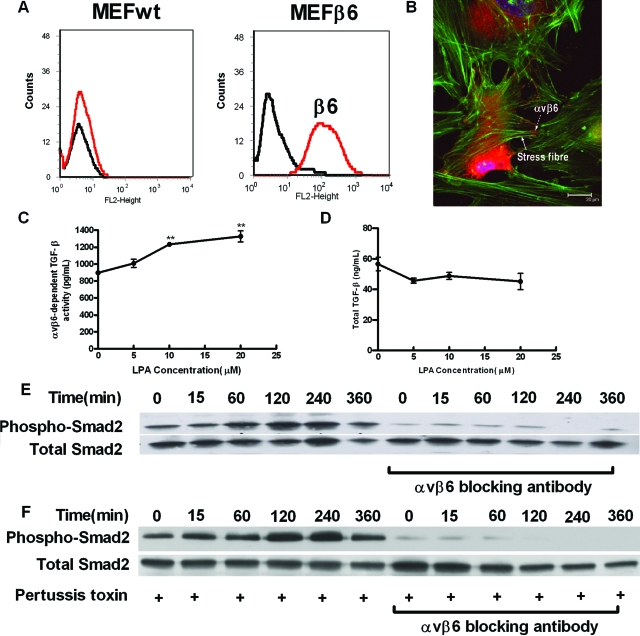
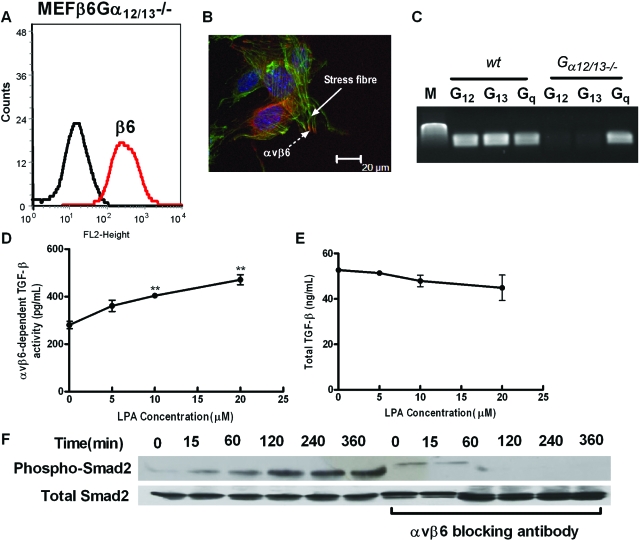
LPA exerts its cellular effects through coupling with downstream small G proteins, including Gαi, Gαq, and Gα12/13. To determine which G protein subunit was involved in LPA induced αvβ6-mediated TGF-β activation, we stably infected wild-type, gαq−/− and gα12/13−/− MEFs with the human β6 integrin subunit. Wild-type MEFs transfected with the β6 integrin subunit (MEFβ6) had high levels of β6 expression on the cell surface (Figure 3A), which were able to form focal contacts, at the termini of actin stress fibers following LPA stimulation (Figure 3B). MEFβ6 cells had high basal αvβ6 integrin-mediated TGF-β activity as assessed by TMLC assay (Figure 3C) and responded to increasing concentrations of LPA with a concentration-dependent increase in αvβ6 integrin-mediated TGF-β activity (Figure 3C). Although MEFβ6 cells had high levels of total TGF-β, they were not affected by LPA stimulation (Figure 3D). Wild-type and knock out MEF’s not transfected with β6 did not activate TGF-β under the conditions used, either at baseline or in response to increasing concentrations of LPA (see supplemental Figure S1, see http://ajp.amjpathol.org). Stimulation of MEFβ6 cells with 10 μmol/L LPA lead to a time-dependent increase on Smad2 phosphorylation that was maximal between 120 and 240 minutes and was inhibited by an αvβ6 blocking antibody (Figure 3E). However, LPA-induced Smad2 phosphorylation could not be inhibited by preincubation of the MEFβ6 cells with pertussis toxin, an inhibitor of Gαi (Figure 3F).
Figure 3.
LPA-induced, αvβ6-mediated TGF-β activity in αvβ6-integrin expressing MEFs is independent of Gαi. A: Wild-type MEFs infected with the wild-type human β6 integrin (MEFβ6) were assessed for cell surface β6 integrin expression by flow cytometry. B: Following stimulation with LPA, MEFβ6 cells were stained with phalloidin (green) and a β6 antibody (red), which demonstrated widespread localization of the αvβ6 integrin (dashed arrow) at the ends of actin stress fibers (solid arrow). C: LPA-induced, αvβ6-dependent, TGF-β activity in MEFβ6 cells was assessed by coculture assay. αvβ6 integrin-mediated TGF-β activity (pg/ml) was calculated. Data presented as mean + SEM. **P < 0.01 in comparison with 0 μmol/L group. D: Total TGF-β levels in response to increasing LPA concentrations were determined. E: The time course of LPA-induced TGF-β activity in MEFβ6 cells was determined by phospho-Smad2 immunoblotting in the presence or absence of αvβ6 blocking antibody. Total Smad2 levels were assessed as control. F: MEFβ6 were pre-treated with 100 ng/ml pertussis toxin for 8 hours and then stimulated with LPA in the absence or presence of αvβ6 blocking antibody. TGF-β activity was determined by immunoblotting for phospho-Smad2. Total Smad2 levels were assessed as controls. All experiments were performed in triplicate and a representative experiment is shown. Data presented as mean + SEM.
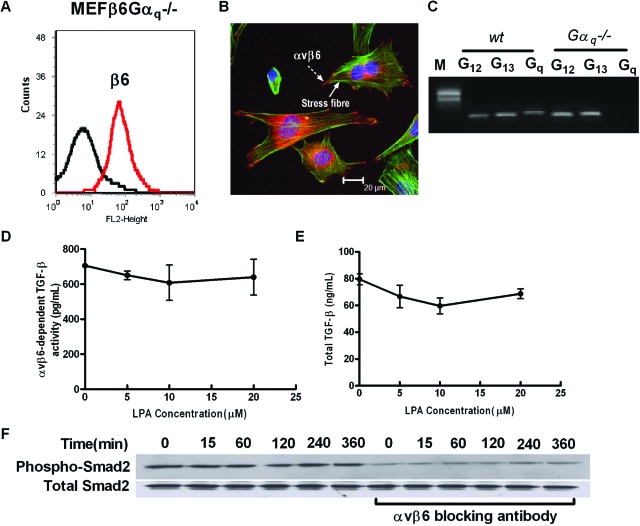
MEFs from gαq−/− mice infected with the β6 integrin subunit (MEFβ6Gαq−/−) also expressed high levels of cell surface β6 integrin (Figure 4A), which following LPA stimulation were located at focal contacts at the termini of actin stress fibers (Figure 4B). The absence of Gαq and presence of Gα12 and Gα13 subunits was confirmed by RT-PCR (Figure 4C). Levels of basal αvβ6 integrin-mediated TFG-β activity were only slightly lower than those of wild-type MEFβ6 and several-fold higher than MEFβ6Gα12/13−/− cells (Figure 4D compared with Figure 3C and Figure 5D). However, unlike MEFβ6 or MEFβ6Gα12/13−/− cells MEFβ6Gαq−/− cells did not increase αvβ6 integrin-mediated TGF-β activity in response to LPA (Figure 4D). Furthermore, stimulation of MEFβ6Gαq−/− with 10 μmol/L LPA did not increase Smad2 phosphorylation (Figure 4F). Levels of total TGF-β were of a similar magnitude to levels in wild-type MEFβ and MEFβ6Gα12/13−/−, and again there was no effect of LPA stimulation (Figure 4E).
Figure 4.
Gαq is necessary for LPA-induced, αvβ6-mediated, TGF-β activity in MEFs expressing αvβ6. A: MEFs null for the Gαq small G proteins infected with the wild-type human β6 integrin (MEFβ6Gαq−/−) were assessed for cell surface β6 integrin expression by flow cytometry. B: LPA-stimulated MEFβ6Gαq−/− were stained with phalloidin (green) and a β6 antibody (red), which demonstrated αvβ6 integrin (dashed arrow) localized to focal contacts at the ends of stress fibers (solid arrow). C: The veracity of the null mutant was confirmed by measuring Gα12, Gα13, and Gαq mRNA levels in wild-type and gαq−/− MEFs by RT-PCR. D: αvβ6-dependent TGF-β activity, following stimulation of MEFβ6Gαq−/− cells with increasing concentrations of LPA, was measured by coculture assay. αvβ6 integrin-mediated TGF-β activity was calculated from a standard curve. Data presented as mean + SEM. E: Total TGF-β levels in response to increasing LPA concentrations were determined. F: The time course of TGF-β activity in response to LPA stimulation was measured by phospho-Smad2 immunoblotting in the presence or absence of αvβ6 blocking antibody. Total Smad2 levels were assessed as control. All experiments were performed in triplicate and a representative experiment is shown. Data presented as mean + SEM.
Figure 5.
The G protein subunits Gα12 and Gα13 do not transduce LPA-induced, αvβ6-mediated, TGF-β activity in MEFs expressing the αvβ6 integrin. A: MEFs null for the Gα12 and Gα13 small G proteins infected with the wild-type human β6 integrin (MEFβ6Gα12/13−/−) were assessed for cell surface β6 integrin expression by flow cytometry, and (B) LPA-stimulated MEFβ6Gα12/13−/− were stained with phalloidin (green) and a β6 antibody (red), which demonstrated localization of the αvβ6 integrin (dashed arrow) in focal contacts at the end of actin stress fibers (solid arrow). C: The veracity of the null mutant was confirmed by measuring Gα12, Gα13, and Gαq mRNA levels in wild-type and gα12/13−/− MEFs by RT-PCR. D: αvβ6-dependent TGF-β activity following stimulation of MEFβ6Gα12/13−/− cells with increasing concentrations of LPA was measured by coculture assay. αvβ6 integrin-mediated TGF-β activity was calculated. Data presented as mean + SEM. **P < 0.01 comparison with 0 μmol/L group. E: Total TGF-β levels in response to increasing LPA concentrations were determined. F: The time course of TGF-β activity in response to LPA stimulation was measured by phospho-Smad2 immunoblotting in the presence or absence of αvβ6-blocking antibody. Total Smad2 levels were assessed as control. All experiments were performed in triplicate and a representative experiment is shown. Data presented as mean + SEM.
MEFs derived from gα12/13−/− mice were infected with the wild-type human β6 integrin subunit (MEFβ6Gα12/13−/−), which was expressed at high levels on their cell surface (Figure 5A), forming focal contacts at the termini of actin stress fibers as observed with wild-type MEFβ6 and MEFβ6Gαq−/− cells (Figure 5B). The presence of the Gαq subunit and absence of the Gα12 and Gα13 subunits were again confirmed by RT-PCR (Figure 5C). Basal levels of αvβ6 integrin-mediated TGF-β activity (Figure 5D) were substantially lower than in wild-type MEFβ6 cells (Figure 3C). However, levels of total TGF-β were similar to wild-type MEFβ6 and MEFβ6Gαq−/− cells (Figure 5E). Nonetheless, stimulation with increasing concentrations of LPA caused concentration-dependent increases in αvβ6 integrin-mediated TGF-β activity (Figure 5D) without affecting total TGF-β levels (Figure 5E). Furthermore, stimulation with 10 μmol/L LPA induced time-dependent Smad2 phosphorylation, which was maximal at 240 to 360 minutes and was completely inhibited by αvβ6 integrin blocking antibody (Figure 5F), as was observed in wild-type MEFβ6 cells.
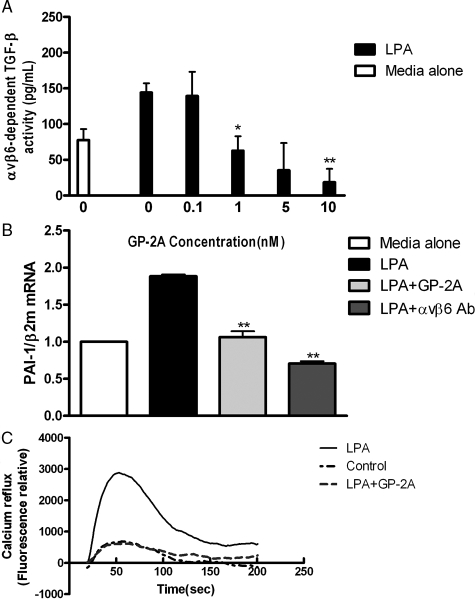
To confirm the central role of Gαq in LPA induced αvβ6 integrin-mediated TGF-β activation in primary epithelial cells, we stimulated NHBE cells in coculture with TMLC cells with 10 μmol/L LPA in the presence of GP antagonist-2A (GP-2A), a specific Gαq inhibitor.42 As would be predicted from our results in MEFs, increasing concentrations of GP-2A inhibited LPA-stimulated αvβ6-dependent TGF-β activity in a concentration-dependent manner (Figure 6A). GP-2A did not inhibit TMLC cell reporter activity even at high concentrations (data not shown). However, to ensure there were no confounding effects of the inhibitor in coculture experiments we assessed the effect of GP-2A on endogenous PAI-1 mRNA levels in NHBE cells following LPA stimulation. 10 μmol/L LPA induced a two-fold increase in NHBE PAI-1 gene expression that was inhibited by both 10 nmol/L GP-2A and an αvβ6 integrin-blocking antibody (Figure 6B). To ensure that GP-2A was inhibiting Gαq in NHBE cells at the concentrations used in these studies, calcium mobilization in the presence of LPA and GP-2A was measured (Figure 6C). LPA induced time-dependent calcium influx, which was completely inhibited by GP-2A confirming its effectiveness at inhibiting Gαq.
Figure 6.
LPA-induced, αvβ6-mediated, TGF-β activation is mediated via Gαq in NHBE cells. A: NHBE cells were stimulated with 10 μmol/L LPA in the presence of increasing concentration of Gαq inhibitor GP-2A, and αvβ6-mediated TGF-β activity were measured by coculture assay. B: NHBE cells were exposed to 10 μmol/L LPA for 1 hour following 2 hours’ pre-incubation with GP-2A or DMEM. TGF-β activity was determined by measuring PAI-1 mRNA levels by real-time RT-PCR. C: NHBE cells were stimulated with LPA either in the presence (bold line) or absence of GP-2A (dotted line), and compared with control cells (dashed line). The time course of calcium influx was measured using fluo4 labeling. All experiments were performed in triplicate and a representative example is shown. Data are presented as the mean + SEM. *P < 0.05 and **P < 0.01 comparison with only LPA stimulation group.
LPA-Induced, αvβ6-Mediated TGF-β Activation is via LPA2
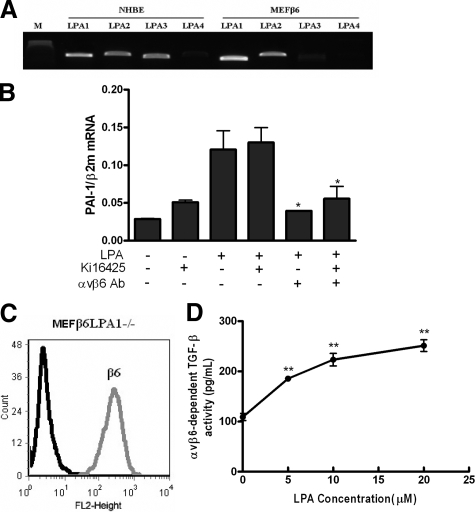
We next sought to determine which LPA receptor was responsible for transducing LPA to αvβ6-mediated TGF-β activation. Having shown that both NHBE and MEFβ6 cells were able to activate TGF-β in an αvβ6 integrin-dependent manner, we first assessed which LPA receptors were present in NHBE and MEFβ6 cells. By RT-PCR LPA1, LPA2, and LPA3 receptors were all expressed in NHBE cells. LPA1 and LPA2 receptors were the predominant receptors expressed in MEFβ6, although there was low level expression of LPA3 (Figure 7A). To examine the role of LPA1 and LPA3, we stimulated NHBE cells with 10 μmol/L LPA in the presence of the LPA receptor inhibitor Ki16425, which selectively antagonizes LPA1 and LPA3,43 and determined TGF-β activation by quantifying PAI-1 mRNA levels by real-time PCR. Ten μmol/L LPA lead to a significant increase in PAI-1 expression that was not inhibited by 10 μmol/L Ki16425, however an αvβ6 blocking antibody reduced PAI-1 mRNA expression to control levels in the presence or absence of Ki16425 (Figure 7B). Similar results were obtained in MEFβ6 cells using Smad2 phosphorylation as a readout of TGF-β activation (data not shown). Furthermore, stimulation with increasing concentrations of LPA in MEFs from lpa1−/− mice infected with the β6 integrin subunit (MEFβ6LPA1−/−), expressing high levels of cell surface β6 integrin (Figure 7C), caused concentration-dependent increases in αvβ6 integrin-mediated TGF-β activity (Figure 7D).
Figure 7.
LPA-induced, αvβ6-dependent, TGF-β activity is not mediated via LPA1 and LPA3. A: Detection of LPA receptors expressed in NHBE and MEFs expressing human β6 integrin (MEFβ6) by RT-PCR. B: NHBE cells were exposed to 10 μmol/L LPA in the presence or absence of an αvβ6 integrin blocking antibody, following pre-incubation with Ki16425 or DMSO control. TGF-β activity was determined by measuring PAI-1 mRNA levels by real-time RT-PCR. Data are presented as the mean + SEM. *P < 0.05 comparison with only LPA stimulation group. C: MEFs null for the LPA1 receptor infected with the wild-type human β6 integrin (MEFβ6LPA1−/−) were assessed for cell surface β6 integrin expression by flow cytometry, and compared with uninfected controls. D: αvβ6-dependent TGF-β activity following stimulation of MEFβ6LPA1−/− cells with increasing concentrations of LPA was measured by coculture assay. αvβ6 integrin-mediated TGF-β activity was calculated from a standard curve. Data presented as mean + SEM. **P < 0.01 comparison with 0 μmol/L group. All experiments were performed in triplicate and a representative example is shown.
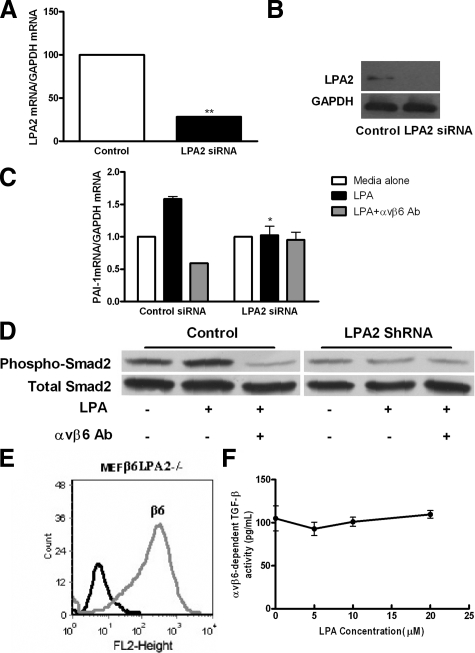
These data suggested that LPA2 would be the receptor mediating LPA to αvβ6 integrin signals. To test this hypothesis, NHBE cells transduced with siRNA specific to human LPA2 were used and αvβ6 integrin-mediated TGF-β activity in response to stimulation with LPA was measured. Real-time PCR and Western blotting confirmed that LPA2 expression was significantly reduced following transduction of NHBE cells with LPA2 siRNA, as compared with non-targeted control cells at both the mRNA and protein level (Figure 8A and 8B respectively). Following transduction of NHBE cells with LPA, siRNA cells were stimulated with 10 μmol/L LPA and PAI-1 gene expression measured by real-time PCR (Figure 8C). LPA increased PAI-1 gene expression in cells transduced with control siRNA that was blocked by an αvβ6 integrin-blocking antibody. Cells transduced with LPA2 siRNA showed no increase in PAI-1 gene expression, and no response to αvβ6 integrin-blocking antibody. Similar experiments were performed in MEFβ6 cells and again LPA failed to induce PAI-1 expression in the absence or presence of αvβ6 antibody in MEFβ6 cells transfected with LPA2 shRNA, as compared with non-targeted control (supplemental Figure S2, see http://ajp.amjpathol.org). Furthermore, LPA-induced Smad2 phosphorylation in MEFβ6 cells was also inhibited by LPA2 shRNA without affecting total Smad2 levels (Figure 8D). To confirm the role of LPA2 as the primary receptor in mediating LPA to integrin signals, MEFs from lpa2−/− mice were infected with the β6 integrin subunit (MEFβ6LPA2−/−), which was again expressed at high levels on the cell surface (Figure 8E). Stimulating MEFβ6LPA2−/− cells with increasing concentrations of LPA did not further enhance αvβ6 integrin-mediated TGF-β activity (Figure 8F). Taken together, these data suggest that LPA2 is the major receptor involved in LPA-induced, αvβ6-mediated, TGF-β activation.
Figure 8.
The LPA2 receptor mediates LPA-induced, αvβ6-dependent TGF-β activity. A: NHBE cells transfected with siRNA against LPA2 demonstrate knockdown of LPA2 RNA expression by real-time RT-PCR, and (B) protein expression by immunoblot. **P < 0.01 comparison with control siRNA. C: Following transfection of NHBE cells with siRNA against LPA2 and control siRNA, αvβ6 integrin-mediated TGF-β activity following stimulation with LPA was assessed by measuring PAI-1 mRNA levels by real-time PCR. *P < 0.05, comparison with media alone group. D: Following transfection of MEFβ6 cells with shRNA against LPA2, TGFβ activation was assessed by phospho-Smad2 immunoblot in the presence or absence of αvβ6 integrin-blocking antibodies. Total Smad2 levels were assessed as control. E: MEFs null for the LPA2 receptor transfected with the wild-type human β6 integrin (MEFβ6LPA2−/−) were assessed for cell surface β6 integrin expression by flow cytometry, and compared with uninfected controls. F: αvβ6-dependent TGF-β activity following stimulation of MEFβ6LPA2−/− cells with increasing concentrations of LPA was measured by coculture assay. αvβ6 integrin-mediated TGF-β activity was calculated. Data presented as mean + SEM All experiments were performed in triplicate and a representative example is shown.
The LPA2 and the αvβ6 Integrin are Co-Expressed during the Development of Bleomycin-Induced Pulmonary Fibrosis and Idiopathic Pulmonary Fibrosis
To determine whether the signal pathway from LPA receptor to αvβ6 integrin may be important in vivo we initially used the bleomycin model of lung fibrosis. We quantified levels of the β6 integrin subunit and LPA2 receptor following lung injury by real time PCR in lung homogenates (Figure 9) and the cellular localization of the these molecules was determined by immunohistochemistry (Figure 10). Bleomycin-induced lung injury lead to a marked increase in mRNA levels of both the β6 integrin subunit and LPA2 receptor in lung homogenates 21 days following injury (Figure 9). In uninjured control lung (Figure 10A), there was very low-level expression of the αvβ6 integrin (Figure 10C) and LPA2 receptor (Figure 10E). However, following lung injury there were areas of established fibrosis after 21 days (Figure 10B) and these regions were associated with increased expression of both αvβ6 integrin (Figure 10D) and the LPA2 receptor (Figure 10F) that colocalized in the lung epithelium. To determine whether a similar effect was observed in patients with idiopathic pulmonary fibrosis, specimens of lung tissue from biopsies showing UIP were analyzed and compared with controls. In normal human lung tissue samples (Figure 11A) αvβ6 integrin (Figure 11C) and LPA2 (Figure 11E) are expressed at low levels. However in areas of fibrosis from patients with IPF (Figure 11B) there is a large up-regulation of αvβ6 integrins in the epithelium (Figure 11D) and also a large up-regulation of LPA2, which localizes both to the epithelium and mesenchymal cells within regions of fibrosis (Figure 11F) in all samples assessed. However there is clear co-expression of both αvβ6 integrins (Figure 11G) and LPA2 (Figure 11H) in epithelial cells at the edge of areas of dense fibrosis. These results suggest that the LPA2 receptor could mediate LPA signaling to αvβ6 integrins during the development of pulmonary fibrosis.
Figure 9.
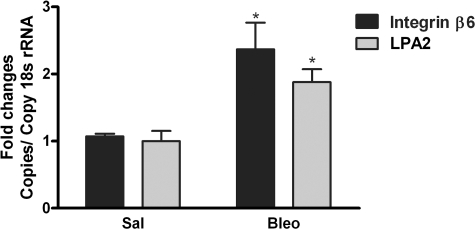
Bleomycin-induced pulmonary fibrosis is associated with increased expression of the LPA2 receptor and the αvβ6 integrin. Lung homogenates were analyzed for LPA2 and β6 integrin subunit gene expression 21 days following bleomycin instillation and compared with control. mRNA was quantified by real-time PCR and normalized to 18s RNA. *P < 0.05 comparison with saline group, n = 3 saline, n = 3 bleomycin.
Figure 10.
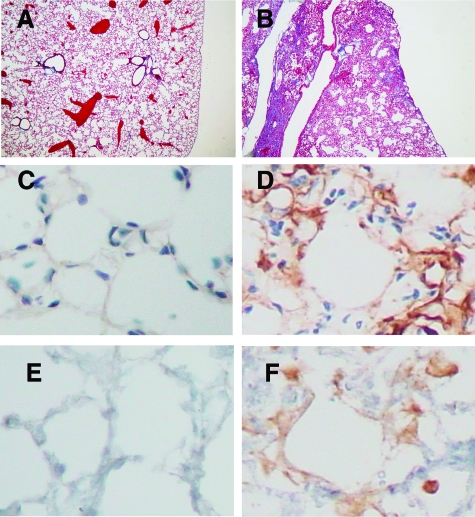
LPA2 receptor and the αvβ6 integrin co-associate in regions adjacent to areas of fibrosis following bleomycin instillation. Paraffin-embedded lung sections were obtained 21 days after bleomycin instillation, or saline control. Immunohistochemistry for the αvβ6 integrin, and LPA2 receptor was performed. Representative sections of control lung (A, C, E) show no immunostaining for αvβ6 integrin (C) or LPA2 (E). In bleomycin-instilled mice, matrix deposition is observed on Masson’s Trichrome staining (B) and there is a robust increase in alveolar αvβ6 integrin expression (D), and in parallel sections LPA2 expression is up-regulated in alveolar cells associated with areas of lung injury and fibrosis (F). Masson’s trichrome; magnification = original × 100. Immunostaining; magnification = original × 400.
Figure 11.
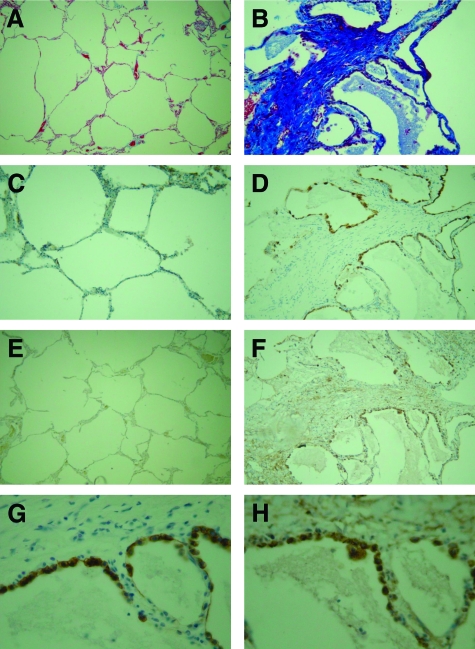
LPA2 receptor and the αvβ6 integrin are increased and co-associate in regions of fibrosis in samples from patients with IPF. Paraffin-embedded lung sections were obtained from patients that had undergone lung biopsy for IPF (n = 7), or non-fibrotic control (n = 4). Masson’s trichrome staining was performed to assess fibrosis (A and B). Immunohistochemistry for the αvβ6 integrin, and LPA2 receptor was performed. Representative sections of control lung (A, C, E) show no immunostaining for αvβ6 integrin (C) or LPA2 (E). In areas of fibrosis from UIP patient sections, matrix deposition was observed on Masson’s Trichrome staining (B). There was a robust increase in alveolar αvβ6 integrin expression (D) and a smaller increase in LPA2 expression in alveolar cells associated with areas of fibrosis (F). However, LPA2 was also up-regulated in mesenchymal cells within regions of fibrosis. Magnification = original × 100. At higher power there was clear colocalization of both (G) the αvβ6 integrin and (H) the LPA2 receptor to alveolar epithelial cells lining areas adjacent to dense fibrosis. (Magnification = original × 400).
Discussion
We have previously shown that during the development of lung injury the αvβ6 integrin must be activated by inflammatory mediators in order for the αvβ6 integrin to activate TGF-β.12,14 Previous data highlighted that thrombin and synthetic agonists of the GPCR PAR1 induce αvβ6 integrin-mediated TGF-β activation in epithelial cells and that this was important in the pathogenesis of acute lung injury.12 Whether the effect of PAR1 agonists on αvβ6 integrin-mediated TGF-β activation was indicative of GPCRs in general, or how GPCR agonists might signal to the αvβ6 integrin to induce TGF-β activation in epithelial cells were unknown. Having shown that PAR1 stimulation of fibroblasts engineered to express the αvβ6 integrin leads to TGF-β activation via RhoA, we used the potent RhoA activator LPA in these studies. We confirmed that LPA is able to induce αvβ6 integrin-mediated TGF-β activation in epithelial cells via a RhoA and Rho kinase pathway. We discovered that the LPA signal to the αvβ6 integrin is mediated via the LPA2 receptor. Furthermore, we demonstrate that agonist-induced, αvβ6 integrin-mediated, TGF-β activity requires Gαq. Finally we show that the expression of LPA2 and αvβ6 integrin is spatially associated in the lung, and these molecules are increased in areas of fibrosis following bleomycin instillation and in regions of fibrosis in patients with usual interstitial pneumonia. These studies indicate that GPCR agonists induce αvβ6 integrin-mediated TGF-β activation in epithelial cells via LPA2, Gαq, RhoA and Rho kinase, and that this pathway could contribute to the pathogenesis of pulmonary fibrosis.
LPA receptors are GPCRs that couple to the heterotrimeric G proteins Gαi, Gαq, and Gα12/13.44 In wild-type MEFs engineered to express the αvβ6 integrin, LPA induced αvβ6-mediated TGF-β activation, which was not inhibited by the Gαi inhibitor pertusis toxin. Previous data in MEFs,12 as well as the data in this study, suggest RhoA is a critical mediator in αvβ6 integrin-mediated TGF-β activation. It is therefore not surprising that Gαi is not central to this pathway, as Gαi is believed to activate Rac1, but not RhoA, via Gβγ-dimers and involvement of phosphoinositide 3-kinase.45,46 It is well established that the G proteins Gα12 and Gα13 can lead to activation of RhoA.47,48 We therefore hypothesized that the LPA to αvβ6 signal would be via a Gα12/13 pathway. Using cells null for the Gα12 and Gα13 proteins again engineered to express the αvβ6 integrin we demonstrated that basal αvβ6 integrin-mediated TGF-β activation was low, consistent with the low basal RhoA activity observed in these cells.49 However, somewhat surprisingly, we found that LPA stimulation of gα12/13 null cells potently induced αvβ6-mediated TGF-β activation. In contrast, there was no response to LPA stimulation in Gαq null cells. The possibility that gαq null cells have constitutively high RhoA activity, and therefore could not be further activated, was considered but thought unlikely. The RhoA activity in gαq null cells has been previously assessed and the basal activity is similar to wild-type MEFs.49 However LPA stimulation of wild-type cells induced higher levels of RhoA activation than stimulation of gαq−/− MEFs.49 These data are consistent with our observations that wild-type and gαq−/− MEFs have similar basal αvβ6 integrin-mediated TGF-β activity, but that only wild-type MEFs can respond to LPA stimulation by increasing TGF-β activation.
To ensure that the observations in MEFs were robust and did not reflect the effects of redundancy or compensation, we stimulated primary epithelial cells with LPA in the presence of a specific inhibitor of Gαq, GP-2A.42,50,51 The finding that GP-2A inhibited αvβ6-dependent TGF-β activity in a concentration-dependent manner supports the hypothesis that Gαq is responsible for LPA-induced αvβ6 integrin-mediated TGF-β. Gαq can activate RhoA in a number of cells including MEFs,49 platelets52 and endothelial cells,53,54,55 although as far as we are aware this is the first report of this effect in primary epithelial cells.
It is intriguing that Gα12/13−/− MEFs engineered to express the αvβ6 integrin have low levels of basal αvβ6-mediated TGF-β activation when compared with both Gαq−/− and wild-type MEFs, despite similar, high levels of total TGF-β. Furthermore, the level of TGF-β generated by fibroblasts is in considerable excess of that which can be activated by the αvβ6 integrin. These observations suggest that the amount of total TGF-β generated by these cells is not limiting the magnitude of LPA-induced, αvβ6- mediated TGF-β activation. It is currently hypothesized that integrin-mediated TGF-β activation is controlled by contractile forces transmitted to the cytoplasmic domain of the integrin that is bound to the small latent TGF-β complex, which in turn is tethered either to the cell surface or extracellular matrix via LTBP1.14,25,26 It is therefore possible that Gα12/13−/− MEFs have low basal αvβ6 integrin mediated TGF-β activity because of reduced basal cell contraction as a consequence of reduced basal RhoA activity.49 However, given the wide variability in basal αvβ6- mediated TGF-β activation among MEF cell lines, it is difficult to draw mechanistic conclusions from this observation.
Having determined that Gαq was central for agonist induced αvβ6 integrin-mediated TGF-β activation, we wanted to determine which LPA receptor was responsible for coupling to Gαq and promoting αvβ6 integrin-mediated TGF-β activation. Using a number of techniques, we highlighted that LPA2 was expressed at a high level in the appropriate cell type (ie, lung epithelial cells) both in vivo and in vitro. Both MEFs from lpa2−/− mice infected with human β6, as well as MEFβ6’s transduced with shRNA directed against LPA2 inhibited LPA-induced, αvβ6 integrin-mediated, TGF-β activation. In contrast, the use of an LPA1 and 3 inhibitor and MEFs from lpa1−/− mice, infected with human β6, both failed to inhibit it. There are five LPA receptors1,2,3,4,5 and mice null for the LPA1 and LPA2 receptor have been generated. LPA1 expression is widespread throughout the body. There is 50% embryological lethality in lpa1−/− mice whereas LPA2 expression is low in normal tissue, and lpa2−/− mice displayed no obvious phenotypic abnormalities.56,57 Recently it has been shown that lpa1−/− mice are protected from bleomycin-induced fibrosis. The proposed mechanism for this is via reduced vascular leak and fibroblast chemotaxis with LPA1 coupling to the small G protein Gαi.33 This would be consistent with our observations that LPA1 is highly expressed in vascular endothelium and submucosal layer of the larger airways (data not shown). LPA has also been shown to induce IL-8-mediated pulmonary inflammation when instilled into the lungs of mice;31 again this is thought to be mediated via LPA1 and Gαi.31,58 It would appear, therefore, that LPA2 maybe responsible for coupling to Gαq in epithelial cells leading to αvβ6 integrin-mediated TGF-β activation, whereas LPA1 may preferentially signal via Gαi in mesenchymal cells.
The effect of LPA release following lung injury could, therefore, be to induce profibrotic signals through both receptors, with LPA2 promoting TGF-β activation and matrix synthesis, and LPA1 promoting inflammation and vascular leak. It is also possible that the effects of LPA could be cell type specific. We have observed high levels of LPA2 in areas of dense fibrosis from patients with IPF, both in epithelial cells associated with increased αvβ6 integrin expression, and also in mesenchymal cells. Recent data has suggested that cell traction in myofibroblasts, a key cell type in the pathogenesis of idiopathic pulmonary fibrosis, leads to αvβ5-mediated TGF-β activation.26 It is also known that other activators of RhoA such as thrombin can both activate TGF-β via the αvβ6 integrin and promote fibrogenesis.12,59,60 It is therefore possible that following lung injury, RhoA activators may act to promote pulmonary fibrosis, through structural cell contraction and TGF-β activation by various different integrins, in a cell-specific manner, although studies to test this hypothesis in vivo have yet to be performed.
Our results demonstrate for the first time that LPA can induce αvβ6-mediated TGF-β activation in human epithelial cells via a Gαq-mediated RhoA activation pathway. We present data that spatially and temporally associates the LPA2 receptor and αvβ6 integrin during the development of lung fibrosis. These data highlight the potential relevance of this pathway to the development of disease.
Supplementary Material
Acknowledgments
The authors thank Dr. Stefan Offermanns for the gift of gαq−/− and gα12/13−/− MEFs, Dr. Jerold Chun for the gift of lpa1−/− and lpa2−/− MEFs, and Dr. Rifkin for the gift of TMLC cells. Thanks also to Dr. Susan Anderson (University of Nottingham) and Dr. Maddy Parsons (Kings College London) for advice and help with immunofluorescence.
Footnotes
Address reprint requests to Dr. Gisli Jenkins, Centre for Respiratory Research, University of Nottingham, Clinical Science Building, Nottingham City Hospital, Hucknal Road, Nottingham, NG5 1PB, UK. E-mail: Gisli.Jenkins@Nottingham.ac.uk.
Supported by Arthritis Research Campaign and Asthma UK to R.G.J., and NIH grants HL53949 and AI024674 to D.S.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of S.M.V.: Stromedix, One Canal Place, Cambridge, MA 02141.
References
- Miyazono K, Heldin CH. Role for carbohydrate structures in TGF-beta 1 latency. Nature. 1989;338:158–160. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990;110:1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- Brown PD, Wakefield LM, Levinson AD, Sporn MB. Physicochemical activation of recombinant latent transforming growth factor-beta’s 1, 2, and 3. Growth Factors. 1990;3:35–43. doi: 10.3109/08977199009037500. [DOI] [PubMed] [Google Scholar]
- Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc. 2006;3:413–417. doi: 10.1513/pats.200601-008AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol. 2008;40:1068–1078. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha (v) beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis M, Reizis B, Melton A, Masteller E, Tang Q, Proctor J, Wang Y, Bernstein X, Huang X, Reichardt L, Bluestone J, Sheppard D. Loss of integrin alpha (v) beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol. 2005;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- Saharinen J, Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins. LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol Biol Cell. 2000;11:2691–2704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991;10:1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes I, Gleizes PE, Metz CN, Rifkin DB. Latent transforming growth factor-beta binding protein domains involved in activation and transglutaminase-dependent cross-linking of latent transforming growth factor-beta. J Cell Biol. 1997;136:1151–1163. doi: 10.1083/jcb.136.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio E, Gaudry C, Gross S, Smith C, Downes S, Griffin M. Regulation of cell surface tissue transglutaminase: effects on matrix storage of latent transforming growth factor-beta binding protein-1. J Histochem Cytochem. 1999;47:1417–1432. doi: 10.1177/002215549904701108. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ali T, Todorovic V, O'Leary JM, Kristina Downing A, Rifkin DB. Amino acid requirements for formation of the TGF-beta-latent TGF-beta binding protein complexes. J Mol Biol. 2005;345:175–186. doi: 10.1016/j.jmb.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Drews F, Knobel S, Moser M, Muhlack KG, Mohren S, Stoll C, Bosio A, Gressner AM, Weiskirchen R. Disruption of the latent transforming growth factor-beta binding protein-1 gene causes alteration in facial structure and influences TGF-beta bioavailability. Biochim Biophys Acta. 2008;1783:34–48. doi: 10.1016/j.bbamcr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Colarossi C, Obata H, Zambuto L, Perle MA, Rifkin DB. Bone abnormalities in latent TGF-β binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF-β bioavailability. J Cell Biol. 2002;156:227–232. doi: 10.1083/jcb.200111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, von Melchner H. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16:2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wu J, Zhu W, Pytela R, Sheppard D. Expression of the human integrin beta6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lung inflammation in beta6 knockout mice. Am J Respir Cell Mol Biol. 1998;19:636–642. doi: 10.1165/ajrcmb.19.4.3293. [DOI] [PubMed] [Google Scholar]
- Annes J, Chen Y, Munger J, Rifkin D. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291(Pt 3):677–680. doi: 10.1042/bj2910677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georas SN, Berdyshev E, Hubbard W, Gorshkova IA, Usatyuk PV, Saatian B, Myers AC, Williams MA, Xiao HQ, Liu M, Natarajan V. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy. 2007;37:311–322. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- Ediger TL, Toews ML. Synergistic stimulation of airway smooth muscle cell mitogenesis. J Pharmacol Exp Ther. 2000;294:1076–1082. [PubMed] [Google Scholar]
- Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, Watkins T, He D, Saatian B, Natarajan V. Protein kinase Cdelta mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- Cerutis DR, Nogami M, Anderson JL, Churchill JD, Romberger DJ, Rennard SI, Toews ML. Lysophosphatidic acid and EGF stimulate mitogenesis in human airway smooth muscle cells. Am J Physiol. 1997;273:L10–L15. doi: 10.1152/ajplung.1997.273.1.L10. [DOI] [PubMed] [Google Scholar]
- Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, Dolinski BM, Pearse BR, Yokota Y, Kawakatsu H, Atakilit A, Sheppard D, Violette SM. Function-blocking integrin alphavbeta6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem. 2004;279:17875–17887. doi: 10.1074/jbc.M312103200. [DOI] [PubMed] [Google Scholar]
- Yang M, Zhong WW, Srivastava N, Slavin A, Yang J, Hoey T, An S. G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the β-catenin pathway. Proc Natl Acad Sci USA. 2005;102:6027–6032. doi: 10.1073/pnas.0501535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res. 2006;32:181–199. doi: 10.1080/01902140600817465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) adopted by the ATS board of directors and ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- Aktories K, Wilde C, Vogelsgesang M. Rho-modifying C3-like ADP-ribosyltransferases. Rev Physiol Biochem Pharmacol. 2004;152:1–22. doi: 10.1007/s10254-004-0034-4. [DOI] [PubMed] [Google Scholar]
- Ikenoya M, Hidaka H, Hosoya T, Suzuki M, Yamamoto N, Sasaki Y. Inhibition of rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J Neurochem. 2002;81:9–16. doi: 10.1046/j.1471-4159.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- Mukai H, Munekata E, Higashijima T. G protein antagonists. A novel hydrophobic peptide competes with receptor for G protein binding, J Biol Chem. 1992;267:16237–16243. [PubMed] [Google Scholar]
- Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, Yagi M, Sato M, Suzuki R, Murooka H, Sakai T, Nishitoba T, Im DS, Nochi H, Tamoto K, Tomura H, Okajima F. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–465. doi: 10.1016/j.semcdb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Vogt S, Grosse R, Schultz G, Offermanns S. Receptor-dependent RhoA activation in G12/G13-deficient cells: genetic evidence for an involvement of Gq/G11. J Biol Chem. 2003;278:28743–28749. doi: 10.1074/jbc.M304570200. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y, Owyang C. The regulatory site of functional GTP binding protein coupled to the high affinity cholecystokinin receptor and phospholipase A2 pathway is on the G beta subunit of Gq protein in pancreatic acini. Biochem Biophys Res Commun. 1995;211:648–655. doi: 10.1006/bbrc.1995.1861. [DOI] [PubMed] [Google Scholar]
- Hunt RA, Bhat GJ, Baker KM. Angiotensin II-stimulated induction of sis-inducing factor is mediated by pertussis toxin-insensitive G(q) proteins in cardiac myocytes. Hypertension. 1999;34:603–608. doi: 10.1161/01.hyp.34.4.603. [DOI] [PubMed] [Google Scholar]
- Moers A, Wettschureck N, Gruner S, Nieswandt B, Offermanns S. Unresponsiveness of platelets lacking both Galpha(q) and Galpha(13). Implications for collagen-induced platelet activation. J Biol Chem. 2004;279:45354–45359. doi: 10.1074/jbc.M408962200. [DOI] [PubMed] [Google Scholar]
- Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J Biol Chem. 2001;276:22614–22620. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J Biol Chem. 2007;282:7833–7843. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Mehta D, Kozasa T, Minshall RD, Malik AB. Protein kinase Calpha-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J Biol Chem. 2003;278:28793–28798. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- Contos JJ, Chun J. Genomic characterization of the lysophosphatidic acid receptor gene, lp(A2)/Edg4, and identification of a frameshift mutation in a previously characterized cDNA. Genomics. 2000;64:155–169. doi: 10.1006/geno.2000.6122. [DOI] [PubMed] [Google Scholar]
- Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol Cell Biol. 2002;22:6921–6929. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatian B, Zhao Y, He D, Georas SN, Watkins T, Spannhake EW, Natarajan V. Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells. Biochem J. 2006;393:657–668. doi: 10.1042/BJ20050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC, Goldsack NR, Marshall RP, McAnulty RJ, Starke R, Purdy G, Laurent GJ, Chambers RC. Direct thrombin inhibition reduces lung collagen, accumulation, and connective tissue growth factor mRNA levels in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2001;159:1383–1395. doi: 10.1016/S0002-9440(10)62525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC, Johns RH, Lasky JA, Shan B, Scotton CJ, Laurent GJ, Chambers RC. Absence of proteinase-activated receptor-1 signaling affords protection from bleomycin-induced lung inflammation and fibrosis. Am J Pathol. 2005;166:1353–1365. doi: 10.1016/S0002-9440(10)62354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.