Abstract
The pentraxin superfamily is a group of evolutionarily conserved proteins that play important roles in the immune system. The long pentraxin PTX3 protein was originally described as able to be induced by pro-inflammatory stimuli in a variety of cell types. In this study, we evaluated the phenotype of Ptx3−/− mice subjected to ischemia followed by reperfusion of the superior mesenteric artery. In reperfused wild-type mice, there was significant local and remote injury as demonstrated by increases in vascular permeability, neutrophil influx, nuclear factor-κB activation, and production of CXCL1 and tumor necrosis factor-α. PTX3 levels were elevated in both serum and intestine after reperfusion. In Ptx3−/− mice, local and remote tissue injury was inhibited, and there were decreased nuclear factor-κB translocation and cytokine production. Intestinal architecture was preserved, and there were decreased neutrophil influx and significant prevention of lethality in Ptx3−/− mice as well. PTX3 given intravenously before reperfusion reversed the protection observed in Ptx3−/− mice in a dose-dependent manner, and PTX3 administration significantly worsened tissue injury and lethality in wild-type mice. In conclusion, our studies demonstrate a major role for PTX3 in determining acute reperfusion-associated inflammation, tissue injury, and lethality and suggest the soluble form of this molecule is active in this system. Therapeutic blockade of PTX3 action may be useful in the control of the injuries associated with severe ischemia and reperfusion syndromes.
The long pentraxin 3 (PTX3) is a member of the pentraxin superfamily, a group of highly conserved proteins characterized by multimeric, usually pentameric structures that stack to form decamers. PTX3 was originally identified as a tumor necrosis factor (TNF)-α-stimulated gene in fibroblasts1 and as a pentraxin family gene from endothelial cells stimulated with interleukin (IL)-1.2 PTX3 is released by a range of cell types, including myeloid dendritic cells, endothelial cells, epithelial cells, mononuclear phagocytes, smooth muscle cells, adipocytes, fibroblasts, synovial cells, and chondrocytes, in response to diverse inflammatory signals, including cytokines, lipopolysaccharide, lipoarabinomannans, and other TLR agonists.3,4 The exact molecular pathways underlying the actions of PTX3 in vivo are not entirely known but several mechanisms have been suggested to explain the actions of the protein, including: i) direct binding to pathogens, thus, functioning as a soluble pattern recognition receptor5; ii) binding and limiting the function of C1q6; and iii) binding to apoptotic cells and modifying their uptake by phagocytic cells.7 A putative PTX3 receptor has also been suggested3 but has not yet been reported.
A few studies have now demonstrated that the concentration of PTX3 is elevated in serum of patients undergoing an ischemic event; the level of PTX3 in serum was increased in patients with unstable angina,8 it was an early indicator of myocardial infarction,9 and it predicted 3-month mortality after an acute myocardial event.10 In experimental animals, there is rapid expression of PTX3 after reperfusion of the ischemic superior mesenteric artery.11 More importantly, overexpression of PTX3, as observed in transgenic mice harboring extra copies of PTX3, was accompanied by an enhancement of lethality and tissue injury after intestinal ischemia and reperfusion (I/R).11 Increased injury and lethality were associated with an exacerbated inflammatory response and production of pro-inflammatory cytokines, including TNF-α. Importantly, administration of a soluble TNFR1 prevented the exacerbated lethality suggesting that overexpression of PTX3 induced exacerbated reperfusion-induced tissue injury and lethality by modulating the production of TNF-α.11 The present study was designed to investigate whether endogenous production of PTX3 would have a disease-modifying effect on reperfusion injury, as described previously in animals overexpressing the protein. We also investigated whether the soluble form of PTX3 was relevant in mediating tissue inflammation and injury in this system. To this end, we evaluated tissue inflammation and injury, systemic production of cytokines, and lethality in wild-type and PTX3-deficient (Ptx3−/−) mice subjected to I/R of the superior mesenteric artery. We then tested whether exogenous administration of PTX3 could alter the tissue injury in Ptx3−/− mice and in wild-type mice.
Materials and Methods
Mice
Generation and characterization of Ptx3−/− mice were described earlier.12 Briefly, gene targeting by homologous recombination was performed to generate mice carrying a null mutation obtained by the deletion of exons 1 and 2 of the Ptx3 gene, thereby preventing any Ptx3 mRNA and PTX3 protein from being synthesized. For all experiments, female Ptx3−/− mice of 6 to 8 weeks of age on a 129/Sv-C57BL/6 mixed background and wild-type controls of the same age and background were used. Mice were maintained in individually ventilated cages housed in a clean, temperature-controlled room, and received sterile food and acidic water ad libitum. The experimental protocols were approved by the Animal Care and Use Committee of Universidade Federal de Minas Gerais.
Ischemia and Reperfusion
Details of the procedure are described elsewhere.11 Mice were anesthetized with urethane (1400 mg/kg, i.p.) and laparotomy was performed. The superior mesenteric artery was isolated and ischemia was induced by totally occluding the superior mesenteric artery for 60 minutes. For measuring percentage of surviving mice, reperfusion was re-established and the number of surviving animals recorded for the indicated time periods. For the other parameters, reperfusion was allowed to occur for 30 minutes (I60R30) and mice were then sacrificed. This time of reperfusion (30 minutes) was chosen based on the presence of significant tissue injury without unduly high mortality rates. Sham-operated animals were used as controls. In a few experiments, recombinant human PTX3 (hPTX3, 1 to 25 mg/kg) or vehicle [phosphate-buffered saline (PBS) with similar amount of bovine serum albumin (BSA)] was given to wild-type or Ptx3−/− mice intravenously 10 minutes before reperfusion. Recombinant human PTX3 was purified as described13 and contained less than 0.125 endotoxin units per ml as determined by the amebocyte lysate assay.
Evaluation of Changes in Vascular Permeability
The extravasation of Evans blue dye into the tissue was used as an index of increased vascular permeability, as previously described.14 Briefly, Evans blue dye (20 mg/kg) was administered intravenously (1 ml/kg) via a tail vein 5 minutes before reperfusion of the ischemic artery. A segment of the duodenum (∼5 cm) or flushed left lung was obtained 30 minutes after reperfusion and allowed to dry at 37°C for 24 hours in a Petri dish. The dry weight of the tissue was recorded and Evans blue extracted using formamide. The amount of Evans blue in the tissue was obtained by comparing the extracted absorbance with that of a standard Evans blue curve read at 620 nm in an enzyme-linked immunosorbent assay plate reader. Results are presented as μg of Evans blue per 100 mg of tissue.
Myeloperoxidase Concentrations
The extent of neutrophil accumulation in the intestine and right lung tissue was measured by assaying myeloperoxidase activity, as previously described.11 Briefly, a portion of duodenum and the flushed right lungs of animals that had undergone I/R injury were removed and snap-frozen in liquid nitrogen. On thawing and processing, the tissue was assayed for myeloperoxidase activity by measuring the change in optical density at 450 nm using tetramethylbenzidine. Results were expressed as the neutrophil index that denotes activity of myeloperoxidase related with casein-elicited murine peritoneal neutrophils processed in the same way.
Measurement of Hemoglobin Concentrations
The determination of hemoglobin concentrations in tissue was used as an index of tissue hemorrhage. After washing the intestines to remove excess blood, a sample of ∼100 mg of duodenum was removed and homogenized in Drabkin’s color reagent according to the instructions of the manufacturer (Analisa, Belo Horizonte, Brazil). The suspension was centrifuged for 15 minutes at 3000 × g and filtered using 0.2-μm filters. The resulting solution was read using an enzyme-linked immunosorbent assay plate reader at 520 nm and compared against a standard curve of hemoglobin.
Measurement of Cytokines in Intestine and Lungs
The concentration of TNF-α, CXCL1 (also known as keratinocyte-derived chemokine, KC), and PTX3 in samples was measured in serum and tissue of animals using commercially available antibodies and according to the procedures supplied by the manufacturer (R&D Systems, Minneapolis, MN). One hundred mg of duodenum or lung of sham-operated and reperfused animals were homogenized in 1 ml of PBS (0.4 mol/L NaCl and 10 mmol/L NaPO4) containing anti-proteases (0.1 mmol/L phenylmethyl sulfonyl fluoride, 0.1 mmol/L benzethonium chloride, 10 mmol/L ethylenediaminetetraacetic acid, and 20 KI aprotinin A) and 0.05% Tween 20. The samples were then centrifuged for 10 minutes at 3000 × g and the supernatant immediately used for enzyme-linked immunosorbent assay assays at a 1:3 dilution in PBS.
Histopathology
Mice were sacrificed after 30 minutes of mesenteric ischemia followed by 60 minutes of reperfusion. Samples of small intestine were removed, promptly rinsed in cold saline solution, and opened along the antimesenteric border and immediately prefixed in 10% buffered formalin phosphate (pH 7.4). The material was then rolled into a spiral with the mucosa facing inward, so as to form a Swiss roll similar to that described by Arantes and Nogueira.15 The rolls were routinely processed for paraffin embedding, sectioned transversely (5 m), and stained with hematoxylin and eosin.
Morphometric Analysis
The mucosal villous height for each coded slide was measured using digitalized images obtained with a ×20 objective from a light microscope (Olympus, Tokyo, Japan) adapted with a digital camera (Media Cybernetics, Silver Spring, MD). The morphometric analysis of the sections was performed on 20 well-oriented villi, arranged like fingers and perpendicular to the muscularis mucosae, by a computerized image analyzer (Image Pro-Express, Media Cybernetics) and reported in μm. The overall architecture of the wall of the small intestine mucosa and aspects of mucosal damage were also evaluated and documented. Sections were evaluated by a single pathologist (F.C.) and the following parameters graded 0 (normal) to 3 (severe): hyperemia, edema, hemorrhage, and neutrophil infiltrate. The scores of each parameter were added to obtain an inflammatory index. Results were expressed as the median of the inflammatory score. The quantification of neutrophil influx in tissue was determined separately using an integration reticule (no. 4740680000000-Netzmikrometer 12.5×; Carl Zeiss, Thornwood, NY). All cells were enumerated in 10 representative and consecutive microscopic high-power fields (×1000) and, at this magnification, each field (integration reticule) had an area of 0.015625 mm2. Descriptive analyses were expressed as mean ± SD of neutrophils per mm2.
Western Blot Analysis
Nuclear extracts were obtained from homogenized intestine, as described previously.16 Briefly, protein was quantified using the Bradford assay reagent from Bio-Rad (Hercules, CA). Nuclear extracts (20 μg) were separated by electrophoresis on a denaturing 10% polyacrylamide-sodium dodecyl sulfate gel and transferred onto nitrocellulose membranes, as previously described.17 Membranes were blocked overnight at 4°C with PBS containing 5% (w/v) nonfat dry milk and 0.1% Tween-20, washed three times with PBS containing 0.1% Tween-20, and then incubated with specific anti-p65/RelA (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) rabbit polyclonal antibodies, in phosphate-buffered saline containing 5% (w/v) BSA and 0.1% Tween-20. After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (1:3000, Santa Cruz Biotechnology). Immunoreactive bands were visualized by using an enhanced chemiluminescence detection system, as described by the manufacturer (GE Healthcare, Piscataway, NJ). Nuclear levels of nuclear factor (NF)-κB p65 were quantified by using densitometric analysis software (LabImage, Leipzig, Germany). Changes in protein levels with respect to control values (wild-type sham) were estimated and the results were expressed as arbitrary units.
Statistical Analysis
Results are shown as the mean ± SEM. Percent inhibition was calculated by subtracting the background levels of Evans blue extravasation or myeloperoxidase (obtained in sham-operated animals) from control and treated animals. Differences were evaluated by using analysis of variance followed by Student-Newman-Keuls posthoc analysis. Results with a P < 0.05 were considered significant. For survival curves, differences between groups were compared using log rank test and considered significant when P < 0.05. For the histopathological analysis, results are presented as median and differences between groups were compared using Kruskal-Wallis.
Results
Enhanced Levels of PTX3 in Wild-Type Mice Subjected to Intestinal I/R
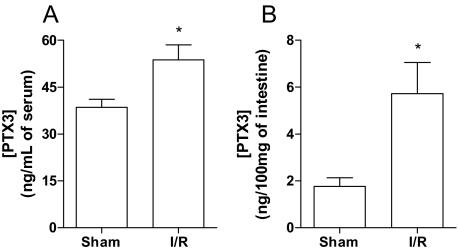
Previous studies from our laboratory have shown that Ptx3 message was enhanced in the intestine after reperfusion of the superior mesenteric artery.11 In agreement with the latter finding, the concentration of PTX3, as determined by enzyme-linked immunosorbent assay, increased significantly in serum and in the intestine of mice subjected to intestinal I/R injury (Figure 1, A and B).
Figure 1.
Enhanced levels of PTX3 in wild-type mice subjected to intestinal I/R. Wild-type mice were sham-operated (Sh) or subjected to 60 minutes of ischemia of the superior mesenteric artery followed by 30 minutes of reperfusion (I/R), when levels of PTX3 were quantified in serum (A) and intestine (B). As shown here, a significant increase of PTX3 protein was detectable after I/R injury when compared with Sh mice in both serum and intestine. *P < 0.05 when compared with sham-operated mice.
Prevention of Reperfusion-Associated Tissue Injury in Ptx3−/− Mice
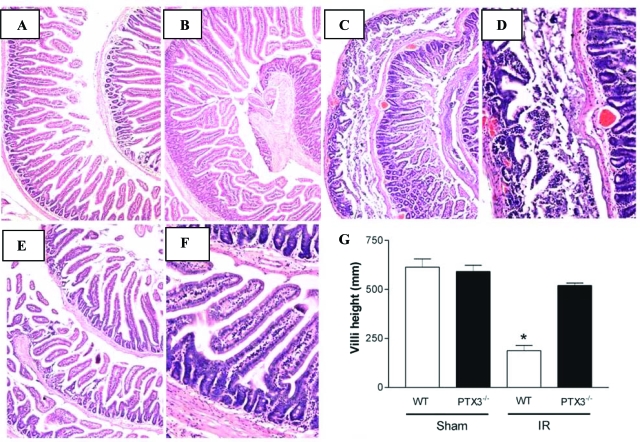
Initial experiments evaluated histopathological changes in the intestine after I/R of the superior mesenteric artery. In wild-type and Ptx3−/− sham-operated mice, the microscopic appearance of the intestinal mucosa was unaltered (Figure 2, A and B). Sixty minutes of ischemia followed by 30 minutes of reperfusion produced marked injury in intestines of wild-type mice (Figure 2, C and D). Macroscopically, wild-type animals showed significant intestinal distension, mural edema, and hemorrhage. Microscopically, there were denuded villi with exposed dilated capillaries, severe architectural distortion, lamina propria disintegration, ulceration, hemorrhagic foci, and marked influx of polymorphonuclear leukocytes (Figure 2D). There was a significant shortening of villi in reperfused wild-type mice, as assessed by morphometric analysis (Figure 2G). Ptx3−/− mice had no major macroscopic changes, and minor microscopic changes, including focal denuded villi with dilated capillaries (Figure 2, E and F). Moreover, morphometric analysis showed prevention of villus shortening in Ptx3−/− mice (Figure 2G).
Figure 2.
Prevention of intestinal injury after I/R injury in Ptx3−/− mice. In wild-type (WT) (A) and Ptx3−/− (B) sham-operated mice the microscopic appearance of the intestinal mucosa was unaltered. C and D: Sixty minutes of ischemia of the superior mesenteric artery followed by 30 minutes of reperfusion produced marked injury in intestines of wild-type mice. Macroscopically wild-type animals showed significant intestinal distension and mural edema. Microscopically, denuded villi with exposed dilated capillaries, severe architectural distortion, lamina propria disintegration, ulceration, and hemorrhage foci were observed. E and F: Ptx3−/− animals did not present macroscopic changes and only minor microscopic changes such as focal denuded villi with dilated capillaries. G: Morphometric analysis of the intestine showed significant villus shortening in wild-type mice subjected to I/R. Ptx3−/− mice had no villus shortening. Results in G are shown as the mean ± SD of six animals in each group. *P < 0.05 when compared with sham-operated or Ptx3−/− mice. Original magnifications: ×40 (A, C, E); ×100 (B, D, F).
Prevention of Reperfusion-Associated Tissue Inflammation and Cytokine Production in Ptx3−/− Mice and Reversal by Administration of PTX3
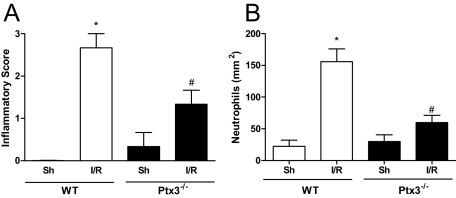
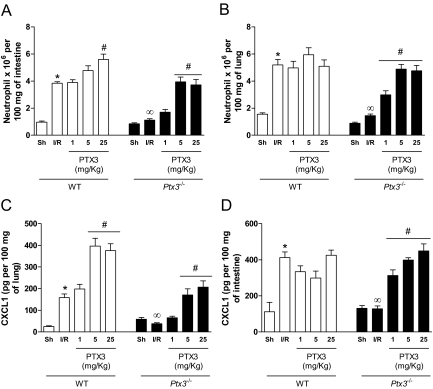
The inflammatory score of the reperfused gut, as assessed by evaluating the degree of hyperemia, edema, hemorrhage, and neutrophil infiltrate, was greatly enhanced in wild-type mice subjected to I/R injury (Figure 3A). In wild-type animals, there was a major influx of neutrophils, as quantified by morphometry (Figure 3B) or by determining tissue levels of myeloperoxidase (see below). Tissue inflammation was greatly reduced in Ptx3−/− mice, as determined by the inflammatory score (Figure 3A) and number of infiltrating neutrophils (Figure 3B).
Figure 3.
Histological assessment of inflammatory score and of neutrophil influx in the intestine after I/R injury in Ptx3−/− and wild-type mice. Wild-type (WT) or Ptx3−/− mice were sham-operated (Sh) or subjected to 60 minutes of ischemia of the superior mesenteric artery followed by 30 minutes of reperfusion (I/R), then sections were graded for inflammatory score (A) or neutrophil influx (B), as described in the Materials and Methods. Neutrophil influx is shown as number of neutrophils per mm2. Results are the mean ± SD of five mice in each group. *P < 0.01 when compared with sham-operated mice; #P < 0.01 when compared with mice subjected to I/R.
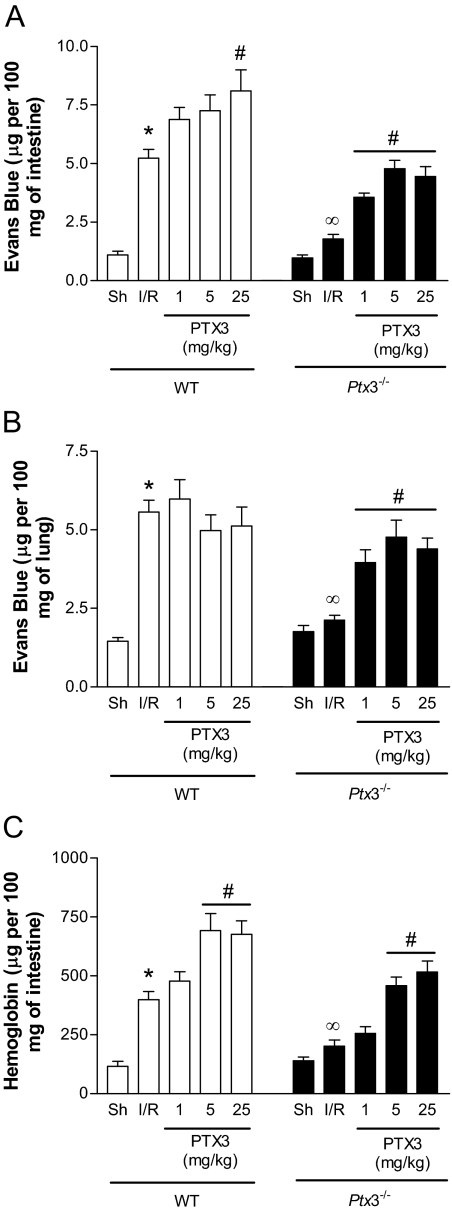
There was significant plasma extravasation, as assessed by the extravasation of Evans blue dye, in the intestine and lungs of wild-type mice submitted to intestinal I/R (Figure 4, A and B). There was also an increase in the intestinal levels of hemoglobin, a marker of hemorrhage in the intestines (Figure 4C). In Ptx3−/− mice, there were markedly lower levels of plasma extravasation in the intestine and lung and less intestinal hemorrhage (Figure 4). Intravenous injection of hPTX3 (1 to 25 mg/kg, 10 minutes before reperfusion) in Ptx3−/− mice restored, in a dose-dependent manner, the plasma extravasation and hemorrhage to levels similar to those observed in wild-type mice (Figure 4).
Figure 4.
Changes in vascular permeability and hemorrhage in the intestine and lungs after I/R injury in Ptx3−/− and wild-type mice. Wild-type (WT) or Ptx3−/− mice were sham-operated (Sh) or subjected to 60 minutes of ischemia of the superior mesenteric artery followed by 30 minutes of reperfusion (I/R), when changes in vascular permeability in the intestine (A) or lungs (B) and hemorrhage in the intestine (C) were evaluated. Recombinant human PTX3 (PTX3, 1 to 25 mg/kg) or vehicle (PBS) was given intravenously to wild-type or Ptx3−/− mice 10 minutes before reperfusion. Changes in vascular permeability are expressed as μg of Evans blue per 100 mg of tissue and hemorrhage as μg of hemoglobin per 100 mg of tissue. Results are the mean ± SEM of five mice in each group. *P < 0.01 when compared with sham; ∞P < 0.01 when comparing wild-type and Ptx3−/− mice subjected to IR; #P < 0.01 when comparing mice given PTX3 with their respective IR control.
A very similar picture was observed when neutrophil influx, as assessed by tissue myeloperoxidase content, was evaluated in the intestine and lungs of reperfused mice. Indeed, there was total inhibition of neutrophil influx into the intestine and lungs of Ptx3−/− mice after intestinal I/R (Figure 5, A and B). Again, administration of exogenous hPTX3 restored neutrophil influx in Ptx3−/− mice to levels similar to those observed in wild-type mice. Several studies have demonstrated that CXCR2-acting chemokines, such as CXCL1, are produced and play a major role in driving neutrophil influx after I/R injury.18,19,20 In our experiments, there was significant production of CXCL1 in the intestine and lungs after reperfusion of the ischemic superior mesenteric artery in wild-type but not in Ptx3−/− mice (Figure 5, C and D). Injection of hPTX3 in Ptx3−/− mice restored the intestinal and pulmonary production of CXCL1 after I/R (Figure 5, C and D).
Figure 5.
Changes in neutrophil influx and CXCL1 production in the intestine and lungs after I/R injury in Ptx3−/− and wild-type mice. Wild-type (WT) or Ptx3−/− mice were sham-operated (Sh) or subjected to 60 minutes of ischemia of the superior mesenteric artery followed by 30 minutes of reperfusion (I/R), when neutrophil influx (A and B) or production of CXCL1 (C and D) were evaluated in the intestine (A and C) or lungs (B and D). Recombinant human PTX3 (PTX3, 1 to 25 mg/kg) or vehicle (PBS) was given intravenously to wild-type or Ptx3−/− mice 10 minutes before reperfusion. Neutrophil influx was evaluated using a myeloperoxidase assay and results are shown as the relative number of neutrophils in 100 mg of tissue and CXCL1 levels as pg of the chemokine per 100 mg of tissue. Results are the mean ± SEM of five mice in each group. *P < 0.01 when compared with sham; ∞P < 0.01 when comparing wild-type and Ptx3−/− mice subjected to IR; #P < 0.01 when comparing mice given PTX3 with their respective IR control.
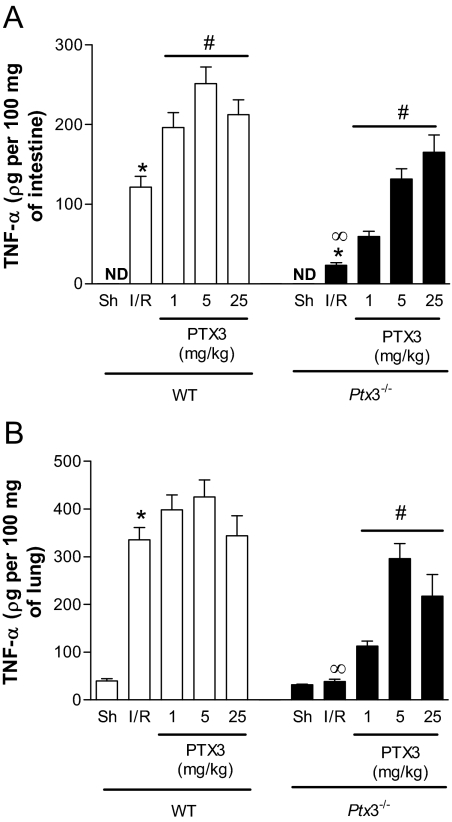
The cytokine TNF-α plays a major role in mediating I/R injury in several organs, including in the intestine.11,21 As seen in Figure 6, A and B, there was a significant production of TNF-α in the lungs and intestine of reperfused wild-type mice. In Ptx3−/− animals, TNF-α production was virtually absent but was restored by injection of recombinant hPTX3 in a dose-dependent manner (Figure 6). Overall, the protection afforded by the Ptx3−/− phenotype was fully reversed by hPTX3 given at the dose of 5 mg/kg.
Figure 6.
Changes in TNF-α production in the intestine and lungs after I/R injury in Ptx3−/− and wild-type mice. Wild-type (WT) or Ptx3−/− mice were sham-operated (Sh) or subjected to 60 minutes of ischemia of the superior mesenteric artery followed by 30 minutes of reperfusion (I/R), when production of TNF-α was evaluated in the intestine (A) or lungs (B). Recombinant human PTX3 (PTX3, 1 to 25 mg/kg) or vehicle (PBS) was given intravenously to wild-type or Ptx3−/− mice 10 minutes before reperfusion. TNF-α levels are shown as pg of the cytokine per 100 mg of tissue. Results are the mean ± SEM of five mice in each group. ND = not detected. *P < 0.01 when compared with sham; ∞P < 0.01 when comparing wild-type and Ptx3−/− mice subjected to IR; #P < 0.01 when comparing mice given PTX3 with their respective IR control.
Our previous results demonstrated that tissue inflammation and injury were enhanced in transgenic mice overexpressing PTX3.11 Consistent with the latter findings, administration of hPTX3 to wild-type mice induced an enhancement of reperfusion-associated plasma extravasation (Figure 3A), hemorrhage (Figure 4C), neutrophil influx (Figure 5A), and production of CXCL1 (Figure 5C) and TNF-α (Figure 6B) in the intestine. Nevertheless, administration of hPTX3 failed to enhance injury induced by reperfusion in the lungs of wild-type mice (Figures 3, 5, and 6).
Decreased Reperfusion-Associated NF-κB Activation in Ptx3−/− Mice
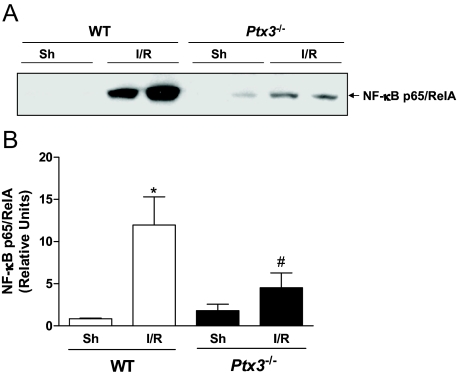
The activation of the transcriptor factor NF-κB plays a major role in driving tissue leukocyte influx and production of mediators of inflammation after I/R injury.16 In a separate series of experiments, we evaluated whether the absence of PTX3 would influence activation of this transcription factor. As seen in Figure 7, there was marked NF-κB activation, as seen by the translocation of p65/Rel A to nuclear extracts, in intestinal tissue of wild-type mice subjected to I/R. In contrast, NF-κB activation was significantly suppressed in Ptx3−/− mice (Figure 7, A and B).
Figure 7.
NF-κB activation in the intestine after I/R injury in Ptx3−/− and wild-type mice. Mice were sham operated (Sh) or subjected to ischemia (60 minutes) and reperfusion (30 minutes) injury of the superior mesenteric artery (I/R). Samples were collected and processed for extraction of nuclear proteins for Western blot analysis, as described in the Materials and Methods. A: Nuclear extracts (20 μg) were fractioned on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membrane, and then probed with anti-p65 antibody. Results shown are representative of at least three experiments with each tissue. B: Densitometric analysis by using LabImage software. Results are expressed as the means ± SEM of three mice in each group. *P < 0.05 when compared with wild-type (WT) sham; and #P < 0.05 when comparing wild-type and Ptx3−/− mice subjected to IR.
Prevention of Reperfusion-Associated Lethality in Ptx3−/− Mice and Reversal by Administration of PTX3
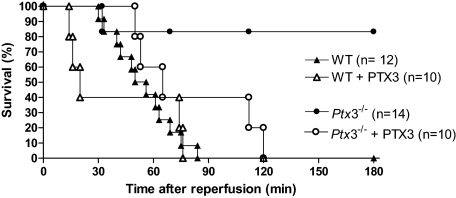
Next, we investigated whether the observed prevention of local (intestine) and remote (lung) tissue injury would be accompanied by prevention of lethality in Ptx3−/− mice. As seen in Figure 8, reperfusion of ischemic mice was accompanied by significant lethality, which was first noted 30 minutes after reperfusion. Although there was 100% death of wild-type animals at 90 minutes after reperfusion, only 20% of Ptx3−/− mice had died by 180 minutes (Figure 8, P < 0.01). Administration of hPTX3 (5 mg/kg) to Ptx3−/− mice induced lethality rates that were similar to those of wild-type mice subjected to intestinal I/R. Finally, more rapid deaths were observed in a significant proportion of wild-type mice given intravenous hPTX3 10 minutes before reperfusion of the ischemic superior mesenteric artery (Figure 8, P < 0.05).
Figure 8.
Survival after I/R injury in Ptx3−/− and wild-type mice. Wild-type (WT) or Ptx3−/− mice were subjected to 60 minutes of ischemia of the superior mesenteric artery followed by reperfusion and the time of death recorded. Recombinant human PTX3 (PTX3, 5 mg/kg) or vehicle (PBS) was given intravenously to wild-type or Ptx3−/− mice 10 minutes before reperfusion. There were significantly (P < 0.01, log rank test) fewer deaths in Ptx3−/− mice subjected to I/R and treatment with PTX3 resulted in significant (P < 0.05, log rank test) loss of protection. Numbers are shown.
Discussion
Intestinal I/R injury is a condition of high morbidity and mortality and may be secondary to several diseases, including abdominal aortic aneurysm, small bowel transplantation, strangulated hernia, and neonatal necrotizing enterocolitis.22 We have previously shown that there is a rapid production of PTX3 in tissues after intestinal I/R injury11 and this was confirmed here by showing elevated levels of PTX3 protein in the intestine and serum of reperfused animals. Elevated levels of PTX3 were also found in serum and correlated with 3-month survival after acute myocardial infarction in humans.9,10 In the present study, we report for the first time that absence of PTX3, as observed in Ptx3−/− mice, is accompanied by greatly diminished tissue inflammation and injury and decreased lethality after reperfusion of the ischemic superior mesenteric artery in mice. The latter data suggest that, although PTX3 is only detected in the low nmol/L range levels in tissues and serum, it is indeed relevant for tissue injury. Importantly, we demonstrate that exogenous PTX3 restored inflammatory responsiveness, tissue injury, and lethality in Ptx3−/− mice and enhanced injury in wild-type mice to levels observed in PTX3-transgenic mice.11 In Ptx3−/− mice, the activation of the transcription factor NF-κB was decreased and so were the levels of the cytokine TNF-α and the chemokine CXCL1. These results clearly show that endogenous PTX3 is fundamental for the cascade of events leading to tissue inflammation and injury after I/R. Moreover, results using exogenous PTX3 suggest that the soluble form of the protein is relevant for the effects observed.
PTX3 is produced rapidly and released by a variety of tissue resident cell types, including macrophages, epithelial cells, and fibroblasts, on exposure to primary inflammatory signals, such as TNF-α and IL-1β, and microbial moieties, such as lipopolysaccharide.1,5,23,24,25 Physical injury has also been shown to induce the release of PTX3, as seen in a model of mechanical ventilation lung injury model. In the latter study, PTX3 release was associated with the severity of injury.26 Not only is PTX3 released during inflammation, but PTX3 may also favor the release of mediators of the inflammatory process. For example, we have previously shown that mice overexpressing PTX3 had enhanced TNF-α production and TNF-α-dependent reperfusion injury and death in mice.11 Furthermore, enhanced production of inflammatory mediators, including TNF-α, was observed after Klebsiella pneumoniae pulmonary infection of mice overexpressing PTX3.27 In the latter system, enhanced production of TNF-α and other mediators caused greater lethality when large inocula were used. In contrast, in experiments conducted using lower inocula of K. pneumonia27 and in a model of cecal and ligation and puncture,28 there was enhanced inflammation in PTX3 transgenic mice and this was associated with optimal anti-bacterial defense and lower lethality rates. In the present study, absence of PTX3 was associated with lower production of both TNF-α and CXCL1. As shown in previous studies by us and by other groups, TNF-α and CXCL1 are essential for neutrophil recruitment, injury, and lethality to occur after intestinal I/R.19,21,29 Hence, the ability of PTX3 to control production of these proteins and consequent neutrophil migration appears to underlie the lack of reperfusion-associated injury and lethality observed in PTX3-deficient mice.
The transcription factor NF-κB is induced in response to oxidative stress and a wide range of stimuli, including pro-inflammatory cytokines, such as TNF-α.30,31,32 On the other hand, NF-κB can induce pro-inflammatory gene expression, including cytokines, adhesion molecules, and chemokines.33,34 The contribution of these transcription factors to the inflammatory response has been demonstrated in several models of acute severe inflammation. Our previous work demonstrated that there is an early activation of NF-κB after reperfusion of the ischemic superior mesenteric artery and that blockade of the activation of NF-κB with a specific inhibitor of thioredoxin was accompanied by prevention of tissue injury and lethality induced by reperfusion.16 Thus, it is possible that part of the mechanism by which PTX3 modulates cytokine production and, consequently, reperfusion injury is secondary to the ability of the molecule to control NF-κB activation.
In the context of infections and sepsis, PTX3 may act as a soluble pathogen recognition receptor (PRR) that binds to the pathogen and to a putative PTX3 receptor on host cells, including macrophages.3,4,35,36 This role of PTX3 to act as soluble PRR is essential in models of fungal infection.5,37 In contrast, acute intestinal I/R does not depend on TLR/Myd-88 function and may also occur in the absence of microbiota.38 Indeed, germ-free mice treated with anti-IL-1038 or 5-lipoxygenase inhibitor39 had reperfusion injury and lethality that was similar to that observed in conventional mice. Hence, it appears that PTX3 is not functioning as a soluble PRR to recognize gut pathogens in this model of intestinal I/R. PTX3 can also directly bind C1q through its pentraxin domain.13 However, the relevance of the interaction between PTX3 and C1q is far from clear because PTX3 can both inhibit and activate the classic complement pathway by binding C1q, depending on the way that it is presented to C1q.40 At least the antifungal function of PTX3 may be independent of C1q.5 In our hands, use of a complement inhibitor based on a recombinant fragment of soluble CR1 prevented I/R injury, but not lethality.41 Hence, inhibition of complement function in the context of reperfusion injury would appear not to explain all of the protective effects observed in Ptx3−/− mice. Additional mechanisms would have to be operative. One possibility is that PTX3 binds to an endogenous molecule(s) expressed during I/R and thereafter controls the production of cytokines and chemokines and subsequent neutrophil influx. The nature of such molecule(s) and the receptor to which PTX3 binds after ligand interaction are questions currently being evaluated in our laboratories.
Recently we showed that PTX3 was expressed in the heart of mice after acute coronary ischemia with or without reperfusion.42 In that system, absence of PTX3, as observed in Ptx3−/− mice, had no effect on infarction size after permanent coronary ligation. On the other hand, infarction size, neutrophil influx, IL-6 production, and no-reflow phenomenon were greatly increased in Ptx3−/− mice submitted to myocardial I/R, a phenotype rescued by treatment with human recombinant PTX3.42 The latter results demonstrate that PTX3 plays a cardioprotective role in reperfused acute myocardial infarction in mice and clearly contrast with the present findings, which show inhibition of intestinal reperfusion injury in absence of PTX3. There are several possible reasons to explain the apparent discrepancy between our findings and those obtained in the heart system. In the murine model of reperfused acute myocardial infarction, inflammation is tightly restricted to the heart and PTX3 expression was not found in other tissues after coronary ligation and reperfusion.42 Moreover, inflammatory parameters are evaluated at much later stages (from 1 day until 13 days after coronary ligation). In the model of intestinal I/R injury, inflammatory injury is also systemic and accompanied by 100% lethality within 2 hours of reperfusion. Hence, it is possible that in acute myocardial infarction, the ability of PTX3 to diminish complement activation and C3 deposition42 may account for its cardioprotective effect. In the intestine, PTX3 is necessary for NF-κB activation, TNF-α production, and for the massive TNF-α-driven inflammatory response that follows reperfusion. It is, thus, clear that PTX3 has different roles in mediating reperfusion injury in the heart (protective, localized response) versus the intestine (deleterious, systemic response) and that the intensity of the inflammatory response may partially explain the relevance of PTX3 in reperfusion injury. A somewhat similar situation is observed in a model of pneumonia caused by Klebsiella pneumonia in which PTX3 is protective when a low inoculum is given but deleterious when a high inoculum is given.27 Increased PTX3 expression correlated to lung injury in response to lipopolysaccharide and high-volume ventilation.26 Thus, it is clear PTX3 may play a detrimental role under certain pathological conditions, especially when inflammation is already severe.
PTX3 is crucial for the production of TNF-α and other mediators in response to pathogens in which occasion it may serve as a pattern recognition receptor.5,27 In the present study, we show that PTX3 also plays a fundamental role in mediating tissue inflammation under sterile conditions (ie, after acute intestinal I/R). Moreover, exogenous PTX3 rescues the phenotype of Ptx3−/− mice. The latter results suggest that PTX3 may function as a soluble molecule that appears to be sensing tissue injury to induce the production of mediators of the inflammatory process, including CXCL1 and TNF-α. The control of the production of mediators of inflammation in part via the control of NF-κB activation appears to underlie the regulatory role of PTX3 in this system. Finally, it is possible that inhibition of the function of PTX3 may be beneficial therapeutically in the context of intestinal reperfusion injury or similar systemic inflammatory syndromes.
Footnotes
Address reprint requests to Mauro Martins Teixeira, Departamento de Bioquimica e Imunologia, Instituto de Ciencias Biológicas, Universidade Federal de Minas Gerais, Av. Antonio Carlos, 6627 Pampulha, 31270-901 Belo Horizonte MG Brazil. E-mail: mmtex@icb.ufmg.br.
Supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), the Fundação do Amparo a Pesquisas do Estado de Minas Gerais (FAPEMIG, Brazil), the Fundação do Amparo a Pesquisas do Estado de São Paulo (FAPESP, Brazil), the European Union FP6 (INNOCHEM, grant number LSHB-CT-2005-518167 and MUGEN, LSHB-CT-2005-005203), the Cariplo Foundation (Nobel project to A.M.), and the National Institutes of Health (grant HD33438 to M.M.M.).
References
- Lee TH, Lee GW, Ziff EB, Vilcek J. Isolation and characterization of eight tumor necrosis factor-induced gene sequences from human fibroblasts. Mol Cell Biol. 1990;10:1982–1988. doi: 10.1128/mcb.10.5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breviario F, d'Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M, Valle GD, Dejana E, Mantovani A, Introns M. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- He X, Han B, Liu M. Long pentraxin 3 in pulmonary infection and acute lung injury. Am J Physiol. 2007;292:L1039–L1049. doi: 10.1152/ajplung.00490.2006. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B, Peri G, Doni A, Vago L, Botto M, De Santis R, Carminati P, Siracusa G, Altruda F, Vecchi A, Romani L, Mantovani A. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- Baruah P, Dumitriu IE, Peri G, Russo V, Mantovani A, Manfredi AA, Rovere-Querini P. The tissue pentraxin PTX3 limits C1q-mediated complement activation and phagocytosis of apoptotic cells by dendritic cells. J Leukoc Biol. 2006;80:87–95. doi: 10.1189/jlb.0805445. [DOI] [PubMed] [Google Scholar]
- Rovere P, Peri G, Fazzini F, Bottazzi B, Doni A, Bondanza A, Zimmermann VS, Garlanda C, Fascio U, Sabbadini MG, Rugarli C, Mantovani A, Manfredi AA. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000;96:4300–4306. [PubMed] [Google Scholar]
- Inoue K, Sugiyama A, Reid PC, Ito Y, Miyauchi K, Mukai S, Sagara M, Miyamoto K, Satoh H, Kohno I, Kurata T, Ota H, Mantovani A, Hamakubo T, Daida H, Kodama T. Establishment of a high sensitivity plasma assay for human pentraxin3 as a marker for unstable angina pectoris. Arterioscler Thromb Vasc Biol. 2007;27:161–167. doi: 10.1161/01.ATV.0000252126.48375.d5. [DOI] [PubMed] [Google Scholar]
- Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, Pizzetti F, Maggioni AP, Moccetti T, Metra M, Cas LD, Ghezzi P, Sipe JD, Re G, Olivetti G, Mantovani A, Latini R. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D, Tarli L, Schweiger C, Fresco C, Cecere R, Tognoni G, Mantovani A, Lipid Assessment Trial Italian Network (LATIN) Investigators Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- Souza DG, Soares AC, Pinho V, Torloni H, Reis LF, Teixeira MM, Dias AA. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol. 2002;160:1755–1765. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan CN, DeMayo J, Demayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–1167. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- Bottazzi B, Vouret-Craviari V, Bastone A, De Gioia L, Matteucci C, Peri G, Spreafico F, Pausa M, D'Ettorre C, Gianazza E, Tagliabue A, Salmona M, Tedesco F, Introna M, Mantovani A. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. 1997;272:32817–32823. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- Souza DG, Coutinho SF, Silveira MR, Cara DC, Teixeira MM. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur J Pharmacol. 2000;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- Arantes RM, Nogueira AM. Distribution of enteroglucagon- and peptide YY-immunoreactive cells in the intestinal mucosa of germ-free and conventional mice. Cell Tissue Res. 1997;290:61–69. doi: 10.1007/s004410050908. [DOI] [PubMed] [Google Scholar]
- Souza DG, Vieira AT, Pinho V, Sousa LP, Andrade AA, Bonjardim CA, McMillan M, Kahn M, Teixeira MM. NF-kappaB plays a major role during the systemic and local acute inflammatory response following intestinal reperfusion injury. Br J Pharmacol. 2005;145:246–254. doi: 10.1038/sj.bjp.0706190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa LP, Silva BM, Brasil BS, Nogueira SV, Ferreira PC, Kroon EG, Kato K, Bonjardim CA. Plasminogen/plasmin regulates alpha-enolase expression through the MEK/ERK pathway. Biochem Biophys Res Commun. 2005;337:1065–1071. doi: 10.1016/j.bbrc.2005.09.154. [DOI] [PubMed] [Google Scholar]
- Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G, Cervellera MN, Di Cioccio V, Cesta MC, Galliera E, Martinez FO, Di Bitondo R, Troiani G, Sabbatini V, D'Anniballe G, Anacardio R, Cutrin JC, Cavalieri B, Mainiero F, Strippoli R, Villa P, Di Girolamo M, Martin F, Gentile M, Santoni A, Corda D, Poli G, Mantovani A, Ghezzi P, Colotta F. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci USA. 2004;101:11791–11796. doi: 10.1073/pnas.0402090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza DG, Bertini R, Vieira AT, Cunha FQ, Poole S, Allegretti M, Colotta F, Teixeira MM. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;143:132–142. doi: 10.1038/sj.bjp.0705862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F, Noris M, Remuzzi G, Benigni A. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int. 2005;67:1753–1761. doi: 10.1111/j.1523-1755.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- Souza DG, Cassali GD, Poole S, Teixeira MM. Effects of inhibition of PDE4 and TNF-alpha on local and remote injuries following ischaemia and reperfusion injury. Br J Pharmacol. 2001;134:985–994. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–3493. [PubMed] [Google Scholar]
- Doni A, Peri G, Chieppa M, Allavena P, Pasqualini F, Vago L, Romani L, Garlanda C, Mantovani A. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur J Immunol. 2003;33:2886–2893. doi: 10.1002/eji.200324390. [DOI] [PubMed] [Google Scholar]
- Han B, Mura M, Andrade CF, Okutani D, Lodyga M, dos Santos CC, Keshavjee S, Matthay M, Liu M. TNFalpha-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J Immunol. 2005;175:8303–8311. doi: 10.4049/jimmunol.175.12.8303. [DOI] [PubMed] [Google Scholar]
- Okutani D, Han B, Mura M, Waddell TK, Keshavjee S, Liu M. High-volume ventilation induces pentraxin 3 expression in multiple acute lung injury models in rats. Am J Physiol. 2007;292:L144–L153. doi: 10.1152/ajplung.00002.2006. [DOI] [PubMed] [Google Scholar]
- Soares AC, Souza DG, Pinho V, Vieira AT, Nicoli JR, Cunha FQ, Mantovani A, Reis LF, Dias AA, Teixeira MM. Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 2006;8:1321–1329. doi: 10.1016/j.micinf.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Dias AA, Goodman AR, Dos Santos JL, Gomes RN, Altmeyer A, Bozza PT, Horta MF, Vilcek J, Reis LF. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol. 2001;69:928–936. [PubMed] [Google Scholar]
- Köksoy C, Kuzu MA, Kuzu I, Ergün H, Gürhan I. Role of tumour necrosis factor in lung injury caused by intestinal ischaemia-reperfusion. Br J Surg. 2001;88:464–468. doi: 10.1046/j.1365-2168.2001.01737.x. [DOI] [PubMed] [Google Scholar]
- Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- Takada Y, Singh S, Aggarwal BB. Identification of a p65 peptide that selectively inhibits NF-kappa B activation induced by various inflammatory stimuli and its role in down-regulation of NF-kappa B-mediated gene expression and up-regulation of apoptosis. J Biol Chem. 2004;279:15096–15104. doi: 10.1074/jbc.M311192200. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Garlanda C, Doni A, Bottazzi B. Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunol. 2008;28:1–13. doi: 10.1007/s10875-007-9126-7. [DOI] [PubMed] [Google Scholar]
- Bottazzi B, Bastone A, Doni A, Garlanda C, Valentino S, Deban L, Maina V, Cotena A, Moalli F, Vago L, Salustri A, Romani L, Mantovani A. The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J Leukoc Biol. 2006;79:909–912. doi: 10.1189/jlb.1005557. [DOI] [PubMed] [Google Scholar]
- Gaziano R, Bozza S, Bellocchio S, Perruccio K, Montagnoli C, Pitzurra L, Salvatori G, De Santis R, Carminati P, Mantovani A, Romani L. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob Agents Chemother. 2004;48:4414–4421. doi: 10.1128/AAC.48.11.4414-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173:4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- Souza DG, Fagundes CT, Amaral FA, Cisalpino D, Sousa LP, Vieira AT, Pinho V, Nicoli JR, Vieira LQ, Fierro IM, Teixeira MM. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol. 2007;179:8533–8543. doi: 10.4049/jimmunol.179.12.8533. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Bottazzi B, Mantovani A, Salvatori G, Kishore U, Schwaeble WJ, Gingras AR, Tzima S, Vivanco F, Egido J, Tijsma O, Hack EC, Daha MR, Roos A. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur J Immunol. 2003;33:465–473. doi: 10.1002/immu.200310022. [DOI] [PubMed] [Google Scholar]
- Souza DG, Esser D, Bradford R, Vieira AT, Teixeira MM. APT070 (Mirococept), a membrane-localised complement inhibitor, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2005;145:1027–1034. doi: 10.1038/sj.bjp.0706286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, Pasqualini F, Latini R, Garlanda C, Mantovani A. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]