Abstract
It is desirable to have an early and sensitive detection marker of autoimmune disease in intact animals. Nuclear factor (NF)-κB is a transcription factor that is associated with inflammatory responses and immune disorders. Previously, we demonstrated that so-called idiotypic-driven T–B cell collaboration in mice doubly transgenic for paired immunoglobulin and T cell receptor transgenes resulted in a systemic autoimmune disease with systemic lupus erythematosus-like features. Here, we investigated NF-κB activation by including an NF-κB-responsive luciferase reporter transgene in this animal model. Triply transgenic mice developed bioluminescence signals from diseased organs before onset of clinical symptoms and autoantibody production, and light emissions correlated with disease progression. Signals were obtained from secondary lymphoid organs, inflamed intestines, skin lesions, and arthritic joints. Moreover, bioluminescence imaging and immunohistochemistry demonstrated that a minority of mice suffered from an autoimmune disease of the small intestine, in which light emissions correlated with antibodies against tissue transglutaminase and gliadin. Detection of luciferase by immunohistochemistry revealed NF-κB activation in collaborating B and T cells, as well as in macrophages. These results demonstrate that bioluminescent in vivo imaging of NF-κB activation can be used for early and sensitive detection of autoimmune disease in an experimental mouse model, offering new possibilities for the evaluation of anti-inflammatory drugs.
Despite intense research efforts, the etiology of most autoimmune diseases remains obscure. Recently, CD4+ T cells that recognize V region (idiotypic, Id) peptides of antibodies have been described in a number of autoimmune diseases in humans1,2,3,4 such as rheumatoid arthritis,3 systemic lupus erythematosus (SLE),1,2 and multiple sclerosis,4 as well as in several murine models of autoimmune disease.5,6,7 However, it has been unclear whether Id-specific CD4+ T cells may actually cause autoimmune disease and by which mechanism they could do so.
B cell receptors (BCRs) spontaneously undergo antigen processing, and B cells display Id-peptides on their major histocompatibility complex (MHC) class II molecules; such complexes activate Id-specific T cells.8,9,10,11,12 Conversely, Id+ B cells can be helped by Id-specific CD4+ T cells and differentiate into antibody10,13 and autoantibody13,14,15 secreting B cells. Such findings have paved the way for the concept of Id-driven T–B collaboration, as first suggested by our group.11,16 Similar models were later proposed by others.6,7 Importantly, Id-driven T–B collaboration requires BCR ligation for the germinal center reaction and isotype switching to occur.13 Therefore, since autoantigens are ubiquitously expressed, B cells with autoreactive BCRs are especially prone to partake in Id-driven T–B collaboration, explaining why this type of T–B collaboration is associated with induction of autoantibodies and autoimmune disease.13,14,15
T cells are tolerant to abundant germline-encoded V region sequences,17,18,19 in part due to deletion in the thymus.10,14 Thus, T cell tolerance restricts the extent of Id-driven T–B collaboration. However, a T cell repertoire exists toward rare V region sequences that depend on somatic mutations or possibly N-region diversity.17,18,19 Thus, low-frequency autoreactive B cells that express uncommon Id could haphazardly encounter Id-specific T cells in peripheral lymphoid tissues, resulting in Id-driven T–B interaction and autoimmunity.6,7,11,13,14,16
Id-driven T–B collaboration and autoimmunity has been studied in mice that are transgenic for both Id+ Ig L-chain and Id-specific T cell receptors (TCRs).10,14 Surprisingly, T cell tolerance toward Id was not complete in such doubly transgenic mice. Thus, a minor population of Id-specific T cells escaped tolerization, expanded as mice aged, and provided Id-driven help to Id+ B cells. Such Id-driven T–B collaboration caused secretion of high levels of IgG antibodies and ultimately severe systemic autoimmunity, including inflammatory bowel disease, arthritis, and kidney and skin diseases.14
NF-κB, originally identified in B cells,20,21 is a central transcription factor in both innate and adaptive immune responses. NF-κB is activated by a plethora of pro-inflammatory cytokines, chemokines, adhesion molecules, and immunoregulatory mediators. Inappropriate regulation of NF-κB has been associated with a number of disorders including arthritis, asthma, and inflammatory bowel disease.20,22
At least two NF-κB signaling pathways exist.20,21 The classical pathway is dependent on the inhibitor of kappa B kinase beta and is involved in cytokine signaling, eg, tumor necrosis factor (TNF)α, interleukin 1, or pathogen recognition (Toll-like receptors) in inflammatory responses and innate immunity. The classical pathway is also triggered by TCR and BCR signaling.20,21 The alternative pathway is dependent on inhibitor of kappa B kinase alpha and is mediated through the NF-κB family members RelB and p52. The alternative pathway is involved in the development, homeostasis, and activation of adaptive immunity through ligands such as LTβ and CD40L.20,21 Thus, NF-κB is involved in several pathways resulting in inflammation.
To monitor NF-κB-associated inflammation in mice undergoing Id-driven autoimmune disease, we have here produced triple transgenic mice expressing both Id+ B cells and Id-specific CD4+ T cells14 as well as a luciferase reporter transgene under NF-κB control.23 On NF-κB activation and nuclear translocation of NF-κB, luciferase activity could be repeatedly monitored in intact mice by whole body bioluminescence imaging. Moreover, results could be corroborated at the cellular level by detection of luciferase protein expression in single cells. The results show that imaging of NF-κB activation permits early detection of subclinical disease as well as tracking of disease development.
Materials and Methods
Mice
The hemizygous λ2315 Ig L-chain transgenic mice [Id+-mice]24 and the hemizygous αβ TCR transgenic mice25 specific for the Id(λ2315)-peptide (amino acids 91 to 101) presented on I-Ed are both on a BALB/c background and have been made homozygous for their transgenes (Id+/+ and TCR+/+ respectively). Mice transgenic for a luciferase (Luc) reporter construct under control of NF-κB responsive elements and with a CBAxC57BL/6 background were back-crossed three times with BALB/c mice. Offspring that were typed as NF-κB-Luc (Luc+/−) were crossed with homozygous TCR transgenic mice (TCR+/+) on a BALB/c background. Resulting litters were typed for MHC haplotype and Luc. Offspring that were TCR+/−Luc+/− H2d/d were further crossed with homozygous Id+/+ mice (BALB/c background), resulting in litters composed of TCR+/−Id+/−Luc+/− experimental mice and TCR−/−Id+/−Luc+/− control mice. All mice were thus H2d/d but had considerable contributions of CBA and C57BL/6 to a predominately BALB/c background. TCR+/−Id−/−Luc+/− control mice were H2d/d homozygous offspring from an independent (TCR+/+ × Luc+/−) cross. Mice were typed for expression of Id+ L-chain and TCR transgenes by flow cytometry and for the NF-κB transgene by injection of luciferin substrate and measurement of in vivo bioluminescence.
Clinical Assessment of Mice
Mice were weighed weekly. Skin disease was scored as positive when skin was clearly inflamed with induration, erythema, loss of hair, scabbing, and scarring. Arthritis entailed swollen joints and redness of overlying skin. Mice with perianal erythema, rectal prolapse, diarrhea, and mucoid/bloody stool were defined as having inflammatory bowel disease.
Enzyme-Linked Immunosorbent Assay
Hamster tissue transglutimase 2 (TG2) and trypsin digested Gliadin, kindly provided by Dr L. Sollid (Oslo), was coated onto 96-well plates, diluted sera was added, and plates were developed by biotinylated rat-anti IgG monoclonal antibody (mAb, BD Pharmingen) essentially as described.14
Measurements of Serum Immunoglobulins and Autoantibodies
Immunoglobulins were detected by enzyme-linked immunosorbent assay as described.14 Diluted sera were added to HEp2 cells (Immuno Concepts) and antinuclear antibodies (ANA) were detected with Alexa Fluor 488 goat anti-IgG2a and Alexa Fluor 546 goat anti-IgG1 (Molecular probes).
Immunohistochemistry
Organs were embedded in optimal cutting temperature compound, and 5-μm frozen sections were mounted on l-polylysine-coated glass slides, air-dried overnight, and blocked with 30% heat-aggregated rat serum. All further solutions contained 30% rat serum. Double staining: Slides were stained with Rabbit anti-luciferase (Sigma-Aldrich St. Louis, MO) in PBS w/30% rat serum. Nuclei were stained with 4,6-diamino-2-phenylindoldi-hydrochloride (DAPI, Molecular Probes, Eugene, OR). Slides were further developed with highly cross-absorbed goat anti-rabbit (H+L)-Alexa 488 (Molecular Probes), followed by signal amplification with donkey anti-goat-Alexa 488 (Molecular Probes). Fluorescein isothiocyanate-conjugated F(ab′)2 goat-anti-mouse-C3 was from Cappel ICN, Costa Mesa, CA. The following mAbs were biotinylated in our lab: transgenic TCR-clonotype-specific GB113,26 anti-CD4 (GK1.5), anti-CD11b (TIB198), anti-MHC class II (TIB120), and 2B6 (anti-Cλ2/3).24 MAbs were purified and biotinylated in our lab and were detected with streptavidin-Cy3 from Amersham Pharmacia Biotech (Piscataway, NJ). Triple staining: Luciferase was stained as described above. In addition, biotinylated 2B6 (anti-Cλ2/3), developed with streptavidin-Alexa 355 (Molecular Probes), was used for detection of Id+ B cells. Biotinylated GB113, developed with streptavidin Cy3, was used for detection of Id-specific T cells. Biotin was blocked by avidin (Sigma-Aldrich) 10 μg/ml, followed by addition of biotin (Sigma-Aldrich). Luciferase-staining could also be detected in acetone or ethanol-fixed slides.
Imaging and Luciferase Measurements
Cohorts of littermates were investigated for NF-κB activity with a luciferin-based bioluminescence assay, from three weeks of age. Isoflurane (2.5%) was used as anesthetic to immobilize the mice, followed by an i.p injection of d-luciferin (120 mg/kg; Biothema, Dalarö, Sweden), dissolved in 200 μl PBS pH 7.8. Immediately afterward, the mice were placed in a light-sealed chamber connected to a sensitive and cooled CCD camera (IVIS-100 Imaging System, Xenogen). Luminescence emitted from the mouse was integrated for 1 minute starting 8 minutes after the injection of luciferin. In some experiments, mice were sacrificed; individual organs were excised from the mice 10 minutes after d-luciferin injection, and imaged. Light emission was analyzed on the Living Image 2.0 software and expressed as photons/sec/cm2/steradian. Organ homogenates were made by homogenizing frozen tissues in lysis buffer (Promega, Madison, WI). The luciferase activity in homogenates was assessed as described previously.23 The luminescent probe L-012 (8-amino-5-chloro-7-phenylpyrido[3,4-day]pyridazine-1,4-(2H,3H)dione),27 Wako Pure Chemical Industries (Osaka, Japan) emits light in presence of oxygen radicals. L-012 was injected i.p. (25 mg/kg in 100 μl ddH2O), and mice were imaged 10 to 15 minutes later in the IVIS-100 Imaging system as described above. In some experiments a microscope with an ultrasensitive CCD camera (Andor iXon DV887, Andor Technology, Belfast, Northern Ireland) was used to image emitted light from sections.
Statistics
The Mann-Whitney test was used throughout the paper to calculate the indicated P values.
Results
Neither Introduction of NF-κB-Luciferase Reporter Transgenes Nor Altered Genetic Background Affect the Course of Autoimmune Disease Caused by Chronic Id-Driven T–B Collaboration
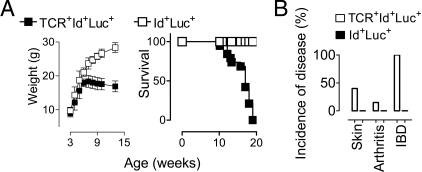
The aim of the present study was to see if NF-κB activation could serve as an early in vivo marker for detection of autoimmune disease elicited by chronic Id-driven T–B collaboration. To this end we bred NF-κB-luciferase reporter transgenes into doubly Id-specific TCR/Id+Ig transgenic mice previously described to develop hypergammaglobulinemia, IgG autoantibodies and lethal SLE-like disease.14 A potential caveat to this approach is that the multiple NF-κB responsive elements could absorb NF-κB and thereby thwart disease development in triply (TCR +Id+NF-κB-Luc+) transgenic mice. Another concern was that in-breeding of the NF-κB-Luc transgenes resulted in triply transgenic mice with substantial amounts of CBA and C57Bl/6 background genes, which could alter the autoimmune disease previously observed in doubly transgenic mice on a pure BALB/c background.14 However, triply transgenic mice developed lethal disease (Figure 1A) with clinical signs (Figure 1B) and development of IgG autoantibodies (see below) that did not differ significantly from that previously observed in doubly TCR+Id+ transgenic mice.14 Thus, CBA and C57BL/6 background genes did not markedly influence disease development, although minor effects cannot be ruled out. Both TCR and Id+ L chain transgenes were needed for disease development since neither TCR+NF-κB-Luc+ nor Id+NF-κB-Luc+ doubly transgenic mice developed disease (Figure 1 and data not shown).
Figure 1.
Weight, survival, and clinical signs in triply transgenic mice. A cohort of 13 TCR+Id+Luc+ triply transgenic mice and 8 Id+Luc+ transgenic control littermates were inspected for disease and weighed weekly. A: Weight (left) and survival (right) of mice. B: Cumulative incidences of skin, joint and inflammatory bowel disease.
Measurement of Luciferase Activity
Triply transgenic mice were injected with the substrate luciferin and photon emissions were externally detected by use of an IVIS 100 apparatus (Xenogen) (Figure 2A–D). Mice that lacked either TCR or Ig L chain transgenes (ie, Id+NF-κB-Luc+ and TCR+NF-κB-Luc+ mice) were used as negative controls; for reasons of simplicity, both these controls are not always shown in the results given below. In some experiments, mice were sacrificed and imaged either after removal of skin (eg, Figure 2E), or after explantation of organs (eg, Figures 2F and 3B). The luciferase activity in organ homogenates23 gave similar results (data not shown). For closer scrutiny of luciferase activity within an organ, luciferin was sometimes dropped onto sections and light observed with an ultrasensitive CCD camera (eg, Figure 2G). To differentiate the cellular origin of the luciferase signal, a staining method was developed that revealed luciferase protein by immunohistochemistry. Using these different multilevel approaches, we below describe organ involvements and cellular origins of NF-κB-dependent light signals in triply transgenic mice suffering from Id-driven autoimmune disease.
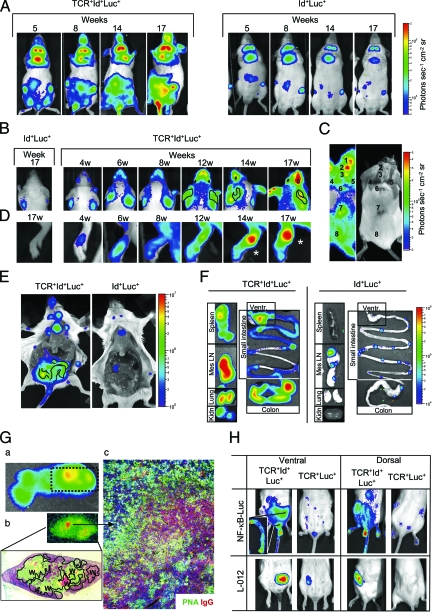
Figure 2.
NF-κB bioluminescence precedes and correlates with disease. A: Photons emitted from ventral aspects of mice with increasing age. Left: a representative TCR+Id+Luc+ mouse investigated at the indicated time-points. Right: an Id+Luc+ control. B–D: Focal disease. A common light intensity bar for all panels in B–D is shown (right). B: Early detection of skin disease on the dorsal aspect of head and upper thorax. Ears and eyes are traced in some frames with a black line. C: A representative 20-week-old triply transgenic mouse with severe skin disease including scarring is shown. Focal areas detected by bioluminescence (left) and photo (right), have been numbered. D: Early signals from the hind paw of a mouse that later developed arthritis. Clinical arthritis was only evident starting from week 14 (asterisks). E: Photons from ventral organs after sacrifice and skin removal. The ventricle and kidney are abbreviated as Vent and Kidn. The surface trace of the colon in the TCR+Id+Luc+ mouse is indicated with black lines. F: Signals from isolated organs postdissection. G: a) A whole spleen from a triple transgenic mouse displaying a focus of high bioluminescence signals (detected by the IVIS100) is shown. b) Light emissions from cross sections of the same spleen with focus were detected by a microscope with an ultrasensitive CCD camera (see Materials and Methods). Light emissions can be seen from the white pulp (W, traced with a black line). c) Immunofluorescence of an area with intense signals (see arrow from b) stained for IgG (red), peanut agglutinin [PNA] (green), and nuclei (blue). An active immune response with PNA+ IgG+ germinal centers (yellow), PNA+ IgG− germinal centers (green), and IgG+ B cells (red) can been seen. H: NF-κB-dependent bioluminescence in triple transgenic treated with L-012; note the colocalization of NF-κB activity and inflammatory (L-012) signals from area of the distal colon. The inset shows NF-κB-dependent light emission postdissection from the distal third of the colon.
Figure 3.
Statistical evaluation of NF-κB bioluminescence signals in triply transgenic mice. A: Ventral surfaces of TCR+Id+Luc+ and Id+Luc+ mice (n = 13, 10 respectively) were visualized and the photons emitted from the indicated areas were measured. Note the different scales on the y axis. *P < 0.05, Mann Whitney. B: Quantification of signals from organs of mice, P values (Mann Whitney) are indicated.
Early Detection of Increased NF-κB Activity in Living Triply Transgenic Mice
Whole animals were repeatedly imaged for several months starting from week 3. Ventral views gave the brightest signals, presumably because such a view allows detection of photons emanating from internal organs (see below). From week 5, triply transgenic mice had a detectable and progressive increase in bioluminescence signals compared with Id+Luc+ mice (Figure 2A). Emission intensities were slightly lower on some days presumably due to luciferin substrate variability, but the anatomical foci and ratio (triply transgenic versus control) of light emissions were robust and reproducible. More specifically, the signals were strongest in the abdominal area, but significant and progressively increasing signals were also obtained from the neck, the thorax, the snout, the perianal region, the proximal tail, the paws, and the inguinal lymph nodes (Figure 3A). The increased signals required both Id+ transgenes and TCR transgenes because both Id+Luc+ mice (Figures 2A and 3A) and TCR+Luc+ mice (not shown) had only low photon emissions.
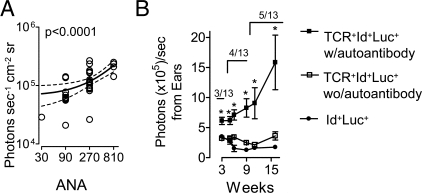
Importantly, the increased bioluminescence signals in triply transgenic mice started approximately at the same time that mice failed to gain weight (Figure 1A), and several weeks before organ-specific clinical signs of disease (see below). In agreement with previous studies in doubly transgenic mice,14 triply transgenic mice had detectable antinuclear IgG serum antibodies first from week 8, which is several weeks delayed compared with the increase in ventral bioluminescence signals observed in intact mice. Sera exhibited a homogenous ANA staining pattern,14 with antibodies predominantly of an anti-dsDNA specificity (L.M., B.B., manuscript in preparation). No IgG autoantibodies were detected in Id+Luc+ controls. ANA titers increased with age of triply transgenic mice (data not shown). When investigated by 10 weeks of age, ANA titers correlated with ventral light emissions (Figure 4A). With advancing age, the ANA-titers as well as ventral bioluminescence signals increased, both parameters rising about twofold by week 14 (data not shown).
Figure 4.
NF-κB bioluminescence correlates with autoantibody levels. A: IgG ANA titers correlated with emitted photons from the ventral sides of 10-week-old triply transgenic mice. A linear regression analysis is shown. B: The presence of IgG skin autoantibodies correlated with bioluminescence from ears measured at the indicated time points. Triply transgenic mice were divided into those positive or negative for serum autoantibodies against skin antigens at any of the indicated time points. The number of autoantibody-positive mice (out of 13) at each time-point is indicated. *P < 0.05, Mann Whitney.
Early Detection of Bioluminescence Signals from Lymphoid Organs
Doubly TCR+Id+ transgenic mice have a pronounced deletion of Id-specific thymocytes due to presentation of Id-peptide/class II molecules in the thymus.10,14 Interestingly, the triply transgenic mice had increased signals from areas corresponding to the thymus, suggesting that negative selection of thymocytes might be associated with activation of NF-κB (Figure 2, A and E).
We have previously described that a few Id-specific T cells escape negative selection in doubly transgenic mice and that these escapees with time expand in the periphery and cause chronic Id-driven T–B collaboration and autoimmune pathology.14 Consistent with this, triply transgenic mice developed increased bioluminescence in cervical and inguinal lymph nodes from 5 weeks of age (Figure 2, A and E). The signals gradually increased with time, suggesting an intensification of Id-driven immune responses with age (Figure 3A). Explanted mesenteric lymph nodes and spleens had strongly increased signals (Figures 2F and 3B). Moreover, foci of intense activity could be detected on spleen sections exposed to luciferin. The activity co-localized with the white pulp (Figure 2G).
Id+ B Cells and Id-Specific T Cells in Synapse Both Express Luciferase Protein
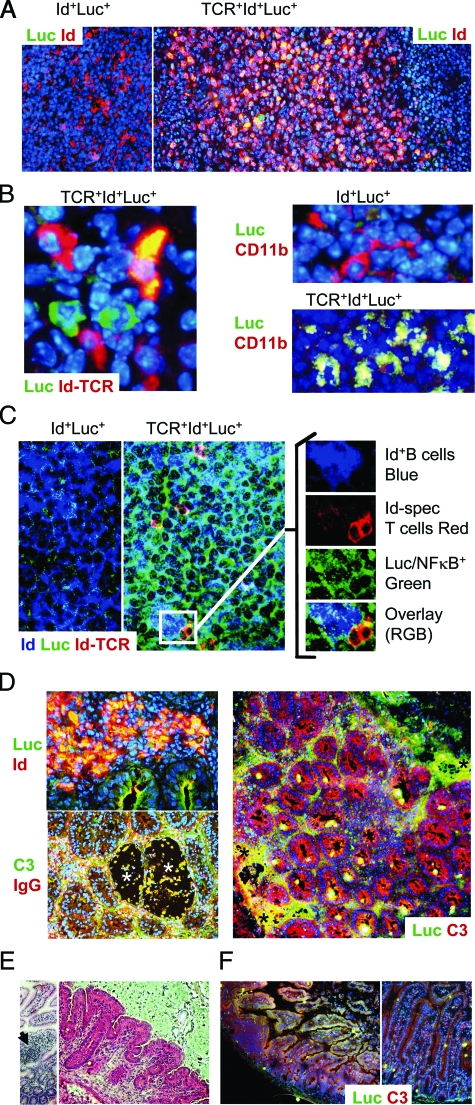
To determine exactly which cells were responsible for such bioluminescence, we developed a staining method for detection of luciferase protein expressed by NF-κB-activated cells. Id+ B cells in lymph nodes and spleens of triply transgenic mice frequently stained positive for luciferase protein, both in follicular and extra-follicular areas (Figure 5A and data not shown). Id-specific T cells also expressed luciferase protein (Figure 5B), as did extra-follicular CD11b+ macrophages (Figure 5B).
Figure 5.
Luciferase protein expression was detected in T cells, B cells, and macrophages, and in inflamed tissues. A–D: Representative examples of immunofluorescence analyses of 15 to 19-week-old mice are shown. A: Lymph node B cell follicles of Id+Luc+ (control) and TCR+Id+Luc+ mice were stained for luciferase protein (green), Id (anti-λ2/3 2B6 mAb, red), and nuclei (DAPI, blue). NF-κB-activated Id+ B cells are yellow. B: Left: Interfollicular area showing Id-specific T cells (TCR-clonotype specific mAb GB113, red), and luciferase (green), and nuclei (DAPI, blue). An NF-κB activated Id-specific T cell (yellow), two non-activated Id-specific cells (red), as well as two NF-κB activated non-Id-specific T cells (green) are seen (T cells expressing exclusively endogenous TCRα-chains are not Id-specific and do not stain with GB11314). Right: interfollicular areas from Id+Luc+ (control) and TCR+Id+Luc+ mice showing CD11b (red) and luciferase (green) and nuclei (DAPI, blue). NF-κB+CD11b+ macrophages are yellow. C: Triple staining of lymph node follicle showing Id+ B cells (anti-λ2/3 2B6 mAb, blue), Id-specific T cells (TCR-clonotype specific mAb GB113, red), and luciferase (green). The inset shows magnification and splitting of channels (right). Several Id+ B cells and Id-specific T cells cluster together, and both cell types are luciferase protein positive. D: Sections of an inflamed colon from a TCR+Id+Luc+ mouse. Left top: High magnification (×400) of area near erosion stained for Id (red), luciferase (green), and nuclei (blue). Id+NF-κB+ B cells are yellow. Left, bottom: Co-localization of IgG (red) and C3 (green) at sites of erosion (asterisks). A cellular infiltrate is seen in the middle left of the panel. Right, low magnification (×100): luciferase (red), and complement C3 (green), nuclei (blue). The colon architecture is effaced by cellular infiltrates and erosions (asterisks). Areas where C3 and luciferase expression co-localize are yellow. E–F: Small intestines of mice with inflammation. E: H&E stain. Left, mononuclear cells infiltrating a villus (arrow). Right, shortening of villi. F: Staining of luciferase (red) and C3 (green) in triply transgenic (left) and doubly transgenic control mice (right).
It has previously been shown that Id-specific T cells activate Id+ B cells by cognate Id-driven collaboration.13,14 To investigate whether Id+ B cells and Id-specific T cells in close proximity both expressed NF-κB, we performed triple staining. Indeed, clusters of Id-specific T cells and Id+ B cells were readily found, and both cell types had activated NF-κB (Figure 5C).
To test whether activation of Id-specific T cells via their TCR was sufficient to stimulate NF-κB and light emissions in vivo, we transferred Id-specific Luc+ T cells into RAG2−/− mice. To avoid background emissions due to homeostatic expansion of T cells in T cell-deficient hosts, we waited 60 days before transfer of either Id+ or Id− splenocytes. After 24 hours, a fourfold increase of light emissions was seen after injection of Id+ cells, while no increase was observed in recipients of Id− cells (L.M., H.C., & B.B., manuscript in preparation). The above findings done in vivo were corroborated by in vitro experiments where Id-specific Luc+ Th2 cell lines emitted light after exposure to Id+, but not Id− B cells (data not shown).
Early NF-κB-Mediated Signals from the Large Intestine
Besides a systemic autoimmune disease with SLE-like features, chronic Id-driven T–B collaboration resulted in inflammatory bowel disease in all mice with massive accumulations of Id-specific T cells, Id+ B cells and macrophages in inflamed colons.14 Consistent with this, triply transgenic mice had a strongly increased signal from the lower part of the abdomen and the perianal area (Figures 2A and 3A). Importantly, light signals from these areas predated the appearance of mucoid bloody diarrhea, a sign of inflammatory bowel disease, by several weeks. On opening of the abdominal cavity, signal patterns demarking the large bowel could be seen (Figure 2E).
Sections revealed transmural inflammation with multiple areas of erosions. Luciferin protein co-localized to areas with inflammation, complement/IgG immune complex deposition and cellular infiltrates, and luciferase activity was readily detected in both Id+ B cells and other cells yet to be defined (Figure 5D). Notably, some mice had bioluminescent stools, indicating shedding of NF-κB-expressing cells (data not shown).
The L-012 probe emits photons when activated by reactive oxygen radicals and has previously been used to visualize degranulation of phagocytes in vitro.27 When triply transgenic mice were injected with this probe, a discrete signal was obtained that corresponded to an area of colon that was also revealed by injection of the luciferin substrate (Figure 2H). The reason why the L-012 probe only gave a signal localized to the colon, and not other sites revealed by the luciferin substrate, could be related to the severe inflammation of the colon and infiltration by activated macrophages.
Early Detection of Vasculitis
We have previously observed that adult doubly transgenic mice often lost distal parts of their tails due to vasculitis.14 Consistent with this, proximal tail signals were in the present experiments significantly increased from week 6 (Figure 3A), although no scarring or auto-amputation due to vasculitic disease was seen before week 20.
Early Detection of Arthritis
Previously, we have described that about 20% of Id+TCR+ mice develop arthritis characterized by pannus formation, cartilage destruction and bone erosion.14 Based on clinical inspection, 15% of the triply transgenic mice in the current study had arthritic disease with metacarpal/tarsal swelling and erythema (Figure 1B). However, nearly half the mice had signals from one or more paws, and the increased signal was significant as early as from week 8 (Figure 3A). In fact, mice that developed marked swelling of joints emitted photons 5 to 8 weeks before clinically obvious disease (Figure 2D, and data not shown).
Early Detection of Skin Disease
Signals were also significantly augmented from the dorsal aspects of the mice, reflecting skin disease in the relative absence of signals from internal organs. From weeks 5 to 8, snout, periorbital, and ear signals were seen (Figure 2B), antedating clinical skin and eye affection by several weeks (Figure 2B). From week 12, marked luminescence signals were found in localized areas in some mice, suggesting skin disease (eg, from ears), although no visual signs of disease (except a few cases of mild erythema) were evident by mere inspection (Figure 2B). From weeks 17 to 19, more intense signals appeared on the dorsal aspects of mice corresponding to sites of more severe disease (Figure 2C). Interestingly, some foci of increased bioluminescence appeared normal by clinical inspection (Figure 2C, see spot 8). In summary, only 5 of 13 mice had clinically obvious skin disease before 20 weeks of age, with inflammation and scarring (Figure 1B). However, already at 6 weeks of age 8/13 had emissions from the facial skin, and by 20 weeks, 12/13 had bright skin signals. Areas of clinical skin disease always had an increased NF-κB signal. These results indicate that NF-κB signal from the skin is a sensitive and early indicator of skin inflammation.
Detection of Kidney Disease
We have previously described that more than a third of Id+TCR+ mice have detectable IgG and C3 depositions in kidney glomeruli.14 However, the glomerulonephritis is relatively mild as compared with that seen in end-stage disease in BWF1 and MRL/lpr mice strains,28 and only very low level proteinuria has been seen (L.M., B.B., unpublished). In the present study, at 20 weeks of age, significantly increased but modest bioluminescence signals were obtained from the dorsal aspects overlying the kidneys of TCR+Id+Luc+ mice (see supplemental Figure S1 at http://ajp.amjpathol.org). Even so, it may be difficult ascribe such relatively weak light emissions to the kidney, because the skin or the perirenal tissue may contribute to the signal. However, on dissection, kidneys of triply transgenic mice exhibited enhanced bioluminescence (Figure 2F).
NF-κB Signals Correlate with IgG Anti-Nuclear and Skin Autoantibodies
We have previously reported that doubly TCR+Id+ mice develop IgG autoantibodies that bind tissue antigens and activate complement at sites of disease.14 It was therefore of interest to test if levels of skin-specific autoantibodies (see supplemental Figure S2 at http://ajp.amjpathol.org) correlated significantly with bioluminescence signals from skin so readily accessible for detection by bioluminescence. This seemed to be the case since mice that harbored anti-skin autoantibodies14 had significantly increased light emissions from the ear (Figure 4B, and supplemental Figure S2 at http://ajp.amjpathol.org).
Hitherto Unrecognized Affection of Small Intestine Revealed by Increased NF-κB Signals
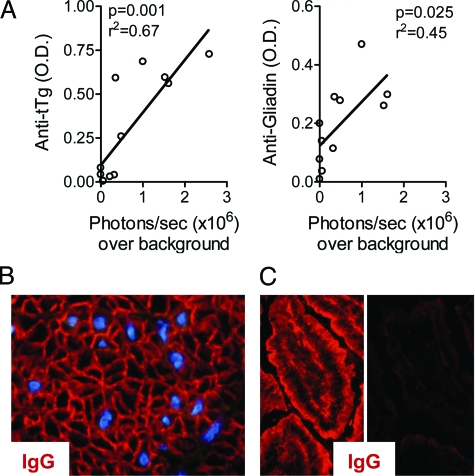
Small intestinal disease has not previously been described under conditions of chronic Id-driven T–B collaboration, and has only rarely been found in other mouse models of autoimmune diseases.29,30,31 It was therefore surprising to observe that 4 of the 13 triply transgenic mice had more than twice-increased photon emissions from the small bowel. The bioluminescence originated from both contiguous segments of the small intestine and from focal areas, the latter probably in part corresponding to increased activity in Peyers’ patches (Figure 2F). Interestingly, in three of these four mice, the light emissions from the small intestine were greater than the signals from the colons. Strikingly, these mice had serum IgG autoantibodies toward tissue transglutaminase (tTG2) and IgG anti-gluten antibodies (Figure 6A) that correlated with signals from the small intestine. Moreover, two of the four had IgG anti-endomycium autoantibodies (Figure 6B). These parameters are all associated with celiac disease in humans. Further, two of these mice had low titers of serum IgG autoantibodies that stained murine small intestinal villi, including epithelial cells (Figure 6C). Lastly, in addition to bioluminescence signals, the mice had inflammation in the small intestines, including cellular infiltrates, flattening of villi, C3 deposition and NF-κB/luciferase expression in a variety of cells (Figure 5, E–F). Thus, the bioluminescence technology directed our attention to a previous overlooked area of pathology, the small intestine, where NF-κB activation correlated with autoantibodies, cellular infiltrates, and disease.
Figure 6.
Antibodies related to intestinal pathology correlate with signals from inflamed small intestines. Sera were screened for antibodies associated with small intestinal pathology. A: Levels of serum anti-transglutaminase 2 (TG2) autoantibodies (left) and anti-Gliadin (right) correlated with NF-κB bioluminescence from small intestines isolated from TCR+Id+Luc+ mice. B: Serum from a 15-week-old triply transgenic mouse with small intestinal light emissions gave an anti-endomycium staining pattern, [IgG (red), and smooth muscle cell nuclei (blue)]. C: Autoantibodies directed toward the small intestine of a BALB/c RAG2−/− mouse. Left: serum from triply transgenic mouse (15 weeks old) with small intestinal signal and disease. Right: negative stain, serum from triply transgenic littermate (15 weeks) without small intestinal disease.
Discussion
We here use bioluminescence to detect and track systemic autoimmune disease in intact mice. The autoimmune disease in this model relies on the pathogenicity of so-called Id-driven T–B collaboration13,14 where B cells present BCR V region antigenic determinants as Id peptides on MHC class II to Id-specific T cells. In such Id-driven T–B collaboration, autoreactive Id+ B cells receive help from Id-specific T cells, undergo the germinal center reaction, and secrete isotype-switched pathogenic autoantibodies.13,14 We have previously described that doubly transgenic offspring from (Id-specific TCR transgenic × Id+ L chain transgenic) mice develop systemic autoimmune disease with SLE-like features.14 Herein we introduced a NF-κB responsive luciferase reporter transgene23 into the model to follow NF-κB activation in triply transgenic mice.
Importantly NF-κB signals were consistently detected several weeks before clinically manifest inflammatory bowel disease, skin disease, and arthritis. Moreover the NF-κB signals preceded the detection of autoantibodies. When disease developed, the clinical severity correlated with the bioluminescence signals. Finally, bioluminescence signals revealed sites of disease that went unnoticed by clinical inspection and autopsy alone. These results indicate that NF-κB mediated bioluminescence is a very sensitive and early indicator of inflammation and disease.
Bioluminescent imaging has a high sensitivity, since as few as 103 to 106 cells can be detected, depending on the anatomical site.32 The technique also has a high specificity, as background light emission is virtually negligible,33 and is low in cost and high-throughput.34 Although advances such as three-dimensional imaging techniques have been developed, and gene activation and expression can be monitored (as in the current paper), bioluminescent imaging is limited by a spatial resolution in the millimeter range. Further, tissues vary in their absorption of light emissions, and pathological processes may influence tissue permeability to light, eg, increased blood content may cause increased light absorption by hemoglobin.35 Despite these limitations, the technology applied to disease models such as the one examined herein should be very useful for studies on early intervention, eg, drug treatment, to prevent or treat autoimmune disease. Moreover, the technology might be extended to human disease in human → mouse xenograft models.
NF-κB activation can result from a plethora of cellular stimuli and signaling pathways.20,21,36 Thus NF-κB mediated light emissions could in the present experiments result from a number of independent pathways and settings, with contributions from (i) baseline, homeostatic NF-κB signals, (ii) Id-specific TCR signaling, (iii) autoantigen-specific BCR signaling, (iv) Id-driven T–B collaboration, and (v) autoantibody secretion, immune complex deposition, recruitment of cells to sites of inflammation, tissue destruction, erosions and exposure to intestinal microflora.
As concerns baseline homeostatic signaling (i) during the first 3 weeks of age, NF-κB signals from triply transgenic were confined to lymph nodes and the thymus and were equivalent to littermate controls. This background level of NF-κB could be caused by responses to homeostatic chemokines such as secondary lymphoid-tissue chemokine (SLC, CCL21), stromal cell-derived factor-1 (SDF-1, CXCL12) or to the TNF family member B-cell activating factor (BAFF).37,38,39
Id-specific TCR signaling (ii) probably contributed to the signal since we show that ligation of the Id-specific receptor induced distinct NF-κB signals (most likely via the classical pathway) in both in vitro assays and in adoptive transfer studies. Moreover, superficial lymph nodes (inguinal and cervical) gave strong signals in triple transgenic mice from 5 weeks of age. Interestingly, triple transgenic mice had a strong bioluminescent signal from the thymi even though thymi are quite small in these mice due to negative selection.10,14 Signaling of Id-specific thymocytes and induction of their apoptosis could be the cause of this signal.
Concerning autoantigen-specific BCR signaling and germinal center formation (iii), this would be expected to cause NF-κB activation.20,21,36 Consistent with this, we show that Id+ B cells express luciferase protein but we do not show whether the expression is due to ligation of their BCR by autoantigens or help from Id-specific T cells or both. However, as antinuclear antibody levels correlated with NF-κB/light emissions and disease progression, the importance of BCR signaling for light emission is suggested. An interesting possibility is that anti-dsDNA Id+ B cells, which are frequent in triply transgenic mice (L.M., B.B., manuscript in preparation), can bind chromatin through both BCR and Toll-like receptor 9 (TLR9),40,41 allowing for NF-κB activation via two separate stimulants of the classical pathway.
It would be expected from the two preceding paragraphs that Id-driven T–B collaboration (iv) significantly contributed to the NF-κB signal. Indeed, by development of a staining method for luciferase protein, we could show by immunohistochemistry that luciferase protein, and hence NF-κB activation, was found in Id+ B cells and Id-specific T cells. Moreover, Id-specific T cells positive for luciferase protein and Id+ B cells often interacted in clusters. Finally, interacting T and B cells in vitro produced a bioluminescence signal. These results demonstrate that Id-driven T–B collaboration directly contributed to light emissions.
As for secondary effects of autoantibody production like complement activation and inflammation (v), it is known that both immune complexes and complement factors activate NF-κB in phagocytes, allowing secretion of TNF, TNF-mediated NF-κB activation, and oxidative burst with release of oxygen radicals by phagocytes.20,21,42 Consistent with this, we found that colons with immune complex and complement deposition displayed intense NF-κB activation, and co-localized with phagocyte oxidative bursts visualized in vivo by use of a specific probe (L-012).27 Moreover, mucosal erosions should expose cells in the underlying tissue to the luminal microflora. Indeed, such areas had intense microscopic expression of luciferase protein, probably in part due to activation of the classical NF-κB pathway by pattern recognition receptors.38
We have previously described that Id-driven T–B collaboration results in a systemic disease with SLE-like features in addition to organ specific affections, eg, of the large bowel, skin, and joints.14 We here report a novel site of involvement, the small intestine, where bioluminescence signals were related to the presence of antibodies. With some exceptions such as graft versus host disease30 and experiments with CD8 intraepithelial T cell epitopes,31 the search for murine models of human small intestinal disease has been difficult. However, multiple examples of inflammatory disease of the colon are well established.29 In the Id-driven T–B autoimmunity model herein, autoantibodies toward tTG2 and IgG anti-gliadin antibodies correlated with light emission from the small bowel. In humans, transglutimase is responsible for the deamidation of glutamine residues of wheat gliadin, a requirement for binding of gluten epitopes to HLA-DQ2 in celiac disease.43 tTG2 is increasingly expressed in inflammatory responses, and is up-regulated in cells undergoing apoptosis.44 Whether antibodies to gliadin and tTG2 are directly associated with induction of small intestinal disease, or are secondary phenomena in this model, remain to be elucidated.
Supplementary Material
Acknowledgments
We thank Hilde Omholt and Peter Hofgaard for expert technical help and Dr. Keith Thompson for discussions and valuable comments.
Footnotes
Address reprint requests to Bjarne Bogen (bjarne.bogen@medisin.uio.no) or Ludvig A. Munthe (Ludvig.Munthe@rr-research.no), Centre for Immune Regulation, Institute of Immunology, University of Oslo and Rikshospitalet Medical Centre, N0027 Oslo, Norway.
Supported by the Norwegian Research Council, The University of Oslo, Medinnova and Rikshospitalet Medical Center.
L.A.M. and B.B. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org.
H.C. and R.B. have ownership in Cgene AS that holds the commercial rights to various strains of NF-κB luciferase mice.
References
- Williams WM, Staines NA, Muller S, Isenberg DA. Human T cell responses to autoantibody variable region peptides. Lupus. 1995;4:464–471. doi: 10.1177/096120339500400608. [DOI] [PubMed] [Google Scholar]
- Dayan M, Segal R, Sthoeger Z, Waisman A, Brosh N, Elkayam O, Eilat E, Fridkin M, Mozes E. Immune response of SLE patients to peptides based on the complementarity determining regions of a pathogenic anti-DNA monoclonal antibody. J Clin Immunol. 2000;20:187–194. doi: 10.1023/a:1006685413157. [DOI] [PubMed] [Google Scholar]
- van Schooten WC, Devereux D, Ho CH, Quan J, Aguilar BA, Rust CJ. Joint-derived T cells in rheumatoid arthritis react with self-immunoglobulin heavy chains or immunoglobulin-binding proteins that copurify with immunoglobulin. Eur J Immunol. 1994;24:93–98. doi: 10.1002/eji.1830240115. [DOI] [PubMed] [Google Scholar]
- Holmoy T, Fredriksen AB, Thompson KM, Hestvik AL, Bogen B, Vartdal F. Cerebrospinal fluid T cell clones from patients with multiple sclerosis: recognition of idiotopes on monoclonal IgG secreted by autologous cerebrospinal fluid B cells. Eur J Immunol. 2005;35:1786–1794. doi: 10.1002/eji.200425417. [DOI] [PubMed] [Google Scholar]
- Singh RR, Kumar V, Ebling FM, Southwood S, Sette A, Sercarz EE, Hahn BH. T cell determinants from autoantibodies to DNA can upregulate autoimmunity in murine systemic lupus erythematosus. J Exp Med. 1995;181:2017–2027. doi: 10.1084/jem.181.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RR, Hahn BH. Reciprocal T–B determinant spreading develops spontaneously in murine lupus: implications for pathogenesis. Immunol Rev. 1998;164:201–208. doi: 10.1111/j.1600-065x.1998.tb01221.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Smith DS, Guth A, Wysocki LJ. A receptor presentation hypothesis for T cell help that recruits autoreactive B cells. J Immunol. 2001;166:1562–1571. doi: 10.4049/jimmunol.166.3.1562. [DOI] [PubMed] [Google Scholar]
- Weiss S, Bogen B. B-lymphoma cells process and present their endogenous immunoglobulin to major histocompatibility complex-restricted T cells. Proc Natl Acad Sci USA. 1989;86:282–286. doi: 10.1073/pnas.86.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991;64:767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- Bogen B, Dembic Z, Weiss S. Clonal deletion of specific thymocytes by an immunoglobulin idiotype. EMBO J. 1993;12:357–363. doi: 10.1002/j.1460-2075.1993.tb05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe LA, Kyte JA, Bogen B. Resting small B cells present endogenous immunoglobulin variable-region determinants to idiotope-specific CD4+ T cells in vivo. Eur J Immunol. 1999;29:4043–4052. doi: 10.1002/(SICI)1521-4141(199912)29:12<4043::AID-IMMU4043>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Snyder CM, Zhang X, Wysocki LJ. Negligible class II MHC presentation of B cell receptor-derived peptides by high density resting B cells. J Immunol. 2002;168:3865–3873. doi: 10.4049/jimmunol.168.8.3865. [DOI] [PubMed] [Google Scholar]
- Munthe LA, Os A, Zangani M, Bogen B. MHC-restricted Ig V region-driven T-B lymphocyte collaboration: B cell receptor ligation facilitates switch to IgG production. J Immunol. 2004;172:7476–7484. doi: 10.4049/jimmunol.172.12.7476. [DOI] [PubMed] [Google Scholar]
- Munthe LA, Corthay A, Os A, Zangani M, Bogen B. Systemic autoimmune disease caused by autoreactive B cells that receive chronic help from Ig V region-specific T cells. J Immunol. 2005;175:2391–2400. doi: 10.4049/jimmunol.175.4.2391. [DOI] [PubMed] [Google Scholar]
- Snyder CM, Aviszus K, Heiser RA, Tonkin DR, Guth AM, Wysocki LJ. Activation and tolerance in CD4+ T cells reactive to an immunoglobulin variable region. J Exp Med. 2004;200:1–11. doi: 10.1084/jem.20031234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen B, Weiss S. Processing and presentation of idiotypes to MHC-restricted T cells. Int Rev Immunol. 1993;10:337–355. doi: 10.3109/08830189309061709. [DOI] [PubMed] [Google Scholar]
- Bogen B, Jorgensen T, Hannestad K. T helper cell recognition of idiotopes on lambda 2 light chains of M315 and T952: evidence for dependence on somatic mutations in the third hypervariable region. Eur J Immunol. 1985;15:278–281. doi: 10.1002/eji.1830150313. [DOI] [PubMed] [Google Scholar]
- Bogen B, Malissen B, Haas W. Idiotope-specific T cell clones that recognize syngeneic immunoglobulin fragments in the context of class II molecules. Eur J Immunol. 1986;16:1373–1378. doi: 10.1002/eji.1830161110. [DOI] [PubMed] [Google Scholar]
- Eyerman MC, Zhang X, Wysocki LJ. T cell recognition and tolerance of antibody diversity. J Immunol. 1996;157:1037–1046. [PubMed] [Google Scholar]
- Karin M, Lin A. NF-[kappa]B at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-B activity. Ann Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Carlsen H, Moskaug JO, Fromm SH, Blomhoff R. In vivo imaging of NF-[kappa]B activity. J Immunol. 2002;168:1441–1446. doi: 10.4049/jimmunol.168.3.1441. [DOI] [PubMed] [Google Scholar]
- Bogen B, Weiss S. A rearranged lambda 2 light gene chain retards but does not exclude kappa and lambda 1 expression. Eur J Immunol. 1991;21:2391–2395. doi: 10.1002/eji.1830211015. [DOI] [PubMed] [Google Scholar]
- Bogen B, Gleditsch L, Weiss S, Dembic Z. Weak positive selection of transgenic T cell receptor-bearing thymocytes: importance of major histocompatibility complex class II. T cell receptor and CD4 surface molecule densities Eur J Immunol. 1992;22:703–709. doi: 10.1002/eji.1830220313. [DOI] [PubMed] [Google Scholar]
- Bogen B, Lauritzsen GF, Weiss S. A stimulatory monoclonal antibody detecting T cell receptor diversity among idiotype-specific, major histocompatibility complex-restricted T cell clones. Eur J Immunol. 1990;20:2359–2362. doi: 10.1002/eji.1830201030. [DOI] [PubMed] [Google Scholar]
- Nishinaka Y, Aramaki Y, Yoshida H, Masuya H, Sugawara T, Ichimori Y. A new sensitive chemiluminescence probe. L-012, for measuring the production of superoxide anion by cells. Biochem Biophys Res Commun. 1993;193:554–559. doi: 10.1006/bbrc.1993.1659. [DOI] [PubMed] [Google Scholar]
- Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- Cornelius EA. Protein-losing enteropathy in the graft-versus-host reaction. Transplantation. 1970;9:247–252. doi: 10.1097/00007890-197003000-00008. [DOI] [PubMed] [Google Scholar]
- Vezys V, Lefrancois L. Inflammatory signals drive organ-specific autoimmunity to normally cross-tolerizing endogenous antigen. J Immunol. 2002;169:6677–6680. doi: 10.4049/jimmunol.169.12.6677. [DOI] [PubMed] [Google Scholar]
- Edinger M, Sweeney TJ, Tucker AA, Olomu AB, Negrin RS, Contag CH. Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia. 1999;1:303–310. doi: 10.1038/sj.neo.7900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt IJ, Gambhir SS. Molecular imaging applications for immunology. Clin Immunol. 2004;111:210–224. doi: 10.1016/j.clim.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Kim SB, Ozawa T, Watanabe S, Umezawa Y. High-throughput sensing and noninvasive imaging of protein nuclear transport by using reconstitution of split Renilla luciferase. Proc Natl Acad Sci USA. 2004;101:11542–11547. doi: 10.1073/pnas.0401722101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissleder R. A clearer vision for in vivo imaging. Nature Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- Ruland J, Mak TW. Transducing signals from antigen receptors to nuclear factor kappaB. Immunol Rev. 2003;193:93–100. doi: 10.1034/j.1600-065x.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Enzler T, Bonizzi G, Silverman GJ, Otero DC, Widhopf GF, Anzelon-Mills A, Rickert RC, Karin M. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403–415. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi G, Montecucco F, Bertolotto M, Dallegri F, Ottonello L. Immune complexes induce monocyte survival through defined intracellular pathways. Ann NY Acad Sci. 2007;1095:209–219. doi: 10.1196/annals.1397.025. [DOI] [PubMed] [Google Scholar]
- Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- Fesus L, Szondy Z. Transglutaminase 2 in the balance of cell death and survival. FEBS Lett. 2005;579:3297–3302. doi: 10.1016/j.febslet.2005.03.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.