Abstract
β-glucosylceramide has been shown to affect natural killer T cell function in models of inflammation. We, therefore, investigated the effects of different β- glycosphingolipids, including β-glucosylceramide, on STAT (signal transducers and activators of transcription) signaling pathways and determined whether these effects were mediated by lipid raft microdomains and/or CD1d molecules. The effects of α- and β-structured ligands on the lipid raft protein flotillin-2 were studied in both natural killer T hybridoma cells and leptin-deficient mice. To determine whether CD1d was involved in the effects of the β-glycosphingolipids, an anti-CD1d blocking antibody was used in a cell proliferation assay system. The downstream effects on the protein phosphorylation levels of STAT1, STAT3, and STAT6 were examined in both immune-mediated hepatitis and hepatoma models. The effects of β-glycosphingolipids on the STAT signaling pathways were found to be dependent on CD1d. Lipid rafts were affected by both the dose and ratio of the β-glycosphingolipids and the acyl chain length, and these effects were followed by downstream effects on STAT proteins. Our results show that β-glycosphingolipids have beneficial effects in natural killer T cell-dependent immune-mediated metabolic and malignant animal models in vivo.
Valpha14 invariant natural killer T (iNKT) cells comprise a unique T cell lineage that shares properties with both natural killer and memory T cells.1 Lymphocytes in this subset recognize CD1d-associated glycosphingolipids via a semi-invariant T cell antigen receptor (TCR), which is composed of an invariant Valpha14-Jalpha18 chain paired preferentially with a restricted set of TCR β chains.2,3 This semi-invariant TCR is specific for glycolipid antigens presented by the major histocompatibility complex class I-like molecule CD1d.3 CD1 proteins restrict the antigen-specific responses of NKT cells in humans and mice. The CD1d-iNKT cell system functions as a sensor for alterations in cellular lipid content, and CD1d-binding ligands can modulate NKT cell responses.4,5,6
During NKT cell development, immature, uncommitted CD4+CD8+ thymocytes that rearrange the semi-invariant TCR are directed to the iNKT cell lineage via interactions with endogenous CD1d-associated glycosphingolipids.1 The developmental program of iNKT cells is regulated by a number of signaling cues that have little or no effect on conventional T cells, including the Fyn/SAP/SLAM pathway, the nuclear factor-κB and T-bet transcription factors, and interleukin (IL)-15.7,8,9 The dependency of NKT cell development on Fyn, a Src family kinase member, distinguishes NKT cells from conventional T cell populations.10,11 Diverse mutations that interfere with lysosomal processing and glycolipid degradation also affect NKT cell development. In general, lysosomal storage diseases can disrupt CD1d antigen presentation, hindering NKT cell development.4,12,13
The sea sponge-derived agent α-galactosylceramide (αGalCer) selectively stimulates NKT cells in vivo.14,15,16 Administration of αGalCer potently activates NKT cells, induces rapid and robust cytokine production, and activates a variety of cells of the innate and adaptive immune systems.17 A truncated αGalCer analogue, (2S,3S,4R)-1-O-(alpha-D-galactopyranosyl)-N-tetracosanoyl-2-amino-1,3,4-nonanetriol (OCH), selectively stimulates NKT cells to produce IL-4 and IL-10. The crystal structure of CD1d complexed with a short-chain synthetic variant of αGalCer revealed the basis of the high specificity of CD1d for microbial ligands and demonstrated the restriction of the α-linkage as a unique pathogen-specific pattern-recognition motif. The differential T helper type 1-like and T helper type 2-like properties of NKT cells arise largely from differences in ligand binding.18,19,20 Once loaded onto the CD1d, the binding of glycosphingolipids by the Valpha14 Jalpha18 chain of the TCR can be paired with the binding of an NK1.1 cell-derived Vbeta chain or any Vbeta8 chain to achieve high-affinity recognition of the glycolipid. This suggests that the iNKT TCR has stricter requirements for recognizing αGalCer, and it is assumed that the most rigid part of αGalCer, the sugar moiety, is critical for the interaction.21
α-Anomeric d-glycosylceramides are not found in mammals, and the mechanism of the effect of β-structured ligands is unknown. The results of recent studies suggest that β-structured glycosphingolipids are potent NKT cell ligands.22 β-glucosylceramide (β-GC), a naturally occurring glycolipid, is a metabolic intermediate in the anabolic and catabolic pathways of glycoglycosphingolipids.23 Its synthesis from ceramide is catalyzed by the enzyme glucosylceramide synthase.22 An inherited deficiency of this lysosomal hydrolase results in high serum levels of β-GC and in Gaucher’s disease. Patients with Gaucher’s disease have altered humoral and cellular immune profiles and a distorted NKT lymphocyte number and function.22 In vitro, CD1d-bound β-GC inhibits NKT cell activation by αGalCer.21 β-galactosylceramide (β-d-GalCer)-deficient mice exhibit normal NKT cell development and function, and cells from these animals can stimulate NKT hybridomas.24 β-d-GalCer (C12) efficiently reduces the number of detectable NKT cells in vivo without inducing cytokine expression.25
Lipid rafts are highly ordered cholesterol and ganglioside-rich platforms abundant in the plasma membrane. These submicroscopic assemblies facilitate and coordinate close interactions between critical signaling molecules to amplify downstream signaling.26 A number of proteins involved in signal transduction copurify with lipid rafts, such as the dually acylated Src-family kinases, LCK and FYN.27 CD1d is also raft-localized and disruption of the lipid raft structures blocks efficient signaling of αGalCer through mCD1d, indicating that lipid rafts are required for efficient signal transduction by CD1d.27,28
The administration of β-GC in vivo attenuates NKT cell-mediated damage in animal models of Con A hepatitis, immune-mediated colitis, and diabetes.29,30,31 Preliminary data suggest that β-GC has a similar role in humans.32 The aims of the present study were to investigate the effects of different β-glycosphingolipids on distinct STAT signaling pathways in vitro and in vivo in the several models, and to determine whether the effects are lipid raft microdomain- and CD1d molecule-dependent in vitro and in some of the tested in vivo models.
Materials and Methods
Effect of β-Structured Ligands on Lipid Raft-Associated Proteins
Cells
The NKT hybridoma cell line DN32.D3, a thymocyte-specific clone (kindly provided by Dr. Albert Bendelac, University of Chicago, Chicago, IL), was used for the isolation of lipid rafts and for the detection of flotillin-2 within the raft domains. Dually acylated Src-family kinases, LCK and FYN, and STAT 1 and 3 proteins were tested in this cell line. Cells were stimulated in tissue culture flasks with 500 ng/ml of β-GC, β-lactosylceramide (β-LC), a 1:1 combination of β-GC and β-LC (β-IGL), or PBS. DN32.D3 cells were stimulated by various β-glycosphingolipids, and cell proliferation was measured by [3H] thymidine incorporation after 72 hours.
Isolation of Lipid Rafts
Flotation studies were performed as described previously. Aliquots of 3 × 107 freshly isolated lymphocytes were washed three times in ice-cold PBS. Cells were lysed in TNE buffer (150 mmol/L NaCl, 25 mmol/L Tris–HCl, and 5 mmol/L EDTA, pH 7.5) containing 1% Triton X-100 for 30 minutes at 4°C. Supernatants were transferred to TLS-55 (Beckman Instruments, Fullerton, CA) centrifuge tubes and adjusted to 35% Nycodenz (Sigma-Aldrich, St. Louis, MO) by adding an equal volume of ice-cold 70% Nycodenz dissolved in TNE buffer. TNE buffer with an 8% to 25% Nycodenz linear step gradient was added above the lysate. Samples were centrifuged at 100,000 ×g for 4 hours at 4°C. Twelve 180-μl fractions were sequentially collected from the top. The presence of GM1 was determined by spotting 10 μl of each fraction onto a Nytran N membrane (Schleicher & Schuell, Germany), which was then blocked with 5% bovine serum albumin in PBS, and reacted with CTxB- horseradish peroxidase (Sigma, 12.5 ng/ml) for 30 minutes followed by four washes with TBS-T. Membranes were developed using Western blot-Luminol Reagent (Santa Cruz Biotechnology, Santa Cruz, CA).
Expression of Raft-Associated Proteins and Transcription Factors
Equal volumes of each gradient fraction were separated by SDS-polyacrylamide gel electrophoresis (10% acrylamide) and subjected to immunoblot analysis. The following antibodies were used: anti-flotillin-2 (1:5000, BD Biosciences, Franklin, NJ), anti-LCK and anti-FYN (1:1000, NeoMarkers, Stratech, Suffolk, UK), and anti-STAT 1 and 3 (1:1000, Cell Signaling, Danvers, MA). Horseradish peroxidase-coupled antibodies (Jackson Immuno Research, Bar Harbor, ME) were used as secondary antibodies and detection was performed with Western blot-Luminol Reagent (Santa Cruz Biotechnology).
Binding of β-Ligands to CD1d Receptors
[3H] Thymidine Incorporation Assays
Assays were performed using CD1d–NKT restricted hybridomas (DN32.D3) as described previously.33 Anti-CD1d blocking antibody (5 μg/ml; BD Biosciences) was used to block CD1d receptors.
Animals and Experimental Groups
β-GC, β-D-GalCer, and β-LC were purchased from Avanti Polar Lipids (Alabaster, AL), dissolved in ethanol, and emulsified in PBS. Ceramide, iGb3, and αGalCer were purchased from Alexis Biochemicals (San Diego, CA).
Eight-week-old male leptin-deficient ob/ob mice were purchased from Jackson laboratories. Animals were housed in the Animal Core of the Hadassah-Hebrew University Medical School. Mice were given standard laboratory chow and water ad libitum and housed under 12-hour light/dark cycles. Animal experiments were performed according to the guidelines of the Hebrew University-Hadassah Institutional Committee for Care and Use of Laboratory Animals, with the committee’s approval. Leptin-deficient ob/ob mice (10/group) received daily intraperitoneal injections of PBS, or 2.5 mg/kg β-GC, β-LC, or a 1:1 combination of both (β-IGL) for 6 weeks.
Follow-Up Parameters
The expression of hepatic flotillin-2 by mice in both the soluble and insoluble fractions was determined as described above. The effect of alterations in STAT pathways on metabolic syndrome was determined by glucose tolerance tests, determination of serum triglyceride levels, and assessment of liver weight and histology. Mice underwent a glucose tolerance test on day 60. Glucose was administered orally (1 g/kg), and serum glucose measurements were performed on tail-vein blood collected every 15 minutes for 3 hours. Glucose levels were measured using a standard glucometer. On day 60, serum triglyceride levels were measured using a spectrophotometer (Cobas DP-25P). For histological analysis, a liver segment from each mouse was fixed in 10% formaldehyde and embedded in paraffin. Five sections (5 μm) were stained with H&E and Oil Red-O for quantitative assessment of hepatic fat and were reviewed by two pathologists in a blinded manner.34
Downstream Effects of β-Ligands on STAT1, STAT3, and STAT6 Pathways
Animals
Male C57Bl/6 mice (11 to 12 weeks old) were obtained from Harlan Laboratories (Jerusalem, Israel).
Induction of Hepatitis by Concanavalin A
Concanavalin A (Con A, MP Biomedical, Irvine, CA) was dissolved in 50 mmol/L Tris (pH 7), 150 mmol/L NaCl, and 4 mmol/L CaCl2 and injected into the tail vein at a dose of 500 μg/mouse (15 mg/kg). Con A hepatitis was induced in C57BL/6 mice (10 mice/group) injected 2 hours prior with 0.5 to 75 μg/mouse of various β-glycosphingolipids, 1 μg/mouse of αGalCer, a combination of both, or 100 μl PBS. Animals were sacrificed 10 hours after the β-glycolipid injections, 8 hours after injection of ConA.
Expression of STAT Proteins
The levels of STAT1, phosphorylated STAT1, and STAT6 were determined by Western blot analysis of splenocytes harvested from mice in all groups. Splenocytes were lysed in 200 μl ice-cold RIPA buffer containing protease inhibitor cocktail (Sigma). Lysates were left on ice for 30 minutes, then centrifuged twice for 10 minutes in 4°C. Supernatants were taken and protein concentrations were determined by Bradford assay (Bio-Rad, Hercules, CA). β- Actin (Abcam, UK) was used as a loading control. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes and blocked with 5% nonfat dry milk. The following antibodies were used: STAT1, STAT6, and phospho-specific STAT1-Tyr701 (Cell Signaling35). Horseradish peroxidase-coupled antibodies (Jackson Immuno Research) were used as secondary antibodies, and detection was performed using Western blot-Luminol Reagent (Santa Cruz Biotechnology).
Isolation of Splenocytes and Intrahepatic Lymphocytes
Splenocytes and intrahepatic lymphocytes were isolated as follows: livers and spleens were placed in RPMI-1640 + fetal bovine serum and spleens were filtered through a 70-μm nylon cell strainer36 and centrifuged (1250 rpm for 7 minutes) to remove the debris. Red blood cells were lysed with 1 ml of cold 155 mmol/L ammonium chloride lysis buffer and immediately centrifuged (1250 rpm for 3 minutes). Splenocytes were then washed and resuspended with 1 ml RPMI + fetal bovine serum (5%). The remaining connective tissue was removed. Cell viability, as assessed by trypan blue staining, was above 90%. For intrahepatic lymphocytes, livers were first filtered through a stainless steel mesh (size 60, Sigma) and the cell suspension was placed in a 50-ml tube for 5 minutes to allow cell debris to settle to the bottom. Lymphoprep (10 ml; Ficoll, Axis-Shield PoC AS, Oslo, Norway) was slowly placed under the same volume of cell suspension in 50-ml tubes. The tubes were then centrifuged at 1800 rpm for 18 minutes. Cells in the interface were collected and moved to new tubes, which were centrifuged again at 1800 rpm for 10 minutes, to obtain a pellet of cells depleted of hepatocytes in a final volume of 250 μl. Approximately 1 × 106 cells/mouse liver were recovered. Following lymphocyte isolation, flow cytometry was performed using 1 × 106 lymphocytes in 100 μl PBS. To determine the percentage of NKT lymphocytes, we used phycoerythrin-conjugated Cy5 anti-mouse CD3 and anti-mouse NK1.1 antibodies (eBioscience, San Diego, CA).
Assessment of the Effect of β-Glycosphingolipids on Liver Damage
Liver Enzymes
Animals were sacrificed 10 hours after the glycolipid injections and 8 hours after the injection of Con A. Sera from individual mice were obtained. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were determined using a Kodak automatic analyzer.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling Staining
The terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay was performed on paraffin-embedded liver sections using a commercially available kit, according to the manufacturer’s instructions (In Situ Cell Death Detection kit, Fluorescein, Roche Diagnostics, Germany). TUNEL-positive cells were characterized morphologically by staining with 4′,6-diamidino-2-phenylindole. Slides were visualized using a Zeiss Axioplan fluorescence microscope (Oberkochen, Germany). Images were collected with a cooled charge-coupled device camera (Quantix Corp., Cambridge, MA) using Image Pro software. For cell counting in tissues processed for TUNEL staining, five standardized microscopic fields (×20 objective) per slide (per animal) were digitally photographed under the fluorescence microscope and then quantified using Total Lab software (Phoretix, Durham, NC).
Alterations of STAT1, STAT4, and STAT6 Phosphorylation Levels Induced by Different Ratios and Doses of β-Glycosphingolipids
Different ratios and doses of β-ligands were investigated in the Con A hepatitis model. Thirteen groups of mice (Table 1), 10 mice per group, were treated with Con A and different ratios and doses of β-glycosphingolipids as described above. STAT1, STAT4, and STAT6 phosphorylation levels were examined. The effects of signaling pathway alterations on NKT lymphocyte distribution were determined by fluorescent-activated cell sorting analysis of intrahepatic and intrasplenic NKT lymphocytes. The effects on immune-mediated hepatitis were evaluated by TUNEL assay, serum interferon-γ levels, and enzyme levels.
Table 1.
Effects of Different Ratios and Doses of β-Glycosphingolipids on Liver Enzyme Activity in the Con A Hepatitis Model (Percentage of Control)
| Group | Treatment | ALT (%) | AST (%) |
|---|---|---|---|
| A | PBS | 100 | 100 |
| B | GC 0.5 mg/mouse | 81.63 | 80.46 |
| C | LC 0.5 mg/mouse | 77.54 | 78.06 |
| D | IGL (0.25 + 0.25) mg/mouse | 63.32 | 81.23 |
| E | GC 1.5 mg/mouse | 58.40 | 61.00 |
| F | LC 1.5 mg/mouse | 62.11 | 65.29 |
| G | IGL 0.75 + 0.75 mg/mouse | 47.17 | 48.42 |
| H | GC 15 mg/mouse | 65.58 | 68.01 |
| I | LC 15 mg/mouse | 66.54 | 64.14 |
| J | IGL 7.5 + .7.5 mg/mouse | 83.62 | 68.73 |
| K | GC 75 mg/mouse | 41.78 | 45.74 |
| L | LC 75 mg/mouse | 64.00 | 65.28 |
| M | IGL 37.5 + 37.5 mg/mouse | 55.22 | 55.35 |
Influence of Acyl Chain Length on the Immunomodulatory Effects of β-Structured Ligands
Athymic BALB/c mice (8 mice/group) were sublethally irradiated and transplanted with Hep3B hepatocellular carcinoma, followed by daily intraperitoneal injections of β-GC or β-LC with 8 or 12 carbons in the acyl chain (β-GC8, β-GC12, β-LC8, or β-LC12, respectively) or PBS for 42 days. The tumor volume in each animal was assessed. To determine the effect of acyl chain length on NKT distribution and intrahepatic CD8 lymphocyte trapping, fluorescent-activated cell sorting analysis was performed on lymphocytes isolated from the spleen and liver.
Statistical Analysis
Comparisons of two independent groups were performed using the Student’s t-test. Multiple comparisons were assessed by the analysis of variance test. All tests applied were two-tailed, and a P value of 0.05 or less was considered to be statistically significant.
Results
β-Glycosphingolipids Affect Lipid Raft-Associated Markers
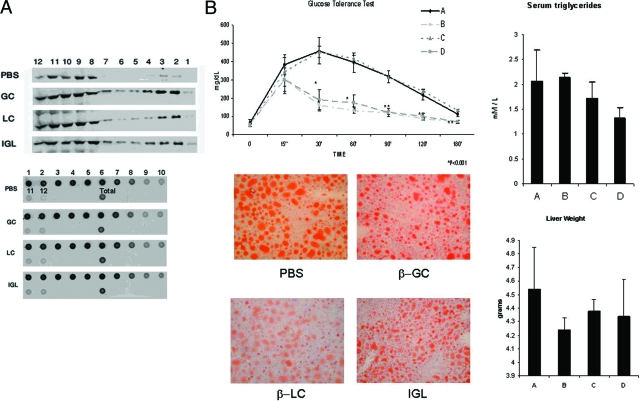
Administration of β-structured glycosphingolipids to NKT hybridoma cells (DN32.D3) in vitro in the presence of antigen presenting cells led to flotillin-2 recruitment to lipid raft fractions (Figure 1A, upper panel). Recruitment of the Src-family kinase LCK was not affected (data not shown). GM1, a lipid raft marker, was detected in cell membrane fractions 1–6, but no significant effects of β-glycosphingolipids on its content were noted (Figure 1A, lower panel).
Figure 1.
A: Effect of β-structured ligands on the lipid raft protein flotillin-2. Administration of β-structured glycosphingolipids to DN32.D3NKT hybridoma cells in the presence of dendritic cells led to prominent flotillin-2 recruitment to raft fractions. The upper panel is a representative Western blot indicating the expression of flotillin-2. The lower panel is a representative dot blot indicating the expression of GM1. The numbers in both panels indicate the fraction number of samples obtained from a Nycodenz linear step gradient. B: Effect of treatment of Ob/Ob mice with β-glycosphingolipids for 6 weeks on glucose intolerance (P < 0.005 for B and D versus A), serum triglycerides (P < 0.005 for D versus A), intrahepatic fat accumulation, and liver weight (P < 0.005 for B, C, and D versus A). Experimental groups are specified in Table 1. Bars shows SD. *P < 0.05, **P < 0.001.
In vivo administration of β-glycosphingolipids to leptin-deficient ob/ob mice increased the expression of hepatic flotillin-2 in both the soluble and insoluble fractions. These alterations in signaling pathways were associated with the alleviation of NKT cell-dependent metabolic derangements in ob/ob mice. β-glycosphingolipids administration ameliorated glucose intolerance (β-GC and β-IGL, Figure 1B), decreased serum triglyceride levels (β-IGL), reduced intrahepatic fat accumulation (β-GC, β-LC, and β-IGL), and decreased liver weight (β-GC, β-LC, and β-IGL).
The Effect of β-Glycosphingolipids Is Dependent on the CD1d Receptor
Proliferation of NKT hybridoma cells (DN32.D3) in the presence of spleen-derived dendritic cells was assessed by [3H]thymidine incorporation assay. The administration of β-IGL stimulated NKT cell proliferation, in contrast with β-GC or β-LC administration alone, which resulted in NKT cell inhibition (122%, 77%, and 76%, respectively, vs. phosphate buffered saline [PBS]-treated cells, P < 0.005; Figure 2). The role of CD1d in this effect was determined by blocking the CD1d receptor with an anti-CD1d antibody, resulting in stimulation of cell proliferation for β-GC and β-LC-treated groups (P < 0.005; Figure 2). αGalCer induced a 50% increase in NKT proliferation, which was blocked by the anti-CD1d antibody.
Figure 2.
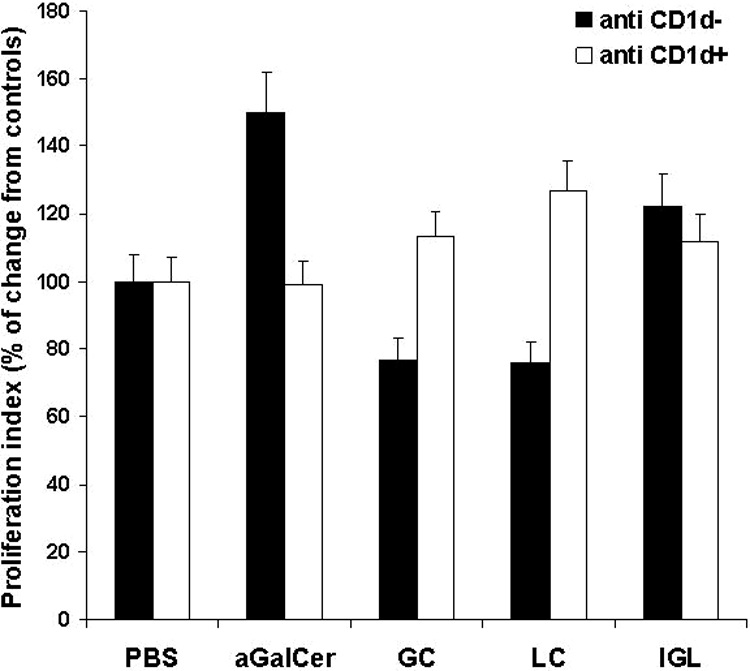
Effect of β-glycosphingolipids with or without anti-CD1d antibody on NKT cell proliferation in vitro. A thymidine incorporation assay was conducted, and the percentage of change from PBS-treated controls is shown.
Downstream Effects of β-Glycosphingolipids on STAT 1, 3, and 6 Pathways, and Liver Injury
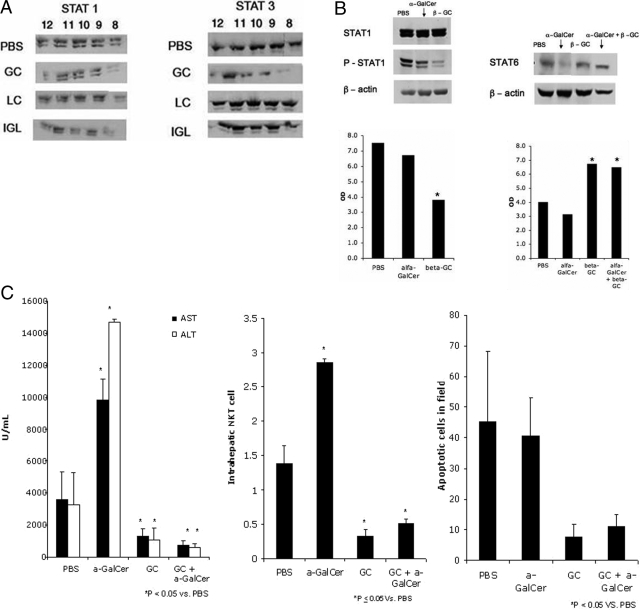
Alterations of raft microdomains by β-ligands was associated with decreased STAT1 levels in these fractions (Figure 3A). STAT3 levels within raft fractions were increased in β-LC- and β-IGL-treated cells, in contrast to a decrease in β-GC-treated cells.
Figure 3.
A: Downstream effects of β-ligands on STAT1 and STAT3 pathways. Alterations in raft microdomains were associated with decreased STAT1 localization in some of the cytosolic raft fractions of β-ligand-treated cells and increased STAT3 localization in raft fractions of β-LC- and β-IGL-treated cells. B: In the mouse Con A hepatitis model, splenic phosphorylated STAT1 decreased following treatment with αGalCer and β-GC. Hepatic STAT6 expression increased following combined treatment with αGalCer and β-GC. C: Serum transaminase activities, NKT cell numbers, and TUNEL quantification of apoptotic cells for control and α- and/or β-glycolipid-treated mice. *P < 0.05.
In the ConA NKT-dependent immune hepatitis model, splenic STAT1 phosphorylation decreased by 11% following αGalCer treatment compared with a 51% decrease in β-GC-treated mice (Figure 3B, P = NS). Administration of β-GC to αGalCer-treated mice, however, significantly increased STAT1 phosphorylation (30%, P < 0.005; data not shown). Hepatic STAT6 expression increased by 62% (Figure 3B, P < 0.005), along with a 112% increase in serum IL-4 levels following treatment with β-GC (data not shown). In contrast, a small decrease in STAT 6 expression was detected following αGalCer treatment. STAT6 phosphorylation was not detectable.
β-GC treatment in ConA hepatitis was associated with a decrease in intrahepatic NKT cell number, in contrast with a significant increase in NKT cell number in αGalCer-treated mice (P < 0.005; Figure 3C). Administration of β-GC to αGalCer-treated mice significantly suppressed the intrahepatic NKT cell number (66% decrease versus control, P < 0.05). These effects of β-GC were associated with protection of the liver from αGalCer-induced, immune-mediated damage. Administration of αGalCer led to increased serum transaminase levels versus the control, in contrast with decreased levels when β-GC was co-administered with αGalCer (increases of 136% and 231% and decreases of 76% and 81% for AST and ALT, respectively, P < 0.005; Figure 3C). β-GC treatment led to a marked decrease in TUNEL-positive hepatocytes compared with the control (82% decrease, P < 0.05; Figure 3C). Administration of β-GC to αGalCer-treated mice led to a 61% decrease in apoptosis (P < 0.05).
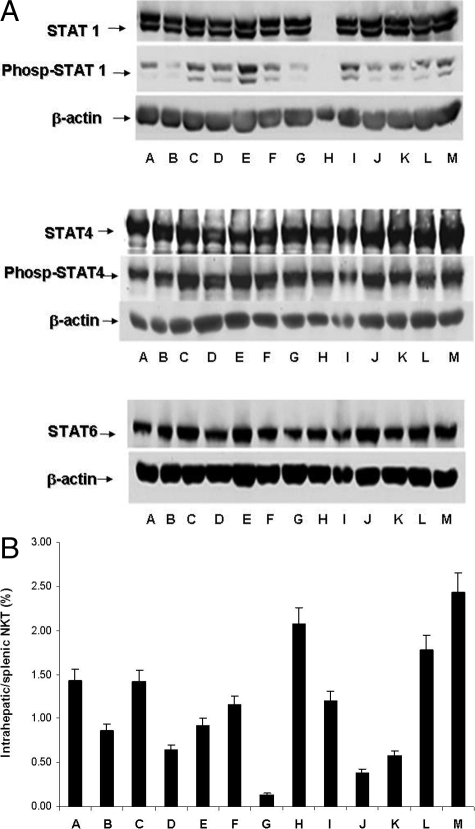
STAT1, STAT4, and STAT6 Phosphorylation Is Dependent on the β-Glycosphingolipid Dose
Different doses of β-glycosphingolipids in the Con A hepatitis model affected STAT1and STAT4 phosphorylation and STAT1, STAT4, and STAT6 expression in the spleen (Figure 4A). Alterations in doses and combinations of β-glycosphingolipids were associated with qualitative alterations in NKT lymphocyte distribution (Figure 4B) and with disease amelioration, as manifested by decreased AST and ALT levels (Table 1). A correlation between the decrease in intrahepatic NKT cells and liver enzymes was noted for mice treated with β-GC (groups B, E, and K) or IGL (groups D and J).
Figure 4.
A: Effects of different ratios and doses of β-ligands on phosphorylated STAT1, STAT4, and STAT6 levels in the mouse Con A hepatitis model. Treatments A–M are specified in Table 1. B: Effects of different ratios and doses of β-ligands on NKT lymphocyte distribution in the mouse Con A hepatitis model. FACS analysis was preformed for CD3/NK1.1 surface markers on lymphocytes in the spleen and in the liver.
A decrease in STAT1 phosphorylation was noted for most β-glycosphingolipid-treated mice. The most significant decrease was noted for group G mice, which correlated with marked suppression of intrahepatic NKT lymphocytes and dramatically decreased serum ALT and AST levels. STAT 4 phosphorylation was up-regulated by β-glycosphingolipid treatment.
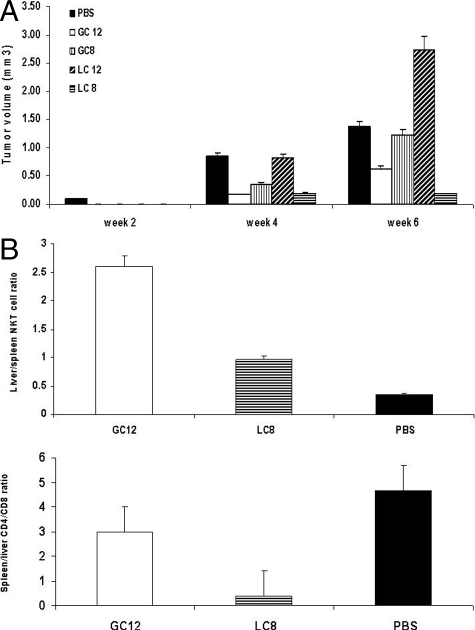
β-Glycosphingolipid Acyl Chain Length Determines the NKT Cell-Mediated Anti-Tumor Effect
Administration of β-GC12 and β-LC8 suppressed hepatocellular carcinoma growth. Maximal tumor volumes were 0.63 (β-GC12) and 0.19 (β-LC8) compared with 1.22 (β-GC8) and 2.72 mm3 (β-LC12) (Figure 5A, P < 0.05). β-GC12 and β-LC8 exerted profound effects on NKT and CD8 lymphocyte distribution, manifested by increased intrahepatic NKT lymphocytes and decreased intrahepatic CD8 T lymphocyte trapping (Figure 5B). The liver/spleen NKT lymphocyte ratios were 2.6 (β-GC12) and 0.97 (β-LC8) as compared with 0.35 (PBS) (P < 0.05). The spleen/liver CD4/CD8 lymphocyte ratios were 3.0 (β-GC12) and 0.39 (β-LC8) compared with 4.68 (PBS) (Figure 5B, P < 0.005). These results suggest that there is a correlation between lipid chain length and the anti-tumor effect of NKT cells.
Figure 5.
A: Influence of β-glycolipid acyl chain length on the anti-tumor effect. Hepatocellular carcinoma- bearing mice were treated with β-ligands for 6 weeks. Tumor volumes were reduced in β-GC12- and β-LC8-treated mice compared with β-GC8- and β-LC12-treated mice. B: β-GC12 and β-LC8 increased intrahepatic NKT lymphocytes and decreased intrahepatic CD8 T lymphocyte trapping.
Discussion
The results of the present study indicated that β-glycosphingolipids affect STAT signaling pathways in CD1d and flotillin-2 raft protein-dependent manners. The β-structure, the alteration of the carbon number in the acyl chain, and the use of several combinations of β-analogues in different doses affected lipid raft structure, followed by downstream effects on STAT proteins. These β-sphingolipid–induced effects on STAT signaling were associated with beneficial effects in several models of NKT cell-dependent, immune-mediated, metabolic, or malignant disorders.
NKT lymphocytes respond to a variety of self ligands and environmental stimuli, including disease-target antigens, antigen-presenting cells, co-stimulatory signals, soluble factors, and effector cells.37,38 The activation and development of iNKT cells are guided by signals from glycolipid metabolic pathways.39,40 iNKT cell function is controlled by affinity thresholds for glycolipid antigens.24
The presentation of a neo-self glycolipid by an infectious assault of antigen-presenting cells activates iNKT cells, releasing pro-inflammatory or anti-inflammatory cytokines.41 Several glycosphingolipids and phospholipids derived from mammalian, bacterial, protozoan, and plant species have been identified as possible natural ligands for NKT cells.18,19,20,42 The semi-invariant αβTCRs can recognize the mammalian glycosphingolipid isoglobotrihexosylceramide (iGb3) and microbial α-glycuronylceramides found in the cell walls of Gram-negative, lipopolysaccharide-negative bacteria.43 iGb3 is candidate molecule recognized by NKT cells under pathophysiologic conditions, such as cancer and autoimmune diseases.43,44 A recent study indicated that iGb3 is unlikely to be an endogenous CD1d lipid ligand determining thymic iNKT selection.45 Although β-GC was unable to activate NKT hybridomas in a cell-free antigen-presentation assay,24 it does have a role in loading natural Vα14Jα18 NKT antigens.
Lipid rafts are highly dynamic, submicroscopic assemblies enriched in glycosphingolipids and cholesterol with saturated acyl chains.46 Specific signaling proteins segregate into lipid rafts, and this process is important for certain signal transduction events in a variety of cell types.47 CD1d, a raft-localized receptor, is essential for raft glycolipid-mediated activation and disruption of the lipid raft structures blocks efficient signaling through mCD1d.28 β-glycosphingolipids are normal constituents of cell membranes.48,49 The results of the present study suggest that β- glycolipid administration is associated with prominent flotillin-2 recruitment to raft fractions in vitro, but does not affect LCK or GM1.
Flotillin-2 is a plasma membrane-associated cytoplasmic protein that is enriched in detergent-insoluble membrane fractions. Lipid raft flotillins are involved in tyrosine kinase activation of protein kinases.50 On T cell stimulation, flotillin-2 in T cells co-localizes with activated glycosylphosphatidylinositol-linked proteins and the Fyn kinase, participating in the assembly of protein complexes essential for signal transduction.51 Increased association of cellular flotillin with lipid raft microdomains during chemokine exposure is important in chemokine receptor signaling and receptor partitioning in lipid rafts.52 Flotillin-2 is an integral component of raft microdomains, which represent the units of function at the cell surface through which ligand-stimulated STAT signaling is initiated.53 Methyl-β-cyclodextrin, which sequesters cholesterol and disrupts plasma membrane rafts, inhibits IL-6- and interferon-γ-induced STAT signaling.
In the present study, downstream effects of β-glycosphingolipids on STAT protein pathways were demonstrated in CD3+NK1.1+ cell-dependent models. Alterations in STAT1 and STAT3 phosphorylation were noted in cytosolic raft fractions in leptin-deficient ob/ob mice. These downstream signaling changes were associated with amelioration of several metabolic derangements. Direct interactions of resident raft proteins, caveolins, and flotillin with insulin-signaling molecules may organize these molecules to ensure accurate transduction of the insulin signal in adipocytes.47,54 β-glycolipid-mediated alterations of STAT1 and STAT6 pathways were also associated with amelioration of αGalCer-induced liver damage. Administration of β-GC in the ConA hepatitis model decreased STAT1 phosphorylation. The administration of β-GC to αGalCer-treated mice also decreased STAT1 phosphorylation. Hepatic STAT6 expression increased following combined β-GC treatment, suggesting the up-regulation of IL-4, an anti-inflammatory cytokine. Different combinations of β-ligands were tested, and the results demonstrated versatile effects of β-analogues on STAT1, STAT4, and STAT6 expression and phosphorylation. These changes in STAT pathways were associated with altered NKT lymphocyte distribution and disease amelioration. Administration of β-GC had significant inhibitory effects on intrahepatic NKT lymphocytes, and administration of β-GC to αGalCer-treated mice suppressed the intrahepatic NKT cell number.
Lipid rafts are required for efficient signal transduction by CD1d and presentation of αGalCer by CD1d to NKT cells is facilitated by lipid rafts.55 Raft disruption inhibits IL-6/STAT3 and interferon-γ/STAT1 signaling, suggesting that almost all signaling by these cytokines is initiated in raft microdomains.56 Blocking the CD1d receptor inhibited NKT cell stimulation by the synthetic ligand αGalCer, but the effect of CD1d blocking on NKT activation via natural β- glycosphingolipids is not known. In the present study, blocking the CD1d receptor stimulated β-glycolipid-induced NKT cell proliferation. Our results suggest that changes in the glycolipid structure may alter their binding affinity. β-glycosphingolipid recognition may depend on CD1d and/or TCR interactions. If glycosphingolipids are similarly bound by CD1d, it is most likely that recognition will occur concomitantly with TCR binding. β-glycosphingolipid binding by CD1d, however, presumably positions the glycolipid where it can be recognized by the TCR. Consequently, if the glycolipid is not “properly” presented by CD1d, it may not be effectively recognized by the TCR. Interestingly, the combination of two β-glycosphingolipids (β-IGL) overcame the CD1d-dependent inhibition of NKT activation induced by either of the β-glycosphingolipids alone.
Optimization of the NKT ligand structure has been suggested as a potential tool to control immunomodulatory effects. The glycolipid sphingosine chain may be important for determining the affinity of the ligand for the CD1 groove in tumor antigen-presenting cells, thereby affecting CD8 and NKT cell activation. The data presented here suggest that the length of the β-glycolipid sphingosine chain is essential for determining the magnitude of CD8 redistribution, in breaking immune tolerance toward tumor antigens, and in suppressing hepatocellular carcinoma growth. Redistribution of lymphocyte subsets is a possible countermeasure to overcome immune-escape mechanisms associated with tumor progression.
iNKT cells are unique in their tendency to simultaneously produce both Th1 and Th2 cytokines. Furthermore, iNKT cells enhance immunity in some disease models, but are reported to suppress immunity in others.24 The present results indicate that administration of β-glycosphingolipids exerts distinct effects on STAT1, STAT4, and STAT6 protein expression on peripheral and intrahepatic CD4+, CD8+, and NKT cell distribution, and on Th1/Th2 cytokines. Thus, by affecting STAT signaling pathways, β-glycosphingolipids alter NKT lymphocyte plasticity, possibly serving as “fine tuners” of the immune system.30
Identifying antigens and determining lipid and carbohydrate recognition requirements for NKT cells will allow for the use of ligand analogues as fine tuners for NKT immune regulation.57,58,59 The data presented here suggest that the structure and dose of β-glycosphingolipids analogues may influence STAT signaling pathways involved in cellular events. The identification of precise structures or combinations of carbohydrates and lipids recognized by CD3+NK1.1+ cells will facilitate the production of biologically relevant epitopes via synthetic chemistry. Approaches that interfere with glycolipid metabolism or that introduce rationally designed glycosphingolipids are attractive means of immunomodulation that may become valuable tools in NKT cell-based immune therapies.
Footnotes
Address reprint requests to Yaron Ilan, M.D., Liver Unit, Department of Medicine, Hebrew University-Hadassah Medical Center, P.O.B 12000, Jerusalem, Israel, IL-91120. E-mail: ilan@hadassah.org.il.
Supported in part by a grant from The Roaman-Epstein Liver Research Foundation (to Y.I.).
G.L., A.B.Y., and D.M.L. contributed equally to this work.
References
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Sandberg JK, Ljunggren HG. Development and function of CD1d-restricted NKT cells: influence of sphingolipids. SAP and sex. Trends Immunol. 2005;26:347–349. doi: 10.1016/j.it.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutkiewicz RR, Lin Y, Cho S, Hwang YK, Sriram V, Roberts TJ. CD1d-mediated antigen presentation to natural killer T (NKT) cells. Crit Rev Immunol. 2003;23:403–419. doi: 10.1615/critrevimmunol.v23.i56.30. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Gapin L. Developmental program of mouse Valpha14i NKT cells. Curr Opin Immunol. 2005;17:122–130. doi: 10.1016/j.coi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- MacDonald HR, Mycko MP. Development and selection of Valpha l4i NKT cells. Curr Top Microbiol Immunol. 2007;314:195–212. [PubMed] [Google Scholar]
- Matsuda JL, Gapin L. Does the developmental status of Valpha14i NKT cells play a role in disease? Int Rev Immunol. 2007;26:5–29. doi: 10.1080/08830180601070211. [DOI] [PubMed] [Google Scholar]
- Dao T, Guo D, Ploss A, Stolzer A, Saylor C, Boursalian TE, Im JS, Sant'Angelo DB. Development of CD1d-restricted NKT cells in the mouse thymus. Eur J Immunol. 2004;34:3542–3552. doi: 10.1002/eji.200425546. [DOI] [PubMed] [Google Scholar]
- Schumann J, Mycko MP, Dellabona P, Casorati G, MacDonald HR. Cutting edge: influence of the TCR Vbeta domain on the selection of semi-invariant NKT cells by endogenous ligands. J Immunol. 2006;176:2064–2068. doi: 10.4049/jimmunol.176.4.2064. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Joyce S. Innate immunity: nKT cells in the spotlight. Curr Biol. 2005;15:R429–R431. doi: 10.1016/j.cub.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr Opin Immunol. 2007;19:186–193. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, Unutmaz D, Van Kaer L, Joyce S. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- Bezbradica JS, Hill T, Stanic AK, Van Kaer L, Joyce S. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc Natl Acad Sci USA. 2005;102:5114–5119. doi: 10.1073/pnas.0408449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- Van Kaer L. Alpha-galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat Rev Immunol. 2005;5:31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- Zajonc DM, Ainge GD, Painter GF, Severn WB, Wilson IA. Structural characterization of mycobacterial phosphatidylinositol mannoside binding to mouse CD1d. J Immunol. 2006;177:4577–4583. doi: 10.4049/jimmunol.177.7.4577. [DOI] [PubMed] [Google Scholar]
- Zajonc DM, Maricic I, Wu D, Halder R, Roy K, Wong CH, Kumar V, Wilson IA. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc DM, Cantu C, 3rd, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu C, 3rd, Benlagha K, Savage PB, Bendelac A, Teyton L. The paradox of immune molecular recognition of alpha-galactosylceramide: low affinity, low specificity for CD1d, high affinity for alpha beta TCRs. J Immunol. 2003;170:4673–4682. doi: 10.4049/jimmunol.170.9.4673. [DOI] [PubMed] [Google Scholar]
- Lalazar G, Preston S, Zigmond E, Ben Yaacov A, Ilan Y. Glycolipids as immune modulatory tools. Mini Rev Med Chem. 2006;6:1249–1253. doi: 10.2174/138955706778742722. [DOI] [PubMed] [Google Scholar]
- Yang YG, Wang H, Asavaroengchai W, Dey BR. Role of interferon-gamma in GVHD and GVL. Cell Mol Immunol. 2005;2:323–329. [PubMed] [Google Scholar]
- Stanic AK, De Silva AD, Park JJ, Sriram V, Ichikawa S, Hirabyashi Y, Hayakawa K, Van Kaer L, Brutkiewicz RR, Joyce S. Defective presentation of the CD1d1-restricted natural Va14Ja18 NKT lymphocyte antigen caused by beta-D-glucosylceramide synthase deficiency. Proc Natl Acad Sci USA. 2003;100:1849–1854. doi: 10.1073/pnas.0430327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo JR, Young HA, Winkler-Pickett RT, Bere EW, Jr, Murphy WJ, Wiltrout RH. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J Immunol. 2004;172:943–953. doi: 10.4049/jimmunol.172.2.943. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Iwama A, Morita Y, Eto K, Ema H, Nakauchi H. Cytokine signaling, lipid raft clustering, and HSC hibernation. Ann NY Acad Sci. 2007;1106:54–63. doi: 10.1196/annals.1392.017. [DOI] [PubMed] [Google Scholar]
- Filipp D, Julius M. Lipid rafts: resolution of the “fyn problem?”. Mol Immunol. 2004;41:645–656. doi: 10.1016/j.molimm.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Park YK, Lee JW, Ko YG, Hong S, Park SH. Lipid rafts are required for efficient signal transduction by CD1d. Biochem Biophys Res Commun. 2005;327:1143–1154. doi: 10.1016/j.bbrc.2004.12.121. [DOI] [PubMed] [Google Scholar]
- Margalit M, Ghazala SA, Alper R, Elinav E, Klein A, Doviner V, Sherman Y, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Glucocerebroside treatment ameliorates ConA hepatitis by inhibition of NKT lymphocytes. Am J Physiol Gastrointest Liver Physiol. 2005;289:G917–G925. doi: 10.1152/ajpgi.00105.2005. [DOI] [PubMed] [Google Scholar]
- Zigmond E, Preston S, Pappo O, Lalazar G, Margalit M, Shalev Z, Zolotarov L, Friedman D, Alper R, Ilan Y. Beta-glucosylceramide: a novel method for enhancement of natural killer T lymphocyte plasticity in murine models of immune-mediated disorders. Gut. 2007;56:82–89. doi: 10.1136/gut.2006.095497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit M, Shalev Z, Pappo O, Sklair-Levy M, Alper R, Gomori M, Engelhardt D, Rabbani E, Ilan Y. Glucocerebroside ameliorates the metabolic syndrome in OB/OB mice. J Pharmacol Exp Ther. 2006;319:105–110. doi: 10.1124/jpet.106.104950. [DOI] [PubMed] [Google Scholar]
- Zigmond E, Lalazar G, Pappo O, Zangen SW, Levy Sklair M, Hemed N, Rabbani E, Itamar R, Ilan Y, Margalit M: Treatment of non-alcoholic steatohepatitis by B-glucosylceramide: a phase I/II clinical study. Hepatology 2006, 44: Supplement, 57th Annual Meeting of the American Association for the Study of Liver Diseases, 180A, Poster #1256 [Google Scholar]
- Trop S, Samsonov D, Gotsman I, Alper R, Diment J, Ilan Y. Liver-associated lymphocytes expressing NK1.1 are essential for oral immune tolerance induction in a murine model. Hepatology. 1999;29:746–755. doi: 10.1002/hep.510290334. [DOI] [PubMed] [Google Scholar]
- Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- Beyan H, Buckley LR, Yousaf N, Londei M, Leslie RD. A role for innate immunity in type 1 diabetes? Diabetes Metab Res Rev. 2003;19:89–100. doi: 10.1002/dmrr.341. [DOI] [PubMed] [Google Scholar]
- Falcone M, Facciotti F, Ghidoli N, Monti P, Olivieri S, Zaccagnino L, Bonifacio E, Casorati G, Sanvito F, Sarvetnick N. Up-regulation of CD1d expression restores the immunoregulatory function of NKT cells and prevents autoimmune diabetes in nonobese diabetic mice. J Immunol. 2004;172:5908–5916. doi: 10.4049/jimmunol.172.10.5908. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells—conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- Hammond KJ, Godfrey DI. NKT cells: potential targets for autoimmune disease therapy? Tissue Antigens. 2002;59:353–363. doi: 10.1034/j.1399-0039.2002.590501.x. [DOI] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Stanic AK, Park JJ, Joyce S. Innate self recognition by an invariant, rearranged T-cell receptor and its immune consequences. Immunology. 2003;109:171–184. doi: 10.1046/j.1365-2567.2003.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M. Glycolipids and phospholipids as natural CD1d-binding NKT cell ligands. Cell Mol Life Sci. 2006;63:1889–1898. doi: 10.1007/s00018-006-6073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. The immunological function of iGb3. Curr Protein Pept Sci. 2006;7:325–333. doi: 10.2174/138920306778018007. [DOI] [PubMed] [Google Scholar]
- Hansen DS, Schofield L. Regulation of immunity and pathogenesis in infectious diseases by CD1d-restricted NKT cells. Int J Parasitol. 2004;34:15–25. doi: 10.1016/j.ijpara.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci USA. 2007;104:5977–5982. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- Bickel PE. Lipid rafts and insulin signaling. Am J Physiol Endocrinol Metab. 2002;282:E1–E10. doi: 10.1152/ajpendo.2002.282.1.E1. [DOI] [PubMed] [Google Scholar]
- Goni FM, Alonso A. Biophysics of sphingolipids I. Membrane properties of sphingosine, ceramides and other simple sphingolipids. Biochim Biophys Acta. 2006;1758:1902–1921. doi: 10.1016/j.bbamem.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Sonnino S, Mauri L, Chigorno V, Prinetti A. Gangliosides as components of lipid membrane domains. Glycobiology. 2007;17:1R–13R. doi: 10.1093/glycob/cwl052. [DOI] [PubMed] [Google Scholar]
- Sugawara Y, Nishii H, Takahashi T, Yamauchi J, Mizuno N, Tago K, Itoh H. The lipid raft proteins flotillins/reggies interact with Galphaq and are involved in Gq-mediated p38 mitogen-activated protein kinase activation through tyrosine kinase. Cell Signal. 2007;19:1301–1308. doi: 10.1016/j.cellsig.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Solomon S, Masilamani M, Rajendran L, Bastmeyer M, Stuermer CA, Illges H. The lipid raft microdomain-associated protein reggie-1/flotillin-2 is expressed in human B cells and localized at the plasma membrane and centrosome in PBMCs. Immunobiology. 2002;205:108–119. doi: 10.1078/0171-2985-00114. [DOI] [PubMed] [Google Scholar]
- Giri B, Dixit VD, Ghosh MC, Collins GD, Khan IU, Madara K, Weeraratna AT, Taub DD. CXCL12-induced partitioning of flotillin-1 with lipid rafts plays a role in CXCR4 function. Eur J Immunol. 2007;37:2104–2116. doi: 10.1002/eji.200636680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal PB, Guo GG, Shah M, Kumar V, Patel K. Cytokine signaling: sTATS in plasma membrane rafts. J Biol Chem. 2002;277:12067–12074. doi: 10.1074/jbc.M200018200. [DOI] [PubMed] [Google Scholar]
- Souto RP, Vallega G, Wharton J, Vinten J, Tranum-Jensen J, Pilch PF. Immunopurification and characterization of rat adipocyte caveolae suggest their dissociation from insulin signaling. J Biol Chem. 2003;278:18321–18329. doi: 10.1074/jbc.M211541200. [DOI] [PubMed] [Google Scholar]
- Lang GA, Maltsev SD, Besra GS, Lang ML. Presentation of alpha-galactosylceramide by murine CD1d to natural killer T cells is facilitated by plasma membrane glycolipid rafts. Immunology. 2004;112:386–396. doi: 10.1111/j.1365-2567.2004.01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal PB. Plasma membrane rafts and chaperones in cytokine/STAT signaling. Acta Biochim Pol. 2003;50:583–594. [PubMed] [Google Scholar]
- Hammond KJ, Kronenberg M. Natural killer T cells: natural or unnatural regulators of autoimmunity? Curr Opin Immunol. 2003;15:683–689. doi: 10.1016/j.coi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Ilan Y, Ohana M, Pappo O, Margalit M, Lalazar G, Engelhardt D, Rabbani E, Nagler A. Alleviation of acute and chronic graft-versus-host disease in a murine model is associated with glucocerebroside-enhanced natural killer T lymphocyte plasticity. Transplantation. 2007;83:458–467. doi: 10.1097/01.tp.0000252783.66886.f3. [DOI] [PubMed] [Google Scholar]
- Wilson MT, Singh AK, Van Kaer L. Immunotherapy with ligands of natural killer T cells. Trends Mol Med. 2002;8:225–231. doi: 10.1016/s1471-4914(02)02325-0. [DOI] [PubMed] [Google Scholar]