Abstract
Hyperhomocysteinemia and β-amyloid (Aβ) overproduction are critical etiological and pathological factors in Alzheimer disease, respectively; however, the intrinsic link between them is still missing. Here, we found that Aβ levels increased and amyloid precursor protein (APP) levels simultaneously decreased in hyperhomocysteinemic rats after a 2-week induction by vena caudalis injection of homocysteine. Concurrently, both the mRNA and protein levels of presenilin-1, a component of γ-secretase, were elevated, whereas the expression levels of β-secretase and presenilin-2 were not altered. We also observed that levels of phosphorylated APP at threonine-668, a crucial site facilitating the amyloidogenic cleavage of APP, increased in rats with hyperhomocysteinemia, although the phosphorylation per se did not increase the binding capacity of pT668-APP to the secretases. The enhanced phosphorylation of APP in these rats was not relevant to either c-Jun N-terminal kinase or cyclin-dependent kinase-5. A prominent spatial memory deficit was detected in rats with hyperhomocysteinemia. Simultaneous supplementation of folate and vitamin-B12 attenuated the hyperhomocysteinemia-induced abnormal processing of APP and improved memory. Our data revealed that hyperhomocysteinemia could increase Aβ production through the enhanced expression of γ-secretase and APP phosphorylation, causing memory deficits that could be rescued by folate and vitamin-B12 treatment in these rats. It is suggested that hyperhomocysteinemia may serve as an upstream factor for increased Aβ production as seen in patients with Alzheimer disease.
Alzheimer’s disease (AD) is a progressive neurological disorder characterized histopathologically by the formation of numerous senile plaques and neurofibrillary tangles. The senile plaques are mainly composed of amyloid-β (Aβ), surrounded by dystrophic neuritis.1 Aβ is generated by the consecutive cleavage of amyloid precursor protein (APP) by two proteases, ie, β-secretase (BACE-1) and γ-secretase (presenilin, PS-1/PS-2).2,3 In the amyloidogenic pathway, cleavage of APP by β-secretase generates an N-terminal soluble fragment (sAPPβ) and beta C-terminal fragment that is sequentially cleaved by γ-secretase to produce the Aβ peptides.4,5,6 Similar to many other toxic insults, Aβ promotes cell death by oxidative damage,7,8 influencing calcium homeostasis,9 activating caspases,10 stimulating protein phosphorylation,11 and causing mitochondrial abnormalities.12 In addition, Aβ fibrils specifically induce neuron dystrophy.13,14 In the cultured rats’ cortical neurons, overexpression of APP induces apoptosis and this apoptosis can be intercepted by γ-secretase inhibitor.15 In transgenic mouse models, Aβ aggregation induces dysfunction of neurites, tau pathology, and neuron death, and Aβ can also damage DNA.16 When APP is overexpressed or abnormally cleaved, Aβ forms toxic oligomers that aggregate into amyloid plaques and are associated with age-related memory impairment.17
It is well known that gene mutation of APP and PS-1 is causative for the increased Aβ production in hereditary AD.18 However, the mechanism leading to the Aβ overproduction in the majority sporadic AD patients is unclear. APP is a phosphoprotein, which have a large N-terminal extracellular domain and a short intracellular C-terminal domain that can be phosphorylated by various protein kinases with well-defined phosphorylation sites.19 Notably, phosphorylation of APP at Thr668 facilitates the amyloidogenic cleavage and the phosphorylated APP is elevated in AD brain.20,21
Epidemiology and clinical investigations have demonstrated that the elevated plasma homocysteine (Hcy) and the occurrence of AD are positively correlated, and thus hyperhomocysteinemia has been proposed to be a strong and independent risk factor of AD.22,23,24,25,26 Hcy is catabolized through the folate and vitamin (vit) B12-dependent remethylation cycle, which provides methyl-group for a number of metabolic steps.27 High Hcy suppresses the cellular levels of S-adenosylmethionine and S-adenosylhomocysteine, and thus inhibits the activity of methyltransferases, which in turn interrupts the methylation of some functional proteins and genes.28 Recently, we have reported that hyperhomocysteinemia can increase prominently the plasma Hcy level and thus induce tau hyperphosphorylation.29 In a hyperhomocysteinemic AD transgenic mouse model, an increased Aβ level in the brain was observed,30 and Hcy could interrupt DNA repair in hippocampal neurons and make the neurons more vulnerable to the amyloid toxicity.31,32,33 Until now, the effects of hyperhomocysteinemia on Aβ production in normal gene background and the underlying mechanisms leading to Aβ overproduction have not been reported, and it is also not known whether the induced hyperhomocysteinemia in adulthood affects the memory of the rats.
In the present study, we produced a hyperhomocysteinemia model in adult rats by injecting Hcy through vena caudalis and investigated the role of hyperhomocysteinemia in Aβ production and the related mechanisms, and as well as the effects on the memory ability of the rats. We found that hyperhomocysteinemia could increase remarkably the Aβ level with concurrent overexpression of PS-1 and hyperphosphorylation of APP at Thr-688, and it also led to spatial memory deficits of the rats. Simultaneous supplementation of folate and vit-B12 could attenuate the hyperhomocysteinemia-induced abnormal APP processing and the memory impairments of the rats.
Materials and Methods
Antibodies and Chemicals
The type, dilution, specificity, and source of the primary antibodies used in this study are listed in Table 1. Secondary antibodies for Western blotting were from Amersham Pharmacia Biotech (Little Chalfort, Buckinghamshire, UK). The bicinchoninic acid protein detection kit and chemiluminescent substrate kit were from Pierce Chemical Company (Rockford, IL). DL-Hcy was from Sigma Chemical CO (St. Louis, MO) and it was dissolved to a final concentration of 400 μg/ml and 1600 μg/ml in saline immediately before injection. Folate (Yabang Aipusen Ltd. Jiangsu, China) and vit-B12 (Yunpeng Ltd. Linfen, Shanxi, China) were dissolved in drinking water of the rats. Other reagents were of the highest quality available and obtained from commercial sources.
Table 1.
Antibodies Employed in this Study and their Properties
| Antibody | Specificity* | Type | Dilution | Source |
|---|---|---|---|---|
| 22C11 | APP 66-81 | Mono- | 1:1000 for WB | Chemicon (Temecula) |
| 369 | APP 650-695 | Poly- | 1:1000 for WB | CalBiochem (Darmstadt, Germany) |
| 6E10 | Aβ 1-17 | Mono- | 1:500 for WB | Santa Cruz (Santa Cruz, CA) |
| 4G8 | Aβ 17-24 | Mono- | 1:1000 for WB | Chemicon (Temecula, CA) |
| PT668 | p-Thr668-APP | Poly- | 1:1000 for WB 1:400 for IP 1:200 for IHC | Cell Signaling (Boston, MA) |
| Aβ | Aβ 1-39 | Poly- | 1:200 for IHC | Cell Signaling (Boston, MA) |
| PS-1 | PS-1 313-334 | Mono- | 1:300 for WB | Chemicon (Temecula, CA) |
| PS-2 | PS-2 C-20 | Poly- | 1:500 for WB | Santa Cruz (Santa Cruz, CA) |
| BACE | BACE C-15 | Poly- | 1:500 for WB | Santa Cruz (Santa Cruz, CA) |
| t-JNK1/2 | total JNK | Mono- | 1:1000 for WB | Cell Signaling (Boston, MA) |
| p-JNK1/2 | pTpY183/185 | Poly- | 1:1000 for WB | Biosource (Camarillo, CA) |
| CDK5 | CDK5 C-8 | Poly- | 1:500 for WB | Santa Cruz (Santa Cruz, CA) |
| p35 | p35 C-19 | Poly- | 1:500 for WB | Santa Cruz (Santa Cruz, CA) |
| b-actin | total actin | Mono- | 1:1000 for WB | Abcam (Cambridge, UK) |
According to the sequence of APP695 of human brain. Mono-, monoclonal; poly-, polyclonal; WB, Western-blotting; IP, immunoprecipitation; IHC, immunohistochemistry.
Animals and Drug Administration
Male Sprague-Dawley rats (3 to 4 months old, 280 ± 20 g) supplied by the Experimental Animal Central of Tongji Medical College were housed with accessible food and water ad libitum. All animal experiments were performed according to the “Policies on the Use of Animals and Humans in Neuroscience Research” revised and approved by the Society for Neuroscience in 1995. Rats were kept in cages under a 12: 12 light-dark cycle with the light on from 7:00 AM to 7:00 pm. For the time course study, we injected the rats by vena caudalis with Hcy (400 μg/kg/day) or saline with the same volume for 3, 7, 14, and 21 days, respectively. According to the results, we selected 14 days of injection because it caused significant Aβ production. Then, we injected the rats with two different dosages of Hcy (400 μg/kg/day or 1600 μg/kg/day) with or without a simultaneous supplement of folate (4 mg/kg/day) and vit-B12 (250 μg/kg/day) through drinking water34 for 14 days. The injection was performed each day from 9:00 AM to 2:00 PM and the animals were sacrificed 24 hours after the final injection following measurement of spatial memory.
Sandwich Enzyme Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay (ELISA) was performed to measure the level of Aβ40 and Aβ42 in hippocampal extracts.35,36 In brief, Aβ40 and Aβ42 in samples were captured respectively with G2-10 and G2-11 (Aβeta, Germany), monoclonal antibodies specific for Aβ40 and Aβ42. The level of Aβ was then detected specifically by antibody biotin-WO2 (Aβeta, Germany), and further developed with horseradish peroxidase-NeutrAvidin (Pierce Rockford, IL). Horseradish peroxidase activity was assayed by color development using 3, 3′, 5, 5′-tetramethylbenzidine microwell peroxidase system (Kirkegaard and Perry Laboratories, Gaithersburg, MD). The results were expressed as ng/mg tissue by referring to standard synthetic control peptides (Sigma, St Louis, MO). The plasma level of homocysteine was measured by high performance liquid chromatography.29
Western Blotting
Rats were decapitated after the spatial memory retention test. The hippocampi were rapidly removed and homogenized at 4°C using a Teflon glass homogenizer in 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 10 mmol/L NaF, 1 mmol/L Na3VO4, 5 mmol/L EDTA, 2 mmol/L benzamidine, and 1 mmol/L phenylmethylsulfonyl fluoride. The extract was mixed with sample buffer (3:1, v/v) containing 200 mmol/L Tris-HCl, pH 7.6, 8% SDS, 40% glycerol, 40 mmol/L dithiothreitol, boiled for 10 minutes, and then centrifuged at 12,000 × g for 10 minutes at 25°C. The supernatant was stored at −80°C for Western blotting analysis. The protein concentration in the supernatant was estimated by bicinchoninic acid kit according to manufacturer’s instructions. The proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk dissolved in TBS-Tween-20 (50 mmol/L Tris HCl, pH 7.6, 150 mmol/L NaCl, 0.2% Tween-20) for 1 hour and probed with primary antibody at 4°C for overnight. Then the blots were incubated with anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase (1:5000) for 1 hour at 37°C, and visualized with enhanced chemiluminescence. The blots were quantitatively analyzed by Kodak Digital Science 1D software (Eastman Kodak Co., New Haven, CT).
Immunohistochemistry
For immunohistochemical studies, rats were sacrificed by overdose chloral hydrate (1 g/kg) and perfused through the aorta with 100 ml 0.9% NaCl followed by 400 ml phosphate buffer containing 4% paraformaldehyde. Brains were removed and postfixed in perfusate overnight and then cut into sections (20 μm) with a vibratome (Leica, Nussloch, Germany; S100, TPI). The sections of rat brain were collected consecutively in PBS for immunohistochemistry staining. Free floating sections were blocked with 0.3% H2O2 in absolute methanol for 30 minutes and nonspecific sites were blocked with bovine serum albumin for 30 minutes at room temperature. Sections were then incubated overnight at 4°C with primary antibodies (see Table 1). After washing with PBS, sections were subsequently incubated with biotin-labeled secondary antibodies for 1 hour at 37°C. The immunoreaction was detected using horseradish peroxidase-labeled antibodies for 1 hour at 37°C and visualized with the diaminobenzidine tetrachloride system (brown color). For each primary antibody, 3 to 5 consecutive sections from each brain were used. The images were observed using a microscope (Olympus BX60, Tokyo, Japan).
Immunoprecipitation
Immunoprecipitation was performed as described.37 In brief, the rat hippocampi were quickly dissected out and homogenized on ice in buffer containing 50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1% (v/v) Triton X-100, 1% (w/v) deoxycholate, 0.1% (w/v) SDS, 10 mmol/L NaF, 1 mmol/L Na3VO4, and 2 μg/ml each of aprotinin, leupeptin, and pepstatin. Then, the hippocampal extracts (about 500 μg total proteins) was immunoprecipitated with anti-pT668-APP at 4°C and shaken overnight, and then protein G agarose (Pierce Chemical Company, Rockford, IL) was added and incubated at 4°C for 2 hours. The agarose beads were collected, washed, and resuspended in 60 μl of sample buffer containing 50 mmol/L Tris-HCl, pH 7.6, 2% SDS, 10% glycerol, 10 mmol/L dithiothreitol, and 0.2% bromophenol blue and boiled for 5 minutes and analyzed by Western blotting.
Reverse Transcription-PCR
Total RNA was extracted from the hippocampus using Trizol reagent according to manufacturer’s instruction (Invitrogen Life Technologies, Carlsbad, CA). Then total RNA (3 μg in 25 μl) was reverse-transcribed and the produced cDNA (1 μl) was used to detect the transcripts. For APP primers were: 5′-AGAGGTCTACCCTGAACTGC-3′ (forward primer), 5′-ATCGCTTACAAACTCACCAAC-3′ (reverse), product is 154bp; for PS-1: 5′-GGATGGGCAGCTAATCTATAC-3′(forward primer), 5′-CCTTCAGCCATATTCACCAAC-3′ (reverse primer) product is 576bp; for PS-2: 5′-GGAGAACGAGGACGACTG-3′ (forward primer), 5′-GAACAAGAAGAGGAGCATCA-3′ (reverse primer), product is 400bp; for BACE-1: 5′-CGGGAGTGGTATTATGAAGTG-3′ (forward primer), 5′-AGGATGGTGATGCGGAAG-3′ (reverse primer), product is 320bp. For glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-ACCACCATGGAGAAGGCTGG-3′ (forward primer), 5′-CTCAGTGTAGCCCAGGATGC-3′ (reverse primer), product is 526bp; for β-actin: 5′-CATCACTATCGGCAATGAGC-3′ (forward primer), 5′-GACAGCACTGTGTTGGCATA-3′ (reverse primer), product is 187bp. The PCR products were separated on 1.0% agarose gels and stained with GoldView. The cDNA bands were visualized under UV transillumination, and the PCR products were semi- quantitatively analyzed by Kodak Digital Science 1D software (Eastman Kodak Company, New Haven, CT). All of the mRNA levels were normalized to β-actin and GAPDH mRNA. Finally, all of them were expressed as relative level against corresponding control.
Morris Water Maze Test
Spatial memory was measured by Morris water maze test.38,39 The temperature of the room and the water was kept at ∼24 ± 2°C. Before each experiment (2 hours), the rats were brought to the site to allow them to be acclimated. For spatial learning, rats were trained in water maze to find a hidden platform for six consecutive days, four trials per day with a 30-s interval from 14:00 to 20:00 pm. On each trial, the rat started from one of the middle of the four quadrants facing the wall of the pool and ended when the animal climbed on the platform. The rats were not allowed to search for the platform more than 60 s, after which they were guided to the platform. Through these training sessions, rats acquired spatial memory about location of the safe platform. The swimming pathways and latencies of the rats to find the hidden platform were recorded each day. The pathway and the length that the rats passed through the previous platform quadrant were recorded by a video camera fixed to the ceiling of the room, 1.5 m from the water surface. The camera was connected to a digital-tracking device attached to an IBM computer loaded with the water maze software. The longer a rat stayed in the previous platform-located quadrant, the better it scored the spatial memory. After the injection of homocysteine for 14 days, the spatial memory retention of the rats was tested again by the maze.
Statistic Analysis
Data were expressed as mean ± SD and analyzed using SPSS 12.0 statistical software (SPSS Inc., Chicago, Illinois). The one-way analysis of variance procedure, followed by least significant difference post hoc tests, was used to determine the statistical significance of differences of the means. To analyze the correlations among the variables, Pearson Correlations were computed with the bivariate correlations procedure.
Results
Hyperhomocysteinemia Increases Aβ40 with Simultaneous Decrease of APP
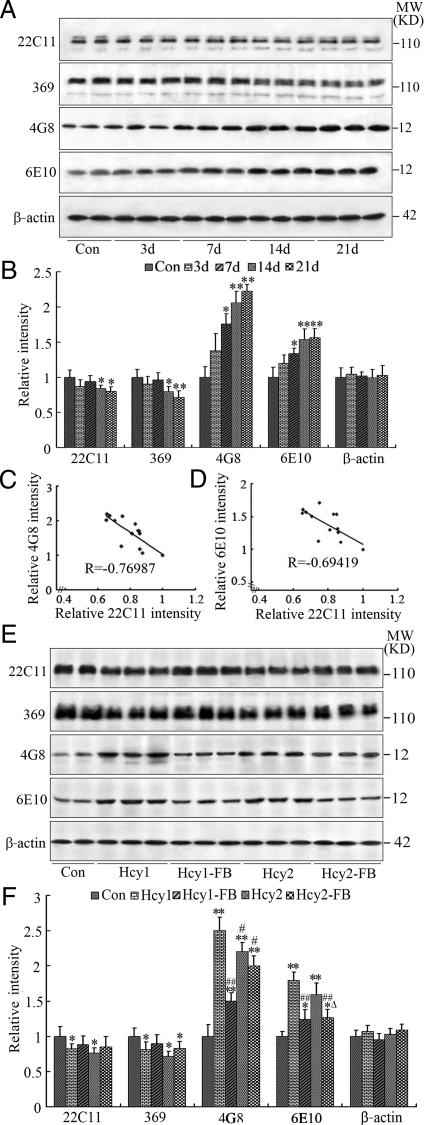
To investigate the effect of high plasma Hcy on APP metabolism/Aβ production, we injected the rats through vena caudalis with Hcy (400 μg/kg/day) or with the same volume of saline for 3, 7, 14, and 21 days. Then, we detected the alterations of APP and Aβ levels using a panel of the antibodies. We observed that the immunoreactivity of Aβ probed by 4G8 and 6E1040,41 increased, whereas the immunoreactivity of full length APP probed by 22C11 and 36942,43 decreased in rats injected with Hcy (Figure 1A and 1B). Furthermore, the decrease of APP and the increase of Aβ were negatively correlated (Figure 1, C and D) (for the specificity of APP and Aβ antibodies, see Table 1). These data demonstrate that increasing Hcy can induce APP cleavage and Aβ overproduction. As no significant difference in immunoreactivity at different time points was detected by saline (vehicle) injection, we only showed the saline control (Con) received at day 0 for the analysis (Figure 1, A and B).
Figure 1.
Hyperhomocysteinemia promotes amyloidogenic cleavage of APP. Homocysteine (Hcy, 400 μg/kg/day) was injected through vena caudalis into the rats for different time periods (n = 6 to 7 for each group) (A, B). The correlation between decrease of APP and increase of Aβ was analyzed by Pearson (C, D). Alternatively, Hcy was injected in different concentrations for 2 weeks (Hcy1 = 400 μg/kg/day, Hcy2 = 1600 μg/kg/day) with or without supplement of folate and Vit-B12 (FB, 4 mg/kg/day or 250 μg/kg/day) (n = 10 for each group) (E, F). The same volume of saline was injected as control (Con). The hippocampal extract was used for Western blotting (A, E) and quantitative analysis (B, F). The relative Aβ level was accessed by 4G8 and 6E10 and the full length APP was measured by 22C11 and 369, all of which were normalized against β-actin. All bands shown in the blots were collected for the calculation of APP and Aβ, and all data were expressed as mean ± SD. *P < 0.05, **P < 0.01 vs control; #P < 0.05, ##P < 0.01 vs Hcy1; ΔP < 0.05, vs Hcy2.
Since the significant alterations were detected at 14 days for all of the 4 APP and Aβ antibodies used, we then injected the rats for 14 days with two different dosages of Hcy (400 μg/kg/day or 1600 μg/kg/day), with or without simultaneous supplementation with folate and vit-B12. We observed that the immunoreactivity of 22C11 and 369 decreased whereas the immunoreactivity of 4G8 and 6E10 increased prominently, and the changes were more remarkable in rats injected with Hcy at 400 μg/kg/day (Figure 1, E and F). When folate and vit-B12 were simultaneously supplied, the Hcy-induced APP dissection and Aβ overproduction at both dosages were partially attenuated (Figure 1, E and F). No further elevation of Aβ production was observed when the concentration of Hcy was raised from 400 μg/kg/day to 1600 μg/kg/day (Figure 1, E and F). The alteration of plasma Hcy was confirmed by high performance liquid chromatography, which demonstrated that the plasma Hcy level increased from 6.2 μmol/L to 8.7 μmol/L and 10.2 μmol/L respectively after administration of 400 and 1600 μg/kg of Hcy for 2 weeks (P < 0.01) and it was restored to 7.3 μmol/L and 7.9 μmol/L by simultaneous administration of folate and vitB-2 (P < 0.01).
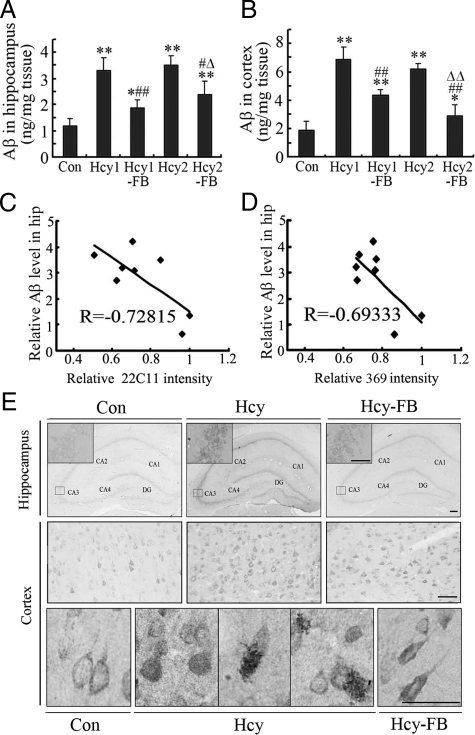
To further confirm the changes of Aβ, we measured the Aβ levels by an ELISA assay using Aβ40- and Aβ42- specific antibodies. We found that the level of Aβ40 was significantly elevated and simultaneous administration of folate and vit-B12 antagonized the elevation in both hippocampus (Figure 2A) and the cortex (Figure 2B) of the rat brains. The decreases of APP (see Figure 1, E and F) and the increases of Aβ were negatively correlated (Figure 2, C and D). We also measured the level of Aβ42, but no obvious difference was observed (data not shown). By immunohistochemical staining, we observed that the increased Aβ40 staining was mainly distributed in the cell bodies of the hippocampus and the cortex, though enhanced extracellular staining of Aβ was also detected in the cortex (Figure 2E). The increased Aβ immunoreactivity was partially attenuated by folate and vit-B12 in both cortex and hippocampus (Figure 2, A, B, and E). These data further confirm that elevation of plasma Hcy can induce cleavage of APP and overproduction of Aβ in the rat brains.
Figure 2.
Hyperhomocysteinemia increases Aβ40 in cortex and hippocampus. The rats received injection of Hcy (Hcy1 = 400 μg/kg/day, Hcy2 = 1600 μg/kg/day) with or without supplement of folate and Vit-B12 (FB, 4 mg/kg/day or 250 μg/kg/day) for 2 weeks, and the same volume of saline was injected as controls (Con). The rats were then sacrificed and the levels of Aβ40 in the hippocampus and cortex were measured by ELISA (A, B) and immunocytochemistry staining (E, 400 μg/kg/day of Hcy and 250 μg/kg/day of FB) by using an Aβ40-specific antibody. The correlation between decrease of APP (see Figure 1, E and F) and increase of Aβ40 measured by ELISA was analyzed by Pearson (C, D). All data expressed as mean ± SD; n = 7 for ELISA; n = 3 for immunocytochemistry, scale bar = 100 μm. *P < 0.05, **P < 0.01 vs control; #P < 0.05, ##P < 0.01 vs Hcy1; ΔP < 0.05, ΔΔP < 0.01 vs Hcy2. CA1-CA4 denote the Cornu Ammonis areas and DG represents the denate gyrus.
Hyperhomocysteinemia Selectively Increases the Expression of PS-1
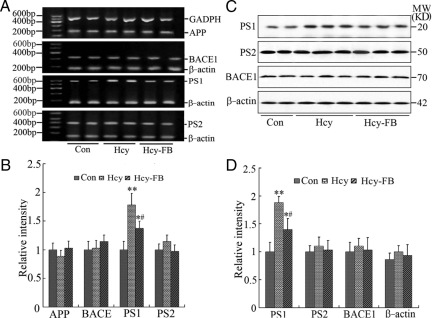
To explore the mechanisms underlying the high Hcy-induced cleavage of APP into Aβ, we first detected the mRNA level of APP and the secretases in the rats, including BACE-1 and the components of γ-secretase, ie, PS-1 and PS-2. We found that the mRNA levels of APP, BACE-1 and PS-2 were not altered after injection of Hcy (400 μg/kg/day) or Hcy plus folate and vit-B12 for 14 days, whereas the mRNA level of PS-1 increased remarkably (P < 0.01, Figure 3, A and B) in the Hcy-injected group and folate/vit-B12 antagonized the elevation (P < 0.05, Figure 3, A and B). We also detected the protein level of APP, BACE-1, PS-1, and PS-2 by Western blotting. In accordance with the altered mRNA level, only an increased PS-1 protein was observed after injection of Hcy and simultaneous injection of folate/vit-B12 suppressed partially the elevation (Figure 3, C and D). These data suggest that the elevated PS-1 may be responsible for the Hcy-induced APP cleavage at γ-site.
Figure 3.
Hyperhomocysteinemia selectively increases the mRNA and protein levels of PS-1. Hcy (400 μg/kg/day) was injected into the rats for 2 weeks with or without supplement of folate and Vit-B12 (FB, 250 μg/kg/day). Then the hippocampus extracts were prepared and the mRNA and protein levels of APP and its secretases, including PS-1, PS-2 and BACE, were measured and quantitatively analyzed by reverse transcription-PCR (A, B) and Western blotting (C, D), respectively. The β-actin and GAPDH were used as internal controls. All data were expressed as mean ± SD; n = 4 for reverse transcription-PCR; n = 6 for Western blotting. *P < 0.05, **P < 0.01 vs control; #P < 0.05 vs Hcy.
Hyperhomocysteinemia Increases the Phosphorylation of APP at Thr668
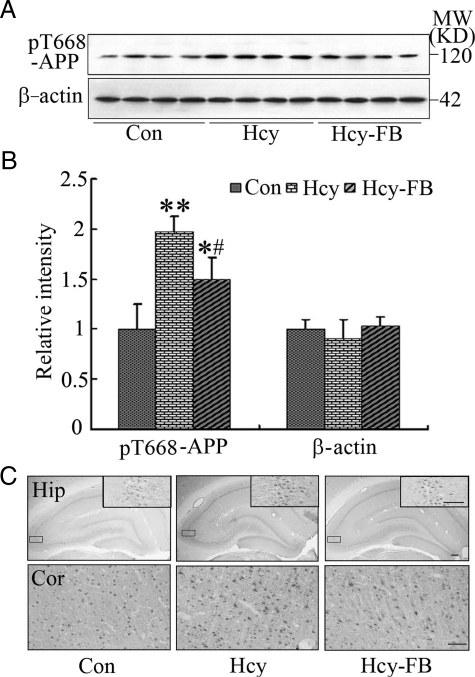
Though a prominent cleavage of APP at β-site was detected by antibody 4G8 and 6E10 as demonstrated in Figure 1, the mRNA and protein levels of BACE-1 did not change (Figure 3), suggesting that the β-cleavage of APP may involve other mechanisms than BACE-1 up-regulation. Previous studies have demonstrated that the phosphorylation of APP at Thr668 (pT668-APP) also affects its cleavage.20,21 Therefore, we detected the phosphorylation status of APP at Thr668 using a phosphorylation site-specific antibody. We found that the level of pT668-APP increased remarkably after injection of Hcy, and folate and vit-B12 could attenuate the phosphorylation of APP (P < 0.05, Figure 4, A and B). By immunohistochemical staining, the phosphorylated APP was detected both in the cortex and the hippocampus of the control rats, and a significantly enhanced staining was detected after injection of Hcy (Figure 4C). The phosphorylation of APP was attenuated by folate and vit-B12 (Figure 4C). These data suggest that the phosphorylation of APP at Thr668 site may also contribute to the Hcy-induced cleavage of APP.
Figure 4.
Hyperhomocysteinemia increases the phosphorylation of APP at Thr668. Hcy (400 μg/kg/day) was injected into the rats for 2 weeks with or without supplement of folate and Vit-B12 (FB, 250 μg/kg/day). Then the hippocampal extracts were prepared and the phosphorylation level of APP at Thr668 (pT668-APP) was measured by Western blotting (A) and quantitative analysis (B), and the expression of pT668-APP was also detected by immunohistochemistry staining in hippocampus (Hip) and cortex (Cor) (C). All data expressed as mean ± SD (n = 5). *P < 0.05, **P < 0.01 vs control; #P < 0.05 vs Hcy. Scale bar = 100 μm.
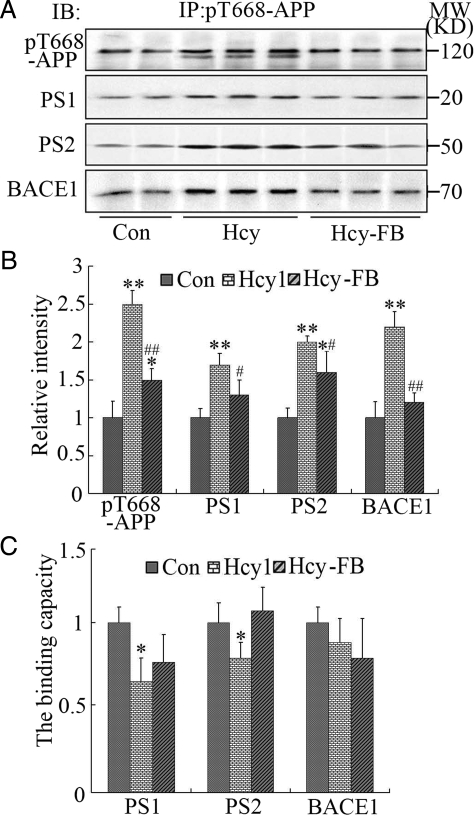
To further explore whether the phosphorylation of APP enhanced its binding capacity to the secretases, we used co-immunoprecipitation. The results showed that the levels of BACE-1, PS-1 and PS-2 in the pellet were increased accompanied by a remarkably increased level of pT668-APP (Figure 5, A and B). However, the increased BACE-1 was no longer present and the levels of PS-1 and PS-2 were even decreased in rats with hyperhomocysteinemia when normalized to pT668-APP (P < 0.05, Figure 5, A and C). These data suggest that the elevated total binding level of the β- and γ-secretases caused by the increased phosphorylation of APP may contribute to the enhanced APP cleavage and Aβ overproduction, but the phosphorylation per se does not increase the binding capacity of the secretases to pT668-APP. We also noticed that a new cleaved band with lower molecular mass of the pT668-APP was only shown in the Hcy-injected rat samples (Figure 5A), which further confirms the enhanced cleavage of the phosphorylated APP induced by high Hcy. Additionally, as compared with the hyperhomocysteinemia rats, the binding capacity of PS-2 to pT668-APP was restored and the binding capacity of PS-1 and BACE-1 to the pT668-APP did not change significantly with supplement of folate/vit-B12 (Figure 5, A–C).
Figure 5.
Phosphorylation of APP increases the total binding level to the secretases without affecting the binding capacity. Hcy (400 μg/kg/day) was injected into the rats for 2 weeks with or without supplement of folate and Vit-B12 (FB, 250 μg/kg/day). The hippocampal extracts were immunoprecipitated (IP) with pT668-APP antibody and the precipitates were analyzed by immunoblotting (IB) using the antibodies against pT668-APP, BACE1, PS1 and PS2 as labeled (A). The relative levels of the pT668-APP and the bound secretases in the precipitates were quantitatively analyzed as indicated (B), and the binding capacity of the secretases to the pT668-APP was calculated by normalized against the level of pT668-APP in each group and expressed by setting the vehicle control group as 1 (C). All data expressed as mean ± SD (n = 5). *P < 0.05, **P < 0.01 vs control; #P < 0.05, ##P < 0.01 vs Hcy.
Hyperhomocysteinemia Decreases the Level of p-JNK and p25
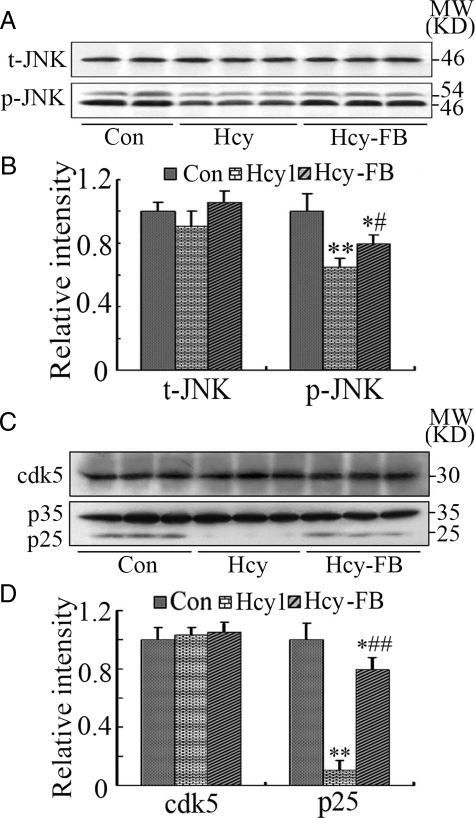
Phosphorylation of APP at Thr668 site can be regulated by glycogen synthase kinases-3β (GSK-3), c-Jun N-terminal kinase (JNK), cyclin-dependent kinase-5 (Cdk-5), and protein phosphatase-2A (PP-2A). We reported previously that the activity of PP-2A decreased and the activity of GSK-3 did not change in the hyperhomocysteinemia rats.29 Here, we measured the expression and the activity-dependent modification of JNK, Cdk-5, and its modulators, p35 and p25. We found that the expression of JNK did not change but the phosphorylated JNK (p-JNK, the active form) decreased significantly after the injection of Hcy, and folate and vit-B12 restored partially the level of p-JNK (Figure 6, A and B). We also observed that the level of p25 (the activator of Cdk-5) decreased remarkably after the injection of Hcy and folate/vit-B12 restored the level of p25, whereas the levels of Cdk-5 and p35 did not change much in Hcy and folate/vit-B12 groups (Figure 6, C and D). These data together ruled out the role of JNK and Cdk-5/p25 in the Hcy-induced hyperphosphorylation of APP.
Figure 6.
Hyperhomocysteinemia inactivates JNK and Cdk-5. Hcy (400 μg/kg/day) was injected into the rats for 2 weeks with or without supplement of folate and Vit-B12 (FB, 250 μg/kg/day). The levels of total JNK (t-JNK) and the phosphorylated (p-JNK, the activated form), and Cdk-5, p35, and p25 were measured by Western blotting (A and C) and quantitatively analyzed (B and D). All data expressed as mean ± SD (n = 5). *P < 0.05. **P < 0.01 vs control; ##P < 0.01 vs Hcy.
Hyperhomocysteinemia Impairs the Spatial Memory of the Rats
To detect the influence of Hcy on spatial memory of the rats, we performed the water maze test. The rats were trained in water maze for six consecutive days to remember the location of the hidden platform. Then, the rats were injected with 400 μg/kg/day of Hcy or NS for 2 weeks with or without simultaneous supplement of folate and vit-B12 and the memory retention was tested at day 15 after the injection. We selected the rats that were able to find the hidden platform within 10 s in a relatively straightforward searching pathway after being trained for 6 days (n = 10 for each group, Figure 7, A and B, pre-), which could ensure a similar starting point for the retention memory test. The latency to find the hidden platform was significantly increased in rats injected with Hcy (Figure 7, A and B, post-), suggesting memory deficit induced by high Hcy. The memory impairment was also detected by removing the platform (Figure 7, C and D), and supplement of folate/vit-B12 efficiently improved the Hcy-induced memory deficits (Figure 7). These results together demonstrated that high plasma Hcy is harmful to the spatial memory of the rats and folate/vit-B12 can effectively attenuate the detrimental effects induced by hyperhomocysteinemia.
Figure 7.
Hyperhomocysteinemia impairs the spatial memory of the rats. Hcy (400 μg/kg/day) was injected for 2 weeks with or without supplement of folate and Vit-B12 (FB, 250 μg/kg/day) after a consecutive training for 6 days in Morris water maze. Then, the spatial memory retention of the rats was measured at day 15 after the injection. The path to find the platform before (pre-) and after (post-) injection (A), the escape latency to find the hidden platform (B), the path swimming in the maze for one minute after removed the platform (C), and the distance swimming in the third quadrant in one minute (D) of the rats were shown. All data expressed as mean ± SD (n = 12). * P < 0.05, ** P < 0.01 vs control.
Discussion
Alzheimer’s disease is the most common cause of dementia, characterized pathologically by numerous neurofibrillary tangles and senile plaques in the selective brain regions. The hyperphosphorylated tau protein is the major protein component of the tangles, whereas Aβ, a fragment of APP, is the major peptide of senile plaques.44 Until now, the upstream factors leading to the formation of tangles and plaques was not fully understood. Epidemiological studies revealed that ∼40% AD patients had high plasma Hcy and the patients with hyperhomocysteinemia displayed more rapid neural atrophy than those with lower levels of Hcy.22,45 Therefore, hyperhomocysteinemia has been proposed to be a strong and independent risk factor of AD.22,23,24,25,26 Based on these observations, we speculate that there must be an intrinsic link between hyperhomocysteinemia and the AD pathologies. To test this hypothesis, we produced a rat model with hyperhomocysteinemia by vena caudalis injection of Hcy, and found that hyperhomocysteinemia could induce tau hyperphosphorylation.29 In the present study, we further investigated the effects of high Hcy on Aβ production and the related mechanisms, and as well as the spatial memory of the rats. We found that hyperhomocysteinemia could increase prominently the Aβ level with prominently increased mRNA and protein levels of PS-1 and hyperphosphorylation of APP at Thr668. A prominent spatial memory deficit was also observed in the rats with hyperhomocysteinemia. By simultaneous supplementation with folate/vit-B12, the abnormal APP processing/Aβ overproduction and the memory deficits of the rats were attenuated, with restoration of the plasma Hcy level.
It is well known that Aβ is produced by cleavage of APP at both the β and γ sites, which are catalyzed respectively by β- and γ-secretases.46,47 The two important components of γ-secretase are PS-1 and PS-2, which may function as the catalytic subunit of the secretase, and overexpression of the two genes increases the Aβ production.48,49 Studies have demonstrated that PS-1 expression is regulated by methylation status of the promoter gene, ie, the methylation at cytosine of the CpG dinucleotide in the PS-1 promoter silences the gene.50,51,52 On the contrary, demethylation of the PS-1 promoter gene stimulates PS-1 expression, which in turn increases the production of Aβ.53 In the present study, we found that the levels of PS-1 mRNA and protein were increased in rats with hyperhomocysteinemia, whereas the levels of PS-2 were not changed, which suggests that high Hcy may selectively disrupt the methylation of PS-1 but not PS-2 promoter gene. A previous study also demonstrated that the expression of PS-2 was not modulated by the methylation of the promoter gene.52 Another study showed that homocysteic acid, an oxidized metabolite of homocysteine, could induce intraneuronal accumulation of Aβ42 with a PS-1-involved mechanism.54 These data together indicate that overexpression of PS-1 may be responsible for the hyperhomocysteinemia-induced cleavage of APP at γ-site. The involvement of a de-regulated methylation in the increased expression of PS-1 was also supported by the results that supplementation of folate/vit-B12 could arrest the overexpression of PS-1. The detailed mechanisms underlying the regulation of methylation on PS-1 expression need further investigation. We also noticed that Aβ40, but not Aβ42, was increased in our rat model, which might be related to the specific cleavage of PS-1 at different γ-sites. Though it is now believed by many in the field that the ratio of Aβ42/Aβ40 seems more important in the pathogenesis of the disease, the toxic effect of Aβ40 to the neurons has also been reported.55
Though the increase of APP cleavage at β-site was prominent, as demonstrated by the prominently increased 4G8 and 6E10 immunoreactions, we did not detect any obvious up-regulation of BACE-1. These results suggest that, instead of BACE-1 activation, there must be other mechanisms, such as phosphorylation of APP, that contribute to the increased β-cleavage of APP in rats with hyperhomocysteinemia. Previous studies revealed that there were eight putative phosphorylation sites in the cytomere of APP, seven of which were phosphorylated in the AD brains,20 Among these sites, the phosphorylation at Thr668 of APP was the most implicated in affecting APP processing.56 Furthermore, the phosphorylation of APP at Thr668 mediates the binding of APP with BACE-1 and thus plays a crucial role in APP metabolism and Aβ production.20 To measure the phosphorylation status of APP, we used a phosphorylation site-specific antibody and did Western blotting and immunohistochemistry. The results revealed that high Hcy could increase APP phosphorylation at the Thr668 site. It has been reported that the conjugation of APP and PS-1 can augment the Aβ production.57 Therefore, we also tested the binding capacity of the phosphorylated APP to the secretases by immunoprecipitation using an anti-pT668-APP antibody. We found that the levels of BACE-1, PS-1, and PS-2 co-precipitated with pT668-APP were all elevated along with the increased pT668-APP in the Hcy-injected group. However, the ratio did not increase when normalized to the increased pT668-APP level as compared with the control rats. These data suggest that the phosphorylation of APP at Thr668 site does not increase the binding capacity of APP to the secretases, although it can increase prominently the total binding level. According to our data, we speculate that the phosphorylation of APP may contribute to the Hcy-induced cleavage of APP through the increased phosphorylation level, thus leading to an increased binding level to the secretases, but not through increasing the binding capacity of pT668-APP to the secretases.
The phosphorylation level of APP at Thr668 is reportedly regulated by GSK-3, JNK, and Cdk-556,58,59,60,61, and PP-2A62,63 We have recently reported that high plasma Hcy inhibits PP-2A and it does not affect the activity of GSK-3β,29 the most widely studied phosphatase and kinase in AD-like tau hyperphosphorylation.64 Here, we measured the activity-dependent modifications of JNK and Cdk-5. We found that the p-JNK (the active form) decreased significantly with no change of total JNK in Hcy-injected rats, suggesting inhibition of JNK by Hcy; we also observed that the Cdk-5 activator (p25) decreased with no change in the total levels of Cdk-5 and p35 in the Hcy-injected rats, suggesting inhibition of Cdk-5. The mechanism underlying the inhibition of JNK and Cdk-5 by high Hcy is currently not understood, but these results can rule out the involvement of JNK and Cdk-5 in the enhanced APP phosphorylation. We can also rule out GSK-3β in the increased phosphorylation of APP, because it is also inactivated in rats with high plasma Hcy.29 As the activity of PP-2A is decreased in the Hcy-injected rats,29 we speculate that the decreased PP-2A may be responsible for the hyperphosphorylation of APP. This is supported by the recent study in which it was demonstrated in the N2a cell line that the decreased methylation of PP-2A was associated with increased phosphorylation of APP at Thr668.63 PP-2A is the most active protein phosphatase in dephosphorylating tau proteins; the decrease of PP-2A also results in tau hyperphosphorylation in the rats.29 Therefore, we propose that hyperhomocysteinemia could induce AD-like Aβ overproduction and tau hyperphosphorylation through demethylation (inactivation) of PP-2A,29 in addition to the effects on PS-1 expression.
Spatial memory decline is an early symptom of AD, and the serum Hcy concentration is an early and susceptive indication of memory impairment.65 Recently, it was reported that hyperhomocysteinemia caused by gestational insufficiency of vitamin B could induce neurobehavioral disabilities of the pups.66 Therefore, we detected the spatial memory ability of the rats. The results revealed that the spatial memory ability of the rats was significantly impaired in rats injected with Hcy, while supplementation of folate and vit-B12 could effectively protect the spatial memory of the rats. Numerous studies have demonstrated that APP overexpression and Aβ overproduction are closely related to memory loss.46,67,68,69,70,71 Additionally, neurofibrillary tangles composed of the abnormally hyperphosphorylated tau is positively correlated with the degree of dementia.72,73 As the hyperhomocysteinemia rats in our study shows both Aβ overproduction and tau hyperphosphorylation,29 we speculate that hyperhomocysteinemia may impair the spatial memory of the rats through inducing Aβ and tau pathologies as found in our studies, and as well as inducing cell apoptosis.66
Hcy is a non-essential sulfur-containing amino acid and it is an intermediate formed during “methionine cycle.”27 In humans, hyperhomocysteinemia is defined as the plasma Hcy level higher than 14 μmol/L.22,74 In Sprague–Dawley rats, the basal plasma level of Hcy is 5.0 μmol/L to 6.2 μmol/L in rats with different ages.75,76 After 2 weeks dietary administration of 0.3% homocysteine-containing diet, the plasma Hcy level rose to 18 μmol/L.76 In our study, the plasma Hcy level of control rats was 6.2 μmol/L and it increased to 8.7 μmol/L and 10.2 μmol/L respectively after administration of 400 and 1600 μg/kg of Hcy.29 As Hcy can penetrate the blood-brain barrier through carrier/receptor-mediated transport,24 and the elevated plasma Hcy compromises the integrity of the blood-brain barrier,77 we therefore speculate that the brain concentration of Hcy should be at least similar to that observed in the blood. Hcy is catabolized through multiple pathways. One of these pathways is the folate/vit-B12-dependent remethylation, in which folate provides the methyl group and vit-B12 functions as a co-enzyme for the methyltransferase.78 Folate and vit-B12 can down-regulate the level of plasma Hcy.79
In summary, we have found in the present study that injection of Hcy increases Aβ production with elevated expression of PS-1 and hyperphosphorylation of APP at Thr668 in both the hippocampus and cortex. Hyperhomocysteinemia also leads to spatial memory deficits of the rats, and simultaneous supplement of folate and vit-B12 attenuates the homocysteine-induced Aβ overproduction and memory deficits. Our in vivo data have provided molecular evidence to disclose the intrinsic link between hyperhomocysteinemia and AD-like Aβ overproduction and memory impairments.
Footnotes
Address reprint requests to Prof. Jian-Zhi Wang, Department of Pathophysiology, Key Laboratory of Neurological Disease of Education Committee of China, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China. E-mail: wangjz@mails.tjmu.edu.cn.
Supported in part by grants from the Committee of Science and Technology of China (2006CB500703, 2006AA02Z4A1), and Natural Science Foundation of China (30731160621, 30872722).
Present address of C.-E.Z. Department of Pathophysiology, Guangzhou Medical University, Guangzhou, China.
References
- Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- Nishitomi K, Sakaguchi G, Horikoshi Y, Gray AJ, Maeda M, Hirata-Fukae C, Becker AG, Hosono M, Sakaguchi I, Minami SS, Nakajima Y, Li HF, Takeyama C, Kihara T, Ota A, Wong PC, Aisen PS, Kato A, Kinoshita N, Matsuoka Y. BACE1 inhibition reduces endogenous abeta and alters APP processing in wild-type mice. J Neurochem. 2006;99:1555–1563. doi: 10.1111/j.1471-4159.2006.04178.x. [DOI] [PubMed] [Google Scholar]
- Vassar R. β-Secretase (BACE) as a drug target for Alzheimer’s disease. Adv Drug Del Rev. 2002;54:1589–1602. doi: 10.1016/s0169-409x(02)00157-6. [DOI] [PubMed] [Google Scholar]
- Iwata N, Saido TC. Amyloid-beta peptide metabolism and Alzheimer’s disease. Nippon Yakurigaku Zasshi. 2003;122:5–14. doi: 10.1254/fpj.122.5. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer’s disease. J Biol Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- Vassar R. Beta-secretase, APP, and abeta in Alzheimer’s disease. Subcell Biochem. 2005;38:79–103. [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994;53:438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Tomaselli KJ, Rydel RE. Calcium-destabilizing and neurodegenerative effects of aggregated beta-amyloid peptide are attenuated by basic FGF. Brain Res. 1993;621:35–49. doi: 10.1016/0006-8993(93)90295-x. [DOI] [PubMed] [Google Scholar]
- Harada J, Sugimoto M. Activation of caspase-3 in beta-amyloid-induced apoptosis of cultured rat cortical neurons. Brain Res. 1999;842:311–323. doi: 10.1016/s0006-8993(99)01808-9. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Lorenzo A, Yeh J, Yankner BA. Beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14:879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci USA. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J, Yeh J, Yankner BA. Beta-amyloid neurotoxicity in human cortical culture is not mediated by excitotoxins. J Neurochem. 1993;61:1565–1568. doi: 10.1111/j.1471-4159.1993.tb13658.x. [DOI] [PubMed] [Google Scholar]
- Pigino G, Pelsman A, Mori H, Busciglio J. Presenilin-1 mutations reduce cytoskeletal association, deregulate neurite growth, and potentiate neuronal dystrophy and tau phosphorylation. J Neurosci. 2001;21:834–842. doi: 10.1523/JNEUROSCI.21-03-00834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienlen-Campard P, Miolet S, Tasiaux B, Octave JN. Intracellular amyloid-beta 1–42, but not extracellular soluble amyloid-beta peptides, induces neuronal apoptosis. J Biol Chem. 2002;277:15666–15670. doi: 10.1074/jbc.M200887200. [DOI] [PubMed] [Google Scholar]
- Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Patho. 2004;165:1289–1300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo A, McCord M, Troen AM, Allison JD, McDonald MP. Impaired spatial memory in APP-overexpressing mice on a homocysteinemia-inducing diet. Neurobiol Aging. 2007;28:1195–1205. doi: 10.1016/j.neurobiolaging.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T. Beta-amyloid cascade: current status and future directions. Clin Neurol. 2000;40:1228–1230. [PubMed] [Google Scholar]
- Gandy SE, Caporaso GL, Buxbaum JD, de Cruz Silva O, Iverfeldt K, Nordstedt C, Suzuki T, Czernik AJ, Nairn AC, Greengard P. Protein phosphorylation regulates relative utilization of processing pathways for Alzheimer beta/A4 amyloid precursor protein. Ann NY Acad Sci. 1993;695:117–121. doi: 10.1111/j.1749-6632.1993.tb23038.x. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kao SC, Lemere CA, Xia W, Tseng HC, Zhou Y, Neve R, Ahlijanian MK, Tsai LH. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 2003;163:83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot N, Santos SF, Feyt C, Morel M, Brion JP, Octave JN. Calcium-mediated transient phosphorylation of tau and amyloid precursor protein followed by intraneuronal amyloid-beta accumulation. J Biol Chem. 2006;281:39907–39914. doi: 10.1074/jbc.M606015200. [DOI] [PubMed] [Google Scholar]
- Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- McCaddon A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 1998;13:235–239. doi: 10.1002/(sici)1099-1166(199804)13:4<235::aid-gps761>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006;580:2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Porcellini E, Licastro F. Homocysteine and folacin as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82:636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Levitt AJ, Karlinsky H. Folate, vitamin B12 and cognitive impairment in patients with Alzheimer’s disease. Acta Psychiatr Scand. 1992;86:301–305. doi: 10.1111/j.1600-0447.1992.tb03270.x. [DOI] [PubMed] [Google Scholar]
- Fowler B. Homocysteine: overview of biochemistry, molecular biology, and role in disease processes. Semin Vasc Med. 2005;5:77–86. doi: 10.1055/s-2005-872394. [DOI] [PubMed] [Google Scholar]
- Zhang CE, Tian Q, Wei W, Peng JH, Liu GP, Zhou XW, Wang Q, Wang DW, Wang JZ. Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging. 2008;29:1654–1665. doi: 10.1016/j.neurobiolaging.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Pacheco-Quinto J, Rodriguez de Turco EB, DeRosa S, Howard A, Cruz-Sanchez F, Sambamurti K, Refolo L, Petanceska S, Pappolla MA. Hyperhomocysteinemic Alzheimer’s mouse model of amyloidosis shows increased brain amyloid β peptide levels. Neurobiol Dis. 2006;22:651–665. doi: 10.1016/j.nbd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Hultberg B, Andersson A, Isaksson A. Hypomethylation as a cause of homocysteine-induced cell damage in human cell lines. Toxicology. 2000;147:69–75. doi: 10.1016/s0300-483x(00)00189-x. [DOI] [PubMed] [Google Scholar]
- Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20:6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Duan J, Murohara T, Ikeda H, Shintani S, Shimada T, Akita T, Egami K, Imaizumi T. Rescue of hypercholesterolemia-related impairment of angiogenesis by oral folate supplementation. J Am Coll Cardiol. 2003;42:364–372. doi: 10.1016/s0735-1097(03)00629-6. [DOI] [PubMed] [Google Scholar]
- Sun X, Cole GM, Chu T, Xia W, Galasko D, Yamaguchi H, Tanemura K, Frautschy SA, Takashima A. Intracellular Aβ is increased by okadaic acid exposure in transfected neuronal and non-neuronal cell lines. NeurobiolAging. 2002;23:195–203. doi: 10.1016/s0197-4580(01)00265-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain abeta distinguish Alzheimer’s disease from normal and pathologic aging. Exp Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Xu YF, Liu YH, Yin J, Li HL, Wang Q, Wang JZ. Peroxynitrite induces Alzheimer-like tau modifications and accumulation in rat brain and its underlying mechanisms. FASEB J. 2006;20:1431–1442. doi: 10.1096/fj.05-5223com. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zhang AH, Li HL, Wang Q, Deng HM, Netzer WJ, Xu H, Wang JZ. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J Neurochem. 2003;87:1333–1344. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- DeGiorgio LA, Manuelidis L, Bernstein JJ. Transient appearance of amyloid precursor protein plaques in the brain of thymectomized rats after human leptomeningeal cell grafts. Neurosci Letters. 2002;322:62–66. doi: 10.1016/s0304-3940(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Sastre M, Calero M, Pawlik M, Mathews PM, Kumar A, Danilov V, Schmidt SD, Nixon RA, Frangione B, Levy E. Binding of cystatin C to Alzheimer’s amyloid β inhibits in vitro amyloid fibril formation. Neurobiol Aging. 2004;25:1033–1043. doi: 10.1016/j.neurobiolaging.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Xu H, Gross RS, Greenfield JP, Hai B, Wang R, Greengard P. Testosterone reduces neuronal secretion of Alzheimer’s β -amyloid peptides. Proc Natl Acad Sci USA. 2000;97:1202–1205. doi: 10.1073/pnas.97.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Lorenzo A, Yankner BA. Methodological variables in the assessmen of beta amyloid neurotoxicity. Neurobiol Aging. 1992;13:609–612. doi: 10.1016/0197-4580(92)90065-6. [DOI] [PubMed] [Google Scholar]
- Gottfties CG, Lehmann W, Regland B. Early diagnosis of cognitive impairment in the elderly with the focus on Alzheimer’s disease. J Neural Transm. 1998;105:773–786. doi: 10.1007/s007020050094. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Sisodia SS, St George-Hyslop PH. Gamma-secretase, notch, abeta and Alzheimer’s disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of γ -secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- Kimberly WT, Wolfe MS. Identity and function of gamma-secretase. J Neurosci Res. 2003;74:353–360. doi: 10.1002/jnr.10736. [DOI] [PubMed] [Google Scholar]
- Bergman Y, Mostoslavsky R. DNA demethylation: turning genes on. J Biol Chem. 1998;379:401–407. doi: 10.1515/bchm.1998.379.4-5.401. [DOI] [PubMed] [Google Scholar]
- Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa S, Fuso A, D'Anselmi F, Cavallaro RA. Presenilin 1 gene silencing by S- adenosylmethionine: a treatment for Alzheimer disease? FEBS Lett. 2003;541:145–148. doi: 10.1016/s0014-5793(03)00277-1. [DOI] [PubMed] [Google Scholar]
- Fuso A, Seminara L, Cavallaro RA, D'Anselmi F, Scarpa S. S-adenosylmethionine/ homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci. 2005;28:195–204. doi: 10.1016/j.mcn.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Ukai W, Jo DG, Xu X, Mattson MP, Nakagawa M, Araki W, Saito T, Yamada T. Homocysteic acid induces intraneuronal accumulation of neurotoxic Abeta42: implications for the pathogenesis of Alzheimer’s disease. J Neurosci Res. 2005;80:869–876. doi: 10.1002/jnr.20514. [DOI] [PubMed] [Google Scholar]
- Heredia L, Lin R, Vigo FS, Kedikian G, Busciglio J, Lorenzo A. Deposition of amyloid fibrils promotes cell-surface accumulation of amyloid beta precursor protein. Neurobiol Dis. 2004;16:617–629. doi: 10.1016/j.nbd.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Iijima K, Ando K, Takeda S, Satoh Y, Seki T, Itohara S, Greengard P, Kirino Y, Nairn AC, Suzuki T. Neuron-specific phosphorylation of Alzheimer’s beta-amyloid precursor protein by cyclin-dependent kinase 5. J Neurochem. 2000;75:1085–1091. doi: 10.1046/j.1471-4159.2000.0751085.x. [DOI] [PubMed] [Google Scholar]
- Dewji NN, Singer SJ. Specific intercellular binding of the beta-amyloid precursor protein to the presenilins induces intercellular signaling: Its significance for Alzheimer’s disease. Proc Natl Acad Sci USA. 1998;95:5055–5060. doi: 10.1073/pnas.95.25.15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly WT, Zheng JB, Town T, Flavell RA, Selkoe DJ. Physiological regulation of the beta-amyloid precursor protein signaling domain by c-Jun N-terminal kinase JNK3 during neuronal differentiation. J Neurosci. 2005;25:5533–5543. doi: 10.1523/JNEUROSCI.4883-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB, Crews L, Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3β signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci. 2007;27:1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder J, Su Y, Liu F, Li B, Zhou Y, Ni B. Divergent roles of GSK-3 and Cdk-5 in APP processing. Biochem Biophys Res Commun. 2003;312:922–929. doi: 10.1016/j.bbrc.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Standen CL, Brownlees J, Grierson AJ, Kesavapany S, Lau KF, McLoughlin DM, Miller CC. Phosphorylation of thr (668) in the cytoplasmic domain of the Alzheimer’s disease amyloid precursor protein by stress-activated protein kinase 1b (Jun N-terminal kinase-3). J Neurochem. 2001;76:316–320. doi: 10.1046/j.1471-4159.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- Holzer M, Brückner MK, Beck M, Bigl V, Arendt T. Modulation of APP processing and secretion by okadaic acid in primary guinea pig neurons. J Neural Transm. 2000;107:451–461. doi: 10.1007/s007020070087. [DOI] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Sontag JM, Diaz-Arrastia R, Ogris E, Dayal S, Lentz SR, Arning E, Bottiglieri T. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci. 2007;27:2751–2759. doi: 10.1523/JNEUROSCI.3316-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol. 2008;85:148–175. doi: 10.1016/j.pneurobio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Blaise SA, Nédélec E, Schroeder H, Alberto JM, Bossenmeyer-Pourié C, Guéant JL, Daval JL. Gestational vitamin B deficiency leads to homocysteine-associated brain apoptosis and alters neurobehavioral development in rats. Am J Pathol. 2007;170:667–679. doi: 10.2353/ajpath.2007.060339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Dahl EE, Overmier JB, Mantyh P, Cleary J. Effects of an exogenous beta-amyloid peptide on retention for spatial learning. Behav Neural Biol. 1994;62:60–67. doi: 10.1016/s0163-1047(05)80059-7. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Overmier JB, Bandyopadhyay S, Babcock D, Cleary J. Reversal of beta-amyloid-induced retention deficit after exposure to training and state cues. Neurobiol Learn Mem. 1996;65:35–47. doi: 10.1006/nlme.1996.0004. [DOI] [PubMed] [Google Scholar]
- Sweeney WA, Luedtke J, McDonald MP, Overmier JB. Intrahippocampal injections of exogenous beta-amyloid induce postdelay errors in an eight-arm radial maze. Neurobiol Learn Mem. 1997;68:97–101. doi: 10.1006/nlme.1997.3770. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Featy MB, Dickson DW. Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol. 1996;40:139–148. doi: 10.1002/ana.410400204. [DOI] [PubMed] [Google Scholar]
- Selhub J, Jacques PF, Bostom AG, D'Agostino RB, Wilson PW, Belanger AJ, O'Leary DH, Wolf PA, Schaefer EJ, Rosenberg IH. Association between plasma homocysteine concentrations andextracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–291. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- Kim JM, Lee H, Chang N. Hyperhomocysteinemia due to short-term folate deprivation is related to electron microscopic changes in the rat brain. J Nutr. 2002;132:3418–3421. doi: 10.1093/jn/132.11.3418. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim HJ, Kim JM, Chang N. Effects of dietary folic acid supplementation on cerebro- vascular endothelial dysfunction in rats with induced hyperhomocysteinemia. Brain Res. 2004;996:139–147. doi: 10.1016/j.brainres.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder G, Roffi M, Pin R, Flammer Y, Lange H, Eberli FR, Meier B, Turi ZG, Hess OM. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med. 2001;345:1593–1600. doi: 10.1056/NEJMoa011364. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Shea TB. Folacin and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]