Abstract
Although recombinant human erythropoietin (rHuEpo) has revolutionized the treatment of anemia, recent clinical trials suggested that rHuEpo use may be associated with decreased survival in cancer patients. Although the expression of erythropoietin (Epo) receptor (EpoR) has been demonstrated in various human cancers, the effect of exogenous Epo on the growth and therapy resistance of EpoR-bearing tumor cells is unclear at present. In the current study, we examined the hypothesis that EpoR may contribute to tumor growth independent of Epo in A2780 human ovarian carcinoma cells. A2780 human ovarian carcinoma cells showed high levels of EpoR expression, but lacked expression of Epo mRNA and biologically active Epo protein under both normoxic and hypoxic conditions. Exogenous Epo did not stimulate EpoR-mediated signaling, proliferation, invasiveness, or resistance to cytotoxic drugs in A2780 cells. In contrast, specific inhibition of EpoR expression using a short hairpin RNA (shRNA) expression plasmid resulted in markedly reduced proliferation and invasiveness in vitro. In addition, inhibition of EpoR expression led to abrogated in vivo ovarian cancer cell growth in a tumor xenograft system and resulted in decreased EpoR signaling. Our findings suggest that EpoR may be constitutively active in some cancer cells in the absence of Epo and provide the first evidence for a potential role of an Epo-independent, EpoR-mediated pathway in the growth of some human cancers.
Erythropoietin (Epo), a glycoprotein hormone produced by the kidney in response to hypoxia,1,2 has been considered to be a specific stimulator of erythropoiesis.1 Epo acts via its receptor (EpoR), a member of the cytokine receptor type I superfamily. Signal transduction on Epo binding takes place because of conformational change leading to activation of EpoR-bound Janus kinase 2 (JAK2)3 and via recruitment and activation of signal transducer-activator of transcription-5 (STAT5), leads to activation of mitogen-activated protein kinases (MAPK), phosphatidylinositol 3-kinase (PI3K), and Akt. Recent evidence suggests that JAK2-independent Epo signaling may also exist.4 EpoR stimulation in erythrocytic progenitors results in the stimulation of proliferation and differentiation, and inhibition of apoptosis.1,5
Until recently, the action of Epo was considered to be restricted to erythropoietic cells. In recent years, however, it has become clear that EpoR is expressed by several other cell types, and various biological effects of Epo, including neuro- and cardioprotection under hypoxia, stimulation of angiogenesis, cell proliferation, and migration, have been demonstrated.6 We and others have recently described that various cancer cells express Epo and EpoR at the gene and protein levels and display functional EpoR signaling.7,8,9,10,11,12,13,14,15,16 Although several studies suggested that Epo may enhance tumor angiogenesis,17,18 cancer cell migration,19 invasiveness,11,13,15 survival,7,8,15,20 and proliferation,9,14,21 other reports contradicted these findings22,23,24 and thus, currently the role for EpoR in cancer biology is unclear.
Recombinant human erythropoietin (rHuEpo) has revolutionized the treatment of anemia in chronic renal failure.25 Anemia is present in many cancer patients at the time of diagnosis and/or as the result of cancer therapy.26 Anemia not only impairs the quality of life of patients, but it leads to tumor hypoxia, resistance to chemo- and radiotherapy,27 and reduces survival.26 Because of the apparent interconnections among anemia, hypoxia, tumor responsiveness to therapy, and outcomes, clinical studies have been conducted in the assumption that correction of anemia will not only alleviate anemia-related symptoms but also improve tumor response to therapy and increase survival.26,28,29 Although rHuEpo treatment was clearly shown to be effective in reducing transfusion requirements and improve quality of life,26,28,29 its effects on survival are controversial. Although a recent meta-analysis of clinical trials indicated no adverse effect,29 the results of several recent clinical trials in patients with breast,30,31 (PREPARE study, http://wwwext.amgen.com/media/media_pr_detail.jsp?year=2007&releaseID=1083091, accessed 01/2009), head and neck,32,33 lung,34 and cervix35 cancers suggested a potential adverse outcome in rHuEpo-treated patients compared with placebo-treated groups, partly because of earlier tumor progression/recurrence, prompting the United States Food and Drug Administration to issue a black box warning for Epo products.36
The hypothesis currently put forward suggests that EpoR may play a role in tumor progression by stimulating proliferation and/or inhibiting apoptosis of cancer cells on Epo binding, and some investigators suggested that the adverse effects are likely attributable to stimulation of EpoR present on cancers cells by rHuEpo treatment.37 In contrast, others, based on in vitro and in vivo data showing lack of a proliferative effect of exogenous Epo despite EpoR expression, argue that whereas EpoR is present in cancer cells, it is not biologically active and not essential for tumor growth.22,23,24,38 However, cancer cells are thought to undergo a continuous cycle of natural selection during which better adapted cells are favored to pass on their genetic information, and they are unlikely to retain biologically inactive metabolic and regulatory pathways. In the present study we studied the role and significance of EpoR expressed in A2780 human ovarian carcinoma cells that are not responsive to exogenous Epo.
Materials and Methods
Cell Culture
The A2780 human ovarian carcinoma cells were provided by Dr. George Coukos (University of Pennsylvania, Philadelphia, PA). CHO cells were purchased from Invitrogen (Carlsbad, CA). UT-7 cells were a gift from Dr. Elaine Dunlop (Belfast City Hospital, Belfast, UK). HepG2 and K562 cells were received from Drs. Jiandong Chen and Mei Huang, respectively (Moffitt Cancer Center, Tampa, FL). Cells were grown in RPMI (GIBCO Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), penicillin (50 IU/ml), and streptomycin sulfate (50 μg/ml) (GIBCO Invitrogen) at 37°C in a humidified atmosphere containing 5% CO2, unless otherwise specified. Media of UT-7 cells were supplemented with 10 U/ml rHuEpo (Epogen, Epoetin α; Amgen Pharmaceuticals, Thousand Oaks, CA) after each passage. To test the short-term effect of rHuEpo treatment on cell lines, subconfluent cultures of A2780 and 1 × 106 UT-7 cells were treated for 5 minutes with rHuEpo in six-well plates after overnight serum (1% FBS) and, in case of UT-7 cells, Epo starvation. Twenty-four hours before hypoxia treatments cells were switched to serum-free medium. Hypoxia treatment of cells was performed in an enclosed chamber (Billups-Rothenberg Inc., Del Mar, CA) flushed with premixed gas mixture (2% O2, 5% CO2, 93% N2) for 6 hours. To test the ability of Epo neutralization to effect the proliferation and survival of A2780 and UT-7 cells, the AB-286-NA Epo neutralization rabbit total IgG (R&D Systems, Minneapolis, MN) was used at a concentration of 30 μg/ml (corresponding to 10 × 50% neutralization dose). A2780 cells were plated in wells of 96-well plates (2000 cells/well) and allowed to adhere overnight. UT-7 cells, growing in suspension culture, were washed three times in culture media lacking Epo and suspended in treatment medium at a density of 2000 cells/100 μl. The medium of adhered A2780 cells was changed to 100-μl treatment medium consisting of 10% FBS in RPMI, 10% FBS in RPMI with 0.4 U/ml Epo, 10% FBS in RPMI with 30 μg/ml neutralizing antibody, or 10% FBS in RPMI with both 0.4 U/ml Epo and 30 μg/ml neutralizing antibody.
Plasmids
Oligonucleotide cDNA inserts encoding short hairpin RNA (shRNA) specific for EpoR were designed using the Insert Design Tool available at the Ambion (Austin, TX) website. The oligonucleotides were annealed and ligated into the pSilencer 4.1-CMV hygro vector (Ambion) containing a modified cytomegalovirus (CMV) promoter for RNA polymerase II-mediated transcription. EpoR shRNA oligonucleotides (top strand, 5′-GATCCCTACAGCTTCTCCTACCAGTTCAAGAGACTGGTAGGAGAAGCTGTAGTTA-3′; bottom strand, 5′-AGCTTAACTACAGCTTCTCCTACCAGTCTCTTGAACTGGTAGGAGAAGCTGTAGG-3′) contained a region specific to bases 362 to 382 of EpoR mRNA (bold), a hairpin loop region, and 5′ and 3′ linker sequences for subcloning into the HindIII and BamHI sites of the pSilencer 4.1-CMV hygro vector. The scrambled negative control shRNA oligonucleotides contained identical hairpin loop and linker sequences but a sequence of DNA not complementary to any known human gene (5′-GACCAGCTTCTCCACAATCAT-3′). The newly created pSilencer 4.1-CMV hygro-EpoR shRNA (pS-EpoR) and pSilencer 4.1-CMV hygro-scrambled shRNA (pS-Neg) vectors were prepared from individual bacterial colonies. Correct orientation and location of the oligonucleotide cloning were confirmed by sequencing. The pBCMGS-Neo expression vector containing human wild-type full-length EpoR was generously provided by Dr. Ajay Verma (Uniformed Services University of the Health Sciences, Bethesda, MD). The sequence validated EpoR coding region was incorporated into pcDNA6.2 D-TOPO vector (Invitrogen) according to the manufacturer’s protocol and used for full-length human EpoR overexpression in A2780 cells.
Transfection of Cells
For stable transfection, A2780 cells were plated in six-well plates at a density of 5 × 105 cells per well and incubated overnight. Cells were transfected with Lipofectamine 2000 (Invitrogen) using 4 μg of the pSilencer 4.1-CMV hygro plasmid containing EpoR shRNA or the scrambled negative control sequence. Stable transfectant cell lines were selected by growth in the presence of 200 μg/ml of hygromycin (Invitrogen). Individual cells were isolated with cloning disks. Thirty-five stable clones expressing the EpoR shRNA were screened for EpoR knockdown using quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blotting. Full-length EpoR-overexpressing pcDNA6.2 plasmids were nucleofected into A2780 cells using an Amaxa Nucleofector and Cell Line Nucleofector Kit V (Amaxa Inc., Gaithersburg, MD) according to the manufacturer’s protocol. Stable EpoR-expressing cells were selected with 10 μg/ml of Blasticidin (Invivogen, San Diego, CA) and were used for experiments after at least 2 weeks of selection.
Quantitative Real-Time RT-PCR Assays
Total RNA was extracted using the RNeasy mini kit (Qiagen, Valencia, CA). For RT-PCR 1 μg of total RNA per sample was reverse-transcribed to cDNA using the SuperScript first-strand synthesis system (Invitrogen). Quantitative real-time PCR was performed on the iCycler real-time detection system (Bio-Rad Laboratories, Hercules, CA) using a total reaction volume of 20 μl containing 5 μl of cDNA template, sense and antisense primers, and 1× iQ SYBR Green Supermix reagents (Bio-Rad Laboratories). Amplifications were performed at 95°C for 3 minutes and for 40 cycles of 30 seconds at 95°C and 30 seconds at 60°C, and SYBR Green melting curve was measured from 55 to 90°C. Gene expression was normalized to either the geometric mean of three endogenous control genes, including β-actin (BACT), succinate dehydrogenase (SDHA), and hypoxanthine phosphoribosyltransferase 1 (HPRT1), or in case of measurements from the same cell lines to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers used were the following: EpoR sense 5′-CGGGCAACTACAGCTTCTCC-3′, antisense 5′-GTAGGCAGCGAACACCAGAA-3′; Epo sense 5′-CTGGAAGAGGATGGAGGTCGG-3′, antisense: 5′-GCTGGGAAGAGTTGACCAACAG-3′; BACT sense 5′-CTACCTCATGAAGATCCTCACCGA-3′, antisense 5′-ACGTAGCACAGCTTCTCCTTAATG-3′; SDHA sense 5′-GGACAACTGGAGGTGGCATTT-3′, antisense 5′-TGTAGTGGATGGCATCCTGGT-3′; HPRT1 sense 5′-CTCCTCCTGAGCAGTCAGCC-3′, antisense 5′-CATCATCACTAATCACGACGCC-3′; GAPDH sense 5′-AACCTGCCAAATATGATGACATCA-3′, antisense 5′-TAGCCCAGGATGCCCTTGAG-3′. Primers were designed using the Beacon Designer software (version 3; Premier Biosoft International, Palo Alto, CA).
Western Blotting
Cells were washed twice with ice cold phosphate-buffered saline (PBS) and lysed in RIPA buffer containing 1× Halt phosphatase and protease inhibitor cocktails (Pierce, Rockford, IL). Tumor xenograft samples were lysed in Tissue Protein Extraction Reagent (T-PER, Pierce) with phosphatase and protease inhibitor cocktails. Whole cell lysates were normalized for protein. Twenty μg of proteins from each sample were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Proteins were detected using antibodies against EpoR (goat polyclonal, 1:500 dilution; R&D Systems), phosphorylated-EpoR (p-EpoR) (rabbit polyclonal, 1:500; Santa Cruz Biotechnology, Santa Cruz, CA), and JAK2 (rabbit polyclonal, 1:1000; Santa Cruz). p-JAK2, STAT5, p-STAT5, Akt, p-Akt, MAPK, p-MAPK rabbit polyclonal antibodies were also obtained from Cell Signaling Technology (Danvers, MA) and used in 1:1000 dilution. As a loading control, horseradish peroxidase-conjugated polyclonal antibodies to β-actin (1:2000) and GAPDH (1:4000) (both from Santa Cruz) were used. Membranes were incubated with the primary antibodies overnight at 4°C; horseradish peroxidase-conjugated bovine anti-rabbit, anti-goat, or anti-mouse pre-absorbed antibodies for secondary staining were purchased from Santa Cruz and used in 1:5000 dilution. Immunoreactive bands were visualized using chemiluminescence (ECL Advanced Western blotting detection system; Amersham-GE Healthcare Bio-Sciences Co., Piscataway, NJ).
Flow Cytometry
Cell surface EpoR expression was assessed by flow cytometry using phycoerythrin-conjugated mouse monoclonal EpoR antibody (FAB307P) and matching isotype-negative control (IC003P) (both from R&D Systems). Cell staining was performed according to the manufacturer’s protocol and measured using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Results were analyzed using the FlowJo software (Tree Star Inc., Ashland, OR).
MTT Assay
Cell viability was assessed using a modified MTT assay (CellTiter 96; Promega, Madison, WI) according to the manufacturer’s recommendations. Briefly, for determination of growth rate cells were plated in 96-well plates at a density of 1000 cells per well in 100 μl of medium containing rHuEpo (0 to 100 U/ml). The amount of viable cells was determined every 24 hours using eight wells per time point. For cytotoxic drug treatment experiments cells were plated at a density of 10,000 cells per well in 96-well plates and incubated at 37°C for 24 hours. The medium in the wells was replaced by 100 μl of serum-free medium, Cells were treated with rHuEpo (0 to 100 U/ml) for 60 minutes, then exposed to taxol (0 to 300 μg/ml, Paclitaxel; Mead Johnson Oncology Products, Princeton, NJ) or cisplatin (0 to 300 μg/ml; Bedford Laboratories, Bedford, OH) and incubated for 24 hours. For color development, 15 μl of dye solution was added to each well and the plates were incubated at 37°C for 4 hours, followed by addition of 100 μl of solubilization/stop solution to each well. Absorbance was recorded at 570 nm using a 96-well plate reader. Eight wells were assayed per treatment condition. All experiments were performed in triplicate. Results were compared using the repeated measures one-way analysis of variance followed by the Tukey-Kramer multiple comparison test, when appropriate. Statistical significance was determined if the two-sided P value of a test was less than 0.05. Computations were performed using the Graphpad Prism software (GraphPad Software, San Diego, CA).
UT-7 Co-Culture Experiments
To test the ability of A2780 cells to produce autocrine/paracrine-acting functional Epo a transwell cell culture system was used. A2780 and HepG2 cells were plated in 24-well plates at a concentration of 1 × 105 cells/well (four wells/cell line). After cells adhered to the culture dishes, transwell inserts (8-μm pore size; Becton Dickinson Labware, Bedford, MA) were placed in the wells and 1 × 104 Epo-dependent UT-7 cells were plated in each insert. The 8-μm pore size was previously found to be sufficient to keep UT-7 cells from crossing the transwell membrane. In the cases of positive and negative controls no cells were plated in the basal compartments, and transwells were either supplied with fully supplemented growth medium containing 0.4 U/ml Epo or no Epo, respectively. Media were changed every 3 days, and transwells were moved into newly assembled 24-well plates containing freshly plated A2780 and HepG2 cells on the 4th day to prevent overgrowth of cells in the basal compartment. Eight days after initiation of the experiments UT-7 cells were washed and resuspended in PBS. The cell suspensions were mixed at a ratio of 1:1 with 0.4% Trypan Blue solution (Sigma-Aldrich, St. Louis, MO), incubated for 2 minutes, and the numbers of viable and nonviable cells were determined using a hemocytometer.
Cell Invasion Assay
Cell invasion assays were performed using 24-well Biocoat Matrigel invasion chambers with an 8-μm pore polycarbonate filter according to the manufacturer’s instructions (Becton Dickinson Labware). Briefly, cells in the growing phase were trypsinized and resuspended at a concentration of 5 × 105 cells/ml. The lower compartments of the plates received 750 μl of either 0.5% FBS or 10% FBS-containing medium. Cells were plated in each insert at a density of 2.5 × 105 cells in medium containing 0.5% FBS and allowed to invade for 48 hours at 37°C in a humidified incubator. Cells that remained inside the inserts at the termination of the experiments were thoroughly wiped off with a cotton swab and invading cells were fixed and stained using Diff-Quick stain solution (Dade Behring, Newark, DE). Invading cells were quantified by counting the number of stained cells in five predetermined fields. Experiments were performed using six inserts per group. Percent invasion was determined by the following formula: % invasion = NM/NC × 100, where NM is the mean number of cells invading through Matrigel insert membrane and NC is mean number of cells invading through control insert membrane. The difference in percent invasion between groups was statistically analyzed using a two-tailed Student’s t-test.
Xenografts
Five-week-old male athymic NCr nude mice were purchased from Taconic, Inc. (Hudson, NY) and housed in a pathogen-free room controlled for temperature and humidity. Animal protocols were performed under approved University of Pennsylvania Institutional Animal Care and Use Committee guidelines. A2780 cells stably transfected with pS-EpoR and pS-Neg vectors were grown to subconfluence, harvested by trypsinization, and 1 × 106 cells were injected subcutaneously into the flank region of mice. Tumor growth was measured twice a week using a caliper and tumor volume was calculated using the following formula: tumor volume = (a x b2)/2, where a is the widest diameter of the tumor and b is the diameter perpendicular to a. Differences in tumor volume between groups were statistically analyzed using a two-tailed Student’s t-test. Animals were sacrificed after 9 weeks and the tumors were excised and weighed. Parts of the tumors were snap-frozen in liquid nitrogen and subsequently used for Western blotting and quantitative real-time RT-PCR assays. The rest of the tumors were fixed for 24 hours in 10% phosphate-buffered formalin and embedded in paraffin for histological examination and immunohistochemical studies.
Immunohistochemistry
Immunohistochemical assays were performed on formalin-fixed, paraffin-embedded sections. For Ki-67 immunostaining the DAKO EnVision+ system horseradish peroxidase kit was used according to the manufacturer’s recommendations. Slides were boiled for 20 minutes in 1× Target retrieval buffer (pH 6.0; DakoCytomation, Carpinteria, CA), and incubated with the antibody (mouse monoclonal, clone Ki-S5, 1:25; DakoCytomation) for 30 minutes at room temperature. For positive control, slides of human tonsil were used. A negative control was done in each case by omission of the primary antibody. For immunohistochemical detection of apoptotic cells the SignalStain cleaved caspase-3 (Asp175) IHC detection kit (Cell Signaling Technology) was used according to the manufacturer’s recommendations. Tumor cells showing nuclear immunoreactivity were counted in10 random areas of tumor tissue away from necrotic regions under high magnification (×400) (corresponding to at least 5000 tumor cells per section). The ratio of Ki-67 and cleaved caspase-3-positive cells were expressed as the percentage of all tumor cells.
Results
A2780 Cells Express High Levels of EpoR, but Not Epo
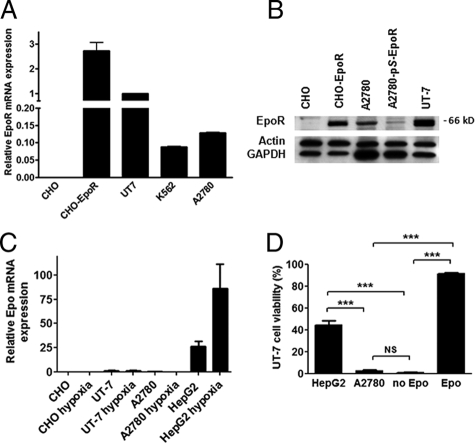
A2780 human ovarian carcinoma cells expressed a higher level of EpoR mRNA as measured by quantitative real-time RT-PCR than known EpoR-expressing K562 cells. Compared with the currently best defined EpoR-expressing and Epo-dependent UT-7 erythroleukemia cells, the level of EpoR mRNA expression in A2780 cells was ∼15% of that seen in UT-7 cells (Figure 1A). CHO cells showed no significant human EpoR mRNA expression (negative control), whereas CHO cells transfected with a plasmid expressing human full-length EpoR showed very high levels of EpoR expression (positive control). Western blotting showed a specific band at ∼66 kDa in A2780 cells, corresponding to EpoR. The specificity of the antibody to detect EpoR was confirmed using CHO cells transfected with human full-length EpoR (Figure 1B, see supplemental Figure S1 for complete blot at http://ajp.amjpathol.org). Epo mRNA expression in A2780 cells was measured by quantitative RT-PCR and compared with known Epo-expressing HepG2 hepatoma cells40 and Epo nonexpressing UT-7 and CHO cells.41 No Epo mRNA expression was found in A2780, UT-7, and CHO cells under either normoxic conditions or after exposure to hypoxia (2% O2) for 6 hours. In contrast, high levels of Epo mRNA expression were detected in HepG2 cells, which were further increased by exposure to hypoxia (Figure 1C). To assess whether A2780 cells produced biologically active Epo, we tested the ability of A2780 and HepG2 cells to promote the survival of Epo-dependent UT-7 cells in a transwell co-culture system. Epo (0.4 U/ml) supported survival of UT-7 cells (91.5 ± 1.0%, mean ± SD, viable cells). Although co-cultured HepG2 cells maintained a 44.4 ± 3.7% (mean ± SD) UT-7 cell viability, viable UT-7 cells were scarcely found in A2780 co-cultures (2.5 ± 0.7%, mean ± SD) or when cells were grown in the absence of Epo (0.8 ± 0.5%, mean ± SD) (Figure 1D). Furthermore, treatment of the cells with a neutralizing anti-Epo antibody completely abrogated 0.4 U/ml Epo-induced UT-7 survival and proliferation, but had no effect on the growth of A2780 cells (see supplemental Figure S2 at http://ajp.amjpathol.org).
Figure 1.
A: A2780 cells express high levels of erythropoietin receptor (EpoR) mRNA. Relative EpoR expression levels are presented relative to the EpoR mRNA level in UT-7 erythroleukemia cells. Quantitative RT-PCR assays were run in triplicate; error bars indicate the SEM. Three independent experiments yielded similar results. B: A2780 ovarian carcinoma cells express EpoR protein. Protein extracts were prepared from A2780 cells, A2780 cells stably transfected with an EpoR-specific shRNA-expressing vector (A2780-pS-EpoR), and UT-7 cells and subjected to Western blotting. As negative and positive controls, CHO cells and CHO cells stably transfected with human full-length EpoR (CHO-EpoR) were used, respectively. A specific immunoreactive band was seen at ∼66 kDa in the positive control CHO-EpoR, A2780, and UT-7 cells, whereas no corresponding band was seen in the negative control CHO cells. Note that A2780-pS-EpoR cells show a marked decrease in the intensity of the specific band at ∼66 kDa. The blots were stripped and reprobed with β-actin and GAPDH as loading controls. For the image of the full blot, please see Supplemental Figure S1 at http://ajp.amjpathol.org. C: Erythropoietin (Epo) mRNA is not expressed in A2780 cells. Real-time quantitative RT-PCR results are presented relative to the EpoR mRNA level in UT-7 cells under normoxic conditions. No significant Epo mRNA expression was seen in A2780 cells, similar to known Epo nonexpressing UT-7 and the negative control CHO cells under either normoxic conditions or after exposure to hypoxia (2% O2) for 6 hours. In contrast, HepG2 cells, which are known to express Epo, showed two orders of magnitude higher Epo mRNA expression levels, which was further elevated by exposure to hypoxia. Quantitative RT-PCR assays were run in triplicate; error bars indicate SEM. Three independent experiments yielded similar results. D: A2780 cells fail to express biologically active Epo and cannot promote the survival of Epo-dependent UT-7 cells. Epo-dependent UT-7 cells were grown in a transwell co-culture system in the presence of HepG2 or A2780 cells. As a positive and negative control, UT-7 cells were also grown in the presence and absence of Epo (0.4 U/ml), respectively. Cell viability was tested after 8 days of culture using the Trypan Blue dye exclusion assay. Four wells were used for each experimental condition, error bars represent SEM. Results were analyzed by pair-wise comparison using the Mann-Whitney test (NS, not significant; P > 0.05) (*P < 0.05; **P < 0.01; ***P < 0.001).
EpoR Protein Expression Is Not Detectable on the Surface of A2780 Cells
EpoR is an integral membrane protein, which is understood to function by serving as a receptor for Epo within the cell membrane. We aimed to assess the expression of EpoR on the surface of UT-7 cells, A2780 cells, and A2780 cells overexpressing full-length human EpoR (A2780-F-EpoR) by flow cytometry using a specific EpoR antibody detecting the N-terminal (extracellular) domain of EpoR. Although high levels of EpoR surface expression was found in UT-7 cells, no significant surface EpoR expression was seen in parent A2780 cells. A2780-F-EpoR showed high levels of EpoR surface expression (see supplemental Figure S3 at http://ajp.amjpathol.org).
Exogenous Epo Shows No Biological Effect in A2780 Cells
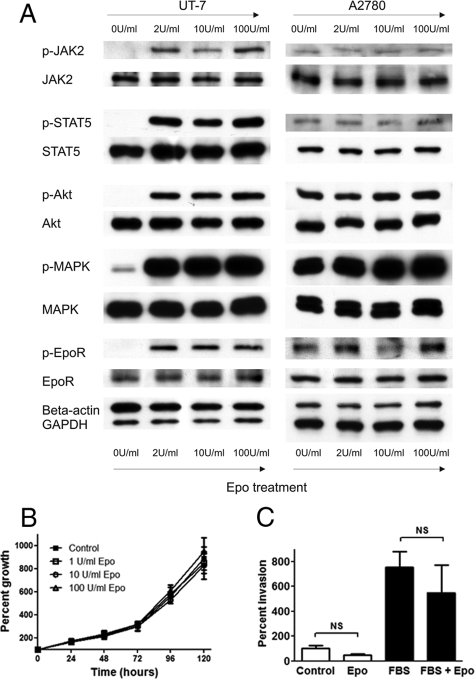
To assess the effect of exogenous Epo on EpoR signaling in A2780 cells, subconfluent cultures were subjected to Epo treatment for 5 minutes after overnight serum starvation. As a positive control, UT-7 cells treated similarly after overnight Epo and serum starvation were used. As expected, Epo treatment stimulated the phosphorylation of EpoR signaling proteins, including EpoR itself, JAK2, STAT5, Akt, and MAPK in UT-7 cells. In contrast, Epo failed to induce increased phosphorylation of EpoR signaling components in A2780 cells (Figure 2A). Interestingly, in contrast with UT-7 cells, increased baseline phosphorylation of all examined EpoR signaling proteins was seen in A2780 cells, even in the absence of Epo. Interestingly, 10-fold overexpression of human full-length EpoR mRNA in A2780 cells resulting in markedly increased EpoR protein expression by Western blotting failed to render A2780-F-EpoR cells Epo-responsive in in vitro phosphorylation studies (pEpoR, pJAK2, pSTAT5, pMAPK) (not shown).
Figure 2.
A: Exogenous erythropoietin (Epo) treatment fails to stimulate downstream Epo receptor (EpoR) signaling in A2780 cells. Exogenous Epo (2 to 100 U/ml) treatment of UT7 erythroleukemia and A2780 ovarian carcinoma cells was performed for 5 minutes after overnight Epo and serum starvation. Strong induction of the phosphorylation of EpoR signaling pathway mediators was found in UT7 cells, whereas exogenous Epo did not stimulate phosphorylation of the same proteins in A2780 cells. Of note, a high baseline level of phosphorylation of EpoR signaling components was seen in A2780 cells even in the absence of exogenous Epo. Data are representative of three independent experiments yielding similar results. B: Exogenous Epo has no effect on the proliferation of A2780 cells. The effect of exogenous Epo (0 to 100 U/ml) on the proliferation of A2780 ovarian carcinoma cells was studied using a modified MTT assay. Eight wells were used for each time point; error bars represent the SEM (P > 0.05, one-way analysis of variance). Three independent experiments yielded similar results. C: Exogenous Epo has no effect on the invasiveness of A2780 cells. The effect of exogenous Epo on the in vitro invasiveness of A2780 ovarian carcinoma cells was examined using a Matrigel invasion assay. Cells were plated in the invasion chambers in culture medium containing 0.5% FBS, whereas the lower compartments contained medium with 0.5% FBS with or without 10 U/ml Epo. As a positive control, 10% FBS (designated as FBS on the figure) was used in the lower compartments (with or without 10 U/ml Epo). Assays were performed using six inserts per treatment group. Results are presented as mean percent invasion from one representative experiment; error bars represent SEM. Three independent experiments yielded similar results.
To examine the effect of exogenous Epo on cell proliferation and sensitivity to cytotoxic drugs in vitro, a modified MTT assay was used. Exogenous Epo had no significant effect either on the proliferation (Figure 2B), or cisplatin and taxol sensitivity of A2780 cells (not shown). To assess the effect of exogenous Epo on the in vitro invasiveness of A2780 ovarian carcinoma cells we used transwell chambers coated with Matrigel. Exogenous Epo had no significant effect on the invasiveness of A2780 cells either in the absence or presence of a FBS concentration gradient (Figure 2C).
EpoR Down-Regulation by shRNA in A2780 Cells Suppresses Cell Proliferation and Invasiveness in Vitro
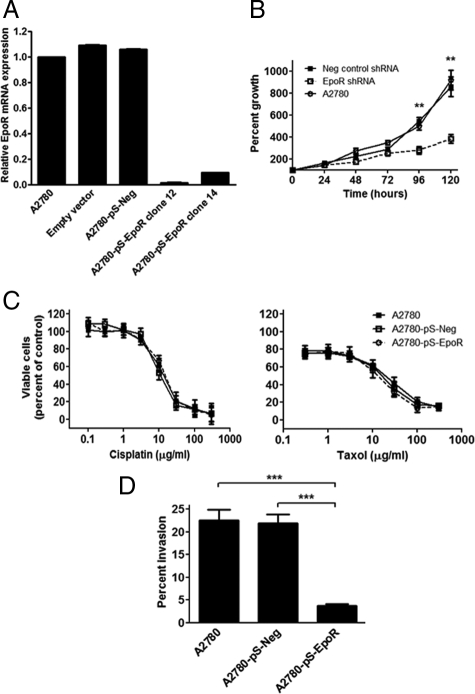
To study the effect of inhibition of EpoR expression in ovarian carcinoma cells we used vector-based RNA interference. The shRNA sequence used was homologous only to EpoR mRNA as confirmed by the nucleotide blast search engine of the National Center for Biotechnology Information. EpoR mRNA level was determined by real-time quantitative RT-PCR in clones of the three different stable transfected cell groups, containing either the empty plasmid, the pSilencer 4.1-CMV hygro plasmid with a scrambled negative control shRNA sequence (A2780-pS-Neg), or the pSilencer 4.1-CMV hygro plasmid with the EpoR shRNA oligonucleotide (A2780-pS-EpoR). Two EpoR shRNA cell clones (clones 12 and 14) showed a more than 90% reduction in EpoR levels compared with the cell lines containing the scrambled negative control shRNA or the empty vector (Figures 1B and 3A).
Figure 3.
A: Erythropoietin receptor (EpoR) was specifically down-regulated in A2780 ovarian carcinoma cells. Greater than 90% reduction in EpoR mRNA expression was found in A2780 cells stably transfected with EpoR-specific shRNA expression plasmids in clones 12 and 14 compared with parent cells and cells transfected with negative scrambled shRNA-expressing vectors (A2780-pS-Neg). Quantitative real-time RT-PCR assays for EpoR were performed in triplicates and expression results were normalized to the geometric mean of BACT, SDHA, and HPRT1 expression. Normalization to GAPDH expression data gave similar results. Error bars indicate the SEM. Three independent experiments yielded similar results. B: Down-regulation of EpoR in A2780 cells results in decreased in vitro cell proliferation. A modified MTT assay was used to determine the proliferation of A2780 cell clones stably transfected with EpoR-specific shRNA expression plasmids (A2780-pS-EpoR). Inhibition of EpoR expression in A2780-pS-EpoR cells resulted in a significantly decreased proliferation rate compared with parent A2780 or A2780 cells transfected with a negative control scrambled shRNA expression plasmid (A2780-pS-Neg). Data are presented as mean percent growth compared with time 0; error bars represent SEM (**P < 0.01, Student’s t-test). C: Down-regulation of EpoR expression does not alter the sensitivity of A2780 cells to the cytotoxic drugs cisplatin and taxol. A modified MTT assay was used to assess the sensitivity of A2780 cells stably transfected with EpoR-specific shRNA expression plasmids (A2780-pS-EpoR) to cis-platin and taxol. Inhibition of EpoR expression in A2780-pS-EpoR cells did not result in altered sensitivity of the cells to the cytotoxic drugs compared with parent A2780 or A2780 cells transfected with a negative control scrambled shRNA expression plasmid (A2780-pS-Neg). Data are presented as the mean percentage of viable cells compared with untreated cells; error bars represent SEM. Three independent experiments yielded similar results. D: Down-regulation of EpoR resulted in decreased invasiveness of A2780 ovarian carcinoma cells in vitro. In vitro invasiveness of A2780 cells stably transfected with EpoR-specific shRNA expression plasmids (A2780-pS-EpoR) was assessed using the Matrigel invasion assay with 10% FBS containing medium as a chemoattractant. Inhibition of EpoR expression in A2780-pS-EpoR cells resulted in significantly decreased invasiveness compared with parent A2780 or A2780 cells transfected with a negative control scrambled shRNA expression plasmid (A2780-pS-Neg). Results are presented as the mean percentage of cells invading through Matrigel-coated compared with control membranes; error bars represent SEM. Three independent experiments yielded similar results. ***P < 0.001.
To examine the effect of reduced EpoR expression on the proliferation of A2780 cells, a modified MTT assay was used. Inhibition of EpoR expression in A2780-pS-EpoR cells producing EpoR shRNA resulted in significantly slower proliferation compared with control A2780 cells or A2780-pS-Neg cells producing a negative control scrambled shRNA (Figure 3B). Exogenous Epo (1 to 100 U/ml) had no significant effect on the proliferation of A2780-pS-EpoR or A2780-pS-Neg cells (not shown).
One of the major problems in the treatment of ovarian cancer is the development of resistance to chemotherapeutic drugs. Because prior studies suggested that exogenous Epo may increase the resistance of some ovarian carcinoma cells to chemotherapeutic drugs,10 we examined whether inhibition of EpoR expression had an effect on the sensitivity of A2780 cells to chemotherapeutic drugs commonly used to treat ovarian cancer. As shown in Figure 3C, we found no significant difference between the effects of cisplatin and taxol in A2780-pS-EpoR cells compared with A2780-pS-Neg or control A2780 cells. To assess the effect of inhibition of EpoR expression in A2780 cells on invasiveness in vitro, transwell chambers coated with Matrigel were used. As shown in Figure 3D, inhibition of EpoR expression in A2780-pS-EpoR cells resulted in significantly decreased invasiveness compared with A2780-pS-Neg or parent A2780 cells.
EpoR-Specific shRNA Abrogates A2780 Ovarian Cancer Cell Growth in Vivo
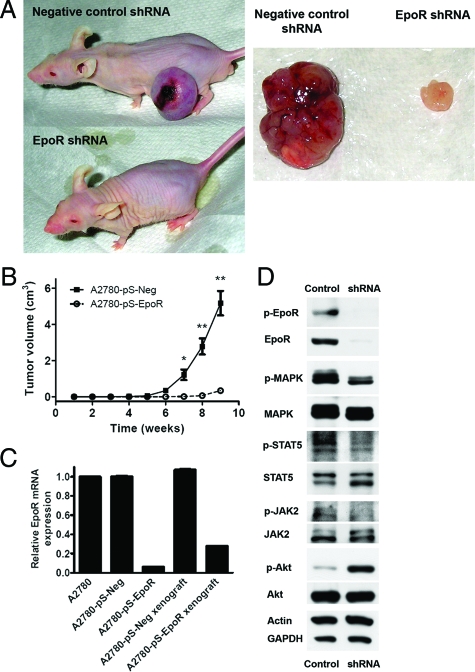
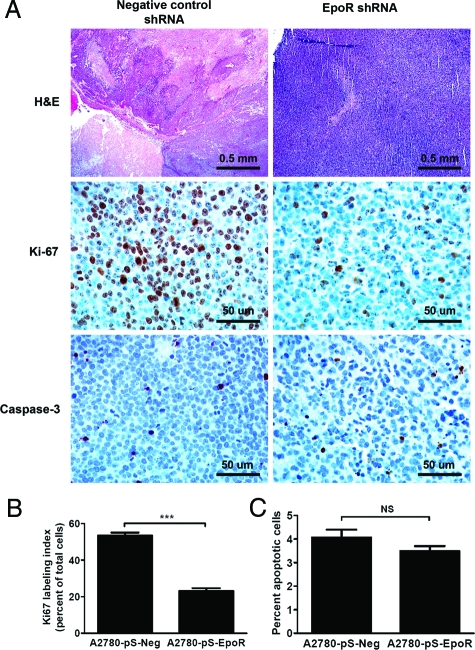
To determine whether inhibition of EpoR expression decreases in vivo tumorigenicity, we conducted a xenograft study using A2780 cells expressing either scrambled negative control or EpoR-specific shRNA. A2780-pS-EpoR cell xenografts showed a significant decrease in tumor size compared with cells expressing a negative control shRNA when injected into NCr nude athymic mice (Figure 4, A and B). A2780-pS-EpoR xenografts showed a significant decrease in EpoR mRNA and protein expression as measured by quantitative RT-PCR and Western blotting, respectively (Figure 4, C and D). Moreover, a marked decrease in the levels of p-JAK2, p-STAT-5, and p-MAPK was also detected in EpoR shRNA-expressing xenografts, whereas p-Akt was increased in these tumors (Figure 4D). On histological analysis control tumors showed large central areas of necrosis consistent with tumor hypoxia (Figure 5A). In contrast, the small tumors formed by A2780-pS-EpoR cells showed only minute foci of necrosis and individual cell death. To compare the proliferative and apoptotic activity in the xenografts we used immunohistochemistry to detect Ki-67 and cleaved caspase-3, respectively. As shown in Figure 5, A and B, the percentage of proliferating, Ki-67-immunoreactive cells was markedly decreased in A2780-pS-EpoR xenografts compared with control tumors. On the other hand, we found no difference in the apoptotic activity (expressed as the percentage of cleaved caspase-3-positive cells) between tumors expressing EpoR-specific and negative control shRNA (Figure 5, A and C).
Figure 4.
A: Down-regulation of EpoR expression in A2780 ovarian carcinoma cells using vector-based RNA inhibition resulted in markedly diminished tumor growth in vivo in a xenograft system. The figure shows representative images of NCr nude mice 9 weeks after implantation of A2780 cells transfected with a negative control scrambled shRNA-expressing plasmid (A2780-pS-Neg cells, negative control shRNA) and A2780 cells transfected with EpoR-specific shRNA-expressing plasmids (A2780-pS-EpoR cells, EpoR shRNA) (n = 5 for each group). The right part of the figure shows representative images of the A2780-pS-Neg and A2780-pS-EpoR xenograft tumors excised from the animals 9 weeks after implantation. B: Down-regulation of EpoR expression resulted in decreased growth of xenograft tumors in vivo. A2780 cells stably transfected with EpoR-specific shRNA-expressing plasmids (A2780-pS-EpoR) showed significantly diminished tumor growth in a mouse xenograft model compared with cells transfected with a negative control scrambled shRNA-expressing plasmid (A2780-pS-Neg). Cells were implanted in the flanks of NCr nude mice and tumor size was measured weekly by calipers. Results are presented as mean tumor volume (n = 5); error bars represent the SEM (*P < 0.05, **P < 0.01; Student’s t-test). C: The expression of EpoR mRNA was down-regulated in xenograft tumors of A2780 cells transfected with EpoR-specific shRNA-expressing plasmids (A2780-pS-EpoR). Quantitative real-time RT-PCR assays for EpoR mRNA expression were performed in triplicate. Expression results were normalized to the geometric mean of BACT, SDHA, and HPRT1 expression levels and expressed relative to EpoR mRNA levels in parent A2780 cells. Error bars represent SEM. Three independent measurements yielded similar results. D: Down-regulation of EpoR expression in A2780 ovarian carcinoma cells resulted in decreased phosphorylation of EpoR-signaling component proteins in xenograft tumors. A2780-pS-EpoR xenografts showed markedly decreased expression of EpoR and p-EpoR proteins by Western blotting verifying the stability of gene down-regulation in vivo. The phosphorylation of MAPK, STAT5, and JAK2 was decreased in A2780-pS-EpoR tumors compared with A2780-pS-Neg controls. Interestingly, an elevation in the phosphorylation status of Akt was found in the tumors showing EpoR down-regulation. Detection of GAPDH and β-actin levels served as loading controls.
Figure 5.
A: H&E-stained slides of the xenografts showed large areas of central necrosis in tumors of A2780 cells transfected with negative control scrambled shRNA expression plasmids (A2780-pS-Neg) consistent with hypoxia, whereas the small tumors formed by A2780 cells transfected with EpoR-specific shRNA-expressing plasmids (A2780-pS-EpoR) showed only minute foci of necrosis and individual cell death. Immunohistochemical assays for Ki-67 and cleaved capsase-3 showed markedly reduced tumor cell proliferation, but no significant change in apoptotic activity in xenografts of A2780-pS-EpoR cells, respectively. B: The proliferation rate as assessed by the Ki-67 index was markedly lower in A2780-pS-EpoR xenografts expressing EpoR-specific shRNA compared with negative control A2780-pS-Neg tumors. Data are presented as mean percent Ki-67-positive tumor cells determined in 10 random areas of the tumors away from necrotic tumor regions; error bars represent the SEM (***P < 0.0001, Mann-Whitney test). C: The rate of apoptotic activity, as determined by immunohistochemistry for cleaved caspase-3, was not significantly different in xenografts of A2780-pS-EpoR cells compared with negative control A2780-pS-Neg tumors. Data are presented as mean percent cleaved caspase-3-positive tumor cells determined in 10 random areas of the tumors away from necrotic tumor regions; error bars represent SEM (NS: P > 0.05, Mann-Whitney test). Original magnifications: ×25 (top); ×200 (middle and bottom).
Discussion
Recombinant human Epo (rHuEpo) has revolutionized the treatment of anemia.25 Recent reports however, describing the expression of EpoR in human tumors and tumor cell lines raised questions regarding the safety of the use of rHuEpo in the treatment of anemia of cancer patients with EpoR-bearing tumors.8,11,15,41 Indeed, the results of recent clinical trials suggesting a potential adverse effect of rHuEpo treatment on patient outcome prompted the United States Food and Drug Administration to issue a black box warning limiting the use of rHuEpo as supportive therapy in human malignancies. The biological mechanisms underlying the observed potential adverse effect of Epo are currently unclear. Although several reports have suggested the involvement of EpoR expressed by tumor cells in providing a growth advantage and increased therapy resistance for tumors, definitive evidence of in vivo biological responsiveness of cancer cells to Epo is currently lacking.22,23,24 The main finding of the current study is that we showed that EpoR expressed by cancer cells may influence tumor growth and cellular functions even in the absence of Epo.
Similar to data reported previously, we detected high levels of EpoR mRNA and protein expression in A2780 ovarian carcinoma cells.10,42 EpoR mRNA was detected at higher levels by quantitative real-time RT-PCR in A2780 cells than in K562 cells, which have been previously shown to express functional EpoR,43 suggesting the potential biological activity of EpoR in A2780 cells. Nevertheless, exogenous Epo had no significant effect on the proliferation, cytotoxic drug sensitivity, and in vitro invasiveness of A2780 cells. Similarly, Epo treatment of A2780 cells failed to stimulate EpoR-related signaling proteins, in contrast to its effect in Epo-dependent UT-7 cells. Importantly, EpoR signaling components showed a high level of baseline phosphorylation in A2780 cells in the absence of Epo, suggesting that EpoR may be active in these cells even in the absence of exogenous Epo. Furthermore, overexpression of full-length human EpoR in A2780 cells also failed to render these cells Epo-responsive despite the presence of EpoR on the surface of cells as detected by flow cytometry. These findings also suggest that EpoR signaling pathways are fully active in A2780 cells at basal EpoR expression.
Given the observed lack of any effect of exogenous Epo on EpoR-bearing A2780 cells, and the previously reported data suggesting the presence of autocrine Epo-EpoR signaling in some cancer cells,7,42,44 we explored the possibility of the existence of such an autocrine Epo-EpoR loop in A2780 cells. Using quantitative real-time RT-PCR, we did not detect significant levels of Epo mRNA in A2780 cells, even under hypoxic conditions. A2780 cells failed to support the survival of Epo-dependent UT-7 cells, indicating the lack of expression/secretion of biologically active Epo by A2780 cells. Furthermore, treatment with a neutralizing anti-Epo antibody failed to abrogate the proliferation of A2780 cells. These results are in contrast to those of Jeong and colleagues,42 who recently reported that blocking endogenous Epo inhibited the proliferation of A2780 cells suggesting the existence of an autocrine Epo-EpoR loop in these cells. Although the reasons for these discrepant results are currently unclear, they may be because of clonal differences between cells obtained from different sources or different culture and treatment conditions. Nevertheless, the lack of Epo mRNA and protein expression as measured by quantitative real-time RT-PCR and biological assays in our studies strongly argues for the lack of significant Epo expression and autocrine Epo signaling in A2780 cells used in our studies. These findings are also supported by the apparent lack of EpoR expression on the surface of A2780 cells as assessed by flow cytometry. Given the high expression level of EpoR, the marked baseline activity of EpoR signaling pathway components, the lack of Epo production and Epo responsiveness of the cells, the apparent lack of surface EpoR signal may be attributed to several mechanisms: faulty processing of the EpoR protein within these cells may lead to its inability to access the cell membrane, or marked down-regulation of surface EpoR may be because of rapid Epo-independent activation. Alternatively, biologically active altered forms of EpoR, not detectable by current reagents typically used to assess full-length EpoR expression levels, may also be expressed by cancer cells.45
To examine the role of EpoR in A2780 cells, we down-regulated its expression by RNA interference using specific shRNA-expressing plasmids. Compared with parent cells and cells expressing a scrambled negative control shRNA, down-regulation of EpoR in A2780 cells resulted in a marked decrease in cell proliferation and invasiveness in vitro. The marked effect of decreased EpoR expression in these assays was in sharp contrast with the lack of effect of exogenous (or endogenous) Epo in these assays. To test the in vivo effects of EpoR down-regulation on tumor growth, A2780 cells were implanted into NCr nude mice. Similar to the in vitro findings, xenografts of A2780-pS-EpoR cells with low levels of EpoR expression showed a marked decrease in tumor growth compared with xenografts of negative control cells. The stability of EpoR down-regulation in the xenografts was verified by real-time quantitative PCR and Western blot analysis. The effect of EpoR down-regulation or tumor growth does not appear to be restricted to the ovarian cancer cells studied in the current work because we have found similar growth inhibitory effect of EpoR knockdown in melanoma cells as well (S.M. Kumar et al, manuscript in preparation). Furthermore, we found a marked decrease in the level of phosphorylation of EpoR itself, as well as EpoR signaling components in A2780-pS-EpoR tumors compared with controls. Interestingly, Akt showed increased phosphorylation in the pS-EpoR tumors. The apparent increase of Akt phosphorylation may be because of a biased selection of viable cells harboring other, possibly compensatory, changes that enable their survival after EpoR down-regulation. Although PI3K-Akt inhibition was shown to have only limited effect on the cytotoxic therapy resistance of ovarian cancer cells in vitro,46 the activation of such compensatory mechanisms may explain the lack of effect of EpoR down-regulation on the cytotoxic drug resistance of A2780 cells.
To further characterize the effects of decreased EpoR expression in the xenograft tumors, immunohistochemical analysis of the tumor tissues was performed. Immunohistochemical studies revealed a significant decrease in the proliferation rate of xenografts of A2780-pS-EpoR cells showing low EpoR expression, whereas no significant difference was found in the rate of apoptosis between xenografts. These results suggest that the difference in tumor growth is predominantly because of decreased proliferation of tumor cells, rather than increased apoptosis.
In summary, we have shown that A2780 human ovarian carcinoma cells expressing high levels of EpoR are insensitive to exogenous Epo and lack an autocrine Epo-EpoR signaling pathway. In contrast to Epo-dependent UT-7 cells, A2780 cells show high baseline levels of phosphorylation of EpoR signaling pathway component proteins. Specific down-regulation of EpoR in A2780 cells resulted in markedly decreased proliferation and invasion in vitro and abrogated tumor growth in vivo. Taken together, these data suggest that EpoR may be constitutively active in these cells and provide the first evidence for the existence of an Epo-independent, EpoR-mediated mechanism involved in the growth of some human cancer cells. Our results may provide an explanation for the discrepant results of various in vitro and in vivo studies examining the effect of Epo on tumor cells44,47 and suggest that tumors may differ from one another in their responsiveness to exogenous Epo despite EpoR expression. It appears that the potential adverse effect of Epo treatment may depend not only on the presence of EpoR, but also on its activation status and the ability of the tumor cells to produce Epo themselves. Other potential explanations for the observed discrepant in vitro and in vivo findings such as the stimulation of a small number of EpoR-bearing tumor initiating cells by Epo,48 or the effect of Epo on tumor microenvironment18 should also be further explored.
Given the widespread clinical use of rHuEpo and the alarming results of clinical trials suggesting a possible adverse effect of rHuEpo in some cancer patients, elucidation of the mechanisms underlying the roles and significance Epo-stimulated and/or Epo-independent EpoR-mediated pathways in the biology of human cancer is highly important. Future studies examining the basis and mechanisms of such pathways will likely significantly enhance our understanding of the complex roles of EpoR in human cancers and may contribute to the development of specific tests to identify cancer patients whose tumors are not stimulated by exogenous Epo and thus can be safely treated with rHuEpo if necessary to maintain hemoglobin levels and improve quality of life.
Supplementary Material
Footnotes
Address reprint requests to Geza Acs, M.D., Ph.D., Moffitt Cancer Center, Room 2071D, 12902 Magnolia Dr., Tampa, FL 33612. E-mail: geza.acs@moffitt.org.
Supported in part by the University of Pennsylvania (University Research Foundation Award) and the Moffitt Cancer Center (research account funds) to G.A.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Jelkmann W. Molecular biology of erythropoietin. Intern Med. 2004;43:649–659. doi: 10.2169/internalmedicine.43.649. [DOI] [PubMed] [Google Scholar]
- Ebert BL, Bunn HF. Regulation of the erythropoietin gene. Blood. 1999;94:1864–1877. [PubMed] [Google Scholar]
- Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Bittorf T, Buchse T, Sasse T, Jaster R, Brock J. Activation of the transcription factor NF-kappaB by the erythropoietin receptor: structural requirements and biological significance. Cell Signal. 2001;13:673–681. doi: 10.1016/s0898-6568(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Miura Y, Miura O, Ihle JN, Aoki N. Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem. 1994;269:29962–29969. [PubMed] [Google Scholar]
- Brines M, Cerami A. Discovering erythropoietin’s extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70:246–250. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- Acs G, Chen M, Xu X, Acs P, Verma A, Koch CJ. Autocrine erythropoietin signaling inhibits hypoxia-induced apoptosis in human breast carcinoma cells. Cancer Lett. 2004;214:243–251. doi: 10.1016/j.canlet.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Acs G, Zhang PJ, McGrath CM, Acs P, McBroom J, Mohyeldin A, Liu S, Lu H, Verma A. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression. Am J Pathol. 2003;162:1789–1806. doi: 10.1016/S0002-9440(10)64314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–3565. [PubMed] [Google Scholar]
- McBroom JW, Acs G, Rose GS, Krivak TC, Mohyeldin A, Verma A. Erythropoietin receptor function and expression in epithelial ovarian carcinoma. Gynecol Oncol. 2005;99:571–577. doi: 10.1016/j.ygyno.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Mohyeldin A, Lu H, Dalgard C, Lai SY, Cohen N, Acs G, Verma A. Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma. Neoplasia. 2005;7:537–543. doi: 10.1593/neo.04685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnon K, Pacary E, Commo F, Antoine M, Bernaudin M, Bernaudin JF, Callard P. Expression of erythropoietin and erythropoietin receptor in non-small cell lung carcinomas. Clin Cancer Res. 2005;11:993–999. [PubMed] [Google Scholar]
- Lai SY, Childs EE, Xi SC, Coppelli FM, Gooding WE, Wells A, Ferris RL, Grandis JR. Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma. Oncogene. 2005;24:4442–4449. doi: 10.1038/sj.onc.1208635. [DOI] [PubMed] [Google Scholar]
- Feldman L, Wang YX, Rhim JS, Bhattacharya N, Loda M, Sytkowski AJ. Erythropoietin stimulates growth and STAT5 phosphorylation in human prostate epithelial and prostate cancer cells. Prostate. 2006;66:135–145. doi: 10.1002/pros.20310. [DOI] [PubMed] [Google Scholar]
- Mohyeldin A, Dalgard CL, Lu H, Mcfate T, Tait AS, Patel VC, Wong K, Rushing E, Roy S, Acs G, Verma A. Survival and invasiveness of astrocytomas promoted by erythropoietin. J Neurosurg. 2007;106:338–350. doi: 10.3171/jns.2007.106.2.338. [DOI] [PubMed] [Google Scholar]
- Arcasoy MO, Amin K, Karayal AF, Chou SC, Raleigh JA, Varia MA, Haroon ZA. Functional significance of erythropoietin receptor expression in breast cancer. Lab Invest. 2002;82:911–918. doi: 10.1097/01.lab.0000020415.72863.40. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell'Era P, Nico B, Roncali L, Dammacco F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- Hardee ME, Cao Y, Fu P, Jiang X, Zhao Y, Rabbani ZN, Vujaskovic Z, Dewhirst MW, Arcasoy MO. Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression. PLoS ONE. 2007;2:e549. doi: 10.1371/journal.pone.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RD, Jo M, Campana WM, Gonias SL. Erythropoietin promotes MCF-7 breast cancer cell migration by an ERK/mitogen-activated protein kinase-dependent pathway and is primarily responsible for the increase in migration observed in hypoxia. J Biol Chem. 2005;280:39273–39277. doi: 10.1074/jbc.M509446200. [DOI] [PubMed] [Google Scholar]
- Pajonk F, Weil A, Sommer A, Suwinski R, Henke M. The erythropoietin-receptor pathway modulates survival of cancer cells. Oncogene. 2004;23:8987–8991. doi: 10.1038/sj.onc.1208140. [DOI] [PubMed] [Google Scholar]
- Westenfelder C, Baranowski RL. Erythropoietin stimulates proliferation of human renal carcinoma cells. Kidney Int. 2000;58:647–657. doi: 10.1046/j.1523-1755.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- Belda-Iniesta C, Perona R, Carpeno Jde C, Cejas P, Casado E, Manguan-Garcia C, Ibanez de Caceres I, Sanchez-Perez I, Andreu FB, Ferreira JA, Aguilera A, Dela PJ, Perez-Sanchez E, Madero R, Feliu J, Sereno M, Gonzalez-Baron M. Human recombinant erythropoietin does not promote cancer growth in presence of functional receptors expressed in cancer cells. Cancer Biol Ther. 2007;6:1600–1605. doi: 10.4161/cbt.6.10.4726. [DOI] [PubMed] [Google Scholar]
- Liu WM, Powles T, Shamash J, Propper D, Oliver T, Joel S. Effect of haemopoietic growth factors on cancer cell lines and their role in chemosensitivity. Oncogene. 2004;23:981–990. doi: 10.1038/sj.onc.1207294. [DOI] [PubMed] [Google Scholar]
- LaMontagne KR, Butler J, Marshall DJ, Tullai J, Gechtman Z, Hall C, Meshaw A, Farrell FX. Recombinant epoetins do not stimulate tumor growth in erythropoietin receptor-positive breast carcinoma models. Mol Cancer Ther. 2006;5:347–355. doi: 10.1158/1535-7163.MCT-05-0203. [DOI] [PubMed] [Google Scholar]
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- Bohlius J, Weingart O, Trelle S, Engert A. Cancer-related anemia and recombinant human erythropoietin—an updated overview. Nat Clin Pract Oncol. 2006;3:152–164. doi: 10.1038/ncponc0451. [DOI] [PubMed] [Google Scholar]
- Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- Hudis CA, Van BS, Chang J, Muenstedt K. rHuEPO and treatment outcomes: the clinical experience. Oncologist. 2004;9(Suppl 5):55–69. doi: 10.1634/theoncologist.9-90005-55. [DOI] [PubMed] [Google Scholar]
- Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennett C, Engert A. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta-analysis. J Natl Cancer Inst. 2005;97:489–498. doi: 10.1093/jnci/dji087. [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, Makhson A, Roth A, Dodwell D, Baselga J, Biakhov M, Valuckas K, Voznyi E, Liu XY, Vercammen E. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4:459–460. doi: 10.1016/s1470-2045(03)01163-x. [DOI] [PubMed] [Google Scholar]
- Henke M, Laszig R, Rube C, Schafer U, Haase KD, Schilcher B, Mose S, Beer KT, Burger U, Dougherty C, Frommhold H. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Hoff C, Sand Hansen H, Specht L, Overgaard M, Grau C, Andersen E, Johansen J, Andersen L, Evensen J. Randomized study of the importance of novel erythropoiesis stimulating protein (Aranesp) for the effect of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC)—the Danish Head and Neck Cancer Group DAHANCA 10 randomized trial. Eur J Cancer Suppl. 2007;5:7. [Abstract 6LB] [Google Scholar]
- Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, Szechtman B, Roa W, Mulroy L, Rudinskas L, Gagnon B, Okawara GS, Levine MN. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25:1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, Abulafia O, Lucci JA, III, Begg AC. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja V, Keegan P, Gootenberg JE, Rothmann MD, Shen YL, Lee KY, Weiss KD, Pazdur R. Continuing reassessment of the risks of erythropoiesis-stimulating agents in patients with cancer. Clin Cancer Res. 2008;14:3242–3247. doi: 10.1158/1078-0432.CCR-07-1872. [DOI] [PubMed] [Google Scholar]
- Henke M, Mattern D, Pepe M, Bezay C, Weissenberger C, Werner M, Pajonk F. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol. 2006;24:4708–4713. doi: 10.1200/JCO.2006.06.2737. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA, Di X, Walker TD, Sawyer ST. Erythropoietin fails to interfere with the antiproliferative and cytotoxic effects of antitumor drugs. Clin Cancer Res. 2006;12:2232–2238. doi: 10.1158/1078-0432.CCR-05-2287. [DOI] [PubMed] [Google Scholar]
- Goldberg MA, Glass GA, Cunningham JM, Bunn HF. The regulated expression of erythropoietin by two human hepatoma cell lines. Proc Natl Acad Sci USA. 1987;84:7972–7976. doi: 10.1073/pnas.84.22.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeval JL, Mitjavila MT, Dusanter-Fourt I, Wendling F, Mayeux P, Vainchenker W. Autocrine stimulation by erythropoietin (Epo) requires Epo secretion. Blood. 1994;84:2649–2662. [PubMed] [Google Scholar]
- Acs G, Xu X, Chu C, Acs P, Verma A. Prognostic significance of erythropoietin expression in human endometrial carcinoma. Cancer. 2004;100:2376–2386. doi: 10.1002/cncr.20244. [DOI] [PubMed] [Google Scholar]
- Jeong JY, Feldman L, Solar P, Szenajch J, Sytkowski AJ. Characterization of erythropoietin receptor and erythropoietin expression and function in human ovarian cancer cells. Int J Cancer. 2008;122:274–280. doi: 10.1002/ijc.23068. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Tanaka T, Yamaoka G, Yamaguchi M, Ohnishi H, Kawanishi K, Takahara J, Irino S. Wortmannin, a specific inhibitor of phosphatidylinositol-3-kinase, inhibits erythropoietin-induced erythroid differentiation of K562 cells. Leukemia. 1996;10:720–726. [PubMed] [Google Scholar]
- Hardee ME, Arcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332–339. doi: 10.1158/1078-0432.CCR-05-1771. [DOI] [PubMed] [Google Scholar]
- Acs G, Paragh G, Xu X, Kumar S: Constitutively active erythropoietin receptor variants in human ovarian and breast cancers. Proceedings of the Annual Meeting of the American Association for Cancer Res, 2008 April 12–16, San Diego, CA. AACR Meeting Abstracts 2008, Abstract 3428 [Google Scholar]
- Mitsuuchi Y, Johnson SW, Selvakumaran M, Williams SJ, Hamilton TC, Testa JR. The phosphatidylinositol 3-kinase/AKT signal transduction pathway plays a critical role in the expression of p21WAF1/CIP1/SDI1 induced by cisplatin and paclitaxel. Cancer Res. 2000;60:5390–5394. [PubMed] [Google Scholar]
- Sinclair AM, Todd MD, Forsythe K, Knox SJ, Elliott S, Begley CG. Expression and function of erythropoietin receptors in tumors: implications for the use of erythropoiesis-stimulating agents in cancer patients. Cancer. 2007;110:477–488. doi: 10.1002/cncr.22832. [DOI] [PubMed] [Google Scholar]
- Phillips TM, Kim K, Vlashi E, McBride WH, Pajonk F. Effects of recombinant erythropoietin on breast cancer-initiating cells. Neoplasia. 2007;9:1122–1129. doi: 10.1593/neo.07694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.