Abstract
Accelerated glycolysis is one of the biochemical characteristics of cancer cells. The glucose transporter isoform 1 (GLUT1) gene encodes a key rate-limiting factor in glucose transport into cancer cells. However, its expression level and functional significance in hepatocellular cancer (HCC) are still disputed. Therefore, we aimed to analyze the expression and function of the GLUT1 gene in cases of HCC. We found significantly higher GLUT1 mRNA expression levels in HCC tissues and cell lines compared with primary human hepatocytes and matched nontumor tissue. Immunohistochemical analysis of a tissue microarray of 152 HCC cases revealed a significant correlation between Glut1 protein expression levels and a higher Ki-67 labeling index, advanced tumor stages, and poor differentiation. Accordingly, suppression of GLUT1 expression by siRNA significantly impaired both the growth and migratory potential of HCC cells. Furthermore, inhibition of GLUT1 expression reduced both glucose uptake and lactate secretion. Hypoxic conditions further increased GLUT1 expression levels in HCC cells, and this induction was dependent on the activation of the transcription factor hypoxia-inducible factor-1α. In summary, our findings suggest that increased GLUT1 expression levels in HCC cells functionally affect tumorigenicity, and thus, we propose GLUT1 as an innovative therapeutic target for this highly aggressive tumor.
Hepatocellular carcinoma (HCC) is the most common primary malignant tumor of the liver and one of the most common tumors worldwide.1 Liver cirrhosis is the main predisposing condition, but the molecular pathogenesis of HCC is still not well understood. Morbidity and mortality correlate directly with surgical resectability of the primary tumor. However, outcome is mostly poor, because the majority of patients are diagnosed at an advanced stage, and only 10 to 20% of HCCs can be resected completely.2,3
In the 1920s Otto Warburg made the observation that tumor cells use glycolysis instead of mitochondrial oxidative phosphorylation for energy production even under oxygen-rich conditions. Recently, the Warburg effect has experienced a revival because it has been shown that aerobic glycolysis governs tumor cell biology.4,5 Previous studies found differences in glycolytic capacity between HCC cells and hepatocytes,6 and positron emission tomography (PET) revealed the fluorine-18-fluorodeoxyglucose uptake value as an independent prognostic factor for HCC.7 Further, higher PET activity was shown to correlate with advanced tumor stages.8
The glucose transporter isoform 1 (GLUT1, also known as SLC2A1; MIM no. 138140) is a key rate-limiting factor in the transport and metabolism of glucose in cancer cells. GLUT1 expression is primarily undetectable in normal epithelial tissues and benign epithelial tumors. However, GLUT1 is overexpressed in a significant proportion of human carcinomas.9,10 The apparent expression of a certain type of glucose transporter suggests an important role for this transporter in tumor biology. Therefore, it has been hypothesized that elevated GLUT1 expression by human carcinomas indicates an increased metabolic state, enhanced utilization of energy, and an associated increase in aggressive, metastatic behavior. Actually, Glut1 protein expression confers poor prognosis in a wide range of solid tumors.11,12
Studies regarding GLUT1 expression in HCC have revealed inconclusive results, and the biological significance of GLUT1 expression in HCC remains unknown.13,14,15,16,17,18,19 Here, we show that GLUT1 expression is increased in a significant number of HCC cell lines and tissues, and high GLUT1 expression correlates with HCC proliferation and invasiveness. Furthermore, we found that siRNA-mediated abrogation of GLUT1 in HCC cell lines inhibits their proliferative and migratory potential. This suggests that increased GLUT1 expression in HCC does not only indicate an increased utilization of energy, which may correlate with an aggressive behavior, but directly causes tumorigenesis. Consequently, GLUT1 may serve as both a prognostic marker and a therapeutic target in HCC.
Materials and Methods
Cells and Cell Culture
The HCC cell lines HepG2 [American Type Culture Collection (Rockville, MD) HB-8065], PLC (American Type Culture Collection CRL-8024), and Hep3B (American Type Culture Collection HB-8064) were cultured as described.20 Primary human hepatocytes (PHHs) were isolated and cultured as previously described.21 Human liver tissue for cell isolation was obtained according to the guidelines of the charitable state-controlled foundation Human Tissue and Cell Research with the patient’s informed consent. Hypoxia was induced by incubation with 2,2′-dipyridyl (DP) (100 μmol/L; Sigma Aldrich, Deisenhofen, Germany) or exposure to 1% O2 for the indicated periods of time. For pharmaceutical inhibition of hypoxia-inducible factor (HIF)-1 activity cells were incubated with 100 μmol/L of 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1; Calbiochem, Darmstadt, Germany)22,23 or 10 nmol/L of echinomycin (Alexis Biochemicals, Lörrach, Germany).24
Human Tissues and HCC Tissue Microarray (TMA)
HCC tissues and corresponding nonneoplastic liver tissues were obtained from HCC patients (child A/B cirrhosis) undergoing surgical resection at the university hospitals Regensburg (n = 85) and Erlangen (n = 67). TMAs were constructed as described.20 Further, tissue samples of 31 patients were immediately snap-frozen, stored at −80°C, and subsequently used for RNA isolation and analysis of mRNA expression. Clinicopathological patient characteristics are summarized in Table 1.
Table 1.
Glut1 Immunoreactivity (IR) in HCC Tissue of 152 Patients in Relation to Clinicopathological Characteristics and Proliferation Rate
| Variable | Categorization | n | % | Glut1 IR-negative | Glut1 IR-positive | P* |
|---|---|---|---|---|---|---|
| Clinicopathological characteristics | ||||||
| Age at diagnosis | ||||||
| <60 years | 57 | 37.5 | 51 | 6 | 0.621 | |
| ≥60 years | 95 | 62.5 | 81 | 14 | ||
| Sex | ||||||
| Female | 22 | 14.5 | 19 | 3 | 1.000 | |
| Male | 130 | 85.5 | 113 | 17 | ||
| Tumor stage | ||||||
| pT1 | 52 | 34.2 | 45 | 7 | 0.023 | |
| pT2 | 44 | 29.0 | 40 | 4 | ||
| pT3 | 48 | 31.6 | 42 | 6 | ||
| pT4 | 4 | 2.6 | 1 | 3 | ||
| nd | 4 | 2.6 | 4 | 0 | ||
| Histological grade | ||||||
| G1 | 57 | 37.5 | 55 | 2 | <0.0001 | |
| G2 | 76 | 50.0 | 68 | 8 | ||
| G3 | 19 | 12.5 | 10 | 9 | ||
| Tumor size | ||||||
| ≤5 cm | 77 | 50.7 | 66 | 11 | 0.588 | |
| >5 cm | 53 | 34.9 | 48 | 5 | ||
| nd | 22 | 14.5 | 18 | 4 | ||
| Cirrhosis | ||||||
| No | 36 | 23.7 | 34 | 2 | 0.348 | |
| Yes | 95 | 62.5 | 83 | 12 | ||
| nd | 21 | 13.8 | 15 | 6 | ||
| Proliferation rate (MIB1 index) | ||||||
| ≤5% | 64 | 42.1 | 61 | 3 | 0.006 | |
| >5% | 81 | 53.3 | 64 | 17 | ||
| 7 | 4.6 | 7 | 0 |
Fisher’s exact test (two-sided); bold face represents P values <0.05.
nd, no data available; IR, immunoreactivity.
Expression Analysis
Isolation of total cellular RNA from cultured cells and tissues and reverse transcription were performed as described previously.25 Quantitative real-time polymerase chain reaction (PCR) was performed with primers specific for GLUT1 (forward: 5′-AACTCTTCAGCCAGGGTCCAC; reverse: 5′-CACAGTGAAGATGATGAAGAC) using LightCycler technology (Roche, Mannheim, Germany).26 Expression of MAZ was analyzed applying the QuantiTect primer assay according to the manufacturer’s instructions (Qiagen, Hilden, Germany).
Protein Analysis
Protein extraction and Western blotting were performed as described elsewhere,27 applying the following primary antibodies: anti-Glut1 (1:600; Lab Vision, Wedel, Germany), anti-HIF-1 α (1:500; Novus Biologicals, Littleton, CO), and anti-β-actin (1:20,000, Sigma). Immunohistochemical staining of 5-μm sections of the TMA blocks was performed using polyclonal anti-Glut1 antibody (1:50) and an indirect immunoperoxidase protocol according to the LSAB2-kit (DAKO, Hamburg, Germany).20 A surgical pathologist (A.H.) performed a blinded evaluation of the stained slides. For negative control, the primary antibody was omitted and IgG isotype control antibodies did not reveal any detectable staining. For analysis of the TMA, positivity for Glut1 was defined as any detectable membranous staining. Glut1 expression in carcinomas, when present, was variable as reported before in other studies,16 ranging from at least 25 to almost 100% of the cells. In contrast, cases designated as Glut1-negative did not reveal any immunohistochemical staining for Glut1. MIB1 was analyzed applying anti-Ki-67 antibody (rabbit monoclonal, clone MIB1, 1:10, final concentration of 5 μg/ml; DAKO). Antibody binding was visualized using AEC-solution (LSAB2-Kit, DAKO). Finally, the tissues were counterstained by hemalaun.
Transfection of HCC Cell Lines
Applying the Lipofectamine plus method (Invitrogen, Carlsbad, CA) small interfering RNA (siRNA; GLUT1 Hs-SLC2A1-5 and GLUT1 Hs-SLC2A1-6; MAZ Hs_MAZ_6 and MAZ Hs_MAZ_8; all from Qiagen) was transiently transfected into HCC cells to deplete GLUT1 or MAZ expression. Transfection efficiency was determined by fluorescence-activated cell sorting analysis applying Alexa Fluor 488-labeled control siRNA (AllStars negative control siRNA; Qiagen).
For luciferase reporter assays, cells were transfected with 0.5 μg of a reporter construct harboring six copies of the hypoxia-responsive element of the human phosphoglycerate kinase (PGK) gene (6×HRE) upstream of a HSV thymidine kinase promoter. To normalize transfection efficiency, 0.2 μg of a pRL-TK plasmid (Promega, Mannheim, Germany) was co-transfected and renilla luciferase activity measured by a luminometric assay (Promega). All transfections were performed in triplicate.
Proliferation and Migration Assays
Cell proliferation was measured using the XTT assay (Roche).25 Further, cell number was determined by microscopic counting after trypsination of cells seeded in six-well plates (six per condition) at different time points. Migration assays were performed as previously described.20
PET
PET using 2-[18F]fluorodeoxyglucose (FDG-PET/CT) was performed by means of a Biograph16 PET/CT scanner (Siemens, Erlangen, Germany). After fasting for 4 to 6 hours to achieve blood glucose values <120 mg/dl, the patients received an intravenous dose of FDG (5 to 10 mCi, 185 to 379 MBq). Whole-body image acquisition from the skull base to the proximal thigh started ∼60 minutes later (axial field 90 cm; seven bed positions for 3 minutes each, one head/neck position). The total time required was ∼20 minutes (40 seconds CT scanning and subsequent CT attenuation-corrected PET scanning, low-dose CT).
Statistical Analysis
Statistical analyses were performed using SPSS version 10.0 (SPSS, Chicago, IL) and GraphPad Prism Software (GraphPad Software, Inc., San Diego, CA). Results are expressed as mean ± SE (range) or percent. P values <0.05 were considered statistically significant. Comparisons between groups were made using the Mann-Whitney test. Contingency table analysis and the two-sided Fisher’s exact test were used to study the statistical association between clinicopathological and immunohistochemical variables.
Results
GLUT1 Expression in HCC
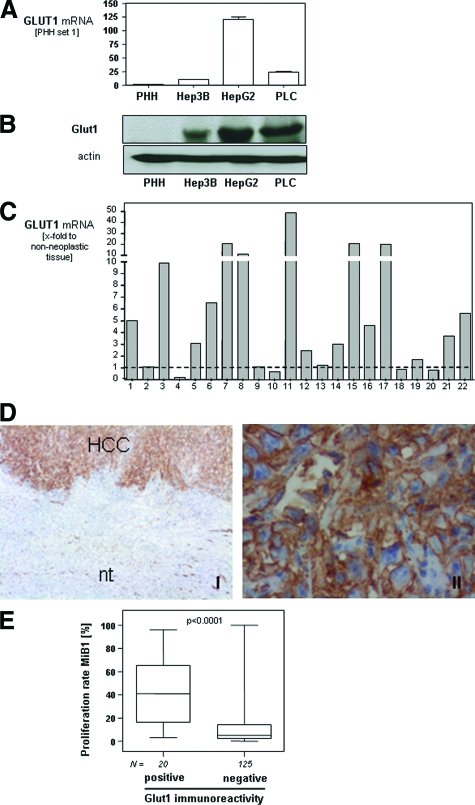
Initially, we analyzed GLUT1 mRNA expression in three different HCC cell lines (HepG2, PLC, and Hep3B) and PHHs by quantitative real-time PCR. In all three HCC cell lines a significantly increased GLUT1 mRNA expression was observed compared with PHHs (Figure 1A). This result was confirmed by Western blotting (Figure 1B).
Figure 1.
GLUT1 expression in HCC. GLUT1 mRNA (A) and protein (B) expression in PHHs and three different HCC cell lines (HepG2, PLC, and Hep3B) analyzed by quantitative real-time PCR (A) and Western blotting (B). Data are given as mean ± SEM (*P < 0.05). C: GLUT1 mRNA expression in 22 human HCC samples in relation to matching nontumorous liver tissue samples. D: Glut1 immunohistochemical staining of human HCC tissue and adjacent nontumorous liver tissue (nt) and higher magnification of the same HCC tissue sample. E: Proliferation rate (analyzed by immunohistochemical staining applying anti-Ki-67 antibodies) in HCC-tissues with positive or negative immunoreactivity to Glut1 applying TMA technology. Original magnifications: ×40 (left); ×400 (right).
Next, we analyzed a panel of 22 paired specimens obtained from patients with HCC. From each HCC patient, RNA was isolated from cancerous tissue and adjacent nontumorous liver tissue, and GLUT1 mRNA expression was measured by quantitative real-time PCR (Figure 1C). In 15 HCC specimens, GLUT1 mRNA expression was increased compared with matched nontumorous tissue. In six patients (nos. 2, 9, 13, and 18 to 20) GLUT1 mRNA expression was not significantly different from control tissue. Only two HCC specimens (nos. 4 and 10) revealed decreased GLUT1 mRNA expression levels compared with nontumorous tissue.
To assess GLUT1 expression in HCC in situ, we performed immunohistochemical staining for Glut1 protein. A representative immunohistochemical staining of a Glut1-positive tumor is presented in Figure 1D. Immunohistochemistry revealed a strong membranous signal in HCC cells (Figure 1DII). In contrast, no Glut1 staining was detectable in nontumorous hepatic tissue.
Next, we analyzed Glut1 expression in a series of 152 HCCs and corresponding nontumorous tissue of the same patients (n = 146) using TMA technology. Investigation of Glut1 protein expression was informative in all HCC and nontumorous tissue samples. In 13.2% (20 of 152) of the HCC, a Glut1 immunosignal was detectable. In contrast, Glut1 expression was found in none of the noncancerous tissue samples (P < 0.0001).
Matched data of mRNA expression and semiquantitative protein expression analyzed on the TMA were available from 31 HCC patients. GLUT1 mRNA expression was significantly higher in HCC cases with positive Glut1 immunosignal (n = 7) compared with cases in which no GLUT1 was detectable (n = 24; 3.4 ± 1.1-fold; P = 0.0015). This finding indicates that highly increased Glut1 expression is accurately detected by immunohistochemistry (IHC). However, lower expression may be below the detection limit of IHC. Herewith, differences between HCC and nontumorous liver may be missed, and probably, Glut1 protein is increased in even more cases than now shown by IHC.
For descriptive data analysis, clinicopathological characteristics were compared with GLUT1 mRNA and protein expression. GLUT1 mRNA expression correlated significantly with tumor stage (r = 0.37, P = 0.039), grading (r = 0.48, P = 0.007), and proliferation rate (MiB-1 index; r = 0.62, P = 0.0002). Immunohistochemistry confirmed these data on the protein level (Table 1). Glut1 expression was significantly associated with higher tumor stage (P = 0.023) and tumor grading (P < 0.0001). Furthermore, Glut1-positive HCCs had a significantly higher proliferation rate (MiB-1 index) compared with Glut1-negative HCCs (P = 0.006, Figure 1E). No correlation was found between Glut1 expression and age, gender, tumor size, and the existence of liver cirrhosis. The etiology of the underlying liver disease was known in only approximately half of the patients (73 of 152). In most cases, HCC had developed in alcohol-related cirrhosis (52 of 73, 71%), and this percentage was similar in the groups of Glut1-negative (45 of 64, 70%) and -positive (7 of 9, 78%) HCCs.
Molecular Mechanisms of GLUT1 Expression in HCC
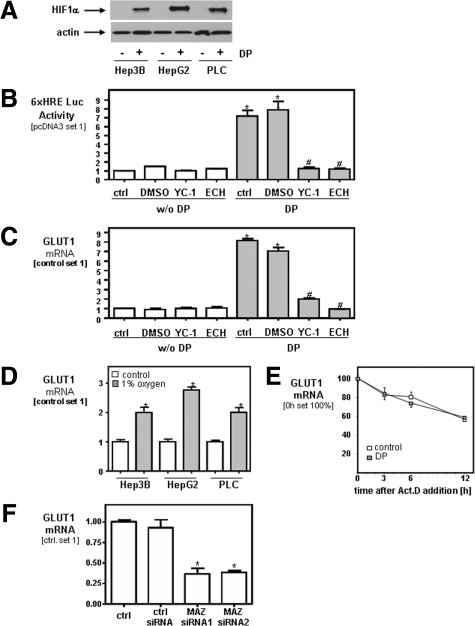
The transcription factor HIF-1α is an important mediator of hypoxic adaptation of tumor cells and controls several genes that have been implicated in tumor growth including GLUT1. Therefore, we analyzed HIF-1α protein expression in three different HCC cell lines (HepG2, PLC, and Hep3B) grown under aerobic conditions in vitro. Interestingly, no HIF-1α expression could be detected in HCC cells by Western blotting (Figure 2A). In contrast, after pharmacological HIF induction by DP, strong HIF-1α protein expression was detected in all three HCC cell lines by Western blotting (Figure 2A).
Figure 2.
Regulation of GLUT1 expression in HCC under aerobic and anaerobic conditions. A: HIF-1α expression in three different HCC cell lines (HepG2, PLC, and Hep3B) with or without pharmacological induction of hypoxia by DP (100 μmol/L) analyzed by Western blotting. B: Luciferase activity in Hep3B cells transiently transfected with a reporter gene driven by six hypoxia-responsive elements (HREs) in relation to cells transfected with control plasmid (pcDNA3). DP (2,2′-dipyridy, 100 μmol/L), YC-1 (100 μmol/L); ECH (echinomycin, 10 nmol/L). * and #, P < 0.05 compared with control and DMSO without DP or with DP, respectively. C: GLUT1 mRNA expression in Hep3B cells with or without pharmacological induction of hypoxia or HIF-1α inhibition analyzed by quantitative real-time PCR. * and #, P < 0.05 compared with control and DMSO without DP or with DP, respectively. D: Analysis of GLUT1 mRNA expression in HCC cells cultured under normoxic conditions or 1% oxygen for 16 hours. *P < 0.05 compared with control. E: Hep3B cells were exposed to normoxic or hypoxic conditions, actinomycin D (Act.D, 7.5 μg/ml) was added, and incubation was continued for 3, 6, and 12 hours. GLUT1 mRNA was analyzed by qPCR. F: Analysis of GLUT1 mRNA expression in Hep3B cell transiently transfected with two different MAZ siRNAs (siRNA1 and siRNA2), and cells transfected with control siRNA and nontransfected HCC cells (ctrl.). *P < 0.05 compared with control. All experiments have been performed at least three times. Data are given as mean ± SEM.
In line with this finding, transfection of Hep3B cells with a luciferase reporter plasmid containing six copies of a functional hypoxia-responsive element (HRE) revealed only baseline activity under aerobic conditions (Figure 2B). In contrast, strong HRE reporter gene activity was observed in Hep3B cells under DP-induced hypoxia, and this activity was completely abrogated by pharmaceutical inhibition of HIF-1α activity with YC-122,23 or echinomycin.24 Similar results were obtained with HepG2 and PLC cells (data not shown). Interestingly, GLUT1 expression was further increased in HCC cells under DP-induced hypoxia, and this induction was strongly repressed by inhibition of HIF-1α activity with YC-1 or echinomycin (Figure 2C). Similarly as DP-induced hypoxia, also culture of HCC cells under hypoxic conditions led to a significant increase of GLUT1 mRNA expression in all three HCC cell lines (Figure 2D).
Previous studies in glia cells have shown that hypoxia alters GLUT1 expression post–transcriptionally by enhancing GLUT1 mRNA stability.28 Therefore, we analyzed GLUT1 mRNA expression in HCC cells at different time point after exposure to DP-induced hypoxia with or without pretreatment with actinomycin D (ActD), an inhibitor of transcription. ActD treatment resulted in a decline of GLUT1 mRNA levels with time indicating a GLUT1 mRNA half-live larger than 12 hours, similarly as previously reported in neurons (Figure 2E).28 No significant difference was found between HCC cells grown under normoxic and hypoxic conditions, indicating that hypoxia does not affect mRNA stability in HCC cells. Together, these data indicate that basal GLUT1 expression in HCC is further increased under hypoxic conditions, and this induction is regulated on the transcriptional level by HIF-1α activation.
In search for the molecular mechanisms that cause the increased expression of GLUT1 in HCC cells under normoxic conditions we performed in silico promotor studies using the Genomatix software. Alignment of the promotor sequence of the murine, rat, and human GLUT1 gene revealed that a binding site for the transcription factor MAZ (Myc-associated zinc finger protein, located 310 bp upstream of the transcriptional start site of the human GLUT1 gene) was highly conserved in all three species. Similarly as previously described29 we found increased MAZ expression in HCC cells compared with PHHs (data not shown), and noteworthy, transient transfection with two different MAZ siRNAs significantly inhibited GLUT1 mRNA expression in Hep3B cells (Figure 2F) as well as in HepG2 and PLC cells (data not shown). In summary, these data indicate that constitutively high GLUT1 mRNA expression in HCC cells under normoxic conditions is at least in part dependent on the transcription factor MAZ, and that GLUT1 mRNA expression is further increased under hypoxic conditions by HIF-1α activation.
Inhibition of GLUT1 Expression in HCC Cells
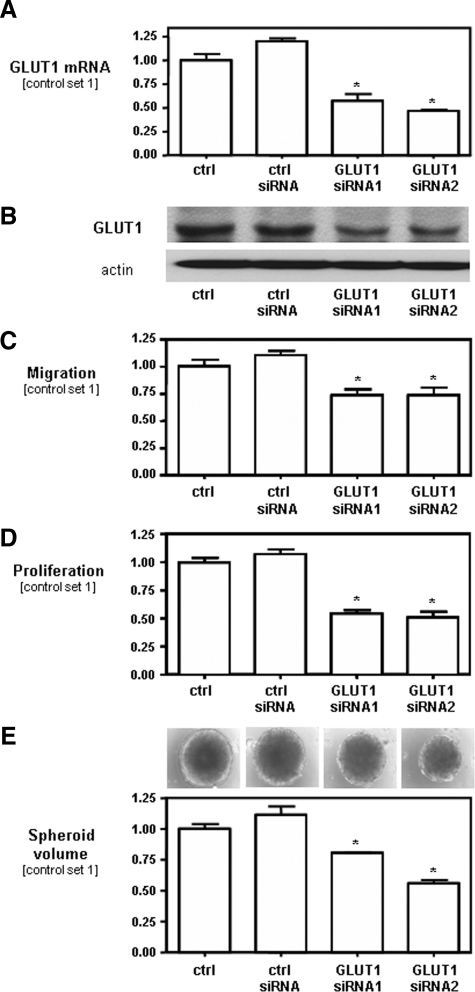
To gain insight into the functional role of increased GLUT1 in HCC, we inhibited GLUT1 expression in HCC cells by transient transfection with two different GLUT1 siRNAs. Quantitative real-time PCR analysis revealed a strong down-regulation of GLUT1 mRNA in Hep3B cells transfected with GLUT1 siRNA (siRNA1 and siRNA2) as compared with Hep3B cells transfected with control siRNA and nontransfected cells, respectively (Figure 3A). Down-regulation of GLUT1 expression in Hep3B cells transfected with GLUT1 siRNA was also confirmed at the protein level (Figure 3B).
Figure 3.
Effect of GLUT1 inhibition on migration and proliferation of HCC cells. Analysis of GLUT1 (A) mRNA and (B) protein expression in Hep3B cells transiently transfected with two different GLUT1 siRNAs (siRNA1 and siRNA2), and cells transfected with control siRNA and nontransfected HCC cells (ctrl.). C: The migratory potential of these cells was assessed by Boyden chamber assays. D: For analysis of proliferation, cells were trypsinized and counted at different time points. Cell number at day 4 is set at 1. E: Growth in a three-dimensional cell culture system was assessed by analysis of the spheroid volume at day 14. All experiments have been performed at least three times. Data are given as mean ± SEM (*P < 0.05 compared with controls).
FACS analysis of Alexa Fluor 488-labeled control siRNA revealed a transfection efficiency of ∼90.6 ± 0.8% (data not shown). Furthermore, we studied the duration of the inhibitory effect of GLUT1 siRNA on GLUT1 mRNA expression in HCC cells and found that GLUT1 mRNA remained reduced for at least 4 days after transfection (data not shown). Comparable results regarding efficiency of transfection and GLUT1 expression with GLUT1 siRNA were obtained in PLC and HepG2 cells (data not shown).
Effect of GLUT1 Inhibition on Migration and Proliferation of HCC Cells
To further characterize the role of GLUT1 in HCC cells, we performed functional in vitro assays with HCC cells by suppressing GLUT1 expression (GLUT1 siRNA1 and GLUT1 siRNA2) in comparison with HCC cells transfected with control siRNA and nontransfected HCC cells. Inhibition of GLUT1 expression caused significantly impaired migration as analyzed in Boyden chamber assays (Figure 3C). Next, we analyzed whether GLUT1 expression affected the proliferation of HCC cells in vitro. HCC cells with suppressed GLUT1 expression grew significantly slower compared with controls cultured in monolayers (Figure 3D). In addition, we compared the growth of HCC cells with suppressed GLUT1 expression and control cells in a three-dimensional cell culture model. Transfection with GLUT1 siRNA resulted in the formation of significantly smaller spheroids compared with spheroids of control cells (Figure 3E).
Effect of GLUT1 on Glucose Uptake and Glycolytic Rate in HCC
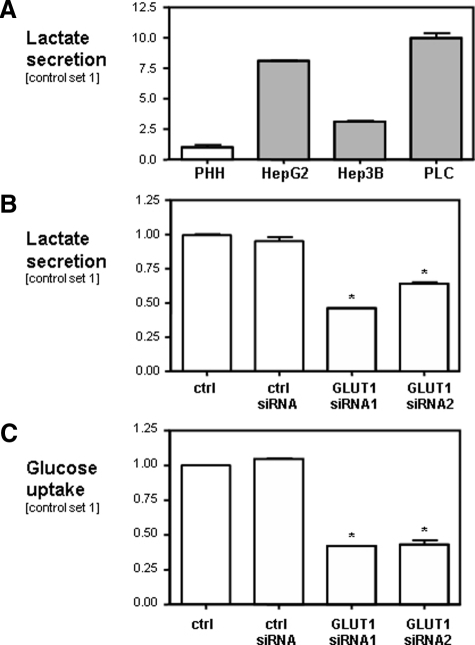
HCC cells secreted significantly more lactate into the supernatant than PHHs (Figure 4A). In Hep3B cells transfected with GLUT1 siRNA lactate secretion was significantly reduced as compared with control cells (Figure 4B). Further, suppression of GLUT1 expression reduced glucose uptake into Hep3B cells significantly (Figure 4C). Similar results for glucose uptake and lactate secretion were obtained for HepG2 and PLC cells after GLUT1 suppression (data not shown). Together, these data indicate that GLUT1 is a rate-limiting factor for the glucose transport and glycolysis in HCC cells in vitro.
Figure 4.
Effect of GLUT1 on rates of glucose uptake and glycolysis in HCC. Analysis of lactate secretion into the supernatant of PHHs and three different HCC cell lines (HepG2, PLC, and Hep3B) (A) and Hep3B cells transiently transfected with two different GLUT1 siRNAs (siRNA1 and siRNA2) (B), cells transfected with control siRNA and nontransfected HCC cells (ctrl.). C: Glucose uptake is reported as glucose utilization per cell within 24 hours. To that end, glucose concentration was measured in the supernatant and cells were counted 24 hours after seedings. All experiments have been performed at least three times. Data are given as mean ± SEM (*P < 0.05 compared with controls).
Discussion
The aim of this study was to analyze the expression and function of GLUT1 in HCC. Previous studies on the expression of GLUT1 in human and murine HCC tissue and cells had revealed discrepant results. In some studies a strong and increased expression of GLUT1 mRNA had been found in HCC as compared with adjacent noncancerous tissue13,14,15 in >80% of cases. Other studies, in contrast, failed to detect Glut1 protein expression by immunohistochemical staining in HCC tissue in most, if not all cases.16,17,18,19 However, these previous studies had been performed in only a small number of cases and GLUT1 expression had been measured only at the mRNA or the protein level.
In the present study, we investigated GLUT1 mRNA and protein expression in 152 HCC cases by quantitative real-time PCR and immunohistochemical analysis, respectively. We found an increased expression of GLUT1 mRNA and protein in all HCC cell lines compared with PHHs. Furthermore, 68.2% of human HCC tissues revealed higher GLUT1 mRNA levels than adjacent noncancerous liver tissue. By immunohistochemical analysis Glut1 was detectable in only 13.2% of the HCC tissues, but in none of the noncancerous liver tissues analyzed. An explanation for the discrepant GLUT1 mRNA and protein expression data may be, that the antibodies and/or the immunodetection methods used here and in the previous studies lacked sufficient sensitivity to detect Glut1 in HCC, in which it was expressed at low level. Supportive for this hypothesis, we found a close correlation between GLUT1 mRNA and protein expression, and only HCCs with the highest GLUT1 mRNA levels revealed a visible Glut1 immunosignal, suggesting that there is a threshold that allows the detection of Glut1 protein by immunohistochemistry. In addition, in all three HCC cell lines analyzed treatment with GLUT1 siRNA significantly suppressed both GLUT1 mRNA and protein expression, further suggesting that GLUT1 expression in HCC is at least in part regulated at the transcriptional level.
Biological Significance of GLUT1 Expression in HCC
The expression of GLUT1 in a significant number of HCCs and its lower or undetectable expression in corresponding normal and benign hepatic tissue indicate that this transporter probably plays an important role in the uptake of glucose by HCC cells. Glucose is a major source of energy, and increased GLUT1 expression may indicate an increased utilization of energy, which in turn may correlate with aggressive behavior of cancer cells. Indeed, GLUT1 overexpression was associated with parameters conferring more aggressive behavior in several solid tumor types.11,12 Actually, we found that GLUT1 expression was a rate-limiting factor for the uptake of glucose and glycolysis in HCC cells. We also showed for the first time, that GLUT1 expression was significantly associated with tumor growth and invasiveness in HCC.
It is well known that proliferation of transformed cells is accompanied by an accelerated uptake and metabolism of glucose.4,5 GLUT1 was shown to be up-regulated during development/embryogenesis, and is more abundant in fetal hepatocytes than in adult hepatocytes.30,31 Hence, one may speculate that the GLUT1 overexpression observed in cancerous tissue fosters rapid tumor growth. Indeed, we have found that inhibition of GLUT1 expression in HCC cells reduced proliferation rate. In addition, it also reduced migratory potential, thus suggesting a direct role of GLUT1 in the tumorigenicity of HCC.
Interestingly, two previous studies have found higher PET activity in advanced HCC stages,7,8 and in a preliminary study we found a positive FDG-PET signal only in two HCC patients with positive Glut1 immunohistochemical staining of the primary tumor but none of the four patients with undetectable Glut1 immunosignal (data not shown). Certainly, this finding has to be confirmed in a larger cohort of patients but puts forward the hypothesis that high GLUT1 expression leads to increased glucose metabolism (as indicated by a positive FDG-PET signal), and herewith, promotes tumorigenicity of HCC also in vivo.
Regulation of GLUT1 in HCC
It has been described that GLUT1 expression is regulated by the transcription factor HIF-1α. HIF-1α is induced in response to stress and hypoxia. In cancerous tissue, it may also be up-regulated under aerobic conditions. Of note, HIF-1α was not detectable in HCC cells in vitro and GLUT1 expression in these cells was not dependent on HIF-1α under aerobic conditions. Interestingly, we newly identified MAZ as regulator of GLUT1 expression. MAZ is a six-Cys2-His2 zinc finger transcription factor, and by performing in silico analysis we found a potential MAZ binding site conserved in the GLUT1 promotor of three different species. In accordance with a previous study we found increased expression of MAZ in HCC,29 and suppression of MAZ in HCC cells inhibited GLUT1 expression under normoxic conditions. Further studies have to be performed to demonstrate whether MAZ regulates GLUT1 directly by promotor binding, however, our data indicate that increased MAZ expression accounts at least in part for the up-regulation of GLUT1 in HCC under normoxic conditions. Induction of hypoxia further induced GLUT1 expression in HCC cells in vitro, and this induction was dependent on HIF-1α. Interestingly, hypoxia-independent overexpression of HIF1α through enhanced PI3 kinase/Akt signaling has been reported as an early event during hepatocancerogenesis.32 Further, HIF-1α expression has been shown to correlate with HCC survival, and to play a role in tumor progression after induction of hypoxia in HCC.33,34
Altogether, these findings suggest that in vivo in addition to GLUT1 overexpression secondary to the tumorigenic transformation of the tumor cells, chronically hypoxic tumor cells will further enhance glucose transport via a HIF-dependent increase of GLUT1 synthesis. It has been shown that hypoxia promotes HCC cell growth and resistance to therapy,34,35 and it is sometimes observed that surviving cells in HCC nodules pretreated with transarterial chemoembolization (TACE) grow faster than those in neighboring nodules, and become resistant to subsequent TACE. The molecular basis of this phenomenon was in part explained by hypoxia-induced hexokinase II (HK II) expression in human HCC cells in a HIF-1α-dependent manner, and this enhanced HK II expression accelerates HCC cell proliferation.35 Based on the results of the present study it can be speculated that hypoxia-induced Glut1 expression increases HCC cell growth and motility and accelerates the progression of HCC.
GLUT1 as a Therapeutic Target
Pharmacological inhibition of glucose metabolism has been shown to exhibit promising anticancer activity in vitro and in vivo, alone or in combination with other therapeutic modalities.36 Inhibition of expression or functionality of GLUT1, rather than inhibiting glucose metabolism in its entirety may more specifically target those cells within the tumor that depend on a high rate of glucose uptake and glycolysis. There has been significant progress in the theoretical and experimental characterization of the crystal structure of Glut1, which may prove useful for the rational design of Glut1-inhibiting agents.37,38 Furthermore, lessons learned from the treatment of patients with Glut1 deficiency may open a way for overcoming potential adverse effects of such agents.38 Studies investigating the use of glucose analogues or glucose conjugates that are likely to be taken up into target cells through Glut1 offer compelling evidence that the difference of Glut1 expression between the brain and tumors is large enough to allow targeting of Glut1. For instance, the PET tracer FDG has recently been investigated in mouse models of breast cancer as a radiomolecular therapy, and doses up to 5 mCi proved to be nonradiotoxic to normal organs.38
In summary, this study revealed increased GLUT1 expression in a subset of HCC and suggests that this increased GLUT1 expression functionally affected proliferation and invasiveness of HCC cells. Herewith, GLUT1 expression in HCC appears as a potential innovative therapeutic target for this highly malignant tumor.
Acknowledgments
We thank Rudolph Jung, Birgitta Ott-Rötzer, and Ruth Schewior for excellent technical assistance; and Peter Ratcliffe (Oxford, UK) and Patrick Maxwell (London, UK) for the kind gift of the 6xHRE reporter plasmid.
Footnotes
Address reprint requests to Claus Hellerbrand, M.D., University of Regensburg, Department of Internal Medicine I, D-93042 Regensburg, Germany. E-mail: claus.hellerbrand@klinik.uni-regensburg.de.
Supported by grants from the German Research Association (to A.K.B. and C.H.), the Medical Faculty of the University of Regensburg (ReForM) (to A.H., T.S.W., O.S., M.K., A.K.B., and C.H.), and BayGene (to P.J.O.).
References
- El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 2007;19:223–229. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Hugo-Wissemann D, Anundi I, Lauchart W, Viebahn R, de Groot H. Differences in glycolytic capacity and hypoxia tolerance between hepatoma cells and hepatocytes. Hepatology. 1991;13:297–303. [PubMed] [Google Scholar]
- Kong YH, Han CJ, Lee SD, Sohn WS, Kim MJ, Ki SS, Kim J, Jeong SH, Kim YC, Lee JO, Cheon GJ, Choi CW, Lim SM. [Positron emission tomography with fluorine-18-fluorodeoxyglucose is useful for predicting the prognosis of patients with hepatocellular carcinoma]. Korean J Hepatol. 2004;10:279–287. [PubMed] [Google Scholar]
- Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, Collins BT, Di Bisceglie AM. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792–797. doi: 10.1016/s0168-8278(00)80248-2. [DOI] [PubMed] [Google Scholar]
- Airley RE, Mobasheri A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy. 2007;53:233–256. doi: 10.1159/000104457. [DOI] [PubMed] [Google Scholar]
- Medina RA, Owen GI. Glucose transporters: expression, regulation and cancer. Biol Res. 2002;35:9–26. doi: 10.4067/s0716-97602002000100004. [DOI] [PubMed] [Google Scholar]
- Cooper R, Sarioglu S, Sokmen S, Fuzun M, Kupelioglu A, Valentine H, Gorken IB, Airley R, West C. Glucose transporter-1 (GLUT-1): a potential marker of prognosis in rectal carcinoma? Br J Cancer. 2003;89:870–876. doi: 10.1038/sj.bjc.6601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver RJ, Woodwards RT, Sloan P, Thakker NS, Stratford IJ, Airley RE. Prognostic value of facilitative glucose transporter Glut-1 in oral squamous cell carcinomas treated by surgical resection; results of EORTC Translational Research Fund studies. Eur J Cancer. 2004;40:503–507. doi: 10.1016/j.ejca.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170:223–230. doi: 10.1016/0006-291x(90)91263-r. [DOI] [PubMed] [Google Scholar]
- Grobholz R, Hacker HJ, Thorens B, Bannasch P. Reduction in the expression of glucose transporter protein GLUT 2 in preneoplastic and neoplastic hepatic lesions and reexpression of GLUT 1 in late stages of hepatocarcinogenesis. Cancer Res. 1993;53:4204–4211. [PubMed] [Google Scholar]
- Su TS, Tsai TF, Chi CW, Han SH, Chou CK. Elevation of facilitated glucose-transporter messenger RNA in human hepatocellular carcinoma. Hepatology. 1990;11:118–122. doi: 10.1002/hep.1840110120. [DOI] [PubMed] [Google Scholar]
- Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Wide expression of the human erythrocyte glucose transporter Glut1 in human cancers. Cancer Res. 1996;56:1164–1167. [PubMed] [Google Scholar]
- Zimmerman RL, Fogt F, Burke M, Murakata LA. Assessment of Glut-1 expression in cholangiocarcinoma, benign biliary lesions and hepatocellular carcinoma. Oncol Rep. 2002;9:689–692. [PubMed] [Google Scholar]
- Zimmerman RL, Burke M, Young NA, Solomides CC, Bibbo M. Diagnostic utility of Glut-1 and CA 15-3 in discriminating adenocarcinoma from hepatocellular carcinoma in liver tumors biopsied by fine-needle aspiration. Cancer. 2002;96:53–57. doi: 10.1002/cncr.10309.abs. [DOI] [PubMed] [Google Scholar]
- Roh MS, Jeong JS, Kim YH, Kim MC, Hong SH. Diagnostic utility of GLUT1 in the differential diagnosis of liver carcinomas. Hepatogastroenterology. 2004;51:1315–1318. [PubMed] [Google Scholar]
- Hellerbrand C, Amann T, Schlegel J, Wild P, Bataille F, Spruss T, Hartmann A, Bosserhoff AK. The novel gene MIA2 acts as a tumour suppressor in hepatocellular carcinoma. Gut. 2008;57:243–251. doi: 10.1136/gut.2007.129544. [DOI] [PubMed] [Google Scholar]
- Weiss TS, Jahn B, Cetto M, Jauch KW, Thasler WE. Collagen sandwich culture affects intracellular polyamine levels of human hepatocytes. Cell Prolif. 2002;35:257–267. doi: 10.1046/j.1365-2184.2002.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YS, Yeo EJ, Choi E, Teng CM, Bae JM, Kim MS, Park JW. Inhibitory effect of YC-1 on the hypoxic induction of erythropoietin and vascular endothelial growth factor in Hep3B cells. Biochem Pharmacol. 2001;61:947–954. doi: 10.1016/s0006-2952(01)00564-0. [DOI] [PubMed] [Google Scholar]
- Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, Park JW. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003;95:516–525. doi: 10.1093/jnci/95.7.516. [DOI] [PubMed] [Google Scholar]
- Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- Hellerbrand C, Muhlbauer M, Wallner S, Schuierer M, Behrmann I, Bataille F, Weiss T, Scholmerich J, Bosserhoff AK. Promoter-hypermethylation is causing functional relevant downregulation of methylthioadenosine phosphorylase (MTAP) expression in hepatocellular carcinoma. Carcinogenesis. 2006;27:64–72. doi: 10.1093/carcin/bgi201. [DOI] [PubMed] [Google Scholar]
- Hellerbrand C, Bataille F, Schlegel J, Hartmann A, Muhlbauer M, Scholmerich J, Buttner R, Hofstadter F, Bosserhoff AK. In situ expression patterns of melanoma inhibitory activity 2 in healthy and diseased livers. Liver Int. 2005;25:357–366. doi: 10.1111/j.1478-3231.2005.01099.x. [DOI] [PubMed] [Google Scholar]
- Schuierer MM, Bataille F, Weiss TS, Hellerbrand C, Bosserhoff AK. Raf kinase inhibitor protein is downregulated in hepatocellular carcinoma. Oncol Rep. 2006;16:451–456. [PubMed] [Google Scholar]
- Bruckner BA, Ammini CV, Otal MP, Raizada MK, Stacpoole PW. Regulation of brain glucose transporters by glucose and oxygen deprivation. Metabolism. 1999;48:422–431. doi: 10.1016/s0026-0495(99)90098-7. [DOI] [PubMed] [Google Scholar]
- Dudas J, Mansuroglu T, Moriconi F, Haller F, Wilting J, Lorf T, Fuzesi L, Ramadori G. Altered regulation of Prox1-gene-expression in liver tumors. BMC Cancer. 2008;8:92. doi: 10.1186/1471-2407-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky LL, Zheng Q, Mink K, Rhoads DB. GLUT-1 and GLUT-2 mRNA, protein, and glucose transporter activity in cultured fetal and adult hepatocytes. Am J Physiol. 1994;267:E88–E94. doi: 10.1152/ajpendo.1994.267.1.E88. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Levitsky LL, Mink K, Rhoads DB. Glucose regulation of glucose transporters in cultured adult and fetal hepatocytes. Metabolism. 1995;44:1553–1558. doi: 10.1016/0026-0495(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yamamoto M, Hashimoto N, Miyakoshi M, Tamakawa S, Yoshie M, Tokusashi Y, Yokoyama K, Yaginuma Y, Ogawa K. Hypoxia-independent overexpression of hypoxia-inducible factor 1alpha as an early change in mouse hepatocarcinogenesis. Cancer Res. 2006;66:11263–11270. doi: 10.1158/0008-5472.CAN-06-1699. [DOI] [PubMed] [Google Scholar]
- Huang GW, Yang LY, Lu WQ. Expression of hypoxia-inducible factor 1alpha and vascular endothelial growth factor in hepatocellular carcinoma: impact on neovascularization and survival. World J Gastroenterol. 2005;11:1705–1708. doi: 10.3748/wjg.v11.i11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZF, Poon RT, To J, Ho DW, Fan ST. The potential role of hypoxia inducible factor 1alpha in tumor progression after hypoxia and chemotherapy in hepatocellular carcinoma. Cancer Res. 2004;64:5496–5503. doi: 10.1158/0008-5472.CAN-03-3311. [DOI] [PubMed] [Google Scholar]
- Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005;42:358–364. doi: 10.1016/j.jhep.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- Salas-Burgos A, Iserovich P, Zuniga F, Vera JC, Fischbarg J. Predicting the three-dimensional structure of the human facilitative glucose transporter glut1 by a novel evolutionary homology strategy: insights on the molecular mechanism of substrate migration, and binding sites for glucose and inhibitory molecules. Biophys J. 2004;87:2990–2999. doi: 10.1529/biophysj.104.047886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A, Bates V, Troy H, Hewitt S, Holbeck S, Chung YL, Phillips R, Stubbs M, Griffiths J, Airley R. Glut-1 as a therapeutic target: increased chemoresistance and HIF-1-independent link with cell turnover is revealed through COMPARE analysis and metabolomic studies. Cancer Chemother Pharmacol. 2008;61:377–393. doi: 10.1007/s00280-007-0480-1. [DOI] [PubMed] [Google Scholar]