Abstract
Corneal neovascularization is one of the leading causes of blindness. The aim of this study was to evaluate the pro-angiogenic role of corneal fibroblast-derived membrane type-1 matrix metalloproteinase (MT1-MMP) on basic fibroblast growth factor (bFGF)-induced corneal neovascularization in vivo and in vitro. Immunohistochemical studies demonstrated that MT1-MMP was expressed in keratocytes and immortalized corneal fibroblast cell lines. Vascular endothelial growth factor protein levels were increased after bFGF-stimulation of wild-type fibroblast cells compared with MT1-MMP knockout fibroblast cells. Corneal vascularization was significantly increased after a combination of bFGF pellet implantation and naked MT1-MMP DNA injection in wild-type mouse corneas compared with either bFGF pellet implantation or naked MT1-MMP DNA-injected corneas. Western blotting analysis of the phosphorylation levels of the key signaling molecules (p38, JNK, and ERK) demonstrated that phosphorylation levels of both p38 and JNK were diminished after bFGF stimulation of MT1-MMP knockout cells compared with wild-type and MT1-MMP knockin cells. These results suggest that MT1-MMP potentiates bFGF-induced corneal neovascularization, likely by modulating the bFGF signal transduction pathway.
The cornea is typically avascular in its normal state. However, corneal neovascularization (NV) occurs in conjunction with several corneal diseases such as infection, injury, and autoimmune reactions and is one of the leading causes of blindness. Recent studies have identified several tyrosine kinases and their corresponding ligands that mediate NV, including basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF).1,2,3
bFGF was first identified as a pro-angiogenic factor and is studied extensively in corneal NV models because it is thought to be a major factor in the induction of corneal NV.4,5,6 bFGF is secreted by corneal epithelial cells, stromal fibroblasts, and endothelial cells, and is localized to the corneal extracellular matrix.7 Low levels of bFGF are produced in unwounded corneas; however, enhanced bFGF production was detected in corneal epithelial cells after injury.8 VEGF was also shown to promote NV in corneal wounding models,9 and cross talk is thought to occur between bFGF and VEGF during corneal NV. For example, bFGF was shown to induce corneal NV by activating the VEGF/VEGFR system10,11 and the systemic administration of anti-VEGF-A neutralizing antibodies dramatically reduces this effect.12
Membrane type-1 matrix metalloproteinase (MT1-MMP) is the first transmembrane-containing matrix metalloproteinase to be identified.13 Based on previous reports using corneal wound-healing models, MT1-MMP mRNA is mainly localized to the corneal stroma.14 During NV, quiescent endothelial cells are activated and migration is facilitated by degrading the extracellular matrix through the action of specific proteases, including MT1-MMP.15,16,17 The importance of the enzymatic function of MT1-MMP in corneal NV was shown using the corneal pocket assay in MT1-MMP-deficient mice.18 Interestingly, the expression of MT1-MMP is up-regulated by bFGF stimulation in prostate carcinoma cell lines,19 and it was also reported that MT1-MMP promotes VEGF secretion.20,21,22,23,24,25
In this study, we developed anti-MT1-MMP antibody to localize and characterize MT1-MMP protein in the mouse cornea. To assess the relationship between MT1-MMP and bFGF during corneal NV, we performed experiments that combined the corneal pocket assay using a bFGF pellet with the injection of naked MT1-MMP DNA. We observed an enhanced phosphorylation of MAP kinases in wild-type and MT1-MMP knockin (KI) cell lines over that of MT1-MMP knockout (KO) cell lines, suggesting a role of MT1-MMP in modulating bFGF-mediated signal transduction pathways.
Materials and Methods
Animals
Eight- to ten-week-old C57BL/6 wild-type mice were used. All animals were treated in accordance with the Animal Care and Use Committee’s guidelines for the University of Illinois at Chicago and The Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Generation of Immortalized Wild-Type and MT1-MMP KO Fibroblast Cell Lines
Wild-type and MT1-MMP KO immortalized mouse corneal cell lines were generated as previously described.26 Briefly, the entire mouse corneal stroma was excised and incubated with Dulbecco’s modified Eagle’s medium (HyClone Laboratories, Logan, UT) containing 3.3 mg/ml of collagenase type II (Sigma-Aldrich, St. Louis, MO) at 37°C with shaking for 90 minutes. Isolated keratocytes were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (HyClone Laboratories) at 37°C in a 5% CO2 humidified atmosphere. Subconfluent stromal fibroblasts were supplemented with a mixture containing polybrene (4 μg/ml) and an equal volume of pZIPTEX virus (containing SV40T antigen). Immortalized mouse MT1-MMP KO corneal fibroblast cell lines were generated from MT1-MMP KO mouse corneal stroma (kindly provided by Dr. Zhongjun Zhou, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, Stockholm, Sweden). Immortalized corneal cells from wild-type or MT1-MMP KO mice were subcloned by a serial dilution method to generate corneal keratocyte cell lines.
Characterization of Corneal Fibroblasts
The corneal fibroblast cell lines were characterized immunohistochemically using anti-keratin AE1/AE3 (ICN, San Francisco, CA) and anti-α-smooth muscle actin (ICN) antibodies.26 Cultured fibroblasts were fixed for 15 minutes in cold (−20°C) methanol and rinsed three times in phosphate-buffered saline (PBS). Fixed cells were incubated in a blocking buffer of 1% bovine serum albumin (BSA, Sigma-Aldrich) and 0.2% Triton X-100 in PBS for 30 minutes at room temperature. Cells were then incubated with the following primary antibodies for 1 hour at room temperature: monoclonal mouse anti-keratin antibodies diluted 1:100 in 1% BSA-PBS or monoclonal mouse anti-α-smooth muscle actin antibodies diluted 1:200 in 1% BSA-PBS. The secondary antibody, fluorescein-conjugated donkey anti-mouse IgG (1:200 dilution of 1.2 μg/μl IgG; Jackson ImmunoResearch Laboratories, West Grove, PA), was applied to the fixed cultured cells for 1 hour at room temperature. Cells were washed three times in PBS and mounted with mounting medium containing propidium iodide (PI; Vector Laboratories, Burlingame, CA) and were subsequently viewed using a fluorescence microscope (Eclipse E 800; Nikon, Tokyo, Japan).
Plasmid Construction
A cDNA encoding the MT1-MMP gene was subcloned into the pCN vector for naked DNA injection and into the pFB vector (Stratagene, La Jolla, CA) for stable cell line generation. For the pFB-MT1-MMP construct, an enhanced green fluorescent protein (EGFP) gene was linked to this construction, along with an internal ribosomal entry site and a 3′ nontranslated region. All constructs were confirmed by DNA sequencing.
Generation of Recombinant Virus in 293T Cells
To generate cell lines stably expressing MT1-MMP, a retroviral expression system was used. Recombinant viruses were generated by co-transfection of 293T cells with either the recombinant EGFP-expressing pFB vector encoding MT1-MMP or an empty vector. Briefly, 2 μl of pFB-MT1-MMP DNA, 1 μg of pVPack Eco, and 1 μg of pVPack gag-pol (Stratagene) were mixed with 16 μl of Enhancer solution and 300 μl of transfection buffer (Qiagen, Valencia, CA). After vortex and incubation for 5 minutes, 60 μl of Effectene transfection reagent (Qiagen) was added and mixed for 10 seconds. The final solution was then incubated for 5 to 10 minutes at room temperature for transfection-complex formation, which was then mixed with 3 ml of conditioned growth medium and added dropwise to 7 ml of fresh conditioned medium in the cell dish. After a 24-hour incubation, the conditioned medium was replaced by Dulbecco’s modified Eagle’s medium and incubated for another 24 hours. The medium obtained from the 2nd and 3rd days after transfection (containing a high amount of virus) was collected and used to infect the corneal fibroblast cell lines from MT1-MMP KO mice. The infected corneal fibroblast cells were incubated at 37°C for 1 to 3 days and monitored daily for the presence of EGFP expression. The resulting EGFP+ MT1-MMP KI cells were sorted by fluorescence-activated cell sorting.
Flow Cytometry and Cell Sorting
The MT1-MMP KI cells were sorted based on the expression of EGFP by the Flow Cytometry Core Facility at the Schepens Eye Research Institute, Harvard Medical School, Boston, MA. Single cell suspensions were prepared and washed with cold PBS, trypsinized, and centrifuged at 1000 rpm for 5 minutes, and subsequently fixed in PBS containing 1% paraformaldehyde. Flow cytometry analysis was performed using the Coulter EPICS XL-MCL flow cytometer (Coulter Electronics Inc., Miami, FL). Cells were sorted using a Coulter ELITE cell sorter to give two cell populations, EGFP-positive and EGFP-negative cells. The EGFP-positive population was then cultured and sorted again. The stability of the expression of EGFP was monitored by flow cytometry and the expression of MT1-MMP was analyzed by Western blotting.
Immunohistochemistry/Immunocytochemistry Staining
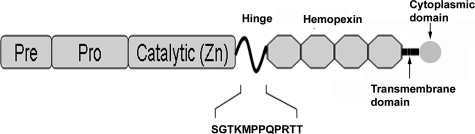
Confocal immunohistochemistry/immunocytochemistry was performed as described previously.27 Primary antibodies pre-incubated (overnight at 4°C) with their specific blocking peptide were used as a negative control. To localize MT1-MMP in fibroblast cells and in mouse corneas, rabbit polyclonal anti-MT1-MMP peptide antibody was generated. The immunogen was a peptide sequence in the MT1-MMP hinge domain (SPTKMPPQPRTT) (Figure 1). The same peptide was used as a blocking peptide (negative control). To detect the expression of MT1-MMP in the cornea, we performed double-immunofluorescence staining with goat anti-vimentin (which may be expressed by corneal stromal cells or other immune cells) and rabbit anti-MT1-MMP antibodies. The VEGF expression in corneal fibroblast cell lines after bFGF stimulation was detected using anti-VEGF antibody (R&D Systems, Inc., Minneapolis, MN). The secondary antibodies used were fluorescein isothiocyanate-conjugated anti-rabbit IgG and Cy5-conjugated anti-goat IgG (Jackson ImmunoResearch Laboratories). PI was used for nuclear staining.
Figure 1.
Schematic representation of MT1-MMP and the location of the amino acid sequence (SGTKMPPQPRTT) that was used to generate the rabbit anti-MT1-MMP antibody.
Corneal Pocket Assay with bFGF and Naked MT1-MMP DNA Injection
Corneal micropellet assays (with 120 ng of bFGF pellets) were performed. The methods we used are described by Kato and colleagues.28 To assess the interaction between bFGF and MT1-MMP, we combined the naked DNA injection technique with the micropocket assay. A ½ inch, 33-gauge needle attached to a 10-μl gas-tight syringe (Hamilton, Reno, NV) was introduced from the micropocket into the corneal stroma, and plasmid (5 μg of pCN or pCN-MT1-MMP) solution was forcibly injected into the stroma to separate corneal lamellae and disperse the plasmid.29 After the DNA was injected, bFGF pellets were inserted as described above. Six mice per group were used for surgery. Combinations of bFGF or blank pellet along with vector or MT1-MMP DNA were introduced into mouse corneas in each group. To evaluate a possible pro-angiogenic effect of MT1-MMP in bFGF-induced corneal NV, we used a low-dose of bFGF (50 ng/pellet) in conjunction with naked DNA injection. Ofloxacin eye drops were applied after the surgery. The eyes were examined and photographed on postoperative days 1, 4, 7, and 10 by slit lamp microscopy (Nikon). Color images were digitized and the images were resolved at 300 pixels/inch. The areas of corneal NV were calculated and analyzed with NIH ImageJ software (National Institutes of Health, Bethesda, MD).
Western Blotting
Wild-type or MT1-MMP KO fibroblast cell lines were plated at a density of 5.0 × 106 cells in a 75-cm2 flask. After stimulation by bFGF (20 ng/ml), cells were lysed by an ice-cold immunoprecipitation assay buffer containing a protease inhibitor and phosphatase inhibitor. Total protein concentration was measured using a protein assay (Bio-Rad, Hercules, CA) and each sample was adjusted to 1.0 mg/ml. Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon P membranes (Millipore, Bedford, MA) that had been blocked with 3% BSA for 60 minutes. The membranes were then incubated overnight with rabbit anti-ERK antibody (1:200 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), mouse anti-phospho-ERK antibody (1:1000; Cell Signaling Technology, Danvers, MA), rabbit anti-p38 antibody (1:1000, Cell Signaling Technology), rabbit anti-phospho-p38 antibody (1:1000, Cell Signaling Technology), rabbit anti-JNK antibody (1:1000, Cell Signaling Technology), rabbit anti-phospho-JNK antibody (1:1000, Cell Signaling Technology), or rabbit anti-VEGF-A antibody (1:1000 dilution, Millipore). The membranes were then incubated with horseradish peroxidase-conjugated donkey anti-rabbit or anti-mouse IgG antibodies (1:20,000 dilution; Amersham Biosciences, Buckinghamshire, UK) for 30 minutes. The membrane was washed with TBST and antigen was detected using ECL solution (Pierce Biotechnology Inc., Rockford, IL).
Reverse Transcriptase-Polymerase Chain Reaction Analysis and Real-Time Polymerase Chain Reaction (PCR) of VEGF-A and MT1-MMP
Total RNA was purified from keratocyte wild-type, KO, and KI cell lines using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Reverse transcription of RNA was synthesized using High-Capacity cDNA reverse transcription kits (Applied Biosystems, Foster City, CA) reagents. The following primer sets were synthesized and used for amplification: MT1-MMP, sense 5′-GCTTTACTGCCAGCGTTC-3′ and antisense 5′-CCCACTTATGGATGAAGCAAT-3′; VEGF-A, sense 5′-CAAAAACGAAAGCGCAAGAAA-3′ and antisense 5′-CGCTCTGAACAAGGCTCACA-3′; GAPDH sense 5′-TTGCCATCAATGACCCCTTCA-3′ and antisense 5′-ATGGGCTTCCTGTTGATGACA-3′. GAPDH mRNAs were used as internal controls. Real time PCRs were performed using the fluorescence detection method using the ABI Prism 7900HT sequence detection system with SYBR Green PCR master mix (Applied Biosystems). The cycling conditions were as follows: activation of Taq enzyme at 50°C for 2 minutes, initial denaturation at 95°C for 10 minutes, followed by 40 amplification cycles of 95°C for 15 seconds, 60°C for 60 seconds. All experiments were performed with triplicate samples. After completion of PCR, ABI PRISM SDS 2.3 software was used to convert the raw data. The amount of VEGF-A and MT1-MMP mRNAs were normalized with GAPDH mRNA.
Statistical Analysis
Results of areas of corneal NV are expressed as mean ± SEM, and statistical analyses were performed using a paired sample t-test. Differences were considered significant when P < 0.05.
Results
Localization and Enhancement of Corneal MT1-MMP Expression After bFGF Pellet Implantation
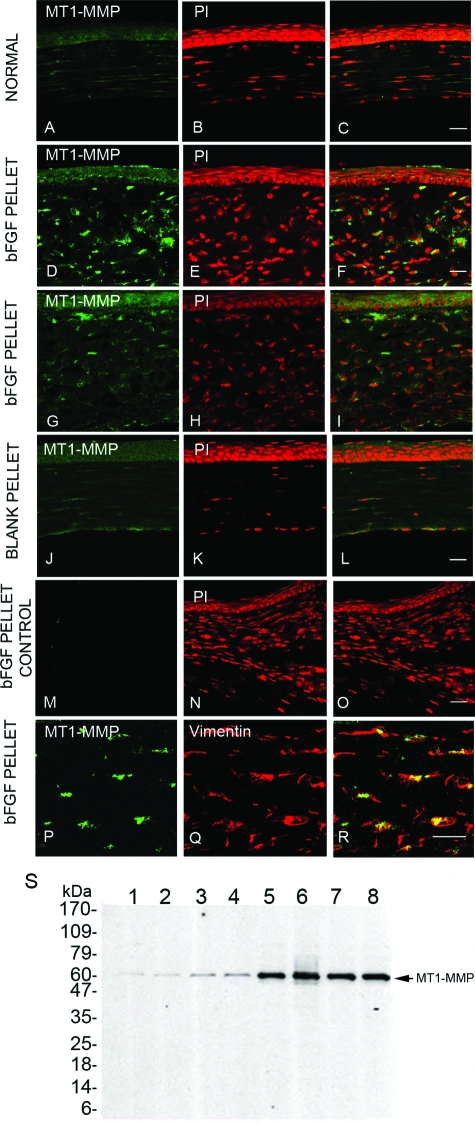
To characterize the localization of MT1-MMP in mouse corneal tissues, we generated anti-MT1-MMP antibody and performed immunohistochemical staining of normal uninjured mouse corneas. We observed weak expression of MT1-MMP in the stromal and epithelial layer of mouse cornea (Figure 2, A–C). The levels of corneal MT1-MMP expression were enhanced at day 7 after bFGF (120 ng) pellet implantation (Figure 2, D–L) when compared with that of blank pellet implantation. The specificity of the MT1-MMP immunostaining was confirmed by pre-incubating the anti-MT1-MMP antibody with the cognate peptide as a control (Figure 2, M–O). bFGF-induced mouse corneal sections were double immunostained using anti-MT1-MMP and anti-vimentin antibodies. The stromal expression of MT1-MMP (Figure 2P) was co-localized to the cells that expressed vimentin (Figure 2, Q and R). In addition, MT1-MMP protein levels were increased in bFGF-treated corneas as compared with untreated corneas (Figure 2S).
Figure 2.
MT1-MMP immunolocalization in normal and bFGF-induced mouse corneas. Normal uninjured mouse eyes were enucleated, frozen in OCT, and corneal sections were stained with anti-MT1-MMP antibody (A) and PI (B); overlay image (C). Seven days after the blank (controls) or 120-ng bFGF pellet implantation, mouse eyes were enucleated and the sections were stained with anti-MT1-MMP antibody (D, G, J and P), PI (E, H, and K), or anti-vimentin antibody (Q); overlay images (F, I, L, O, and R). Antibody pre-incubated with cognate peptide was used to stain the bFGF pellet-inserted cornea as a control (M, N, and O). S: Lysates from individual bFGF-stimulated mouse corneas5,6,7,8 and untreated controls1,2,3,4 were immunoblotted with anti-MT1-MMP antibody. Scale bars = 20 μm.
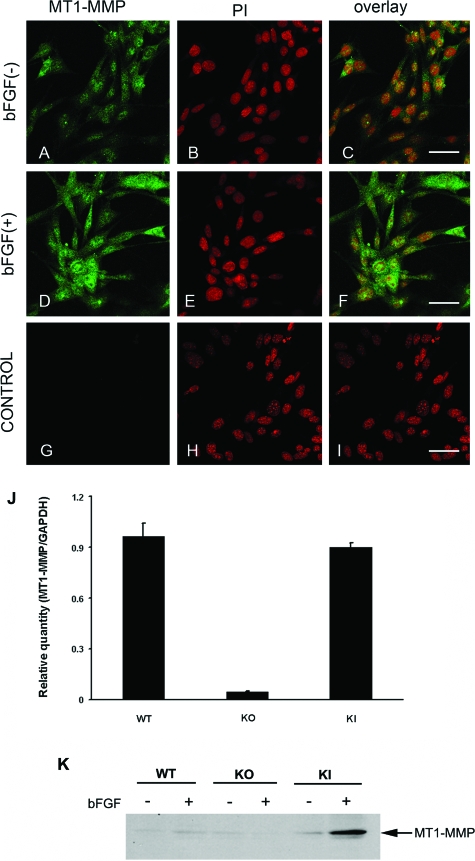
We performed immunofluorescence staining on corneal fibroblast cell lines, and found that MT1-MMP was expressed in cultured corneal fibroblast cells (Figure 3, A–C). Additionally, the levels of MT1-MMP immunostaining in corneal fibroblast cells were enhanced on stimulation with 20 ng/ml of bFGF for 48 hours (Figure 3, D–I). MT1-MMP mRNA levels of these cell lines were determined by real-time PCR (Figure 3J). MT1-MMP protein levels in these cell lines with or without bFGF stimulation are shown in Figure 3K.
Figure 3.
MT1-MMP immunolocalization in corneal fibroblast cell lines. Unstimulated wild-type (WT) corneal fibroblast cell lines were immunostained with anti-MT1-MMP antibody (A) and PI (B). Wild-type corneal fibroblast cells stimulated with bFGF (20 ng/ml) were immunostained with anti-MT1-MMP antibody (D) and PI (E); overlay images (C, F, and I). Antibody preincubated with cognate peptide was used as a control (G–I). J: Real-time PCR analysis of MT1-MMP expression in wild-type, MT1-MMP KO, and MT1-MMP KI keratocytes was performed. K: Western blot analysis was performed using MT1-MMP antibody in wild-type, MT1-MMP KO, and MT1-MMP KI keratocytes treated with and without bFGF. Scale bars = 50 μm.
VEGF-A Expression in Wild-Type and MT1-MMP KO Cell Lines
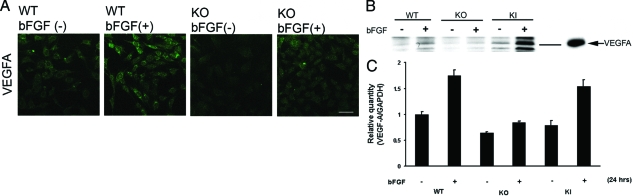
VEGF-A expression levels were determined in wild-type and MT1-MMP KO fibroblasts using immunostaining and Western blot analysis. Cells were starved for 18 hours and stimulated with 20 ng/ml of bFGF for 48 hours, then immunostained with anti-VEGF-A antibodies (Figure 4A). The intensity of anti-VEGF-A antibody immunostaining was enhanced in bFGF-stimulated wild-type fibroblast cells, but staining was observed to a lesser extent in MT1-MMP KO cells. Similarly, using Western blot analysis, we confirmed that VEGF-A expression was up-regulated by bFGF stimulation in wild-type fibroblast cells, however the enhancement of VEGF-A expression in bFGF-stimulated MT1-MMP KO cells was less notable (Figure 4B). bFGF enhanced VEGF-A mRNA expression in wild-type and KI keratocytes but to a lesser extent in KO keratocytes by real-time PCR (Figure 4C).
Figure 4.
VEGF-A expression in corneal fibroblasts. A: Wild-type (WT) and KO corneal fibroblasts were starved for 18 hours and treated with 20 ng/ml of bFGF for 48 hours. They were then fixed, permeabilized, and stained with antibody to VEGF-A. Corneal fibroblasts were treated with bFGF in the same way, and the cell lysates were subjected to Western blot analysis with antibodies to VEGF-A (B; VEGF-A control is shown) as well as real-time PCR analysis (C). Scale bar = 20 μm.
MT1-MMP Expression After Injection of Plasmid DNA
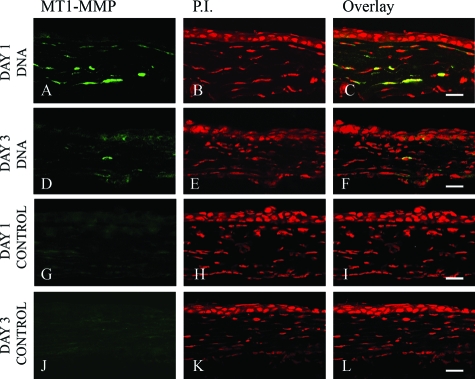
Naked DNA injection has been used routinely for transient expression of exogenous proteins in mouse corneas. To assess the feasibility and efficiency of this technique, intrastromal protein expression was confirmed by immunohistochemical staining. Twenty-four hours after injection of MT1-MMP DNA or vector DNA into the mouse cornea, sections were immunostained with anti-MT1-MMP antibody. Our data showed that keratocytes injected with MT1-MMP DNA expressed MT1-MMP proteins (Figure 5, A–F). Increased levels of MT1-MMP protein were not detected in the vector-injected corneas (Figure 5, G–L). The expression of MT1-MMP diminished 3 days after naked DNA injection (Figure 5, D–F).
Figure 5.
MT1-MMP protein expression after naked MT1-MMP or vector DNA injection. Either MT1-MMP-encoding DNA (A–F) or empty vector DNA (G–L) was injected into mouse corneas, and the eyes were enucleated at days 1 and 3 and the corneal sections were stained with anti-MT1-MMP antibody (A, D, G, and J) and PI (B, E, H, and K); overlay images (C, F, I, and L). Data are representative of three independent experiments. Scale bars = 20 μm.
Potentiating Effects of MT1-MMP on bFGF-Induced Corneal NV in Vivo
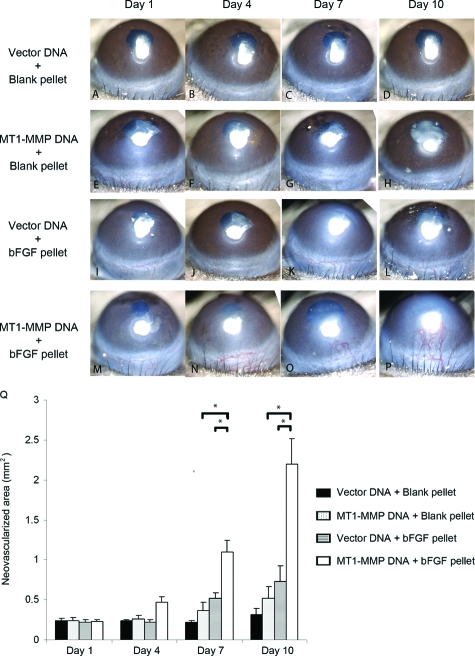
We combined corneal micropocket implantation of blank or bFGF pellets with naked vector or MT1-MMP DNA injection and the results of corneal NV are shown in Figure 6. At days 7 and 10 after implantation/injection, the areas of NV in the corneas that had been both injected with MT1-MMP DNA and implanted with bFGF pellets (Figure 6, M–P) were significantly higher than the groups with the MT1-MMP DNA injection (Figure 6, E–I) or the bFGF pellet implantation alone (Figure 6, I–L; summarized in Figure 6Q).
Figure 6.
Enhanced bFGF-induced corneal NV after a combination of bFGF pellet implantation and naked MT1-MMP DNA plasmid injection. The blank pellets were implanted immediately after MT1-MMP DNA (E–H) or vector control DNA (A–D) was injected into corneal stroma. Likewise, the bFGF pellets were implanted immediately after MT1-MMP DNA (M–P) or vector control DNA (I–L) was injected into corneal stroma. Photographs were taken on days 1, 4, 7, and 10 after surgery. Q: Graphic representation of at least five independent experiments (mean ± SEM, *P < 0.05).
MT1-MMP Induces the Activation of Three MAP Kinase
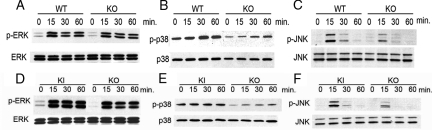
In the keratocyte cells lines, the levels of three MAP kinases (ERK, p38, and JNK) known to be phosphorylated by bFGF stimulation were also assessed.30,31 Wild-type and MT1-MMP KO corneal fibroblast cells were stimulated with bFGF and the total protein levels and phosphorylation status of the three MAP kinases was evaluated in cell extracts using Western blot (Figure 7). We found that phosphorylation levels of these MAP kinases were enhanced in response to bFGF stimulation in corneal fibroblast cells, whereas the total protein levels were unchanged (Figure 7, A–C). MT1-MMP KI and KO fibroblast cell lines were stimulated with bFGF and cell lysates were subsequently analyzed by Western blot analysis (Figure 7, D–F). The phosphorylation levels of p38 in KI were higher than those of KO keratocytes (Figure 7E).
Figure 7.
Activation of MAP kinases by bFGF stimulation in wild-type (WT) and MT1-MMP KO cell lines. MT1-MMP KO and wild-type cell lines were serum-starved for 18 hours and treated with bFGF for 15, 30, or 60 minutes. The cell lysate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with subsequent Western blot analysis with antibodies against phospho-ERK1/2, ERK1/2 (A), phospho-p38, p38 (B), or phospho-JNK and JNK (C). Comparison of the phosphorylation of MAP kinases on bFGF stimulation in MT1-MMP KO and KI cell lines. Cell lines were serum-starved for 18 hours and stimulated with bFGF for 15, 30, and 60 minutes. Cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis with subsequent Western blot analysis with antibodies against phospho-ERK1/2, ERK1/2 (D), phospho-p38, p38 (E), or phospho-JNK and JNK (F).
Discussion
In this study, we investigated the relationship between bFGF and MT1-MMP in corneal NV. The expression levels of both MT1-MMP14 and bFGF8 are known to be increased in corneal wound healing. We have demonstrated that bFGF causes an increased expression of MT1-MMP in the cornea. We and others have previously demonstrated that an increase in MT1-MMP enhances VEGF production and cleavage of ECM, which may facilitate corneal NV. Taken together, these data may suggest that bFGF induction of MT1-MMP expression may in part be responsible for the role of bFGF in NV. The interaction between bFGF and MT1-MMP may occur in both stromal and vascular endothelial cells. Additionally, because MT1-MMP is expressed in tumor cells,32 the interaction between MT1-MMP and bFGF may play a similar role in the enhancement of tumor growth and vascularization. Based on these findings, we further examined the function of MT1-MMP in corneal keratocyte/ fibroblasts.
In agreement with our previous report detailing the localization of MT1-MMP mRNA in rat corneas. We showed that MT1-MMP protein is localized to the keratocyte in normal and neovascularized corneas.14 By using corneal fibroblast cell lines, the expression levels of MT1-MMP are enhanced by bFGF stimulation. Our data are in agreement with Udayakumar and colleagues,19 who show an up-regulation of MT1-MMP protein on bFGF stimulation in a prostate carcinoma cell line.
Zhou and colleagues33 reported that MT1-MMP is required for bFGF-induced corneal NV. Therefore, to investigate the interaction between MT1-MMP and bFGF, we have used a corneal NV model. This model combines naked MT1-MMP DNA injection with corneal pocket implantation of bFGF because implantation is a method that is typically used to assay the effects of bFGF on NV in the avascular background.5 After MT1-MMP plasmid injection into mouse corneal stroma, we could detect the expression of MT1-MMP protein. When combined with the corneal pocket assay, MT1-MMP DNA injections significantly promoted bFGF-induced NV in comparison with the independent effect generated by bFGF stimulation or DNA injection alone. We therefore hypothesize that MT1-MMP potentiated the effect of bFGF-induced corneal NV by modulating the bFGF-mediated signal transduction pathways.
Three MAP kinase homologues, ERK, JNK, and p38, are known to be phosphorylated by bFGF stimulation.30,31,34 Additionally, expression of MT1-MMP activates ERK, which is important for MT1-MMP-dependent cell migration.34,35 These reports correspond with our present data, which demonstrate increased pERK expression on bFGF stimulation in MT1-MMP KI cell lines compared with wild-type cell lines. On the other hand, no difference in pERK expression was observed on comparison of MT1-MMP KO and wild-type cell lines.
The difference in p38 and JNK activation was evident when comparing wild-type and KO cell lines in this study. Disruption of the p38 gene results in embryonic lethality because of severe defects in placental NV.36 The reports of p3837,38,39 and JNK40 involvement in VEGF expression suggest a relationship between MAP kinases and bFGF-induced NV. The central role of VEGF and its receptor in vascular endothelial cell proliferation and NV is already established.41 Activation of MAP kinases by various cellular stresses increases VEGF mRNA stability and subsequently enhances VEGF protein production.42 These findings and reports imply a mechanism of MT1-MMP modulating bFGF signal transduction pathways and subsequently regulating VEGF-A expression. Interestingly, overexpression of MT1-MMP in tumors enhances NV in vivo by stimulating VEGF-A synthesis from the tumor cells.22,23,24,25 In agreement with this finding is our observation that VEGF-A expression is diminished in MT1-MMP KO cells when compared with that of wild-type fibroblast cells. The enhancement of VEGF synthesis requires the catalytic activity and cytoplasmic domain of MT1-MMP.25 This report shows the potentiating function of MT1-MMP on bFGF-induced corneal NV mediated through intracellular signal transduction pathways.
We show that MT1-MMP has an effect on bFGF-induced signal transduction via activation of MAP kinases in corneal NV. Based on our data, along with previously published data, we can envision a complicit interaction between MT1-MMP, bFGF, and VEGF-A in corneal NV. Further study is necessary to more clearly define the interaction between MT1-MMP and the upstream regulatory molecules involved in bFGF-induced MAP kinases during corneal NV. Investigating the mode of action by which MT1-MMP affects signal transduction is valuable for identifying potential targets for the treatment of not only corneal NV but also NV-related disorders such as diabetic retinopathy, macular degeneration, and cancer.
Footnotes
Address reprint requests to Dimitri T. Azar, M.D., Chairman, Department of Ophthalmology and Visual Sciences, Illinois Eye and Ear Infirmary, University of Illinois at Chicago, 1855 West Taylor St., Chicago, IL 60612. E-mail: dazar@uic.edu.
Supported by the National Institutes of Health (grants EY10101 and EY 001792 to D.T.A. and EY14048 to J.H.C.).
References
- Tallquist MD, Soriano P, Klinghoffer RA. Growth factor signaling pathways in vascular development. Oncogene. 1999;18:7917–7932. doi: 10.1038/sj.onc.1203216. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- Kwon YS, Kim JC. Inhibition of corneal neovascularization by rapamycin. Exp Mol Med. 2006;38:173–179. doi: 10.1038/emm.2006.21. [DOI] [PubMed] [Google Scholar]
- Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- Adamis AP, Meklir B, Joyce NC. In situ injury-induced release of basic-fibroblast growth factor from corneal epithelial cells. Am J Pathol. 1991;139:961–967. [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I. A heparin-binding angiogenic protein—basic fibroblast growth factor—is stored within basement membrane. Am J Pathol. 1988;130:393–400. [PMC free article] [PubMed] [Google Scholar]
- Benelli U, Bocci G, Danesi R, Lepri A, Bernardini N, Bianchi F, Lupetti M, Dolfi A, Campagni A, Agen C, Nardi M, Del Tacca M. The heparan sulfate suleparoide inhibits rat corneal angiogenesis and in vitro neovascularization. Exp Eye Res. 1998;67:133–142. doi: 10.1006/exer.1998.0512. [DOI] [PubMed] [Google Scholar]
- Epstein RJ, Stulting RD, Hendricks RL, Harris DM. Corneal neovascularization. Pathogenesis and inhibition. Cornea. 1987;6:250–257. doi: 10.1097/00003226-198706040-00004. [DOI] [PubMed] [Google Scholar]
- Tille JC, Wood J, Mandriota SJ, Schnell C, Ferrari S, Mestan J, Zhu Z, Witte L, Pepper MS. Vascular endothelial growth factor (VEGF) receptor-2 antagonists inhibit VEGF- and basic fibroblast growth factor-induced angiogenesis in vivo and in vitro. J Pharmacol Exp Ther. 2001;299:1073–1085. [PubMed] [Google Scholar]
- Auguste P, Gursel DB, Lemiere S, Reimers D, Cuevas P, Carceller F, Di Santo JP, Bikfalvi A. Inhibition of fibroblast growth factor/fibroblast growth factor receptor activity in glioma cells impedes tumor growth by both angiogenesis-dependent and -independent mechanisms. Cancer Res. 2001;61:1717–1726. [PubMed] [Google Scholar]
- Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 1998;12:1075–1095. [PubMed] [Google Scholar]
- Ye HQ, Maeda M, Yu FS, Azar DT. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Invest Ophthalmol Vis Sci. 2000;41:2894–2899. [PubMed] [Google Scholar]
- Chantrain CF, Henriet P, Jodele S, Emonard H, Feron O, Courtoy PJ, DeClerck YA, Marbaix E. Mechanisms of pericyte recruitment in tumour angiogenesis: a new role for metalloproteinases. Eur J Cancer. 2006;42:310–318. doi: 10.1016/j.ejca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003;10:136–141. doi: 10.1097/00062752-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Udayakumar TS, Nagle RB, Bowden GT. Fibroblast growth factor-1 transcriptionally induces membrane type-1 matrix metalloproteinase expression in prostate carcinoma cell line. Prostate. 2004;58:66–75. doi: 10.1002/pros.10293. [DOI] [PubMed] [Google Scholar]
- Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez BG, Matias-Roman S, Albar JP, Sanchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J Biol Chem. 2001;276:37491–37500. doi: 10.1074/jbc.M104094200. [DOI] [PubMed] [Google Scholar]
- Toth M, Hernandez-Barrantes S, Osenkowski P, Bernardo MM, Gervasi DC, Shimura Y, Meroueh O, Kotra LP, Galvez BG, Arroyo AG, Mobashery S, Fridman R. Complex pattern of membrane type 1 matrix metalloproteinase shedding. Regulation by autocatalytic cells surface inactivation of active enzyme. J Biol Chem. 2002;277:26340–26350. doi: 10.1074/jbc.M200655200. [DOI] [PubMed] [Google Scholar]
- Langlois S, Gingras D, Beliveau R. Membrane type 1-matrix metalloproteinase (MT1-MMP) cooperates with sphingosine 1-phosphate to induce endothelial cell migration and morphogenic differentiation. Blood. 2004;103:3020–3028. doi: 10.1182/blood-2003-08-2968. [DOI] [PubMed] [Google Scholar]
- Sounni NE, Roghi C, Chabottaux V, Janssen M, Munaut C, Maquoi E, Galvez BG, Gilles C, Frankenne F, Murphy G, Foidart JM, Noel A. Up-regulation of vascular endothelial growth factor-A by active membrane-type 1 matrix metalloproteinase through activation of Src-tyrosine kinases. J Biol Chem. 2004;279:13564–13574. doi: 10.1074/jbc.M307688200. [DOI] [PubMed] [Google Scholar]
- Azar DT, Casanova FH, Mimura T, Jain S, Chang JH. Effect of MT1-MMP deficiency and overexpression in corneal keratocytes on vascular endothelial cell migration and proliferation. Curr Eye Res. 2008;33:954–962. doi: 10.1080/02713680802461106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PC, Ye H, Maeda M, Azar DT. Immunolocalization and gene expression of matrilysin during corneal wound healing. Invest Ophthalmol Vis Sci. 1999;40:20–27. [PubMed] [Google Scholar]
- Kato T, Chang JH, Azar DT. Expression of type XVIII collagen during healing of corneal incisions and keratectomy wounds. Invest Ophthalmol Vis Sci. 2003;44:78–85. doi: 10.1167/iovs.01-1257. [DOI] [PubMed] [Google Scholar]
- Stechschulte SU, Joussen AM, von Recum HA, Poulaki V, Moromizato Y, Yuan J, D'Amato RJ, Kuo C, Adamis AP. Rapid ocular angiogenic control via naked DNA delivery to cornea. Invest Ophthalmol Vis Sci. 2001;42:1975–1979. [PubMed] [Google Scholar]
- Hefti MA, Harder BA, Eppenberger HM, Schaub MC. Signaling pathways in cardiac myocyte hypertrophy. J Mol Cell Cardiol. 1997;29:2873–2892. doi: 10.1006/jmcc.1997.0523. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Abe M, Sato Y. Roles of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in the signal transduction of basic fibroblast growth factor in endothelial cells during angiogenesis. Jpn J Cancer Res. 1999;90:647–654. doi: 10.1111/j.1349-7006.1999.tb00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Browder T, Palmblad J. Angiogenesis research: guidelines for translation to clinical application. Thromb Haemost. 2001;86:23–33. [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras D, Bousquet-Gagnon N, Langlois S, Lachambre MP, Annabi B, Beliveau R. Activation of the extracellular signal-regulated protein kinase (ERK) cascade by membrane-type-1 matrix metalloproteinase (MT1-MMP). FEBS Lett. 2001;507:231–236. doi: 10.1016/s0014-5793(01)02985-4. [DOI] [PubMed] [Google Scholar]
- Takino T, Miyamori H, Watanabe Y, Yoshioka K, Seiki M, Sato H. Membrane type 1 matrix metalloproteinase regulates collagen-dependent mitogen-activated protein/extracellular signal-related kinase activation and cell migration. Cancer Res. 2004;64:1044–1049. doi: 10.1158/0008-5472.can-03-1843. [DOI] [PubMed] [Google Scholar]
- Mudgett JS, Ding J, Guh-Siesel L, Chartrain NA, Yang L, Gopal S, Shen MM. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci USA. 2000;97:10454–10459. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YS, Park YG, Kim BK, Han SY, Jee YH, Han KH, Lee MH, Song HK, Cha DR, Kang SW, Han DS. Angiotensin II stimulates the synthesis of vascular endothelial growth factor through the p38 mitogen activated protein kinase pathway in cultured mouse podocytes. J Mol Endocrinol. 2006;36:377–388. doi: 10.1677/jme.1.02033. [DOI] [PubMed] [Google Scholar]
- Zetser A, Bashenko Y, Edovitsky E, Levy-Adam F, Vlodavsky I, Ilan N. Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and Src activation. Cancer Res. 2006;66:1455–1463. doi: 10.1158/0008-5472.CAN-05-1811. [DOI] [PubMed] [Google Scholar]
- Wang M, Crisostomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol. 2006;291:R880–R884. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- Tokuda H, Hirade K, Wang X, Oiso Y, Kozawa O. Involvement of SAPK/JNK in basic fibroblast growth factor-induced vascular endothelial growth factor release in osteoblasts. J Endocrinol. 2003;177:101–107. doi: 10.1677/joe.0.1770101. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- Pagès G, Berra E, Milanini J, Levy AP, Pouyssegur J. Stress-activated protein kinases (JNK and p38/HOG) are essential for vascular endothelial growth factor mRNA stability. J Biol Chem. 2000;275:26484–26491. doi: 10.1074/jbc.M002104200. [DOI] [PubMed] [Google Scholar]