Abstract
Objectives
We examined associations between anthropometric measures (body mass index [BMI], waist circumference [WC], waist-to-hip ratio [WHR], waist-to-height ratio [WHtR]) and risk of incident cardiovascular disease (CVD, including nonfatal myocardial infarction, nonfatal ischemic stroke, cardiovascular death).
Background
Controversy exists regarding the optimal approach to measure adiposity, and the utility of BMI has been questioned.
Methods
Participants included 16,332 men in the Physicians’ Health Study (mean age 61, 1991) and 32,700 women in the Women’s Health Study (mean age 61, 1999). We used Cox proportional hazards models to determine relative risks (RR) and 95% confidence intervals (CI) for developing CVD according to self-reported anthropometric indices.
Results
A total of 1505 CVD cases occurred in men, and 414 occurred in women (median follow-up, 14.2 and 5.5 years, respectively). While WHtR demonstrated statistically the strongest associations with CVD and best model fit, CVD risk increased linearly and significantly with higher levels of all indices. Adjusting for confounders, the RR (CI) for CVD was 0.58 (0.32–1.05) for men with the lowest WHtR (<0.45) and 2.36 (1.61–3.47) for the highest WHtR (≥0.69; versus WHtR 0.49-<0.53). Among women, the RR (95% CI) was 0.65 (0.33– 1.31) for those with the lowest WHtR (<0.42) and 2.33 (1.66–3.28) for the highest WHtR (≥0.68; versus WHtR 0.47- <0.52).
Conclusions
WHtR demonstrated statistically the best model fit and strongest associations with CVD. However, as compared to BMI, differences in cardiovascular risk assessment using other indices were small and likely not clinically consequential. Our findings emphasize that higher levels of adiposity, however measured, confer increased risk of CVD.
Keywords: obesity, cardiovascular disease, epidemiology
We face an epidemic of overweight and obesity which affects more than a billion adults worldwide.1 While multiple health organizations recommend using body mass index (BMI) for the identification of overweight and obese individuals,2, 3 uncertainty exists regarding the optimal approach to measure adiposity, and the utility of BMI has been questioned.4, 5 Using BMI as the current standard measure of adiposity can result in misclassification of risk among certain populations and may not adequately describe adiposity in relation to cardiovascular risk.
Anthropometric indices of central adiposity and body composition, including the waist circumference (WC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR), may more accurately identify groups at risk for adverse health consequences of excess weight.2, 6–8 However, the relative utility of various anthropometric measures in assessing cardiovascular risk remains unclear.9, 10 Furthermore, while some studies have explored the associations between anthropometric indices and cardiovascular risk beyond BMI, analytic approaches and results have been inconsistent and most studies have not directly compared multiple indices.10
We therefore examined the associations between various anthropometric parameters of adiposity and the risk of incident cardiovascular disease (CVD) in prospective cohorts of more than 49,000 men and women.
METHODS
Study Populations
We used data from two prospective cohorts of health professionals in the US to examine the associations between various anthropometric indices and cardiovascular risk. Baseline and follow-up information was self-reported and collected through mailed questionnaires every 6 months for the first year and annually thereafter. Details of the study methods and results have been described elsewhere.11–15
Physicians’ Health Study (PHS)
Study subjects included participants in the PHS, a completed randomized trial of aspirin and beta carotene in the primary prevention of CVD and cancer.11, 12 This trial included 22,071 apparently healthy male physicians, age 40–84 years in 1982, without a history of CVD or other major illnesses.
Analyses were limited to men who remained in the cohort at 9 years (n=20,889), the time at which waist and hip circumferences were requested. We excluded men who did not return the 9-year questionnaire (n=183), those missing information on waist (n=2097), hip (n=2135), or BMI (n=623), those with WC or hip circumferences <20 in. or >70 in. (n=28), and men with a history of CVD, coronary revascularization procedures, or angina prior to the 9-year questionnaire (n=1780), leaving 16,332 men for our analyses.
Women’s Health Study (WHS)
Study subjects also included participants in the WHS, a completed randomized trial of aspirin and vitamin E in the primary prevention of CVD and cancer.13–15 This trial included 39,876 female health professionals, age ≥45 years in 1993 without a history of CVD or other major illnesses.
Analyses were limited to women who remained in the cohort at 6 years (n=39,135), the only time at which waist and hip circumferences were requested. We excluded women who did not return the 6-year questionnaire (n=2070), those missing information on waist (n=3099), hip (n=3103), or BMI (n=758), those with WC <20 in. (n=4), and women with a history of CVD, coronary revascularization procedures, or angina prior to the 6-year questionnaire (n=492), leaving 32,700 women for our analyses.
Exposures
We calculated BMI from self-reported weight (kg) divided by the square of the height (m). To evaluate BMI over the range of “normal” and “overweight,” we categorized BMI as <20.0, 20.0- <22.5, 22.5- <25.0, 25.0- <27.5, 27.5- <30.0, 30.0- <35.0, ≥35.0 kg/m2. We also assessed BMI as a continuous variable (per standard deviation unit, SD). We used baseline height and weight reported with the 9-year (PHS) or 6-year (WHS) questionnaire to calculate BMI.
Participants were asked to report circumferences using a paper tape measure supplied with the questionnaire. Instructions requested that participants (1) measure their WC at the level of the umbilicus, (2) measure their hip circumference as the largest circumference between the umbilicus and the thigh, and (3) record the measurements to the nearest quarter inch. WHR was calculated by dividing the WC by the hip circumference and WHtR by dividing the WC by the baseline height. We evaluated WC, WHR, and WHtR in seven categories defined by the percentile distributions of participants in the corresponding seven BMI categories and as a continuous variable, per SD unit. Self-reports of anthropometric measures have been validated in other health professional cohorts.16
Covariates
From the PHS, covariates assessed at 9 years included age (5-year categories), physical activity (rarely/never, 1–2, 3–4, 5–7 days/week), and history of cancer, diabetes, elevated cholesterol (≥240 mg/dL or history of cholesterol-lowering medication use), or hypertension (self-reported systolic blood pressure [BP] ≥140 mmHg, diastolic BP ≥90 mmHg, or antihypertensive medication use). Other covariates were obtained at the most recent prior questionnaire: smoking at 5 years (never, past, current), alcohol consumption at 7 years (rarely/never, 1–3 drinks/month, 1–6 drinks/week, ≥1 drink/day), and, from baseline, parental history of myocardial infarction before the age of 60.
From the WHS, covariates at 6 years included age (5-year categories) and history of cancer, history of diabetes, elevated cholesterol (≥240 mg/dL, cholesterol-lowering medication use, or elevated cholesterol diagnosed by a clinician), or hypertension (self-reported systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, hypertension diagnosed by a clinician, or history of antihypertensive treatment). We measured smoking (never, past, current) at baseline, updating the variable through year six. Other covariates were obtained at the most recent prior questionnaire: postmenopausal hormone use (never, past, current) at 5 years, alcohol consumption at 4 years (rare/never, 1–3 drinks/month, 1–6 drinks/week, ≥1 drink/day), and, from baseline, physical activity (≤1, 2–3, ≥4 times/week), parental history of myocardial infarction before the age of 60, highest level of education (less than a bachelor’s degree, bachelor’s degree, master’s/doctorate degree), and race (white, black, other). Dietary information was obtained at baseline from a 161-item standardized food frequency questionnaire,17 and nutrient intake was adjusted for total energy intake using the residual method.18 Dietary variables included cereal fiber, folate, glycemic load, trans fat, polyunsaturated-to-saturated fat ratio, and omega-3 fatty acids (all in quintiles).19
Outcome
We defined incident major CVD as first nonfatal myocardial infarction, nonfatal ischemic stroke, or fatal CVD (defined as fatal myocardial infarction, fatal ischemic stroke, sudden death, or any deaths related to ischemic heart disease; International Classification of Diseases-Ninth Revision, ICD-9, codes 410–414, 430–438, 798).
Confirmation of all endpoints required review of available medical records by an endpoints committee of physicians who used standardized criteria. Myocardial infarctions were confirmed by elevated plasma levels of cardiac enzymes or diagnostic electrocardiograms. Fatal myocardial infarction was confirmed based on autopsy reports, symptoms, circumstances of death, and history of coronary heart disease. Nonfatal stroke was defined as a focal neurological deficit of vascular mechanism and sudden onset that lasted more than 24 hours. Fatal stroke was documented with evidence of a cerebrovascular mechanism using all available information, including death certificates and medical records. Brain imaging and clinical information were used to distinguish between types of stroke. The interobserver agreement on the classification of major stroke subtypes was excellent in both the PHS (κ=0.81)20 and WHS (κ=0.96).21
Deaths were identified through systematic searches of the National Death Index (NDI). We identified cohort members who died before March 31, 2006, and obtained their death certificates from state agencies. Trained nosologists classified causes of death according to ICD-9 codes in conjunction with the “Automated Classification of Medical Entities Decision Tables” to select the underlying cause of death. The reliability of the NDI for epidemiologic purposes among female health professionals has been previously validated (98% sensitivity, ~100% specificity).22
Analysis
We compared participant characteristics according to BMI categories by chi-square tests for categorical variables and analysis of variance for continuous variables. We calculated Pearson correlations (r) among the indices. For each index, we used Cox proportional hazards models to compute hazard ratios as the measure of the relative risks (RRs) and 95% confidence intervals (CIs) for CVD. Person-years of follow-up were calculated as the time from exposure assessment to development of the first endpoint of interest, censoring, or end of follow-up (March 31, 2006), whichever occurred first.
We considered three multivariable-adjusted models. Model 1 adjusted for potential confounders. In the PHS, these included age, physical activity, smoking, alcohol consumption, and parental history of myocardial infarction before the age of 60. In the WHS, these included age, physical activity, smoking, alcohol consumption, parental history of myocardial infarction before the age of 60, postmenopausal hormone use, race, education, and the dietary factors.
Anthropometric indices were evaluated by (1) comparing the strengths of the associations with CVD, and (2) comparing model fit as assessed by log-likelihoods. All log-likelihood comparisons involved models with constant sample sizes. For indices demonstrating the strongest associations and best model fit, we examined a second model adjusting for the confounders in Model 1 plus BMI. We compared nested models with and without BMI using the likelihood ratio test (LRT). In these models, the RRs for the indices reflect associations with CVD beyond those conveyed by BMI.
We examined a third model adjusting for the variables in Model 1 plus possible mediators of the association between adiposity and CVD (diabetes, elevated cholesterol, and hypertension). The RRs from this model reflect associations between the indices and CVD beyond those mediated through the overt development of these intermediates.
In sub-analyses, we excluded those with a history of smoking or cancer, those who developed the outcome or were censored early during follow-up (first 4 years in the PHS, 2 years in the WHS, given the shorter duration of follow-up in the WHS), and those with an absolute weight change ≥5% over the year preceding exposure assessment, to reduce bias due to potential confounding by smoking and preexisting disease.23 In other analyses, we excluded persons with BMI ≥35.0 kg/m2, given potential misclassification of risk at the highest BMI.
In stratified and joint models, we explored interactions between anthropometric indices and BMI, age, physical activity, or smoking status. To assess for statistically significant effect modification, we used the LRT contrasting age-adjusted models with and without interaction terms of interest.
We assessed for linear and curvilinear trends in the RR across categories of indices by including the relevant indices in models as continuous or quadratic variables, respectively, assigning median values to each category and comparing models using the LRT. Two-sided P-values were reported in all analyses. P-values <0.05 were considered statistically significant. All data analyses were performed using SAS Software Version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Participant characteristics are shown according to baseline BMI categories in Table 1. Men with higher BMI were younger, had higher WC, WHR, and WHtR indices, and were more likely to have a history of hypertension, diabetes, and high cholesterol. They were also more likely to smoke, consumed less alcohol, and were less physically active. Leaner women were more likely to be current smokers, to use postmenopausal hormones, had lower levels of trans-fat and omega-3 fatty acid intake, and had higher levels of polyunsaturated-to-saturated fat, glycemic load, folic acid, and cereal fiber intake.
Table 1.
| Table 1(a). Characteristics of the 16,332 men in the PHS according to BMI at 9 years | ||||||
|---|---|---|---|---|---|---|
| Body Mass Index (kg/m2) |
P* | |||||
| <22.5 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | ≥30.0 | ||
| Number of men | 2789 | 5545 | 4893 | 2006 | 1099 | |
| Mean age ± SD | 63.1 ± 10.0 | 61.7 ± 8.9 | 61.1 ± 8.3 | 60.9 ± 7.8 | 59.7 ± 7.5 | <0.001 |
| Mean weight, kg (lb) | 67.6 (150.2) | 75.4 (167.5) | 82.6 (183.6) | 91.5 (203.4) | 103.1 (229.1) | <0.001 |
| Mean BMI (kg/m2) | 21.3 | 23.8 | 26.1 | 28.6 | 32.6 | <0.001 |
| Mean WC ± SD (in) | 34.3 ± 2.3 | 36.4 ± 2.3 | 38.6 ± 2.4 | 41.3 ± 2.6 | 44.8 ± 3.7 | <0.001 |
| Mean WHR ± SD | 0.92 ± 0.07 | 0.93 ± 0.06 | 0.95 ± 0.06 | 0.97 ± 0.06 | 0.98 ± 0.07 | <0.001 |
| Mean WHtR ± SD | 0.49 ± 0.03 | 0.52 ± 0.03 | 0.55 ± 0.03 | 0.58 ± 0.04 | 0.64 ± 0.05 | <0.001 |
| Hypertension†(%) | 29.5 | 33.4 | 39.3 | 46.1 | 57.4 | <0.001 |
| Diabetes mellitus (%) | 3.3 | 3.3 | 4.1 | 4.2 | 9.6 | <0.001 |
| High cholesterol ‡(%) | 20.4 | 22.7 | 24.7 | 24.7 | 23.0 | <0.001 |
| Prior cancer (%) | 5.4 | 5.2 | 4.3 | 4.7 | 3.7 | 0.06 |
| Smoking status (%) | <0.001 | |||||
| Never | 53.7 | 52.0 | 48.1 | 47.6 | 43.2 | |
| Past | 39.2 | 42.2 | 45.3 | 45.5 | 48.5 | |
| Current | 7.1 | 5.8 | 6.6 | 6.9 | 8.3 | |
| Exercise ≥3 times/week (%) | 50.5 | 47.4 | 40.5 | 32.4 | 26.3 | <0.001 |
| Alcohol intake ≥1 drink/d (%) | 21.2 | 18.7 | 17.6 | 16.7 | 11.7 | <0.001 |
| Parental history of MI at <60 yrs (%) | 9.1 | 8.7 | 8.7 | 10.4 | 11.2 | 0.02 |
| Table 1(b). Characteristics of the 32,700 women in the WHS according to BMI at 6 years | ||||||
|---|---|---|---|---|---|---|
| Body Mass Index (kg/m2) |
P* | |||||
| <22.5 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | ≥30.0 | ||
| Number of women | 6792 | 7421 | 6849 | 4160 | 7478 | |
| Mean age ± SD | 61.2 ± 7.6 | 60.9 ± 7.0 | 60.9 ± 6.9 | 60.7 ± 6.7 | 59.7 ± 6.3 | <0.001 |
| Mean weight, kg (lb) | 56.1 (124.7) | 63.7 (141.6) | 70.4 (156.4) | 77.3 (171.7) | 91.8 (204.1) | <0.001 |
| Mean BMI (kg/m2) | 20.9 | 23.8 | 26.3 | 28.8 | 34.5 | <0.001 |
| Mean WC ± SD (in) | 29.7 ± 2.9 | 32.5 ± 3.0 | 35.0 ± 3.2 | 37.5 ± 3.3 | 41.9 ± 4.5 | <0.001 |
| Mean WHR ± SD | 0.79 ± 0.07 | 0.82 ± 0.07 | 0.84 ± 0.07 | 0.86 ± 0.07 | 0.87 ± 0.07 | <0.001 |
| Mean WHtR ± SD | 0.46 ± 0.04 | 0.50 ± 0.05 | 0.54 ± 0.05 | 0.58 ± 0.05 | 0.65 ± 0.07 | <0.001 |
| Hypertension†(%) | 25.4 | 32.0 | 40.7 | 48.1 | 62.3 | <0.001 |
| Diabetes mellitus (%) | 1.7 | 2.5 | 3.6 | 5.7 | 12.7 | <0.001 |
| High cholesterol ‡(%) | 27.9 | 32.9 | 35.5 | 39.9 | 39.6 | <0.001 |
| Prior cancer (%) | 4.1 | 3.8 | 3.6 | 3.4 | 3.3 | 0.16 |
| Postmenopausal hormone use (%) | <0.001 | |||||
| Never | 24.6 | 22.8 | 24.7 | 26.4 | 30.8 | |
| Past | 12.9 | 12.6 | 13.6 | 14.1 | 14.4 | |
| Current | 62.5 | 64.6 | 61.6 | 59.5 | 54.7 | |
| Smoking status (%) | <0.001 | |||||
| Never | 52.1 | 51.6 | 51.5 | 51.3 | 52.8 | |
| Past | 36.1 | 39.6 | 39.5 | 40.6 | 40.2 | |
| Current | 11.8 | 8.8 | 9.0 | 8.1 | 6.9 | |
| Exercise ≥4 times/week (%) | 16.7 | 12.4 | 10.5 | 9.4 | 6.3 | <0.001 |
| Alcohol intake ≥1 drink/d (%) | 6.8 | 5.7 | 4.7 | 3.9 | 2.4 | <0.001 |
| Dietary intake§(%) | ||||||
| Trans-fat (≥3.0 g/d) | 16.3 | 17.0 | 18.9 | 20.0 | 25.0 | <0.001 |
| N-3 fatty acid (≥1.7 g/d) | 18.6 | 19.5 | 19.0 | 21.8 | 20.7 | <0.001 |
| PUFA/saturated fat (≥0.7) | 22.3 | 20.6 | 19.2 | 19.3 | 15.8 | <0.001 |
| Glycemic load (≥135/d) | 24.8 | 22.2 | 18.5 | 17.6 | 15.2 | <0.001 |
| Folic acid (≥618µg/d) | 21.8 | 20.7 | 19.6 | 18.1 | 16.7 | <0.001 |
| Cereal fiber (≥5.4 g/d) | 21.3 | 19.8 | 19.7 | 18.9 | 19.3 | 0.01 |
| Parental history of MI before age 60 years (%) | 11.0 | 11.6 | 12.7 | 14.8 | 14.0 | <0.001 |
| Race/ethnicity (%) | <0.001 | |||||
| Black/African American | 0.6 | 1.1 | 2.2 | 3.0 | 3.3 | |
| White/Non-Hispanic | 95.2 | 95.3 | 94.4 | 93.9 | 93.6 | |
| Other/unknown | 4.1 | 3.6 | 3.3 | 3.1 | 3.0 | |
| Highest level of education (%) | <0.001 | |||||
| Less than bachelor’s degree | 51.0 | 52.5 | 56.5 | 57.3 | 61.3 | |
| Bachelor’s degree | 24.0 | 25.1 | 23.8 | 24.2 | 21.7 | |
| Master’s degree or doctorate | 24.9 | 22.4 | 19.7 | 18.4 | 17.0 | |
BMI = body mass index; WHtR = waist-to-height ratio; WC = waist circumference; WHR = waist-to-hip ratio.
P-values are from Pearson chi square tests for categorical variables and analysis of variance for continuous variables.
Hypertension defined as systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg, or antihypertensive medication use prior to 9 years).
High cholesterol defined as level ≥240 mg/dl or lipid-lowering medication use prior to 7 years.
BMI = body mass index; WHtR = waist-to-height ratio; WC = waist circumference; WHR = waist-to-hip ratio.
P-values are from Pearson chi square tests for categorical variables and analysis of variance for continuous variables.
Hypertension defined as systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg, or diagnosis of hypertension by a clinician prior to 6 years.
High cholesterol defined as a diagnosis of high cholesterol [≥240 mg/dl] or cholesterol-lowering medication use prior to 6 years.
Levels refer to highest quintile of energy-adjusted dietary intake.
BMI most strongly correlated with WC (r=0.78 for men, 0.82 for women) and WHtR (r=0.80, 0.84), as well as weight (r=0.86, 0.93).
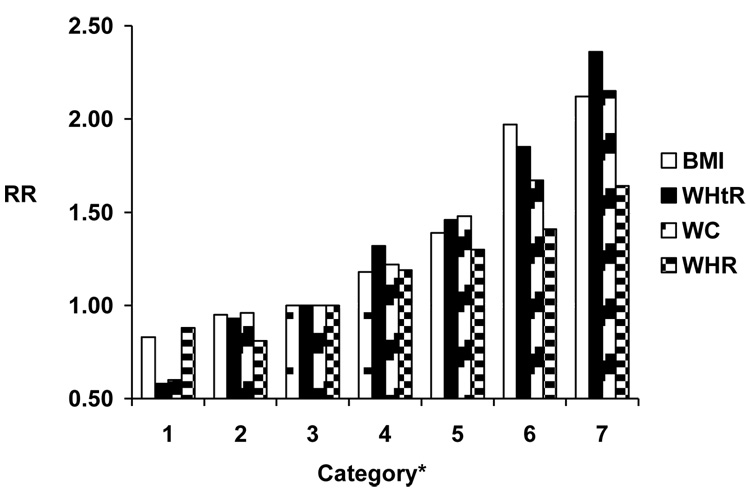
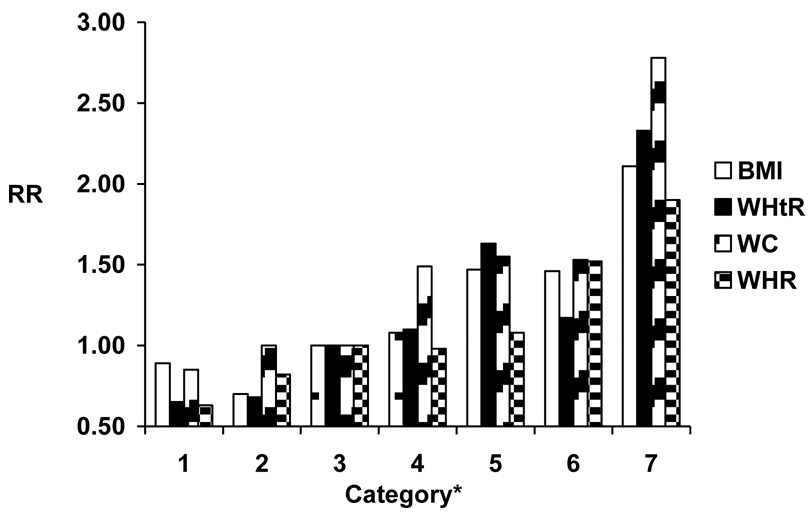
A total of 1505 cases of major CVD occurred in men after a median (SD) follow-up of 14.2 (3.4) years (606 nonfatal myocardial infarctions, 604 nonfatal ischemic strokes, and 295 fatal CVD deaths), and 414 cases (174 nonfatal myocardial infarctions, 182 nonfatal ischemic strokes, and 58 fatal CVD cases) occurred in women after 5.5 (0.9) years. For all indices, higher values were associated linearly with increasing risk of CVD, among both men and women and in both age- and multivariable-adjusted models (Table 2,Table 3). Overall, associations with CVD did not vary substantially among the indices, although associations were somewhat weaker for WHR, particularly among men (Figure 1). We also found similar measures of model fit among the various indices.
Table 2.
| Table 2 - Relative risks (95% confidence intervals) for cardiovascular disease according to categories of anthropometric indices among 16,332 men | |||||||
|---|---|---|---|---|---|---|---|
| (a) | |||||||
| Body Mass Index (kg/m2) |
|||||||
| <20.0 | 20.0–22.4 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | ≥35.0 | |
| No. of men | 294 | 2495 | 5545 | 4893 | 2006 | 925 | 174 |
| No. of cases | 25 | 205 | 456 | 454 | 217 | 126 | 22 |
| Rate (No./1000 p-y) | 7.5 | 6.6 | 6.4 | 7.3 | 8.6 | 11.2 | 10.8 |
| Age-adjusted | 0.88 | 0.96 | 1.00 | 1.21 | 1.46 | 2.12 | 2.35 |
| (0.59–1.32) | (0.81–1.13) | (1.06–1.37) | (1.24–1.72) | (1.74–2.58) | (1.53–3.61) | ||
| −2logL=28105 | |||||||
| Model 1* | 0.83 | 0.95 | 1.00 | 1.18 | 1.39 | 1.97 | 2.12 |
| (0.55–1.24) | (0.80–1.12) | (1.04–1.35) | (1.18–1.64) | (1.61–2.41) | (1.36–3.30) | ||
| −2logL=27669 | |||||||
| Model 1+WHtR† | 1.08 | 1.06 | 1.00 | 1.05 | 1.14 | 1.49 | 1.42 |
| (0.70–1.67) | (0.89–1.27) | (0.91–1.21) | (0.92–1.39) | (1.14–1.95) | (0.81–2.46) | ||
| −2logL=27648 | |||||||
| Model 1+WC† | 0.99 | 1.01 | 1.00 | 1.09 | 1.20 | 1.65 | 1.70 |
| (0.65–1.53) | (0.85–1.21) | (0.95–1.26) | (0.99–1.46) | (1.27–2.15) | (0.99–2.94) | ||
| −2logL=27657 | |||||||
| LRT for Model 1 vs Model 1+WHtR: P=0.002 | |||||||
| LRT for Model 1 vs Model 1+ WC: P=0.07 | |||||||
| Table 2(b) | |||||||
|---|---|---|---|---|---|---|---|
| Waist-to-Height Ratio |
|||||||
| <0.45 | 0.45-<0.49 | 0.49-<0.53 | 0.53-<0.58 | 0.58-<0.62 | 0.62-<0.69 | ≥0.69 | |
| No. of men | 288 | 2454 | 5558 | 4921 | 2034 | 900 | 177 |
| No. of cases | 11 | 150 | 412 | 520 | 251 | 132 | 29 |
| Rate (No./1000 p-y) | 2.9 | 4.7 | 5.8 | 8.4 | 10.1 | 12.3 | 14.6 |
| Age-adjusted | 0.56 | 0.92 | 1.00 | 1.33 | 1.54 | 1.99 | 2.60 |
| (0.31–1.03) | 0.76–1.11) | (1.17–1.51) | (1.32–1.81) | (1.64–2.42) | (1.78–3.79) | ||
| −2logL=28086 | |||||||
| Model 1* | 0.58 | 0.93 | 1.00 | 1.32 | 1.46 | 1.85 | 2.36 |
| (0.32–1.05) | (0.77–1.12) | (1.16–1.50) | (1.25–1.72) | (1.51–2.26) | (1.61–3.47) | ||
| −2logL=27657 | |||||||
| Model 1+BMI† | 0.56 | 0.92 | 1.00 | 1.29 | 1.33 | 1.48 | 1.73 |
| (0.30–1.04) | (0.75–1.13) | (1.11–1.49) | (1.09–1.62) | (1.13–1.94) | (1.05–2.83) | ||
| −2logL=27648 | |||||||
| LRT for Model 1 vs Model 1+BMI: P=0.18 | |||||||
| Table 2(c) | |||||||
|---|---|---|---|---|---|---|---|
| Waist Circumference (in) |
|||||||
| 22.0–31.25 | 31.5–34.25 | 34.5–37.25 | 37.5–40.75 | 41.0–43.5 | 43.75–48.0 | 48.25–62.0 | |
| No. of men | 283 | 2475 | 5285 | 5056 | 2090 | 950 | 193 |
| No. of cases | 12 | 169 | 403 | 508 | 259 | 128 | 26 |
| Rate (No./1000 p-y) | 3.3 | 5.3 | 5.9 | 8.0 | 10.1 | 11.2 | 11.9 |
| Age-adjusted | 0.59 | 0.95 | 1.00 | 1.25 | 1.57 | 1.81 | 2.32 |
| (0.33–1.05) | (0.79–1.14) | (1.10–1.42) | (1.34–1.83) | (1.48–2.21) | (1.56–3.45) | ||
| −2logL=28104 | |||||||
| Model 1* | 0.60 | 0.96 | 1.00 | 1.22 | 1.48 | 1.67 | 2.15 |
| (0.33–1.06) | (0.80–1.15) | (1.07–1.40) | (1.27–1.74) | (1.37–2.05) | (1.44–3.21) | ||
| −2logL=27671 | |||||||
| Model 1+BMI† | 0.61 | 0.97 | 1.00 | 1.16 | 1.28 | 1.24 | 1.38 |
| (0.33–1.10) | 0.80–1.18) | (1.01–1.34) | (1.06–1.55) | (0.95–1.62) | (0.83–2.27) | ||
| −2logL=27657 | |||||||
| LRT for Model 1 vs Model 1+BMI: P=0.03 | |||||||
| Table 2(d) | |||||||
|---|---|---|---|---|---|---|---|
| Waist-to-Hip Ratio |
|||||||
| <0.83 | 0.83-<0.89 | 0.89-<0.94 | 0.94-<0.99 | 0.99-<1.03 | 1.03-<1.11 | ≥1.11 | |
| No. of men | 269 | 2491 | 5593 | 4870 | 2010 | 924 | 175 |
| No. of cases | 16 | 143 | 443 | 507 | 251 | 122 | 23 |
| Rate (No./1000 p-y) | 4.6 | 4.4 | 6.1 | 8.4 | 10.2 | 11.2 | 10.8 |
| Age-adjusted | 0.83 | 0.80 | 1.00 | 1.20 | 1.36 | 1.51 | 1.69 |
| (0.50–1.36) | (0.66–0.97) | (1.06–1.36) | (1.16–1.59) | (1.23–1.84) | (1.11–2.58) | ||
| −2logL=28133 | |||||||
| Model 1* | 0.88 | 0.81 | 1.00 | 1.19 | 1.30 | 1.41 | 1.64 |
| (0.53–1.45) | (0.67–0.98) | (1.04–1.35) | (1.11–1.52) | (1.15–1.73) | (1.07–2.52) | ||
| −2logL=27694 | |||||||
| Table 2(e). Multivariable model adjusting for the variables in Model 1* plus history of diabetes, hypertension, or elevated cholesterol | |||||||
|---|---|---|---|---|---|---|---|
| Category |
|||||||
| BMI (kg/m2) | <20.0 | 20.0–22.4 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | ≥35.0 |
| −2logL=27513 | 0.86 | 0.98 | 1.00 | 1.12 | 1.30 | 1.65 | 1.65 |
| (0.57–1.29) | (0.83–1.16) | (0.98–1.28) | (1.10–1.53) | (1.34–2.02) | (1.06–2.58) | ||
| WHtR | <0.45 | 0.45-<0.49 | 0.49-<0.53 | 0.53-<0.58 | 0.58-<0.62 | 0.62-<0.69 | >0.69 |
| −2logL=27505 | 0.62 | 0.97 | 1.00 | 1.25 | 1.32 | 1.58 | 1.90 |
| (0.34–1.12) | (0.80–1.17) | (1.10–1.43) | (1.13–1.56) | (1.29–1.93) | (1.29–2.80) | ||
| WC (in) | 22.0–31.25 | 31.5–34.25 | 34.5–37.25 | 37.5–40.75 | 41.0–43.5 | 43.75–48.0 | 48.25–62.0 |
| −2logL=27514 | 0.64 | 0.98 | 1.00 | 1.18 | 1.36 | 1.46 | 1.61 |
| (0.36–1.14) | (0.82–1.18) | (1.03–1.34) | (1.16–1.60) | (1.19–1.79) | (1.07–2.40) | ||
| WHR | <0.83 | 0.83-<0.89 | 0.89-<0.94 | 0.94-<0.99 | 0.99-<1.03 | 1.03-<1.11 | >1.11 |
| −2logL=27525 | 0.88 | 0.85 | 1.00 | 1.13 | 1.20 | 1.26 | 1.60 |
| (0.53–1.45) | (0.70–1.02) | (0.99–1.28) | (1.03–1.41) | (1.03–1.55) | (1.04–2.46) | ||
BMI = body mass index; WHtR = waist-to-height ratio; WC = waist circumference; WHR = waist-to-hip ratio.
Model 1 adjusts for age, physical activity, smoking, alcohol consumption, and parental history of myocardial infarction before the age of 60 years.
“Model 1 + WHtR,” “Model 1 + WC,” “Model 1 + BMI” refer to models adjusting for the variables in Model 1 plus waist-to-height ratio, waist circumference, or body mass index in 7 categories at 9 years, respectively.
| Table 3 - Relative risks (95% confidence intervals) for cardiovascular disease according to categories of anthropometric indices among 32,700 women | |||||||
|---|---|---|---|---|---|---|---|
| (a) | |||||||
| Body Mass Index (kg/m2) |
|||||||
| <20.0 | 20.0–22.4 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | ≥35.0 | |
| No. of women | 1512 | 5280 | 7421 | 6849 | 4160 | 4938 | 2540 |
| No. of cases | 20 | 44 | 82 | 82 | 66 | 72 | 48 |
| Rate (No./1000 p-y) | 2.6 | 1.6 | 2.1 | 2.3 | 3.0 | 2.8 | 3.6 |
| Age-adjusted | 1.03 | 0.75 | 1.00 | 1.10 | 1.50 | 1.47 | 2.22 |
| (0.63–1.69) | (0.52–1.08) | (0.81–1.49) | (1.09–2.08) | (1.07–2.03) | (1.55–3.18) | ||
| −2logL=8282 | |||||||
| Model 1* | 0.89 | 0.70 | 1.00 | 1.08 | 1.47 | 1.46 | 2.11 |
| (0.54–1.46) | (0.49–1.02) | (0.80–1.47) | (1.06–2.03) | (1.06–2.01) | (1.46–3.05) | ||
| −2logL=8163 | |||||||
| Model 1+WHtR† | 1.24 | 0.82 | 1.00 | 0.98 | 1.27 | 1.18 | 1.38 |
| (0.70–2.19) | (0.56–1.22) | (0.71–1.36) | (0.88–1.84) | (0.78–1.78) | (0.81–2.34) | ||
| −2logL=8144 | |||||||
| Model 1+WC† | 1.04 | 0.77 | 1.00 | 1.00 | 1.30 | 1.18 | 1.38 |
| (0.59–1.82) | (0.52–1.13) | (0.73–1.38) | (0.90–1.88) | (0.79–1.77) | (0.83–2.30) | ||
| −2logL=8151 | |||||||
| LRT for Model 1 vs. Model 1+WHtR: P=0.004 | |||||||
| LRT for Model 1 vs. Model 1+WC: P=0.06 | |||||||
| Table 3(b) | |||||||
|---|---|---|---|---|---|---|---|
| Waist-to-Height Ratio |
|||||||
| <0.42 | 0.42-<0.47 | 0.47-<0.52 | 0.52-<0.57 | 0.57-<0.61 | 0.61-<0.68 | ≥0.68 | |
| No. of women | 1524 | 5347 | 7305 | 6891 | 4076 | 5013 | 2544 |
| No. of cases | 9 | 35 | 78 | 85 | 75 | 68 | 64 |
| Rate (No./1000 p-y) | 1.1 | 1.3 | 2.0 | 2.4 | 3.5 | 2.6 | 4.9 |
| Age-adjusted | 0.65 | 0.67 | 1.00 | 1.10 | 1.61 | 1.19 | 2.42 |
| (0.33–1.30) | (0.45–1.00) | (0.81–1.49) | (1.17–1.21) | (0.86–1.65) | (1.74–3.37) | ||
| −2logL=8265 | |||||||
| Model 1* | 0.65 | 0.68 | 1.00 | 1.10 | 1.63 | 1.17 | 2.33 |
| (0.33–1.31) | (0.46–1.02) | (0.81–1.49) | (1.19–2.25) | (0.84–1.63) | (1.66–3.28) | ||
| −2logL=8150 | |||||||
| Model 1+BMI† | 0.60 | 0.69 | 1.00 | 1.03 | 1.43 | 0.96 | 1.78 |
| (0.28–1.28) | (0.45–1.06) | (0.74–1.42) | (0.99–2.07) | (0.63–1.46) | (1.08–2.93) | ||
| −2logL=8144 | |||||||
| LRT for Model 1 vs Model 1+BMI: P=0.46 | |||||||
| Table 3(c) | |||||||
|---|---|---|---|---|---|---|---|
| Waist Circumference (in) | |||||||
| 20.0–27.0 | 27.25–30.0 | 30.25–33.25 | 33.5–36.5 | 36.75–38.75 | 39.0–43.75 | 44.0–55.0 | |
| No. of women | 1480 | 5114 | 7112 | 7158 | 3743 | 5426 | 2667 |
| No. of cases | 10 | 42 | 63 | 99 | 55 | 80 | 65 |
| Rate (No./1000 p-y) | 1.3 | 1.6 | 1.7 | 2.7 | 2.8 | 2.8 | 4.7 |
| Age-adjusted | 0.86 | 0.99 | 1.00 | 1.49 | 1.56 | 1.55 | 2.94 |
| (0.44–1.68) | (0.67–1.46) | (1.08–2.04) | (1.09–2.24) | (1.11–2.15) | (2.08–4.16) | ||
| −2logL=8272 | |||||||
| Model 1* | 0.85 | 1.00 | 1.00 | 1.49 | 1.55 | 1.53 | 2.78 |
| (0.43–1.65) | (0.68–1.49) | (1.09–2.05) | (1.08–2.23) | (1.09–2.14) | (1.95–3.97) | ||
| −2logL=8157 | |||||||
| Model 1+BMI† | 0.87 | 1.06 | 1.00 | 1.37 | 1.33 | 1.22 | 2.08 |
| (0.42–1.80) | (0.70–1.61) | (0.98–1.91) | (0.90–1.99) | (0.81–1.85) | (1.27–3.40) | ||
| −2logL=8151 | |||||||
| LRT for Model 1 vs Model 1+BMI: P=0.39 | |||||||
| Table 3(d) | |||||||
|---|---|---|---|---|---|---|---|
| Waist-to-Hip Ratio |
|||||||
| <0.72 | 0.72-<0.77 | 0.77-<0.82 | 0.82-<0.86 | 0.86-<0.89 | 0.89-<0.95 | ≥0.95 | |
| No. of women | 1506 | 5234 | 7454 | 6846 | 4203 | 4921 | 2536 |
| No. of cases | 8 | 39 | 76 | 75 | 53 | 95 | 68 |
| Rate (No./1000 p-y) | 1.0 | 1.4 | 1.9 | 2.1 | 2.4 | 3.7 | 5.2 |
| Age-adjusted | 0.60 | 0.77 | 1.00 | 1.01 | 1.11 | 1.59 | 2.06 |
| (0.29–1.24) | (0.53–1.14) | (0.73–1.39) | (0.78–1.58) | (1.18–2.16) | (1.48–2.86) | ||
| −2logL=8277 | |||||||
| Model 1* | 0.64 | 0.82 | 1.00 | 0.98 | 1.08 | 1.52 | 1.90 |
| (0.31–1.32) | (0.56–1.21) | (0.71–1.35) | (0.76–1.53) | 1.12–2.06) | (1.37–2.65) | ||
| −2logL=8165 | |||||||
| Table 3(e). Multivariable model adjusting for the variables in Model 1* plus history of diabetes, hypertension, or elevated cholesterol | |||||||
|---|---|---|---|---|---|---|---|
| Index Category, Relative Risk (95% CI) |
|||||||
| BMI (kg/m2) | <20.0 | 20.0–22.4 | 22.5–24.9 | 25.0–27.4 | 27.5–29.9 | 30.0–34.9 | ≥35.0 |
| −2logL=8106 | 0.95 | 0.75 | 1.00 | 1.03 | 1.31 | 1.18 | 1.51 |
| (0.58–1.56) | (0.52–1.08) | (0.76–1.40) | (0.94–1.82) | (0.85–1.63) | (1.03–2.21) | ||
| WHtR | <0.42 | 0.42-<0.47 | 0.47-<0.52 | 0.52-<0.57 | 0.57-<0.61 | 0.61-<0.68 | ≥0.68 |
| −2logL=8095 | 0.70 | 0.72 | 1.00 | 1.01 | 1.43 | 0.95 | 1.66 |
| (0.35–1.40) | (0.48–1.08) | (0.74–1.38) | (1.03–1.97) | (0.68–1.33) | (1.17–2.37) | ||
| WC (in) | 20.0–27.0 | 27.25–30.0 | 30.25–33.25 | 33.5–36.5 | 36.75–38.75 | 39.0–43.75 | 44.0–55.0 |
| −2logL=8102 | 0.90 | 1.07 | 1.00 | 1.40 | 1.37 | 1.25 | 2.00 |
| (0.46–1.76) | (0.72–1.58) | (1.02–1.92) | (0.95–1.97) | (0.89–1.76) | (1.38–2.90) | ||
| WHR | <0.72 | 0.72-<0.77 | 0.77-<0.82 | 0.82-<0.86 | 0.86-<0.89 | 0.89-<0.95 | ≥0.95 |
| −2logL=8104 | 0.71 | 0.88 | 1.00 | 0.93 | 0.96 | 1.28 | 1.51 |
| (0.34–1.47) | (0.60–1.30) | (0.67–1.28) | (0.67–1.37) | (0.94–1.75) | (1.08–2.12) | ||
BMI = body mass index; WHtR = waist-to-height ratio; WC = waist circumference; WHR = waist-to-hip ratio.
Model 1 adjusts for age, physical activity, smoking, alcohol consumption, parental history of myocardial infarction before the age of 60 years, postmenopausal hormone use, race, education, and dietary factors.
“Model 1 + WHtR,” “Model 1 + WC,” “Model 1 + BMI” refer to models adjusting for the variables in Model 1 plus waist-to-height ratio, waist circumference, or body mass index in 7 categories at 6 years, respectively.
Figure 1.
Figure 1a. Relative risk (RR) of cardiovascular disease according to anthropometric indices among men Model adjusts for age, physical activity, smoking, alcohol consumption, and parental history of myocardial infarction before the age of 60.
*BMI (body mass index) categories: <20.0, 20.0-22.4, 22.5-24.9, 25.0-27.4, 27.5-29.9, 30.0-34.9, ≥35.0 kg/m2. WHtR (waist-to-height ratio) categories: <0.45, 0.45-<0.49, 0.49-<0.53, 0.53-<0.58, 0.58-<0.62, 0.62-<0.69, ≥0.69. WC (waist circumference) categories: 22.0-31.25, 31.5-34.25, 34.5-37.25, 37.5-40.75, 41.0-43.5, 43.75-48.0, 48.25-62.0 in. WHR (waist-to-height ratio) categories: <0.83, 0.83-<0.89, 0.89-<0.94, 0.94-<0.99, 0.99-<1.03, 1.03-<1.11, ≥1.11.
Figure 1b. Relative risk (RR) of cardiovascular disease according to anthropometric indices among women Model adjusts for age, physical activity, smoking, alcohol consumption, parental history of myocardial infarction before the age of 60 years, postmenopausal hormone use, race, education, and dietary factors.
*BMI (body mass index) categories: <20.0, 20.0-22.4, 22.5-24.9, 25.0-27.4, 27.5-29.9, 30.0-34.9, ≥35.0 kg/m2. WHtR (waist-to-height ratio) categories: <0.42, 0.42-<0.47, 0.47-<0.52,0.52-<0.57, 0.57-<0.61, 0.61-<0.68, ≥0.68. WC (waist circumference) categories: 20.0-27.0, 27.25-30.0, 30.25-33.25, 33.5-36.5, 36.75-38.75, 39.0-43.75, 44.0-55.0 in. WHR (waist-to-height ratio) categories: <0.72, 0.72-<0.77, 0.77-<0.82, 0.82-<0.86, 0.86-<0.89, 0.89-<0.95,≥0.95.
Among men, WHtR demonstrated the strongest gradient in the association with CVD, followed by WC, BMI, and WHR (Figure 1a, Table 2b). Measures of model fit were overall similar, with the best fit (lowest log-likelihood) seen using the WHtR (Table 2). Results were generally similar for women. Both WHtR and WC demonstrated the strongest gradient in the association with CVD, with weaker associations for BMI and WHR (Figure 1b, Table 3b). Measures of model fit were overall similar, with the best fit seen using the WHtR (Table 3). We found similar results examining indices as continuous variables (data not shown).
Adding BMI to models with WHtR did not significantly improve model fit (P=0.18 for men, 0.46 for women). Although the RRs for WHtR were attenuated after additionally adjusting for BMI, higher WHtR remained associated with increased risk of CVD (Table 2b,Table 3b). By contrast, adding WHtR to models with BMI did improve model fit and substantially attenuated the associations (Table 2a,Table 3a).
Additionally adjusting for the potential intermediates in the association between adiposity and CVD generally attenuated the RRs for the various indices, however, the overall associations remained comparable and statistically significant (Table 2e,Table 3e).
We found similar results excluding participants with BMI ≥35.0 kg/m2 (data not shown).
Higher WHtR remained associated with increased risk of CVD after excluding persons with a history of smoking or cancer, unstable weight during the year prior to WHtR assessment, or with ≤4 (PHS) or ≤2 (WHS) years of follow-up (data not shown). By contrast, higher BMI was less strongly associated with increasing risk of CVD in this subgroup with a history of smoking or cancer or unstable weight. The RR (95% CI) was 1.43 (0.91–2.26) among obese (BMI ≥30.0 kg/m2) men, and 1.68 (0.90–3.14) among obese women, as compared to persons with BMI <25.0 kg/m2.
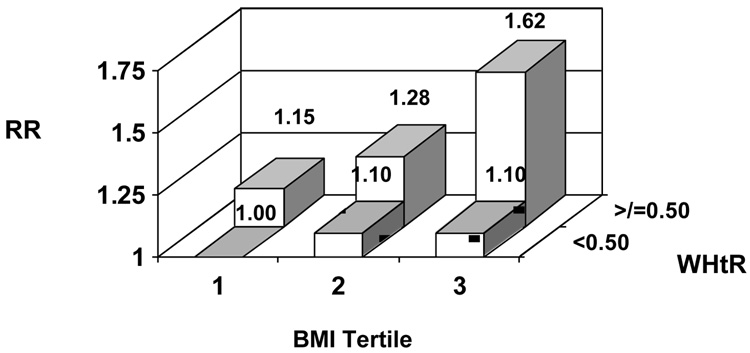
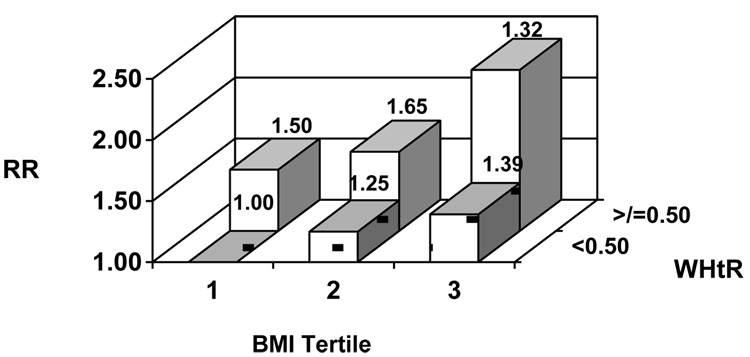
Examining the joint effects of BMI and WHtR, higher WHtR appeared strongly associated with CVD risk within BMI categories (Figures 2a, b). In analyses stratified by BMI (</≥ 25.0 kg/m2), we did not find evidence for effect modification by BMI (LRT P=0.74 for men, 0.88 for women).
Figure 2.
Figure 2a. Relative risk (RR) of cardiovascular disease according to body mass index (BMI) and waist-to-height ratio (WHtR) among men Model adjusts for age, physical activity, smoking, alcohol consumption, and parental history of myocardial infarction before the age of 60.
Figure 2b. Relative risk (RR) of cardiovascular disease according to body mass index (BMI) and waist-to-height ratio (WHtR) among women Model adjusts for age, physical activity, smoking, alcohol consumption, parental history of myocardial infarction before the age of 60 years, postmenopausal hormone use, race, education, and dietary factors.
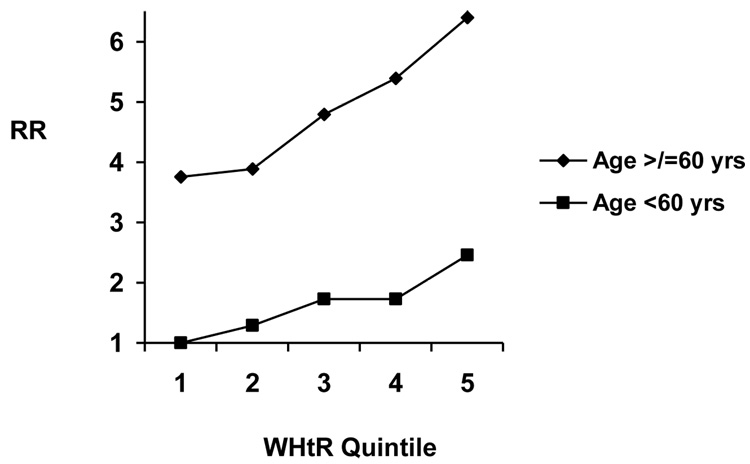
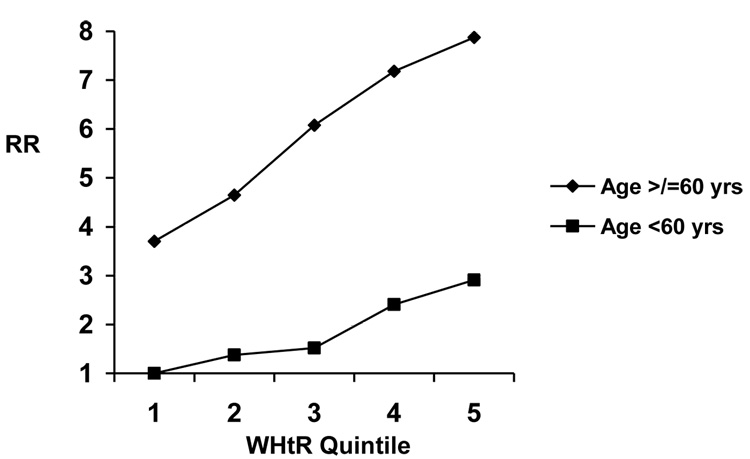
Higher WHtR and BMI were associated linearly with increased CVD risk among both younger and older men and women (Table 4). Although associations for both WHtR and BMI appeared stronger among younger participants, this finding was not statistically significant. Examining the joint effects of age and WHtR, those ≥60 years consistently had the highest RR of CVD, across all WHtR categories, as compared to younger persons (Figures 3a, b). We also did not find evidence for effect modification by physical activity (LRT P=0.25 for men, 0.51 for women) or smoking status (LRT P=0.99 for men, 0.96 for women).
| Table 4(a) - Relative risks (and 95% confidence intervals) for cardiovascular disease according to waist-to-height ratio and age among men | |||||
|---|---|---|---|---|---|
| Waist-to-Height Ratio Quintiles |
|||||
| <0.50 | 0.50-<0.52 | 0.52-<0.54 | 0.54-<0.58 | ≥0.58 | |
| Age <60 years | 1892 | 1700 | 1508 | 1444 | 1383 |
| No. of cases | 56 | 65 | 81 | 78 | 109 |
| Model 1*(n=7840) | 1.00 | 1.24 (0.86–1.77) | 1.59 (1.13–2.25) | 1.61 (1.14–2.29) | 2.20 (1.57–3.07) |
| −2logL=6752 | |||||
| Age ≥60 years | 1390 | 1574 | 1712 | 1852 | 1877 |
| No. of cases | 141 | 166 | 223 | 267 | 319 |
| Model 1*(n=8307) | 1.00 | 1.00 (0.79–1.25) | 1.18 (0.96–1.47) | 1.33 (1.08–1.64) | 1.57 (1.28–1.92) |
| −2logL = 19209 | |||||
|
Body Mass Index Quintiles (kg/m2) |
|||||
| <22.8 | 22.8-<24.3 | 24.3-<25.6 | 25.6-<27.4 | ≥27.4 | |
| Age <60 years | 1454 | 1534 | 1644 | 1618 | 1677 |
| No. of cases | 41 | 62 | 73 | 87 | 126 |
| Model 1*(n=7840) | 1.00 | 1.39 (0.94–2.07) | 1.50 (1.02–2.21) | 1.71 (1.18–2.49) | 2.30 (1.61–3.29) |
| −2logL = 6751 | |||||
| Age ≥60 years | 1839 | 1702 | 1623 | 1674 | 1567 |
| No. of cases | 222 | 200 | 214 | 230 | 250 |
| Model 1*(n=8307) | 1.00 | 1.05 (0.87–1.28) | 1.15 (0.95–1.40) | 1.29 (1.07–1.56) | 1.58 (1.31–1.91) |
| −2logL = 19213 | |||||
| Table 4(b) - Relative risks (and 95% confidence intervals) for cardiovascular disease according to waist-to-height ratio and age among women | |||||
|---|---|---|---|---|---|
| Waist-to-Height Ratio Quintile | |||||
| <0.47 | 0.47-<0.52 | 0.52-<0.56 | 0.56-<0.62 | ≥0.62 | |
| Age <60 years | 4018 | 3736 | 3457 | 3327 | 3442 |
| No. of cases | 13 | 17 | 18 | 28 | 36 |
| Model 1*(n=17,973) | 1.00 | 1.25 (0.60–2.57) | 1.38 (0.67–2.83) | 2.06 (1.06–4.01) | 2.44 (1.27–4.68) |
| −2logL = 2058 | |||||
| Age >60 years | 2466 | 2847 | 3080 | 3234 | 3093 |
| No. of cases | 31 | 45 | 63 | 78 | 85 |
| Model 1*(n=14,709) | 1.00 | 1.24 (0.78–1.96) | 1.60 (1.03–2.46) | 1.91 (1.26–2.92) | 2.15 (1.41–3.28) |
| −2logL= 5570 | |||||
|
Body Mass Index Quintiles (kg/m2) |
|||||
| <22.5 | 22.5-<24.7 | 24.7-<27.1 | 27.1-<30.7 | ≥30.7 | |
| Age <60 years | 3492 | 3543 | 3438 | 3562 | 3945 |
| No. of cases | 8 | 15 | 24 | 27 | 38 |
| Model 1*(n=17,973) | 1.00 | 1.77 (0.75–4.19) | 2.79 (1.25–6.23) | 2.93 (1.32–6.50) | 3.55 (1.63–7.73) |
| −2logL = 2054 | |||||
| Age ≥60 years | 3044 | 2960 | 3137 | 3016 | 2563 |
| No. of cases | 54 | 51 | 57 | 73 | 67 |
| Model 1*(n=14,709) | 1.00 | 1.13 (0.77–1.66) | 1.22 (0.83–1.78) | 1.67 (1.16–2.40) | 1.93 (1.32–2.81) |
| −2logL = 5572 | |||||
Model 1 adjusts for age, physical activity, smoking, alcohol consumption, and parental history of myocardial infarction before the age of 60 years. P-value from LRT comparing age-adjusted models with and without interaction terms between age (</≥60 years) and WHtR (quintiles) = 0.26.
Model 1 adjusts for age, physical activity, smoking, alcohol consumption, parental history of myocardial infarction before the age of 60 years, postmenopausal hormone use, race, education, and dietary factors. P-value from LRT comparing age-adjusted models with and without interaction terms between age (</≥60 years) and WHtR (quintiles) = 0.70
Figure 3.
Figure 3a. Relative risk (RR) of cardiovascular disease according to age and waist-to-height ratio (WHtR) among men Model adjusts for age, physical activity, smoking, alcohol consumption, and parental history of myocardial infarction before the age of 60.
Figure 3b. Relative risk (RR) of cardiovascular disease according to age and waist-to-height ratio (WHtR) among women Model adjusts for age, physical activity, smoking, alcohol consumption, parental history of myocardial infarction before the age of 60 years, postmenopausal hormone use, race, education, and dietary factors.
DISCUSSION
In these prospective cohorts of men and women, we found linear associations between higher adiposity measures and risk of incident CVD. While all indices demonstrated generally similar associations with CVD and measures of model fit, the WHtR most consistently showed the strongest associations and statistically best model fit. Overall, however, we did not find substantial or likely clinically meaningful differences between BMI and WHtR. These results were similar for both men and women.
Our findings remained materially unaltered after accounting for multiple confounders and potential intermediates in the pathway between adiposity and CVD, suggesting that the increased risk conferred by higher measures of adiposity may not solely be mediated by the development of diabetes, hypertension, or high cholesterol. Associations between indices and CVD risk did not vary substantially by age, physical activity, or smoking status. Furthermore, associations between WHtR and CVD were attenuated but remained significant after adjusting for BMI, suggesting that much, but not all, of the risk conferred by a higher WHtR is reflected by BMI.
Previous studies on anthropometric indices and cardiovascular risk have shown conflicting results. Both increased WC and WHR have been associated with higher coronary risk.7, 8, 24 Studies have been inconsistent, however, when comparing the various indices.
Although WC may be correlated more strongly with visceral fat than the WHR,9 particularly among the elderly,10 WC has not been a consistently stronger predictor of cardiovascular risk.25 In a cohort of male health professionals, WHR was particularly associated with coronary risk among the elderly, whereas BMI was more strongly associated among younger individuals.7 Conversely, in the Nurses’ Health Study, both WHR and WC were associated with coronary risk after adjustment for BMI, with stronger associations among younger women.8 Furthermore, while some suggest that WHR is comparably or more strongly associated with CVD, as compared to WC and BMI,6, 26 others have shown stronger associations for WC.27–29 WHtR has been more strongly associated with cardiovascular risk factors, such as hypertension, hyperglycemia, hypertriglyceridemia, and the metabolic syndrome, than BMI or WC in selected populations, primarily among Asian populations.30–36
Multiple biologic mechanisms have been implicated in mediating the adverse health effects of excess adiposity, however, the exact pathways are unknown.10 Visceral fat may be more sensitive to lipolysis, as compared to subcutaneous fat, thereby preferentially increasing circulating free fatty acid levels.10 Other proposed mechanisms involve secretion of adipokines, which may differ by fat storage site.10
Although our study has several strengths, including the prospective design, large sample size and number of outcome events, long duration of follow-up, measurement of multiple potential confounders, and confirmation of CVD events after medical record review, certain limitations should be considered. First, we used self-reported information on exposures, which may contribute to misclassification. However, validation studies of other health professional cohorts have demonstrated the reliability of self-reported information on anthropometric indices and cardiovascular risk factors.16, 37 Moreover, due to the prospective design, we expect that such potential misclassification would likely have yielded underestimates of effect.
Second, residual confounding may have occurred with self-reported information on comorbid conditions and potential mediators. However, we analyzed multiple biologically relevant confounders, and such residual confounding would not be expected to alter substantially the observed associations. Third, our study was limited to female health professionals and mostly Caucasian male physicians in the US, which may limit the generalizability of our results to other populations. However, our study population’s homogeneity in income, educational attainment, and access to medical care may reduce confounding due to socioeconomic factors.
In conclusion, we found that higher measures of both overall and central adiposity confer greater risk of subsequent CVD in both men and women, regardless of the index chosen. Current clinical guidelines using BMI to define overweight and obesity may miss identifying persons at “normal” BMI levels with increased CVD risk related to central fat distribution. However, while the WHtR and central fat distribution may particularly reflect CVD risk, we found differences between BMI and WHtR in associations with CVD and model fit to be small and likely not clinically consequential. Given its ease of measurement and current standard use in the classification of overweight and obesity, BMI may remain the most clinically practical measure of adiposity. Our findings emphasize that higher levels of adiposity, however measured, confer overall greater risk of CVD.
Acknowledgment
We are indebted to the participants in the Physician's Health Study and the Women’s Health Study for their outstanding commitment and cooperation and to the entire Physicians' Health Study and Women’s Health Study staff for their expert and unfailing assistance.
FUNDING SOURCES
This work was supported by grants CA-34944, CA-40360, and CA-47988 from the National Cancer Institute, grants HL-26490, HL-34595, and HL-43851 from the National Heart, Lung, and Blood Institute, and grant 5 T32 AG000158-18 from the National Institute on Aging, Bethesda, MD.
ABBREVIATIONS
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- LRT
likelihood ratio test
- PHS
Physicians’ Health Study
- SD
standard deviation
- RR
relative risk
- WC
waist circumference
- WHR
waist-to-hip ratio
- WHtR
waist-to-height ratio
- WHS
Women’s Health Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: none
REFERENCES
- 1.Curbing the obesity epidemic. Lancet. 2006;367:1549. doi: 10.1016/S0140-6736(06)68664-9. [DOI] [PubMed] [Google Scholar]
- 2.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S–209S. [PubMed]
- 3.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva: WHO; WHO Technical Report Series no. 894. (ISBN Number 92 4 120894 5) 2000 [PubMed]
- 4.James WP. Assessing obesity: are ethnic differences in body mass index and waist classification criteria justified? Obes Rev. 2005;6:179–181. doi: 10.1111/j.1467-789X.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- 5.Franzosi MG. Should we continue to use BMI as a cardiovascular risk factor? Lancet. 2006;368:624–625. doi: 10.1016/S0140-6736(06)69222-2. [DOI] [PubMed] [Google Scholar]
- 6.Walker SP, Rimm EB, Ascherio A, Kawachi I, Stampfer MJ, Willett WC. Body size and fat distribution as predictors of stroke among US men. Am J Epidemiol. 1996;144:1143–1150. doi: 10.1093/oxfordjournals.aje.a008892. [DOI] [PubMed] [Google Scholar]
- 7.Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 8.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 9.Rankinen T, Kim SY, Perusse L, Despres JP, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999;23:801–809. doi: 10.1038/sj.ijo.0800929. [DOI] [PubMed] [Google Scholar]
- 10.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 11.Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed]
- 12.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 14.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 15.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 16.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127:188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 18.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000;71:1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 20.Berger K, Kase CS, Buring JE. Interobserver agreement in the classification of stroke in the physicians' health study. Stroke. 1996;27:238–242. doi: 10.1161/01.str.27.2.238. [DOI] [PubMed] [Google Scholar]
- 21.Atiya M, Kurth T, Berger K, Buring JE, Kase CS. Interobserver agreement in the classification of stroke in the Women's Health Study. Stroke. 2003;34:565–567. doi: 10.1161/01.str.0000054159.21017.7c. [DOI] [PubMed] [Google Scholar]
- 22.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 23.Gelber RP, Kurth T, Manson JE, Buring JE, Gaziano JM. Body mass index and mortality in men: evaluating the shape of the association. Int J Obes (Lond) 2007 doi: 10.1038/sj.ijo.0803564. [DOI] [PubMed] [Google Scholar]
- 24.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001;25:1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- 25.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 26.Folsom AR, Kushi LH, Anderson KE, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 27.Baik I, Ascherio A, Rimm EB, et al. Adiposity and mortality in men. Am J Epidemiol. 2000;152:264–271. doi: 10.1093/aje/152.3.264. [DOI] [PubMed] [Google Scholar]
- 28.Reeder BA, Senthilselvan A, Despres JP, et al. The association of cardiovascular disease risk factors with abdominal obesity in Canada. Canadian Heart Health Surveys Research Group. CMAJ. 1997;157 Suppl 1:S39–S45. [PubMed] [Google Scholar]
- 29.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh SD, Yoshinaga H. Waist/height ratio as a simple and useful predictor of coronary heart disease risk factors in women. Intern Med. 1995;34:1147–1152. doi: 10.2169/internalmedicine.34.1147. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh SD, Yoshinaga H. Abdominal fat distribution and coronary heart disease risk factors in men-waist/height ratio as a simple and useful predictor. Int J Obes Relat Metab Disord. 1995;19:585–589. [PubMed] [Google Scholar]
- 32.Hsieh SD, Muto T. Metabolic syndrome in Japanese men and women with special reference to the anthropometric criteria for the assessment of obesity: Proposal to use the waist-to-height ratio. Prev Med. 2006;42:135–139. doi: 10.1016/j.ypmed.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh SD, Muto T. The superiority of waist-to-height ratio as an anthropometric index to evaluate clustering of coronary risk factors among non-obese men and women. Prev Med. 2005;40:216–220. doi: 10.1016/j.ypmed.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Bosy-Westphal A, Geisler C, Onur S, et al. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int J Obes (Lond) 2006;30:475–483. doi: 10.1038/sj.ijo.0803144. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs FD, Gus M, Moreira LB, et al. Anthropometric indices and the incidence of hypertension: a comparative analysis. Obes Res. 2005;13:1515–1517. doi: 10.1038/oby.2005.184. [DOI] [PubMed] [Google Scholar]
- 36.Esmaillzadeh A, Mirmiran P, Azizi F. Comparative evaluation of anthropometric measures to predict cardiovascular risk factors in Tehranian adult women. Public Health Nutr. 2006;9:61–69. doi: 10.1079/phn2005833. [DOI] [PubMed] [Google Scholar]
- 37.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]