BACKGROUND
The use of thoracic duct drainage for immunosuppression in transplantation has been previously reported by several groups, including our own (9). Maximum benefit requires prolonged lymphocyte depletion rather than temporary diversion. The success in establishing prolonged thoracic duct drainage has been variably reported as 40 per cent by Franksson and associates (3), 50 per cent by Traeger and co-workers (10), 55 per cent by Murray and collaborators (7) , 60 per cent by Fish and colleagues (2) and 80 per cent by Johnson and co-authors (5). With the use of the techniques to be described and using readily available materials, we have been able to establish prolonged thoracic duct drainage in 86 of the 90 patients upon whom it was attempted.
MATERIALS AND METHODS
The anatomy of the thoracic duct and the embryologic explanation for its many variations has been described by Davis (1). Of particular interest are those variations of the terminal portion, reported by Greenfield and Gottlieb (4). In 1 to 5 per cent, the thoracic duct terminates on the right side of the neck. The usual termination is in the region of the left angulus venosus, emptying into either the subclavian or the internal jugular vein.
Five per cent of the thoracic ducts enter the left innominate vein. In 77 to 89 per cent of the cadavers, the thoracic duct ends in a single trunk. When multiple ducts join the venous system, all but the one cannulated must be totally interrupted at operation to prevent collateral drainage away from the fistula. Another anatomic variant of surgical interest is the thoracic duct island or insula produced by the divergence and reconvergence of main thoracic duct trunks. Connections with left cervical and subclavian lymphatic branches further complicate these thoracic duct insulae. Careful dissection of the branches and ligation of these tributaries will, eventually, yield a confluence capable of accommodating a drainage catheter that the individual branches would not.
A transverse left supraclavicular incision is used to approach the cervical thoracic duct. The clavicular head of the sternocleidomastoid muscle is divided. The internal jugular vein is dissected for at least 2 centimeters to its junction with the subclavian vein. Division and ligation of small branches of the internal jugular vein, at this time, permits considerable retraction on the internal jugular later without injury. The omohyoid fascia is opened, and occasionally, the omohyoid muscle is partially divided. Dissection is begun along the anterior surface of the subclavian vein and proceeds posteriorly along its superior surface near the angulus venosus until the thoracic duct is identified. The duct is ligated and divided at its venous junction.
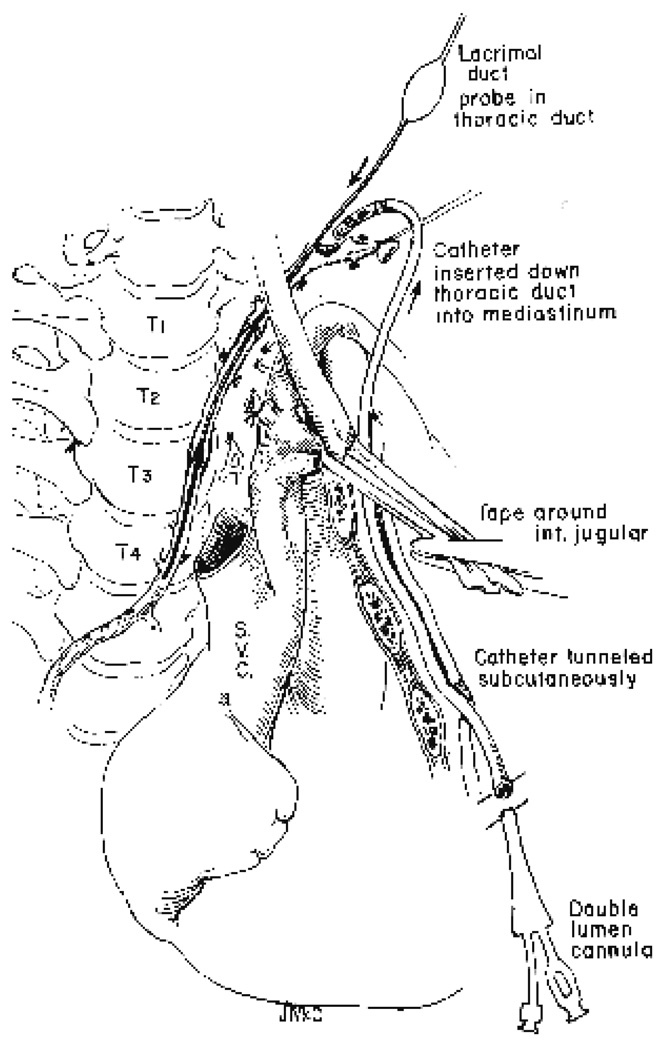
Once ligated, the thoracic duct dilates, facilitating circumferential dissection. Lymphatic tributaries coming from the left arm and upper part of the neck are ligated and divided from the main trunk, but branches going toward the thorax are preserved, since they may be contributors to an unsuspected insula. Usually, it is necessary to dissect the thoracic duct for a distance of several centimeters until it begins its descent into the superior mediastinum. Only in this way can the collection catheter be manipulated through the multiple valves. Anterior and medial retraction of the internal jugular vein provides good exposure as the duct passes inferiorly near the esophagus. When the descent of the main thoracic duct is identified, it is opened and probed with lacrimal duct probes and calibrated to accommodate comfortably the largest possible catheter (Fig. 1). The effective use of the probes is a particularly important step if the catheter is to be inserted for any distance. The repeated passage of probes of graded size can rupture, obstructing valve leaflets, and can dilate stricture-like narrowings. Most commonly, two or three such narrowed areas are encountered before the thoracic duct reaches the superior mediastinum.
FIG. 1.
Left cervical approach to the thoracic duct. Division of the thoracic duct near its terminus at the angulus venosus permits dissection and probing preparatory to insertion of the catheter.
After completing the probing and dilating maneuvers, the tip of a double lumen catheter is tunneled into the wound and advanced a minimum of 3, and preferably 10, centimeters down the duct. Swan-Ganz catheters have ideal cannula characteristics. They are manufactured in various sizes. Sizes 4, 5, 6 and 7 have all been acceptable. With the flow directed catheter, the rubber balloon covering the proximal aperture must be removed. The relative stiffness of the catheters has caused erosions when a catheter tip abbutted an angulation in the thoracic duct. To avoid this problem, the catheters have, more recently, been withdrawn 1 centimeter after placement rather than leaving them at their maximum depth of insertion.
Torsion on the mobilized portion of the duct will occlude flow, but a stabilizing device at the catheter tip has not been necessary as long as the catheter tip is advanced beyond the mobilized portion of duct into the mediastinum. A silk ligature is then used to cinch the mobilized duct around the catheter. Since this ligature may eventually cut through the duct, a long subcutaneous tunnel helps prevent a cutaneous lymph fistula.
A large central venous catheter is inserted for lymph return. A branch of the external or the internal jugular vein is usually used, although a purse-string suture in the internal jugular or the divided stump of the thoracic duct has been used. Finally, careful layered closure of the sternocleidomastoid, platysma and skin is mandatory to prevent a cutaneous lymph fistula.
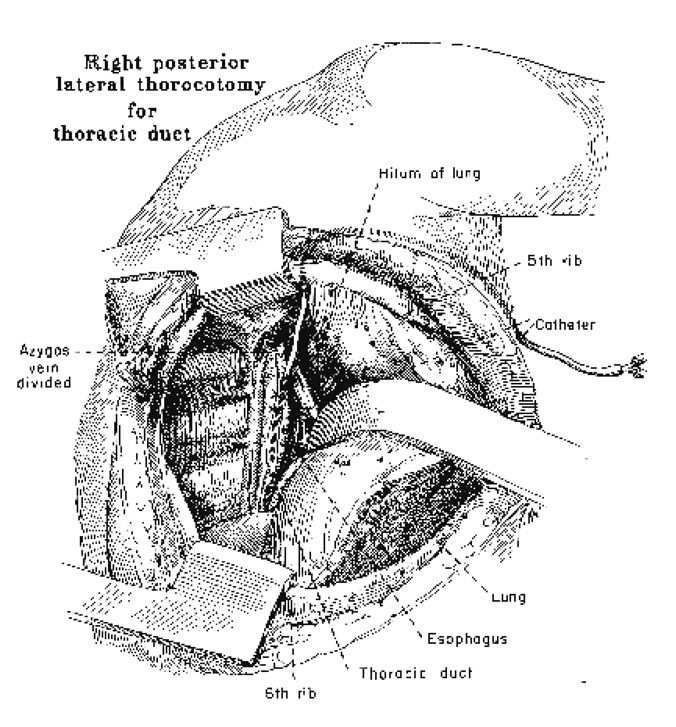
If the cervical thoracic duct cannot be located or has been used before, a right thoracic approach may be indicated for thoracic duct cannulation (Fig. 2). Through a fifth interspace right posterolateral thoracotomy, the esophagus is dissected anteriorly just inferior to the azygos vein. Almost directly posterior to the esophagus usually one but, occasionally, more thoracic trunks can be identified. The largest duct can easily be cannulated and other branches ligated. The collecting catheter enters the chest anteriorly at the second interspace, curves over the hilus of the lung and descends posteriorly to enter the duct. The mediastinal pleura is carefully repaired to prevent a chylothorax.
FIG. 2.
Right transthoracic approach to the thoracic duct, Reflection of the esophagus anteriorly permits cannulation of the thoracic duct just inferior to the azygos vein.
The thoracic duct cannula requires prompt and continuous care, beginning before the wound is closed. An infusion at 20 milliliters per hour of normal saline solution, to which has been added 2 U.S.P. units of heparin per milliliter, has been effective in preventing clotting in the catheter, even when the lymph has been bloody. The small side lumen has customarily been used for infusion of the heparinized saline solution, while lymph has been collected at the larger distal lumen.
The wound and catheter exit sites are maintained according to our parenteral hyperalimentation catheter protocol of daily dressings and local antibiotic ointment. Systemic antibiotic prophylaxis for staphylococci is also provided. Lymph collection is in a closed system using blood collection bags prepared without any anticoagulant additives. The heparin irrigant is sufficient to avoid any coagulation in the bags. At the beginning, collection was in standard blood collection bags to which a transfer pack was attached. Similar, but more satisfactory, specially made, collection bags are now commercially available. After the lymph bags are centrifuged at 4,000 revolutions per minute for ten minutes, the cell free supernatant is pressed into the appendage bag and refrigerated until reinfusion. The cell sediment consists almost entirely of small lymphocytes. Approximately 85 per cent of the removed lymph is retrieved for reinfusion.
The collection for one day is processed, and the lymph is reinfused continuously by the central venous catheter throughout the following day. Most patients who are receiving an unrestricted oral regimen do not require replacement of the volume disparity between collected and returned lymph. If vasomotor instability develops from plasma volume deficit, the difference is made up with normal saline solution or lactated Ringer's solution. The lymph is cultured two times per week. If bacterial growth is found, lymph infusions are stopped, and the lymph drainage is replaced entirely with electrolyte solution until three successive negative lymph cultures are obtained. The exchange of electrolyte solution for discarded lymph has been possible for as long as two months in prospective kidney recipients without harmful effects, other than a gradual fall of serum proteins. However, when patients are being prepared for renal transplantation with thoracic duct drainage, dialysis care must be more intensive than usual to prevent hypotension, and supplemental albumin is often necessary. Some patients with end-stage liver disease and ascites also have required special care with fluid replacement, since the thoracic duct drainage may be as much as 1 liter per hour.
Reasonable daily care has enabled a single catheter to function through the entire period of lymphoid depletion in the vast majority of patients. Operative revision, with or without catheter replacement, is undertaken promptly if the catheter has become plugged, dislodged or broken. This has been done under both local and general anesthesia and has, almost invariably, been successful.
To terminate thoracic duct drainage, heparin irrigation is stopped and the collection bag elevated by daily increments until there has been no flow for a day, after which the catheter is removed. Rarely, lymph accumulated in the subcutaneous tissue or a fistula formed as the bag was raised. In these situations, the catheter was pulled and a cutaneous lymph fistula allowed to close slowly for several days. In no patient was an operation required for a persistent fistula.
RESULTS
Thoracic duct drainage was successful for 21 to 119 days in 86 of 90 patients in whom it was attempted. Sixty-one were kidney homograft recipients, 26 were liver recipients and three had heart or pancreas transplants. The average daily lymph output in those with renal disease averaged nearly 5 liters (9), while those with liver disease produced even more (8). Approximately 2 billion lymphocytes per day are removed. Reoperation and revision of the thoracic duct fistula were necessary in 15 of the 86 patients.
Thoracic duct drainage could not be provided in four patients, despite a secondary transthoracic attempt in two. In one patient, the entire thoracic duct clotted after liver transplantation. Three renal recipients had unfavorable anatomy with multiple ducts too small to permit cannulation. In the first 30 patients, three failures occurred.
There was no mortality directly attributable to thoracic duct drainage. Bacterial growth in the collected lymph was documented in one-quarter of 86 patients, but septicemia was unusual. Revision of antibiotic coverage and substitution of lymph replacement with electrolyte solution were effective treatment. In one patient with a transthoracic catheter, empyema developed, and decortication was required.
Minor complications included local accumulations of lymph in the neck, transient edema of the face or left arm, temporary Horner's syndrome in two patients, one example of transient right chylothorax following removal of the cannula and one retained subcutaneous catheter which was removed later.
In three kidney recipients, ascites developed for a few days after removal of the catheter. The presumed chyloperitoneum promptly receded with diuretic therapy.
DISCUSSION
The profound immunodepression caused by thoracic duct drainage has been known for nearly 20 years, yet the procedure has not been widely used in transplantation, apparently largely because of its unreliability and presumed inconvenience. Experience reported herein has demonstrated that almost all organ recipients can have whatever benefits accrue from this kind of lymphoid depletion. Furthermore, the patients have been able to be mobile within the limits imposed by a rolling intravenous stand for infusion with a basket for collecting bags at its base. As many as 20 patients at a time have had lymph fistulas in place. The principles of success have included deep insertion of a double lumen catheter which allows simultaneous infusion and collections as well as meticulous nursing care.
The most effective use to which thoracic duct drainage can be put for transplantation is under study. Evidence of Walker and associates (11), Traeger and colleagues (10) and our own experience (9) has suggested the importance of treatment before transplantation. The changes in immunologic reactivity, caused by thoracic duct drainage and the time curve of these changes have been particularly well documented by Machleder and Paulus (6) in patients treated for autoimmune disorders.
SUMMARY
Prolonged thoracic duct drainage as an immunosuppressive adjunct was accomplished in 96 per cent of organ recipients upon whom it was attempted.
REFERENCES
- 1.Davis HK. A statistical study of the thoracic duct in man. Am. J. Anat. 1914;17:211. [Google Scholar]
- 2.Fish JC, Sarles HE, Remmers AR., Jr Thoracic duct fistulas in man. Surg. Gynecol. Obstet. 1970;1 31:869. [PubMed] [Google Scholar]
- 3.Franksson C, Lundgren G, Magnusson G, Ringden O. Drainage of thoracic duct lymph in renal transplant patients. Transplantation. 1976;21:133. doi: 10.1097/00007890-197602000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Greenfield J, Gottlieb MI. Variations in the terminal portion of the human thoracic duct. Arch. Surg. 1956;73:955. doi: 10.1001/archsurg.1956.01280060055012. [DOI] [PubMed] [Google Scholar]
- 5.Johnson HK, Niblack GD, T Allen MB, Richie RE. Immunologic preparation for cadaver renal transplant by thoracic duct drainage. Transplant Proc. 1977;9:1499. [PubMed] [Google Scholar]
- 6.M Achleder HI, Paulus H. Clinical and immunological alterations observed in patients undergoing long-term thoracic duct drainage. Surgery. 1978;84:157. [PubMed] [Google Scholar]
- 7.Murray JE, Wilson RE, Tilney NL, et al. Five years' experience in renal transplantation with immunosuppressive drugs; survival. function. complications. and the role of lymphocyte depletion by thoracic duct fistula. Ann. Surg. 1968;168:416. doi: 10.1097/00000658-196809000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Koep LJ, Weil R, III, et al. Thoracic duct drainage in organ transplantation; will it permit better immunosuppression? Transplant Proc. 1979;9:276. [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Weil R, III, Koep LJ, et al. Thoracic duct fistula and renal transplantation. Ann. Surg. doi: 10.1097/00000658-197910000-00007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traeger J, T Ouraine JL, Archimbaud JP, et al. Thoracic duct drainage and antilymphocyte globulin for renal transplantation in man. Kidney Int. 1978;13 Suppl. 8:103. [PubMed] [Google Scholar]
- 11.Walker WE, Niblack GD, Richie RE, et al. Use of thoracic duct drainage in human renal transplantation. Surg. Forum. 1977;28:316. [PubMed] [Google Scholar]