Abstract
Our ability to prepare an action in advance allows us to respond to our environment quickly, accurately, and flexibly. Here, we used event-related fMRI to measure human brain activity while subjects maintained an active state of preparedness. At the beginning of each trial, subjects were instructed to prepare a pro- or anti-saccade to a visual cue that was continually present during a long and variable preparation interval, but to defer the saccade’s execution until a go signal. The deferred saccade task eliminated the mnemonic component inherent in memory-guided saccade tasks and placed the emphasis entirely on advance motor preparation. During the delay while subjects were in an active state of motor preparedness, BOLD signal in the frontal cortex showed: 1) a sustained elevation throughout the preparation interval; 2) a linear increase with increasing delay length; 3) a bias for contra- rather than ipsiversive movements; 4) greater activity when the specific metrics of the planned saccade were known compared to when they were not; 5) increased activity when the saccade was directed towards an internal versus an external representation (i.e., anti-cue location). These findings support the hypothesis that both the human frontal and parietal cortices are involved in the spatial selection and preparation of saccades.
Keywords: saccade, antisaccade, fMRI, preparatory set, oculomotor control, attention, intention
INTRODUCTION
Over the past 25 years, neuroscientists have relied on the memory-guided saccade task to investigate sensory, motor, and mnemonic functions (Bruce and Goldberg 1985; Curtis et al. 2004; Funahashi et al. 1989; Gnadt and Andersen 1988; Hikosaka and Wurtz 1983; Lawrence et al. 2005; Schluppeck et al. 2006; Srimal and Curtis in press). In this task, a sensory cue is briefly flashed in the periphery and the primate is required to ‘hold in mind’ the location of the cue, after which an eye-movement is generated to the remembered location. This paradigm is designed to separate in time the physiological responses to the sensory, delay, and motor components, so that they can be analyzed independently, uncontaminated by each of the other components. For example, persistent neural activity during the memory delay, after the visual cue has extinguished but before the memory-guided saccade has been generated, may represent the mnemonic mechanism that bridges in time the past sensory event and the future contingent response (Fuster 2001).
However, one critical problem arises when trying to infer the nature of persistent activity during the memory interval. Namely, does the delay activity represent a retrospective code of the sensory cue’s location or does it instead represent a prospective code of the forthcoming movement (Calton et al. 2002; Curtis and D’Esposito 2006; Curtis et al. 2004; D’Esposito et al. 2000; Dickinson et al. 2003; Funahashi et al. 1993; Snyder et al. 1997; Srimal and Curtis in press; Takeda and Funahashi 2004)? The type of motor effector the primate plans to use can modulate persistent activity and thus the activity may represent the ‘intention’ to make a specific motor response (for review, see (Snyder et al. 2000)). Another question is whether neural activity only persists when the planned response is guided by memory? In other words, would the observed monkey single unit (Snyder et al. 2000) and functional magnetic resonance imaging (fMRI) signals (Curtis 2006) persist even when there is no memory component and the movement is externally guided? To address this question, we used an oculomotor deferred saccade task (DST), in which the sensory cue is continuously present throughout the delay interval, rather than merely being transiently flashed prior to the delay interval. This paradigm eliminates the memory component (i.e., the need to remember the location of the saccade target) and places the emphasis squarely upon advance motor preparation (Evarts et al. 1984; Requin et al. 1990; Riehle and Requin 1993). Consequently, in the present study, fMRI-BOLD activity increases can be argued to represent a prospective motor plan and/or a “preparatory set.”
A second and related question is whether or not such signals would persist even when a future saccadic eye movement is planned to an internal rather than to an external representation of the target. This issue was addressed by requiring subjects to plan either a pro-saccade (i.e., look toward the target) or an anti-saccade (Hallett 1978; Munoz and Everling 2004). In the anti-saccade task, subjects prepared saccades to an internal representation of the mirrored location of the target. Third, we tested whether delay signals represent a general form of “preparatory set,” (i.e, a readiness to act upon the visual target) versus the preparation of the specific metrics of the planned saccade. To do so, subjects performed trials in which the target was present (known DST) or not present until after (unknown DST) the preparatory delay interval. Fourth, we tested whether brain areas with preparatory activity have spatial selectivity by comparing activity during the preparation of contraversive and ipsiversive movements. Electrophysiologists have repeatedly demonstrated a bias in which neuronal activity represents contraversive saccade plans (Bruce et al. 2004; Platt and Glimcher 1997; Schall 1991).
Therefore, we stipulated several a priori criteria that must be met before we can strongly conclude that an area codes for the planning of a specific saccade. Most importantly, it must exhibit activity increases during the preparatory delay interval that are greater when the precise saccade metrics are known to the subject and greater prior to contraversive movements.
METHODS
Subjects
Twelve neurologically healthy participants (4 females, 8 males, between age 21 and 35, 10 right-handed) were recruited for participation and were paid for their time. All subjects had normal or corrected to normal vision. Subjects gave written informed consent and all procedures were in compliance with the safety guidelines for fMRI research and approved by the human subjects Institutional Review Board at New York University.
Behavioral procedures
The experimental stimuli were controlled by E-Prime (Psychology Software Tools, Inc., PA) and projected (Eiki LC-XG100) into the bore of the scanner on a screen that was viewed by the subjects through an angled mirror.
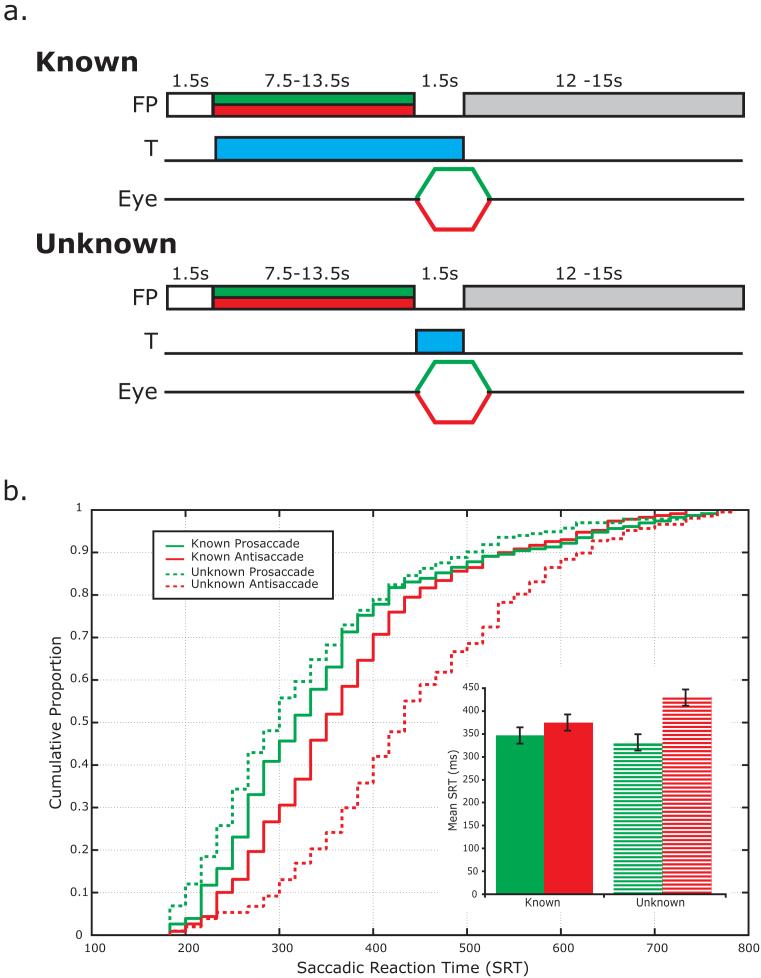
Each subject performed 8 runs of the deferred-saccade task (DST) yielding 144 total trials. The schematic of the experiment is illustrated in Figure 1a. In the known DST, a trial began when the gray fixation dot turned white to alert the subject that a new trial was beginning. Then, the fixation dot changed color to either green (pro-saccade) or red (anti-saccade) to instruct the subject as to whether or not they were to look toward (pro-saccade) or away from (anti-saccade) the target. Simultaneously, a target (1° cyan square) appeared anywhere between 5-15° left or right and 4-5° above or below the central fixation point (white dot). Subjects continued to maintain fixation during a long, variable, and unpredictable delay (7.5, 9, 10.5, 12 or 13.5 seconds) during which they were instructed to plan the eye movement and remain in a ready-state to execute the eye movement as soon as the fixation point disappeared. Trials were separated by an intertrial interval (ITI) between 12 – 15 seconds to allow the hemodynamic response to return to baseline.
Figure 1.
a. Schematic of the deferred saccade task (DST). In the known DST, a trial began when the gray fixation dot turned white to alert the subject that a new trial was beginning. Then, the dot changed color (green = pro-saccade or red = anti-saccade) to instruct the subject to look toward (pro-saccade) or away from (anti-saccade) the target. Simultaneously, a target (T; 1° cyan square) appeared in the periphery. Subjects continued to maintain fixation during a long, variable, and unpredictable delay during which they were instructed to plan the eye movement and remain in a ready-state to execute the eye movement as soon as the fixation point disappeared. In contrast, in the unknown condition the subject was instructed via the color cue that it was a pro- or anti-saccade trial but because the target was not presented until after the delay, they did not know the spatial goal of the saccade and thus could not plan the metrics of the saccade. b. Cumulative distribution plot of saccadic response times (SRTs) measured during the fMRI scanning session. There are three noteworthy observations: 1) SRTs were in the ‘normal’ saccadic range, i.e., 200 – 500 ms; 2) pro-saccades had shorter latencies than anti-saccades; and 3) this disparity between pro- and anti-saccade latencies was much greater in the unknown condition. The inset is a bar graph of mean (±S.E.) SRTs for each trial type.
In contrast, in the unknown condition the subject was instructed via the color cue that it was a pro- or anti-saccade trial but because the target was not presented until after the delay, the spatial goal of the saccade was not known and thus the precise metrics of the saccade could not be used (Fig. 1a). Otherwise, all timing and specifics were the same. Known and unknown blocks were performed alternately with the order of blocks counterbalanced across subjects.
Oculomotor methods
Eye position was monitored in the scanner at 60 Hz with an infrared videographic camera equipped with a telephoto lens (ASL 504LRO; Applied Sciences Laboratories, Bedford, MA; modified with a Sony HAD CCD) that focused on the right eye viewed from a flat surface mirror mounted inside the RF coil. Nine-point calibrations were performed at the beginning of the session and between runs when necessary. Eye-movement data were calibrated then transformed to degrees of visual angle using a third-order polynomial algorithm that fit eye positions to known spatial positions and scored offline with in-house software (GRAPES). Saccadic reaction times were estimated with semiautomatic routines that relied on the velocity of the eye reaching about 30°/second to determine the onset of saccades. We visually inspected each trial to validate the saccade onset time determined by the routine.
We excluded from analysis a total of 126 trials (an average of 4.5% of trials) in which subjects did not strictly comply with task instructions. The vast majority of these were breaks in fixation during the delay periods. We also excluded from analysis trials in which subjects made saccade errors. With regard to errors (i.e., pro-saccades on anti-saccade trials or vice versa), subjects made errors on 4.3% of known anti-saccade trials, 12.3% of unknown anti-saccade trials, 2.0% of known pro-saccades, and 1.1% of unknown pro-saccade trials. These trials were separately modeled in the GLM to remove any variance in BOLD signal associated with these epochs, but because they were so infrequent they were not further analyzed.
MRI procedures
MRI data were collected using a 3 T head-only scanner (Allegra; Siemens, Erlangen, Germany) at the Center for Brain Imaging at New York University. Images were acquired using custom radio frequency coils (NM-011 transmit head-coil and NMSC-021 four-channel phased array receive coil; NOVA Medical, Wakefield, MA) placed over lateral frontal and parietal cortices. During each fMRI scan, a series of volumes were acquired using a T2*-sensitive echo planar imaging pulse sequence (repetition time, 1500 ms; echo time, 30 ms; flip angle 75°; 24 slices; 3 × 3 × 3 mm3 voxels; 192 × 192 FOV). High-resolution (1 mm isotropic voxels) MP-RAGE three-dimensional T1-weighted scans were acquired for anatomical registration, segmentation, and display. To minimize head motion, subjects were stabilized with foam padding around the head.
fMRI data preprocessing and surface based statistical analysis
Post hoc image registration was used to correct for residual head motion [MCFLIRT (motion correction using FMRIB’s Linear Image Registration Tool)]. Additional preprocessing of the fMRI data were as follows. We bandpass filtered the time series of each voxel (0.01 to 0.33 Hz) to compensate for the slow drift typical in fMRI measurements (Biswal and Hyde 1997; Zarahn et al. 1997), divided the time series of each voxel by its mean intensity to convert to percent signal modulation and compensate for the decrease in mean image intensity with distance from the receive coil, and spatially smoothed the data to arrive at smoothness of 6mm at FWHM.
We modeled each within-trial event (i.e., instruction + target, delay, and response) for each trial type (i.e., known/unknown x pro-/anti-saccade) separately. Since the instruction and target were presented simultaneously in the known condition, these were modeled as a single event. The encoding of the instruction + target and the generation of the motor response were short transient events and were thus modeled with an impulse time-locked to the event convolved with a canonical hemodynamic response function (HRF) (Polonsky et al. 2000). The preparatory delay spanned 7.5 - 13.5 seconds and was modeled very well by the linear combination of a zero-order polynomial (i.e., boxcar) and a first-order polynomial (i.e., linear ramp). Both delay regressors spanned the delay period and were time-shifted by 4000 ms to account for the hemodynamic lag. This time-shift resulted in the least correlation among regressors. The parameter estimates from the first-order polynomial were used to estimate delay period activity at the group level because at the individual subject level they predicted significant delay period activity, which was confirmed by plotting the time series of the voxels identified by this parameter. Each of the independent variable regressors were entered into a modified general linear model (GLM) (Worsley and Friston 1995) for statistical analysis using VoxBo (http://www.voxbo.org).
For each subject, we used Caret (http://brainmap.wustl.edu/caret) for anatomical segmentation, gray-white matter surface generation, flattening, and multi-fiducial deformation mapping to the PALS atlas (Van Essen 2005). Registering subjects in a surface space using precise anatomical landmark constraints (e.g., central sulcus, sylvian and calcarine fissures, etc.) results in greater spatial precision of the alignment compared to standard volumetric normalization methods (Van Essen 2005). Further, statistical maps for contrasts of interest were created using the beta-weights estimated from each subject’s GLM. These maps were then deformed into the same atlas space, and t-statistics were computed for each contrast. We used a nonparametric statistical approach based on permutation tests to help address the problem of multiple statistical comparisons (Holmes 1996; Nichols 2002). First, we constructed a permuted distribution of clusters of neighboring surface nodes with t-values > 3.0. We chose a primary t-statistic cutoff of 3.0 because it is strict enough that intense focal clusters of activity would pass but not so strict that diffuse large clusters of activity are lost. In the case of a one-sample comparison, where measured values are compared to the test value of 0, the signs of the beta values for each node were randomly permuted for each subject’s surface, prior to computing the statistic. One thousand iterations, N, of this procedure were performed to compute a permutation distribution for each statistical test performed. Then, we ranked the resulting suprathreshold clusters by their area. Finally, corrected p-values at α = 0.05 for each suprathreshold cluster were obtained by comparing their area to the area of the top 5% of the clusters in the permuted distribution, where the critical suprathreshold cluster size, C, at a t-score threshold of t > 3.0 was C = Nα+1. The permutation tests controlled for Type I error by allowing us to formally compute the probability that an activation of a given magnitude could cluster together by chance.
Region-of-Interest (ROI) time series procedures
We used ROI-based analyses of the time courses of BOLD signal change. First, on each subject’s high resolution anatomical scans, we traced around grey matter of several ROIs including the superior precentral sulcus (sPCS), paracentral sulcus (paraCS), intraparietal sulcus (IPS), and transverse parietal sulcus (tPS). These ROIs were selected because of consistent activations from past studies and the preliminary investigations of the current single-subject data. The sPCS was defined as the dorsal segment of the precentral sulcus at the junction of the superior frontal sulcus. The paraCS was defined as the descending sulcus along the dorsal medial wall just anterior to the central lobule. The IPS was defined as the sulcus that divides the superior and inferior parietal lobules. The tPS was defined as the descending sulcus on the medial wall of the parietal lobe posterior to the cingulate and anterior to the posterior occipital sulcus. Next, within each ROI, we selected the 20 voxels (540 mm3) with the strongest main effect of the linear combination of all the task covariates. These voxels showed some consistent deviation from baseline during the task without being biased by any task component. Using a combined structural-functional criteria to select voxels for study is similar to the way electrophysiologists first identify neurons that respond to the task and then subject those neurons to further study.
We plotted the time series of BOLD responses, averaged across voxels within an ROI and averaged across subjects from analogous ROIs, time-locked to the presentation of the instruction cue or the signal to generate the saccade. In both cue-locked and response-locked plots, the average signal was baselined against the average response of the last two TRs before the trial began. For cue-locked plots, the average plot data only included the TRs up to the end of the delay so as not to contaminate the estimation with activity evoked by the motor response after the delay. This allowed for us to combine data from the different delay lengths. The error bands were computed by taking the average of each individual’s standard error, which appropriately estimates the mean of the within-subject variance.
Contraversive effect
To test hypotheses about lateralized activity, we combined activity from both hemispheres of each ROI in the following way. Contraversive activity was defined as left ROI activity when the planned saccade was directed into the right visual field plus right ROI activity when the saccade was directed towards the left visual field. Similarly, ipsiversive activity was defined as left ROI/left saccade direction plus right ROI/right saccade direction. Although we find the same results when we analyze each hemisphere ROI separately, this procedure allowed us to pool data across hemispheres to increase our statistical power.
RESULTS
Oculomotor results
By concurrently measuring eye movements we were able to characterize the distribution of saccadic response times (SRTs). About 90% SRTs fell in the normal range of visually guided saccades, i.e., 200 – 500 ms (Figure 1b). A repeated measures ANOVA (condition [Known AS, Known PS, Unknown AS, Unknown PS] by delay length [7.5,9,10.5,12,13.5 s]) showed that the conditions differed significantly by SRT, F(3,9)=21.4, p<0.0001. Importantly, the SRTs did not differ as a function of the delay length (F[4,8]=0.9, p>0.5) and repeated measures ANOVAs run separately for Known and Unknown trials confirmed that SRTs were not affected by the interaction between delay length and the type of saccade (i.e, AS, PS), Known: F(4,8)=1.7, p>0.22, Unknown: F(4,8)=1.2, p>0.36. Since SRTs were comparable across the delays used in the study, we collapsed across the delays in subsequent analyses. All follow-up pairwise comparisons were significantly different from one another at p<0.05 level (Mean±SE for Known AS = 374±19 ms, Known PS = 349±18 ms, Unknown AS = 434±19 ms, Unknown PS = 321±17 ms) except Known and Unknown PS trials (p=0.3). The longer latencies for antisaccade compared to prosaccades presumably reflects the extra processes required for this type of movement (e.g., inversion of visually guided saccade vector). The difference in SRTs between anti- and pro-saccades was significantly diminished when the target metrics were known in advance (∼25ms vs. ∼112ms) suggesting that much of the computational differences happen during planning rather than execution of the saccade. Overall, the SRT data support our assumption that subjects did indeed prepare saccades in advance during the delay when the location of the target was known.
Surface-based statistical tests
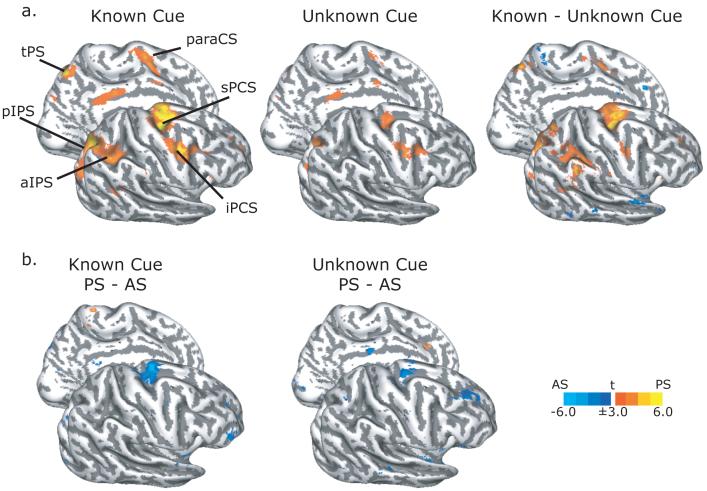
Group statistical maps of activity specific to processing the instruction cue (i.e., pro-/anti-saccade) and the visual target in the known DST are shown in Figure 2 and listed in Table 1. We observed BOLD activation during the cue epoch in the known condition in the sPCS, iPCS, and paraCS in the frontal cortex and along the posterior and lateral segments of the IPS in the parietal cortex bilaterally (Figure 2a, left). We observed less activation when the visual target was not shown until after the delay in the unknown trials (Figure 2a, middle and right). The increased activation in these frontal and parietal areas could be due to several additional factors occurring during the early part of the trial in the known condition. Namely, visual stimulation evoked by the target cue’s appearance, visual-motor selection, inhibition of the tendency to saccade to the abruptly onset visual target, and the planning of the instructed saccade. At the cue epoch, the sPCS bilaterally were the only areas that were significantly more active during antisaccade trials compared to prosaccade trials (Figure 2b). Even in the unknown trials, when the specific metrics of the saccade could not be planned, the sPCS was significantly more active during antisaccades than prosaccades (Figure 2b, right). No areas were more active for prosaccades that antisaccades at this trial epoch.
Figure 2. Surface-based statistics from the instruction cue epoch.
a. During the known trials (left), processing the instruction cue and early visual target evoked BOLD activation in sPCS, iPCS, paraCS, and IPS. Similar areas were activated during the unknown trials (middle), when only the instruction cue was present. A comparison of known minus unknown trials revealed larger activations in dorsal frontal and lateral parietal oculomotor areas (right). b. Antisaccade (AS) trials evoked more activity than prosaccade (PS) trials mainly in the sPCS during both known (left) and unknown (right) trials. BOLD activations rendered on the gray-white matter surface of the right lateral and left medial inflated hemispheres. Dark gray overlay indicates sulci, while light grey indicates gyri. Abbreviations: sPCS = superior precentral sulcus; iPCS = inferior precentral sulcus; MFG = middle frontal gyrus; IFG = inferior frontal gyrus; paraCS = paracentral sulcus; pIPS = posterior intraparietal sulcus; aIPS = anterior intraparietal sulcus; tPS = transverse parietal sulcus.
Table 1.
Peak MNI volumetric coordinates for active foci.
| Peak MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Known Cue | Unknown Cue | ||||||
| Region | Hemi | x | y | z | x | y | z |
| sPCS | L | -30.1 | -13.3 | 49.9 | -31.0 | -14.1 | 51.5 |
| R | 25.8 | -7.9 | 51.1 | 22.9 | -9.2 | 52.6 | |
| iPCS | L | -44.1 | -2.1 | 34.5 | -43.6 | -1.9 | 35.3 |
| R | 44.0 | -0.4 | 41.0 | 41.6 | -0.1 | 39.3 | |
| paraCS | L | -7.8 | -10.2 | 52.4 | -7.9 | -7.9 | 55.5 |
| R | 3.1 | -6.8 | 59.1 | 5.2 | -10.0 | 59.2 | |
| aIPS | L | -36.3 | -47.3 | 47.3 | |||
| R | 39.4 | -44.4 | 45.1 | ||||
| pIPS | L | -29.3 | -65.6 | 44.4 | -31.6 | -68.9 | 42.1 |
| R | 26.9 | -64.4 | 43.2 | 25.8 | -63.9 | 41.7 | |
| tPS | L | -5.5 | -65.4 | 48.0 | |||
| R | 6.1 | -56.3 | 46.8 | ||||
| Known Delay | Unknown Delay | ||||||
|---|---|---|---|---|---|---|---|
| Region | Hemi | x | Y | Z | X | Y | z |
| sPCS | L | -33.2 | -13.4 | 51.6 | -29.1 | -13.3 | 49.9 |
| R | 25.7 | -10.0 | 51.1 | 25.3 | -9.4 | 50.9 | |
| iPCS | L | -45.7 | -2.6 | 33.7 | -43.1 | -1.8 | 36.3 |
| R | 42.5 | -0.1 | 39.9 | 40.2 | 0.7 | 34.7 | |
| paraCS | L | -7.9 | -4.2 | 56.4 | -6.9 | -11.0 | 57.8 |
| R | 2.5 | -13.8 | 55.0 | 1.4 | -17.0 | 64.2 | |
| IPS | L | -30.6 | -63.9 | 37.5 | -33.0 | -55.5 | 42.8 |
| R | 25.6 | -70.2 | 32.2 | 26.2 | -70.4 | 31.7 | |
| Known Response | Unknown Response | ||||||
|---|---|---|---|---|---|---|---|
| Region | Hemi | X | Y | Z | X | Y | Z |
| sPCS | L | -33.6 | -13.0 | 51.2 | -32.7 | -10.6 | 48.7 |
| R | 27.6 | -10.1 | 51.2 | 22.0 | -13.6 | 56.6 | |
| iPCS | L | -45.2 | -2.7 | 36.3 | -46.6 | -0.8 | 31.2 |
| R | 45.3 | 0.0 | 37.5 | 45.4 | -2.3 | 46.7 | |
| MFG | L | -28.1 | 30.7 | 35.3 | |||
| R | 36.3 | 32.9 | 33.7 | 34.5 | 33.4 | 34.5 | |
| IFG | L | -48.5 | 25.2 | 3.7 | -48.8 | 21.3 | 4.5 |
| R | 52.7 | 21.4 | 14.5 | ||||
| paraCS | L | -7.9 | -4.1 | 54.9 | -7.3 | -8.3 | 59.2 |
| R | 1.1 | -18.5 | 67.2 | 1.1 | -17.0 | 66.7 | |
| aIPS | L | -39.1 | -49.5 | 39.9 | -40.0 | -54.9 | 39.4 |
| R | 36.7 | -42.5 | 43.3 | 3.26 | -44.7 | 46.7 | |
| pIPS | L | -26.2 | -78.8 | 29.5 | |||
| R | 24.6 | -63.9 | 39.6 | 27.7 | -74.3 | 27.0 | |
| SPL | L | -21.4 | -68.7 | 52.8 | -28.2 | -61.1 | 52.9 |
| R | 16.1 | -69.3 | 53.5 | 23.0 | -61.1 | 54.9 | |
| tPS | L | -6.1 | -64.8 | 48.5 | -5.6 | -66.2 | 48.8 |
| R | 5.2 | -51.1 | 55.3 | 5.7 | -49.3 | 48.3 | |
Abbreviations: sPCS = superior precentral sulcus; iPCS = inferior precentral sulcus; MFG = middle frontal gyrus; IFG = inferior frontal gyrus; paraCS = paracentral sulcus; pIPS = posterior intraparietal sulcus; aIPS = anterior intraparietal sulcus; SPL = superior parietal lobule; tPS = transverse parietal sulcus.
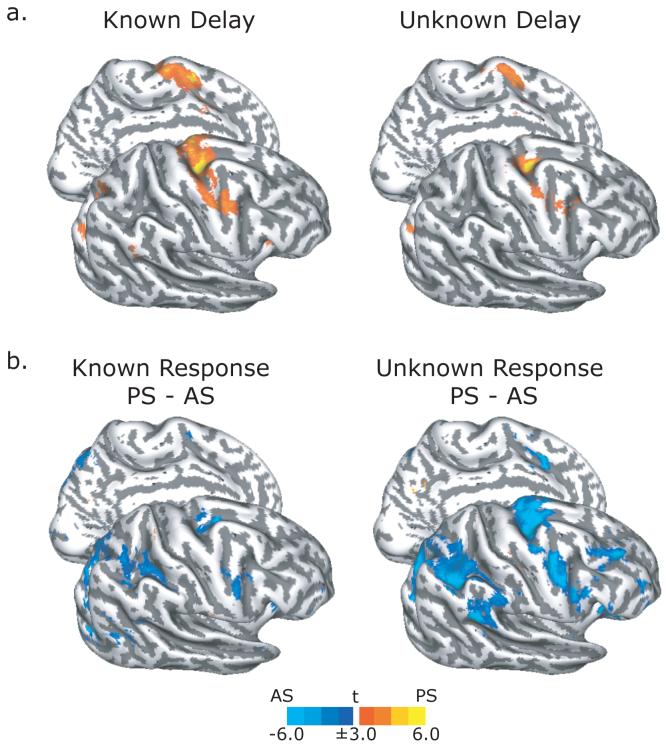
Similarly, the sPCS, iPCS, paraCS, and the posterior ascending segment of the IPS were significantly active during the delay period for both known and unknown trials (Figure 3a, Table 1). As can be seen in the statistical maps shown in Figure 3a, the activity is greater during the delay for the known compared to unknown trials. However, a direct comparison failed to reveal any significant differences at the corrected level of threshold (see Methods). Dropping the threshold to an uncorrected value of p<0.05, nonetheless, revealed active clusters in the sPCS and IPS. Looking at the contrast in individual subjects indicated much variability in the pattern of activation from this contrast, especially in the IPS where the anatomical folding patterns were quite variable. This was less so for the sPCS but again the activation in the sPCS was just below statistical thresholds. As we will see below, time series analyses of ROIs defined in individual subjects were more sensitive to these differences.
Figure 3. Surface-based statistics from the preparatory delay and saccadic response epochs.
a. During the known trials (left), advanced saccade preparation evoked BOLD activation in sPCS, iPCS, paraCS, and IPS. Similar areas were activated during the unknown trials (right), when the specific saccade metrics could not be planned. Although the amount of BOLD activation appears greater during known trials compared to unknown trials, this contrast did not yield any significant clusters. b. At the saccadic response epoch, antisaccade (AS) trials evoked more activity than prosaccade (PS) trials in the sPCS, iPCS, and IPS during both known (left) and unknown (right) trials.
We then compared BOLD activation between pro- and anti-saccade trials during the response period. Not surprisingly, we found that the entire cortical oculomotor network was highlighted by this contrast (Figure 3b, Table 1) with anti-saccades showing greater activation than prosaccades in all areas. The differences appear to be greater during the unknown condition compared to the known condition. Moreover, the dorsolateral prefrontal cortex and dorsal anterior cingulate showed a larger BOLD response during antisaccades compared to prosaccades, but only in the unknown condition (Figure 3b). Perhaps when the antisaccade can be planned during the delay on known trials, fewer resources are needed at the response epoch compared to the unknown trials when one must inhibit looking at the target, transform the target’s vector by 180°, and finally plan the saccade to the internal representation of that location.
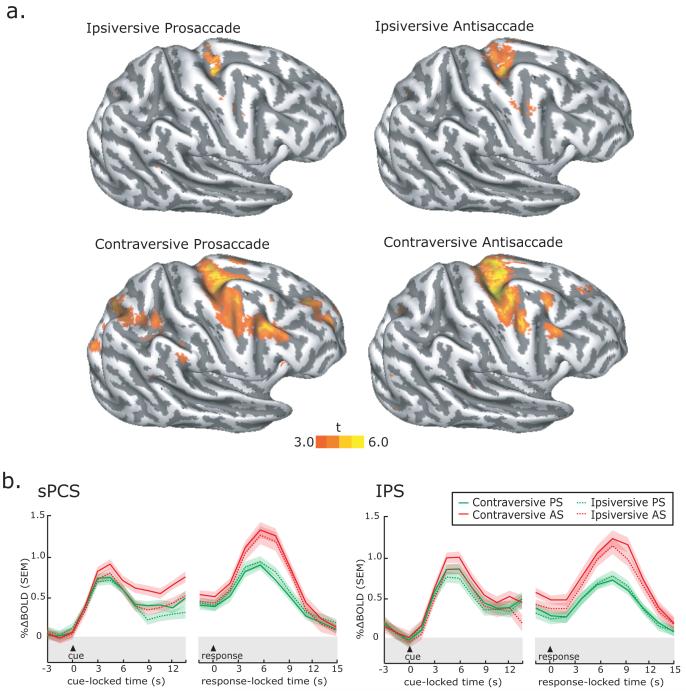
Finally we focused on the preparatory delay period of the known trials so we could test if the side of the visual stimulation or the direction of the forthcoming planned saccade biased the activations we observed. During the planning of prosaccades, the side of visual stimulation is the same as the direction of the saccade (e.g., visual target on left, prepare saccade to left). Indeed, there was greater activation in the right hemisphere when the visual target and motor plan were to the left of fixation (Figure 4a left column). During the planning of antisaccades, the side of visual stimulation is opposite the direction of the saccade (e.g., visual target on right and saccade to the left). The laterality bias can actually be addressed by comparing the hemisphere-by-side interaction. At least in the frontal cortex along the PCS, there was greater activation in the hemisphere opposite the direction of the motor plan (i.e., in the contraversive hemisphere) compared to the side of visual stimulation. At the whole brain level, there was not enough power to test these differences directly (e.g., contraversive AS > ipsiversive AS) since the number of trials is too small [i.e., (144 total trials) / (2 conditions known/unknown) / (2 saccades PS/AS) / (2 directions contra/ipsiversive) / (2 hemispheres) = 9 trials.] As we will see below and plotted in Figure 4b, we were able to collapse across hemispheres in the time series analyses to boost our power and we find clear support for the trends in a contraversive bias we see in the data at the whole brain level shown in Figure 4a.
Figure 4. Contraversive bias in activity during saccade preparation.
a. Surface-based statistics from the delay period show that the preparation of contraversive, compared to ipsiversive, saccades during the known delay periods evoked greater activity; activity in the hemisphere opposite the visual field toward which the saccade was planned was greater. b. Trial-averaged BOLD time courses aligned on the presentation of the instruction cue during the known trials and the generation of the saccadic response. BOLD signal in sPCS, but not IPS, was larger in the hemisphere opposite the direction of the planned saccade for both antisaccade and prosaccade known trials. These effects cannot be attributed to visual stimulation, because on antisaccade trials the visual target appeared in the ipsilateral hemifield. Each line represents the mean across subjects and the error bands are the mean of each subject’s standard error.
Region of interest time series analyses
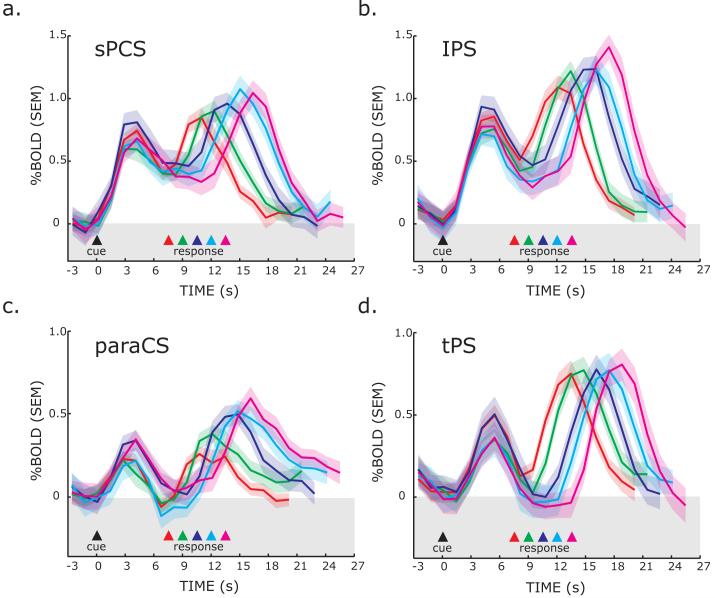
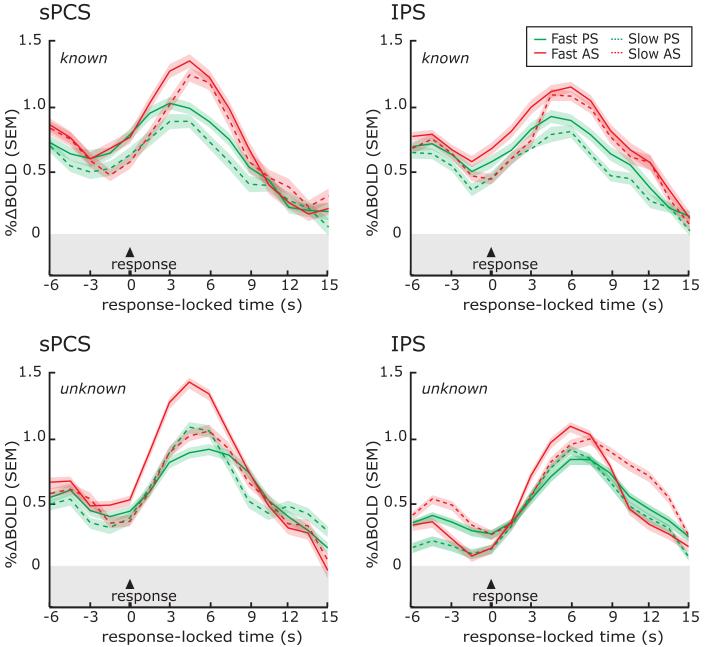
Next, we plotted the time series of BOLD responses from frontal and parietal ROIs to test several hypotheses. First, a region that plays a critical role in saccade preparation should show activity above baseline throughout the preparatory delay irrespective of the length of the delay. For each ROI, we plotted the BOLD time course data for each of the five different delay lengths of the known DST, time-locked to the presentation of the instructional cue (Figure 5). Initially, a transient response can be seen time-locked to the instruction cue and visual target. Then, the sPCS and IPS ROIs show activity above baseline that spans the entire delay, even when the subjects were in an active state of motor preparedness for nearly 14 seconds. Notice that in the late phase of the delay period, activity begins to increase or ramp-up well before the end of the delay, and well before the saccade was generated. The presaccadic ramping is even more pronounced if one figures in the hemodynamic delay of about 2 seconds (i.e., shifting the BOLD time course to the left). Finally, one can see another later robust transient response time-locked to the instructed saccade that is staggered in time in the order of the delay length. Moreover, the magnitudes of the saccade related responses are also staggered by delay length, with larger responses following longer delays. To quantify this effect, for each subject we computed the slope of a line fit through the peak responses at each delay. The slopes were significantly greater than zero for the sPCS (p<0.00001, slope=0.045), IPS (p<0.001, slope=0.059), and paraCS (p<0.02, slope=0.020), but not tPS (p=0.2, slope=0.012) ROIs. For example, the sPCS response after the delay tended to increase in magnitude by 0.045% with every 1.5 seconds increase in the delay period. This can be accounted for by the integration of the ramping activity during the delay with a fixed magnitude response at the saccade period. The signal in tPS did not sustain throughout the delay period and activity in the paraCS slowly climbed above baseline during the latter half of the delay period. Together, the time course data reveal robust delay period activity in the sPCS and IPS when subjects are preparing a saccadic response. The activity during the preparatory delay cannot be attributed to processing the instruction or generating the saccade.
Figure 5. Trial-averaged BOLD time courses aligned on the presentation of the instruction cue during known trials.
Separate lines represent the different delay lengths, where the time of the saccade is indicated by the colored triangles and dotted lines. The data plotted is for combined contraversive pro- and anti-saccades. The peaks of the responses are staggered according to the preparatory delay length. Importantly, the sPCS and IPS ROIs showed evidence of building activity regardless of the length of the delay, even at very long delays. Each line represents the mean across subjects and the error bands are the mean of each subject’s standard error.
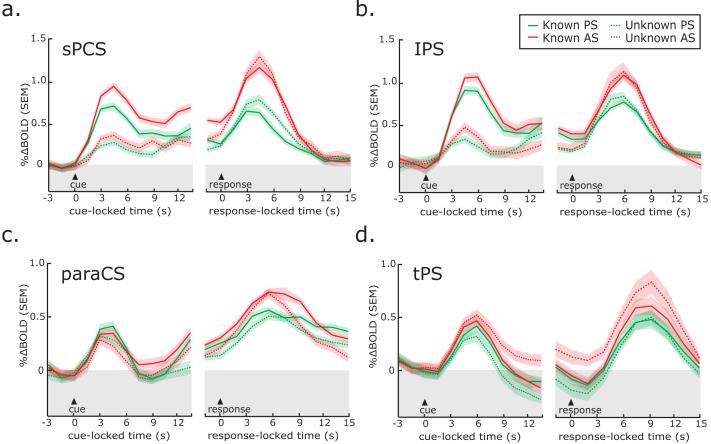
Second, we tested the hypothesis that an area that is involved in saccade planning should show greater activity when the specific movement can be planned than when it cannot. To test this, we plotted the BOLD time courses from the ROIs separately for the known and unknown saccade conditions (Figure 6). We collapsed the time course data across the different delay lengths by time-locking the data to the instruction cue and averaging trials up to the end of the delay. We also plotted the data time-locked to the instruction to generate the saccade. To test for significance, we computed t-statistics (α=0.05, one-tailed) comparing the magnitude of BOLD time course data during the delay period, using as the metric of analysis the average of the time points beginning at 6 seconds to the end of the delay. During the preparatory delay period, BOLD activity in the sPCS and IPS was greater throughout the delay in the known compared to unknown condition for both regions (all p’s<0.05). No significant differences were found in the paraCS and tPS.
Figure 6. Trial-averaged BOLD time courses time-locked to the presentation of the instruction cue and the generation of the saccadic response.
BOLD time courses in sPCS (a) and IPS (b) are generally larger for antisaccades than prosaccades and larger during known trials than unknown trials. No clear differences were found in the paraCS (c) and tPS (d) ROIs. Each line represents the mean across subjects and the error bands are the mean of each subject’s standard error.
Third, we tested the hypothesis that an area involved in saccade planning should show greater activity during the preparation of saccades that were contraversive (i.e., saccades toward the visual field opposite the hemisphere of the ROI). As can been seen in the averaged BOLD time courses from known trials in Figure 4b, activity was greater in the sPCS during the delay periods when subjects were preparing contraversive saccades. On prosaccade trials, the average sPCS signal was higher during the delay in the hemisphere opposite the planned saccade, t(11)=4.3, p<0.05. However, this difference could be due to a visual response evoked by the visible target in the contralateral hemifield. Our hypothesis can be better tested with data from antisaccade trials, where the side of visual stimulation and saccade planning are disassociated. On antisaccade trials, the preparatory signal is higher in the hemisphere opposite the planned saccade, not visual target, t(11)=5.4, p<0.05). Note that as soon as the saccade was generated, the contraversive bias was lost. Besides during saccade preparation, we have also observed lateralized biases in BOLD signals during memory-guided saccade delay periods (Curtis and D’Esposito 2006; Srimal and Curtis in press) and during the maintenance of spatially directed attention (Ikkai and Curtis in press). As in the current study, such spatial biases were lost when the memory-guided saccade was generated. This may be due to cross hemisphere interactions between homotopic brain areas that nullify the differences that can be observed before the saccade is generated. Indeed, FEF neurons that code for conflicting saccade vectors are thought to inhibit one another around the time of saccade execution (Schlag et al. 1998; Seidemann et al. 2002), which would evoke BOLD responses that were together non-spatially selective for saccade direction. One implication is that a “push-pull” mechanism may be active only around the time of the saccade and not during saccade preparation. The other ROIs, including the IPS (Figure 4b right), did not show significant visual field (e.g., contralateralized) or motor response (e.g., contraversive) biases.
DISCUSSION
The goals of the current study were to identify candidate human cortical areas involved in advanced saccade preparation and test whether physiological changes in these candidate areas are consistent with several predictions about neural activity underlying saccade preparation. In summary, we identified cortical areas in frontal and parietal cortex whose neural activity persisted as long as subjects were in an active state of maintaining a saccade plan. BOLD activity in the sPCS and IPS ramped up as the motor plan evolved. This preparatory activity was greater when the direction of the forthcoming saccade was known compared to when only the type of movement (i.e., pro-, anti-saccade) that would be later executed was known. Moreover, activity in the sPCS was greater for contraversive saccade plans. We will discuss each of these findings in greater detail and in relation to existing work.
Ramping activity in the human frontal and parietal cortex
Single-unit recordings from monkeys have demonstrated that spike rates increase in saccade neurons in the frontal eye field (FEF) and lateral intraparietal (LIP) area prior to saccades and build up to a maximum when the saccade is finally triggered (Andersen et al. 1992; Bruce and Goldberg 1985). It is thought that these cortical neurons are important for specifying the metrics or spatial goals of saccades. In order to identify homologous activity in humans using a non-invasive technique, we used fMRI to measure cortical activity while subjects prepared to make saccades. Importantly, with our design we were able to separately measure activity during preparation and execution. Based on the surface-based statistical maps (Figure 3a) of activity during the preparatory epoch, we found robust activations in the sPCS and IPS bilaterally. These same areas were also active when the saccade was finally executed. We confirmed the effects in these statistical maps by plotting the trial-averaged time series data from several individually defined frontal and parietal ROIs. Consistent with the preparatory delay period surface maps, we found that BOLD activity in the sPCS and IPS persisted above baseline until the saccade was generated (Figure 4b). Activity in the sPCS and IPS began to increase, or ramp up, during the preparatory delay prior to the instruction to generate the saccade even when the amount of preparation time was randomly varied in duration (Figure 5). Therefore, the activity we measured is related to saccade preparation and not to the processing of the instruction or the generation of the saccade itself. We conclude that the delay period BOLD signals we measured reflect neural activity of the human homologues of the monkey FEF and LIP.
Prospective coding of saccade metrics
If these delay period BOLD signals do indeed reflect the activity of neurons involved in saccade planning, then we can make several predictions. First, we expected that activity would be greater when the precise metrics of the upcoming saccade were known. Indeed, the sPCS and IPS were more active when subjects could prepare a specific saccade compared to when they could not (Figures 3a and 6), consistent with the hypothesis that the BOLD signals during the preparatory period reflect the activity of saccade-specific neurons in the human frontal and parietal cortex. Although much smaller in magnitude, we did observe significant activity during the trials in which subjects did not know the spatial goal of the upcoming saccade in both sPCS and to a much smaller extent in posterior IPS. Such activity likely reflects general, non-spatial, anticipation of a future action akin to a “preparatory set” (Evarts et al. 1984). In previous fMRI studies, Connolly, Goodale, Munoz and colleagues (Connolly et al. 2007; Connolly et al. 2005; Connolly et al. 2002) have used a gap saccade task to show that activity in the human PCS, but not IPS, ramps up in general anticipation that a saccade will be required shortly. In this task, the fixation point disappears for a variable duration (0, 2, or 4 seconds) before the visual saccade target is presented and BOLD activity builds as the gap duration lengthens. The current study’s unknown condition is most similar to the gap saccade paradigm and we too show strong evidence of preparatory delay period activity in PCS even when no spatial information about the saccade is available. In contrast to the gap saccade studies, we also find that the IPS is active during the preparatory delay, even when the spatial direction of the saccade is unknown. The magnitude of the effect is very small relative to when the saccade’s direction is known, but it is reliable and consistent with other reports of nonspatially specific saccade preparation (Astafiev et al. 2003; Brown et al. 2007; Curtis et al. 2005; Curtis and D’Esposito 2003b; DeSouza et al. 2003). Moreover, these results are consistent with monkey electrophysiological work showing that parietal area LIP neurons increase in spike rate in anticipation of making a saccade even in the absence of the spatial direction of the saccade (Dickinson et al. 2003; Stoet and Snyder 2004). Similarly, our results in the PCS, the putative human FEF, are consistent with nonspatial preparatory signals recorded in monkey FEF neurons (Lawrence and Snyder 2006).
Although we did not test to see if these preparatory signals were effector-specific, other studies have done so and are relevant here. Within the FEF, surprisingly, there is not convincing evidence for effector-specificity for eye compared to forelimb movements during preparatory delays in humans (Astafiev et al. 2003; Connolly et al. 2007; Connolly et al. 2000; Levy et al. 2007) or monkeys (Lawrence and Snyder 2006); neural activity precedes planned saccades and forelimb movements. Within the monkey parietal cortex, area LIP, like the FEF, has traditionally been thought of as an oculomotor region (Andersen et al. 1992), but it too shows activity preceding planned eye and forelimb movements (Dickinson et al. 2003). Importantly, these signals, which may be related to “preparatory set,” can often be observed when the specific movement cannot be prepared because its spatial goal is not available. The current study provides additional evidence that the putative human FEF and LIP is involved in such a simple “preparatory set.” In both monkey FEF and LIP, however, signals are often greater when the spatial goal of the movement is known and the metrics of the movement can be prepared. It is thought that non-spatially specific and spatially specific information combines linearly in these regions (Dickinson et al. 2003; Lawrence and Snyder 2006). Our data contribute to this literature by showing that indeed activity in the putative human FEF and LIP is greater when spatial information is available for motor planning; activity in sPCS and IPS was greater during the preparatory delay during the known compared to unknown trials (Figure 6).
Second, we expected that the field of the visual cue or the direction of the planned saccade would spatially bias neural activity. Specifically, we tested the hypothesis that activity would be greater in the hemisphere opposite the hemifield of the visual target guiding the saccade, i.e., a visual contralateralized bias. We tested the alternative hypothesis that activity would be biased by the direction of the planned saccade, i.e., a motor contraversive bias. The maintenance of a planned pro-saccade evoked delay period activity that was greater in the hemisphere contralateral to the visual cue in the sPCS, a finding that is consistent with both the visual and motor bias hypotheses. To differentiate between these two hypotheses, we relied on signal evoked during the planning of anti-saccades, where the visual stimulus and saccade plan were in opposite hemifields. We found that differential activity was consistent with the contraversive hypothesis; namely, that activity was greater in the hemisphere opposite the direction of the saccade, but not during the visual cue guiding the saccade (Figure 3). Similar conclusions have been made by Medendorp and colleagues (Medendorp et al. 2006; 2005) regarding a region of the IPS that they refer to as “retIPS,” for retinotopic IPS, which had contralateralized memory-guided saccade activity. In these studies, the authors demonstrated that retIPS primarily stores saccade goals as opposed to the visual cues specifying the saccade directions (also see (Gottlieb and Goldberg 1999; Platt and Glimcher 1997). Our data support their claims and further extend them to the putative human FEF, which has not been demonstrated previously. Indeed, the response field (RF) of monkey FEF neurons is often located in the contralateral visual field (Bruce and Goldberg 1985; Marrocco 1978; Schall 1991; Tehovnik and Sommer 1997). Moreover, electrical stimulation of the human FEF induces saccades to the contralateral visual field (Blanke et al. 1999) and lesions disrupt contraversive saccades (Gaymard et al. 1999; Rivaud et al. 1994). Therefore, our data provide new convergence with existing monkey and human studies.
Implications for the nature of persistent activation during spatial working memory delays
Persistent neural activity during the retention interval of memory guided saccade tasks has been most consistently found in the sPCS and IPS, presumably human homologues of monkey areas FEF and LIP (for reviews see (Curtis 2006; Postle 2006)). Since the precise metrics of the forthcoming saccade are known during these memory delays, we have argued that a component of these signals may originate from neurons whose activity codes for the spatial goal of planned saccades (Curtis 2006; Curtis and D’Esposito 2006; Curtis et al. 2004). Such a prospective code is different from a retrospective code (e.g., the color, shape, or position of a past stimulus) that is most usually associated with theories of working memory (Curtis and D’Esposito 2003a; Fuster 2001; Srimal and Curtis in press). Here, we show that BOLD signals persist in the frontal and parietal cortex throughout the delay interval even when working memory is not required since the target is continuously visible (i.e., there is no need to remember the location of the saccade target). Therefore, the delay period activity that is often interpreted as the signature of working memory maintenance may in fact rely on mechanisms related to prospective motor planning or spatial attention to bridge the temporal gap between a stimulus and its contingent response.
Patterns of activity in frontal and parietal cortex are often strikingly similar during visuospatial working memory, but the two cortices may make distinct contributions. One hypothesis suggests that the frontal cortex represents prospective information, while the parietal cortex represents retrospective information (Fuster 2001). Our recent work has generally supported this distinction (Connolly et al. 2007; Connolly et al. 2005; Connolly et al. 2002; Curtis 2006; Curtis and D’Esposito 2006; Curtis et al. 2004), but with the caveat that the distinction is relative. For instance, we show here that both sPCS and IPS are active when maintaining a saccade plan, just as they are when subjects maintain spatial information in working memory (Curtis and D’Esposito 2006; Curtis et al. 2004; Srimal and Curtis in press), and just as they are when subjects maintain covert attention (Ikkai and Curtis in press). If we assume attention is directed to the location of an external target (e.g., a visible cue) or an internal target (e.g., a memorized cue or transformed anti-cue), then attentional factors may be the commonality between spatial working memory and saccade planning (Awh et al. 2006; Corbetta et al. 2002; Postle et al. 2006; Srimal and Curtis in press; Theeuwes et al. 2005). Similarly, all of the effects that we have called saccade planning could be mediated through attention mechanisms. For instance, attention could be fixed at the location of the planned saccade. Area FEF and LIP may contain populations of neurons that form topographic maps of prioritized space (Goldberg et al. 2002; Serences and Yantis 2006; Thompson et al. 2005). A read-out of such a map of prioritized locations could be used to guide eye-movements, whether they are visually or memory guided, as well as guide our attention.
Prosaccades versus antisaccades
We generally found greater BOLD responses to anti- compared to pro-saccade trials, in agreement with other fMRI studies (Brown et al. 2006; Brown et al. 2007; Connolly et al. 2002; Cornelissen et al. 2002; Curtis and D’Esposito 2003b; DeSouza et al. 2003; Ford et al. 2005). As a side note, spike rate prior to antisaccades compared to prosaccades is lower in FEF neurons (Everling and Munoz 2000). The most likely reason for the discrepancy is methodological; BOLD signal is sensitive not only to neural spiking but to synaptic activity (Logothetis and Wandell 2004), making it potentially sensitive to post-synaptic potentials related to inhibition. Nevertheless, several differences in task requirements are likely responsible for increased activity on antisaccade trials. One important difference is response selection demands. On antisaccade trials, the saccade vector must be flipped 180° from the visually cued location. The difference in saccadic response times between pro- and anti-saccades was much greater in the unknown compared to known condition (∼25 ms vs. ∼112 ms), indicating that response selection took place as soon as the visual cue was given, before the preparatory delay in the known condition, but after it in the unknown condition. Consistent with this hypothesis, we found greater BOLD differences between anti- and pro-saccades early in the trial (i.e., cue period) in the known condition and later in the trial at the response epoch in the unknown condition. These two contrasts resulted in greater activity in the sPCS and IPS for antisaccades (Figures 2b and 3b). An alternative, but not mutually exclusive, hypothesis is that the differences emerge from implementing a “preparatory set” or the task rule (Evarts et al. 1984; Miller and Cohen 2001). We find support for this hypothesis as well. In the unknown condition, when the specific response cannot be selected, small increases in BOLD were found in the sPCS and IPS as has been reported elsewhere (Astafiev et al. 2003; Connolly et al. 2007; Connolly et al. 2002; Medendorp et al. 2006; 2005). Therefore, both rule implementation and response selection effects are reflected in the frontal and parietal cortex. These differences cannot be due to differences in inhibition, as in the known condition, because saccades to the abrupt onset of the visual cue must be inhibited on both pro- and anti-saccade trials. Indeed, when subjects were instructed to select the transformed response in the known condition, antisaccades were only slightly delayed compared to prosaccades. At the response epoch of the unknown condition, antisaccade trials required inhibition of the “reflex-like” saccade to the cue, but prosaccade trials did not. The dorsal anterior cingulate and the dorsolateral prefrontal cortex were active during this BOLD contrast, suggesting their involvement in saccade inhibition (Brown et al. 2006; Curtis et al. 2005). However, other factors such as performance or conflict monitoring could have also contributed to the difference.
Summary
In the current set of experiments, we demonstrated that neural activity in the frontal and parietal cortex persisted while subjects were in an active state of maintaining a saccade plan. This activity predicted whether the subject knew the specific metrics of the saccade, whether the saccade was directed towards an internal representation (i.e., anti-cue location), and the direction of the planned movement. These findings are in accord with observations in homologous brain regions in the monkey and together support the hypothesis that both the human frontal and parietal cortices are involved in the spatial selection and preparation of saccades.
ACKNOWLEDGEMENTS
We thank Marcus Lauer, Adam Riggall, Riju Srimal, Akiko Ikkai, Trent Jerde, Souheil Inati, and Keith Sanzenbach for assistance. This work was supported by grants from the NIH R01 EY016407 and the Seaver Foundation.
REFERENCES
- Andersen RA, Brotchie PR, Mazzoni P. Evidence for the lateral intraparietal area as the parietal eye field. Curr Opin Neurobiol. 1992;2:840–846. doi: 10.1016/0959-4388(92)90143-9. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Hyde JS. Contour-based registration technique to differentiate between task-activated and head motion-induced signal variations in fMRI. Magn Reson Med. 1997;38:470–476. doi: 10.1002/mrm.1910380315. [DOI] [PubMed] [Google Scholar]
- Blanke O, Morand S, Thut G, Michel CM, Spinelli L, Landis T, Seeck M. Visual activity in the human frontal eye field. Neuroreport. 1999;10:925–930. doi: 10.1097/00001756-199904060-00006. [DOI] [PubMed] [Google Scholar]
- Brown MR, Goltz HC, Vilis T, Ford KA, Everling S. Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. Neuroimage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brown MR, Vilis T, Everling S. Frontoparietal Activation with Preparation for Antisaccades. J Neurophysiol. 2007 doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Friedman HR, Kraus MS, Stanton GB. The primate frontal eye field. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. The MIT Press; Cambridge, MA: 2004. pp. 1428–1448. [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Calton JL, Dickinson AR, Snyder LH. Non-spatial, motor-specific activation in posterior parietal cortex. Nat Neurosci. 2002;5:580–588. doi: 10.1038/nn0602-862. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Cant JS, Munoz DP. Effector-specific fields for motor preparation in the human frontal cortex. Neuroimage. 2007;34:1209–1219. doi: 10.1016/j.neuroimage.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Desouza JF, Menon RS, Vilis T. A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. J Neurophysiol. 2000;84:1645–1655. doi: 10.1152/jn.2000.84.3.1645. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Goltz HC, Munoz DP. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J Neurophysiol. 2005;94:605–611. doi: 10.1152/jn.00830.2004. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Cornelissen FW, Kimmig H, Schira M, Rutschmann RM, Maguire RP, Broerse A, Den Boer JA, Greenlee MW. Event-related fMRI responses in the human frontal eye fields in a randomized pro- and antisaccade task. Exp Brain Res. 2002;145:270–274. doi: 10.1007/s00221-002-1136-3. [DOI] [PubMed] [Google Scholar]
- Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139:173–180. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D’Esposito M. Canceling planned action: an FMRI study of countermanding saccades. Cereb Cortex. 2005;15:1281–1289. doi: 10.1093/cercor/bhi011. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003a;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95:3923–3927. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003b;15:409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D’Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci. 2004;24:3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Ballard D, Zarahn E, Aguirre GK. The role of prefrontal cortex in sensory memory and motor preparation: an event-related fMRI study. Neuroimage. 2000;11:400–408. doi: 10.1006/nimg.2000.0571. [DOI] [PubMed] [Google Scholar]
- DeSouza JF, Menon RS, Everling S. Preparatory set associated with prosaccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol. 2003;89:1016–1023. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- Dickinson AR, Calton JL, Snyder LH. Nonspatial saccade-specific activation in area LIP of monkey parietal cortex. J Neurophysiol. 2003;90:2460–2464. doi: 10.1152/jn.00788.2002. [DOI] [PubMed] [Google Scholar]
- Evarts E, Shinoda Y, Wise S. Neurophysiological Approaches to Higher Brain Functions. Wiley; New York: 1984. [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. J Neurophysiol. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud-Pechoux S, Pierrot-Deseilligny C. The frontal eye field is involved in spatial short-term memory but not in reflexive saccade inhibition. Exp Brain Res. 1999;129:288–301. doi: 10.1007/s002210050899. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bisley J, Powell KD, Gottlieb J, Kusunoki M. The role of the lateral intraparietal area of the monkey in the generation of saccades and visuospatial attention. Ann N Y Acad Sci. 2002;956:205–215. doi: 10.1111/j.1749-6632.2002.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Goldberg ME. Activity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nat Neurosci. 1999;2:906–912. doi: 10.1038/13209. [DOI] [PubMed] [Google Scholar]
- Hallett P. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Ikkai A, Curtis CE. Cortical activity time-locked to the shift and maintenance of spatial attention. Cereb Cortex. doi: 10.1093/cercor/bhm171. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence BM, Snyder LH. Comparison of effector-specific signals in frontal and parietal cortices. J Neurophysiol. 2006;96:1393–1400. doi: 10.1152/jn.01368.2005. [DOI] [PubMed] [Google Scholar]
- Lawrence BM, White RL, 3rd, Snyder LH. Delay-period activity in visual, visuomovement, and movement neurons in the frontal eye field. J Neurophysiol. 2005;94:1498–1508. doi: 10.1152/jn.00214.2005. [DOI] [PubMed] [Google Scholar]
- Levy I, Schluppeck D, Heeger DJ, Glimcher PW. Specificity of human cortical areas for reaches and saccades. J Neurosci. 2007;27:4687–4696. doi: 10.1523/JNEUROSCI.0459-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Marrocco RT. Saccades Induced by Stimulation of the Frontal Eye Fields: interaction with voluntary and reflexive eye movements. Brain Research. 1978;146:23–34. doi: 10.1016/0006-8993(78)90215-9. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T. Directional selectivity of BOLD activity in human posterior parietal cortex for memory-guided double-step saccades. J Neurophysiol. 2006;95:1645–1655. doi: 10.1152/jn.00905.2005. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T. Remapping the remembered target location for anti-saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:734–740. doi: 10.1152/jn.01331.2004. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nichols TEaH AP. Nonparametric permutation tests for functional neuroimaging : a primer with examples. Human Brain Mapping. 2002;15:1–15. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Responses of intraparietal neurons to saccadic targets and visual distractors. J Neurophysiol. 1997;78:1574–1589. doi: 10.1152/jn.1997.78.3.1574. [DOI] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat Neurosci. 2000;3:1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Idzikowski C, Sala SD, Logie RH, Baddeley AD. The selective disruption of spatial working memory by eye movements. Q J Exp Psychol (Colchester) 2006;59:100–120. doi: 10.1080/17470210500151410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requin J, Lecas JC, Vitton N. A comparison of preparation-related neuronal activity changes in the prefrontal, premotor, primary motor and posterior parietal areas of the monkey cortex: preliminary results. Neurosci Lett. 1990;111:151–156. doi: 10.1016/0304-3940(90)90360-l. [DOI] [PubMed] [Google Scholar]
- Riehle A, Requin J. The predictive value for performance speed of preparatory changes in neuronal activity of the monkey motor and premotor cortex. Behav Brain Res. 1993;53:35–49. doi: 10.1016/s0166-4328(05)80264-5. [DOI] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot-Deseilligny C. Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res. 1994;102:110–120. doi: 10.1007/BF00232443. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields. J Neurophysiol. 1991;66:559–579. doi: 10.1152/jn.1991.66.2.559. [DOI] [PubMed] [Google Scholar]
- Schlag J, Dassonville P, Schlag-Rey M. Interaction of the two frontal eye fields before saccade onset. J Neurophysiol. 1998;79:64–72. doi: 10.1152/jn.1998.79.1.64. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci. 2006;26:5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidemann E, Arieli A, Grinvald A, Slovin H. Dynamics of depolarization and hyperpolarization in the frontal cortex and saccade goal. Science. 2002;295:862–865. doi: 10.1126/science.1066641. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: a review. Vision Res. 2000;40:1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- Srimal R, Curtis CE. Persistent neural activity during the maintenance of spatial position in working memory. Neuroimage. doi: 10.1016/j.neuroimage.2007.08.040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoet G, Snyder LH. Single neurons in posterior parietal cortex of monkeys encode cognitive set. Neuron. 2004;42:1003–1012. doi: 10.1016/j.neuron.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Takeda K, Funahashi S. Population vector analysis of primate prefrontal activity during spatial working memory. Cereb Cortex. 2004;14:1328–1339. doi: 10.1093/cercor/bhh093. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Electrically evoked saccades from the dorsomedial frontal cortex and frontal eye fields: a parametric evaluation reveals differences between areas. Exp Brain Res. 1997;117:369–378. doi: 10.1007/s002210050231. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Olivers CN, Chizk CL. Remembering a location makes the eyes curve away. Psychol Sci. 2005;16:196–199. doi: 10.1111/j.0956-7976.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D’Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]