Abstract
During the last decade, evidence has been accumulated in our laboratories (24, 25, 37–40) that substances termed hepatotrophic factors in portal venous blood can specifically influence liver function as well as the size, chemical composition and dividing capability of the hepatocytes. In this report, the role of these portal hepatotrophic factors will be examined in the acute regeneration process that follows partial hepatectomy.
METHODS
Animal Groups
Seventy mongrel dogs, weighing 8 to 29 kilograms, contributed to the final data. An approximately equal number of experiments failed because of thrombosis of the vascular reconstructions, premature death of the dog from a variety of other complications or because of failure to produce alloxan diabetes.
The various operations, biopsies and sacrifice procedures were performed under anesthesia with pentobarbital sodium supplemented with phencyclidine hydrochloride (Sernylan®) and succinylcholine chloride (Anectine Chloride®).
Group 1
Eleven normal dogs were anesthetized, group 1A. After obtaining several grams of tissue from one of the right and one of the left liver lobes for the various analyses, the dogs were sacrificed (Fig. 1a). These same dogs were the normal controls in another recent publication (40).
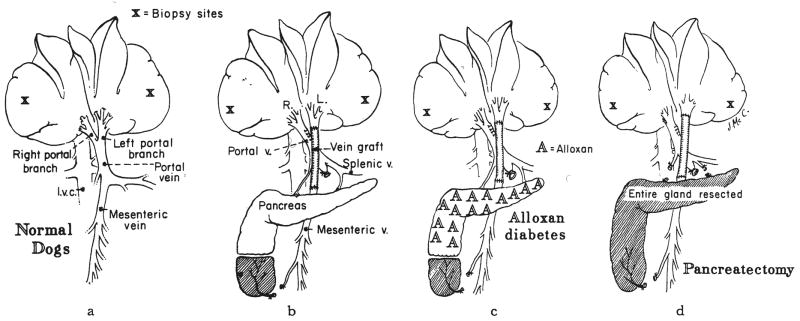
Fig. 1.
A series of control procedures not involving partial hepatectomy. The dogs either were normal or had been submitted to acute splanchnic division, a, Group 1—normal dogs, with or without sham operation. b, Group 2—dogs in which the left liver lobes were perfused with intestinal blood and in which the right liver lobes received pancreaticogastroduodenosplenic venous blood. Explanation of partial removal of inferior lobe of pancreas is given in text. c, Group 3—same operation as in b, but in dogs with alloxan-induced diabetes. d, Group 4—same operation as in b, but with total pancreatectomy. The duodenum and common duct were left intact.
Six additional normal dogs of group 1B were submitted to a sham operation that was designed to simulate the procedure in group 2. The portal vein and its bifurcation were dissected free as well as the more inferior junction of the superior mesenteric and splenic veins. The superior mesenteric vein was occluded for 20 to 30 minutes after which the tip of the inferior lobe of the pancreas was resected as shown in Figure 1b. Four days later, the dogs were sacrificed, and tissue was obtained for both the histopathologic and the autoradiographic studies.
Group 2
Five dogs had a procedure called splanchnic division, which diverts the nutrient rich intestinal venous blood into the left liver lobes through a reversed external jugular vein graft (37) and which provides perfusion of the right liver lobes by the hormone rich pancreaticogastroduodenosplenic venous blood (Fig. 1b). The tail of the inferior lobe of the pancreas was resected (Fig. 1b) to prevent the experimental artifact that would have been caused by drainage of this small piece of pancreatic tissue into the intestinal vein and, from there, into the left liver lobes.
Group 3
Stable diabetes mellitus was induced with a single injection of 70 to 80 milligrams of alloxan—mesoxalyl urea—per kilogram of body weight. The diabetes mellitus was managed by daily subcutaneous injections of 10 to 15 units of neutral protamine Hagedorn insulin. Three to eight weeks later, two dogs had the same splanchnic division procedure as those in group 2 (Fig. 1c). Insulin therapy was continued in the three and five day periods of postoperative observation.
Group 4
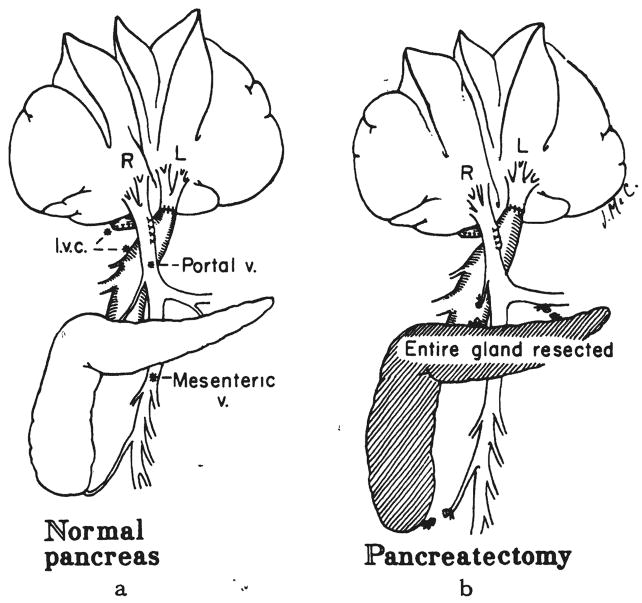
Six dogs had total pancreatectomy with preservation of the duodenum and common duct at the same operation as splanchnic venous division (Fig. 1d). Three of the dogs were given insulin subcutaneously for the three to four day period of postoperative study, group 4A. The diabetes mellitus of the remaining three dogs was not treated, group 4B.
Group 5
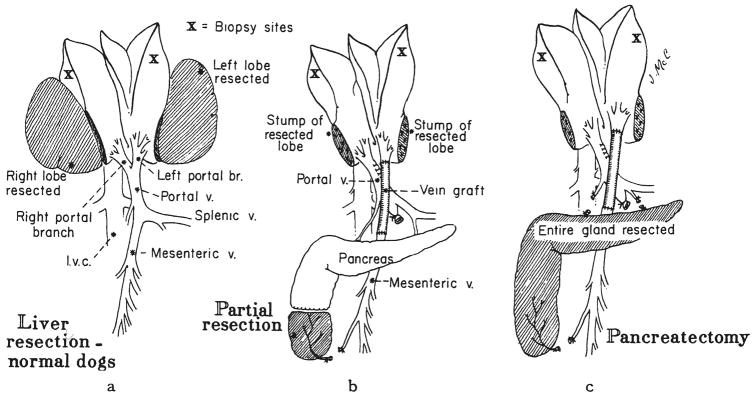
Four normal dogs had complete removal of the most right and the most left liver lobes (Fig. 2a). These two lobes make up an estimated 30 per cent of the total liver weight. The dogs were sacrificed four days later.
Fig. 2.
Procedures involving hepatectomy or acute splanchnic division. a, Groups 5 and 6— hepatectomy without splanchnic division. The extent of hepatectomy was 30 per cent in group 5 and 60 per cent in group 6. b, Groups 7 and 8—splanchnic division was carried out plus 30 per cent, group 7, or 60 per cent, group 8, hepatectomy. c, Group 9—splanchnic division, total pancreatectomy and 30 per cent hepatectomy on the same day.
Group 6
Four dogs had resection of the two most leftward liver lobes, the most right liver lobe and the tip of a small lobe located along the left side of the vena cava. The estimated resection was 60 per cent. The dogs were sacrificed three days later.
Group 7
Six dogs had the same splanchnic division procedure as those in group 2. In addition, a 30 per cent hepatectomy was performed (Fig. 2b). The dogs were sacrificed four to five days later.
Group 8
Five dogs had the same splanchnic division procedure as those in group 2, plus a 60 per cent hepatectomy. The dogs were sacrificed after two or three days.
Group 9
Four dogs had total pancreatectomy on the same day as a 30 per cent hepatectomy and splanchnic division (Fig. 2c). During the four days between operation and sacrifice, two dogs were treated subcutaneously with insulin, group 9A. Two dogs were not treated, group 9B.
Group 10
Five dogs had partial portacaval transposition (Fig. 3a). The left portal branch was detached and anastomosed end-to-end to the suprarenal inferior vena cava. Thus, the left lobes were perfused with systemic venous blood, including the renal and adrenal effluent, whereas the right lobes were exposed to the total nonhepatic splanchnic venous return. The dogs were sacrificed five days after operation, except for one dog that was observed for seven days.
Fig. 3.
Procedures involving acute partial portacaval transposition without partial hepatectomy. a, Group 10— partial portacaval transposition with systemic venous blood to left lobes. b, Group 11—same as in a, plus total pancreatectomy.
Group 11
Three dogs had simultaneous partial portacaval transposition and total pancreatectomy (Fig. 3b). Postoperatively, they were not treated with insulin during the four or five days of the experiment.
Group 12
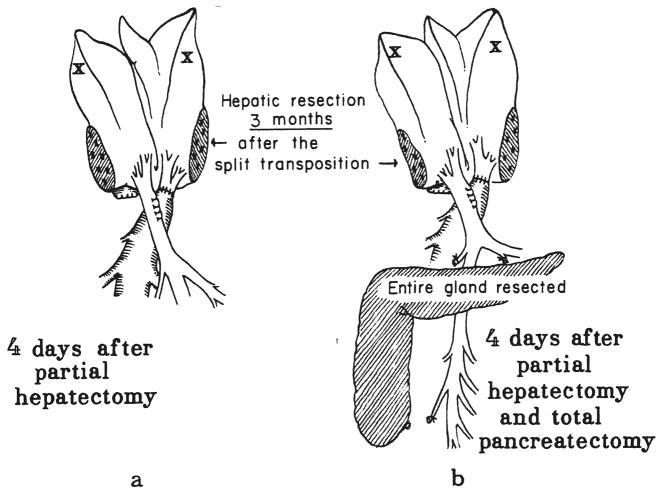
Five dogs underwent partial portacaval transposition. From 89 to 95 days later, reoperation was performed. The expected hypertrophy of the right lobes and the atrophy of the left lobes were found. The most far right and the most far left lobes were removed (Fig. 4a), for an estimated 30 per cent resection. After four more days, the dogs were sacrificed.
Fig. 4.
Chronic partial portacaval. transposition plus delayed hepatectomy. a, Group 12—30 per cent hepatectomy three months after transpositon. b, Group 13—30 per cent hepatectomy and total pancreatectomy three months after transposition.
Group 13
Four dogs had the same 30 per cent hepatectomy as those in group 12, 89 to 95 days after partial portacaval transposition. In addition, the pancreas was totally removed (Fig. 4b). Subcutaneous insulin was given for the next four days until sacrifice.
Deoxyribonucleic Acid Synthesis
About two hours before sacrifice, the 70 dogs were given (CH3-3H) thymidine by the intravenous route. The specific activity was 6.4 curies per millimole. Because doses of 1.5 or 4.5 millicuries of (CH3-3H) thymidine were used at different phases of the investigation, but without consideration of body weight, the dose spectrum was broad, ranging from 0.05 to 0.41 millicurie per kilogram of body weight.
At the time of sacrifice, liver biopsies were quick-frozen and kept at minus 20 degrees C. until the analyses were completed. Extraction and purification of deoxyribonucleic acid were carried out according to the method of Schneider and Greco (32) with minor modifications as described elsewhere (40). In essence, the method involves first removing the ribonucleic acid and acid soluble fractions with sodium hydroxide and cold perchloric acid, respectively. Deoxyribonucleic acid is then extracted from the residue, including protein and lipid, with hot perchloric acid. The deoxyribonucleic acid content was measured by the diphenylamine method of Burton (5), using calf thymus deoxyribonucleic acid as the standard.
The in vivo incorporation of (CH3-3H) thymidine into deoxyribonucleic acid was estimated by measuring the specific activity of the deoxyribonucleic acid obtained by extraction. The details of the counting were described in another publication (40). The results of the radioactivity uptake were expressed as counts per minute per 10 micrograms of deoxyribonucleic acid.
Pathologic Studies
Samples of liver tissue were fixed in 10 per cent normal buffered Formalin® (aqueous solution of formaldehyde). Frozen sections were cut and stained with hematoxylin and eosin, Gordon and Sweet’s silver impregnation method for reticulin fibers, Perls’ Prussian blue method for iron, tri-chrome for collagen and fibrin, periodic acid-Schiff method for glycogen and Pearse’s method for ceroid and lipofuscin.
Other paraffin sections were covered by stripping film. The autoradiographs were exposed for 28 to 64 days, developed and then stained through the emulsion with Ehrlich’s hematoxylin and eosin by Pelc’s method.
Additional small hepatic samples were initially fixed in glutaraldehyde solution and then postfixed in osmic acid and embedded in Epon® (synthetic embedding medium). Half micron and ultrathin sections were cut. The former were stained with azure II for examination in the light microscope, while the latter were stained with lead citrate and examined in a Philips 300 electron microscope.
The size of the hepatocytes was determined on hematoxylin and eosin stained sections by a method previously described (37). In essence, the technique consists of tracing out large numbers of hepatocytes on standard thickness paper, cutting out the silhouettes and weighing them. The weights were designated as size units. Midzonal hepatocytes also were used for measuring rough endoplasmic reticulum length per area of cytoplasm by the morphometric method of Loud (21).
RESULTS
Normal Dogs
Deoxyribonucleic acid synthesis
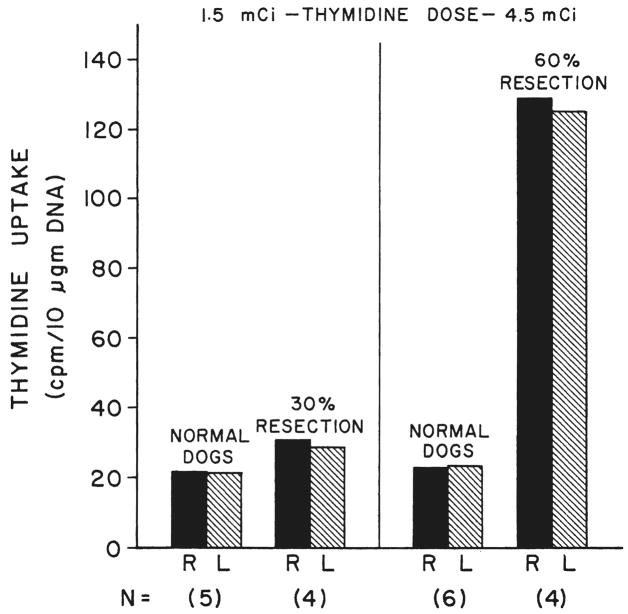
Thymidine uptake was equal in the right and left lobes of the livers of the 11 normal dogs of group 1A. The average specific activity was not much different at the doses of 1.5 and 4.5 millicuries (Fig. 5). A 30 per cent hepatic resection resulted in a slight bilateral increase in the specific activity of the residual hepatic tissue of the dogs of group 5 that were administered 1.5 millicuries of tritiated thymidine. After a 60 per cent hepatectomy, the dogs of group 6 that were given 4.5 millicuries of tritiated thymidine had a sixfold increase in specific activity that was equal on both sides (Fig. 5).
Fig. 5.
Tritiated thymidine uptake expressed as specific activity in the right, R, and left, L, liver lobes of normal dogs with and without hepatic resections. Two doses of [CH3-3H] thymidine were used. Five of the normal dogs from group 1 received 1.5 millicuries; the other six were given 4.5 millicuries. The four dogs of group 5 had 30 per cent liver resections; four days later, they received the smaller thymidine dose, Another four dogs, group 6, were sacrificed three days after 60 per cent resection; they received the larger thymidine dose. Note that 30 per cent resection caused only a slight increase in thymidine uptake, whereas the 60 per cent resection produced a sixfold increase in specific activity. N, Number of experiments.
Histopathology and autoradiography
There were no significant differences in the sizes of either the lobules or the hepatocytes in the right and left liver lobes of the 11 normal dogs in group 1A. All of these livers lacked fat and nine contained normal amounts of glycogen and appeared normal ultrastructurally. The other two livers were deficient in glycogen. Autoradiographs of the livers of the dogs in group 1A showed similar low numbers of labeled hepatocytes in the right and left lobes (Table I). The findings were the same in the dogs of group 1B after the sham operation, as shown in Table I.
TABLE I.
COMPARISONS OF HEPATOGYTE SIZE AND CELL DIVISION BY AUTORADIOGRAPHY IN SPLANCHNICDIVISION EXPERIMENTS
| Statistical comparisons between groups, p values | Hepatocyte size, size units |
No. of labeled hepatocytes per 1,000 hepatocytes |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| R. vs. L. | R. vs. L. | ||||||||
| Group | Description | No. exp. | Right | p value | Left | Right | p value | Left | |
| 1A | Normal dogs | 11 | 0.176 ± 0.041 | NS | 0.175 ± 0.047 | 1.6 ± 0.5 | NS | 1.5 ± 0.4 | |
| 1B | Normal sham | 6 | 0.172 ± 0.043 | NS | 0.172 ± 0.048 | 1.7 NS ± 1.1 | 1.8 ± 0.9 | ||
| 5 | 30 per cent resection | 4 | 0.186 ± 0.034 | NS | 0.192 ± 0.010 | 5.3 ± 1.7 | NS | 5.5 ± 1.4 | |
| 5 vs. 1 | NS | NS | <0.001 | <0.001 | |||||
| 6 | 60 per cent resection | 4 | 0.200 ± 0.072 | NS | 0.198 ± 0.020 | 14.6 ± 1.4 | NS | 14.2 ± 1.7 | |
| 6 vs. 1 | NS | NS | <0.001 | <0.001 | |||||
| 6 vs. 5 | NS | NS | <0.001 | <0.001 | |||||
| 2 | Splanchnic division | 5 | 0.218 ± 0.071 | <0.02 | 0.109 ± 0.039 | 16.5 ± 2.5 | <0.001 | 5.4 ± 1.1 | |
| 2 vs. 1 | NS | <0.02 | <0.001 | <0.001 | |||||
| 7 | Splanchnic division + 30 per cent resection | 6 | 0.254 ± 0.072 | <0.01 | 0.188 ± 0.069 | 17.2 ± 1.5 | <0.001 | 9.9 ± 2.0 | |
| 7 vs. 5 | NS | NS | <0.001 | <0.01 | |||||
| 7 vs. 2 | NS | NS | NS | <0.01 | |||||
| 8 | Splanchnic division + 60 per cent resection | 5 | 0.285 ± 0.081 | <0.05 | 0.218 ± 0.033 | 24.7 ± 5.0 | <0.01 | 14.8 ± 2.8 | |
| 8 vs. 6 | NS | NS | <0.01 | NS | |||||
| 8 vs. 2 | NS | <0.01 | <0.02 | <0.001 | |||||
| 8 vs. 7 | NS | NS | <0.01 | <0.01 | |||||
| 3 | Splanchnic division + alloxan diabetes + insulin | 2 | 0.167 ± 0.005 | NS | 0.163 ± 0.007 | 1.9 ± 1.0 | <0.02 | 9.6 ± 1.3 | |
| 3 vs. 1 | NS | NS | NS | <0.001 | |||||
| 3 vs. 2 | NS | NS | < 0.001 | <0,01 | |||||
| 4A | Splanchnic division+ pancreatectomy+ insulin | 3 | 0.168 ± 0.023 | NS | 0.165 ± 0.028 | 1.9 ± 1.0 | <0.01 | 8.8 ± 1.8 | |
| 4Avs. 1 | NS | NS | NS | < 0.001 | |||||
| 4Avs. 2 | NS | NS | < 0.001 | <0.02 | |||||
| 4B | Splanchnic division + pancreatectomy, no insulin | 3 | 0.147 ± 0.043 | NS | 0.144 ± 0.041 | 1.8 ± 1.1 | NS | 1.7 ± 0.7 | |
| 4Bvs. 1 | NS | NS | NS | NS | |||||
| 4B vs. 2 | NS | NS | <0.001 | <0.01 | |||||
| 4B vs. 4A | NS | NS | NS | <0.01 | |||||
| 4AandB | Splanchnic division + pancreatectomy | 6 | 0.158 ± 0.033 | NS | 0.155 ± 0.033 | 1.9 ± 0.9 | NS | 5.3 ± 4.1 | |
| 4 vs. 1 | NS | NS | NS | <0.01 | |||||
| 4 vs. 2 | NS | NS | <0.001 | NS | |||||
| 9A | Splanchnic division + pancreatectomy + 30 per cent resection + insulin | 2 | 0.188 ± 0.028 | NS | 0.189 ± 0.042 | 4.8 ± 0.7 | NS | 10.3 ± 1.8 | |
| 9Avs. 5 | NS | NS | NS | <0.05 | |||||
| 9A vs. 4A | NS | NS | <0.05 | NS | |||||
| 9B | Splanchnic division + pancreatectomy + 30 per cent resection, no insulin | 2 | 0.181 ± 0.053 | NS | 0.181 ± 0.058 | 4.9 ± 0.4 | NS | 4.8 ± 0.1 | |
| 9Bvs. 5 | NS | NS | NS | NS | |||||
| 9B vs. 4B | NS | NS | <0.05 | <0.01 | |||||
| 9B vs. 9A | NS | NS | NS | NS | |||||
| 9A and B | Splanchnic division + pancreatectomy + 30 per cent resection | 4 | 0.184 ± 0.035 | NS | 0.185 ± 0.043 | 4.9 ± 0.5 | NS | 7.6 ± 3.3 | |
| 9 vs. 7 | NS | NS | <0.001 | NS | |||||
| 9 vs. 4 | NS | NS | <0.001 | NS | |||||
Mean ± standard deviation.
NS, Not significant.
A 30 per cent hepatic resection in the dogs of group 5 resulted, after four days, in a slight, but nonsignificant, enlargement of the hepatocytes in all lobes (Table I) and in an accumulation of vacuoles of various sizes in the liver cell cytoplasm. In one dog, large fat globules were present in the centrilobular hepatocytes. The amount of stainable glycogen was reduced. The mitotic index was normal, but autoradiographs (Table I) showed that the number of labeled hepatocytes was slightly and significantly raised, p<0.001. Ultrastructurally, the hepatocytes contained more autophagosomes and less glycogen than normal, and the cisternae of the rough endoplasmic reticulum were often dilated.
Three days after 60 per cent hepatectomy in group 6, the hepatocytes were enlarged in both sides of the liver, and their cytoplasm contained many vacuoles and some fat. The amount of stainable glycogen was reduced. Both the mitotic index and the number of labeled hepatocytes (Table I) were markedly raised. Electron microscopic examination of the hepatocytes confirmed the decrease in the amount of cytoplasmic glycogen and showed that the hepatocytes contained many autophagosomes and increased numbers of lipid droplets. Dilatation of the cisternae of the rough endoplasmic reticulum was marked.
Splanchnic Division
Deoxyribonucleic acid synthesis
The absolute specific activities in the dog livers submitted to splanchnic division were so variable from experiment to experiment that data analysis was limited to comparisons between the right and left lobes, using an analytic technique whereby the side with the greater specific activity was assigned a value of 100 per cent. A proportionally smaller percentage was calculated for the liver side with less specific activity.
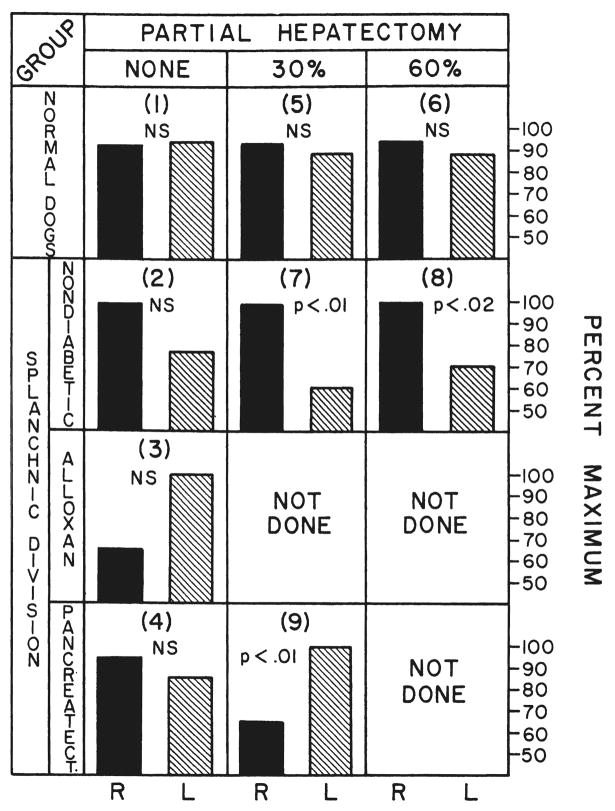
The results are summarized in Figure 6. After splanchnic division, the predominant deoxyribonucleic acid synthesis in the dogs of group 2 was in the right lobes which were perfused with pancreaticogastroduodenosplenic blood, although the lobar differences were not significant. However, this effect was accentuated in groups 7 and 8 by partial hepatectomy and reached statistical significance. The dominance of the right lobes in deoxyribonucleic acid synthesis was eliminated by alloxan-induced diabetes, group 3, or after pancreatectomy, group 4. The most clearcut results were in the dogs of group 9 submitted to 30 per cent hepatic resection plus total pancreatectomy (Fig. 6). The predominant deoxyribonucleic acid synthesis was transferred to the left lobes to a statistically significant degree, p<0.01.
Fig. 6.
Relative thymidine uptake of the right, R, and left, L, liver lobes after splanchnic division. In each experiment, the liver lobe side having the greater specific activity was assigned a value of 100 per cent and a proportionally lower percentage calculated for the other side of the liver. The numbers in parentheses correspond to the dog groups described in the section on methods. Statistical comparisons between the right and left lobes in each group are indicated by p values or by NS, not significant. For comparison, the results are given in the top row from the normal dogs of group 1 and the dogs of groups 5 and 6 after hepatic resection. Note that the advantage in thymidine uptake enjoyed by the right lobes which received pancreaticogastroduodenosplenic blood after splanchnic division in groups 2, 7 and 8 was abolished in groups 3 and 4 or even transferred to the left lobes in group 9 by the creation of diabetes, either with alloxan or by pancreatectomy.
Histopathology and autoradiography
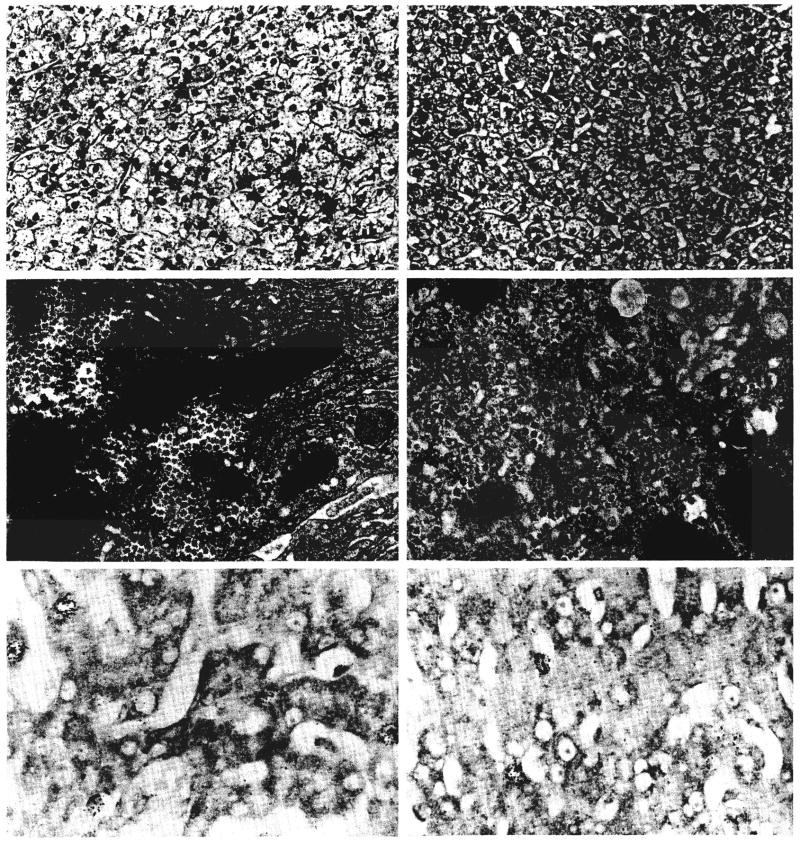
Five days after splanchnic division in the dogs of group 2, the hepatocytes in the left liver lobes, which had received the nutrient rich intestinal venous blood, had undergone a number of changes (Fig. 7). They had decreased in size (Table I), were irregular in shape and were depleted of glycogen. The lobules were shrunken, and the Kupffer cells were increased in number and size and contained hemosiderin. There were no fat globules visible by light microscopy. Ultrastructurally, the rough endoplasmic reticulum was reduced in amount and disorganized, and some of the cisternae were dilated. There was also proliferation of the smooth endoplasmic reticulum, glycogen granules were less frequent than normal and the number of small fat vacuoles was increased. The right liver lobes, which had been perfused by the hormone rich pancreaticogastroduodenosplenic blood, had larger lobules and hepatocytes than were present in the normal dogs of group 1. The enlarged hepatocytes contained normal quantities of glycogen and were free of fat. Binucleate liver cells and proliferating bile ductules were present, and the mitotic index was raised. Ultrastructurally, the enlarged liver cells were essentially normal. Autoradiography showed about three times as many labeled hepatocytes in the enlarged right lobes as in the smaller left lobes (Table I).
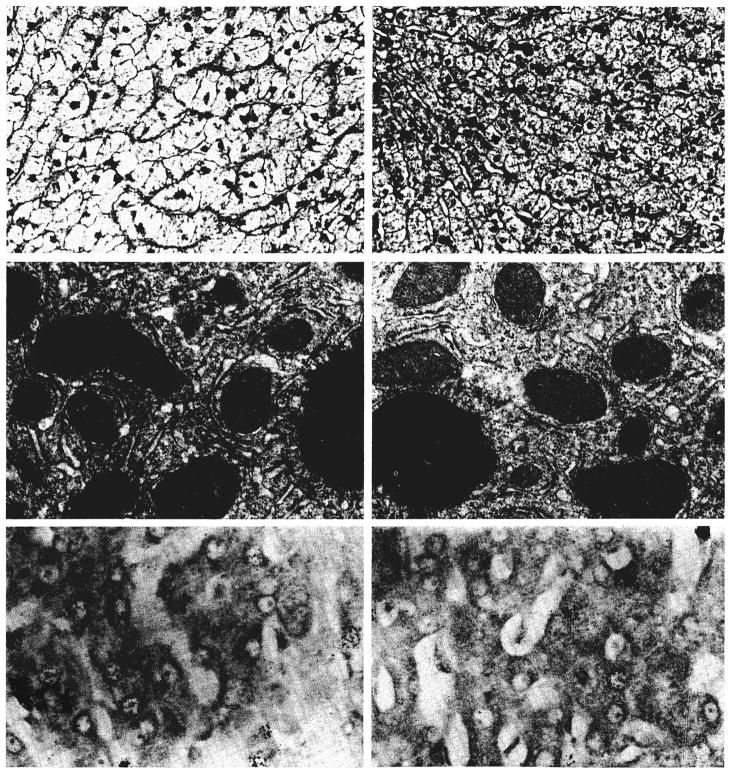
Fig. 7.
Changes in a liver three days after a splanchnic division experiment of group 2 as shown by light microscopy, electron microscopy and autoradiography. The hepatocytes in the nutrient enriched lobes, panels on right, are atrophic; depleted of glycogen and rough endoplasmic reticulum and contain increased smooth endoplasmic reticulum when compared with the enlarged and ultrastructurally normal liver cells in the hormone influenced lobes, panels on left. The rate of cell division, as indicated by autoradiography, is increased on both sides of the liver, but particularly in the hormone influenced lobes. Upper panels, hematoxylin and eosin, ×120; middle panels, electron micrography, ×1,700; and lower panels, autoradiography, ×300.
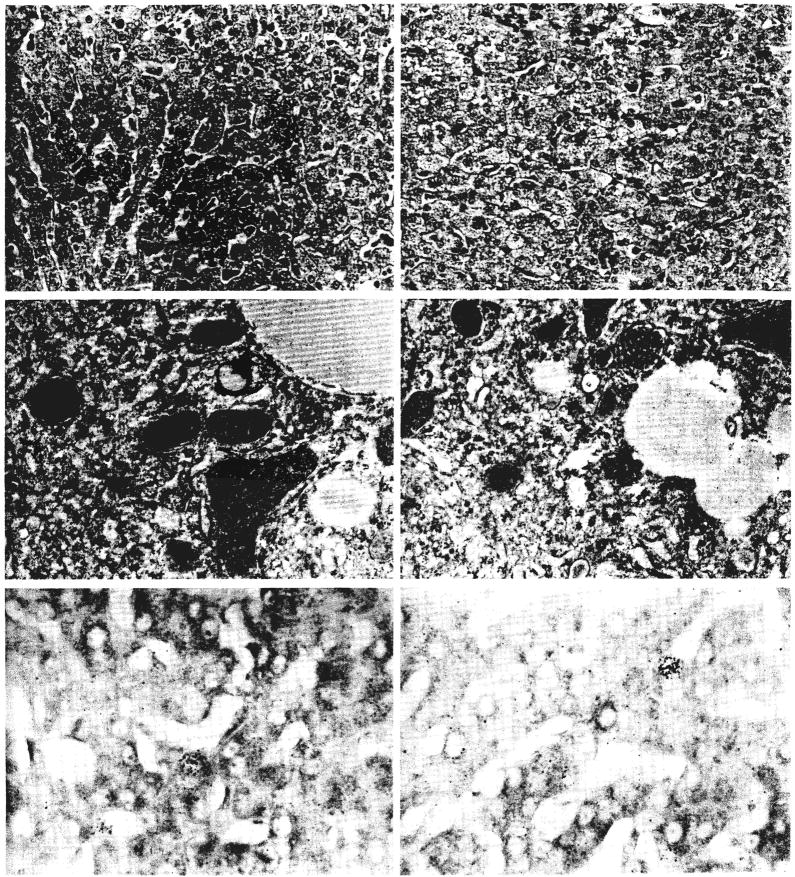
The addition of a 60 per cent partial hepatectomy to splanchnic division in group 8 resulted in the kind of histologic appearance shown in Figure 8. There was marked enlargement of the lobules and hepatocytes on both sides of the liver (Table I). The left lobe hepatocytes, instead of being atrophic, were as large as the liver cells on that side after 60 per cent resection alone. Right lobe hepatocytes were larger than after simple resection. The enlarged right lobar hepatocytes contained less glycogen than normal, and in four of the five livers, light microscopy showed globules of fat in the cytoplasm of the cells on the right side. In two of these livers, fat also was present in the hepatocytes of the left lobes. Binucleate liver cells were present, and the mitotic index was raised on both sides of the liver. Autoradiography showed more labeled hepatocytes on both sides of the liver than after splanchnic division alone (Table I). The numbers of radioactive nuclei on the left sides of the livers were roughly the same as after the 60 per cent resection alone. The numbers on the right side were higher than after either 60 per cent resection or splanchnic division alone. Ultrastructurally, the hepatocytes on both sides contained fewer glycogen granules than normal and increased numbers of autophagosomes and lipid droplets. The amount of rough endoplasmic reticulum was normal, but the cisternae were a little dilated.
Fig. 8.
Changes in a liver three days after a splanchnic division and total pancreatectomy experiment of group 4B as shown by light microscopy, electron microscopy and autoradiography. The hepatocytes on both sides of the liver are atrophic and contain excess cytoplasmic fat. The amount of glycogen and rough endoplasmic reticulum is reduced: The rate of cell division, as indicated by autoradiography, is bilaterally normal. Upper panels, hematoxylin and eosin, ×120; middle panels, electron micrography, ×1,700; and lower panels, autoradiography, ×300.
Similar, but much milder, changes were produced by the combination of 30 per cent partial hepatectomy and splanchnic division, which was used for group 7 (Table I).
The difference in size of the hepatocytes in the left versus the right lobes, brought about by splanchnic division, did not occur in the seven diabetic dogs of groups 3 and 4 (Table I and Fig. 9). After two to five days, the hepatocytes in the right and left lobes were almost equal in size and smaller than those of normal dogs. The hepatocyte atrophy was most obvious in the three dogs of group 4B which were not treated with insulin; these dogs also showed abundant fat in the cytoplasm of the hepatocytes. The livers of the other dogs in groups 3 and 4 were free of fat visible by light microscopy.
Fig. 9.
Changes in a liver three days after a splanchnic division and 60 per cent hepatectomy experiment of group 8 as shown by light microscopy, electron microscopy and autoradiography. The hepatocytes on both sides of the liver are enlarged and contain normal amounts of rough endoplasmic reticulum, increased numbers of autophagosomes and lipid droplets and less glycogen than normal. The rate of cell division, as indicated by autoradiography, is higher on both sides of the liver than after splanchnic division alone and is particularly increased in the hormone influenced lobes, panel on left. Upper panels, hematoxylin and eosin, ×120; middle panels, electron micrography; ×1,700; and lower panels, autoradiography, ×300.
Ultrastructurally, the hepatocytes on both sides of these livers in diabetic groups 3 and 4 showed varying degrees of disruption and loss of rough endoplasmic reticulum, an increase in the amount of smooth endoplasmic reticulum, a loss of glycogen particles and increased numbers of lipid droplets. Autoradiography showed normal numbers of labeled hepatocytes in both sides of the livers of the three diabetic dogs of group 4B that were not given insulin. In the dogs treated with insulin, there were increased numbers of radioactive liver cells in the left lobes.
The addition of the 30 per cent hepatectomy in the diabetic dogs of group 9 was associated with slightly larger hepatocytes both sides of the liver. The hepatocyte size was similar to that seen after liver resection alone in group 5. The numbers of radioactive cells were raised on both sides of the livers in group 9 (Table I), but the number of labeled hepatocytes was highest in the left lobes of the insulin-treated dogs of group 9A. The ultra-structure of the hepatocytes did not differ significantly from that seen in the livers of the diabetic dogs of groups 3 and 4.
Partial Portacaval Transposition
Deoxyribonucleic acid synthesis
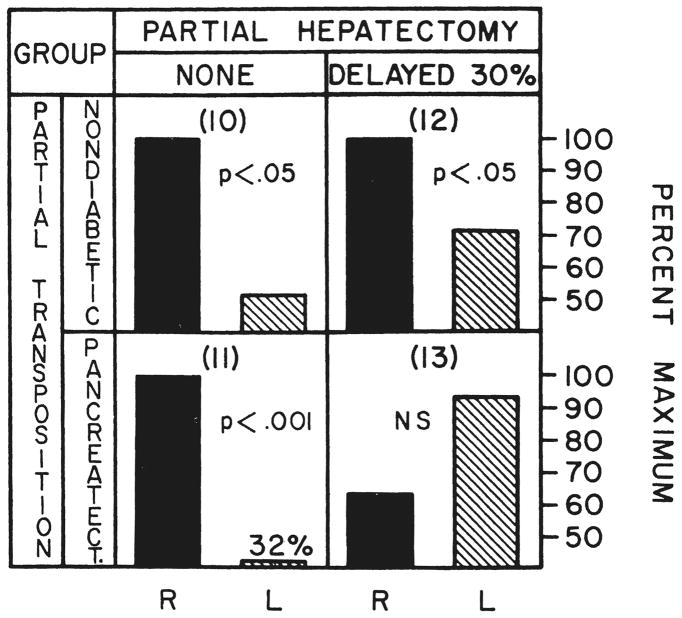
The right liver lobes of the dogs of group 10 which were perfused for four or five days after operation with the total splanchnic venous return had significantly greater deoxyribonucleic acid synthesis than the left lobes which were receiving the venous return from the kidneys and hindquarters (Fig. 10). This right lobar dominance was not changed by the absence of the pancreas in the dogs of group 11 (Fig. 10).
Fig. 10.
Relative thymidine uptake of the right, R, and left, L, liver lobes after partial portacaval transposition. The method of calculation, the nomenclature and the statistical comparisons are the same as in Figure 6. The right liver lobes, which in this preparation received the total splanchnic venous return, had significantly greater deoxyribonucleic acid synthesis, groups 10, 11 and 12, except when pancreatectomy was superimposed upon delayed 30 per cent hepatic resection, group 13.
Three months following partial portacaval transposition and four days after 30 per cent hepatectomy, group 12, deoxyribonucleic acid synthesis was again significantly greater in the right liver lobes. The addition of pancreatectomy at the time of 30 per cent hepatectomy in group 13 switched the predominant deoxyribonucleic acid synthesis to the left lobes, although the lateralization to the left did not achieve significance (Fig. 10).
Histopathology and autoradiography
Four to five days after partial portacaval transposition in the dogs of group 10, the hepatocytes in the left liver lobes, which had received systemic blood, had decreased in size (Table II) and had become depleted of glycogen. The lobules were smaller than normal and the Kupffer cells were increased in size and number and contained hemosiderin. Ultrastructurally, there was loss of rough endoplasmic reticulum, glycogen granules were scarce and there were increased numbers of small lipid droplets. The right liver lobes, which had received splanchnic venous blood, had larger lobules and hepatocytes than were present in the normal dogs of group 1. Ultrastructurally, the enlarged cells were essentially normal. Autoradiographs showed more labeled hepatocytes in the right lobes than in the left lobes (Table II).
TABLE II.
COMPARISONS OF HEPATOCYTE SIZE AND CELL DIVISION BY AUTORADIOGRAPHY IN PARTIAL PORTAGAVAL TRANSPOSITION
| Statistical comparisons between groups, p values | Hepatocyte size, size units |
No. of labeled hepatocytes per. 1,000 hepatocytes |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| R. vs. L. | R. vs. L. | ||||||||
| Group | Description | No. exp. | Right | p value | Left | Right | p value | Left | |
| 1A | Normal dogs | 11 | 0.176 ± 0.041 | NS | 0.175 ± 0.047 | 1.6 ± 0.5 | NS | 1.5 ± 0.4 | |
| 1B | Normal sham | 6 | 0.172 ± 0.043 | NS | 0.172 ± 0.048 | 1.7 ± 1.1 | NS | 1.8 ± 0.9 | |
| 5 | 30 per cent resection | 4 | 0.186 ± 0.034 | NS | 0.192 ± 0.010 | 5.3 ± 1.7 | NS | 5.5 ± 1.4 | |
| 5 vs. 1A | NS | NS | <0.001 | <0.001 | |||||
| 10 | Partial transposition | 5 | 0.211 ± 0.066 | <0.02 | 0.123 ± 0.031 | 13.7 ± 4.5 | <0.01 | 2.3 ± 0.9 | |
| 10 vs. 1A | NS | <0.05 | <0.001 | <0.05 | |||||
| 11 | Partial transposition + pancreatectomy | 3 | 0.147 ± 0.020 | NS | 0.114 ± 0.014 | 4.1 ± 3.1 | NS | 1.9 ± 1.6 | |
| 11 vs. 1A | NS | <0.05 | <0.05 | NS | |||||
| 11 vs. 10 | NS | NS | <0.05 | NS | |||||
| 12 | Partial transposition + 30 per cent resection | 5 | 0.206 ± 0.043 | <0.05 | 0.136 ± 0.029 | 12.4 ± 3.6 | <0.01 | 6.5 ± 1.3 | |
| 12 vs. 5 | NS | <0.01 | <0.01 | NS | |||||
| 12 vs. 10 | NS | NS | NS | < 0.001 | |||||
| 13 | Partial transposition + 30 per cent resection + pancreatectomy + insulin | 4 | 0.209 ± 0.036 | NS | 0.197 ± 0.037 | 5.2 ± 1.0 | <0.05 | 10.1 ± 3.2 | |
| 13 vs. 12 | NS | <0.05 | <0.01 | NS | |||||
The presence of untreated diabetes in the dogs of group 11 changed some of these findings. The enlargement of the hepatocytes in the right lobes failed to occur, and the number of radioactive thymidine-labeled hepatocytes in these lobes was lower than after uncomplicated portacaval transposition, although still greater than on the left side (Table II).
Three months after partial portacaval transposition and four days following 30 per cent hepatectomy, group 12, the hepatocytes of the right lobes were larger than the left, and the incidence of labeled hepatocytes was also greater on that side. The ultrastructure of the liver cells in the right and left lobes did not differ significantly from that seen after partial portacaval transposition alone (40). The addition of diabetes treated with insulin, group 13, was associated with enlargement of the previously atrophic hepatocytes in the left lobes to a size greater than normal and similar to those in the right lobes. The enlarged hepatocytes contained many lipid droplets. Autoradiography showed more labeled hepatocytes in the left lobes than in the right lobes. Ultrastructurally, the rough endoplasmic reticulum was dilated in the hepatocytes of both lobes and reduced in amount in the cells of the left lobes. There were fewer than normal glycogen granules in all lobes.
DISCUSSION
In a classic article published 55 years ago, Rous and Larimore (31) raised the possibility that substances in portal venous blood prevented atrophy and promoted regeneration. In discussing the atrophic effects of portal branch ligation upon the regions of rabbit liver thus deprived of splanchnic blood and the concomitant hypertrophy found in the rest of the liver, they suggested “… that the liver has no essential activity—none on which its maintenance depends—that it (sic) is not intimately connected with substances derived from organs drained by the portal system.”
Rous and Larimore (31) did not have evidence in direct support of their suggestion, nor was substantiation provided by subsequent workers including Mann (23) during the next 40 years. Instead, articles by Child (7) and Fisher (10, 11, 12) and their associates led to the almost universal acceptance of the alternative view that the portal component of total hepatic blood flow was important primarily in proportion to its quantity and that the quality of the blood was not significant.
During the last decade, the pendulum has moved back toward the position that portal venous blood has special properties which profoundly affect hepatocellular structure and function. The revival of the qualitative concept came from our investigations with revascularization of auxiliary liver grafts (24, 25, 39). In these experiments, coexisting whole livers or two portions of the same liver were given different kinds of portal venous inflow. The hepatic tissue perfused with venous return from the splanchnic organs underwent hypertrophy, hyperplasia and glycogenation in comparison with the hepatic tissue deprived of this advantage. The confirmation by other workers of these observations has been summarized elsewhere (37, 38, 40).
Results of recent studies have indicated the nature of the hepatotrophic substances. The essence of their influence is the interreaction of hormones generated by splanchnic organs and delivered straight to the liver and with a presumably augmented significance because of the concentrated flux of nutritional substrate in the same venous blood (37, 38, 40). Both by inference and by direct testing, insulin was concluded to be the single most important hepatotrophic factor, although by no means the only one (37, 38, 40). This hormonal and biochemical concept has helped to explain earlier observations by Marchioro (25), Pouyet (27), and Ranson (29) and their associates that were made in experiments in which splanchnic venous flow was divided between two livers or two liver fragments.
Although its probable relation to liver regeneration has been obvious, the hepatotrophic concept as it has evolved in our laboratories has been based on chronic experiments not involving partial hepatectomy. The acute studies herein reported were undertaken in diabetic and nondiabetic dogs, of which some were submitted to subtotal hepatic resection. The same double liver fragment models which originally made it possible to study hepatotrophic effects in chronic experiments were adapted for the acute investigations. The advantage of these preparations is that hepatotrophic factors apparently are exhausted or greatly diminished by exposure to one hepatic fragment and, therefore, are relatively unavailable to the other competing liver tissue, even when the latter had a quantitatively good blood supply from nonsplanchnic origins. The clearing of insulin by the liver has been particularly well studied, as was recently reviewed by Field (9). An unmasking of the role of portal blood constituents is thereby made feasible to an extent not possible if portal blood is bypassed around a single liver but eventually returned to it in diluted form as with Eck’s fistula or t[he portacaval transposition of Child.
Nondiabetic dogs submitted to splanchnic division or partial portacaval transposition showed the remarkable rapidity with which liver tissue was altered after it was denied exposure to either the total splanchnic venous return or its pancreaticogastroduodenosplenic component. Within four or five days, the hepatocyte atrophy was almost as pronounced as we have reported (24, 37, 40) in the same kinds of experiments after two months. Deglycogenation, depletion or distortion of the rough endoplasmic reticulum, fatty vacuolization and other structural changes also were advanced within four or five days. The ultrastructural abnormalities in these shrunken hepatocytes were similar to those reported by Reaven and his colleagues (30) in rat livers within three days after the production of the insulinopenia of alloxan diabetes. At the same time, hypertrophy of the healthy contralateral liver cells had occurred.
These differential effects upon the right and left liver lobes were drastically altered by diabetes mellitus in the splanchnic division experiments. A similar modification of results was caused by the addition of pancreatectomy to partial portacaval transposition. The crucial observation was that the lobes previously protected either by pancreaticogastroduodenosplenic or by total splanchnic flow now partly, or completely, lost this advantage. The profoundly influential effects of iatrogenic diabetes mellitus were similar to those reported by us previously over the much longer period of two months (38,40).
A special note should be made about the effect of the blood flow deviations upon over-all hepatocyte replication. In all double liver fragment experiments not involving diabetes or hepatectomy, the deprivation of one portion of the liver of qualitatively optimal blood set into motion a low grade proliferative response Which could be consistently detected by autoradiography, which did not occur in the sham operated upon controls and which was shown in a previous report (40) to persist through the first two postoperative months. Although this heightened mitotic activity was predominantly in the hypertrophic liver lobes perfused by the total splanchnic return or its pancreaticogastroduodenal portion, cell division in the disadvantaged hepatic tissue that was undergoing atrophy was also raised above control levels. The atrophy itself may have provided an impetus for cell renewal, even though a full expression of response was possible only in the optimally perfused lobes. In the experiments which did not involve hepatectomy, diabetes mellitus blunted the increase in cell proliferation on both sides, even when insulin treatment was provided. The low grade hyperplastic response was eliminated if exogenous insulin was withheld. Parenthetically, these experiments provided an example of the kind of dissociation that can occur between hyperplasia and hypertrophy, as has been noted by ourselves (38, 40) and by Weinbren (44), Malamud (22), Sigel (35, 36) and Price (28) and their associates. The combination of simultaneous low grade hyperplasia and severe atrophy has even been recorded in dog and baboon livers after the performance of an Eck fistula (38).
The pace of changes in the livers of nondiabetic dogs submitted to splanchnic division or partial portacaval transposition was as rapid as described in rabbits by Rous and Larimore (31) after portal branch ligation and by Weinbren and his co-workers (44) after simple portacaval shunt in rats, but the involutional changes in their experiments could have been primarily due to a loss of the quantity of portal inflow instead of a reduction in its quality. Indeed, this was the interpretation placed on their own observations by Weinbren and his group (44). In our double liver preparations, both of the hepatic fragments retained a portal inflow. Nevertheless, Weinbren and his associates (44) have suggested that portal flow disparities between the liver fragments in such experiments could have accounted for all the hepatotrophic effects described by us (37) in an earlier publication. In the light of all the evidence now available, this criticism is not tenable.
There is little point in debating exactly how much flow through the portal vein was provided to the two liver sides in these specific experiments, since such measurements were not made. Suffice it to say that it is probably impossible to compensate liver tissue for the loss of splanchnic venous blood flow with any amount of replacement, however great, if the diverted splanchnic blood is being delivered to another and competing liver fragment. This was demonstrated in our laboratories by Marchioro and his associates (24) who arterialized portal vein branches to dog liver lobes after detaching these branches from the main portal vein. Atrophy of the altered lobes was not thereby prevented, even though the arterialized portal flow was, undoubtedly, several times normal, as Zuidema and his associates have reported (47). Supernormal portal flow in rapidly atrophying auxiliary livers or liver fragments that were revascularized by portacaval transposition has also been reported by Marchioro (24) and Daloze (8) and their associates. Earlier work by us (42) and by Heer and his co-authors (15) had already shown that the total blood flow of whole livers revascularized by portacaval transposition was essentially normal. Finally, Pouyet and his colleagues (27) have calculated in their version of the splanchnic division procedure that the intestinal component of splanchnic venous return was greater than the fraction from the pancreaticogastroduodenosplenic area, even though the latter has a much greater hepatotrophic effect.
Such tedious discussions and counterarguments about potential artifacts introduced by blood flow disparities in double liver fragment models no longer serve a useful purpose, since they have been rendered moot by our acute experiments herein described as well as by our reported chronic experiments (40). This work showed that differential hepatotrophic effects found in the two coexisting liver fragments were canceled or remarkably diminished by diabetes mellitus. In both the long and short term experiments, the results with stable alloxan-induced diabetes were particularly enlightening, since alloxan is not known or suspected to change splanchnic flow. Thus, the only conceivable explanation for the results was that a fundamental change in the relative chemical environment of the two liver sides had been wrought by removal of endogenous insulin. Dramatic consequences of the removal or addition of insulin or other hormones have also been shown by Leffert (18, 19) in hepatocyte monolayer cultures in which flow factors did not exist at all. Earlier, the role of insulin in supporting the growth and replication of other kinds of cells had been well established by Lieberman and Ove (20).
By using cell division as an end point, it was interesting to compare the results in the dogs of group 3 which were submitted to splanchnic division plus alloxan-induced diabetes with those obtained in the dogs of group 4 which had splanchnic division and total pancreatectomy. As in chronic animals reported earlier (38, 40), there was not a potent or even identifiable further effect of the total loss of the pancreas compared with the consequences caused by the simpler hormone defect of alloxan-induced diabetes. This meant that glucagon and other substances from the pancreas singly or together did not have a hepatotrophic role approaching in importance that of insulin. These observations have greatly weakened our own earlier speculation (37) which was subsequently supported by Bucher and Swaffield (4) that the insulin to glucagon ratio might be more important than insulin alone. Our more recent findings are also a frontal challenge to the contention by Price (28) and Whittemore (45) and their colleagues that glucagon is the principal hepatotrophic factor.
Our emphasis on insulin as the single most important hepatotrophic influence and, for that matter, the only easily identifiable one in our test system, does not imply that it is the sole factor. In a number of recent publications (37, 38, 40, 41), information was reviewed favoring multiple portal and extraportal factors. These presumably include other hormones than insulin and probably also nutrients.
The acute experiments herein reported have further strengthened the multifactorial hypothesis as it applies to hepatocyte mitosis. In the splanchnic division experiments of groups 3 and 4, the creation of diabetes eliminated the dominance of cell division in the previously favored right lobes and transferred this to the left, providing there was treatment with subcutaneous exogenous insulin which was eventually distributed to both fragments. However, if the replacement insulin was omitted as in group 4B, the rate of cell division became bilaterally equal. Confirmatory observations were made in the dogs of groups 9 and 13, which were submitted to total pancreatectomy plus 30 per cent hepatic resections. In all these dogs with diabetes of one kind or another, the combination of exogenous insulin plus intestinal blood apparently supported cell renewal in liver tissue to a greater extent than exogenous insulin plus gastroduodenosplenic or inferior vena caval blood. A subtle, but significant, hepatotrophic effect of intestinal venous blood was seemingly unmasked by the special conditions of these experiments. Analogous conclusions have been reached from observations after a two month interval (37, 40).
Until now, the discussion has focused almost entirely upon double liver fragment preparations exclusive of partial hepatectomy. The addition of a 30 or 60 per cent hepatic resection in normal, and otherwise unaltered, dogs evoked the well known changes of healthy hepatocyte hyperplasia and hypertrophy through the whole liver. In nondiabetic dogs with splanchnic division or partial portacaval transposition, these findings were most prominent in the optimally perfused liver lobes. However, the lobes deprived of total splanchnic flow or its pancreaticogastroduodenosplenic component also participated in the regeneration efforts, although at a lower rate. It was interesting that the disadvantaged lobes appeared generally more healthy than in the companion nonhepatectomy experiments, as if these lobes had benefited or been provided protection by something about the over-all regeneration response. In the same kinds of experiments, the hepatic regeneration response was markedly inhibited bilaterally if diabetes was superimposed and, to an even greater extent, if insulin therapy was omitted postoperatively. A. clearer demonstration of the participation of portal blood factors in regeneration and the permissive role of insulin could hardly be imagined. Yet, the same collection of experiments has shown the inexorability of regeneration and its lack of total dependence upon any single known factor. The work of Younger (46) and Short (34) and their associates has made the same point.
In the 11 years since the first papers from our laboratory reopened the possibility that portal blood contents affect liver structure and function, a number of workers have tested the role of hepatotrophic substances in the acute regeneration after liver resection and often with conflicting conclusions. After a first negative report by Lee and Edgington (16) consistent support for the concept of hepatrophic portal factors has come from surgical research laboratories of the University of California, San Diego, by Lee (17), Chandler (6), Sgro (33) and Broelsch (1) and their associates with studies using double liver fragment preparations or techniques of selective visceral extirpation. Fisher (10, 11, 12), an earlier opponent of the hepatrophic hypothesis, became one of its adherents (13), although incorrectly localizing the liver supporting splanchnic factors to the intestine (14), as has been shown by our studies and those of Sgro (33) and Broelsch (1) and their associates and of Poirier and Cahow (26). The contention of Price (28) and Whittemore (45) and their associates that glucagon is the principal heptotrophic substance now seems indefensible for reasons already stated. Weinbren, who long ago showed that liver regeneration can occur after Eck fistula (43), remains the major spokesman against the role of portal blood factors in regeneration (44).
A recent article by Bucher and Swaffield (4) is apt to have a conciliatory effect on these divergent points of view. These authors had previously minimized the role of portal blood factors in the governance of regeneration (3) but in their later work was described an important role by insulin and glucagon as regulators of the rate and extent of the regeneration process. Although our data reported herein and elsewhere (40) have de-emphasized the contribution of glucagon relative to that of insulin, our broader view of regeneration as a complex event under multifactorial control could hardly be more elegantly stated than was done at an earlier time by Bucher and Malt (2) who said, “… nor is there any basis for choosing between a unitary agent or trigger mechanism versus an interplay of several effectors with shifts in balance according to circumstances.”
SUMMARY
The acute influence of portal blood hepatotrophic factors upon the canine liver and upon hepatic regeneration was studied after surgical operations which provided qualitatively different portal venous perfusion to the right and left liver lobes. With one such procedure called splanchnic division, the nutrient rich venous return from the intestines was directed to the left lobes, whereas the hormone rich blood from the pancreas and other splanchnic organs of the upper part of the abdomen passed to the right lobes. Within three to five days, the rate of cell division on both liver sides was increased as judged by autoradiography, but the hormone influenced right lobes exhibited hypertrophy and hyperplasia relative to the nutrient enriched left lobes. In the latter, the hepatocytes underwent pronounced atrophy, deglycogenation, depletion or distortion of the rough endoplasmic reticulum, fatty vacuolization and other structural changes. When 30 or 60 per cent hepatic resection was carried out at the same time as splanchnic division, the regeneration of the hormone dominated hepatic tissue after three to five days was greater than that of the hepatic tissue receiving the intestinal venous effluent, as judged by multiple criteria, although both liver sides participated in the regeneration process.
The advantage enjoyed by the right liver lobes in relation to the left liver lobes both in the resting or in the regenerating state after splanchnic division was reduced or eliminated by pre-existing alloxan-induced diabetes or after concomitant total pancreatectomy. Similar, but less complete, observations about the effect of pancreatectomy were made in dogs submitted to the procedure of partial portacaval transposition, in which all the splanchnic venous blood passed to the right lobes, whereas the left lobes were revascularized with systemic venous blood from the vena cava.
These observations have added to the recent torrent of evidence that insulin is the most easily demonstrable and, therefore, probably the most important specific hepatotrophic factor in portal venous blood. At the same time, further subtle support has been added to our previously proposed hypothesis that multiple other hormonal and possibly nonhormonal factors from the splanchnic viscera and other sources also contribute to the essence of the hepatotrophic effects. These effects were evident and quite advanced within a few days. A prominent hepatotrophic role of glucagon was not identifiable.
Acknowledgments
The work was supported by research grants from the Veterans Administration; by Grant Nos. AI-AM-08898 and AM-07772 from the National Institutes of Health; by Grant Nos. RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, the National Institutes of Health, and by a grant from the Medical Research Council of Great Britain.
Contributor Information
T. E. Starzl, Denver, Colorado
K. A. Porter, London, England
N. Kashiwagi, Denver, Colorado
C. W. Putnam, Denver, Colorado
References
- 1.Broelsch CE, Lee S, Charters AC, III, et al. Regeneration of liver isografts transplanted in continuity with splanchnic organs. Surg Forum. 1974;25:394. [PubMed] [Google Scholar]
- 2.Bucher NLR, Malt RA. Regeneration of Liver and Kidney. Boston: Little, Brown & Co; 1971. The nature of the problem; p. 18. [Google Scholar]
- 3.Bucher NLR, Swaffield MN. Regeneration of liver in rats in the absence of portal splanchnic organs and a portal blood supply. Cancer Res. 1973;33:3189. [PubMed] [Google Scholar]
- 4.Idem. Regulation of hepatic regeneration in rats by synergistic action of insulin and glucagon. Proc Natl Acad Sci U S A. 1975;72:1157. doi: 10.1073/pnas.72.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton K. A study of the conditions and mechanisms of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandler JG, Lee S, Krubel R, et al. The inter-liver competition portal blood in regeneration of auxiliary liver transplants. Surg Forum. 1971;22:341. [PubMed] [Google Scholar]
- 7.Child CG, Barr D, Holswade GR, Harrison CS. Liver regeneration following portacaval transposition in dogs. Ann Surg. 1953;138:600. doi: 10.1097/00000658-195310000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daloze PM, Huguet C, Groth CG, Stoll F. Blood flow in auxiliary canine liver homografts. J Surg Res. 1969;9:10. doi: 10.1016/0022-4804(69)90003-1. [DOI] [PubMed] [Google Scholar]
- 9.Field JB. Extraction of insulin by liver. Annu Rev Med. 1973;24:309. doi: 10.1146/annurev.me.24.020173.001521. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Fisher ER, Lee S. Experimental evaluation of liver atrophy and portacaval shunt. Surg Gynecol Obstet. 1967;125:1253. [PubMed] [Google Scholar]
- 11.Fisher B, Fisher ER, Saffer E. Investigations concerning the role of humoral factor in liver regeneration. Cancer Res. 1963;23:914. [PubMed] [Google Scholar]
- 12.Fisher B, Russ C, Updegraff H, Fisher ER. Effect of increased hepatic blood flow upon liver regeneration. Arch Surg. 1954;69:263. doi: 10.1001/archsurg.1954.01270020129015. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Szuch P, Fisher ER. Evaluation of a humoral factor in liver regeneration utilizing liver transplants. Cancer Res. 1971;31:322. [PubMed] [Google Scholar]
- 14.Fisher B, Szuch P, Levine M, et al. The intestine as a source of a portal blood factor responsible for liver regeneration. Surg Gynecol Obstet. 1973;137:210. [PubMed] [Google Scholar]
- 15.Heer FW, Silvius DE, Harper HA. The relation between hepatic blood flow and function in normal dogs and dogs with cirrhosis after portacaval shunt or transposition. Surg Forum. 1960;9:282. [PubMed] [Google Scholar]
- 16.Lee S, Edgington TS. Liver transplantation in the rat. Surg Forum. 1966;17:220. [PubMed] [Google Scholar]
- 17.Lee S, Keiter JE, Rosen H, et al. Influence of blood supply on regeneration of liver transplants. Surg Forum. 1969;20:369. [PubMed] [Google Scholar]
- 18.Leffert HL. Growth control of differentiated fetal rat hepatocytes in primary monolayer culture—VII, hormonal control of DNA synthesis and its possible significance to the problem of liver regeneration. J Cell Biol. 1974;62:792. doi: 10.1083/jcb.62.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Idem. Hepatocellular growth control. In: Newberne P, Hutler W, editors. Workshop on Rat Liver Pathology. New York: American Lesevier Publishing Co; in press. [Google Scholar]
- 20.Lieberman I, Ove P. Growth factors for mammalian cells in culture. J Biol Chem. 1959;234:2754. [PubMed] [Google Scholar]
- 21.Loud AV. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol. 1968;37:27. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malamud D, Gonzalez EM, Chiu H, Malt RA. Inhibition of cell proliferation by azathioprine. Cancer Res. 1972;32:1226. [PubMed] [Google Scholar]
- 23.Mann FC. Restoration and pathological reactions of the liver. Vol. 11. J. Mt. Sinai Hosp; N. Y.: 1944. p. 65. [Google Scholar]
- 24.Marchioro TL, Porter KA, Brown BI, et al. The effect of partial portacaval transposition on the canine liver. Surgery. 1967;61:723. [PMC free article] [PubMed] [Google Scholar]
- 25.Marchioro TL, Porter KA, Dickinson TC, et al. Physiologic requirements for auxiliary liver homotransplantation. Surg Gynecol Obstet. 1965;121:17. [PMC free article] [PubMed] [Google Scholar]
- 26.Poirier RA, Cahow CE. Role of the small intestine in liver regeneration. Am Surg. 1974;40:555. [PubMed] [Google Scholar]
- 27.Pouyet M, Berard Ph, Ruckebusch Y, et al. Derivations portohepatiques selectives origine pancreatique du facteur hepatotrophiquc portal. Ann Chir. 1969;23:393. [PubMed] [Google Scholar]
- 28.Price JB, Jr, Takeshige K, Max MH, Voorhees AB., Jr Glucagon as the portal factor modifying hepatic regeneration. Surgery. 1972;72:74. [PubMed] [Google Scholar]
- 29.Ranson JHC, Garcia-Moran M, Becker F, Localio SA. Influence of the source of portal vein blood on dog auxiliary hepatic allograft function in recipients with hepatic insufficiency. Surg Forum. 1971;22:345. [PubMed] [Google Scholar]
- 30.Reaven EP, Peterson DT, Reaven GM. The effect of experimental diabetes mellitus and insulin replacement on hepatic ultrastructure and protein synthesis. J Clin Invest. 1973;52:248. doi: 10.1172/JCI107181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rous P, Larimore LD. Relation of the portal blood to liver maintenance. J Exp Med. 1920;31:609. doi: 10.1084/jem.31.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider WC, Greco AE. Incorporation of pyrimidine deoxyribonucleosides into liver lipids and other components. Biochem Biophys Acta. 1971;228 :610. doi: 10.1016/0005-2787(71)90725-8. [DOI] [PubMed] [Google Scholar]
- 33.Sgro JG, Charters AC, Chandler JB, et al. Site of origin of the hepatotrophic portal blood factor involved in liver regeneration. Surg Forum. 1973;24:377. [PubMed] [Google Scholar]
- 34.Short J, Brown RF, Husakova A, et al. Induction of deoxyribonucleic acid synthesis in the liver of the intact animal. J Biol Chem. 1972;247:1757. [PubMed] [Google Scholar]
- 35.Sigel B. The extracellular regulation of liver regeneration. J Surg Res. 1969;9:387. doi: 10.1016/0022-4804(69)90084-5. [DOI] [PubMed] [Google Scholar]
- 36.Sigel B, Baldia LB, Menduke H, Feigl P. Independence of hyperplastic and hypertrophic responses in liver regeneration. Surg Gynecol Obstet. 1967;125:95. [PubMed] [Google Scholar]
- 37.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin hormonal nature and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:179. [PMC free article] [PubMed] [Google Scholar]
- 38.Starzl TE, Lee I-Y, Porter KA, Putnam CW. The influence of portal blood upon lipid metabolism in normal and diabetic dogs and baboons. Surg Gynecol Obstet. 1975;140:381. [PMC free article] [PubMed] [Google Scholar]
- 39.Starzl TE, Marchioro TL, Rowlands DT, Jr, et al. Immunosuppression after experimental clinical homotransplantation of the liver. Ann Surg. 1964;160:411. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starzl TE, Porter KA, Kashiwagi N, et al. The effect of diabetes mellitus on portal blood hepatotrophic factors in dogs. Surg Gynecol Obstet. 1975;140:549. [PMC free article] [PubMed] [Google Scholar]
- 41.Starzl TE, Putnam CW. Experience in Hepatic Transplantation. Philadelphia: W. B. Saunders Co; 1969. Metabolic considerations in animals; pp. 475–491. [Google Scholar]
- 42.Starzl TE, Scanlon WA, Shoemaker WC, et al. Studies on hepatic blood flow and the rate of Bromsulphalein clearance in dogs with portacaval transposition. Surgery. 1962;52:660. [PMC free article] [PubMed] [Google Scholar]
- 43.Weinbren KF. The portal blood supply and regeneration of the rat liver. Br J Exp Pathol. 1955;36:583. [PMC free article] [PubMed] [Google Scholar]
- 44.Weinbren KF, Washington SLA, Smith CY. The response of the rat liver to alterations in total portal blood flow. Br J Exp Pathol. 1975;56:148. [PMC free article] [PubMed] [Google Scholar]
- 45.Whittemore AD, Kasuyal M, Voorhees AB, Jr, Price JB., Jr Hepatic regeneration in the absence of portal viscera. Surgery. 1975;77:419. [PubMed] [Google Scholar]
- 46.Younger LR, King J, Steiner DF. Hepatic proliferative response to instilin in severe alloxan diabetes. Cancer Res. 1966;26:1408. [PubMed] [Google Scholar]
- 47.Zuidema GD, Gaisford WD, Abell MR, et al. Segmental portal arteralization of canine liver. Surgery. 1953;53:689. [PubMed] [Google Scholar]