Abstract
To assess the endemicity of tick-borne pathogens in New Hampshire, we surveyed adult tick vectors. Pathogens were more prevalent in areas of high tick density, suggesting a correlation between tick establishment and pathogen endemicity. Infection rates in ticks correlated with disease frequency in humans.
Keywords: Vector-borne infections, Lyme disease, Ixodes spp., ticks, Borrelia burgdorferi, Anaplasma phagocytophilum, Babesia microti, New Hampshire, dispatch
Along the borders of the northeastern and the upper midwestern United States, black-legged ticks (Ixodes scapularis) are invading new areas (1–3). Because this tick is the principal vector of a number of human pathogens, defining and monitoring its possible expansion are imperative. Little information is available about I. scapularis invasions, including the relative rates of pathogen carriage as vectors expand their range and establish locally enzootic cycles. To assess the endemicity of 3 tick-borne pathogens (Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti) throughout New Hampshire, we surveyed adult I. scapularis vectors.
The Study
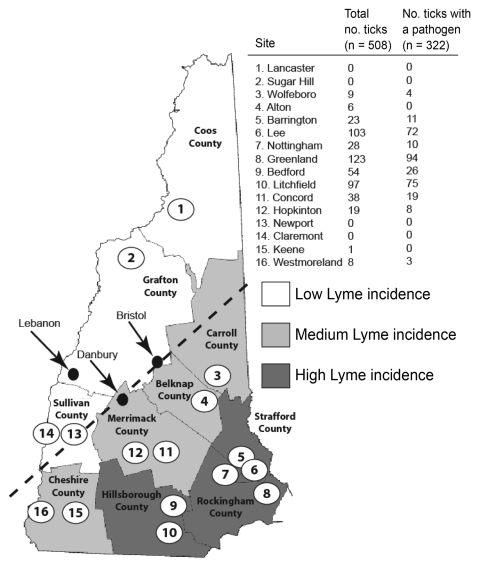
During the fall of 2007, we established 16 sampling sites in the 10 counties of New Hampshire (Figure 1). Levels of reported human Lyme disease had varied among the counties in 2006. We categorized each site as high Lyme disease incidence (HLI), medium Lyme disease incidence (MLI), or low Lyme disease incidence (LLI) according to the reported number of Lyme disease cases per 100,000 persons in 2006 (4). As in the neighboring states of Massachusetts (Xu et al., unpub. data) and Maine (5), in New Hampshire, ticks are most abundant in coastal counties. The HLI sites (Strafford, Rockingham, and Hillsborough counties) were along the coast and had 37–104 reported Lyme disease cases per 100,000 persons; the MLI sites (Carroll, Belknap, Merrimack, and Cheshire counties) bordered the coastal counties and had 10–19 cases per 100,000 persons; and the LLI sites (Coos, Grafton, and Sullivan counties) were the most inland and had 5–12 Lyme disease cases per 100,000 persons.
Figure 1.
Results of Ixodes scapularis tick sampling and pathogen screening in New Hampshire. County names are shown in bold and sampling sites across counties of low (white), medium (light gray), and high (dark gray) Lyme disease incidence are numbered. Dashed line indicates the leading edge of the expanding I. scapularis range. Arrows and black dots indicate cities referred to in the discussion. Numbered open circles identify the locations of the 16 sample sites.
We collected adult ticks rather than the conventionally sought nymphs (6–8) because we believe adults best indicate the pathogen pool in an area. Transovarial transmission of B. burgdorferi is negligible, and I. scapularis ticks acquire infection by feeding on infected hosts as larvae or as nymphs. Because larval ticks feed during late summer and early autumn, they are more likely to feed on migratory animals, such as birds; nymphal ticks feeding in early summer are likely to feed on resident hosts. Hence, adult ticks are more likely to have taken at least 1 resident blood meal from the site of origin, and their Borrelia infection rates (and genotype frequencies) are more representative of endemicity.
We visited each site twice within 1 month (October or November 2007) and sampled each site at each visit for ≈25 minutes. A total of 509 adult ticks were collected by drag-sampling vegetation. As expected, ticks were most abundant at HLI sites and moderate at MLI sites; no ticks were found at the 3 LLI sites, including 2 sites (Newport and Claremont) that were close to where ticks have previously been reported (9). Likewise, we did not find ticks on 6 deer carcasses at hunter check stations in nearby towns (Newport, Danbury, and Bristol).
Ticks were transported to the laboratory, where they were frozen with liquid nitrogen and pulverized in Eppendorf tubes with plastic pestles (Kontes, Vineland, NJ, USA). DNA was extracted using Epicenter Master Complete DNA & RNA Purification Kits (Epicenter Technologies, Madison, WI, USA). Two duplex real-time–PCR reactions were developed (G. Xu et al., unpub. data) by using oligonucleotide primers and Taqman probes for real-time detection of total tick DNA and B. burgdorferi (duplex 1) and A. phagocytophilum–B. microti (duplex 2). The most common pathogen found was B. burgdorferi, followed by B. microti and A. phagocytophilum (Table). A total of 322 (63%) ticks carried at least 1 pathogen, and 40 (8%) ticks carried 2 pathogens. The prevalence of ticks positive for both B. burgdorferi and B. microti was greater than that of ticks positive for both B. burgdorferi and A. phagocytophilum and accounted for most (78%) coinfections. Neither of the observed coinfections (B. burgdorferi–A. phagocytophium, B. burgdorferi–B. microti) differed significantly from its expected random occurrence (contingency table analysis p = 0.487, χ2 p = 0.926).
Table. Pathogen prevalence and coinfection in 2 regions of different tick density, New Hampshire*.
| Infection | No. ticks collected |
||
|---|---|---|---|
| HLI sites | MLI sites | Total | |
| Single infection | |||
| Borrelia burgdorferi | 237 | 29 | 266 |
| Anaplasma phagocytophilum | 1 | 0 | 1 |
|
Babesia microti |
13 |
2 |
15 |
| Multiple infections | |||
| B. burgdorferi + A. phagocytophilum | 9 | 0 | 9 |
| B. burgdorferi + B. microti | 28 | 3 | 31 |
| A. phagocytophilum + B. microti | 0 | 0 | 0 |
| B. burgdorferi + A. phagocytophilum + B. microti | 0 | 0 | 0 |
*HLI, high Lyme disease incidence; MLI, medium Lyme disease incidence.
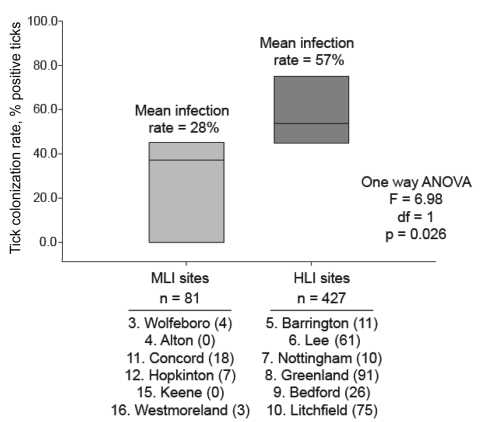
We found a significantly greater percentage of B. burgdorferi–infected ticks from HLI sites than from MLI sites (Figure 2). B. burgdorferi was twice as common in ticks from the HLI sites. Similarly, B. microti was more likely to be sampled from HLI sites (5.0%) than from MLI sites (2.7%), although this difference was not significant. Linear regression showed a strong correlation (R2 = 0.90) between the entomologic risk index (total number of ticks × proportion of ticks infected [6,8]) in this study and the incidence of human cases of Lyme disease by county in New Hampshire in 2007.
Figure 2.
Analysis of variance (ANOVA) of Borrelia burgdorferi prevalence in Ixodes scapularis ticks isolated from New Hampshire counties of medium (MLI) and high (HLI) incidence of Lyme disease.
Conclusions
I. scapularis was initially found in New Hampshire in 1985, near the town of Lebanon (9). Lebanon is located in a region where we were unable to sample ticks (Figure 1). It is tempting to hypothesize that I. scapularis was once more abundant in western counties. In 2001, A.T. Eaton noticed a distribution of ticks similar to what we found (10), suggesting that the current distribution has been stable for at least 7 years. Our result that high tick density areas had the highest overall prevalence of pathogens and the highest prevalence of coinfected ticks supports the finding of Hamer et al., who reported a significant difference in the rates of pathogen carriage between recently invaded and I. scapularis–endemic areas (1). The rates of infection we found are similar to those found by Swanson et al. from 5 other northeastern states (11). According to their meta-analysis, ≈40% (± 13%) of 2,109 adult and nymphal ticks were infected with B. burgdorferi, 21% (± 17%) with A. phagocytophilum, and 9% (± 8%) with either B. microti or B. divergens.
A noteworthy exception is the prevalence of A. phagocytophilum. We detected this pathogen at only 1 site (10 positive ticks from Greenland on 2 independent visits). Human anaplasmosis has been a reportable disease in New Hampshire for at least a decade (12), and cases have been rare (0 or 1 reported per year during 1998–2006). However, human anaplasmosis increased substantially in 2007 (3 cases) and 2008 (9 cases).
Human babesiosis appears to be following a similar trend (2 cases in 2005, 3 cases in 2006 and 2007, and 9 cases in 2008), although the disease has been reportable only since 2005. Lastly, we found a strong correlation between entomologic risk index and the incidence of human Lyme disease. This result contrasts starkly with a lack of correlation found by Falco et al. between the abundance of adult female ticks and reported cases of erythema migrans (a common clinical presentation of Lyme disease) in southern New York State (7). Improved reporting of Lyme disease by clinicians to state health officials may be responsible for this discrepancy. The data presented here suggest that ERI estimates using adult ticks are accurate proxies for the yearly incidence of human Lyme disease in regions where Lyme disease is endemic.
Acknowledgments
We thank the New Hampshire State Health Department for its support in this research. Also, we thank the local health officers who participated in this study, particularly the following who provided invaluable insight during selection of the sampling sites: Seacoast Family YMCA staff (Greenland), Abenaki Ski Area staff (Wolfeboro), Forest Society Conservation Center staff (Concord), Peter Lamb, Lloyd Draper, Bryan Boyers, Michael Kennard, Margo Connors, and Ron Wert.
This work was supported by grant GM60759 (to S.M.R.) from the National Institutes of Health (NIH).
Biography
Dr Walk is currently an NIH research fellow in the Department of Internal Medicine at the University of Michigan. At the time of this study, he was a postdoctoral research fellow in the Department of Plant, Soil, and Insect Sciences at the University of Massachusetts. His research focuses on the evolutionary ecology of bacterial pathogens that cause human illness, particularly environmental and host dynamics that govern pathogen emergence in natural populations.
Footnotes
Suggested citation for this article: Walk ST, Xu G, Stull JW, Rich SM. Correlation between tick density and pathogen endemicity, New Hampshire. Emerg Infect Dis [serial on the Internet]. 2009 April [date cited]. Available from http://www.cdc.gov/EID/content/15/4/585.htm
References
- 1.Hamer SA, Roy PL, Hickling GJ, Walker ED, Foster ES, Barber CC, et al. Zoonotic pathogens in Ixodes scapularis, Michigan. Emerg Infect Dis. 2007;13:1131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurtenbach K, Hanincová K, Tsao JI, Margos G, Fish D, Ogden NH. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol. 2006;4:660–9. 10.1038/nrmicro1475 [DOI] [PubMed] [Google Scholar]
- 3.Ogden NH, Lindsay LR, Hanincová K, Barker IK, Bigras-Poulin M, Charron DF, et al. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks, and Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. [erratum in Appl Environ Microbiol 2008;74:3919]. Appl Environ Microbiol. 2008;74:1780–90. 10.1128/AEM.01982-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.New Hampshire Department of Health and Human Services. New Hampshire tick-borne disease bulletin: 2007. update [cited 2009 Feb 18]. Available from http://www.dhhs.nh.gov/DHHS/CDCS/lymedisease.htm
- 5.Rand PW, Lacombe EH, Dearborn R, Cahill B, Elias S, Lubelczyk CB, et al. Passive surveillance in Maine, an area emergent for tick-borne diseases. J Med Entomol. 2007;44:1118–29. 10.1603/0022-2585(2007)44[1118:PSIMAA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 6.Diuk-Wasser MA, Gatewood AG, Cortinas MR, Yaremych-Hamer S, Tsao J, Kitron U, et al. Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. J Med Entomol. 2006;43:166–76. 10.1603/0022-2585(2006)043[0166:SPOHIS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 7.Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D, et al. Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans. Am J Epidemiol. 1999;149:771–6. [DOI] [PubMed] [Google Scholar]
- 8.Stafford KC, Cartter ML, Magnarelli LA, Ertel SH, Mshar PA. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol. 1998;36:1240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson JF, Magnarelli LA, McAninch JB. Ixodes dammini and Borrelia burgdorferi in northern New England and upstate New York. J Parasitol. 1987;73:419–21. 10.2307/3282102 [DOI] [PubMed] [Google Scholar]
- 10.Eaton AT. Biology and control of ticks in New Hampshire. Durham (NH): University of New Hampshire Cooperative Extension; 2001. [cited 2009 Feb 18]. Available from http://extension.unh.edu/resources/files/Resource000528_Rep550.pdf
- 11.Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708–27. 10.1128/CMR.00011-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.New Hampshire Department of Health and Human Services. Communicable disease bulletin. November 2008. [cited 2009 Feb 18]. Available from http://www.dhhs.nh.gov/DHHS/CDCS/lymedisease.htm