Abstract
Introduction:
A significant percentage of smokers attempting cessation lapse to smoking within a matter of days, and current models of relapse devote insufficient attention to such early smoking lapse. Studies attempting to relate severity of nicotine withdrawal symptoms to short-term smoking cessation outcomes have yielded equivocal results. How one reacts to the discomfort of nicotine withdrawal and quitting smoking (i.e., distress tolerance) may be a more promising avenue of investigation with important treatment implications.
Methods:
The present investigation examined distress tolerance and early smoking lapse using a prospective design. Participants were 81 adult daily smokers recruited through newspaper advertisements targeted at smokers planning to quit smoking without assistance (i.e., no pharmacotherapy or psychosocial treatment; 42 males and 39 females; mean age = 42.6 years, SD = 12.20).
Results:
As hypothesized, both greater breath-holding duration and carbon dioxide–enriched air persistence were associated with a significantly lower risk of smoking lapse following an unaided quit attempt. These effects were above and beyond the risk associated with levels of nicotine dependence, education, and history of major depressive disorder, suggesting that distress tolerance and task persistence may operate independently of risk factors such as nicotine dependence and depressive history. In contrast to expectation, persistence on the Paced Auditory Serial Addition Test (a psychological challenge task) was not a significant predictor of earlier smoking lapse.
Discussion:
These results are discussed in relation to refining theoretical models of the role of distress tolerance in early smoking lapse and the utility of such models in the development of specialized treatment approaches for smoking cessation.
Introduction
An increased level of theoretical and empirical attention has focused on better understanding the nature of early smoking lapse in the context of smoking cessation (Brown, Lejuez, Kahler, Strong, & Zvolensky, 2005). This scientific work has been stimulated, at least in part, by the observation that a significant percentage of smokers attempting cessation lapse to smoking within a matter of days and very few of these individuals recover to achieve abstinence (Brown, Kahler, Niaura, et al., 2001; Cook, Gerkovich, O'Connell, & Potocky, 1995; Doherty, Kinnunen, Militello, & Garvey, 1995; Garvey, Bliss, Hitchcock, Heinold, & Rosner, 1992). It also rests on the larger recognition that the general population contains significant numbers of at-risk, recalcitrant smokers (Augustson & Marcus, 2004) and that targeted efforts to develop specialized treatment for such subgroups of smokers have been inadequate (Brown, 2003; Irvin & Brandon, 2000; Lichtenstein & Glasgow, 1992; Niaura & Abrams, 2002; Shiffman, 1993).
Research has begun to provide insight into the nature of early smoking lapse (typically defined in such work as an instance of smoking; e.g., even a puff) by focusing on individual differences in the (in)ability to tolerate negative affect—termed distress tolerance (Brown et al., 2005). We use the term distress tolerance to convey a behavioral tendency to continue to pursue a goal despite encountering various states of affective discomfort, which may be in response to perceived physical or psychological distress (Brown, Lejuez, Kahler, & Strong, 2002). Interest in behavioral reactions to affective discomfort arises in part from research, suggesting that negative affect is the “motivational basis” of the nicotine withdrawal syndrome (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). This work is particularly relevant to early smoking lapse, given that withdrawal symptoms begin within hours of quitting and peak within the first few days (Hughes, Higgins, & Hatsukami, 1990; Shiffman & Waters, 2004) and that negative affect during nicotine withdrawal is related to smoking outcome (see Baker, Brandon, & Chassin, 2004 and Baker, Piper, et al., 2004 for reviews). Within this context, models of early lapse posit that it is not just the severity or intensity of nicotine withdrawal (characterized by negative affect) but also how an individual responds to discomfort and distress that predicts early smoking lapses (Brown et al., 2005). That is, early lapsers may be characterized by a tendency toward negative affect during nicotine withdrawal and also an inability to tolerate such negative affect. Thus, whereas many smokers quit smoking successfully despite high levels of discomfort, a low threshold for tolerating such unavoidable types of distress would be associated with increased smoking behavior (i.e., the smoking response temporarily ameliorates the experiential discomfort; Baker, Piper, et al., 2004; Parrott, 1999) and increased difficulty quitting smoking (Brown et al., 2005).
A number of studies have provided data consistent with a distress tolerance perspective on early lapse. In some of the earliest studies within this domain, Hajek and colleagues (Hajek, 1991; Hajek, Belcher, & Stapleton, 1987; West, Hajek, & Belcher, 1989) found consistent positive associations between duration of breath holding, as an index of tolerance for physical discomfort, and duration of abstinence from smoking among daily smokers. Building from such work, we examined current, daily smokers (N = 32) who were classified according to whether they had not previously quit for more than 24 hr compared with those who had quit for at least 3 months (Brown et al., 2002). Using laboratory tasks that tapped distress tolerance, defined as persistence on psychologically distressing (Paced Auditory Serial Addition Task [PASAT]; Lejuez, Kahler, & Brown, 2003) and physical (carbon dioxide [CO2]–enriched air inhalation and breath-holding duration) challenge provocation tests designed to generate physical symptoms of interoceptive distress, we examined the relationship between termination of a task prior to its scheduled endpoint and the two smoking groups. Results indicated that immediate relapsers were more likely to terminate the CO2 and PASAT challenges and had a shorter duration of breath-holding than delayed relapsers (Brown et al., 2002).
These findings are noteworthy in that the two groups did not differ in terms of their smoking history or nicotine dependence level. A subsequent investigation by Brandon et al. (2003) evaluated the predictive utility of persistence on behavioral tasks to relapse following treatment for smoking (N = 144). Prior to treatment, participants completed a variety of behavioral persistence tasks, including an anagram persistence task (APT) and a mirror-tracing persistence task (MTPT). Participants were divided into three groups: (a) treatment noncompleters, (b) lapsers (those who completed treatment but relapsed to smoking), and (c) abstainers (those who sustained abstinence until follow-up). MTPT was a significant predictor of sustained abstinence, whereas the APT was not; this effect was apparent after controlling for level of nicotine dependence and gender. Additionally, MTPT persistence increased monotonically across treatment noncompleters, lapsers, and continuous abstainers. Collectively, a variety of studies are consistent with the emerging perspective that distress tolerance is an important factor in smoking lapse. Such a conclusion is strengthened by research that suggests parallel psychological constructs such as sensitivity to anxiety-related states (anxiety sensitivity; Brown, Kahler, Zvolensky, et al., 2001; Zvolensky et al., 2007), a tendency to avoid emotional states (e.g., thought or emotional suppression; Hayes, Wilson, Gifford, Follette, & Strosahl, 1996; Salkovskis & Reynolds, 1994), and emotional reactivity (Zvolensky, Feldner, Eifert, & Brown, 2001) may be related to problems in quitting successfully.
Although work on distress tolerance is promising, it is limited in a number of key respects. First, all but one of the completed investigations addressing such processes have focused on an a priori basis on distress tolerance and early smoking lapse (Brown et al., 2002). Thus, a limited database is focused expressly on early smoking lapse guided by using distress tolerance theory and research. Second, although the Brown et al. (2002) results were promising, they were limited by the use of retrospective reports of the duration of past smoking quit attempts. This assessment tactic has well-known challenges in terms of being influenced by reporting biases, memory distortions, and recall effects more generally (McNally, 1998). Accordingly, it would be important to extend the results of Brown et al. (2002) using a prospective measurement approach. Third, the participants in the earlier investigation by Brown et al. (2002) were not necessarily interested in quitting smoking. To the extent that distress tolerance is an important explanatory construct for early smoking lapse, it needs to be generalizable to smokers interested in quitting so that specialized interventions could be applied to them. It would therefore be important for future work to evaluate the predictive validity of distress tolerance among smokers motivated for, and willing to engage in, a “serious” quit attempt. Finally, in previous work, limited attention has been given to evaluating the incremental validity of distress tolerance in terms of early smoking lapse relative to other theoretically relevant factors (e.g., levels of nicotine dependence). To have more confidence in the concept of distress tolerance, an evaluation is needed to determine whether it uniquely explains a process underlying early lapse or is simply predictive due to shared relationships with other known risk factors.
The present investigation aimed to extend past research on distress tolerance and early smoking lapse. Participants included adult daily smokers recruited through the community and planning to quit smoking without assistance (i.e., no pharmacotherapy or psychosocial treatment). After a comprehensive assessment of psychological and smoking factors, participants were followed for 28 days as they made an unaided quit attempt. Based on extant theory (Brown et al., 2005) and research (Brown et al., 2002), we hypothesized that smokers with high levels of distress tolerance would be characterized by lower levels of depressive symptoms, anxiety sensitivity, and stress reactivity. Further, we hypothesized that smokers who evidenced prequit persistence on physical and psychological challenge tasks (breath-holding duration, CO2 persistence, and PASAT persistence) would have lower risk of lapse to smoking even after controlling for the risk associated with other factors related to smoking lapse in this sample.
Methods
Participants
Participants were 81 adult smokers recruited through newspaper advertisements targeted at smokers planning to quit smoking without assistance (i.e., no pharmacotherapy or psychosocial treatment). The sample included 42 males and 39 females, all aged 19–65 years (M = 42.6; SD = 12.20); 88.5% of participants were White, 3.8% Black, 2.6% Hispanic, and 5.1% indicated belonging to an “Other” racial/ethnic group.
Participants who responded to the study advertisement were given a thorough explanation of the study and screened for inclusion criteria. Of the 380 callers who responded to the advertisement, 72 callers did not want to participate, 182 callers could not be contacted to schedule an appointment, and 45 decided not to participate after the initial screening assessment. For inclusion in the study, participants were required to (a) be a regular smoker for at least 1 year, (b) be currently smoking an average of at least 10 cigarettes/day, (c) report motivation to quit of at least 5 on a 10-point scale, (d) be interested in making a serious quit attempt in the next month, (e) have not decreased the number of cigarettes by more than half in the past 3 months, and (f) be between the ages of 18 and 65 years. Exclusion criteria included (a) having a current Axis I disorder including psychoactive substance abuse or dependence (excluding nicotine dependence) within the past 6 months (n = 67); (b) currently using psychotropic medication (n = 127), including bupropion; (c) having a history of significant medical illness, such as cardiovascular, endocrine, pulmonary (e.g., chronic obstructive pulmonary disease, emphysema, and a history of asthma), neurologic, gastrointestinal, renal, immunologic, or other systemic illness, or being deemed as currently unhealthy in the context of a complete medical examination (n = 116); (d) having limited mental competency and the inability to give informed, voluntary, written consent to participate; (e) currently using nicotine replacement therapy or bupropion or intending to use nicotine replacement therapy or bupropion during their quit attempt (n = 6); and (f) using other tobacco products. Additional participants were ruled out for not meeting the age requirement (n = 50), not meeting regular smoking criteria (n = 28), insufficient motivation to quit within the next month (n = 8), or participant scheduling difficulties (n = 12). The study was approved by the Butler Hospital Institutional Review Board.
Procedure
Interested and eligible participants were instructed to select the day on which they would attempt to quit smoking (quit day) and were scheduled for the baseline assessment session. The baseline assessment was required to occur at least 3 days, but no more than 14 days, prior to the participant's selected quit day.
Baseline assessment session.
The baseline assessment included written informed consent followed by a diagnostic screening interview using the Structured Clinical Interview: Non-Patient Version for DSM-IV (SCID-NP; First, Spitzer, Gibbon, & Williams, 1995) and a medical examination provided by the study physician. Individuals who met exclusionary criteria for the SCID-NP or the medical examination were paid US$25 for their time and given a self-help booklet on smoking cessation prepared by the National Cancer Institute (Clearing the Air; U.S. Department of Health and Human Services [USDHHS], 1995). After a 15-min break, during which participants were given the option to smoke a cigarette, eligible individuals completed the laboratory challenge tasks described in detail below.
Quit week.
After finishing the challenge tasks, participants were reminded of their quit day and instructed to quit smoking by midnight on the night before their quit day. At this point, they were paid $25 for their time and given a copy of Clearing the Air (USDHHS, 1995). Further, participants were told that they were to come to the laboratory on their quit day and 3 and 7 days after quitting between noon and 8 p.m. to verify abstinence using carbon monoxide (CO) analysis (10 ppm cutoff, using a CMD/CO Carbon Monoxide Monitor Model 3110; Spirometrics, Inc., Auburn, Maine) and to complete self-report measures of withdrawal symptoms, mood, and depressive symptoms (see below for details). They were informed that they would receive $25 for completing self-report measures on each of the three quit-week visits. Further, if CO readings verified that they had abstained from smoking for at least 12 hr prior, they would earn $30 at the quit-day assessment, $40 at the 3-day assessment, and $50 at the 7-day assessment. This type of contingent payment program (Heil, Tidey, Holmes, Badger, & Higgins, 2003) was used to provide incentive to participants for making a quit attempt on their chosen quit day and to keep a large number of participants abstinent long enough to acquire data on withdrawal symptoms, mood, and depressive symptoms. We did not expect this fixed schedule payment program to have a significant impact on abstinence durations beyond this quit-week assessment period when the contingency would end (Roll, Higgins, & Badger, 1996).
Follow-up assessments.
Following quit day, participants were scheduled to return to the laboratory between noon and 8 p.m. on days 3, 7, 14, and 28 postcessation to verify abstinence and complete self-report measures of withdrawal symptoms, mood, and depressive symptoms. In addition to CO verification of abstinence, saliva was gathered for cotinine analysis on days 7, 14, and 28. No incentives for abstinence following the quit week were provided, and participants were paid $20 for completing each of the 14- and 28-day follow-ups regardless of their smoking status. This schedule of assessments was used to concentrate measurements during the intervals of primary interest to the study and during the time when withdrawal symptoms are most intense (Hughes et al., 1990). Based on guidelines from the Society for Research on Nicotine and Tobacco Subcommittee on Biochemical Verification (Hughes et al., 2003), participant reports of abstinence for at least 1 day were verified during in-person interviews with expired CO (less than 10 ppm) at all follow-up contacts; reports of abstinence for at least 7 days were verified using salivary cotinine (cutoff of 15 ng/ml) at the 7-, 14-, and 28-day follow-up visits. Participants who did not provide CO verification or saliva for cotinine analysis or whose biochemical measures contradicted self-report were considered lapsed at the last session at which they had verified abstinence.
Materials and apparatus
The screening portion of the baseline assessment session was conducted in a 15′ by 12′ office and the challenge tasks occurred in a 12′ by 8′ experimental room, both located in Butler Hospital (Providence, Rhode Island). For the challenge tasks, participants sat at a desk supporting a Dell Pentium computer, a color monitor, and a mouse. The experimenters sat in an adjacent room containing all other experimental equipment, including an apparatus for providing participants with either room air or a mixture of 20% CO2 (20% CO2, 21% O2, 59% N2). CO2 was stored in a cylinder (101 cm height) and fed through a 5-cm by 5-cm hole via aerosol tubing from the experimenter room to a positive-pressure Downs’ continuous positive airway pressure mask worn by the participant. An automated apparatus (described in Lejuez, Forsyth, & Eifert, 1998) was used for CO2 delivery. The apparatus, which was controlled by the experimenter, utilized a selector switch to deliver either room air or CO2-enriched air to the participant. A one-way mirror allowed the experimenters to observe all session events.
Diagnostic and screening measures
Psychiatric screen.
The SCID-NP (First et al., 1995) was used to assess current (i.e., diagnostic exclusions) and lifetime prevalence of Axis I diagnoses. To limit experimenter bias, the clinician who administered the SCID-NP did not conduct the laboratory challenge tasks.
Measures of smoking history and nicotine dependence
Smoking history.
Smoking history and pattern were assessed with the Smoking History Questionnaire (SHQ; Brown et al., 2002), which includes items pertaining to smoking rate, age at initiation, and years of being a daily smoker. The SHQ has been used successfully in previous studies (Brown et al., 2002; Zvolensky, Lejuez, Kahler, & Brown, 2004).
Severity of dependence.
The Fagerström Tolerance Questionnaire (FTQ) was used as a continuous measure of nicotine dependence (Fagerström, 1978). Specifically, we administered the FTQ and scored it as the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). The FTND has shown good internal consistency, positive relationships with key smoking variables (e.g., salivary cotinine; Heatherton et al., 1991), and high degrees of test–retest reliability (Pomerleau, Carton, Lutzke, Flessland, & Pomerleau, 1994).
Smoking cessation self-efficacy.
We used the Smoking Cessation Self-Efficacy Scale (SSC; Velicer, Diclemente, Rossi, & Prochaska, 1990) to measure confidence in remaining abstinent. Specifically, the well-established SSC includes nine different situations (e.g., “when I am extremely anxious or stressed”) and asks respondents to indicate whether they would smoke in such situations, with extent of confidence in such judgments rated on a 5-point Likert-type scale (1 = not at all confident to 5 = extremely confident). In the present study, we used the negative affect subscale (SSC-N) to index confidence in abstaining from smoking when experiencing negative affect.
Laboratory challenge tasks
We counterbalanced the order of the laboratory challenge tasks across participants according to a Latin square. Regardless of task order, the sessions began with baseline assessment of a series of single-item self-report questions, rated on a Likert scale ranging from 0 (none) to 100 (extreme), to assess moment-to-moment levels of (a) anxiety, (b) irritability, (c) difficulty concentrating, (d) bodily discomfort, (e) urge to smoke, and (f) frustration. These items were selected on the basis of their theoretical relevance for sensitivity to physical and psychological distress. Items (a), (b), (c), and (f) were summed to attain a general dysphoria score to assess psychological distress. The physical discomfort and urge to smoke items were evaluated separately.
Following the single-item self-report questions was a 10-min physiological adaptation period. A Biopac MP100 system was used to digitally record physiological data at a sample rate of 10 samples/s across all channels using Biopac's Acknowledge Software. Raw electrocardiogram data were collected using the Biopac ECG100B electrocardiogram amplifier, with disposable Ag–AgCl electrodes aligned in a standard configuration (right and left of sternum just below the clavicle). Raw data were converted to beats per min to obtain heart rate. Skin conductance levels, converted to microsiemens, were obtained using the Biopac GSR100B electrodermal activity amplifier with the TSD103A Ag–AgCl electrodes placed on the middle segment of the middle and ring fingers. Blood pressure was measured automatically with a self-inflating adult-size cuff attached to the Dinamap Compact Blood Pressure Monitor (Model BP; Critikon) on the participant's nondominant arm. The concentration of CO2 in the participant's expired air was measured using the RI-555 Infrared Gas analyzer (CEA Instruments), which measures up to 100% CO2 concentration. Although we measured physiological responsivity, no significant effects in such factors were observed in predicting early lapse. (Contact the senior author for details about these findings.)
Challenge tasks were separated by a 15-min break, followed by a second presentation of the baseline single-item questions and 10-min adaptation period. For heart rate and skin conductance, a baseline reading was taken for the average of 5–8 min to limit adaptation effects at the start of the baseline and anticipatory effects at the end of the baseline and the start of the challenge tasks. For blood pressure, a baseline reading was taken once during the baseline assessment from 8 to 9 min.
Physical challenges.
Breath-holding and CO2 inhalation: At the start of this challenge, participants were asked to take a deep breath and hold it for as long as they could, while the duration of breath holding was recorded with a stopwatch. Following the breath-holding procedure and a 2-min recovery period, the CO2 challenge began. The CO2 challenge task lasted 15 min and included two CO2 presentations set to occur at 7 and 12 min. The first presentation lasted 25 s, and the participant determined the length of the final presentation. That is, once the final presentation had begun, participants could press a button on the provided computer keyboard to terminate the presentation immediately. Unbeknownst to participants, if a button press was not made within 60 s (i.e., maximum duration), the presentation terminated automatically. Physiological and self-report responding was assessed in a similar fashion as for the PASAT, with the experimental reading occurring during the first 10 s following the first CO2 presentation for heart rate and skin conductance. The experimental blood pressure reading occurred immediately after the 10-s period in which skin conductance and heart rate were collected. Similar to that for the PASAT, the single-item self-report questions followed the blood pressure reading. Repeated-measures analyses of physiological responding to the CO2 challenge indicated that participants showed significant increases in skin conductance (mean difference = 1.99; SD = 2.68; p < .05) and blood pressure (systolic mean difference = 4.2; diastolic mean difference = 3.60) in response to the first presentation of CO2. However, after the CO2 presentation was completed, participants did not report significantly increased dysphoria (mean difference = 1.90; SD = 40.04; p > .05), physical discomfort (mean difference = 2.6; SD = 17.83; p > .05), or cigarette craving (mean difference = 0.90; SD = 17.80; p > .05) compared with the baseline ratings. We found no associations between lapse status and physiological or self-reported physical discomfort or urge to smoke responses to the CO2.
Psychological challenge.
PASAT: As the psychological challenge, we used a modified computer version of the PASAT (Lejuez et al., 2003), which was developed for the assessment of information processing and capacity in patients with head trauma (Gronwall, 1977). The PASAT was used in the present study because it has been shown to produce elevated levels of stress (Deary et al., 1994). A thorough description of this computer version is provided by Lejuez et al. (2003).
The PASAT was set to last up to 20 min. In the task, sequential numbers were flashed on the screen. Participants summed the most recent number with the previous number and used the computer's mouse to click on the correct answer using a keyboard provided on the computer screen. After providing each sum, the participant was to ignore that sum and add the following number to the most recently presented number (e.g., 4 + 3 [correct response = 7] + 6 [=9] + 1 [=7], etc.).
The first level of the PASAT provided a 3-s latency between number presentations (i.e., low difficulty). The second level provided a 1.5-s latency between number presentations (i.e., medium difficulty). The final level provided a 1-s latency between number presentations (i.e., high difficulty). The first level lasted for 3 min and the second level for 5 min. Following a 2-min rest period, the final level lasted up to 10 min, with the participant having an escape option. Specifically, participants were informed that once the final level had begun, they could terminate exposure to the task at any time by pressing a button on the provided computer keyboard. To provide incentive to complete the task to the best of their abilities, participants were told that they would receive a gift certificate worth $5 if their total number of points earned was greater than the average score of the other participants. It was explained further that this “target” score could not be determined until the end of the study participation, but in the event that the participant's score exceeded the target score, the gift certificate would be sent in the mail about 4 weeks after participation. These instructions were intended to produce some incentive for continuing the task while being weak enough not to create ceiling effects in the duration of endurance across participants. All subjects were mailed the $5 payment following participation.
For analyses, we compared baseline and one experimental physiological reading. The experimental reading for heart rate and skin conductance included the first 10 s of the second level of difficulty on the PASAT to capture responses at a high level of difficulty not confounded by the “quit” option in the third presentation. The experimental reading for blood pressure included the first minute during the rest period following the end of level 2 and the beginning of level 3. After this blood pressure reading, the participant again completed the single-item self-report questions, thereby also providing a baseline and an experimental reading for analysis purposes.
Repeated-measures analyses of physiological responding to the PASAT indicated that participants showed significant increases in skin conductance (mean difference = 2.92; SD = 2.23; d = .67, p < .05) and blood pressure (systolic mean difference = 2.85; diastolic mean difference = 3.01) in response to the first two levels of the PASAT prior to being given the option to terminate the task. After completing the first two PASAT levels, participants reported significantly increased dysphoria (mean difference = 77.5, SD = 76.91; p < .01), as well as increased physical discomfort (mean difference = 2.9; SD = 12.72; p < .05) and cigarette craving (mean difference = 7.24, SD = 14.10; p < .01) compared with the baseline ratings. We found no associations between lapse status and physiological or self-report responses to the PASAT (p values > .05).
Measures of affective symptomatology and vulnerability
Affective symptomatology and vulnerability to negative affective states were measured using self-report measures of depressive symptoms, fear of anxiety-related sensation, and a general vulnerability to negative affect.
Depressive symptoms.
The Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977) is a well-established measure of depressive symptoms. Respondents indicate how often within the past week they experienced the symptom in question, responding “rarely or none of the time,” “some or little of the time,” “occasionally or a moderate amount of the time,” and “most or all of the time.”
Fear of anxiety-related sensations.
The Anxiety Sensitivity Index (ASI) is a 16-item questionnaire in which respondents indicate on a 5-point Likert-type scale (0 = very little to 4 = very much) the degree to which they fear the negative consequences of anxiety symptoms (Reiss, Peterson, Gursky, & McNally, 1986). Factor analysis of this scale indicates that it has a hierarchical structure, with three first-order factors titled anxiety sensitivity–physical concerns, mental incapacitation concerns, and social concerns and a single, higher order general factor (Zinbarg, Barlow, & Brown, 1997). The ASI has demonstrated good internal consistency and good psychometric properties (Peterson & Reiss, 1992).
Negative affectivity.
To assess vulnerability to negative affect (also referred to as negative emotionality or negative affectivity), we used the stress reaction scale (SR) of the Multidimensional Personality Questionnaire (MPQ; Tellegen, 1982). The MPQ has strong psychometric properties and good behavioral genetic data from twin studies (Krueger, 2000).
Data analyses
We first examined time to termination (i.e., persistence) on each of the laboratory challenge tasks and the intercorrelations among persistence on each task. To extend our previous analyses (Brown et al., 2002), we tested whether length of longest previous quit attempt was associated with greater persistence on these tasks. We also examined the associations between persistence on the challenge tasks and both smoking (dependence, smoking rate, years of regular smoking) and demographic variables (age, education, gender). We did not expect significant relationships with these variables, but given the novelty of the persistence tasks, we wanted to characterize their relationships with other variables that may be relevant to smoking cessation.
We conducted correlational analyses to examine the relationship between distress tolerance and depressive symptoms, anxiety sensitivity, and stress reactivity. To test the effects of distress tolerance on relapse to smoking, we conducted discrete time survival analyses predicting day of first lapse to smoking. We first examined the effect of persistence on each task independently. We then entered the variables simultaneously into the analyses to gauge the relative strength of associations with quitting behavior. We also created a composite variable from the persistence tasks to see how accurately we could predict lapsing using the combined information from each task.
Results
Task persistence
On average, participants terminated the PASAT after 266.5 s (SD = 198.3). Two participants had missing data on this variable due to procedural problems in the task administration. This variable had some truncation (i.e., a ceiling effect) as 11 participants (13.9%) went the full 600 s. The median time to termination was 210 s. Mean breath-holding duration was 41.1 s (SD = 16.3) with a median of 38 s. On average, participants terminated the CO2 challenge after 44.1 s (SD = 17.1). Four participants had missing data on this variable due to procedural problems in the task administration. This variable had significant truncation as 33 participants (42.9%) went the full 60 s.
Correlation analyses (Spearman's rank-order correlations were used with the nonnormal CO2 persistence data) revealed that breath-holding duration was significantly correlated with greater persistence on the CO2 challenge, rS(77) = .27, but the positive correlation with PASAT persistence did not reach significance, r(79) = .17, p = .14. PASAT persistence was positively correlated with CO2 persistence, rS(77) = .20, although this correlation also did not reach significance, p = .09. Breath holding was significantly and positively correlated with duration of longest previous quit attempt (log-transformed to correct positive skewness), r(81) = .24. CO2 and PASAT persistence were not significantly correlated with longest previous quit attempt, rS = –.12 and .17, respectively. None of the persistence measures were significantly correlated with age, years of education, measures of affective symptomatology and vulnerability (CES-D, ASI, MPQ-SR, major depressive disorder [MDD] history), self-efficacy (SSC-N), or smoking-related variables including FTND score, cigarettes smoked per day, and number of years of regular smoking. However, men showed significantly greater breath-holding duration than women, Mmen = 46.6 s (SD = 15.3), Mwomen = 34.9 s (SD = 15.2), and this difference represented a large effect, d = .77. Men also showed greater persistence on the CO2 challenge according to Wilcoxon’s rank-sum test, medianmen = 52 s; medianwomen = 39 s.
Smoking outcome
Of the 81 participants who completed baseline assessments, we obtained verified follow-up data on the day of initial lapse or verified abstinence for 77 (95.1%) participants. Of these, 16 (20.8%) successfully remained abstinent for the full 28-day follow-up. Of the 61 participants who slipped, 25 (41.0%) slipped within the first day and a total of 50 (82.0%) slipped within the first week.
Prior to running survival analyses with the persistence tasks as predictors, we examined other covariates that might predict outcome in this sample. These included age, years of education, measures of affective symptomatology and vulnerability (CES-D, ASI, MPQ-SR, MDD history), self-efficacy (SSC-N), or smoking-related variables including FTND score, cigarettes smoked per day, and number of years of regular smoking. Of these, only level of dependence (FTND), level of education, and having a history of MDD significantly predicted survival to initial smoking lapses. We therefore controlled for these variables in our analyses. We also controlled for gender, given its relation to persistence on breath-holding and the CO2 challenge, although it was not significantly associated with survival (p = .20).
In the first step of the analysis, we entered FTND, gender, education, and history of MDD into the model simultaneously. Having a higher FTND score was associated with a slightly lower risk of lapsing, RR = 0.88, p = .05. Greater education, coded in years, was associated with a low risk of lapsing, RR = 0.77, p = .001. Having a history of MDD, compared with having no history of MDD, was associated with a significantly greater risk of lapsing during the 28-day follow-up, RR = 1.96, p = .013.
In the next set of analyses, we entered each persistence task separately into the proportional hazards regression model along with FTND, gender, education, and history of MDD. In the first model, the effect of PASAT persistence was nonsignificant, RR = 1.0, p = .93. In the second model, greater breath-holding duration, measured in seconds, was significantly associated with reduced risk of lapsing, RR = 0.98, p = .035. Because of the severely truncated distribution of the CO2 persistence variable, we dichotomized this variable as high vs. low using a median split prior to analysis. High CO2 persistence was associated with a significantly lower risk of lapsing relative to low CO2 persistence, RR = 0.55, p = .031. Finally, given that both breath-holding and CO2 persistence were significantly associated with survival to a first lapse, we entered both of these variables simultaneously into the model. So that both variables would be on a similar metric, we dichotomized breath-holding persistence for this analysis. In this model, high breath-holding persistence was significant, RR = 0.52, p = .045, and the effect of high CO2 persistence was of similar magnitude and approached significance, RR = 0.61, p = .082, suggesting that both measures might have unique effects on outcome.
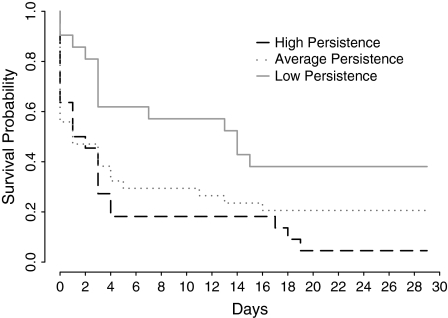
In the final set of outcome analyses, we created a composite score for breath-holding and CO2 persistence by adding the two dichotomized variables to form three groups: low persistence (low persistence on both tasks; n = 24), average persistence (high persistence on one of the tasks; n = 35), and high persistence (high persistence on both tasks; n = 22). Continuous abstinence rates at 7 days were 16.6%, 28.6%, and 59.1% across the respective groups and dropped to 4.1%, 20.6%, and 36.4%, respectively, by 28 days. These outcomes are shown in Figure 1. Proportional hazards models conducted using dummy codes for persistence group and controlling for significant covariates (FTND, years of education, and having a history of MDD) indicated that those low in persistence had 3.27 times higher risk of lapsing during follow-up relative to those with high persistence, p = .006. Those with low persistence had 1.29 times greater risk of lapsing relative to those with average persistence, a difference that was not significant, p = .411. However, those with average persistence still had 2.54 times the risk of lapsing relative to those with high persistence (p = .011).
Figure 1.
Plot of the survival probability of participants in the days following a quit attempt, by persistence group.
Discussion
An increased level of scientific attention has focused on better understanding the nature of early lapse to smoking. Emerging work suggests that early lapsers may be characterized by a tendency toward negative affect during nicotine withdrawal and by an inability to tolerate such negative affect (Brown et al., 2005). Building from such research, our aim was to replicate and extend past research on distress tolerance and early smoking lapse by exploring whether prequit persistence on physical and psychological challenge tasks would be significantly associated with reduced risk of lapsing, using a prospective design.
Consistent with expectation, greater breath-holding duration and CO2 persistence were associated singularly with a significantly lower risk of smoking lapse following an unaided quit attempt. These effects were above and beyond the risk associated with levels of nicotine dependence, education, and history of MDD. These results suggest that distress tolerance and task persistence may operate independently of risk factors such as nicotine dependence and depressive history. Thus, smoking cessation interventions designed to affect distress tolerance may complement the effects of more traditional pharmacological approaches using nicotine replacement that are intended to ameliorate nicotine withdrawal.
In contrast to expectation, the PASAT was not a significant predictor of early smoking lapse. This finding does not replicate that observed using the PASAT in the earlier study by Brown et al. (2002). Additionally, Brandon et al. (2003) found that persistence to a different psychological challenge, mirror tracing, prospectively predicted time to smoking lapse. At this time, further work is needed to sort out the specificity of task persistence under physical and/or psychological challenges in relation to smoking outcome.
A follow-up analysis was completed to further evaluate the unique associations between breath-holding duration, CO2 persistence, and smoking outcomes in one model. In this analysis, high breath-holding persistence remained a significant predictor of smoking lapse, and the effect of high CO2 persistence (similar magnitude of an effect) approached traditional levels of statistical significance (p = .08). We then conducted complimentary outcome analyses, categorizing participants on these two challenge tasks as low, medium, or high on task persistence based on being above the median in persistence on none, one, or two of the tasks, respectively. These analyses yielded empirical evidence that smokers low in persistence to these challenge tasks were significantly more likely to lapse during follow-up relative to those with high persistence. No statistically significant effects were evident between those with low persistence and average persistence. These findings indicate that distress tolerance effects are most readily apparent at the high and low ends of a putative distress tolerance continuum.
It will be useful for future work to identify the mechanisms underlying such effects to further refine our understanding of early lapse processes within a distress tolerance conceptual framework. For example, smokers with low levels of distress tolerance skills may be more highly reactive to the early (interoceptive) signs of nicotine withdrawal and, hence, lapse to smoking sooner than others to help cope with such distress (Baker, Piper et al., 2004). Overall, the present results are consistent with theoretical models of distress tolerance and early smoking lapse (Brown et al., 2005) and suggest that tolerance to distress as evidenced by persistence to laboratory challenge tasks is indeed a clinically relevant process.
Findings of the present study can conceptually guide the development of specialized intervention strategies for smokers who tend to lapse early in their quit attempts. For example, there may be merit in devising specialized treatment approaches that can modify distress tolerance prior to quitting so that these high-risk smokers can maintain longer periods of smoking abstinence and, by extension, corresponding degrees of ultimate success in quitting. Examples of strategies for such specialized treatment programs may be drawn from cognitive–behavioral methods such as interoceptive exposure and from acceptance and commitment therapy (Hayes, 2004), with its focus on acceptance as “the undefended ‘exposure’ to thought, feelings, and bodily sensations as they are directly experienced to be” (p. 21) while behaving according to one's goals and values (i.e., refraining from smoking). Drawing from distress tolerance theory and research, an overarching purpose in this type of work would be to provide smokers with a new model for interpreting and managing internal distress and emergent withdrawal symptoms. The goal would not be to attempt the (perhaps impossible) task of eliminating emotion-laden withdrawal sensations but rather to ensure acceptance or reinterpretation of these internal cues, so as not to allow them to precipitate smoking lapse (Brown et al., 2005). Brown et al. (2008) have developed and described a distress tolerance smoking cessation treatment with these clinical treatment targets; the use of this approach in smokers with a history of early smoking lapse has had promising preliminary findings. Though not focused on early lapse, conceptually related strategies have been used successfully for other populations, including smokers with anxiety problems (Zvolensky, Lejuez, Kahler, & Brown, 2003) and patients with anxiety disorder and benzodiazepine dependence (Otto, Hong, & Safren, 2002).
A number of limitations of the present investigation and points for future direction should be considered. First, the present investigation used a sophisticated prospective design to examine the nature of task persistence variables in regard to early smoking lapse. A natural next step would be to use an experimental design to manipulate task persistence and examine associations with early smoking lapse. For example, researchers could randomly assign participants to brief intervention groups focused on changing distress tolerance and then examine the corresponding impact of such training on smoking lapse following a designated quit attempt. This type of work would bolster the conclusions that can be drawn about distress tolerance and smoking lapse. Second, a striking finding was that none of the persistence measures were related to history of MDD or current depressive symptoms. These data suggest that depressive proneness is not related to the present laboratory-based indices of distress tolerance. Because participants were excluded from the investigation if they had a current Axis I disorder (excluding nicotine dependence), it is unclear if this lack of a relationship would be evident for other types of psychopathology or affect-based vulnerability variables. Future work could seek to better understand the nature of distress tolerance by studying it in relation to a broader range of psychopathology and other psychological factors. This type of work would help explicate the parameters of distress tolerance and tie such understanding to other variables of known relevance to problems in smoking cessation. Finally, the present sample was comprised of a relatively homogeneous (e.g., primarily White) group of psychologically healthy adult smokers who volunteered to participate in the study for monetary reward. To rule out potential self-selection bias among persons with these characteristics and increase the generalizability of these findings, it will be important for researchers to draw from other populations and use recruitment tactics other than those used here.
Together, the present findings uniquely extend previous work documenting an association between distress tolerance and early smoking lapse among adult daily smokers. Results suggest that elevations in physical-based indices of distress tolerance may increase the probability of early lapse and that this association is not attributable to other theoretically relevant factors. These findings provide further evidence that distress tolerance is an important construct in terms of smoking cessation.
Funding
National Cancer Institute (CA88297).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors thank Melanie Poisson and Ovide Pomerleau for their contributions to this work. All research was conducted at Butler Hospital, Warren Alpert Medical School of Brown University.
References
- Augustson E, Marcus S. Use of the current population survey to characterize subpopulations of continued smokers: A national perspective on the “hardcore” smoker phenomenon. Nicotine & Tobacco Research. 2004;6:621–629. doi: 10.1080/14622200410001727876. [DOI] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review of Psychology. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. Journal of Abnormal Psychology. 2003;112:448–456. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Brown RA. Comorbidity treatment: Skills training for coping with depression and negative moods. In: Abrams DB, Niaura RS, Brown RA, Emmons KM, Goldstein MG, Monti PM, editors. The tobacco dependence treatment handbook: A guide to best practices. New York: Guilford Press; 2003. [Google Scholar]
- Brown RA, Kahler CW, Niaura R, Abrams DB, Sales SD, Ramsey SE, et al. Cognitive-behavioral treatment for depression in smoking cessation. Journal of Consulting and Clinical Psychology. 2001;69:471–480. doi: 10.1037//0022-006x.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Zvolensky MJ, Lejuez CW, Ramsey SE. Anxiety sensitivity: Relationship to negative affect smoking and smoking cessation in smokers with past major depressive disorder. Addictive Behaviors. 2001;26:887–899. doi: 10.1016/s0306-4603(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111:180–185. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ. Distress tolerance and early smoking lapse. Clinical Psychology Review. 2005;25:713–733. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, et al. Distress tolerance treatment for early lapse smokers: Rationale, program description and preliminary findings. Behavior Modification. 2008;32:302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MR, Gerkovich MM, O'Connell KA, Potocky M. Reversal theory constructs and cigarette availability predict lapse early in smoking cessation. Research in Nursing & Health. 1995;18:217–224. doi: 10.1002/nur.4770180305. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Ebmeier KP, MacLeod KM, Dougall N, Hepburn DA, Frier BM, et al. PASAT performance and the pattern of uptake of -super(99m)tc-exametazime in brain estimated with single photon emission tomography. Biological Psychology. 1994;38:1–18. doi: 10.1016/0301-0511(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: Relationship to relapse and predictors. Psychopharmacology. 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: A report from the normative aging study. Addictive Behaviors. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Gronwall DMA. Paced Auditory Serial-Addition Task: A measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Hajek P. Individual differences in difficulty quitting smoking. British Journal of Addiction. 1991;86:555–558. doi: 10.1111/j.1360-0443.1991.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Hajek P, Belcher M, Stapleton J. Breath-holding endurance as a predictor of success in smoking cessation. Addictive Behaviors. 1987;12:285–288. doi: 10.1016/0306-4603(87)90041-4. [DOI] [PubMed] [Google Scholar]
- Hayes SC. Acceptance and commitment therapy and the new behavior therapies: Mindfulness, acceptance, and relationship. In: Hayes SC, Follette VM, Linehan MM, editors. Mindfulness and acceptance. New York: The Guilford Press; 2004. [Google Scholar]
- Hayes SC, Wilson KG, Gifford EV, Follette VM, Strosahl K. Experiential avoidance and behavioral disorders: A functional dimensional approach to diagnosis and treatment. Journal of Consulting and Clinical Psychology. 1996;64:1152–1168. doi: 10.1037//0022-006x.64.6.1152. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A Revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heil SH, Tidey JW, Holmes HW, Badger GJ, Higgins ST. A contingent payment model of smoking cessation: Effects on abstinence and withdrawal. Nicotine & Tobacco Research. 2003;5:205–213. doi: 10.1080/1462220031000074864. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami D. Effects of abstinence from tobacco: A critical review. In: Kozlowski LT, Annis HM, Cappell HD, Glaser FB, Goodstadt MS, Israel Y, Kalant H, Sellers EM, Vingilis ER, editors. Research advances in alcohol and drug problems. Vol. 10. 1990. pp. 317–398. [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Irvin JE, Brandon TH. The increasing recalcitrance of smokers in clinical trials. Nicotine & Tobacco Research. 2000;2:79–84. doi: 10.1080/14622200050011330. [DOI] [PubMed] [Google Scholar]
- Krueger RF. Phenotypic, genetic, and nonshared environmental parallels in the structure of personality: A view from the multidimensional personality questionnaire. Journal of Personality and Social Psychology. 2000;79:1057–1067. doi: 10.1037//0022-3514.79.6.1057. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Forsyth JP, Eifert GH. Devices and methods for administering carbon dioxide-enriched air in experimental and clinical settings. Journal of Behavior Therapy and Experimental Psychiatry. 1998;29:239–248. doi: 10.1016/s0005-7916(98)00018-4. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Kahler CW, Brown RA. A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor: Implications for behavioral assessment. The Behavior Therapist. 2003;26:290–293. [Google Scholar]
- Lichtenstein E, Glasgow RE. Smoking cessation: What have we learned over the past decade? Journal of Consulting and Clinical Psychology. 1992;60:518–527. doi: 10.1037//0022-006x.60.4.518. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Experimental approaches to cognitive abnormality in posttraumatic stress disorder. Clinical Psychology Review. 1998;18:971–982. doi: 10.1016/s0272-7358(98)00036-1. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams DB. Smoking cessation: Progress, priorities, and prospectus. Journal of Consulting and Clinical Psychology. 2002;70:494–509. doi: 10.1037//0022-006x.70.3.494. [DOI] [PubMed] [Google Scholar]
- Otto MW, Hong JJ, Safren SA. Benzodiazepine discontinuation difficulties in panic disorder: Conceptual model and outcome for cognitive-behavior therapy. Current Pharmaceutical Design. 2002;8:75–80. doi: 10.2174/1381612023396726. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? American Psychologist. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Reiss S. 2nd ed. Worthington, OH: International Diagnostic Systems; 1992. The anxiety sensitivity index manual. [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerström Tolerance Questionnaire and the Fagerström Test for Nicotine Dependence. Addictive Behaviors. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behavior Research Therapy. 1986;24(1):1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–504. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkovskis PM, Reynolds M. Thought suppression and smoking cessation. Behaviour Research and Therapy. 1994;32:193–201. doi: 10.1016/0005-7967(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Smoking cessation treatment: Any progress? Journal of Consulting and Clinical Psychology. 1993;61:718–722. doi: 10.1037//0022-006x.61.5.718. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Unpublished manuscript, University of Minnesota, Minneapolis; 1982. Brief manual for the multidimensional personality questionnaire; pp. 1010–1031. [Google Scholar]
- U.S. Department of Health and Human Services. Clearing the air: How to quit smoking. And quit for keeps. 1995. (NIH Publication No. 95–1647): Public Health Service, National Institutes of Health, National Cancer Institute. [Google Scholar]
- Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: An integrative model. Addictive Behaviors. 1990;15:271–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Belcher M. Time course of cigarette withdrawal symptoms while using nicotine gum. Psychopharmacology. 1989;99:143–145. doi: 10.1007/BF00634470. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE, Barlow DH, Brown TA. Hierarchical structure and general factor saturation of the anxiety sensitivity index: Evidence and implications. Psychological Assessment. 1997;9:277–284. [Google Scholar]
- Zvolensky MJ, Bernstein A, Cardenas SJ, Colotla VA, Marshall EC, Feldner MT. Anxiety sensitivity and early relapse to smoking: A test among Mexican daily, low-level smokers. Nicotine & Tobacco Research. 2007;9:483–491. doi: 10.1080/14622200701239621. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Feldner MT, Eifert GH, Brown RA. Affective style among smokers: Understanding anxiety sensitivity, emotional reactivity, and distress tolerance using biological challenge. Addictive Behaviors. 2001;26:901–915. doi: 10.1016/s0306-4603(01)00242-8. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Lejuez CW, Kahler CW, Brown RA. Integrating an interoceptive exposure-based smoking cessation program into the cognitive-behavioral treatment of panic disorder: Theoretical relevance and clinical demonstration. Cognitive and Behavioral Practice. 2003;10 (348–358) [Google Scholar]
- Zvolensky MJ, Lejuez CW, Kahler CW, Brown RA. Nonclinical panic attack history and smoking cessation: An initial examination. Addictive Behaviors. 2004;29:825–830. doi: 10.1016/j.addbeh.2004.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.