Abstract
The role of the conserved Focal Adhesion Kinase (FAK) family of protein tyrosine kinases (PTKs) in the development and physiological functions of the CNS has long been an area of interest among neuroscientists. In this report, we observe that Drosophila mutants lacking Fak56 exhibit a decreased life span, accompanied by a bang-sensitive phenotype, which is characterised by sensitivity to mechanical and high-frequency electrical stimulation. Fak56 mutant animals display lower thresholds and higher rates of seizures in response to electroconvulsive stimuli, and direct measurements of action potential conduction in larval segmental nerves demonstrate a slowed propagation speed and failure during high-frequency nerve stimulation. In addition, neuromuscular junctions in Fak56 mutant animals display transmission blockade during high-frequency activity as a result of action potential failure. Endogenous Fak56 protein is abundant in glial cells ensheathing the axon bundles, and structural alterations of segmental nerve bundles can be observed in mutants. Manipulation of Fak56 function specifically in glial cells also disrupts action potential conduction and neurotransmission, suggesting a glial component in the Fak56 bang-sensitive phenotype. Furthermore, we show that increased intracellular calcium levels result in the dephosphorylation of endogenous Fak56 protein in Drosophila cell lines, in parallel with our observations of highly variable synaptic potentials at a higher Ca2+ level in Fak56 mutant larvae. Together these findings suggest that modulation of Fak56 function is important for action potential propagation and Ca2+-regulated neuromuscular transmission in vivo.
Keywords: Fak56, calcium, tyrosine phosphorylation, neuromuscular junction, glia
Introduction
Focal Adhesion Kinase (FAK) and Pyk2 are the founder members of a eukaryotic family of cytoplasmic non-receptor protein tyrosine kinases (PTKs) (for reviews see Avraham et al., 2000; Mitra et al., 2005). FAK is strongly expressed in the developing CNS and is implicated in the promotion of neurite outgrowth and regulation of neuronal cell migration, whereas Pyk2 expression is elevated in adult neurons, showing high abundance at the post-synaptic density, a microscopic structure associated with the post-synaptic membrane that contains a variety of signalling proteins (Girault et al., 1999; Husi et al., 2000; Xiong & Mei, 2003). In Drosophila, the sole FAK family member, Fak56 (Fox et al., 1999; Fujimoto et al., 1999; Palmer et al., 1999), shows extensive similarities with both mammalian FAK and Pyk2 and is highly expressed in the CNS and at muscle attachment sites (Fox et al., 1999; Grabbe et al., 2004). The lethality in mice caused by loss of FAK implies that FAK performs an essential function during development (Ilic et al., 1995). However, Fak56 null mutant flies are surprisingly viable and fertile with no obvious defects during development (Grabbe et al., 2004).
In Drosophila, certain types of genetic mutations cause neurological defects characterised by a sensitivity to mechanical and electrical stimuli, referred to as “bang sensitivity” (Ganetzky and Wu, 1982b). When stimulated, bang-sensitive mutant flies suffer from cycles of seizures and temporary paralysis, similar in many ways to the symptoms displayed by epileptic patients (Kuebler & Tanouye, 2000; Lee & Wu, 2002). Common to all known bang-sensitive mutants is a failure of the giant fiber (GF) pathway, often involving failure at the peripherally synapsing interneuron and motorneurons, features that can be recorded electrophysiologically (Pavlidis & Tanouye, 1995; Kuebler & Tanouye, 2000). The genes identified in causing bang-sensitive behaviour do not appear to share common features, but have rather shown to be involved in cellular structures and pathways as diverse as phospholipid biosynthesis, ion transport, transcription, Ca2+ release and mitochondrial protein synthesis (Royden et al., 1987; Pavlidis & Tanouye, 1995; Zhang et al., 1999; Trotta et al., 2004; Fergestad et al., 2006).
Here we report that Fak56CG1 mutant Drosophila exhibit a decreased life span accompanied by a bang sensitivity that can be induced by mechanical and high-frequency electrical stimulation. Abundant Fak56 protein expression in glial cells, together with experiments manipulating Fak56 function specifically in glia, suggests that defective Fak56 in glial cells can lead to altered motor axon excitability, a phenotype previously described in other bang-sensitive mutants (Ganetzky and Wu, 1982b; Trotta et al., 2004; Fergestad et al., 2006). Furthermore, we demonstrate that stimulation of calcium influx in Drosophila cell lines results in the dephosphorylation of Fak56 on tyrosine and in parallel, that Fak56CG1 mutant flies display Ca2+-dependent defects in synaptic transmission at larval neuromuscular junctions (NMJs). Our data demonstrating neuromuscular defects involving nerve conduction, synaptic transmission, and seizure induction suggests that Fak56 plays an important role in the regulation of nervous system functions in Drosophila.
Materials and Methods
Drosophila stocks
Standard Drosophila husbandry procedures were employed. Flies were raised and crossed at room temperature unless otherwise stated. The control strain used was white1118. The Fak56CG1 and Fak56CG2 mutants have been described previously (Grabbe et al., 2004). ControlRev1 and ControlRev2 are revertant control strains recovered as perfect excisions of the P line KG00304 (Bloomington #13080; Roseman et al. 1995). In order to remove a shaking phenotype unrelated to the Fak56 locus, Fak56CG1 was back crossed to white1118 and Fak56CG1 mutant lines and control lines were reestablished based on bang sensitivity. The presence of the Fak56CG1 mutation was subsequently confirmed by PCR. Multiple Fak56CG1 mutant lines and control lines were used in all experiments. For overexpression studies, repo-GAL4 (Bloomington #7415; Lee & Jones 2005) was used to induce the expression of UAS:Fak56Y430F in glial cells.
Behavioral assays
For life span tests, flies were collected within 24 hours after eclosion and aged at 25°C. Females and males were kept separately and flies were transferred to new vials with fresh food every 3 days. Testing for bang sensitivity was performed on flies 2−4 hours post-eclosion (unless otherwise specified). Flies were collected for two hours and rested 2 hours after CO2 exposure before testing. Individual flies were mechanically stimulated by manual banging for 10 seconds and the recovery time from paralysis was monitored.
Adult Giant Fiber Pathway Electrophysiology
Electroconvulsive seizure responses were studied in the giant fiber (GF) pathway responsible for the jump-and-flight escape response (Tanouye & Wyman, 1980) in tethered flies. The motor output from a pair of cervical GF neurons that receive inputs from various sources, including the visual system (Trimarchi & Schneiderman, 1995), can be monitored in indirect flight muscles (DLM a-f) and jump muscles (TTM). Methods for electroconvulsive seizure recording and characterization in the GF pathway have been described previously (Lee & Wu, 2002). Tungsten electrodes (uninsulated) were used for stimulation and recording. A pair of stimulating electrodes were positioned in the eyes, a recording electrode into DLM a, and a reference electrode into the abdomen. After rest for at least 30 min of tethered flies, a high-frequency electroconvulsion stimulus train (200 Hz, 0.1 msec pulses) was used to induce seizure. Responses were picked up with an AC preamplifier (DAM-5A, WPI, New Haven, CT, USA) (filter bandwidth from 0.1 Hz to 30 kHz, reference grounded) and recorded with pulse code modulation (DR-384, Neuro Data, New York, NY, USA) on videotape at a sampling rate of 44 kHz. Four stimulus levels were used for seizure induction. Each increment of intensity was achieved by doubling stimulus train duration or stimulus voltages (1 = 50 V, 0.5 s, 2 = 50 V and 1.0 s, 3 = 50 V, 2.0 s, 4 = 100 V, 2 s). After the 200 Hz electroconvulsive stimulus, test pulses of 24V for 0.1 msec (or higher), sufficient to evoke short-latency responses (Engel & Wu, 1996) were delivered at 1 Hz to monitor response failure and recovery of the GF pathway. An interval of at least 10 min was allowed between presentations of electroconvulsive stimuli to avoid the effect of refractoriness. Flies were 2−10 days old. All physiological experiments were performed at room temperature.
Larval Motor Axon Action Potential and Synaptic Potential Recordings
The larval neuromuscular preparation for recording axonal action potentials and excitatory junctional potentials (EJPs) has been described previously (Jan & Jan, 1976; Wu et al., 1978; Ueda & Wu, 2006). Briefly, third instar larvae were dissected in Ca2+ free HL3 saline containing (in mM) NaCl (70), KCl (5), MgCl2 (20), NaHCO3 (10), trehalose (5), sucrose (115), HEPES (5), and pH 7.2 (Stewart et al., 1994) to minimize muscle contractions. The visceral organs were removed to expose the body-wall muscles and the nervous system. The segmental nerve was cut near the ventral ganglion. Action potential and EJP recordings were performed in modified HL3.1 (Feng et al., 2004) containing (mM) NaCl (150), KCl (5), CaCl2 (0.1), MgCl2 (4), NaHCO3 (10), treharose (5), sucrose (7.5), HEPES (5), and pH 7.2. The increased sodium concentration facilitates extracellular action potential recording. To evoke nerve action potentials, the segmental nerves were stimulated through the cut end with a suction electrode (10 μm I.D.) at 3 times threshold voltage with a duration of 0.1 ms. Single unit action potentials were recorded from the Type-Is motor axon innervating muscle 4 at the nerve entry point where individual axons can be distinguished. Action potential signals were picked up by a differential AC-amplifier (DAM-5A, WPI) with high-frequency cutoff at 10 K Hz.
The Ca2+ concentration for EJP recordings is specified in the results. Intracellular glass microelectrodes were filled with 3 M KCl and had a resistance of ∼60 MΩ. EJPs were recorded from muscles 1 and 9 with a DC preamplifier (M701, WPI). Data were stored on VCR tapes with a Pulse Code Modulator (DR-384, Neuro Data) and were digitized with pClamp 5 (Axon Instruments, Inc, Burlingame, CA, USA) and analyzed in an IBM-compatible computer. TTX was purchased from Sigma (St. Louis, MO, USA).
Tissue Culture and Cell Stimulation
Drosophila ML-BG2-c6 (from Drosophila Genomics Resource Center, Indiana) cells were cultured at 25°C in modified Shields and Sangs M3 medium (Sigma, St. Louis, MO, USA), supplemented with 10% heat-inactivated fetal calf serum, 50 units/ml Penicillin/Streptomycin (both GIBCO (Invitrogen), Carlsbad, CA, USA) and 10 μg/ml insulin (Sigma #I-9278). Drosophila Schneider cells (S2 cells) were cultured at 25°C in 1x Schneider medium (GIBCO) supplemented with 10% heat-inactivated FCS, 50 units/ml Penicillin/Streptomycin, 50μg/ml Gentamycin and 6mM Glutamine (all GIBCO). For stimulation experiments, non-adhesive plastic dishes were precoated for 60 min with 200 μg/ml crude extracellular matrix, prepared from Kc167 cells as previously described (Takagi et al., 2000), diluted in PBS. 107 ML-BG2-c6 or S2 cells were plated on precoated dishes 4 hours prior to stimulation. Cell stimulations were performed at room temperature, using 6 μM Ionomycin (Sigma), for the times indicated.
Immunoprecipitation, Immunoblotting and Immunohistochemistry
After stimulation, cells were washed three times with ice-cold PBS prior to lysis in Lysis Buffer (100 mM NaCl, 50 mM Tris, pH 7.5, 1 % Triton X-100, 1 mM EDTA, 1 mM EGTA, 10 mM Benzamidine, 15 mM MgCl2, 1 mM DTT, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF and Complete inhibitors (Roche, Basel, Switzerland, Cat. No.1873580). Lysates were cleared by centrifugation and protein concentrations determined using the Bio-Rad protein assay. Immunoprecipitations were carried out with commercial 4G10-antiphosphotyrosine beads (Upstate Biotechnology, Lake Placid, NY, USA) or anti-Fak56 antibodies crosslinked to Protein A beads over night at 4°C and washed five times with Wash Buffer (5 mM MgCl2, 1 % Triton X-100, 1 X PBS). Lysates or immunoprecipitates were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). Membranes were blocked in 5% milk or 5% BSA (in 1 X PBS, 0.1% Tween-20) for 1 hour prior to incubation with primary antibodies overnight, and ECL detection (Amersham Pharmacia Biotech, Uppsala, Sweden). Rabbit anti-Fak56 was used at 1:1500 (Palmer et al., 1999), rabbit anti-phospho-FAK397 was used at 1:1000 (Biosource, (Invitrogen), Carlsbad, CA, USA) and PY-PLUS mAb cocktail at 1:500 (Zymed (Invitrogen), Carlsbad, CA, USA). For immunohistochemistry, third instar larvae were dissected in HL3.1 saline and fixed in 100% ice-cold methanol for 5 minutes. Affinity-purified guinea pig anti-Fak56 antibody was used at 1:300 (a generous gift from Dr. R. Hynes), followed by a FITC-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at 1:50. Morphology of NMJs including synaptic boutons and motor axon bundles in close proximity was visualized by Texas Red-conjugated anti-HRP antibody (Jackson ImmunoResearch Laboratories) at 1:50. Images were collected using a BioRad 1024 confocal microscope (Bio-Rad, Hercules, CA, USA). The z-stacked images were obtained using Image J software (NIH image, Bethesda, MD, USA) and processed with Photoshop (Adobe Systems, Inc., San Jose, CA, USA).
Results
Fak56 mutant animals display a reduced lifespan
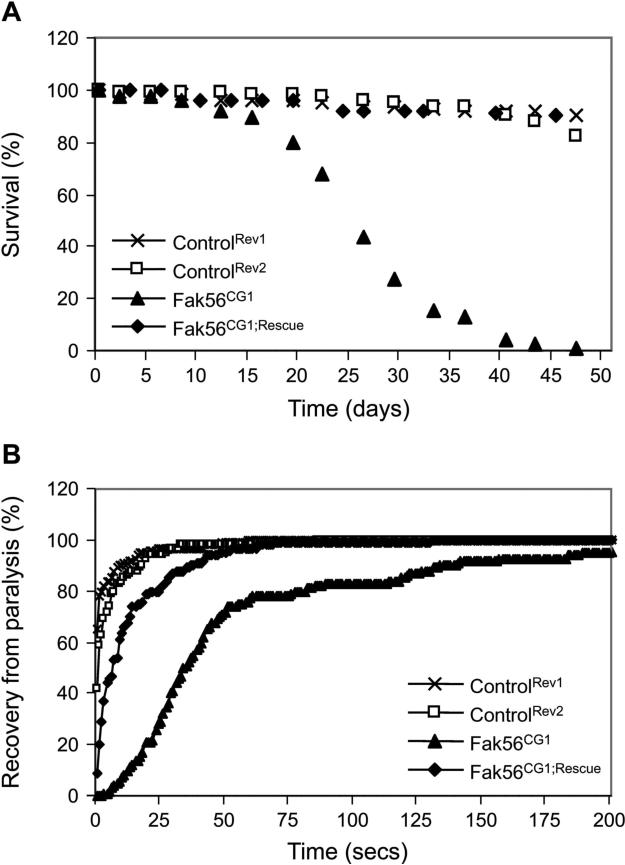
The role of the FAK family of PTKs in the nervous system has long been an area of interest among neuroscientists. FAK has been shown to play a role in establishing neural connections and differentiation in the mouse (Beggs et al., 2003), while Pyk2 is implicated in regulating synaptic plasticity during adult life (Lev et al., 1995; Siciliano et al., 1996; Tian et al., 2000). In contrast to these observations in vertebrates, no obvious developmental defects in the nervous system are observed during embryonic development in Drosophila Fak56 mutants (Grabbe et al., 2004). However, we found that Fak56 mutant flies have a significantly shorter life span (Figure 1A). In the absence of Fak56 protein, flies had an average life span of ∼25 days at 25°C, compared to at least ∼66 days in control flies. This reduction in life span could be entirely attributed to the loss of Fak56, since reintroduction of Fak56 in the Fak56CG1 mutant background using a genomic rescue construct reverted the phenotype. Furthermore, this reduction of life span was not observed in flies from revertant control lines, which were derived from the same P element containing chromosome as the Fak56CG1 mutant.
FIG. 1. Loss of Fak56 reduces life span and induces bang sensitivity in Drosophila melanogaster.
(A) Loss of Fak56 reduces life span in Drosophila melanogaster. Flies of the genotypes indicated were aged at 25°C and the number of surviving flies counted every fifth day. Food was changed every third day. A clear reduction of the life span could be observed in Fak56CG1 mutants. This reduced life span could be rescued by the introduction of a Fak56genomic rescue transgenic construct and was not observed in revertant control flies. 120 flies were analysed for each tested genotype. (B) Loss of Fak56 results in a bang sensitivity phenotype. Flies of the genotypes indicated were aged for 2−4 hours and assayed for paralytic behaviour in response to mechanical shock. Recovery times from paralysis after mechanical shock were measured for each individual fly. Fak56CG1 mutant flies were significantly more sensitive to mechanical stress than control flies. Bang sensitivity was not observed in revertant control flies, and could be partially reversed by the expression of a Fak56genomic rescue construct. 100 flies were analysed for each tested genotype.
Fak56 mutants are bang sensitive
Other published fly mutants which display a reduced life span include the bang-sensitive mutations in the Na+/K+ ATPase alpha subunit (Palladino et al., 2003). We found this intriguing since Fak56CG1 mutant flies appear to be temporarily paralyzed when transferred onto new food. We thus subjected Fak56CG1 mutant flies to a range of analyses and found that they exhibited paralytic behaviors in response to mechanical stress (Figure 1B). Upon stimulation mutant flies responded by entering a bang sensitivity cycle characterized by a long paralytic recovery period, lasting from a few seconds up to several minutes, resembling ATPase alpha subunit mutants. However, this behavior pattern was distinct from other classical bang-sensitive mutants, which exhibit seizure following paralysis (Ganetzky & Wu, 1982b; Kuebler & Tanuye, 2000; Lee & Wu, 2002). By ageing Fak56 flies for different periods of time prior to exposure to mechanical shock, we found that the bang sensitivity caused by the absence of Fak56 protein was strongest immediately after eclosion and declined with age (data not shown). At already 2−3 days post eclosion, Fak56CG1 flies were obviously less sensitive to mechanical shock and the bang-sensitive effect was almost undetectable after 3 weeks. This interesting observation suggests a physiological adaptation to the defects caused by loss of Fak56.
Fak56 mutant animals display increased seizure susceptibility
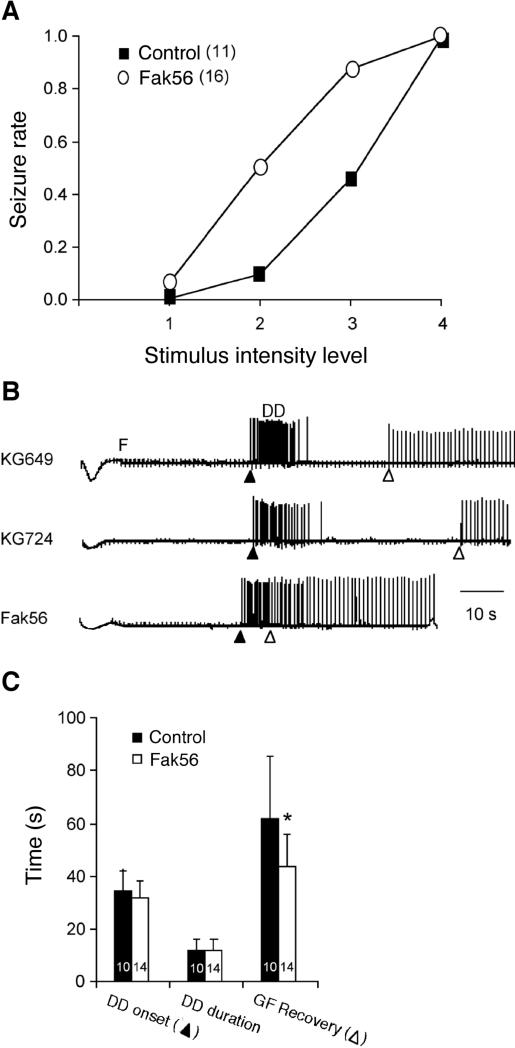
In classical bang-sensitive mutants, the typical seizure-paralysis-recovery repertoire induced by mechanical stress can be correlated to the physiological response to high-frequency electroconvulsive brain stimulation, i.e. response failure of the giant fiber (GF) pathway followed by a delayed seizure discharge (DD) detectable in thoracic flight (DLM) and jump (TTM) muscles (Pavlidis & Tanouye, 1995; Kuebler & Tanouye, 2000; Lee & Wu, 2002). We found a clear increase in seizure susceptibility in Fak56CG1 mutant animals, indicated by a lower stimulus threshold for seizure discharge (Figure 2A), consistent with an increased sensitivity to mechanical stress in Fak56CG1 mutant flies. Recordings of DLM responses to electrical brain stimulation of mutant Fak56CG1 and control flies showed no detectable difference in DD onset or duration (Figure 2B and C). Therefore, motor pattern generation of the DLM flight circuit is not disrupted to the extent of some other classical bang-sensitive mutants (Lee & Wu, 2002; 2006). However, recovery from failure was significantly faster in Fak56CG1 mutants than in control animals (Figure 2B and C), indicating potential alterations in axonal conduction and/or synaptic transmission along the giant fiber pathway.
FIG. 2. Increased seizure susceptibility in Fak56 mutants.
(A) Increased seizure susceptibility in Fak56CG1 mutants. Electroconvulsive shocks (200 Hz, 0.1 ms pulses) were delivered across the brain at different intensity levels (see Materials and Methods) to induce seizure in flies. Fak56CG1 mutants showed higher rate of seizures compared to revertant control lines. Numbers of flies examined are indicated. (B, C) Electroconvulsive seizure pattern in Fak56CG1 and control lines. Electroconvulsive shocks and test pulses (1 Hz, 0.1 ms) were delivered across the fly brain to evoke dorsal longitudinal muscle (DLM) responses (examples shown in B). The characteristic DLM seizure pattern consisted of a delayed discharge (DD) and a failure period (F) of the giant fiber pathway. Fak56CG1 mutants and revertant control flies showed similar DD onset time (filled triangle) and duration. However, recovery from giant fiber pathway failure (open triangle) was faster in mutants than in control animals. Fly numbers are indicated. * indicates p < 0.05, t-test.
Defects in action potential conduction during high-frequency nerve stimulation of Fak56 mutants
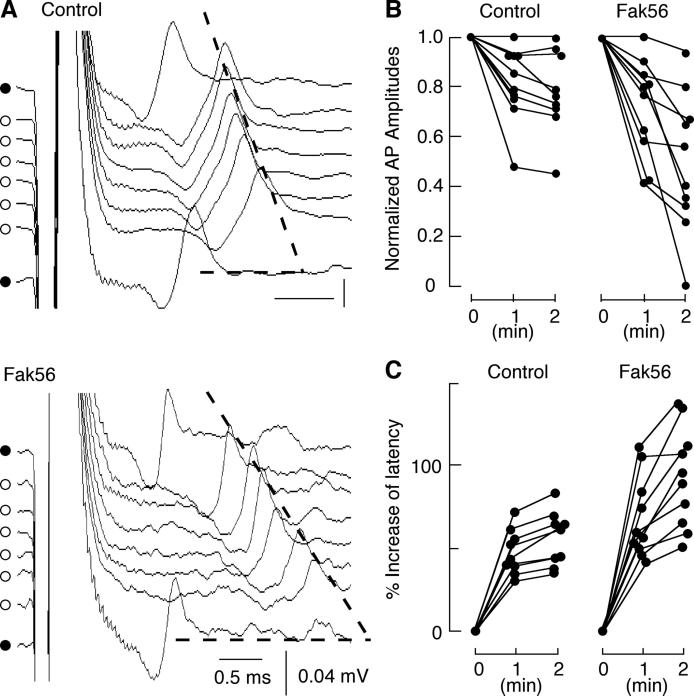
In order to examine neuronal properties during high-frequency activity in Fak56CG1 mutant animals, we used larval neuromuscular preparations for monitoring neuronal excitability and synaptic transmission. The simple stereotypic innervation pattern of identified muscle cells facilitates this analysis, allowing us to recorded action potentials from an identified motor axon (type Is axon innervating muscle 4, Figure 3A). When low-frequency stimulation (≤ 0.5 Hz) was applied to the segmental nerve, action potentials with stable waveforms and latency were recorded, both in wild type and Fak56CG1 mutant larvae. However, when high-frequency stimulation (15 Hz) was applied, the latency increased and the amplitude decreased progressively (Figure 3A). Interestingly, the changes in action potential amplitude (Figure 3B) and latency (Figure 3C) were more severe in Fak56CG1 mutant, as compared to control animals (p < 0.01, T-test). In the extreme case, action potentials in Fak56CG1 decayed to undetectable levels (Figure 3B), indicating that absence of the Fak56 protein disrupts a cellular mechanism critical for maintaining repetitive nerve firing.
FIG. 3. Reduced amplitude and increased latency of action potentials in an identified motor axon during high-frequency stimulation.
(A) Action potentials of type Is motor axons recorded extracellularly from the nerve entry point to muscle 4. High-frequency tetanic stimuli (15 Hz) were applied for two minutes. Action potentials at 20, 40, 60, 80, 100, and 120 s after the onset of the 15 Hz stimulation are presented from top to down (○). Action potentials in response low-frequency test pulses (≤ 0.5 Hz) are shown before and after tetanus for comparison (•). Note that amplitude decreased and latency increased more significantly in Fak56CG1 compared to control animals (shallower slope of dotted line in Fak56CG1). Averaged traces from three to ten sweeps are shown. (B) Alterations in action potential amplitudes during tetanic stimulation in individual axons. The amplitudes at 1 and 2 min were normalized to those before tetanus. Data points from the same axon are connected with line segments. The decrease in amplitudes in Fak56CG1 flies was more pronounced than in controls after 2 min of stimulation (p < 0.01, t-test). (C) Changes in latency during tetanus in individual axons. Data points from the same axon are connected with line segments. The latency increase in Fak56CG1 was greater than control after 2 min of stimulation (p < 0.01, t-test).
Decreased frequency response in synaptic transmission in Fak56 mutants
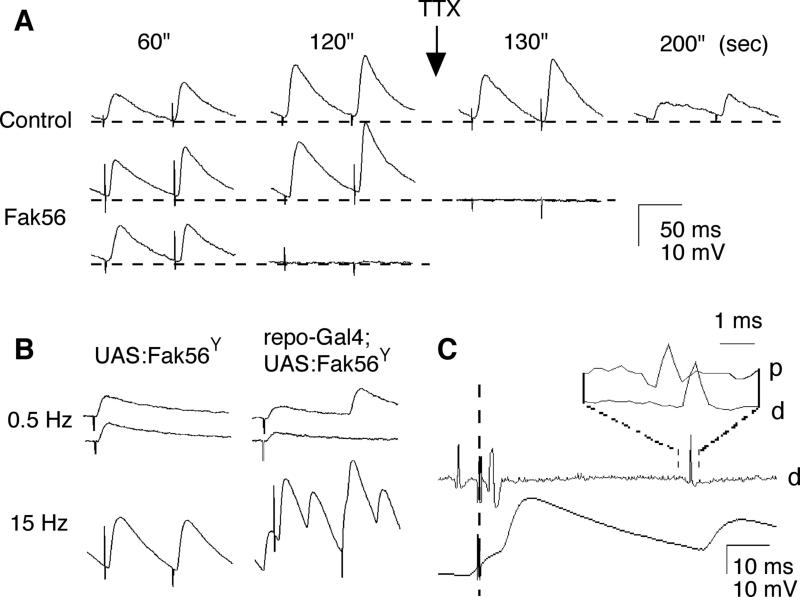
The altered capability of repetitive firing in Fak56CG1 larvae was further indicated by the recording of EJPs from muscles 1 or 9 during high-frequency neuromuscular transmission (Figure 4). A Ca2+ channel blocker, Co2+ (0.2 mM), was added to the recording saline to suppress muscle contraction during high-frequency stimulation. Upon single nerve stimulation (≤ 0.5 Hz), both control and Fak56CG1 produced quantal-sized EJPs with intermittent release failure, due to the antagonistic effect of Co2+ on Ca2+ influx (data not shown). Subsequently, we applied high-frequency stimulation (15Hz) to the segmental nerve for two minutes. Control larvae displayed a continuous increase in EJP size due to frequency-dependent facilitation of synaptic transmission (Figure 4A, upper traces). Fak56CG1 larvae showed a similar facilitation process, but strikingly, abrupt transmission failure could occur even though the EJP size continued to facilitate before the failure (Figure 4A, bottom trace). Such an abrupt EJP failure suggests a blockade of action potential propagation since it could be more frequently induced by weakening Na+ action potential mechanisms as a result of local application of TTX (1 μM) at the NMJ (Figure 4A). During TTX paralysis, control larvae showed a progressive decline in EJP size and a total block of synaptic transmission was not established several minutes after TTX application (Figure 4A, upper trace). In contrast, EJPs in Fak56CG1 larvae abruptly dropped out within tens of seconds following TTX application (Figure 4A, middle trace).
FIG. 4. Decreased axonal excitability in Fak56 mutant animals and increased axonal excitability upon ectopic expression of Fak56Y430F in glial cells.
(A) Abrupt failure of EJPs during tetanus in Fak56CG1. EJPs in a Fak56CG1 larvae abruptly dropped out following 1 min of 15 Hz stimulation (1 out of 5 preparations), whereas EJPs in control larvae persisted over 2 min tetanus (n=5). Sudden failure of full-sized EJPs in Fak56CG1 during tetanus was more frequently induced within seconds after application of 1 μM TTX at the NMJ (2 out of 5 preparations). In contrast, EJPs in control larvae gradually declined in amplitude over minutes during TTX paralysis (n=5). The recording saline contained 0.3 mM Ca2+ and 0.2 mM Co2+. The times stated above the traces indicate the duration of stimulation at 15 Hz. (B) Supernumerary EJPs triggered by high-frequency stimulation (15 Hz) in larvae overexpressing a dominant negative Fak56 (Fak56Y430F) protein in glial cells. Representative EJPs triggered by the stimulation of low (0.5 Hz) and high (15 Hz) frequency. Larvae expressing the dominant negative Fak56Y430F protein in glial cells displayed supernumerary EJPs with increased frequency in response to high-frequency stimulation, in comparison to control flies. (C) Supernumerary action potentials triggering supernumerary EJPs. The upper trace indicates action potentials recorded extracellularly from the segmental nerve, the lower trace indicates EJPs from the muscle cell. The time of nerve stimulation is indicated by a broken line. Note that, in addition to the compound action potential triggered by nerve stimulation, there were supernumerary spikes followed by supernumerary EJPs. The inset demonstrates an orthodromic conduction of supernumerary action potentials, as evidenced by the detection of an action potential at a proximal location (p) prior to a distal location (d), along the axon.
Modulation of Fak56 in glial cells induces nerve hyperexcitability
In order to understand the role of Fak56 in nerve action potential propagation and neurotransmission, we utilised the GAL4/UAS system (Brand & Perrimon, 1993) to express a mutated form of Fak56, Fak56Y430F, in either pre- or postsynaptic compartments. We used C155-GAL4, repo-GAL4, and mef2-GAL4 in the attempt to disrupt Fak56 function in neurons, glia, and muscle cells, respectively. Since the mutation in Y430 prevents the phosphorylation of this residue, Fak56Y430F is predicted to have significant functional consequences. While we were unable to detect any severe alterations in synaptic function upon expression of Fak56Y430F in either muscles or neurons (data not shown), disruption of Fak56 function in glial cells resulted in a striking hyperexitability at Drosophila NMJs. Animals expressing Fak56Y430F in glial cells displayed supernumerary EJPs in response to a single nerve stimulus, when compared to control animals (Figure 4B). Similar hyperexcitability, although to a lesser extent, was also observed with another glial driver, gliotactin-GAL4 (data not shown). Expression of Fak56Y430F in glial cells was confirmed by anti-FAK56 staining (see below).
By simultaneously recording the action potentials in presynaptic motor axons together with postsynaptic muscle EJP responses, it was clear that multiple peaks of EJPs (Figure 4C, lower trace) were triggered by supernumerary axonal action potentials following single stimuli (upper trace). These supernumerary action potentials were of axonal origin, unrelated to activity in synaptic terminal, and were propagating orthodromically, as evidenced by the earlier detection at a proximal location (p) than a more distally located position (d) along the axon (Figure 4C, expanded traces in inset; cf. Ganetzky & Wu, 1982a; Ueda & Wu, 2006). In conclusion, these results indicate that expression of Fak56Y430F in glial cells leads to an increase in axonal excitability, resulting in an alteration of synaptic transmission. The axonal hyperexcitability in larvae expressing Fak56Y430F in glial cells contrasts with the hypoexcitability observed in Fak56CG1 mutants (Figures 3 and 4). It has previously been shown that Fak56 overexpression leads to integrin mutant-like phenotypes, whereas the Fak56CG1 null mutant shows no developmental defect (Grabbe et al., 2004). While Fak56Y430F is predicted to function as a dominant negative, we cannot rule out the possibility that it is still able to perform a scaffolding function within glial cells and thus induce hyperexitability.
Fak56 protein is abundant in glial cells
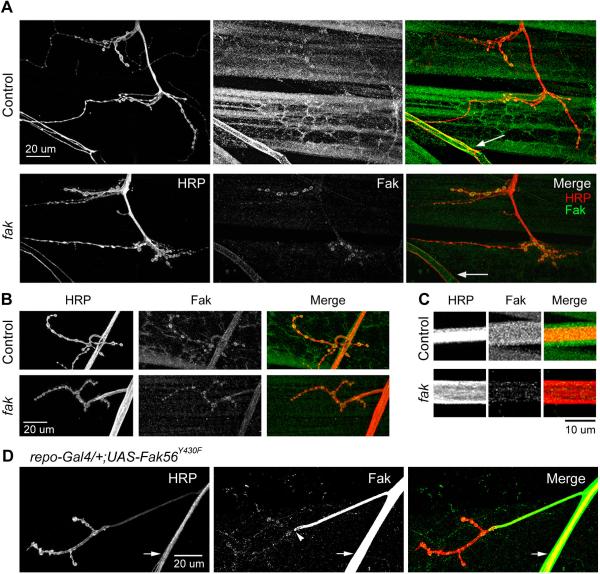
In order to investigate the relevance of a role of endogenous Fak56 in glial cells we investigated the expression pattern of Fak56 protein in larval neuromuscular preparations. In wild type larvae, Fak56 immunoreactivity is evident in the synaptic boutons of the NMJs, as well as in somatic muscles, trachea, and segmental nerve bundles (Figure 5A and B). In Fak56CG1 mutant larvae, Fak56 immunoreactivity is significantly reduced except for a remaining staining in synaptic boutons (Figure 5A and B, see discussion). When counterstaining segmental nerves with a pan-neuronal marker (anti-HRP antibodies; Budnik et al., 1990) and performing examinations at a higher magnification, we found Fak56 immunoreactivity enriched in an area surrounding the axon bundle, not recognised by anti-HRP antibodies (Figure 5C, upper panel). This observation strongly suggests a high expression of Fak56 in the glial cells that wrap the axon bundle and is consistent with a role of Fak56 in glial cells in supporting efficient action potential propagation (Figure 4B and C). These findings are further reinforced by the similar expression profiles of endogenous Fak56 and overexpressed Fak56Y430F proteins (compare Figure 5D (arrows) with 5C, upper panel). Note that overexpression of Fak56Y430F driven by glia-restricted repo-GAL4 demarcates the boundary of glial cells to the nerve entry point, excluding the synaptic boutons in the nerve terminals (Figure 5D, arrowheads). In Fak56CG1 mutant larvae Fak56 protein is strongly reduced in this glial population and the axon bundle itself appears to be wider than in control animals (Figure 5C, lower panel). These findings indicate a role of Fak56 in establishing the structural integrity of the segmental nerves, thus contributing to the proper shielding around axon bundles. This conclusion is also in agreement with a recently published finding where a requirement for Fak56 was shown in the organisation of the surface glial cells that enclose the optic stalk, the structure formed by photoreceptor axons as they project from the eye disc into their targets in the optic lobe (Murakami et al., 2007).
FIG. 5. Localization of Fak56 in larval neuromuscular preparations.
(A) Fak56 is abundant in the synaptic boutons of larval NMJs, detected by anti-Fak56 antibodies. In the figure NMJs 12 and 13 are visualized by antibodies recognizing the pan-neuronal marker HRP (left). Fak56 expression (middle) is also detected in somatic muscles and tracheal tissue (arrows) in a control larva (upper panel). In Fak56CG1 mutant animals, the expression of Fak56 is significantly reduced in somatic muscles and trachea, but not in NMJ synaptic boutons (lower panel). (B) Fak56 protein is abundant in the axon bundles in control larvae (upper panel, showing axons innervating muscle 4). In Fak56CG1 mutant animals the level of Fak56 protein in axon bundles is significantly reduced (lower panel). (C) Fak56 is strongly expressed in glial sheaths surrounding axon bundles. As shown in (B), Fak56 immunoreactivity is detected in a control larva around the axon bundle, beyond the area recognized by the pan-neuronal marker, anti-HRP antibody (upper panel). In Fak56CG1 mutant larvae, the diameter of axon bundles appear to be wider, with no or little expression of Fak56 around the bundle (lower panel). (D) Overexpression of a dominant negative Fak56 variant (FakY430F) in the glial population, driven by repo-GAL4, can be detected by anti-Fak56 antibodies. Importantly, the induced dominant negative Fak56 transgene is distributed around axon bundles (arrow) in a pattern similar to that of endogenous Fak56 (shown in C). The arrowhead indicates the Fak56 overexpression boundary demarcating NMJ regions of the glial-ensheathed axon and presynaptic boutons devoid of glial processes.
Ca2+-dependent abnormal neurotransmission in Fak56 mutants
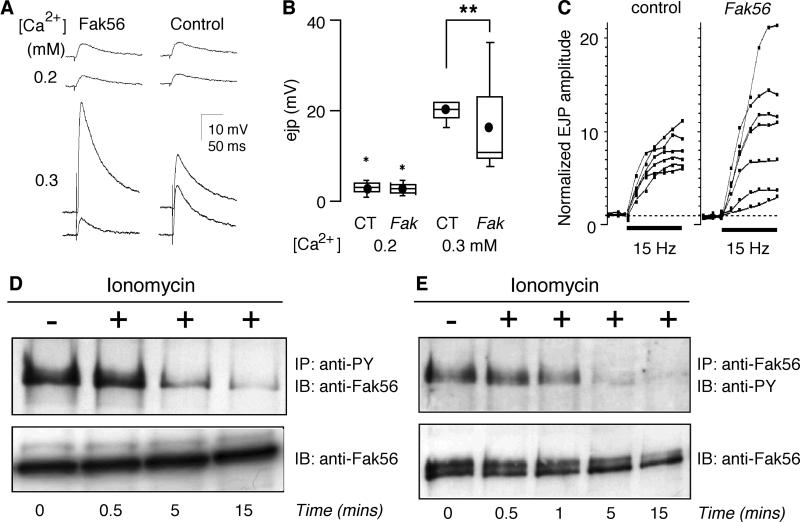
It is known that the intracellular Ca2+ concentrations in neurons and glial cells are increased during neuronal activities (Fellin et al., 2006), especially by repetitive firing or at increased extracellular Ca2+ levels. In light of this it is interesting to note that a more subtle alteration of synaptic transmission in Fak56 CG1 mutant animals is a Ca2+-dependent increase in the variability of EJP size among different larvae, both regarding the response to a single nerve stimuli (Figure 6A and B) as well as the facilitation levels upon 15 Hz repetitive stimulation (Figure 6C). Upon single nerve stimulation, EJP amplitudes in control larvae were tightly regulated at each Ca2+ concentration. In contrast, Fak56CG1 mutant larvae displayed a significant scatter of EJP sizes when Ca2+ concentrations were increased from 0.2 to 0.3 mM (Figure 6B). Furthermore, the level of facilitation during high-frequency stimulation was highly variable among Fak56CG1 mutant larvae, in striking contrast to the predictable facilitation index among different control larvae (Figure 6C). Such activity-dependent facilitation of EJP size is known to be caused by presynaptic accumulation of intracellular Ca2+ during high-frequency action potential invasion to the nerve terminal. Taken together, these results suggest a defect in Ca2+-dependent regulation of synaptic transmission in Fak56CG1 larvae.
FIG. 6. Increased Ca2+ levels enhance variability of EJP amplitudes in Fak56 mutant animals and modulate the tyrosine phosphorylation status of Fak56 protein.
(A and B) Ca2+-dependent variability in EJP amplitudes in Fak56CG1 larvae. Representative traces of EJPs (triggered at 0.5 Hz) recorded at 0.2 mM and 0.3 mM Ca2+. Each trace was recorded from individual muscle cells, showing abnormal variability in mutant EJP amplitudes (A), quantified in box plots for 0.2 mM and 0.3 mM Ca2+ (B). Note the great scattering of EJP amplitude at 0.3 mM, but not 0.2 mM, Ca2+ in Fak56CG1 mutant animals. ** indicates p < 0.01 (F-test). (C) Larger variability in EJP facilitation in Fak56 mutants. The EJP facilitation time course during 15 Hz tetanus stimulation is shown for individual muscle fibers. Note that the extent of facilitation was more uniform in control larvae and highly variable in mutants. (D and E) Increased levels of intracellular calcium modulate the tyrosine phosphorylation status of Fak56. BG2-c6 cells were plated for 4 hours on plastic dishes precoated with crude extracellular matrix prepared from Kc167 cells, before stimulation with 6 μM ionomycin for the times indicated. (D) Tyrosine phosphorylated proteins were immunoprecipitated with 4G10-coupled beads and Fak56 was detected by immunoblotting with anti-Fak56 antibodies (upper panel). Lysates were additionally analysed by anti-Fak56 immunoblotting (lower panel). (E) Fak56 protein was immunoprecipitated with anti-Fak56 antibodies and immunoblotted using antiphosphotyrosine antibodies (upper panel). Lysates were additionally analysed by anti-Fak56 immunoblotting (lower panel).
Ca2+-dependent regulation of Fak56
Having established that Fak56CG1 mutants display Ca2+-dependent defects in synaptic transmission, we moreover wished to investigate Ca2+-dependent properties of the Fak56 protein. Previous work has shown that the activity of FAK family kinases is modulated in response to stimuli inducing an increase of intracellular calcium levels (Lev et al., 1995; Siciliano et al., 1996; Avraham et al., 2000; Tian et al., 2000). Using the Drosophila BG2-c6 cell line derived from the central nervous system of 3rd instar Drosophila larvae (Takagi et al., 2000), we could see a clear and rapid dephosphorylation of Fak56 on tyrosine in response to increased levels of intracellular Ca2+ induced by ionomycin (Figure 6D). We also observed a similar dephosphorylation of endogenous Fak56 protein in response to ionomcyin in a second Drosophila cell line, S2 cells (Supplementary Figure 1). Surprisingly, these results are in direct opposition to observations in mammalian cells, where the FAK family member Pyk2 has been reported to be strongly phosphorylated on tyrosine in response to increased intracellular Ca2+. In order to confirm that we were observing a decrease in tyrosine phosphorylation on Fak56 and not an indirect effect, we additionally immunoprecipitated endogenous Fak56 followed by antiphosphotyrosine immunoblotting (Figure 6E). It is clear that the Drosophila Fak56 protein is dephosphorylated on tyrosine in response to increased calcium levels. This is further confirmed by the accompanying anti-Fak56 immunoblot (Figure 6E, lower panel) in which a loss of lower mobility forms, presumably reflecting phosphorylated forms of Fak56, can be observed. These biochemical results propose the interesting possibility that lack of Fak56 phosphorylation causes irregularities in synaptic transmission, which normally is tightly controlled in correlation to intracellular Ca2+ concentrations (Figure 6A, B, and C).
Discussion
The high degree of conservation of FAK family PTKs throughout evolution implies their functional importance. Surprisingly, the single Drosophila Fak56 protein is not critical for fruitfly survival, since Fak56CG1 null mutants are viable and fertile (Grabbe et al., 2004). In this study we demonstrate a role of Fak56 in the glial sheath, which supports action potential conduction along axons. Flies mutant in the Fak56 gene exhibit a decrease in life span and an increased sensitivity to mechanical stress- and electrical shock-induced paralysis (Figures 1 & 2). Notably, some known bang-sensitive mutants display increased bang-sensitivity with increasing age, while others show a greater sensitivity when young and appear to adapt with age to compensate (Pavlidis et al., 1994; Toivonen et al., 2001; Zhang et al., 2002), as we observe for Fak56CG1 mutants. Potentially, age-dependent neurodegeneration and adaptive mechanisms may be involved in producing these differences. It will be important to investigate the functional similarities and distinctions between these two types of bang-sensitive mutants by constructing relevant Fak double mutants to study genetic interactions with these bang-sensitive mutations.
We examined the larval neuromuscular preparation for defects in axonal conduction, synaptic transmission and muscle physiology by standard electrophysiological techniques (Jan & Jan, 1976; Wu et al., 1978; Prokop, 1999). Signal transmission at the synapses is mediated by exocytosis of neurotransmitters, triggered by Ca2+ influx at the arrival of an action potential along the motor axon. The localization of endogenous Fak56 protein by immunostaining (Figure 5) as well as targeted expression of the Fak56Y430F transgenic protein in glia (Figure 4B and C), together with observations in Fak56CG1 mutant animals, suggest that Fak56 has an important function in the glial cells which surround motor axons and support action potential conduction and hence neurotransmission at the Drosophila NMJ (Figures 3 and 4A). The consequence of targeted expression of Fak56Y430F protein in glia is consistent with a previous report of hyperexcitability at larval NMJs resulting from glial defects (Huang & Stern, 2002). These findings may provide foundations for further studies of previously unsuspected functions for Fak56 in the central nervous system.
The increased sensitivity of Fak56 mutants to mechanical stress (Figure 1) is consistent with a weakened action potential conduction along the axon during high-frequency firing (Figures 3 and 4). However, the DLM response to electrical brain stimulation involves other circuit components in addition to the identified GF and DLM motorneurons. Therefore, it will require further investigations to determine how altered glial cell function in the relevant CNS regions contributes to the bang-sensitive behaviour displayed by Fak56CG1 mutants (Figures 1 and 2).
In addition to altered structural integrity of the segmental nerve (Figure 5C), defects in the Fak56 glial sheath can also affect the cable properties of the axon and thus action potential propagation (Figure 3). For example, the altered packing of the glial sheath can modify length constant of the axon and the resultant poor cable property may explain the observed a slower action potential conduction speed and conduction failure at a higher firing frequency (Figure 3). The associated EJP blockade at subthreshold doses of TTX is consistent with this idea (Fig. 4A). In contrast, glial expression of Fak56Y430F (to interfere with tyrosine phosphorylation) enhanced nerve excitability, causing axonal multiple firing and supernumerary ejps (Fig. 4BC). It is also possible that Fak56 mutations affect external ionic maintenance by glial cells or influence ion channel expression, kinetics, and localization in the axon. For instance, the hyperexcitability in larvae with targeted expression of FAK56Y430F in glia can be explained if axonal Na+ channel expression is increased or if external K+ concentration is increased.
FAK family kinases are implicated in adhesion-mediated signaling downstream of integrins. It is interesting to note that the Drosophila αPS3 integrin subunit, encoded by the volado locus, has been shown to play a role in olfactory learning and memory (Grotewiel et al., 1998), and in synaptic transmission and plasticity at larval neuromuscular junctions (Rohrbough et al., 2000). It will be important to determine the role of glial cells in these integrin-related functions. Recent findings by Murakami and co-workers also show a requirement for Fak56 in glial cells during normal development of the optic stalk (Murakami et al., 2007).
Interestingly, the two members of the mammalian FAK family of PTKs show alternating expression patterns in the developing CNS, where FAK is abundantly expressed in the embryonic brain and downregulated at birth (Menegon et al., 1999). In a complementary fashion Pyk2 expression is low in the embryonic CNS but is dramatically enhanced in the forebrain around birth, enriched in postsynaptic densities (Menegon et al., 1999; Husi et al., 2000). Multiple studies have implicated Pyk2 in the regulation of synaptic plasticity. High-frequency stimulation that cause membrane depolarisation and Long Term Potentiation (LTP) also increase Pyk2 phosphorylation (Huang et al., 2001).
In light of the observed role of Fak56 in maintaining nerve conduction and neurotransmission, it is tempting to speculate that Drosophila Fak56 is more closely related to the second FAK family member, Pyk2. Previous analysis of Fak56CG1 mutants (Grabbe et al., 2004). The essential functions ascribed to mammalian FAK might be performed by another family of PTKs or adhesion molecules in Drosophila, e.g., members of the Src family. We show here that Fak56, in opposition to mammalian Pyk2, is dephosphorylated on tyrosine residues in response to increased levels of intracellular Ca2+, induced either by the calcium ionophore ionomycin (Figure 6D and E) or by KCl-induced membrane depolarization (data not shown). A Ca2+-dependant modulatory role for Fak56 was also suggested by increased transmission variability in Fak56 mutants either at a higher external Ca2+ level or during activity-dependent accumulation of intracellular Ca2+ (Figure 4).
Our extensive efforts in Drosophila cell lines have not revealed obvious modifications in the phosphorylation status or protein-protein interactions with putative targets of Fak56 phosphorylation (such as paxillin and ERK). Thus, to date, the molecular mechanism by which loss of Fak56 function induces the observed behavioral and physiological phenotypes remains elusive. It is known that focal adhesions provide attachment sites for actin cytoskeleton and interestingly, a recent screen for bang-sensitive mutants has identified another cytoskeleton linked protein, a filamin actin-binding protein (Zhang et al., 2002). It will be of interest to see whether future screens will allow the identification of additional Fak56-related signaling/cytoskeleton proteins. The study presented here opens a door to further investigations of evolutionary conserved in vivo roles of FAK family kinases in the nervous system. In particular, it will be interesting to elucidate the potential role of glial FAK in certain neurological diseases involving weakening of nerve conduction.
Supplementary Material
Supplementary FIG. S1. Fak56 is dephosphorylated on tyrosine in response to calcium in S2 cells. Increased levels of intracellular calcium induce tyrosine dephosphorylation of Fak56. S2 cells were plated for 4 hours on plastic dishes precoated with crude extracellular matrix prepared from Kc167 cells, before stimulation with 6μM ionomycin for the times indicated. Tyrosine phosphorylated proteins were immunoprecipitated with 4G10-coupled beads and Fak56 was detected by immunoblotting with anti-Fak56 antibodies (upper panel). Lysates were additionally analysed by anti-Fak56 immunoblotting (lower panel).
Acknowledgements
The authors would like to thank John B. Thomas for initially pointing out the Fak56 bang-sensitive phenotype. We would like to thank Xuxuan Wan for helping establish experimental and control lines. We would also like to thank members of the both RHP and CFW laboratories for fruitful discussions during the course of this work. RHP is a Swedish Cancer Foundation Research Fellow and was supported by the Swedish Research Council (621−2003−3399), the Carl Tryggers Foundation and the Åke Wibergs Foundation. CFW is supported by National Institutes for Health Grants NS18500 and NS26528.
Abbreviations
- FAK
Focal Adhesion Kinase
- PTKs
protein tyrosine kinases
- NMJs
neuromuscular junctions
- EJPs
excitatory junctional potentials
- GF
giant fiber
- TTX
tetrodotoxin
Footnotes
Supplementary material
The following supplementary material may be found on http://www.blackwell-synergy.com Fig. S1. Fak56 is dephosphorylated on tyrosine in response to calcium in S2 cells.
References
- Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JE, Wu CF. Altered habituation of an identified escape circuit in Drosophila memory mutants. J Neurosci. 1996;16:3486–3499. doi: 10.1523/JNEUROSCI.16-10-03486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Haydon PG. Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiology (Bethesda, Md. 2006;21:208–215. doi: 10.1152/physiol.00161.2005. [DOI] [PubMed] [Google Scholar]
- Feng Y, Ueda A, Wu CF. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenet. 2004;18:377–402. doi: 10.1080/01677060490894522. [DOI] [PubMed] [Google Scholar]
- Fergestad T, Bostwick B, Ganetzky B. Metabolic disruption in Drosophila bang-sensitive seizure mutants. Genetics. 2006;173:1357–1364. doi: 10.1534/genetics.106.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GL, Rebay I, Hynes RO. Expression of DFak56, a Drosophila homolog of vertebrate focal adhesion kinase, supports a role in cell migration in vivo. Proc Natl Acad Sci U S A. 1999;96:14978–14983. doi: 10.1073/pnas.96.26.14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto J, Sawamoto K, Okabe M, Takagi Y, Tezuka T, Yoshikawa S, Ryo H, Okano H, Yamamoto T. Cloning and characterization of Dfak56, a homolog of focal adhesion kinase, in Drosophila melanogaster. J Biol Chem. 1999;274:29196–29201. doi: 10.1074/jbc.274.41.29196. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J Neurophysiol. 1982a;47:501–514. doi: 10.1152/jn.1982.47.3.501. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Indirect Suppression Involving Behavioral Mutants with Altered Nerve Excitability in DROSOPHILA MELANOGASTER. Genetics. 1982b;100:597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Costa A, Derkinderen P, Studler JM, Toutant M. FAK and PYK2/CAKbeta in the nervous system: a link between neuronal activity, plasticity and survival? Trends Neurosci. 1999;22:257–263. doi: 10.1016/s0166-2236(98)01358-7. [DOI] [PubMed] [Google Scholar]
- Grabbe C, Zervas CG, Hunter T, Brown NH, Palmer RH. Focal adhesion kinase is not required for integrin function or viability in Drosophila. Development. 2004;131:5795–5805. doi: 10.1242/dev.01462. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF. CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- Huang Y, Stern M. In vivo properties of the Drosophila inebriated-encoded neurotransmitter transporter. J Neurosci. 2002;22:1698–1708. doi: 10.1523/JNEUROSCI.22-05-01698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler D, Tanouye MA. Modifications of seizure susceptibility in Drosophila. J Neurophysiol. 2000;83:998–1009. doi: 10.1152/jn.2000.83.2.998. [DOI] [PubMed] [Google Scholar]
- Lee BP, Jones BW. Transcriptional regulation of the Drosophila glial gene repo. Mech Dev. 2005;122:849–862. doi: 10.1016/j.mod.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Lee J, Wu CF. Electroconvulsive seizure behavior in Drosophila: analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants. J Neurosci. 2002;22:11065–11079. doi: 10.1523/JNEUROSCI.22-24-11065.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Wu CF. Genetic modifications of seizure susceptibility and expression by altered excitability in Drosophila Na+ and K+ channel mutants. J. Neuophysiology. 2006;96:2465–2478. doi: 10.1152/jn.00499.2006. [DOI] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Menegon A, Burgaya F, Baudot P, Dunlap DD, Girault JA, Valtorta F. FAK+ and PYK2/CAKbeta, two related tyrosine kinases highly expressed in the central nervous system: similarities and differences in the expression pattern. Eur J Neurosci. 1999;11:3777–3788. doi: 10.1046/j.1460-9568.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Murakami S, Umetsu D, Maeyama Y, Sato M, Yoshida S, Tabata T. Focal adhesion kinase controls morphogenesis of the Drosophila optic stalk. Development. 2007;134:1539–1548. doi: 10.1242/dev.001529. [DOI] [PubMed] [Google Scholar]
- Palladino MJ, Bower JE, Kreber R, Ganetzky B. Neural dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J Neurosci. 2003;23:1276–1286. doi: 10.1523/JNEUROSCI.23-04-01276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RH, Fessler LI, Edeen PT, Madigan SJ, McKeown M, Hunter T. DFak56 is a novel Drosophila melanogaster focal adhesion kinase. J Biol Chem. 1999;274:35621–35629. doi: 10.1074/jbc.274.50.35621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P, Ramaswami M, Tanouye MA. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell. 1994;79:23–33. doi: 10.1016/0092-8674(94)90397-2. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Tanouye MA. Seizures and failures in the giant fiber pathway of Drosophila bang-sensitive paralytic mutants. J Neurosci. 1995;15:5810–5819. doi: 10.1523/JNEUROSCI.15-08-05810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A. Integrating bits and pieces: synapse structure and formation in Drosophila embryos. Cell Tissue Res. 1999;297:169–186. doi: 10.1007/s004410051345. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Grotewiel MS, Davis RL, Broadie K. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci. 2000;20:6868–6878. doi: 10.1523/JNEUROSCI.20-18-06868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman RR, Johnson EA, Rodesch CK, Bjerke M, Nagoshi RN, Geyer PK. A P element containing suppressor of hairy-wing binding regions has novel properties for mutagenesis in Drosophila melanogaster. Genetics. 1995;141:1061–1074. doi: 10.1093/genetics/141.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royden CS, Pirrotta V, Jan LY. The tko locus, site of a behavioral mutation in D. melanogaster, codes for a protein homologous to prokaryotic ribosomal protein S12. Cell. 1987;51:165–173. doi: 10.1016/0092-8674(87)90144-9. [DOI] [PubMed] [Google Scholar]
- Siciliano JC, Toutant M, Derkinderen P, Sasaki T, Girault JA. Differential regulation of proline-rich tyrosine kinase 2/cell adhesion kinase beta (PYK2/CAKbeta) and pp125(FAK) by glutamate and depolarization in rat hippocampus. J Biol Chem. 1996;271:28942–28946. doi: 10.1074/jbc.271.46.28942. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol [A] 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Ui-Tei K, Hirohashi S. Adhesion-dependent tyrosine phosphorylation of enabled in Drosophila neuronal cell line. Biochem Biophys Res Commun. 2000;270:482–487. doi: 10.1006/bbrc.2000.2458. [DOI] [PubMed] [Google Scholar]
- Tanouye MA, Wyman RJ. Motor outputs of giant nerve fiber in Drosophila. J Neurophysiol. 1980;44:405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- Tian D, Litvak V, Lev S. Cerebral ischemia and seizures induce tyrosine phosphorylation of PYK2 in neurons and microglial cells. J Neurosci. 2000;20:6478–6487. doi: 10.1523/JNEUROSCI.20-17-06478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivonen JM, O'Dell KM, Petit N, Irvine SC, Knight GK, Lehtonen M, Longmuir M, Luoto K, Touraille S, Wang Z, Alziari S, Shah ZH, Jacobs HT. Technical knockout, a Drosophila model of mitochondrial deafness. Genetics. 2001;159:241–254. doi: 10.1093/genetics/159.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JR, Schneiderman AM. Different neural pathways coordinate Drosophila flight initiations evoked by visual and olfactory stimuli. J Exp Biol. 1995;198:1099–1104. doi: 10.1242/jeb.198.5.1099. [DOI] [PubMed] [Google Scholar]
- Trotta N, Rodesch CK, Fergestad T, Broadie K. Cellular bases of activity-dependent paralysis in Drosophila stress-sensitive mutants. J Neurobiol. 2004;60:328–347. doi: 10.1002/neu.20017. [DOI] [PubMed] [Google Scholar]
- Ueda A, Wu CF. Distinct frequency-dependent regulation of nerve terminal excitability and synaptic transmission by IA and IK potassium channels revealed by Drosophila Shaker and Shab mutations. J Neurosci. 2006;26:6238–6248. doi: 10.1523/JNEUROSCI.0862-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B, Jan LY, Jan YN. A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc Natl Acad Sci U S A. 1978;75:4047–4051. doi: 10.1073/pnas.75.8.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WC, Mei L. Roles of FAK family kinases in nervous system. Front Biosci. 2003;8:s676–682. doi: 10.2741/1116. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tan J, Reynolds E, Kuebler D, Faulhaber S, Tanouye M. The Drosophila slamdance gene: a mutation in an aminopeptidase can cause seizure, paralysis and neuronal failure. Genetics. 2002;162:1283–1299. doi: 10.1093/genetics/162.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YQ, Roote J, Brogna S, Davis AW, Barbash DA, Nash D, Ashburner M. stress sensitive B encodes an adenine nucleotide translocase in Drosophila melanogaster. Genetics. 1999;153:891–903. doi: 10.1093/genetics/153.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary FIG. S1. Fak56 is dephosphorylated on tyrosine in response to calcium in S2 cells. Increased levels of intracellular calcium induce tyrosine dephosphorylation of Fak56. S2 cells were plated for 4 hours on plastic dishes precoated with crude extracellular matrix prepared from Kc167 cells, before stimulation with 6μM ionomycin for the times indicated. Tyrosine phosphorylated proteins were immunoprecipitated with 4G10-coupled beads and Fak56 was detected by immunoblotting with anti-Fak56 antibodies (upper panel). Lysates were additionally analysed by anti-Fak56 immunoblotting (lower panel).