SUMMARY
Early during neuromuscular development acetylcholine receptors (AChRs) accumulate at the center of muscle fibers, precisely where motor growth cones navigate and synapses eventually form. Here, we show that Wnt11r binds to the zebrafish unplugged/MuSK ectodomain to organize this central muscle zone. In the absence of such zone, prepatterned AChRs fail to aggregate and, as visualized by live cell imaging, growth cones stray from their central path. Using inducible unplugged/MuSK transgenes we show that organization of the central muscle zone is dispensable for the formation of neural synapses, but essential for AChR prepattern and motor growth cone guidance. Finally, we show that blocking non-canonical dishevelled signaling in muscle fibers disrupts AChR prepatterning and growth cone guidance. We propose that Wnt ligands activate unplugged/MuSK signaling in muscle fibers to restrict growth cone guidance and AChR prepatterns to the muscle center through a mechanism reminiscent of the planar cell polarity pathway.
Keywords: axonal guidance, motoneuron, synaptogenesis, muscle specific kinase, wnt11, neuromuscular junction, unplugged, zebrafish
INTRODUCTION
Formation of functional neuromuscular synapses requires the interplay between presynaptic nerves and postsynaptic muscle components (Burden, 2002; Sanes and Lichtman, 2001). In vertebrates, a hallmark of neuromuscular synapses is the accumulation of acetylcholine receptors (AChRs) in a narrow, central region of muscle fibers, in apposition to nerve terminals. Development of neuromuscular synapses requires nerve-derived agrin to counteract the acetylcholine-mediated dispersal of AChR clusters (Lin et al., 2005; Misgeld et al., 2005). This leads to the removal of aneural AChR clusters and the stabilization of nerve terminal associated AChR clusters, i.e. nascent synapses. Postsynaptic differentiation also requires the muscle specific receptor tyrosine kinase MuSK, a component of the MuSK/Lrp4 agrin receptor, to promote AChR clustering and activate AChR gene expression (DeChiara et al., 1996; Glass et al., 1996; Kim et al., 2008; Zhang et al., 2008).
Even before motor axons contact muscle fibers, AChR clusters are localized to the central region of muscle, independent of nerve contact or nerve derived agrin (Lin et al., 2001; Yang et al., 2001). This AChR prepattern requires MuSK function, and recent studies suggest that ectopic MuSK expression is sufficient to establish AChR prepatterning (Kim and Burden, 2008). Upon contact with motor axons, pre-existing AChR clusters are incorporated into prospective neuromuscular synapses (Flanagan-Steet et al., 2005; Panzer et al., 2006). Thus, formation of neuromuscular synapses can be divided into two phases. An early phase when AChRs first cluster in the center of muscle fibers, precisely where motor growth cones will navigate, and a later phase, when growth cones have made contact with muscle fibers and neural AChR clusters become incorporated into functional neuromuscular synapses (Lin et al., 2001).
Over the past decades, many of the molecular players and mechanisms involved in the later phase of neuromuscular synapse development have been discovered, while the molecules and mechanisms underlying the early phase are not well understood (Burden, 2002; Burden et al., 2003; Kummer et al., 2006; Sanes and Lichtman, 2001). For example, what is the role of nerve independent postsynaptic differentiation, i.e. AChR prepattern during normal synaptogenesis? Similarly, what initiates AChR prepattern and determines its central muscle location? Here, we provide compelling evidence that in zebrafish embryos wnt11r is required to confine navigating growth cones to the center of muscle fibers, and to initiate AChR prepattern. We show that wnt11r and unplugged interact genetically, that Wnt11r binds Unplugged/MuSK through its frizzled like cysteine rich domain (CRD) in vitro, that in the embryo wnt11r binds exclusively to the unplugged receptor expression domain in an unplugged/MuSK dependant manner, and that non-canonical dishevelled signaling in muscle fibers is required for Unplugged/MuSK function. Together, our data provide strong evidence that Wnt ligands activate unplugged/MuSK signaling in muscle fibers to organize a central muscle zone, and thereby spatially restrict growth cone guidance and AChR accumulation through a mechanism reminiscent of the planar cell polarity pathway.
RESULTS
unplugged SV1 is required for AChR prepattern
In the zebrafish embryo, the first AChR prepattern forms on adaxial muscle cells, initially located on the medial surface of somites (Flanagan-Steet et al., 2005; Panzer et al., 2005). As motor growth cones enter the muscle, migratory adaxial cells delaminate from the medial surface, and lateral fast muscle fibers invade the space on the medial somite surface (Supplemental Fig. 1A, B and Cortes et al., 2003). Motor growth cones then contact medial fast muscle fibers, and form neural en passant synapses at sites previously marked by prepatterned AChRs (Flanagan-Steet et al., 2005; Panzer et al., 2005). A group of 2-5 non-migratory adaxial cells, termed muscle pioneers, remain on the medial myotome surface, and upon contact with motor growth cones, form the first neuromuscular junctions (NMJs) (Supplemental Fig. 1B and Flanagan-Steet et al., 2005; Liu and Westerfield, 1992). Here, we focus on the formation of the adaxial cell AChR prepattern.
We have previously shown that unplugged null mutants lack all AChR prepatterning and en passant neuromuscular junctions, and that they display specific axonal guidance defects (Lefebvre et al., 2007; Zhang et al., 2004). The zebrafish unplugged locus encodes two MuSK isoforms: the unplugged Full-Length (FL) isoform which is essential for the formation of neuromuscular junctions, and the unplugged Splice Variant 1 (SV1) isoform, which is essential for axonal guidance, independent of rapsyn (Fig. 1A and Zhang et al., 2004). To examine the role of both isoforms during AChR prepatterning in adaxial fibers, we first analyzed the expression patterns of unplugged FL and SV1 using isoform specific probes. Before and during the time of AChR prepatterning, unplugged FL is weakly expressed while unplugged SV1 is strongly expressed in adaxial cells (Supplemental Fig. 1C-F). Thus, unplugged SV1 expression is consistent with a role in adaxial AChR prepattern.
Figure 1. UnpSV1 controls AChR prepatterning.
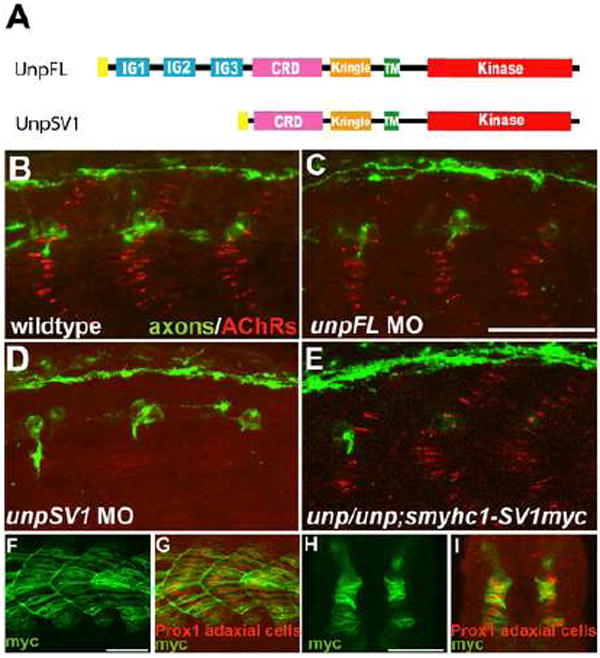
(A) Domain structure of the Unplugged protein isoforms. (B-E) Lateral views of caudal segments in 17 hpf embryos stained for motor axons (green, znp-1/SV2) and AChRs (red, α-BTX). (B) In wildtype embryos, AChRs are prepatterned in a central band along the dorsal and ventral myotome before the first growth cones approach. (C) UnpFL MO injection does not affect AChR prepattern. (D) UnpSV1 MO injection causes complete absence of AChR prepattern. (E) UnpSV1 expression in adaxial cells restores AChR prepattern in unplugged mutant embryos. (F-G) Lateral views and cross-sectional views (H, I) of 17 hpf Tg(smyhc1:UnpSV1myc) embryos stained with anti-myc (green) and anti-Prox1 (red), which labels the nuclei of adaxial cells. Scale bars: 50 μm.
Besides their expression patterns, the two unplugged isoforms also differ in their ectodomain composition. While the FL isoform contains three immunoglobulin-like (Ig) domains in addition to the CRD and the kringle domain, the SV1 ectodomain lacks the Ig domains and only consists of a unique signal sequence followed by the CRD and the kringle domain (Fig.1 and Zhang et al., 2004). To determine which of the two unplugged/MuSK isoforms is critical to initiate adaxial AChR prepatterning in vivo, we used a set of morpholinos previously shown to affect one but not the other isoform (Lefebvre et al., 2007; Zhang et al., 2004). Morpholino-mediated knockdown of unplugged FL and SV1 revealed that unplugged SV1 but not FL is essential for prepatterning, consistent with their differential expression patterns (Fig. 1B-D). To confirm that the unplugged SV1 receptor is indeed responsible for AChR prepatterning, we generated transgenic lines in which myc-tagged unplugged SV1 is expressed under the control of a slow myosin heavy chain (smyhc1) promotor specific for adaxial cells (Fig. 1F-I and Elworthy et al., 2008). When crossed into the unplugged mutant background, the presence of Tg(smyhc1:unpluggedSV1-myc) in unplugged(br307/br307) embryos fully restored adaxial AChR prepattern (Fig. 1E). Thus, similar to its requirement in axonal pathfinding, the unplugged SV1 receptor which lacks the Ig domains is responsible for AChR prepattern, consistent with the idea that in vivo both processes share a common signaling mechanism.
unplugged/MuSK restricts growth cone migration to a central muscle zone
We then asked if unplugged/MuSK co-ordinates axonal pathfinding and AChR prepatterning. While we had previously shown that in the zebrafish embryo unplugged/MuSK is critical for axonal guidance after the period of AChR prepatterning (Zhang and Granato, 2000), we decided to examine axonal pathfinding at an earlier stage during the period of AChR prepatterning. For this, we used the Hb9 transgenic line, in which motoneurons express GFP (Flanagan-Steet et al., 2005). We imaged pioneering wildtype and unplugged mutant growth cones, as they exited from the spinal cord and entered the muscle territory, traversing the central muscle zone. In wildtype embryos, the first motor growth cone to exit from the spinal cord is caudal primary (CaP), and in 50% of the hemisegments it is accompanied by variable primary (VaP), which tightly fasiculates with CaP (Eisen et al., 1990). Confocal time lapse imaging confirmed that once they exited from the spinal cord, wildtype CaP and CaP/VaP growth cones pioneered a tight and narrow path (n=5 growth cones from five embryos, Fig. 2A, C, Suppl. Movie 1 and Eisen et al., 1986).
Figure 2. unplugged restricts navigating growth cones to a central muscle zone.
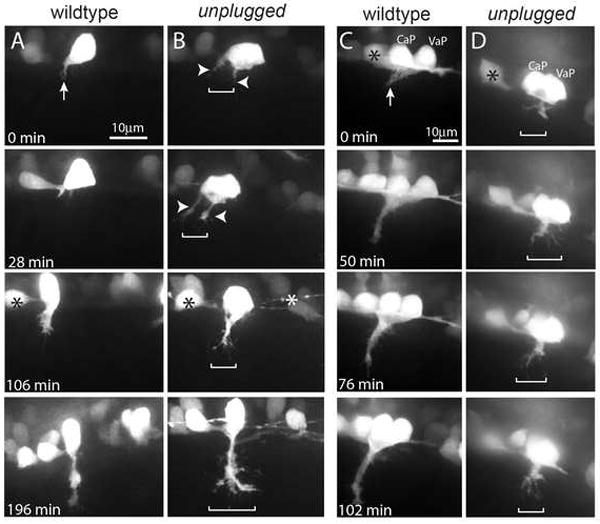
(A-D) Still images from time lapse movies showing the initial migration of single CaP axons (A, B), or CaP/VaP pair axons (C, D) from the spinal cord into the myotome. Arrows point to the single wildtype CaP growth cone (A) and to the tightly fasciculated wildtype CaP/VaP growth cones (C). In contrast unplugged CaP neurons form extensive filopodia and even multiple growth cones (arrowheads) that occupy a broader area (brackets, B). Similary, mutant CaP/VaP growth cones appear defasciculated and occupy a broader area compared to wildtype. Asterisks indicate interneurons also labeled by the Tg(Hb9:GFP) transgene.
In contrast, unplugged CaP and VaP growth cones displayed aberrant growth cone morphologies as they traversed the muscle territory (Fig. 2B, D and Suppl. Movie 1, 2; n=8/8 growth cones from eight embryos). Frequently, unplugged CaP formed excessive filapodia, sometimes even multiple distinct and transient growth cones, which spread and occupied a much wider path no longer restricted to the muscle center (Fig. 2B). Similarly, when CaP and VaP neurons pioneered the path simultaneously, their growth cones invaded lateral muscle territory they usually avoid (Fig. 2D and Suppl. Movie 2). Because the unplugged/MuSK gene is only expressed and functions in muscle cells, we conclude that unplugged/MuSK dependent cues produced by muscle cells confine growth cones to a narrow path in the central muscle region. Thus, live cell imaging demonstrates that during pathfinding unplugged/MuSK limits the muscle territory accessible to growth cones, consistent with the idea that its primary role is to organize a common central muscle zone to which pioneering growth cones and the first AChR clusters are restricted.
The non-canonical wnt11r ligand plays a role in AChR prepatterning and axonal guidance
We next asked which signaling pathway might activate the unplugged/MuSK receptor to organize a central muscle zone. We had previously shown that the unpluggedp31CD mutant allele carries a missense mutation that changes one of the ten conserved cysteines in the CRD, the Wnt binding domain of frizzled receptors (Zhang et al., 2004). Unplugged/MuSK belongs to a small group of non-frizzled CRD containing proteins, including the ROR receptors (Xu and Nusse, 1998), and RORs have recently been shown to directly interact through their CRD with Wnts (Hikasa et al., 2002; Oishi et al., 2003). Furthermore, Wnts play critical roles in synapse formation in the mammalian CNS, in Drosophia and in C. elegans (reviewed in: Speese and Budnik, 2007). One attractive idea is that Wnt signaling via the unplugged/MuSK receptor may induce the formation of a central zone along the anterior-posterior axis of developing muscle. We therefore reasoned that non-canonical Wnt family members known to induce such cellular polarity would be excellent candidates. Expression pattern analysis of published non-canonical Wnt genes during the relevant developmental window (17-24 hpf) identified several candidate Wnt genes, including pipetail (ppt) /wnt5a (Rauch et al., 1997), silberblick (slb)/wnt11(Heisenberg et al., 2000), and wnt11r (Matsui et al., 2005). Analysis of ppt/wnt5a and slb/wnt11 mutants did not reveal any axonal or synaptic defects (Supplemental Fig. 2A-C).
Analysis of wnt11r mRNA expression in 20 somite stage embryos revealed strong signals in the spinal cord and in the dorso-lateral somites, just adjacent to unplugged/MuSK expressing dorsal adaxial cells, consistent with previously published data (Supplemental Fig. 2D-G and Groves et al., 2005; Matsui et al., 2005). Thus, wnt11r is expressed at the right time and place to initiate unplugged/MuSK signaling in adaxial cells, at least in dorsal adaxial cells. We next tested the role of wnt11r, using a previously published translation initiation blocking morpholino (Matsui et al., 2005), and a newly designed splice blocking morpholino. This second morpholino targets the exon 3 donor splice site, predicted to cause a frameshift-induced premature stop codon after amino acid 67 (Fig. 3A; for details see Material and Methods). As determined by RT-PCR, injection of the splice blocking morpholino caused an almost complete reduction of wnt11r transcript (Fig. 3A). Knockdown of wnt11r using either of the two morpholinos did not affect specification, migration or differentiation of adaxial muscle fibers (Supplemental Fig. 2J, K). Importantly, orientation of muscle fibers, which in chick embryos is thought to be controlled by a wnt/PCP pathway (Gros et al., 2008), was completely unaffected (Supplemental Fig. 2J, K). Instead, we observed two prominent phenotypes: axonal stalling and branching at 27hpf (28% of hemisegments, n=851, Fig. 3B-H), and a strong reduction of adaxial AChR prepattern (Fig. 3I-L). While AChR prepattern was severely affected, later developing neural AChR clusters developed, albeit their size was slightly reduced (Fig. 3G, H). Finally, we also noticed a reduction of chondroitin sulfate proteoglycans (CSP) localization at the somite choice point (Supplemental Fig. 2H, I), identical to what we previously observed in unplugged/MuSK mutants (Zhang et al., 2004). Thus, morpholino knockdown of wnt11r causes unplugged/MuSK like CSP, axonal and AChR prepattern phenotypes.
Figure 3. wnt11r is critical for axonal guidance and AChR prepatterning.
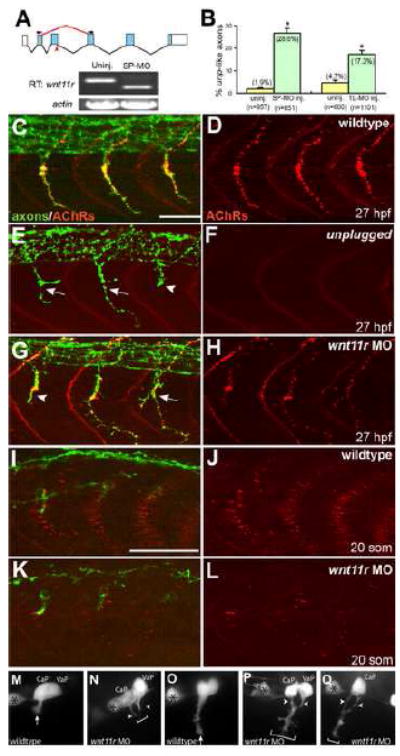
(A) The splice morpholino (SP-MO) targets the splice donor site of the wnt11r exon 3 (red arrow), and MO-induced aberrant splicing is shown in red. RT-PCR analyses of uninjected and wnt11r SP-MO injected embryos (arrows indicate the position of PCR primers). (B) Quantification of wnt11r MOs injected embryos. TL-MO, translation initiation mopholino. Per embryo, twenty hemisegments were analyzed; n=hemisegments. Results are expressed as the mean of multiple injection experiments ±s.e.m., (*p<0.001, t test). (C-L) Wildtype, unplugged and wnt11r MO injected embryos at 27hpf (C-H), and at the 20-somite stage (I-L), stained for motor axons (znp-1, green) and AChR clusters (α-BTX, red). (E, F) In contrast to wildtype, unplugged embryos display characteristic stalling (arrowhead) and branches (arrows) at the choice point, and lack all AChR clusters. (G, H) Injection of wnt11r MO causes unplugged like axonal stalling (arrowhead), branching (arrow), and a strong reduction of AChR prepatterning (K, L). Note that the size and intensity of neural AChR clusters is reduced in wnt11r 27 hpf morphants (H). (M-Q) Time-lapse images of Hb9-GFP labeled wildtype (M, O) and wnt11r morphant CaP and VaP axons (N, P, Q), as they exit from the spinal cord (M, N), and as they reach the somitic choice point (O-Q). Asterisks indicate the cell body of interneurons. (M, O) Wildtype CaP and VaP neurons extended one growth cone (arrow). Note the broad area (brackets) the two defasciculated wnt11r morphants CaP/VaP growth cones occupy (arrowheads in N, P, Q), compared to wildtype (M, O). Scale bars: 50 μm.
To determine if wnt11r is also critical earlier to restrict motor growth cones to the central muscle zone, we imaged pioneering motor growth cones in wnt11r morpholino injected Tg(Hb9:GFP) embryos. In these embryos, the number and position of GFP positive motoneurons was indistinguishable from wildtype (Fig. 3M-Q; wnt11r: n=4/6 growth cones from six morphants). However, as they entered the muscle, wnt11r morphant growth cones strayed away from the central zone and formed excessive filapodia (Fig. 3M-Q). Thus, wnt11r morphants display AChR prepattern and axonal guidance defects identical to those observed in unplugged/MuSK mutants, suggesting that wnt11r acts through unplugged/MuSK.
Wnt11r binds to Unplugged/MuSK
The similarity between the wnt11r morphant and the unplugged/MuSK mutant phenotypes suggested that both genes play roles in the same process. To test this we first examined if both genes interact genetically. For this we injected a suboptimal dose of wnt11r morpholino into wildtype embryos, which induced unplugged like axonal defects in 13.3% hemisegments (n=977 hemisements; Supplemental Figure 3A). unplugged heterozygous embryos do not display any phenotypes, and injection of these embryos with an suboptimal wnt11r MO dose, significantly increased the number of axonal phenotype to 23.3% (n=1179 hemisegments; Supplemental Figure 3A). Moreover, using the same approach, we also observed an increase in AChR prepaptterning defects, demonstrating that wnt11r and unplugged/MuSK interact genetically (Supplemental Figure 3B, C).
The genetic interaction results further suggested that both genes play roles in the same process. One attractive hypothesis first suggested by Burden et al is that secreted Wnt proteins directly bind the unplugged/MuSK receptor through its CRD (Burden, 2000). We therefore examined whether Wnt11r protein can physically associate with the extracellular region of Unplugged. We initially focused on the unplugged SV1 isoform, because it is required for axon guidance and AChR prepatterning in vivo. GST-Unplugged fusion proteins, consisting of the unplugged SV1 extracellular domain tagged with GST at the N-terminus (GST-SV1-ECD) were coupled to Glutathione-Sepharose, and then mixed with conditioned medium containing secreted Wnt11r-FLAG. Wnt11r proteins bound to GST-Unplugged were then detected by anti-FLAG immunoblotting. As shown in Fig. 4A, Wnt11r-FLAG binds to the extracellular Unplugged SV1 region in vitro, suggesting that the extracellular domain of Unplugged associates with Wnt11r.
Figure 4. Wnt11r binds to UnpSV1 and overepressions of wnt11r and unpSV1 increase prepatterning.
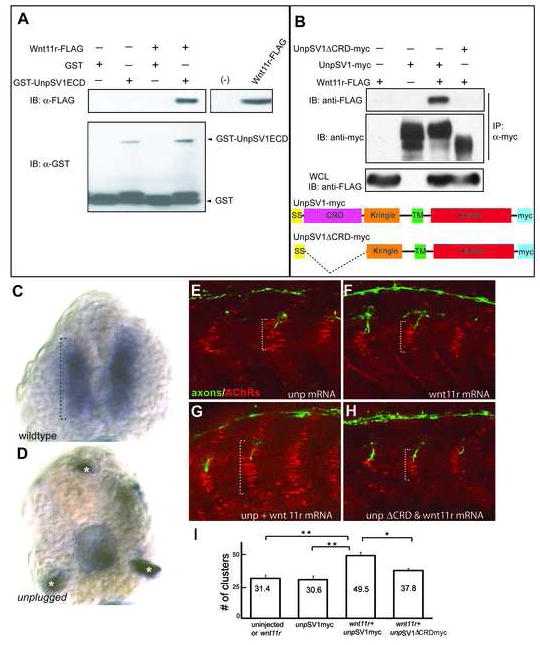
(A) Binding of Wnt11r to the extracellular domain (ECD) of UnpSV1 in vitro. GST-UnpSV1ECD fusion proteins, coupled to glutathione sepharose, were mixed with conditioned media containing secreted Wnt11r-FLAG. Amounts of GST-UnpSV1 and Wnt11r-FLAG used in analysis were assessed by anti-GST (lower panel) and anti-FLAG (right panel) immunoblotting (IB), respectively. Amounts of Wnt11r-FLAG bound were evaluated by anti-FLAG IB (upper panel). (B) Coimmunoprecipitation of UnpSV1 with Wnt11r in 293T cells. 293T cells were cotransfected with Wnt11r-FLAG and UnpSV1-myc or its CRD deletion mutant. Whole cell lysates (WCL) were subjected to anti-FLAG IB to determine the expression of Wnt11r-FLAG (lower panel). The UnpSV1-bound Wnt11r was assessed by IB of the anti-myc immunoprecipitate (upper panel). Schematic diagrams of constructs used in the experiments. SS: signal sequence. (C and D) Cross sections of 20 somite stage embryos injected with purified Wnt11r-FLAG protein. (C) In wildtype embryos, Wnt11r binds to adaxial cells as highlighted by the brackets. Binding is abolished in unplugged mutants(asterisks in D mark non-specific staining). (E-H) Wildtype embryos were injected with mRNAs as indicated. The domain of AChR prepatterning (brackets) was expanded in embryos co-injected with wnt11r and unpSV1myc mRNAs, and was dependent on the CRD domain (G, H). (I) Co-overexpression of wnt11r and unpSV1 significantly increases the number of prepatterned clusters/hemisegment (n=5-18 hemisegments per bar, average=10). Results are expressed as the average of different injection experiments (t test, **p<0.01, *p<0.05). Amounts of mRNA (ng/embryo): wnt11r-FLAG, 0.3; SV1myc, 0.5; SV1ΔCRDmyc, 0.5. AChR cluster size distribution was not altered.
We next examined the physical association between Wnt11r and Unplugged in more detail. Since Unplugged/MuSK proteins contain the CRD known to function as the Wnt-binding sites of Frizzled proteins, we tested whether the unplugged CRD is required for Wnt11r binding. Myc-tagged full length Unplugged SV1 (Unplugged SV1-myc) and myc-tagged Unplugged with the CRD deleted (unplugged SV1 ΔCRD-myc) were cotransfected into 293T cells with FLAG-tagged Wnt11r (Wnt11r-FLAG). Cell lysates were processed for immunoprecipitation with anti-Myc antibody followed by Western blotting with anti-FLAG antibody. Wnt11r bound to full length Unplugged SV1 but not to Unplugged SV1ΔCRD, demonstrating that the Unplugged CRD is required for Wnt11r binding (Fig. 4B). The unplugged FL isoform which is similar to mammalian MuSK also binds Wnt11r, albeit more weakly (Supplemental Fig. 4A).
To determine if Wnt11r binds Unplugged/MuSK in vivo, we examined Wnt11r-FLAG binding in embryos. For this we affinity purified Wnt11r-FLAG protein from supernatant of transfected 293T cells, and injected the soluble protein into the yolk sac of 15 somite stage live embryos (just prior to the onset of AChR prepatterning). The injected protein is transported in the extracellular spaces throughout the entire embryos, and is exposed to the surface of all cells (Lefebvre et al., 2004). In 20 somite stage wildtype embryos, we detected Wnt11r-FLAG binding on adaxial cells, coinciding exclusively with the unplugged/MuSK expression domain (Fig. 4C, compare to Supplemental Fig. 1D). In unplugged mutant embryos binding of Wnt11r-FLAG was completely abolished (Fig. 4D). Thus, together our results demonstrate that unplugged/MuSK has properties of a receptor for Wnt proteins.
Finally, we asked if wnt11r plays a permissive or an inductive role in AChR prepatterning by testing if wnt11r overexpression is sufficient to induce ectopic AChR prepattern. Injection of mRNA encoding for wnt11r or unpluggedSV1 into wildtype embryos revealed no difference in AChR prepattern (Fig. 4E, F, I). In contrast, co-injection of both wnt11r and unpluggedSV1 mRNAs induced ectopic AChR clusters (Fig. G, I). To tested whether the unplugged CRD is required in vivo for wnt11r induced AChR prepatterning, we also co-injected wnt11r and unplugged SV1ΔCRD (lacking the Wnt binding domain), which did not increased AChR prepattern (Fig. H, I). Thus, our results show that unplugged/MuSK and wnt11r are both are mutually required for induction of the AChR prepattern, consistent with a ligand-dependent mode of action.
Blocking dishevelled function in adaxial cells causes unplugged- like phenotypes
Next, we asked if signaling downstream of wnt11r and unplugged/MuSK requires the obligate Wnt intracellular effector dishevelled. Recent studies have shown that the kinase domain of MuSK interacts with dishevelled through its DEP domain, critical for activation of the non-canonical Wnt pathway (Luo et al., 2002). We first used the yeast two-hybrid system to confirm that the zebrafish Unplugged kinase domain interacts with zebrafish Dishevelled (Supplemental Fig. 5A). We then used a truncated form of dishevelled, XDsh–DEP+, shown to specifically block non-canonical Wnt signaling in flies, Xenopus, and zebrafish (Axelrod et al., 1998; Heisenberg et al., 2000; Wallingford et al., 2000). To avoid interference with earlier developmental processes, we used the smyhc1 promotor to target expression of myc-tagged XDsh–DEP+ specifically to adaxial cells, and then generated transient transgenic embryos expressing Myc- XDsh–DEP+ in a small, stochastic subset of adaxial cells.
Analysis of transient transgenic embryos revealed unplugged-like axonal phenotypes in somitic segments expressing Myc-Dsh–DEP+ in dorsal but not in ventral adaxial cells (Fig. 5A, B and data not shown), consistent with the observation that unplugged function is required only in dorsal adaxial cell to guide motor axons (Zhang and Granato, 2000). Furthermore, the frequency of pathfinding defects, up to 36%, correlated with the number of Myc-Dsh–DEP+ positive dorsal adaxial cells (Fig.5 C, D). Analysis of the AChR prepattern revealed that expression of Myc- Dsh–DEP+ in individual adaxial fibers coincided with a marked reduction of clustered AChRs (Fig.5 E-F’). Finally, expression of Myc-Dsh–DEP+ did not affect specification, migration or differentiation of adaxial muscle fibers (Supplemental Fig. 5 B, C), suggesting that the AChR and axonal phenotypes are the primary result of blocking non-canonical Wnt signaling. Thus, blocking Wnt downstream signaling in adaxial cells recapitulates two main phenotypes characteristic for unplugged/MuSK mutants, consistent with the idea that cell-autonomous, non-canonical Wnt signaling in adaxaial cells is critical for axonal guidance and AChR prepatterning.
Figure 5. Inhibition of the non-canonical Dsh pathway in adaxial fibers.
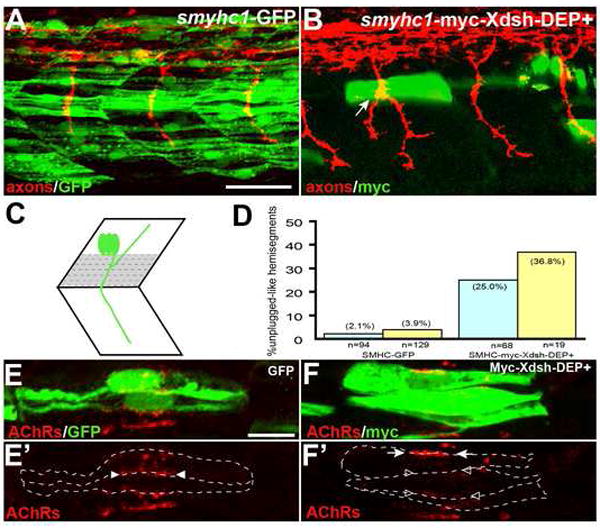
(A) Stochastic expression of Tg(smyhc1:GFP) in adaxial muscle (green) does not affect motor axons (red). (B) Expression of Tg(smyhc1:myc-XDsh-DEP+) (green) in adaxial fibers dorsal to the choice point causes unplugged like pathfinding defects (arrow). (C) Location of the dorsal 6-7 adaxial cells (in grey) used for scoring. (D) Analysis of axonal phenotypes. (n=hemisegments; blue, hemisegments with 2 adaxial cells expressing the transgene; yellow, hemisegments with 3 or more adaxail cells expressing the transgene). (E-F’) Confocal images of adjacent adaxial muscle pioneers expressing the smyhc1-GFP or smyhc1-myc-Xdsh-DEP+ transgene. Only AChR clusters between two adjacent transgene positive adaxial cells were analyzed (outlined by dashed lines). Tg(smyhc1:GFP) expressing adaxial cells form AChR clusters (arrowheads in E’), while Tg(smyhc1:myc-XDsh-DEP+ expression disrupts AChR clusters between transgene expressing cells (F’, open arrowhead); note that this does not affect adjacent, non-transgenic cells which formed normal AChR clusters (F’, arrows). For each transgene, four embryos with GFP or Myc-Dsh-DEP+ positive adaxial cells were analyzed. Prepatterned clusters were reduced in all Myc-Dsh-DEP+ expressing embryos. Scale bars: A, 50 μm; E, 10 μm.
Synapses form in the absence of AChR prepattern
Our results show that unplugged/MuSK and wnt11r play critical roles in initiating the AChRs prepattern. These prepatterned AChR clusters can be incorporated into neuromuscular junctions, but is AChR prepattern essential for synapse formation? To answer this question, we generated multiple inducible unplugged/MuSK transgenic lines, in which the heat shock protein 70 (hsp70l) promotor (Halloran et al., 2000) drives ubiquitous expression of myc-tagged unplugged FL or myc-tagged unplugged SV1. We then crossed these lines into unplugged/MuSK null mutants and confirmed that in the absence of heat shock treatment Tg(hsp70l:unplugged FL-myc); unplugged(br307/br307) or Tg(hsp70l:unplugged SV1-myc); unplugged(br307/br307) embryos lacked all AChR prepattern and neuromuscular synapses (Supplemental Fig. 6A-D).
We then used continuous heat shock treatment to induce expression of Tg(hsp70l:unplugged FL-myc) or Tg(hsp70l:unplugged SV1-myc) in unplugged(br307/br307) embryos, starting several hours before the first AChR clusters become detectable. Heat shock treatment (see Material and Methods for details) was applied until 27 hpf, at which time point growth cones have migrated past the somitic choice point and have formed neural synapses. Analysis of these embryos revealed that unplugged SV1-myc transgene expression completely restored AChR prepattern but failed to induce neural AChR clusters (Fig. 6A-D), consistent with previous observations that the Ig domains absent in the SV1 isoform ectodomain are critical for agrin responsiveness leading to the formation of neuromuscular synapses (Zhou et al., 1999). As predicted, expression of the unplugged FL-myc transgene in unplugged embryos almost completely restored neural AChR clusters (Fig. 6E, F). In these ‘rescued’ embryos, AChR cluster size was slightly reduced, but most AChR clusters were precisely apposed to axonal varicosities, identical to wildtype synapses (Supplemental Fig. 6E-F’). This confirms that the extracellular Ig domains of the unplugged/MuSK receptor are critical for the late stage of synapse formation, when AChRs become incorporated into functional neuromuscular synapses.
Figure 6. Neuromuscular synapses form in the absence of AChR prepattern.
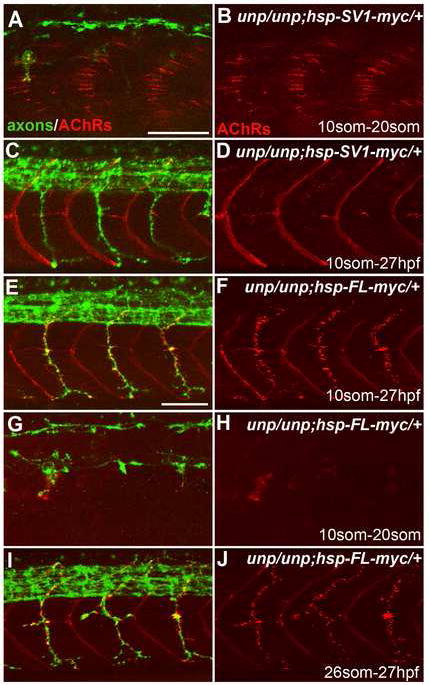
20-somite stage (A-B, G-H) or 27 hpf (C-F and I-J) embryos after heat shock treatment. (A, B) Tg(hsp70l:UnpSV1-myc;unplugged) embryos received heat shock from the 10- to 20-somite stage, which rescued AChR prepattern. (C, D) Similar heat shock treatment (10-somite to 27 hpf) also restored motor axon pathfinding, but not neuromuscular synapses. (E, F) The same heat shock treatment rescued motor axons and neuromuscular synapses in Tg(hsp70l:UnpFL-myc; unplugged) embryos. (G, H) In contrast, heat shock between the 10- and 20-somite stage failed to rescue AChR prepattern in Tg(hsp70l:UnpFL-myc;unplugged) embryos. (I, J) Heat shock treatment of same embryos between the 26-somite stage and 27 hpf, i.e. after the time period of prepatterning, was sufficient to rescue neuromuscular synapses. Scale bars: 50 μm.
However, we noticed that heat shock treated Tg(hsp70l:unplugged FL-myc; unplugged(br307/br307) embryos displayed very little or no AChR prepattern (Fig. 6G, H). We confirmed the absence of detectable adaxial AChR prepattern in three independent transgenic lines, suggesting that this was not due to the influence of chromatin neighboring the transgene integration site. Furthermore, Western Blot analysis of Tg(hsp70l:unplugged FL-myc; unplugged(br307/br307)) embryos showed that heat shock treatment induced high levels of myc-tagged protein, comparable to the levels in Tg(hsp70l:unplugged SV1-myc; unplugged(br307/br307)) embryos (Supplemental Fig. 6H). Finally, expression of unpluggedFL under the control of the adaxial specific promotor [Tg(smyhc1:unpluggedFL-myc)] in unplugged(br307/br307) embryos also failed to restore adaxial AChR prepattern (Supplemental Fig. 4B, C). These experiments indicate a potential negative role for the Ig domains on the AChR prepattern, but more importantly suggest that in vivo neural synapses can form in the absence of prepatterned AChRs.
To exclude the possibility that in heat shock treated Tg(hsp70l:unplugged FL-myc; unplugged(br307/br307)) embryos adaxial AChR prepattern was present but not detectable, e.g. due to small AChR cluster size, we repeated the experiment but started heat shock treatment after the time period of AChR prepatterning, when motor axons have extended well into the periphery (26 somite stage, ~22hpf). Analysis of these embryos at 27hpf revealed the characteristic unplugged axonal defects, and the presence of neural AChR clusters (Fig. 6I, J). Although these ‘rescued’ AChR clusters were abundant, they were smaller in size and less precisely aligned with the axons, when compared to wildtype (Fig. 6I, J and Supplemental Fig. 6G, G’). Nonetheless, heat shock treated Tg(hsp70l:unplugged FL-myc; unplugged(br307/br307)) embryos were fully motile, suggesting that AChR clusters represent functional neuromuscular synapses. Thus, functional neuromuscular synapses can develop in the absence of AChR prepattern, and in the absence of unplugged/MuSK function during the early, nerve independent phase. This suggests that nerve-muscle interactions at the late phase of synapse formation can compensate for the absence of an AChR prepattern, and that these interactions are sufficient to generate neuromuscular synapses in vivo.
DISCUSSION
The role of Wnt signaling in synapse formation
Recent studies in C. elegans, Drosophila and in the mammalian CNS have revealed critical roles for Wnt ligands in synapse formation (Hall et al., 2000; Klassen and Shen, 2007; Packard et al., 2002). At the mammalian neuromuscular junction, the precise role of Wnt signaling is less clear. In vitro, Wnt-1 has no influence on AChR clustering (Luo et al., 2002), but can regulate MuSK expression in cultured myotubes (Kim et al., 2003). Recent studies using cultured myotubes show that Wnt3 increases agrin dependent AChR clustering (Henriquez et al., 2008), and several downstream components of the Wnt pathway, including β-catenin, Dishevelled, APC, PAK and JNK have also been implicated in this process (Luo et al., 2002; Wang et al., 2003; Zhang et al., 2007). More recently, the low-density lipoprotein receptor-related protein 4 (LRP4) whose extracellular domains is similar to the Wnt co-receptor LRP5/6 proteins, has been shown to function as MuSK coreceptor binding nerve released Agrin, and thus promoting neural AChR clusters (Kim et al., 2008; Zhang et al., 2008). Interestingly, LRP4 is also required for AChR prepattern and the accumulation of MuSK protein at presumptive synapses, supporting a role for Wnt signals in the early phase of NMJ development (Weatherbee et al., 2006). However, it has remained unclear if and which Wnt ligand(s) can activate the early, nerve independent AChR prepattern.
Our results provide four compelling lines of evidence that Wnt ligands signal through unplugged/MuSK to initiate the early, nerve independent phase of synapse development. First, morpholino mediated reduction of wnt11r causes severe AChR prepatterning defects, as well as unplugged like axonal defects already as growth cones navigate towards the AChR prepattern. Second, wnt11r and unplugged/MuSK interact genetically, suggesting that they function in the same pathway. Third, in vitro, Wnt11r binds to Unplugged in a CRD dependent manner, and in vivo Wnt11r-FLAG binding sites precisely outline the unplugged/MuSK expression domain, i.e. adaxial muscle, in a unplugged dependant fashion, suggesting that Unplugged/MuSK has the properties of a wnt11r receptor. Fourth, blocking the dishevelled dependent non-canonical Wnt pathway in adaxial cells also causes defects in AChR prepatterning and axonal pathfinding. Together, these data suggest that in response to Wnt ligands muscle cells enable an unplugged/MuSK signaling cascade that restricts growth cones and AChR prepattern to a common muscle zone.
During Drosophila NMJ development, Wnt-1 (Wg) is secreted presynaptically to regulate synapse development (Speese and Budnik, 2007), raising the question of the relevant Wnt11r source in the zebrafish embryo. Although wnt11r is expressed in the spinal cord, it is unlikely that motor nerves are the source of wnt11r. AChR prepattern is visible well before motor growth cones approach (Supplemental Fig. 1), and in mammals AChR prepatterning has been shown to be independent of nerve contacts and signals (Lin et al., 2001; Yang et al., 2001). Based on its spatial mRNA expression, wnt11r is likely secreted by cells in the dorso-lateral somites (Supplemental Fig. 2D-F), adjacent to pre-migratory dorsal adaxial cells in which unplugged function is required (Zhang and Granato, 2000). Interestingly, Wnt11r secreted from these dorso-lateral somitic cells induces AChR prepattern on ventral adaxial cells (Fig. 3K), at a distance of about 10 cell diameters, reminiscent of the well-studied long-range action of Drosophila wingless (Zecca et al., 1996). While Wnt proteins are hydrophobic and probably membrane associated, after secretion, Wnts can diffuse at a rate of up to 50μm in 30 minutes and can act as long-range signals up to 20 cell diameters away (Strigini and Cohen, 2000; Wodarz and Nusse, 1998). More recently, it has become clear that long-range activation is likely mediated by Wnt proteins uniquely packed for long-range signaling (reviewed in: Bartscherer and Boutros, 2008). In the embryo, Wnt11r-FLAG binds to dorsal and ventral adaxial muscle (Fig. 4), consistent with the idea that wnt11r dependent formation of AChR prepattern in ventral adaxial cells might be mediated by a long-range signaling mechanism.
However, given the presence of residual AChR prepatterning and low penetrance of axonal pathfinding defects in wnt11r morphants, it is also possible that additional Wnt ligands, possibly expressed in other tissues, activate the unplugged receptor in more ventral adaxial cells. Although our understanding of how Wnt signals direct activation of unplugged/MuSK is only beginning to emerge, together our data provide compelling evidence that during the early phase of synapse formation, Wnt signals through the unplugged receptor organize a central muscle zone to which navigating motor growth cones and nascent AChR prepattern are confined.
AChR prepattern is dispensable for NMJ formation
In zebrafish, prepatterned AChR clusters can be incorporated into prospective neuromuscular synapses upon contact with motor axons (Flanagan-Steet et al., 2005; Panzer et al., 2006), and in mice aneural AChR clusters per se are not required for synapse formation (Lin et al., 2008; Vock et al., 2008), but it has remained unclear if AChR prepattern itself is essential for synapse formation. Our results demonstrate that activation of unplugged/ MuSK in unplugged mutants after the period of AChR prepatterning results in the unexpected presence of almost wildtype-like, functional neuromuscular synapses. This demonstrates that AChR prepatterning is dispensable for subsequent synapse formation. However, we can not exclude the possibility that during normal development, AChR prepatterning facilitates or serves as an initial scaffold for future synapses, as pre-existing AChR clusters are incorporated into neuromuscular junctions (Flanagan-Steet et al., 2005; Panzer et al., 2005). It is also possible that high levels of unplugged/MuSK protein from the hsp70l transgene compensate for the absence of prepatterned AChRs. For example, expression of constitutively active MuSK leads to self-aggregation, and these aggregates colocalize with AChR clusters, even in the absence of agrin (Jones et al., 1999).
Independent of how ‘late’ unplugged/MuSK activation induces neuromuscular synapses, our results provide insights to a longstanding question. In the now widely accepted ‘myocentric’ model, the muscle determines the future site of synaptogenesis. It has also been long known that motoneurons can form synapses with cultured muscle cells lacking an AChR prepattern, suggesting that such prepattern might not be essential (Anderson and Cohen, 1977; Frank and Fischbach, 1979). Our results demonstrate that in the embryo, functional synapses can develop in the complete absence of the initial AChR prepattern, and suggest that during the late phase of synapse formation, synapses form de novo at sites where the nerve releases Agrin to locally activate MuSK, or possibly by local MuSK autoactivation (Kim and Burden, 2008; Lin et al., 2008). Importantly, while AChRs prepattern is dispensable, e.g. by late expression of unplugged/MuSK, the organization of a central muscle zone is essential to restrict growth cones, as ‘late’ unplugged/MuSK expression fails to rescue the axonal pathfinding defects (Fig. 6). Thus, the central zone determines the muscle territory accessible to motor axons, and thereby the sites of neuromuscular synapses.
The role of unplugged/MuSK in synapse formation
What is the role of unplugged/MuSK in presynaptic development? In MuSK-/-mice, nerve processes are not restricted to the central region of the muscle, but are present throughout the muscle (DeChiara et al., 1996). This exuberant axonal growth has been attributed to the absence of MuSK dependent muscle-derived signals, which normally stop axonal growth and induce presynaptic differentiation (DeChiara et al., 1996), but more recent analyses reveal the presence of axonal branching before the formation of AChR clusters (Lin et al., 2008). While at later stages unplugged/MuSK mutant embryos also display excessive branching (Zhang and Granato, 2000), our time-lapse analysis reveals dramatic defects earlier during axonal pathfinding (Fig. 2). Like mammalian MuSK, unplugged expression is undetectable in motoneurons, and chimera analyses have shown that unplugged/MuSK functions in adaxial muscle to guide motor axons (Zhang and Granato, 2000). Thus, already very early on unplugged/ MuSK dependent, muscle derived signals restrict growth cones to the central muscle zone. This raises the possibility that the later observed exuberant axonal growth is a consequence of the earlier guidance defects, although we cannot exclude the possibility that unplugged/ MuSK provides several independent signals.
Our analyses reveal identical guidance and AChR defects in wnt11r morphants, and in combination with in vitro binding data this suggests that wnt11r activates unplugged/MuSK to organize a central muscle zone, thereby confining pre- and postsynaptic processes to a common, narrow domain. Intriguingly, only overexpression of Wnt11r and Unplugged induces ectopic AChRs, suggesting that wnt11r by itself is not sufficient to induce AChR prepatterning (Fig. 4), but that additional, wnt11r independent mechanisms, e.g. to localize the unplugged/MuSK receptor, are also critical. Based on our data, we propose a model in which during the early phase of synaptogenesis Wnt activates via unplugged/MuSK a dishevelled signaling pathway in muscle, similar to the planar cell polarity pathway to define the position of subcellular components along the anterior-posterior axis (Fig. 7A, B). We propose that one branch of this pathway acts through rapsyn to accumulate AChR clusters to the central zone, thereby generating an AChR prepattern. This is consistent with the requirement of rapsyn in mouse and fish, as in its absence the AChR prepattern fails to form (data not shown and Lin et al., 2001). Rapsyn is not required for axonal guidance and presynaptic development (Zhang et al., 2004), while dishevelled is, suggesting a second, rapsyn-independent branch, downstream of dishevelled, to confine presynaptic growth (Fig. 7A). Such a rapsyn-independent branch is also supported by live imaging, demonstrating that AChR clusters per se are not required for growth cones guidance (Panzer et al., 2005).
Figure 7. unplugged/MuSK signaling during synapse formation.
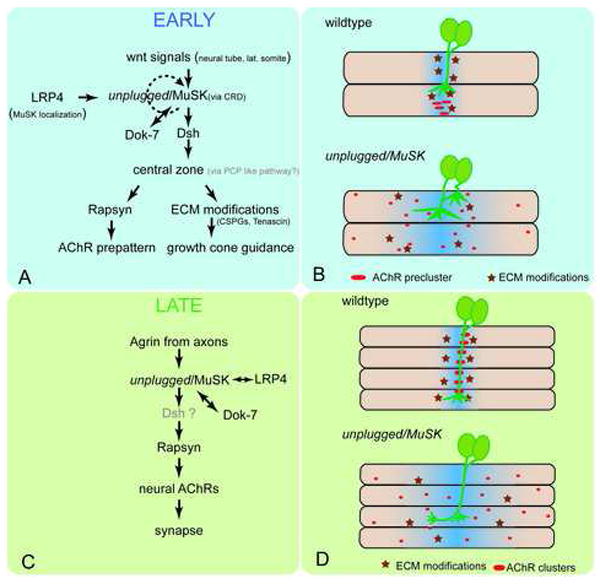
Signaling during the early (A, B) and late (C, D) phase of neuromuscular synapse formation. (A, B) Early during synapse formation, Wnt signals act through unplugged/MuSK receptor to establish a central muscle zone, possibly through a Dsh-dependant, PCP like pathway. LRP4 is essential for MuSK localization, and Dok-7 for MuSK activation (Okada et al., 2006). Ligand dependent unplugged/MuSK activation may rapidly become ligand-independent. One branch of this pathway requires rapsyn to cluster AChRs (red ovals in B) in a central prepattern, while through a rapsyn-independent mechanism, e.g. modifications of the ECM components (dark red stars in B), growth cones are restricted to the central zone. In the absence of Wnt or unplugged/MuSK, rapsyn is not activated and thus AChRs are dispersed throughout the muscle and navigating growth cones extend into lateral muscle territory. Blue shades indicate the central zone. (C, D) During the late phase, nerve-derived agrin signals through unplugged/MuSK and LRP4 to recruit rapsyn, which stabilizes neural AChRs and promotes synapse development. (D) In the absence of unplugged/MuSK, rapsyn is not recruited and thus AChR cluster are not stabilized in the central zone. Absence of unplugged also causes rapsyn-independent pathfinding defects, possibly through the lack of ECM modifications. Note that in the absence of a central muscle zone at the early stages, no AChR prepattern forms, but that local agrin secretion from the axon and late expression of unplugged/MuSK appears sufficient to induce neural AChRs and subsequently functional synapses.
How does unplugged/MuSK signaling restrict growth cones to the central muscle zone? We have previously shown that unplugged /MuSK mutants lack a specific expression domain of two extracellular matrix (ECM) components along the anterior and posterior boundaries of the central muscle zone (Schweitzer et al., 2005; Zhang et al., 2004). This is highly significant because both components are produced by adaxial cells and because only their adaxial expression domain is altered in unplugged/MuSK mutants but not in other motor axon guidance mutants examined (Schweitzer et al., 2005; Zhang et al., 2004). Both of these ECM components, Tenascin and Chondroitin sulfate proteoglycans (CSP), have been implicated in axonal repulsion (Becker et al., 2003; Masuda et al., 2004), and here we show that wnt11r morphants also exhibit defects in CSP localization (Supplemental Fig. 2), consistent with a model by which these and/or additional wnt11r and unplugged/MuSK dependent ECM modifications may restrict the axonal path of navigating growth cones to the central muscle zone.
Together, our data suggest a compelling model for the role of unplugged/MuSK in the early, nerve independent phase of synapse formation (Fig. 7A, B), preceding the better understood nerve/agrin dependent late phase (Fig.7 C, D). We propose that unplugged/MuSK engages a dishevelled-dependent signaling pathway in muscle cells to organize a central muscle zone, essential to confine navigating motor growth cones and nascent AChR prepattern to the center of muscle fibers. More importantly, we also propose that this process is initiated –at least in part- by Wnt signals. We identify wnt11r as a potential unplugged/MuSK ligand, but because of the incomplete penetrance of the wnt11r morphant phenotype, it is possible that additional, functionally redundant ligands, and/or compensatory mechanisms exist. For example, Kim and Burden have recently proposed an elegant model by which ligand independent MuSK activation in the mouse embryo is sufficient for AChR prepattern and presynaptic development (Kim and Burden, 2008). We propose that this complex process is initially ligand dependent- as overexpression of only wnt11r and unplugged/MuSK induces ectopic AChR clusters- but that it might rapidly become ligand independent due to a positive feedback loop (Jones et al., 1999; Moore et al., 2001). Elevating MuSK expression in early myofibers even slightly above the endogenous levels, as was done in the Kim and Burden study, may bypass the initial ligand dependency we observe. Alternatively, species-specific differences dictated by anatomical and/or developmental restrictions, such as muscle fiber length and speed of NMJ formation, may account for divergent mechanisms of unplugged/MuSK activation. Nonetheless, our results provide the first evidence that Wnt ligands are critical for initiating synapse formation, and that Wnts can bind the unplugged/MuSK receptor. We propose that Wnt stimulation engages a dishevelled-dependent signaling cascade to establish polarity within the plane of the muscle, thereby registering AChR clusters with advancing growth cones, possibly through a mechanism reminiscent of the planar polarity pathway.
Materials and Methods
Whole-mount inmmunocytochemistry, wnt11r-FLAG in vivo staining
Embryos were fixed and stained as described in (Zeller et al., 2002). For labeling of AChRs, embryos were permeabilized in 1mg/ml collegenase (Sigma) in phosphate buffer for 6-8 minutes, rinsed in 1xPBS and incubated with AlexaFluor conjugated α-bungarotoxin (Molecular Probes, Eugene, OR) as described by (Lefebvre et al., 2004). Antibodies and dilutions were used as follows: znp-1 (1:200, DSHB), SV2 (1:50, DSHB), myc (9E10, 1:1000, Covance), Prox1 (1:200), F59 (1:20, DSHB), and anti-chondriotin sulfate (CS56, 1:200, Sigma). Embryos were imaged with LSM510 (Zeiss) and LCS (Leica) confocal microscopes.
Quantification of AChR clusters: Confocal images were projected into a single plane and converted to a 16-bit image using Metamorph. A region of interest was drawn around the border of each somitic segment. AChR clusters were counted using the ‘count nuclei’ function, with the minimum/maximum length set to 5/ 100 pixels, respectively and, a minimum average intensity of 60 above background. The results were exported to Microsoft Excel for statistical analysis.
Wnt11r-FLAG was affinity purified from transfected HEK cells, and injected into live embryos as previously reported for αBTX (Lefebvre et al., 2004) and detailed in the Supplemental Material section.
Morpholino and mRNA injections
3-4 nanograms of wnt11r translation blocking MO (wnt11r TL-MO) (Matsui et al., 2005) were injected into one-cell embryos. A splice-blocking MO (wnt11r SP-MO, 5’TTTTTCTCAGTAACTCACCTCGTTC3’) was designed against the splice donor site of exon 3. 6-7 nanograms of wnt11r SP-MO were injected into the embryos at one-cell stage. For RT-PCR analysis, cDNA templates were synthesized from five 24-hpf embryos. PCR Primers were: 5’-TCCTCACATTCCTGCTCCTGTC-3’ (forward) and 5’-TCTTCATCTTCATTGGGGCATC-3’ (reverse). mRNA was in vitro transcribed from linearized constructs using SP6 mMessage mMachine Kit (Ambion), and injected into embryos at the 1- to 2-cell stage.
In vitro GST pull-down assay
Wnt11r-FLAG conditioned medium from transfected 293T cells was incubated with GST proteins and GST-UnpSV1ECD fusion proteins expressed in E.coli and absorbed to Glutathione Sepharose 4B, and after washing eluted proteins were detected with anti-FLAG antibody (1:1000, Sigma) and anti-GST antibody (1:5000, Sigma) on Western Blots as detailed in the Supplemental Method section.
Transient transfection, co-immunoprecipitation, and western blotting
Transient transfection and immunoprecipitation were carried out as previously described (Lu et al., 2004) with some modifications as detailed in the Supplemental Method section.
Transgenes
Transgenic lines were generated by microinjection of DNA as previously described (Thermes et al., 2002). The lines generated in this studies are: Tg(hsp70l:unpSV1-myc)p1, Tg(hsp70l:unpFL-myc)p1, Tg(smyhc1:unpSV1-myc)p1 and Tg(smyhc1:unpFL-myc)p1 in accordance with ZFIN nomenclature.
Heat-shock condition
The embryos from the cross of unpluggedtbr307/tbr307; hsp70l:SV1(FL)-myc/+ to unpluggedtbr307/tbr307 were kept at 28°C to the desired stage before the heatshock. Each pair of embryos was then placed in 100 μl E3 medium in a single well of 96-well PCR plate. Embryos were heat-shocked at 38°C for 35 minutes at 2.5 hours intervals until they reached the appropriate stage. Transgenic embryos were identified from the control siblings by genotyping using the following primers: 5’TGACCAGATGCTCAAATCTGGTCTTTC3’ (forward) and 5’ATTAAGCTAGCGGTGAGGTCGCCCTA3’(reverse).
Live imaging
16 to 20 somite embryos were mounted in MatTek glass bottom culture dishes using 1.2% NuSieve GTG agarose prepared in Ringers plus Tricane, and image stacks taken every 2 minutes using a Perkin Elmar UltraView spinning disk confocal equipped with a 63x lens. Growth cones were analyzed based on their morphologies during pathfinding.
Plasmid construction
Standard molecular biology methods were used to generate unplugged FL, SV1, wnt11r, Dsh plasmids for protein expression, yeast two hybrid and in situ hybridization as outlined in the Supplemental Methods section.
In situ hybridization
Fluorescent in situ hybridizations were performed according to (Downes et al., 2002) and (Schneider and Granato, 2006). Probes compelementary to 5’UTR sequence of UnpSV1 (nt 1-340) or UnpFL unique coding sequence (nt 664-1012) were used. For wnt11r, probes complementary to wnt11r full-length sequences were used.
Supplementary Material
Acknowledgments
We would like to thank Drs. Ingham and Elworthy for sharing the smyhc1 promotor, Dr. Meyer for providing the Hb9 promotor and the Hb9:GFP line before publication, Andrea Stout from the CDB imaging core, members of the Bashaw laboratory for technical advise, and members of the Granato laboratory for comments. We would also like to thank Dr. Gilmour (EMBL) and members of his laboratory for help with live cell imaging. This work was supported by grants from the National Science Foundation (M. G.) and the National Institute of Health (M. G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson MJ, Cohen MW. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977;268:757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes & development. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartscherer K, Boutros M. Regulation of Wnt protein secretion and its role in gradient formation. EMBO reports. 2008;9:977–982. doi: 10.1038/embor.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CG, Schweitzer J, Feldner J, Becker T, Schachner M. Tenascin-R as a repellent guidance molecule for developing optic axons in zebrafish. J Neurosci. 2003;23:6232–6237. doi: 10.1523/JNEUROSCI.23-15-06232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden SJ. Wnts as retrograde signals for axon and growth cone differentiation. Cell. 2000;100:495–497. doi: 10.1016/s0092-8674(00)80685-6. [DOI] [PubMed] [Google Scholar]
- Burden SJ. Building the vertebrate neuromuscular synapse. J Neurobiol. 2002;53:501–511. doi: 10.1002/neu.10137. [DOI] [PubMed] [Google Scholar]
- Burden SJ, Fuhrer C, Hubbard SR. Agrin/MuSK signaling: willing and Abl. Nat Neurosci. 2003;6:653–654. doi: 10.1038/nn0703-653. [DOI] [PubMed] [Google Scholar]
- Cortes F, Daggett D, Bryson-Richardson RJ, Neyt C, Maule J, Gautier P, Hollway GE, Keenan D, Currie PD. Cadherin-mediated differential cell adhesion controls slow muscle cell migration in the developing zebrafish myotome. Developmental cell. 2003;5:865–876. doi: 10.1016/s1534-5807(03)00362-9. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Downes GB, Waterbury JA, Granato M. Rapid in vivo labeling of identified zebrafish neurons. Genesis. 2002;34:196–202. doi: 10.1002/gene.10120. [DOI] [PubMed] [Google Scholar]
- Eisen JS, Myers PZ, Westerfield M. Pathway selection by growth-cones of identified motoneurons in live zebra fish embryos. Nature. 1986;320:269–271. doi: 10.1038/320269a0. [DOI] [PubMed] [Google Scholar]
- Eisen JS, Pike SH, Romancier B. An identified motoneuron with variable fates in embryonic zebrafish. J Neurosci. 1990;10:34–43. doi: 10.1523/JNEUROSCI.10-01-00034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elworthy S, Hargrave M, Knight R, Mebus K, Ingham PW. Expression of multiple slow myosin heavy chain genes reveals a diversity of zebrafish slow twitch muscle fibres with differing requirements for Hedgehog and Prdm1 activity. Development. 2008;135:2115–2126. doi: 10.1242/dev.015719. [DOI] [PubMed] [Google Scholar]
- Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development. 2005 doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- Frank E, Fischbach GD. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979;83:143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, et al. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Gros Jrm, Serralbo O, Marcelle C. WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature. 2008 doi: 10.1038/nature07564. [DOI] [PubMed] [Google Scholar]
- Groves JA, Hammond CL, Hughes SM. Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development. 2005;132:4211–4222. doi: 10.1242/dev.01958. [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Halloran MC, Sato-Maeda M, Warren JT, Su F, Lele Z, Krone PH, Kuwada JY, Shoji W. Laser-induced gene expression in specific cells of transgenic zebrafish. Development. 2000;127:1953–1960. doi: 10.1242/dev.127.9.1953. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Henriquez JP, Webb A, Bence M, Bildsoe H, Sahores M, Hughes SM, Salinas PC. Wnt signaling promotes AChR aggregation at the neuromuscular synapse in collaboration with agrin. Proc Natl Acad Sci U S A. 2008;105:18812–18817. doi: 10.1073/pnas.0806300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H, Shibata M, Hiratani I, Taira M. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development. 2002;129:5227–5239. doi: 10.1242/dev.129.22.5227. [DOI] [PubMed] [Google Scholar]
- Jones G, Moore C, Hashemolhosseini S, Brenner HR. Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J Neurosci. 1999;19:3376–3383. doi: 10.1523/JNEUROSCI.19-09-03376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Xiong WC, Mei L. Regulation of MuSK expression by a novel signaling pathway. J Biol Chem. 2003;278:38522–38527. doi: 10.1074/jbc.M305058200. [DOI] [PubMed] [Google Scholar]
- Kim N, Burden SJ. MuSK controls where motor axons grow and form synapses. Nat Neurosci. 2008;11:19–27. doi: 10.1038/nn2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 Is a Receptor for Agrin and Forms a Complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol. 2006;16:74–82. doi: 10.1016/j.conb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Lefebvre JL, Jing L, Becaficco S, Franzini-Armstrong C, Granato M. Differential requirement for MuSK and dystroglycan in generating patterns of neuromuscular innervation. Proc Natl Acad Sci U S A. 2007;104:2483–2488. doi: 10.1073/pnas.0610822104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL, Ono F, Puglielli C, Seidner G, Franzini-Armstrong C, Brehm P, Granato M. Increased neuromuscular activity causes axonal defects and muscular degeneration. Development. 2004;131:2605–2618. doi: 10.1242/dev.01123. [DOI] [PubMed] [Google Scholar]
- Lin S, Landmann L, Ruegg MA, Brenner HR. The role of nerve- versus muscle-derived factors in mammalian neuromuscular junction formation. J Neurosci. 2008;28:3333–3340. doi: 10.1523/JNEUROSCI.5590-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- Lin W, Dominguez B, Yang J, Aryal P, Brandon EP, Gage FH, Lee KF. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Liu DWC, Westerfield M. Clustering of Muscle Acetylcholine Receptors Requires Motoneurons in Live Embryos, But Not in Cell Culture. J Neurosci. 1992;12:1859–1866. doi: 10.1523/JNEUROSCI.12-05-01859.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Luo ZG, Wang Q, Zhou JZ, Wang J, Luo Z, Liu M, He X, Wynshaw-Boris A, Xiong WC, Lu B, Mei L. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron. 2002;35:489–505. doi: 10.1016/s0896-6273(02)00783-3. [DOI] [PubMed] [Google Scholar]
- Masuda T, Fukamauchi F, Takeda Y, Fujisawa H, Watanabe K, Okado N, Shiga T. Developmental regulation of notochord-derived repulsion for dorsal root ganglion axons. Mol Cell Neurosci. 2004;25:217–227. doi: 10.1016/j.mcn.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Matsui T, Raya A, Kawakami Y, Callol-Massot C, Capdevila J, Rodriguez-Esteban C, Izpisua Belmonte JC. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes & development. 2005;19:164–175. doi: 10.1101/gad.1253605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld T, Kummer TT, Lichtman JW, Sanes JR. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:11088–11093. doi: 10.1073/pnas.0504806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C, Leu M, Muller U, Brenner HR. Induction of multiple signaling loops by MuSK during neuromuscular synapse formation. Proc Natl Acad Sci U S A. 2001;98:14655–14660. doi: 10.1073/pnas.251291598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Okada K, Inoue A, Okada M, Murata Y, Kakuta S, Jigami T, Kubo S, Shiraishi H, Eguchi K, Motomura M, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science. 2006;312:1802–1805. doi: 10.1126/science.1127142. [DOI] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzer JA, Gibbs SM, Dosch R, Wagner D, Mullins MC, Granato M, Balice-Gordon RJ. Neuromuscular synaptogenesis in wild-type and mutant zebrafish. Dev Biol. 2005 doi: 10.1016/j.ydbio.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Panzer JA, Song Y, Balice-Gordon RJ. In vivo imaging of preferential motor axon outgrowth to and synaptogenesis at prepatterned acetylcholine receptor clusters in embryonic zebrafish skeletal muscle. J Neurosci. 2006;26:934–947. doi: 10.1523/JNEUROSCI.3656-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch GJ, Hammerschmidt M, Blader P, Schauerte HE, Strahle U, Ingham PW, McMahon AP, Haffter P. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harbor symposia on quantitative biology. 1997;62:227–234. [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Granato M. The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration. Neuron. 2006;50:683–695. doi: 10.1016/j.neuron.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Schweitzer J, Becker T, Lefebvre J, Granato M, Schachner M, Becker CG. Tenascin-C is involved in motor axon outgrowth in the trunk of developing zebrafish. Dev Dyn. 2005;234:550–566. doi: 10.1002/dvdy.20525. [DOI] [PubMed] [Google Scholar]
- Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends in neurosciences. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Wingless gradient formation in the Drosophila wing. Curr Biol. 2000;10:293–300. doi: 10.1016/s0960-9822(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mechanisms of development. 2002;118:91–98. doi: 10.1016/s0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Vock VM, Ponomareva ON, Rimer M. Evidence for muscle-dependent neuromuscular synaptic site determination in mammals. J Neurosci. 2008;28:3123–3130. doi: 10.1523/JNEUROSCI.5080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, Jing Z, Zhang L, Zhou G, Braun J, Yao Y, Wang ZZ. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat Neurosci. 2003;6:1017–1018. doi: 10.1038/nn1128. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Anderson KV, Niswander LA. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development. 2006;133:4993–5000. doi: 10.1242/dev.02696. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annual review of cell and developmental biology. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Xu YK, Nusse R. The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Curr Biol. 1998;8:R405–406. doi: 10.1016/s0960-9822(98)70262-3. [DOI] [PubMed] [Google Scholar]
- Yang X, Arber S, William C, Li L, Tanabe Y, Jessell TM, Birchmeier C, Burden SJ. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. doi: 10.1016/s0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- Zeller J, Schneider V, Malayaman S, Higashijima S, Okamoto H, Gui J, Lin S, Granato M. Migration of zebrafish spinal motor nerves into the periphery requires multiple myotome-derived cues. Dev Biol. 2002;252:241–256. doi: 10.1006/dbio.2002.0852. [DOI] [PubMed] [Google Scholar]
- Zhang B, Luo S, Dong XP, Zhang X, Liu C, Luo Z, Xiong WC, Mei L. Beta-catenin regulates acetylcholine receptor clustering in muscle cells through interaction with rapsyn. J Neurosci. 2007;27:3968–3973. doi: 10.1523/JNEUROSCI.4691-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 Serves as a Coreceptor of Agrin. 2008;60:285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Granato M. The zebrafish unplugged gene controls motor axon pathway selection. Development. 2000;127:2099–2111. doi: 10.1242/dev.127.10.2099. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lefebvre JL, Zhao S, Granato M. Zebrafish unplugged reveals a role for muscle-specific kinase homologs in axonal pathway choice. Nat Neurosci. 2004;7:1303–1309. doi: 10.1038/nn1350. [DOI] [PubMed] [Google Scholar]
- Zhou H, Glass DJ, Yancopoulos GD, Sanes JR. Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations. J Cell Biol. 1999;146:1133–1146. doi: 10.1083/jcb.146.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


