Abstract
One proposed mechanism of patellofemoral pain, increased stress in the joint, is dependent on forces generated by the quadriceps muscles. Describing causal relationships between muscle forces, tissue stresses, and pain is difficult due to the inability to directly measure these variables in vivo. The purpose of this study was to estimate quadriceps forces during walking and running in a group of male and female patients with patellofemoral pain (n=27, 16 female; 11 male) and compare these to pain-free controls (n=16, 8 female; 8 male). Subjects walked and ran at self-selected speeds in a gait laboratory. Lower limb kinematics and electromyography (EMG) data were input to an EMG-driven musculoskeletal model of the knee, which was scaled and calibrated to each individual to estimate forces in 10 muscles surrounding the joint. Compared to controls, the patellofemoral pain group had greater co-contraction of quadriceps and hamstrings (p=0.025) and greater normalized muscle forces during walking, even though the net knee moment was similar between groups. Muscle forces during running were similar between groups, but the net knee extension moment was less in the patellofemoral pain group compared to controls. Females displayed 30-50% greater normalized hamstring and gastrocnemius muscle forces during both walking and running compared to males (p<0.05). These results suggest that some patellofemoral pain patients might experience greater joint contact forces and joint stresses than pain-free subjects.
Keywords: Muscle force, Patellofemoral Pain
Introduction
Patellofemoral pain is one of the most common disorders of the knee, accounting for 25% of all knee injuries seen in sports medicine clinics (Devereaux and Lachmann, 1984). Participation in sports and daily activities may be adversely affected by patellofemoral pain (Fulkerson, 2002), and many people continue to have problems even after a full treatment program (Blond and Hansen, 1998). The incidence of patellofemoral pain is greater in women compared to men, particularly in young athletes (Baker, 1997; DeHaven and Lintner, 1986; Taunton et al., 2002; Thomee et al., 1995). The causes of patellofemoral pain and the gender disparity remain unclear.
Quadriceps muscle forces play a crucial role in determining the medial-lateral force balance, contact force, and pressure distribution of the patellofemoral joint (Dhaher and Kahn, 2002; Elias et al., 2006; Lee et al., 2002). It has been suggested that an imbalance between the vastus medialis and vastus lateralis forces causes abnormal tracking of the patella (Farahmand et al., 1998; Lieb and Perry, 1968), resulting in reduced contact areas, increased stresses, and patellofemoral pain (Fulkerson and Shea, 1990). Physical therapy interventions for patients with patellofemoral pain often focus on altering recruitment of the medial and lateral components of the vasti (Cowan et al., 2003). However, describing causal relationships between muscle forces, tissue stresses, and pain has proven difficult, due to the inability to directly measure these variables in vivo. The effect of changing the distribution of vasti muscle forces on patellofemoral joint mechanics has been explored using cadaveric experiments (Lee et al., 2002; Lin et al., 2004; Powers et al., 1998) and mathematical models (Dhaher and Kahn, 2002; Elias et al., 2004). While providing valuable information, these studies rely on simplified muscle force estimates and do not account for differences in muscle forces between subjects. There remains a paucity of data describing variation in muscle forces at the knee during walking and running.
Several methods can be used to estimate muscle forces. One approach is to distribute load based upon physiological cross-sectional area (Dhaher and Kahn, 2002; Elias et al., 2004) and/or moment arms of muscles (Brechter and Powers, 2002). Static optimization schemes have also been developed to distribute the net joint moment to individual muscles or muscle groups (Crowninshield and Brand, 1981; Seireg and Arvikar, 1973), but rely on a priori assumptions regarding muscle activation and do not account for individual recruitment patterns. Another approach to estimate muscle forces uses electromyography (EMG) to approximate the neural command and a musculoskeletal model to estimate muscle forces (Buchanan et al., 2004, 2005; Lloyd and Besier, 2003). This scheme is appealing to investigate pathological conditions, such as patellofemoral pain, because it accounts for individual muscle recruitment patterns and co-contraction strategies (Lloyd and Besier, 2003).
The purpose of this study was to use an EMG-driven musculoskeletal model of the knee to estimate muscle forces during walking and running in a group of male and female patients with patellofemoral pain and compare these forces with a group of pain-free control subjects. It was hypothesized that the relative contribution of the vastus medialis muscle would be less in the patellofemoral pain group compared to pain-free controls during walking and running. We also expected subjects to utilize similar quadriceps activation strategies during walking and running, hence the relative contribution of the quadriceps to the net knee moment during walking was hypothesized to be the same as during running.
Methods
Subjects
Twenty-seven individuals with patellofemoral pain (16 female; 11 male) and 16 pain-free controls (8 female; 8 male) participated in this study (Table 1). Patients were diagnosed with patellofemoral pain by a single physician and were accepted into the study if they had pain originating from the patellar region and reproducible pain with at least two of the following functional activities; stair ascent or descent, squatting, kneeling, prolonged sitting, or isometric quadriceps contraction (Brechter and Powers, 2002). Four of the 27 patients presented with bilateral pain. Subjects were excluded if they showed signs of patella tendonitis, or if they reported having previous history of knee surgery, history of traumatic patellar dislocation, or any neurological involvement that would influence gait. None of the patients in this study demonstrated any evidence of knee ligamentous laxity, which was evaluated using standard orthopaedic tests (Reider, 1999).
Table 1.
Mean ± SD age, height, and body mass of subjects.
| Controls | Patellofemoral Pain | |||
|---|---|---|---|---|
| Males (n=8) | Females (n=8) | Males (n=11) | Females (n=16) | |
| Age (yrs) | 27.2 ± 3.0 | 28.8 ± 4.7 | 30.5 ± 4.5 | 28.7 ± 4.6 |
| Height (m) | 1.79 ± 0.07 | 1.66 ± 0.05 | 1.78 ± 0.09 | 1.68 ± 0.06 |
| Mass (kg) | 74.2 ± 4.2 | 58.3 ± 4.6 | 72.4 ± 12.5 | 62.7 ± 10.0 |
The pain-free control group was also screened to ensure that no subjects had previous traumatic injury or knee pathology and could perform the activities described above without pain. Patellofemoral pain subjects completed an Anterior Knee Pain questionnaire (Kujala et al., 1993) to evaluate subjective symptoms and functional limitations of their pain.
Data Collection
Subjects performed at least three trials of walking and running at a self-selected pace and squatting in a motion analysis laboratory. Retro-reflective markers were placed on lower limb landmarks (Kadaba et al., 1990) and three-dimensional marker trajectories measured at 60 Hz using a 6-camera motion capture system (Motion Analysis Corporation, Santa Rosa, CA). Ground reaction forces and electromyographic signals were simultaneously measured at 2400 Hz from a force plate (Bertec Corporation, Columbus, OH) and 16-channel EMG system (MotionLab Systems, Baton Rouge, LA). Surface EMG electrodes were placed on seven muscles crossing the knee: vastus medialis (vasmed), vastus lateralis (vaslat), rectus femoris (rf), medial hamstrings (semimem), lateral hamstrings (bifemlh), medial gastrocnemius (medgas) and lateral gastrocnemius (latgas). For the patellofemoral pain patients, EMG data were taken from the symptomatic, or most painful knee. For the control subjects, the selected knee for EMG data was chosen at random. Subjects performed maximum isometric muscle contractions to elicit maximum activation of knee extensors, knee flexors, and ankle plantar flexors. To perform maximum isometric contractions of the knee extensors and flexors, subjects sat on a chair with the knee at ~80 degrees of flexion and were instructed to extend or flex their knee against the resistance of the tester. Maximum isometric ankle plantar flexion was determined for each subject during a single-leg calf raise with the knee near full extension. Marker trajectories and force plate data were low-pass filtered using a zero-lag fourth-order Butterworth filter with a cut-off frequency of 15 Hz. Spatiotemporal gait parameters including walking and running speed, strike lengths, and cadence were calculated from marker trajectories. Standard Newton-Euler inverse dynamics calculations were performed (Crowninshield and Brand, 1981) using custom-written Matlab code to calculate lower limb joint kinematics and kinetics.
EMG-Driven Musculoskeletal Model
The EMG-driven musculoskeletal model used raw EMG and joint kinematics to estimate individual muscle forces and joint moments (Buchanan et al., 2005; Lloyd and Besier, 2003). The model consisted of: 1) an Anatomical Model to estimate muscle-tendon lengths and moment arms, 2) an EMG-to-Activation Model to represent muscle activation dynamics, 3) a Hill-type Muscle Model to account for muscle-tendon dynamics and estimate force in the muscle-tendon unit, and 4) a Calibration Process to obtain a set of subject-specific model parameters.
A generic musculoskeletal model of the lower limb (Delp et al., 1990) was scaled using Software for Interactive Musculoskeletal Modeling (Delp and Loan, 1995) to fit each subjects’ anthropometry using data from a static motion capture trial. The musculoskeletal model included 10 muscle-tendon actuators: semimem, semiten, bifemlh, biceps femoris short head (bifemsh), vaslat, vasmed, vastus intermedius (vasint), rf, medgas, and latgas. Tensor fascia latae, gracilis, sartorius, plantaris and popliteus were not included in this model due to their small physiological cross-sectional areas. Hip, knee, and ankle kinematics were used as input to the model to determine muscle-tendon lengths and flexion-extension moment arms for each of the tasks performed. Only the flexion-extension moment arms were necessary for this analysis, as the generation of flexion-extension moments at the knee is provided almost entirely by muscles, thus enabling a valid comparison between the models’ prediction of the net joint moment and that calculated from inverse dynamics.
Raw EMG data were high-pass filtered using a zero-lag fourth-order recursive Butterworth filter (30 Hz) and full wave rectified and filtered using a Butterworth low-pass filter (6 Hz). Muscle activity was then normalized to the maximum contraction values for each muscle and input to a recursive filter to account for electromechanical delay, activation dynamics, and tissue filtering characteristics (Lloyd and Besier, 2003). A single-parameter model was used to estimate the neural activation, taking into account the potential nonlinearities between EMG and muscle force (Manal and Buchanan, 2003). Activation was determined from 7 of the 10 muscles for which EMG data was collected. Activation of vasint was estimated as an average of the vasmed and vaslat; semiten activation was assumed to be the same as semimem (Lloyd and Buchanan, 1996) and bifemsh activation was assumed to be the same as bifemlh.
Muscle-tendon lengths and activations were input to a modified Hill-type muscle model to estimate muscle force (Lloyd and Besier, 2003). Tendon was modeled using a non-linear function, normalized to slack length and FMAX (Zajac, 1989). Muscle forces were multiplied by their respective flexion-extension moment arms and summed to determine the net knee joint flexion-extension moment.
Muscle and activation parameters (n=14) were calibrated to an individual using an optimization routine to minimize the difference between the joint moment estimated by the EMG-driven model and the moment calculated from inverse dynamics for three calibration trials (walk, run, and static squat). Three strength parameters were used to scale the FMAX values of the quadriceps, hamstrings, and gastrocnemius muscle groups between 0.5 and 2.0 times their initial scaled values. The nonlinear activation parameter, A, was also allowed to vary between 0 and 0.12 for each muscle (Manal and Buchanan, 2003), and a global electromechanical delay term was used to improve temporal synchronization.
Following calibration, further walking and running trials were predicted by the model without adjusting the muscle or activation parameters. Model predictions were compared to inverse dynamics moments using a squared Pearson product-moment correlation (R2). Muscle forces were normalized by the scaled value of FMAX for each muscle to facilitate the comparison of forces between individuals of different size. Force and moment data were also time normalized to 100 points across the stance phase of gait using a cubic spline. A co-contraction index was determined using the normalized filtered EMG data for the quadriceps and hamstring muscles (Besier et al., 2003) and the distribution of quadriceps muscle forces to produce the net extension moment was calculated and expressed as a percentage.
Statistical Analysis
The following variables were calculated for statistical analysis: spatiotemporal gait parameters – walking and running speed, stride length, and cadence; knee joint moments - peak net knee extension moment; muscle forces – peak normalized muscle forces and average normalized muscle forces at heel strike, weight acceptance, and peak push off; co-contraction index and quadriceps force distribution at heel strike, weight acceptance, and peak push off. Heel strike was defined as the value at 0% of the stance phase. Weight acceptance was defined as the first 15% of the stance phase and peak push off was the mean value 5% on either side of the peak knee extension moment. These variables were compared between male and female patellofemoral pain subjects and pain-free controls using separate two-way ANOVA’s (gender x pain) for walking and running data. To compare quadriceps muscle force distribution between walking and running, a two-way ANOVA was also performed (walk-run x pain). Scheffé post-hoc tests were used to determine significant interactions when main effects were present.
Results
Spatiotemporal Gait Characteristics
Patellofemoral pain subjects walked and ran with similar speed, stride length, and cadence to the control group (Table 2). Males had greater stride length and lower cadence compared to females during walking and running (p=0.015). However, when normalized by height, speeds and stride lengths were similar across all subjects.
Table 2.
Mean ± SD spatiotemporal data for all subjects during walking and running.
| Walking | gender | Speed (m/sec) | Stride Length (m) | Cadence (steps/min) | Normalized Speed (speed/ht) | Normalized Stride Length (stride length/ht) |
|---|---|---|---|---|---|---|
| Controls | male | 1.49 ± 0.12 | 1.61 ± 0.13 | 112 ± 6 | 0.83 ± 0.06 | 0.90 ± 0.07 |
| female | 1.43 ± 0.15 | 1.47 ± 0.15 | 117 ± 6 | 0.86 ± 0.08 | 0.88 ± 0.07 | |
| Patellofemoral Pain | male | 1.52 ± 0.14 | 1.61 ± 0.12 | 113 ± 8 | 0.85 ± 0.11 | 0.91 ± 0.07 |
| female | 1.52 ± 0.20 | 1.52 ± 0.15 | 120 ± 7 | 0.90 ± 0.11 | 0.91 ± 0.09 | |
| Running | ||||||
| Controls | male | 2.67 ± 0.29 | 2.22 ± 0.19 | 146 ± 5 | 1.49 ± 0.14 | 1.24 ± 0.10 |
| female | 2.64 ± 0.36 | 2.04 ± 0.37 | 158 ± 9 | 1.59 ± 0.20 | 1.23 ± 0.21 | |
| Patellofemoral Pain | male | 2.65 ± 0.23 | 2.14 ± 0.13 | 150 ± 11 | 1.49 ± 0.17 | 1.20 ± 0.09 |
| female | 2.65 ± 0.28 | 2.00 ± 0.19 | 161 ± 9 | 1.58 ± 0.17 | 1.19 ± 0.13 | |
Pain Scores
Based on the Anterior Knee Pain Scale (Kujala et al., 1993), more than half of the patellofemoral pain subjects (56%) reported no pain or discomfort during walking, while 44% of subjects indicated they would experience pain after walking more than 2 km in distance. Approximately 90% of the patellofemoral pain subjects reported having pain during running and the mean total Anterior Knee Pain Score for the patellofemoral pain group was 70 ± 10.
Knee Joint Moments
Patellofemoral pain subjects produced similar flexion-extension moments at the knee as the pain-free control subjects during walking (Figure 1a). Knee moments during walking were similar between males and females with patellofemoral pain; however, female control subjects produced ~40% less net knee extension moment than the male controls during walking (Figure 1b, p=0.0025). During running, the patellofemoral pain subjects produced 13% less peak knee extension moment compared to pain-free controls (Figure 1c, p=0.041). Females produced less knee extension moment compared to males during running (1.9 Nm/kg compared with 2.2 Nm/kg, respectively, p=0.009). Post-hoc analysis revealed that the difference between males and females was significant for the patellofemoral pain subjects (females < males; Figure 1d, p=0.029), but not for the control group (Figure 1d).
Figure 1.
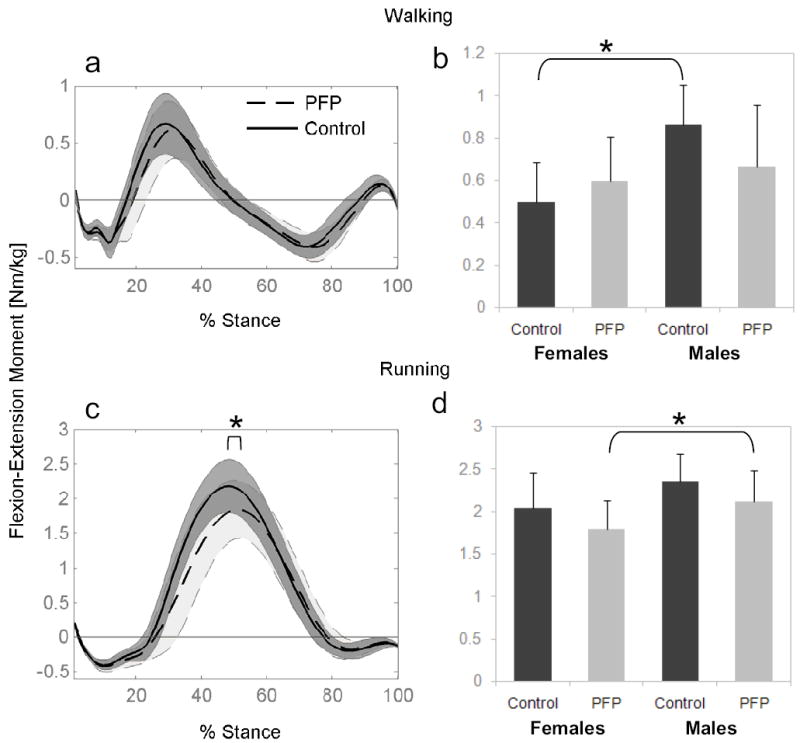
Mean knee flexion-extension moment (± one SD - shaded region) estimated using inverse dynamics during walking (a) and running (c) for Control and Patellofemoral Pain (PFP) subjects. Internal extension moment is positive. Peak extension moments are also shown for males and females during walking (b) and running (d). * indicates difference in peak moments between groups (p<0.05).
Normalized Muscle Forces
The EMG-driven model predicted knee flexion-extension moments close to those calculated from inverse dynamics (walking: R2=0.81 ± 0.09 and running: R2=0.89 ± 0.07). Muscle parameters following scaling and calibration are shown in Table 3. Absolute muscle forces during walking and running are provided as supplemental data. Differences in muscle activation strategies during walking resulted in the variation in normalized muscle forces in pain-free controls and patellofemoral pain groups (Figure 2). Patellofemoral pain subjects produced ~30% greater normalized peak forces in vastus lateralis (p=0.032) and vastus intermedius (p=0.044) compared with the control group. Normalized hamstring muscle forces were also greater in the patellofemoral pain subjects during walking compared with the control group. Peak semitendinosus force was 25% greater in the pain group, compared to the controls (p=0.044) and semimembranosus force at weight acceptance was 20% greater in the pain group, compared to control (p=0.042). Patellofemoral pain subjects also produced 33% greater peak force in the medial gastrocnemius muscle during walking compared with the control group (p<0.001).
Table 3.
Mean [SD] muscle parameters following scaling of the generic musculoskeletal model and calibration of the tendon slack lengths.
| Muscle | Optimal Fiber Length (cm) | Tendon Slack Length (cm) | Fmax (N) | Pennation Angle (deg) |
|---|---|---|---|---|
| Semimembranosus | 9.26 [4.55] | 35.65 [4.64] | 1104.4 [70.6] | 15 |
| Semitendinosus | 21.36 [1.38] | 27.17 [1.8] | 348.61 [22.5] | 5 |
| Biceps Femoris Long Head | 11.64 [0.74] | 35.39 [2.27] | 765.47 [48.4] | 0 |
| Biceps Femoris Short Head | 18.37 [1.12] | 12.22 [0.79] | 426.8 [26] | 23 |
| Rectus Femoris | 9.21 [0.51] | 38.96 [2.23] | 854.09 [47.2] | 5 |
| Vastus Medialis | 9.73 [0.57] | 13.66 [0.81] | 1415.4 [83] | 5 |
| Vastus Intermedius | 9.55 [0.56] | 14.91 [0.88] | 1498.4 [87.7] | 3 |
| Vastus Lateralis | 9.17 [0.54] | 17.09 [1.01] | 2043 [119.9] | 5 |
| Medial Gastrocnemius | 4.50 [0.33] | 39.53 [2.91] | 1112.2 [80.6] | 17 |
| Lateral Gastrocnemius | 6.40 [0.46] | 37.66 [2.77] | 487.86 [35.3] | 8 |
Calibrated parameters for electromechanical delay (149 ± 28 ms), strength coefficient for quadriceps (0.98 ± 0.32), hamstrings (1.21 ± 0.42), and gastrocnemius (1.56 ± 0.27).
Figure 2.
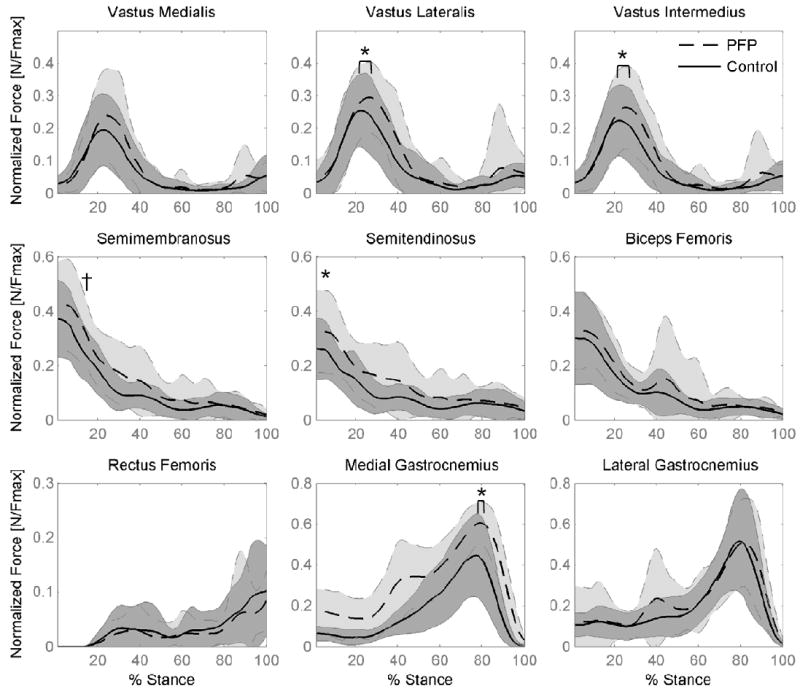
Mean muscle forces for pain-free Control (solid line) and Patellofemoral Pain subjects (PFP – dashed line) during stance phase of walking (shaded region = one SD). Forces are normalized by the maximum isometric muscle force (Fmax) for each muscle. * indicates a difference between PFP and pain-free control group in peak force (p < 0.05). † indicates a difference between PFP and pain-free control group at weight acceptance.
Gender effects were significant for several muscles during the early stages of stance phase during walking. Females produced greater peak normalized hamstring and gastrocnemius muscle forces compared to males (semiten 35%↑, p=0.006; bifem 29%↑, p=0.034; medgas 29%↑, p<0.001; latgas 52%↑, p<0.001), and hamstring muscle forces remained higher in the females through to weight acceptance (15% stance) compared with males (semimem 23%↑, p=0.02; semiten 36%↑, p=0.003; bifem 20%↑, p=0.021).
Muscle forces predicted for running showed large standard deviations within patellofemoral pain and control groups (Figure 3). When normalized by FMAX, there were fewer differences between pain and control groups compared to walking. Only the peak medial gastrocnemius force was different between groups, being 30% greater in the patellofemoral pain group compared to controls (p < 0.002). During peak push off of running, gender differences were similar to walking, with females producing greater normalized hamstring and gastrocnemius muscle forces compared to males (semimem 47%↑, p=0.049; semiten 53%↑, p=0.029; medgas 33%↑, p=0.046; latgas 51%↑, p=0.004).
Figure 3.
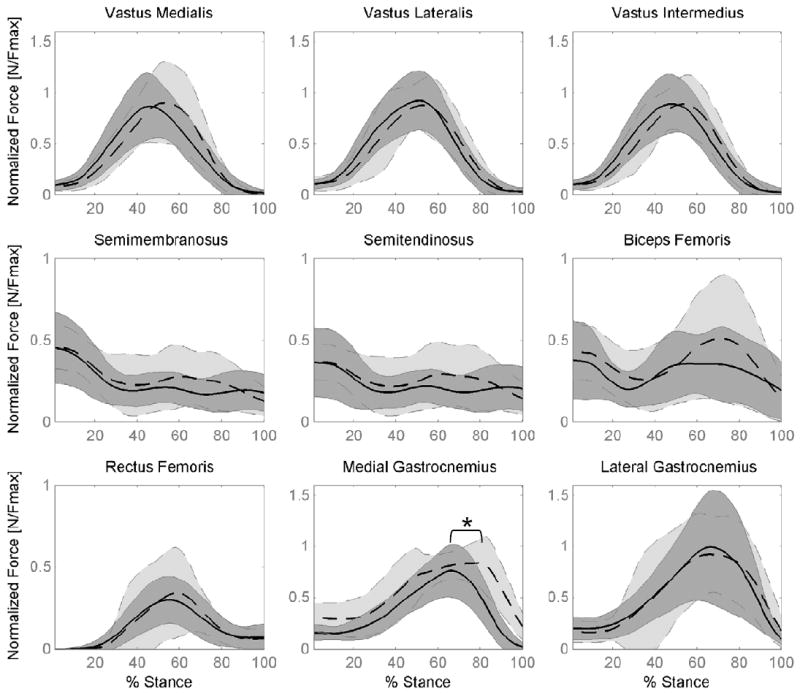
Mean muscle forces for pain-free Control (solid line) and Patellofemoral Pain subjects (PFP – dashed line) during stance phase of running (shaded region = one SD). Forces are normalized by the maximum isometric muscle force (Fmax) for each muscle. * indicates difference in peak force between PFP and pain-free control group (p < 0.05).
Co-contraction Index
The patellofemoral pain group had significantly greater co-contraction of quadriceps and hamstrings at heel strike during walking, indicated by the co-contraction index (0.14 vs. 0.09, p=0.025, Figure 4). This corresponded with the increased peak hamstring muscle forces in the pain group. Qualitatively, the level of co-contraction during running remained higher throughout the stance phase compared to walking (up until 60% of the stance phase).
Figure 4.
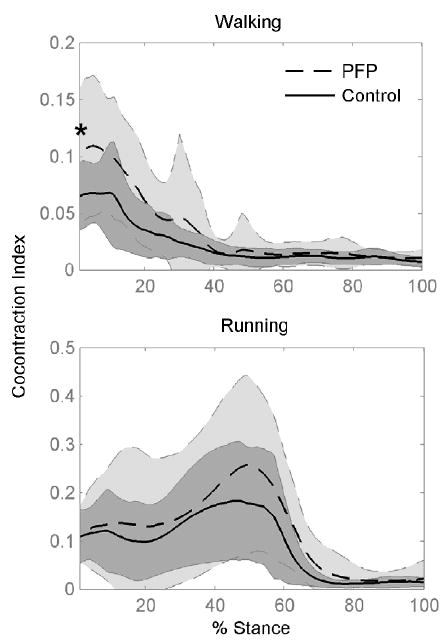
Relative co-contraction of quadriceps and hamstring muscles for pain-free Control and Patellofemoral Pain (PFP) subjects during walking and running. Co-contraction index was calculated as the product of the ratio of quadriceps-to-hamstrings muscle activity and the net activation of these muscles. Shaded regions show one standard deviation for each group. * indicates difference at heel strike between PFP and pain-free control group (p =0.025).
Quadriceps Force Distribution
Patellofemoral pain and control subjects displayed similar contributions from each of the quadriceps muscles at heel strike, weight acceptance and at peak push off during both walking and running (Figure 5). Across stance, there was greater variation in the contributions from each quadriceps muscle during walking (Figures 5a and b) compared with running (Figures 5c and d). Until mid-stance, vastus lateralis provided the largest contribution (~45 ± 10%), followed by vastus intermedius (~29 ± 3%), and vastus medialis (~26 ± 7%). The contribution from vastus medialis increased from 21% during walking to 26% during running at peak push off (p=0.002). Conversely, the contribution from vastus lateralis decreased from 45% during walking to 39% during running at peak push off (p=0.002). Rectus femoris was responsible for only ~5 ± 2% of the total knee extension moment at peak push off and had little contribution during weight acceptance.
Figure 5.
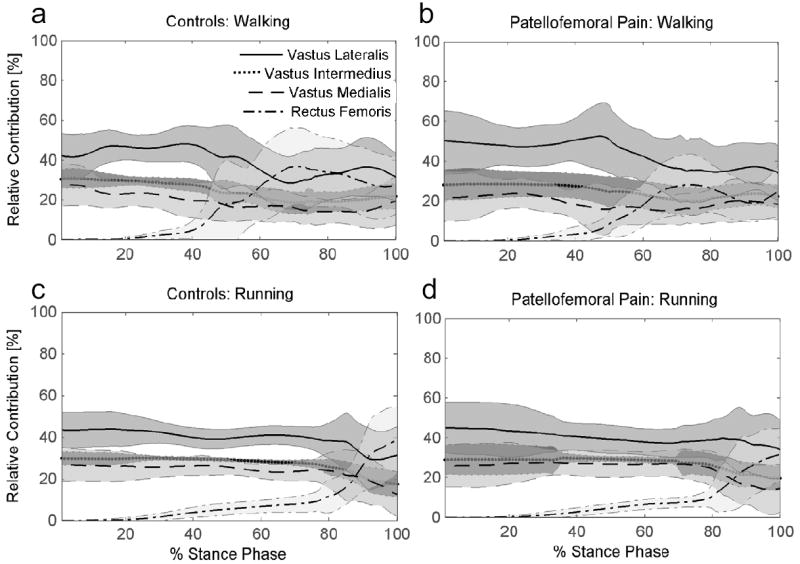
Relative contributions of the quadriceps muscles to the net quadriceps moment during walking and running for pain-free Controls and patients with Patellofemoral Pain.
Discussion
The purpose of this study was to estimate muscle forces during walking and running in a group of patellofemoral pain patients and a group of pain-free subjects. We hypothesized that the relative contribution of the vastus medialis would be less in the patellofemoral pain group compared to pain-free subjects during walking and running. Our results do not support this hypothesis, suggesting that subjects with patellofemoral pain produce a knee extension moment using the same distribution of quadriceps forces as pain-free individuals during walking and running. We also hypothesized that the relative contribution from the quadriceps during walking would be similar to running. This was found to be the case during early stance; however, vastus medialis increased its contribution and vastus lateralis decreased its contribution from walking to running at peak push off.
Using EMG as input to a musculoskeletal model revealed two important findings. Firstly, although the distribution of quadriceps muscle forces was similar between groups, patellofemoral pain subjects had greater co-contraction of hamstring and quadriceps muscles and greater normalized muscle forces during walking compared to pain-free subjects. Secondly, the normalized muscle forces during running in the patellofemoral pain group were similar to those in the pain-free group, even though the patellofemoral pain group produced a net knee extension moment that was less than the controls. It is not known whether these muscle force distributions are an adaptation to pain or if they are causative, but one could argue that increased co-contraction around heel strike might improve knee joint stability and help to align the patella within the trochlear groove. On the other hand, increased muscle forces during peak push off would have a detrimental effect of increasing joint contact forces.
Compared to males, females in this study displayed greater normalized hamstring and gastrocnemius muscle forces during both walking and running. Females have smaller medio-lateral knee dimensions (Conley et al., 2007) and less volitional torsional stiffness compared to males (Wojtys et al., 2003) and might compensate for this mechanical disadvantage by increasing force production in medial and lateral hamstrings and gastrocnemius. As a consequence, females may also experience greater normalized joint contact forces compared to males, which could account for the increased incidence of patellofemoral pain in females. To examine the relationship between muscles forces, joint geometry, contact forces and tissue stresses, muscle forces from this study will be used as input to subject-specific finite element models of the patellofemoral joint (Besier et al., 2005).
Much attention is given to the role of the oblique fibers of the vastus medialis in stabilizing the patella because of their potential to displace the patella medially near full knee extension. Vastus medialis has a bipartite nerve supply capable of separately innervating the upper lateral portion and the middle and lower portion of the muscle (Thiranagama, 1990). However, whether these oblique fibers are activated independently during functional activities is debated. In the current study, EMG electrodes were placed over the middle portion of the vastus medialis to measure the activation of the largest portion of muscle fibers. Contrary to our hypothesis, we did not see any differences between patellofemoral pain patients and pain-free controls in the relative contribution from the vastus medialis and vastus lateralis to the knee moment during walking or running. These findings support EMG studies that have shown the quadriceps to act in concert to produce knee extension (Basmajian et al., 1972; Lieb and Perry, 1971), and this strategy does not appear to be altered in patients who experience patellofemoral pain. Zhang et al. (2003) investigated load sharing among the quadriceps during submaximal isometric tasks and found the vastus intermedius to contribute the most to the total knee extension moment, followed by vastus lateralis, rectus femoris, and vastus medialis. Direct comparison to these data is difficult because the current study did not use fine-wire electrodes to estimate the activation of vastus intermedius. However, the consistent contribution of the quadriceps muscles to produce a net knee extension moment during both walking and running support the conclusion made by Zhang et al. (2003) that the quadriceps must act in a coordinated fashion to balance ‘off-axis’ moments and forces produced from the complex arrangement of the individual muscle fibers. During running, when large knee extension moments were generated, vastus medialis increased its contribution and vastus lateralis reduced its contribution compared to walking.
The current model did not include several smaller muscles that crossed the knee, such as tensor fascia latae, gracilis, or sartorius. These muscles have small physiological cross sectional area compared to the quadriceps, hamstrings, and gastrocnemius muscles, so they do not contribute as much to the knee flexion-extension moment. However, these muscles have moment arms that support varus-valgus rotations and have been shown to be selectively activated to support these moments (Lloyd and Buchanan, 2001). Although the aim of this study was to investigate the main contributors to the flexion-extension moment, patients with patellofemoral pain may also demonstrate adaptations in these smaller muscles to support varus-valgus moments at the knee.
Several clinical hypotheses can be derived from this study. Patients with patellofemoral pain might be experiencing pain from increased joint contact forces due to co-contraction of quadriceps and hamstring muscles and greater normalized muscle forces during walking and running. Females may be more prone to patellofemoral pain and long-term osteoarthritis due to increased muscle force production during walking and running compared to males. Interventions designed to reduce co-contraction such as machine-based weight training or EMG biofeedback might be effective in reducing joint contact forces and pain in these subjects. Future work will investigate how the differences in muscle forces found in this study will influence cartilage and joint stresses.
Acknowledgments
Financial support for this study was obtained from the Department of Veterans Affairs, Rehabilitation R&D Service (grant # A2592R), the National Institute of Health (1R01-EB005790 and U54 GM072970), and Stanford Regenerative Medicine (1R-90DK071508).
Footnotes
Conflict of Interest Statement The authors do not have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker CL., Jr Lower extremity problems in female athletes. J Med Assoc Ga. 1997;86:193–196. [PubMed] [Google Scholar]
- Basmajian JV, Harden TP, Regenos EM. Integrated actions of the four heads of quadriceps femoris: An electromyographic study. Anat Rec. 1972;172:15–20. doi: 10.1002/ar.1091720102. [DOI] [PubMed] [Google Scholar]
- Besier TF, Gold GE, Beaupre GS, Delp SL. A modeling framework to estimate patellofemoral joint cartilage stress in vivo. Med Sci Sports Exerc. 2005;37:1924–1930. doi: 10.1249/01.mss.0000176686.18683.64. [DOI] [PubMed] [Google Scholar]
- Besier TF, Lloyd DG, Ackland TR. Muscle activation strategies at the knee during running and cutting maneuvers. Med Sci Sports Exerc. 2003;35:119–127. doi: 10.1097/00005768-200301000-00019. [DOI] [PubMed] [Google Scholar]
- Blond L, Hansen L. Patellofemoral pain syndrome in athletes: A 5.7-year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64:393–400. [PubMed] [Google Scholar]
- Brechter JH, Powers CM. Patellofemoral joint stress during stair ascent and descent in persons with and without patellofemoral pain. Gait Posture. 2002;16:115–123. doi: 10.1016/s0966-6362(02)00090-5. [DOI] [PubMed] [Google Scholar]
- Buchanan TS, Lloyd DG, Manal K, Besier TF. Neuromusculoskeletal modeling: Estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech. 2004;20:367–395. doi: 10.1123/jab.20.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TS, Lloyd DG, Manal K, Besier TF. Estimation of muscle forces and joint moments using a forward-inverse dynamics model. Med Sci Sports Exerc. 2005;37:1911–1916. doi: 10.1249/01.mss.0000176684.24008.6f. [DOI] [PubMed] [Google Scholar]
- Conley S, Rosenberg A, Crowninshield R. The female knee: Anatomic variations. J Am Acad Orthop Surg. 2007;15(Suppl 1):S31–36. doi: 10.5435/00124635-200700001-00009. [DOI] [PubMed] [Google Scholar]
- Cowan SM, Bennell KL, Hodges PW, Crossley KM, McConnell J. Simultaneous feedforward recruitment of the vasti in untrained postural tasks can be restored by physical therapy. J Orthop Res. 2003;21:553–558. doi: 10.1016/S0736-0266(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Crowninshield RD, Brand RA. A physiologically based criterion of muscle force prediction in locomotion. J Biomech. 1981;14:793–801. doi: 10.1016/0021-9290(81)90035-x. [DOI] [PubMed] [Google Scholar]
- Crowninshield RD, Brand RA. The prediction of forces in joint structures: Distribution of intersegmental resultants. In: Miller DI, editor. Exercise and sport science reviews. Vol. 9. Philadelphia: Franklin Institute Press; 1981. pp. 159–181. [PubMed] [Google Scholar]
- DeHaven KE, Lintner DM. Athletic injuries: Comparison by age, sport, and gender. Am J Sports Med. 1986;14:218–224. doi: 10.1177/036354658601400307. [DOI] [PubMed] [Google Scholar]
- Delp SL, Loan JP. A graphics-based software system to develop and analyze models of musculoskeletal structures. Computers in Biology & Medicine. 1995;25:21–34. doi: 10.1016/0010-4825(95)98882-e. [DOI] [PubMed] [Google Scholar]
- Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Transactions on Biomedical Engineering. 1990;37:757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- Devereaux MD, Lachmann SM. Patello-femoral arthralgia in athletes attending a sports injury clinic. Br J Sports Med. 1984;18:18–21. doi: 10.1136/bjsm.18.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher YY, Kahn LE. The effect of vastus medialis forces on patello-femoral contact: A model-based study. J Biomech Eng. 2002;124:758–767. doi: 10.1115/1.1516196. [DOI] [PubMed] [Google Scholar]
- Elias JJ, Bratton DR, Weinstein DM, Cosgarea AJ. Comparing two estimations of the quadriceps force distribution for use during patellofemoral simulation. J Biomech. 2006;39:865–872. doi: 10.1016/j.jbiomech.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Elias JJ, Cech JA, Weinstein DM, Cosgrea AJ. Reducing the lateral force acting on the patella does not consistently decrease patellofemoral pressures. Am J Sports Med. 2004;32:1202–1208. doi: 10.1177/0363546503262167. [DOI] [PubMed] [Google Scholar]
- Farahmand F, Senavongse W, Amis AA. Quantitative study of the quadriceps muscles and trochlear groove geometry related to instability of the patellofemoral joint. J Orthop Res. 1998;16:136–143. doi: 10.1002/jor.1100160123. [DOI] [PubMed] [Google Scholar]
- Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. The American Journal of Sports Medicine. 2002;30:447–456. doi: 10.1177/03635465020300032501. [DOI] [PubMed] [Google Scholar]
- Fulkerson JP, Shea KP. Disorders of patellofemoral alignment. J Bone Joint Surg Am. 1990;72:1424–1429. [PubMed] [Google Scholar]
- Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8:383–392. doi: 10.1002/jor.1100080310. [DOI] [PubMed] [Google Scholar]
- Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9:159–163. doi: 10.1016/s0749-8063(05)80366-4. [DOI] [PubMed] [Google Scholar]
- Lee TQ, Sandusky MD, Adeli A, McMahon PJ. Effects of simulated vastus medialis strength variation on patellofemoral joint biomechanics in human cadaver knees. J Rehabil Res Dev. 2002;39:429–438. [PubMed] [Google Scholar]
- Lieb FJ, Perry J. Quadriceps function. An anatomical and mechanical study using amputated limbs. J Bone Joint Surg Am. 1968;50:1535–1548. [PubMed] [Google Scholar]
- Lieb FJ, Perry J. Quadriceps function. An electromyographic study under isometric conditions. J Bone Joint Surg Am. 1971;53:749–758. [PubMed] [Google Scholar]
- Lin F, Wang G, Koh JL, Hendrix RW, Zhang LQ. In vivo and noninvasive three-dimensional patellar tracking induced by individual heads of quadriceps. Med Sci Sports Exerc. 2004;36:93–101. doi: 10.1249/01.MSS.0000106260.45656.CC. [DOI] [PubMed] [Google Scholar]
- Lloyd DG, Besier TF. An emg-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J Biomech. 2003;36:765–776. doi: 10.1016/s0021-9290(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Lloyd DG, Buchanan TS. A model of load sharing between muscles and soft tissues at the human knee during static tasks. J Biomech Eng. 1996;118:367–376. doi: 10.1115/1.2796019. [DOI] [PubMed] [Google Scholar]
- Lloyd DG, Buchanan TS. Strategies of muscular support of varus and valgus isometric loads at the human knee. J Biomech. 2001;34:1257–1267. doi: 10.1016/s0021-9290(01)00095-1. [DOI] [PubMed] [Google Scholar]
- Manal K, Buchanan TS. A one-parameter neural activation to muscle activation model: Estimating isometric joint moments from electromyograms. J Biomech. 2003;36:1197–1202. doi: 10.1016/s0021-9290(03)00152-0. [DOI] [PubMed] [Google Scholar]
- Powers CM, Lilley JC, Lee TQ. The effects of axial and multi-plane loading of the extensor mechanism on the patellofemoral joint. Clin Biomech (Bristol, Avon) 1998;13:616–624. doi: 10.1016/s0268-0033(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Reider B. The orthopaedic physical examination. 1. Elsevier; Philadelphia, PA: 1999. Pages. [Google Scholar]
- Seireg A, Arvikar RJ. A mathematical model for evaluation of forces in lower extremeties of the musculo-skeletal system. J Biomech. 1973;6:313–326. doi: 10.1016/0021-9290(73)90053-5. [DOI] [PubMed] [Google Scholar]
- Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36:95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiranagama R. Nerve supply of the human vastus medialis muscle. J Anat. 1990;170:193–198. [PMC free article] [PubMed] [Google Scholar]
- Thomee R, Renstrom P, Karlsson J, Grimby G. Patellofemoral pain syndrome in young women. I. A clinical analysis of alignment, pain parameters, common symptoms and functional activity level. Scand J Med Sci Sports. 1995;5:237–244. [PubMed] [Google Scholar]
- Wojtys EM, Huston LJ, Schock HJ, Boylan JP, Ashton-Miller JA. Gender differences in muscular protection of the knee in torsion in size-matched athletes. J Bone Joint Surg Am. 2003;85-A:782–789. doi: 10.2106/00004623-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Zajac FE. Muscle and tendon: Properties, models, scaling, and application to biomechanics and motor control. Critical Reviews in Biomedical Engineering. 1989;17:359–411. [PubMed] [Google Scholar]
- Zhang LQ, Wang G, Nuber GW, Press JM, Koh JL. In vivo load sharing among the quadriceps components. J Orthop Res. 2003;21:565–571. doi: 10.1016/S0736-0266(02)00196-1. [DOI] [PubMed] [Google Scholar]


