Abstract
The addition of various polymers to pulmonary surfactants improves surface activity in experiments both in vitro and in vivo. Although the viscosity of surfactants has been investigated, the viscosity of surfactant polymer mixtures has not. In this study, we have measured the viscosities of Survanta and Infasurf with and without the addition of polyethylene glycol, dextran or hyaluronan. The measurements were carried out over a range of surfactant concentrations using two concentrations of polymers at two temperatures. Our results indicate that at lower surfactant concentrations, the addition of any polymers increased the viscosity. However, the addition of polyethylene glycol and dextran to surfactants at clinically used concentrations can substantially lower viscosity. Addition of hyaluronan at clinical surfactant concentrations slightly increased Infasurf viscosity and produced little change in Survanta viscosity. Effects of polymers on viscosity correlate with changes in size and distribution of surfactant aggregates and the apparent free volume of liquid as estimated by light microscopy. Aggregation of surfactant vesicles caused by polymers may therefore not only improve surface activity as previously shown, but may also affect viscosity in ways that could improve surfactant distribution in vivo.
Keywords: pulmonary surfactant, viscosity, dextran, polyethylene glycol, hyaluronan
Introduction
The viscosity of lung surfactant is dependent on its molecular composition, its microstructure, the interactions between components and the environmental conditions. Viscosity is believed to influence the rate, extent and uniformity of distribution of surfactant in the lungs [1-3]. The early stage of surfactant distribution, occurring in the upper one-third of approximately 18 generations or bifurcations of the airway system, is rapid and controlled mainly by surfactant bulk viscosity. A lower value allows a more uniform and rapid distribution of the instilled surfactant with less initial loss due to coating of the upper airways [1, 4, 5]. The distribution of surfactant into the small airways and the alveoli, occurring in the remaining dozen or so generations, is controlled by differences in surface tension of various fluids and components in the pulmonary system (Marangoni flow) [5], or by surface viscosity, not by bulk viscosity and therefore not studied.
Our previous work and that of others have shown the benefits in vitro and in vivo of adding an ionic polymer like hyaluronan (HA), or nonionic polymers like polyethylene glycol (PEG) or dextran to exogenous surfactant. Benefits include improvements in surface activity and lung function [6-14]. Although the viscosity of a variety of surfactants has been studied [3, 15], changes in viscosity after addition of polymers have not been investigated. Of concern is whether added polymers would increase viscosity of therapeutic surfactants to the degree that distribution of surfactant in the lung might be affected. For this reason, we measured surfactant viscosity in the presence or absence of the polymers that we have found to be beneficial including those concentrations of surfactant and polymer that may be clinically relevant. In the event that some of these mixtures have lower viscosity than the surfactant itself, then the lowered viscosity may be a contributing factor to the in vivo improvements in lung function previously attributed to enhanced surface activity.
Survanta and Infasurf were selected not only because they are both in widespread clinical use but also because their viscosities differ appreciably, as previously reported [15]. Our aim was to measure and characterize the viscous properties of therapeutic surfactants with and without polymers under a range of conditions. Light microscopy of the different mixtures was carried out to find whether correlations existed between viscosity and the microstructure of surfactant dispersions.
Methods
Materials
Survanta (Ross Laboratories, Columbus, OH) and Infasurf (Forest Laboratories, New York, NY) were obtained through hospital pharmacies. PEG (10kD) and dextran (10kD) were purchased from Sigma Chemical (St. Louis, MO). HA (250kD), produced by streptococci, was obtained from GlycoMed Research, Inc. (Hastings-on-Hudson, NY). The buffer used was 0.9% NaCl with 2.5 mM HEPES and 2.5 mM CaCl2 adjusted to a pH of 7.0.
Procedures
Kinematic viscosity measurements were made with Cannon-Manning semi-microviscometers (State College, PA) sizes 100, 150 and 200. The sizes correspond to different ranges of viscosity with some overlap that were suitable for our measurements. Time required for fluid to drain by gravity through a viscometer tube was measured, and this time was converted to a value of kinematic viscosity using a calibration constant supplied by the manufacturer and verified in our laboratory using standard fluids. The kinematic viscosity can be converted to dynamic (absolute) viscosity by multiplying by the density of the fluid. This method of determining viscosity was chosen because of its relevance to distribution of surfactant in the upper airways that is controlled by gravity as well as by airflow upon inhalation. Measurements were also made using different sized viscometer tubes for surfactant mixtures and no significant differences occurred. This confirms the assumption that the kinematic viscosity varies slowly with shear rate in this regime. The surfactants were used as supplied (Survanta 25mg phospholipid/mL; Infasurf 35mg/mL) or diluted with the buffer defined in Materials above to concentrations of 1.25, 2.5, 5, or 12.5, and for Infasurf, 25mg/mL. The concentrations (w/v), and molecular weights of polymers used were those found effective in previous studies; that is, PEG 10kD, 5%; dextran 10kD, 5%; and HA 250kD, 0.25% [7-9, 16]. The viscosity of mixtures with half these polymer concentrations was also measured. In addition, buffer with higher polymer concentrations (without surfactant) was studied to aid in understanding the dependence of viscosity on free volume of liquid. Polymers in dry powder form were combined with the surfactant at indicated concentrations, then dispersed by Vortex in 5 second bursts until uniform in appearance. Mixtures were maintained at room temperature for approximately 60 minutes, with a brief Vortex again before usage. Measurements were made after the viscometer with sample was placed in a temperature-maintained circulating water jacket for 10 minutes at 23 or 37°C. Three measurements were carried out for each sample mixture and results averaged.
Microscopy
Samples of Infasurf (35 and 12.5mg/mL) and Survanta (25 and 12.5mg/mL) were examined with and without the addition of polymers. Dextran, PEG or HA was added in concentrations of 5%, 5% and 0.25% respectively. Ten microliters of surfactant or surfactant polymer mixture, preincubated for 20 minutes at either 23 or 37°C, were sandwiched between a slide and a cover glass and examined by light microscopy. Photomicrographs were first examined to find whether flocculation was similar to previously reported results with similar polymer-surfactant mixtures studied using freeze fracture electron microscopy or spectroscopy [17, 18]. Images were then processed to improve contrast and converted into two-component (surfactant aggregates and apparent free volume) two-dimensional maps. Five fields per surfactant mixture were processed, measured and averaged to calculate the apparent fraction occupied by aggregates or by free volume using ImageJ Java software (http://rsbweb.nih.gov/ij/). The average size of surfactant aggregates was estimated by measuring the length of at least 50 aggregates taken from a number of fields per suspension.
Experimental data acquisition and analysis
The viscosity measurements and microscopic analysis of surfactant structures were repeated with several independent surfactant batches that showed similar qualitative behavior. Results shown are representative experiments, where different measures are compared for samples from the same surfactant batch, after averaging three different determinations. The data were analyzed by one or two-way repeated measures of variance (ANOVA) using SigmaStat software (SPSS Science Chicago, IL). Comparisons between pairs of groups were done using the Tukey test for multiple comparisons. A p value ≤ 0.05 was considered statistically significant. Errors in the experimental determination of viscosity in low viscosity suspensions (<10 cSt) were of the order of a few percent and are not indicated in the figures.
Results
Viscosity of Infasurf in the absence or presence of polymers
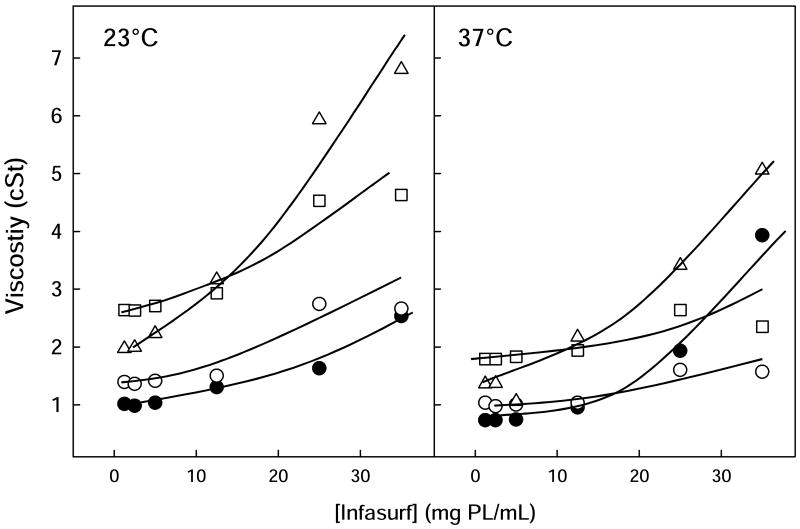
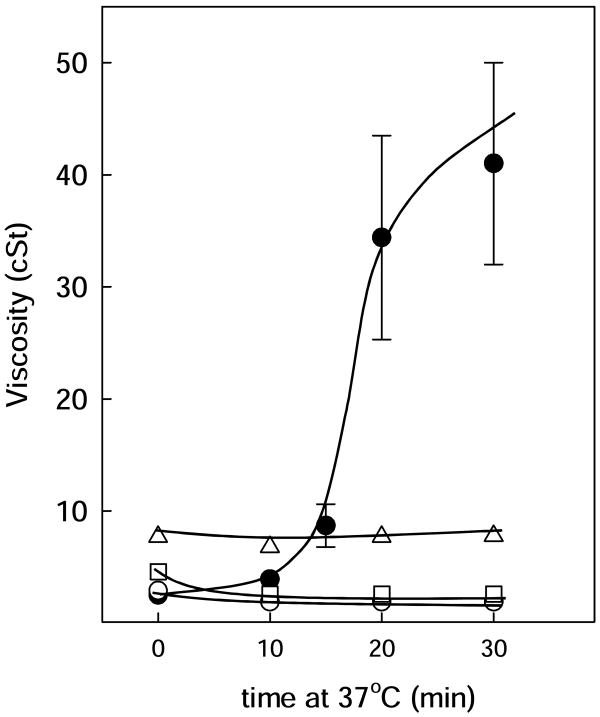
Viscosity of Infasurf was temperature and concentration dependent as shown in fig. 1 and Table 1. At lipid concentrations below 10mg/mL, the viscosity of Infasurf suspensions was only slightly higher than that of the buffer alone (Table 2), but the viscosity increased substantially at the highest concentrations of Infasurf tested, 25 or 35mg/mL. Interestingly, this increase in viscosity was markedly greater at 37 than at 23°C, as was previously noted by King et al. [3, 15]. In fact, the low initial viscosity of Infasurf suspensions at 35mg/mL at 23°C increased significantly with incubation time at 37°C, so that after 20 minutes, the viscosity rose by an order of magnitude to 34 cSt (fig 2).
Figure 1. Viscosity of Infasurf in the absence and presence of polymers.
Viscosity of Infasurf alone (black circles) or with dextran 5% (open circles), PEG 5% (squares) or 0.25% HA (triangles) versus phospholipid concentration, determined at 23°C (left panel) or 37°C (right panel). Lines are visual guides.
Table 1. Viscosity and aggregation state of surfactants with and without polymers.
| Surfactant | Viscosity (cSt) | Aggregation | % apparent free volume | |||
|---|---|---|---|---|---|---|
| 23°C | 37°C | 23°C | 37°C | 23°C | 37°C | |
| Infasurf 35mg/mL | 2.5±0.3 | 3.9±0.5 | + | - | 38±3 | n.d. |
| + Dextran | 2.7±0.1 | 1.6±0.1 | ++ | ++ | 47±3 | 55±6 |
| + PEG | 4.6±0.1 | 2.4±0.1 | +++ | +++ | 63±6 | 62±7 |
| + HA | 6.8±0.1 | 5.1±0.1 | ++ | ++ | 46±5 | 30±5 |
| Survanta 25mg/mL | 92.7±5.4 | 32.5±4 | - | - | <1 | <1 |
| + Dextran | 18.2±0.5 | 12.3±0.1 | +++ | ++ | 29±12 | n.d. |
| + PEG | 6.2±0.1 | 3.9±0.1 | +++ | +++ | 49±11 | 52±6 |
| + HA | 87.8±0.4 | 32.7±0.3 | - | - | <1 | <1 |
Values are means ± s.d. after 3 determinations (viscosity) or averaging 5 frames (free volume) n.d. indicates not determined. More + indicates greater aggregation.
Table 2. Viscosity of polymer-containing media.
| Viscosity (cSt)* | ||
|---|---|---|
| 23°C | 37°C | |
| Buffer | 1.1 | 0.8 |
| + dextran 2.5% | 1.2 | 0.9 |
| + dextran 5% | 1.4 | 1.0 |
| + dextran 10% | 2.6 | 1.9 |
| + dextran 15% | 3.1 | 2.3 |
| + PEG 2.5% | 1.2 | 0.9 |
| + PEG 5% | 2.4 | 1.6 |
| + PEG 10% | 5.1 | 3.8 |
| + PEG 15% | 11.2 | 8.4 |
| + HA 0.125% | 1.7 | 1.2 |
| + HA 0.25% | 2.4 | 1.6 |
values are means using 3 independent measurements, s.d. were in all cases on the order of 1%.
Figure 2. Effect of temperature on the viscosity of Infasurf with and without polymers.
Viscosity of Infasurf alone (black circles) or with dextran 5% (open circles), PEG 5% (squares) or 0.25% HA (triangles) versus time of incubation at 37°C. Time=0 corresponds to 23°C. Lines are visual guides.
When PEG, dextran or HA was added to Infasurf at various concentrations, differing effects were observed. At low surfactant concentrations, addition of polymers increased viscosity to values approximating those of the corresponding polymer-containing buffer as determined in the absence of surfactant (Table 2). Dilute Infasurf with polymer mixtures increased in viscosity in the following order: pure surfactant<dextran<HA<PEG, both at 23 and 37°C with all values quite low. However, at or near concentrations of surfactants used clinically, polymers produced effects on Infasurf viscosity that could not be predicted from the differences in viscosity of the polymer solutions themselves or from dilute Infasurf with polymer mixtures.
At 23°C, 5% dextran or 5% PEG increased the viscosity of the highest concentrations of Infasurf to around 2.5 and 4.5 cSt, respectively, significantly higher than the viscosity measured for a corresponding buffer polymer solution without Infasurf (p<0.001). HA increased viscosity of Infasurf to around 7 cSt for surfactant concentrations of 25 or 35mg/mL, three times higher than the viscosity of HA with buffer (p<0.001). When measurements were made of polymers with highly concentrated Infasurf at 37°C, HA increased the viscosity of Infasurf to around 5 cSt, but PEG and dextran both produced a substantial decrease on measured viscosity, markedly lower than that of Infasurf in the absence of polymers at the same temperature (p<0.001). A striking observation was that the presence of any of the three polymers eliminated the increase of viscosity that occurred in Infasurf after incubation at 37°C (see fig. 2). For example, after 30 minutes incubation of 35mg/mL Infasurf with PEG, dextran or HA, viscosity values were 2.5, 2.0 or 7.5 cSt, respectively compared to 41 cSt for Infasurf alone.
Viscosity of Survanta in the absence or presence of polymers
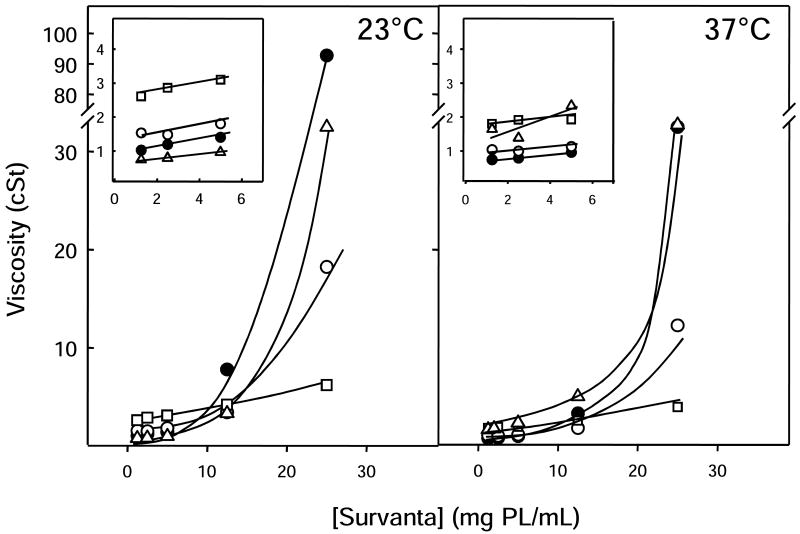
The viscosity of Survanta was highly concentration dependent, with the largest increase occurring between 12.5 and 25mg/mL: at 12.5mg/mL, the viscosity was 8 cSt at 23°C and 3 cSt at 37°C compared to 90 cSt and 32 cSt respectively at 25mg/mL. Survanta had consistently higher viscosity than Infasurf at equal concentration at 23 or 37°C, and at the highest concentrations, the viscosity value of Survanta greatly exceeded that of Infasurf (fig. 3). Also, in contrast to Infasurf, Survanta viscosity at any concentration was always lower at 37°C than at 23°C and did not change with time at either temperature.
Figure 3. Viscosity of Survanta in the absence and presence of polymers.
Viscosity of Survanta alone (black circles) or with dextran 5% (open circles), PEG 5% (squares) or 0.25% HA (triangles) versus phospholipid concentration, determined at 23°C (left panel) or 37°C (right panel). Inserts: rescaling of the plots at low Survanta concentrations. Lines are visual guides.
Addition of polymer to Survanta caused effects on viscosity that depended on the concentration of surfactant also, but were somewhat different than observed for Infasurf. PEG and dextran increased the viscosity of dilute Survanta at 23°C from around 1 to about 1.5 and 2.5 cSt, respectively. However, for concentrations of surfactant above 10mg/mL, the addition of these two polymers produced a considerable reduction of viscosity at 23°C. At 37°C, PEG and dextran had qualitatively similar effects on Survanta viscosity, increasing it slightly at low surfactant concentrations but reducing it markedly (p<0.001) at surfactant concentrations higher than 10mg/mL (fig. 3). When HA was added to dilute Survanta, its viscosity was slightly reduced at 23°C and slightly increased at 37°C, with values at or below 2 cSt. At high Survanta concentrations, such as those used clinically, HA produced little change in viscosity, at either temperature (fig. 3 and Table 1).
When lower concentrations of dextran or PEG (2.5% w/v) were added to either surfactant, the effects on viscosity were qualitatively similar to those found with the higher concentrations used above (data not shown).
Effect of polymers on aggregation of Infasurf and Survanta
Microscopy studies were carried out on Infasurf at 12.5 and 35mg/mL and on Survanta at 12.5 and 25mg/mL. Results at 12.5mg/mL were qualitatively similar to the higher concentrations and are not shown. Figure 4 shows photomicrographs of Infasurf 35mg/mL in the absence or presence of polymers preincubated at 23 or 37°C. Significant differences in aggregation are apparent. At 23°C, Infasurf suspensions were composed of small homogeneously distributed, spherically shaped surfactant aggregates, with an average diameter of 2.1±0.8 μm (see contrast-increased detail in Infasurf picture, fig. 4). These aggregates were completely intermingled and occasionally associated to form dispersed clusters. The apparent free liquid area as projected in two-dimensional pictures (see corresponding two-dimensional map, fig. 4), occupies 35-40% of the total area. These surfactant-free areas are extrapolated into three dimensions and termed free volume.
Figure 4. Microstructure of Infasurf in the absence and presence of polymers.
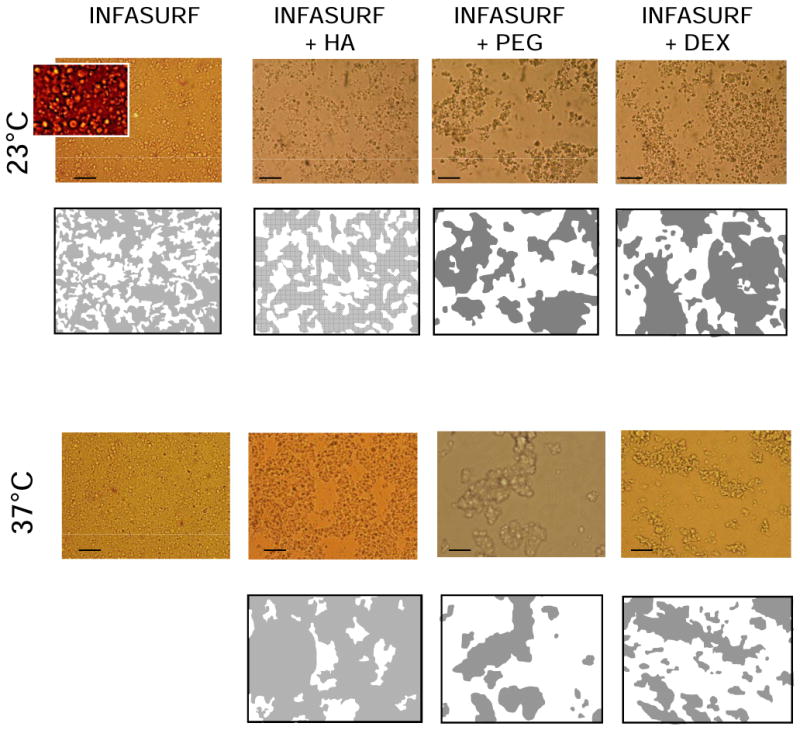
Micrographs of Infasurf suspensions at 35mg/mL, in the absence or presence of dextran 5%, PEG 5% or HA 0.25% preincubated at 23°C (upper pictures) or 37°C (lower pictures). Slides were taken under an optical microscope as described in Methods with scale bars representing 20 μm. Insert in the Infasurf picture was magnified an additional three times and contrast-enhanced to illustrate the aggregate structure of this surfactant. Below the micrographs are two dimensional maps representing the projection of the apparent volume occupied by either surfactant aggregates (grey) or free liquid (white).
On addition of PEG or dextran, Infasurf suspensions became more heterogeneous, with surfactant aggregates forming into large relatively compact clusters of 40±22 or 49±26 μm average dimension producing approximately 65% or 50% free volume respectively (see Table 1). Infasurf in the presence of HA also yielded a partial clustering of surfactant components, producing around 45% free volume. However, in contrast to PEG or dextran, HA-containing Infasurf exhibited interconnected complexes, forming a continuous network throughout the entire field.
The microscopic structure of Infasurf alone changed significantly with time at 37°C compared with 23°C. Surfactant complexes at physiological temperature lost most of their optical contrast and presented a rather uniform appearance with almost no free volume after 20 minutes. In the presence of polymers, Infasurf complexes also showed less contrast at 37 than at 23°C, but these structures were of similar size and occupied similar volume fractions as those observed at 23°C. In the presence of PEG or dextran, Infasurf aggregates were large and dispersed in a continuous free volume, while in the presence of HA, all the aggregates were interconnected with only around 30% dispersed free volume (fig. 4 and Table 1).
Microscope data on the aggregation of Survanta at 25mg/mL, in the absence or presence of polymers, is shown in figure 5. In contrast to Infasurf, Survanta by itself did not show, either at 23 or 37°C, the presence of discrete identifiable structures, but appeared as a continuous matrix with no clear free volume. Addition of PEG or dextran induced a clustering effect on Survanta suspensions, both at 23 and 37°C. PEG was more effective at inducing this effect, causing Survanta to form large aggregate structures (whose polymorphic nature precluded quantification of average size), with about 50% free volume, either at 23 or 37°C. Addition of dextran also induced clusters of large complexes in Survanta, generating around 30% free volume at 23°C. At 37°C, dextran produced clustering of surfactant complexes but the optical contrast of the resulting aggregates was too low to allow calculation of the free volume, although it appeared to be less than that with PEG. Unlike results with PEG or dextran, the addition of HA to Survanta did not produce observable changes, with the HA Survanta mixtures having the same homogeneous and continuous matrix-like structures seen in the absence of polymer.
Figure 5. Microstructure of Survanta in the absence and presence of polymers.
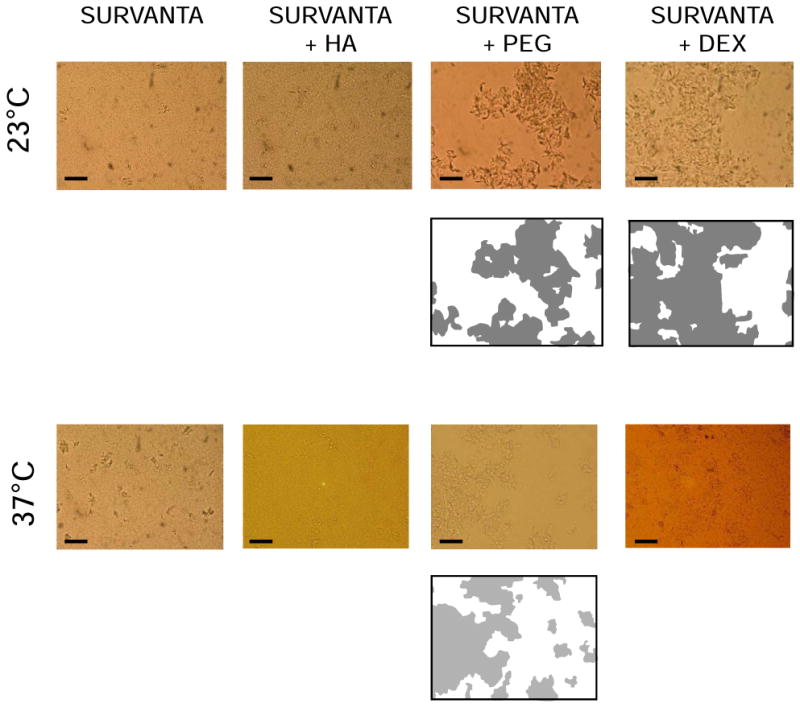
Micrographs of Survanta suspensions at 25mg/mL, in the absence or in the presence of dextran 5%, PEG 5% or HA 0.25% preincubated at 23°C (upper pictures) or 37°C (lower pictures). Slides were taken under an optical microscope as described in Methods with scale bars representing 20 μm. Below some of the micrographs a two dimensional map represents the projection of the apparent volume occupied by surfactant aggregates (grey) or free liquid (white).
Discussion
Our results indicate that at the same concentration, viscosity of Survanta was always higher than that of Infasurf. This finding has been attributed to differences in composition and structure [3, 15]. Survanta contains palmitic acid, tripalmitin and additional DPPC but no cholesterol and much less SP-B compared to Infasurf [19]. Survanta viscosity rose by an order of magnitude as concentration increased from 12.5 to 25mg/mL at either 23°C or 37°C. The large increase in viscosity for Infasurf at 37°C, was also observed by King et al. [3, 15]. This effect grew to an order of magnitude increase in viscosity after 20 minutes at 37°C. These large changes in viscosity can not be explained by a simple extrapolation from lower concentrations and support the hypothesis of substantial morphological and rheological changes occurring at higher concentrations and temperature.
A major finding of this study is that when either surfactant at clinically used concentrations had polymer added, the viscosity was decreased or had little change. At a concentration of 25mg/mL, the addition of dextran or PEG lowered the viscosity of Survanta by approximately an order of magnitude. At high Infasurf concentrations, the addition of dextran, PEG, or HA reversed the higher viscosity observed at 37°C relative to 23°C. The rapid increase of Infasurf viscosity with time at 37°C is also eliminated by addition of any of the three polymers.
The finding that lower concentrations of non-ionic polymers (2.5% w/v) yielded qualitatively similar results as those at higher concentration (5% w/v) implies that there is no evident polymer threshold concentration for the observed effects, at least down to half concentration. Since higher amounts of polymer in surfactant can lead to increased osmotic pressure, causing lung edema [20, 21], the observation that lower concentrations of PEG or dextran can reduce viscosity may prove useful for eventual fine-tuning of clinical surfactant polymer mixtures.
We have found that the addition of PEG or dextran to the surfactants causes lipid aggregation and clustering of aggregates that may be due to depletion forces caused by the exclusion of polymer from the vicinity of surfactant aggregates [22-25]. This clustering creates an increase of surfactant-free space (free volume) within the surfactant suspension (fig. 4, 5) and also promotes adsorption of large surfactant components to the air-liquid interface thereby improving resistance of surfactant to inactivation by various inhibitors [11-14]. Surfactant suspensions, with or without polymers, can be considered as complex colloids composed of a solid-like phase with different sized particle structure and an aggregate-free liquid volume. At low surfactant concentrations, the rheological behavior of the suspensions is dominated by the free liquid phase, and the viscosity approaches that of the buffer plus polymer, if present. At higher surfactant concentrations, those relevant to clinical use, surfactant suspensions with low viscosity have a dominant continuous surfactant-free liquid phase (free volume), while more viscous suspensions have an interconnected matrix of surfactant lipid/protein complexes. Surfactant viscosity would then be expected to follow a behavior similar to that already described for other colloidal suspensions, including an exponential increase in viscosity at colloidal densities above a certain threshold, associated with close particle packing [26]. Infasurf suspensions at 23°C had similar proportions of surfactant aggregates and free volume, while after incubation at 37°C, the entire suspension became more homogeneous and the free volume diminished in parallel with the progressive increase in viscosity. This observation supports an association between changes in microscopic structures of surfactant suspensions and their rheological behavior. The reduction of surfactant viscosity caused by polymers like PEG or dextran correlates with their ability to change a diffuse three-dimensional surfactant matrix into a mostly fluid suspension (large free volume) of large aggregate clusters. This clustering occurred with the addition of PEG or dextran to the surfactants, but not with HA, in agreement with results from freeze fracture electron microscopy [18] and measurements of turbidity [17]. A similar mechanism may lie behind the low viscosities associated with the large aggregation of surfactants caused by addition of other polymers like chitosan [27]. Determination of viscosity of buffered solutions with different polymer concentrations, without surfactant, is shown in Table 2. These data illustrate that much of the decrease in viscosity with addition of polymer at high surfactant concentration is attributable to the generation of large free volume. This is especially evident in the case of PEG where the viscosity at 10% polymer concentration alone is close to that of surfactant plus 5% polymer. Ten percent is approximately the effective polymer concentration since the free volume is close to 50%. Adding HA to the surfactants produced changes in the microscopic structure that were of a different nature than changes induced by PEG or dextran, and were associated with no clear decrease in viscosity. It has been shown that HA can interact with phospholipids to create large fibril-like networks [28, 29]. Our microscope observations indicate that surfactant with HA produces a reorganization of surfactant components to form an interconnected matrix of structures that occupies the whole suspension. This was particularly evident when HA was added to Infasurf (see figure 4). Such a matrix or network could stabilize the surfactant structures within the suspension by sequestering the liquid portion. Stabilization could prevent the homogenizing effect that occurs in polymer-free Infasurf at 37°C. The differing viscosity effects on Infasurf or Survanta when PEG or dextran is added compared to addition of HA support the previously reported hypothesis that non-ionic and anionic polymers produce beneficial effects upon addition to surfactant by different mechanisms [7, 8, 18].
The surface viscosity of Survanta has been previously studied as a function of surface pressure [30, 31]. At low compression ratios, when Survanta films contain a significant fraction of fluid phase, the films showed low surface viscosity that escalated to very high values when the compression-promoted solid phase reached the percolation limit, i.e., enough to interconnect the whole film. Our results suggest that a similar correlation may exist between bulk viscosity of surfactant suspensions and their aggregation state.
In summary, our results have shown that the addition of polymer to Infasurf and Survanta can either lower the viscosity (PEG and dextran) or have little effect on it (HA) at clinically used concentrations and/or physiological temperature. This finding not only answers the initial concern that the addition of polymer might increase viscosity to a degree that would adversely affect distribution in the upper airways but also suggests that the reduction of viscosity with polymer added could in fact aid in rapid and uniform surfactant distribution when introduced as a bolus in the trachea. This viscosity decrease could be responsible, in part, for the beneficial effects previously reported in vivo [8-10, 16]. It has also been shown that aggregation and clustering of surfactant lipids due to addition of PEG or dextran correlates with a decrease of viscosity.
Acknowledgments
We thank Drs. J. Clements, J. Goerke and A. Lu for useful comments and criticisms. Supported by NIH HLBI RO1 HL 66410 and grant BIO2003-03130 from Spanish Ministry of Science (to J. P. –G.). J. P. –G. also acknowledges support as a Visiting Professor in UCSF by a Fellowship from Universidad Complutense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cassidy K, Bull J, Glucksberg M, Dawson C, Haworth S, Hirschl R, Gavriely N, Grotberg J. A rat lung model of instilled liquid transport in the pulmonary airways. J Appl Physiol. 2001;90:1955–1967. doi: 10.1152/jappl.2001.90.5.1955. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J, Molthen R, Dawson C, Haworth S, Bull J, Glucksberg M, Grotberg J. Effect of ventilation rate on instilled surfactant distribution in the pulmonary airways of rats. J Appl Physiol. 2004;97:45–56. doi: 10.1152/japplphysiol.00609.2003. [DOI] [PubMed] [Google Scholar]
- 3.King D, Wang Z, Kendig J, Palmer H, Holm B, Notter R. Concentration-dependent, temperature-dependent non-Newtonian viscosity of lung surfactant dispersions. Chem Phys Lipids. 2001;112:11–19. doi: 10.1016/s0009-3084(01)00150-5. [DOI] [PubMed] [Google Scholar]
- 4.Espinosa F, Kamm R. Bolus dispersal through the lungs in surfactant replacement therapy. J Appl Physiol. 1999;86:391–410. doi: 10.1152/jappl.1999.86.1.391. [DOI] [PubMed] [Google Scholar]
- 5.Halpern D, Jensen O, Grotberg J. A theoretical study of surfactant and liquid delivery into the lung. J Appl Physiol. 1998;85:333–352. doi: 10.1152/jappl.1998.85.1.333. [DOI] [PubMed] [Google Scholar]
- 6.Cui X, Tashiro K, Matsumoto H, Tsubokawa Y, Kobayashi T. Aerosolized surfactant and dextran for experimental acute respiratory distress syndrome caused by acidified milk in rats. Acta Anaesthesiologica Scandinavica. 2003;47:853–860. doi: 10.1034/j.1399-6576.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu K, Goerke J, Clements J, Taeusch H. Hyaluronan decreases surfactant inactivation in vitro. Pediatr Res. 2005;57:237–241. doi: 10.1203/01.PDR.0000150726.75308.22. [DOI] [PubMed] [Google Scholar]
- 8.Lu K, Goerke J, Clements J, Taeusch H. Hyaluronan reduces surfactant inhibition and improves rat lung function after meconium lung injury. Pediatr Res. 2005;58:206–210. doi: 10.1203/01.PDR.0000169981.06266.3E. [DOI] [PubMed] [Google Scholar]
- 9.Lu K, Robertson B, Taeusch H. Dextran or polyethylene glycol added to Curosurf for treatment of meconium lung injury in rats. Biol Neonate. 2005;88:46–53. doi: 10.1159/000084458. [DOI] [PubMed] [Google Scholar]
- 10.Lu K, Taeusch H, Robertson B, Goerke J, Clements J. Polyethylene glycol/surfactant mixtures improve lung function after HCl and endotoxin lung injuries. Am J Respir Crit Care Med. 2001;164:1531–1536. doi: 10.1164/ajrccm.164.8.2104016. [DOI] [PubMed] [Google Scholar]
- 11.Taeusch H, Lu K, Goerke J, Clements J. Nonionic polymers reverse inactivation of surfactant by meconium and other substances. Am J Respir Crit Care Med. 1999;159:1391–1395. doi: 10.1164/ajrccm.159.5.9808047. [DOI] [PubMed] [Google Scholar]
- 12.Tashiro K, Kobayashi T, Robertson B. Dextran reduces surfactant inhibition by meconium. Acta Paediatrica. 2000;89:1439–1445. doi: 10.1080/080352500456615. [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Lu J, Chiu I, Leung K, Chan Y, Zhang L, Policova Z, Hair L, Neumann A. Poly(ethylene glycol) enhances the surface activity of a pulmonary surfactant. Colloids and Surfaces B: Biointerfaces. 2004;36:167–176. doi: 10.1016/j.colsurfb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, Ohta K, Tashiro K, Nishizuka K, Chen WM, Ohmura S, Yamamoto K. Dextran restores albumin - inhibited surface activity of pulmonary surfactant extract. J Appl Physiol. 1999:1178–1184. doi: 10.1152/jappl.1999.86.6.1778. [DOI] [PubMed] [Google Scholar]
- 15.King D, Wang Z, Palmer H, Holm B, Notter R. Bulk shear viscosities of endogenous and exogenous lung surfactants. Am J Physiol Lung Cell Mol Physiol. 2002;282:L277–284. doi: 10.1152/ajplung.00199.2001. [DOI] [PubMed] [Google Scholar]
- 16.Lu K, Taeusch H, Robertson B, Goerke J, Clements J. Polymer-surfactant treatment of meconium-induced acute lung injury. Am J Respir Crit Care Med. 2000;162:623–628. doi: 10.1164/ajrccm.162.2.9909099. [DOI] [PubMed] [Google Scholar]
- 17.Taeusch H, Dybbro E, Lu K. Pulmonary surfactant adsorption is increased by hyaluronan or polyethylene glycol. Colloids Surf B Biointerfaces. 2008;162:243–249. doi: 10.1016/j.colsurfb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Braun A, Stenger P, Warriner H, Zasadzinski J, Lu K, Taeusch H. A freeze-fracture TEM and small angle X-ray diffraction study of the effects of albumin, serum, and polymers on clinical lung surfactant microstructure. Biophys J. 2007;93:1–17. doi: 10.1529/biophysj.106.095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco O, Perez-Gil J. Biochemical and pharmacological differences between preparations of exogenous natural surfactant used to treat Respiratory Distress Syndrome: Role of the different components in an efficient pulmonary surfactant. Eur J Pharmacol. 2007;568:1–15. doi: 10.1016/j.ejphar.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 20.Campbell H, Bosma K, Brackenbury A, McCaig L, Yao L, Veldhuizen R, Lewis J. Polyethylene glycol (PEG) attenuates exogenous surfactant in lung-injured adult rabbits. American Journal Respiratory & Critical Care Medicine. 2001;165:475–480. doi: 10.1164/ajrccm.165.4.2106109. [DOI] [PubMed] [Google Scholar]
- 21.Dehority W, Lu K, Clements J, Goerke J, Pittet J, Allen L, Taeusch H. Polyethylene glycol-surfactant for lavage lung Injury in rats. Pediatr Res. 2005;58:913–918. doi: 10.1203/01.PDR.0000182581.39561.01. [DOI] [PubMed] [Google Scholar]
- 22.Kuhl T, Guo Y, Aldefer J, Berman A, Leckband D, Israelachvili J, Hui S. Direct measurement of polyethylene glycol induced depletion attraction between lipid bilayers. Langmuir. 1996;12:3003–3014. [Google Scholar]
- 23.Meyuhas D, Lichtenberg D. Effect of water-soluble polymers on the state of aggregation, vesicle size, and phase transformations in mixtures of phosphatidylcholine and sodium cholate. Biophys J. 1996;71:2613–2622. doi: 10.1016/S0006-3495(96)79453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyuhas D, Nir S, Lichtenberg D. Aggregation of phospholipid vesicles by water-soluble polymers. Biophys J. 1996;71:2602–2612. doi: 10.1016/S0006-3495(96)79452-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zasadzinski JA, Alig TF, Alonso C, de la Serna JB, Perez-Gil J, Taeusch HW. Inhibition of pulmonary surfactant adsorption by serum and the mechanisms of reversal by hydrophilic polymers: theory. Biophys J. 2005;89:1621–1629. doi: 10.1529/biophysj.105.062646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady J. The rheological behavior of concentrated colloidal dispersions. J Chem Phys. 1993;99:567–581. [Google Scholar]
- 27.Zuo Y, Alolabi H, Shafiei A, Kang N, Policova Z, Cox P, Acosta E, Hair M, Neumann W. Chitosan enhances the in vitro surface activity of dilute lung surfactant preparations and resists albumin induced inactivation. Pediatric Research. 2006;60:125–130. doi: 10.1203/01.pdr.0000227558.14024.57. [DOI] [PubMed] [Google Scholar]
- 28.Jacoboni I, Valdre U, Mori J, Quaglino D, Pasquali-Ronchetti I. Hyaluronic acid by atomic force microscopy. J Struct Biol. 1999;126:52–58. doi: 10.1006/jsbi.1999.4090. [DOI] [PubMed] [Google Scholar]
- 29.Pasquali-Ronchetti I, Quaglino D, Mori G, Bacchelli B, Ghosh P. Hyaluronan-phospholipid interactions. J Struct Biol. 1997;120:1–10. doi: 10.1006/jsbi.1997.3908. [DOI] [PubMed] [Google Scholar]
- 30.Alonso C, Alig T, Yoon J, Bringezu F, Warriner H, Zasadzinski J. More than a monolayer: relating lung surfactant structure and mechanics to composition. Biophys J. 2004;87:4188–4202. doi: 10.1529/biophysj.104.051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso C, Waring A, Zasadzinski J. Keeping lung surfactant where it belongs: protein regulation of two-dimensional viscosity. Biophys J. 2005;89:266–273. doi: 10.1529/biophysj.104.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]