Abstract
Background
Brain-derived neurotrophic factor (BDNF) is an activity-dependent secreted protein that is critical to organization of neuronal networks and synaptic plasticity, especially in the hippocampus. We tested hypothesis that reduced CSF BDNF is associated with age-related cognitive decline.
Methodology/Principal Findings, and Conclusions/Significance
CSF concentration of BDNF, Aβ42 and total tau were measured in 128 cognitively normal adults (Normals), 21 patients with Alzheimer's disease (AD), and nine patients with Mild Cognitive Impairment. Apolipoprotein E and BDNF SNP rs6265 genotype were determined. Neuropsychological tests were performed at baseline for all subjects and at follow-up visits in 50 Normals. CSF BDNF level was lower in AD patients compared to age-matched Normals (p = 0.02). CSF BDNF concentration decreased with age among Normals and was higher in women than men (both p<0.001). After adjusting for age, gender, education, CSF Aβ42 and total tau, and APOE and BDNF genotypes, lower CSF BDNF concentration was associated poorer immediate and delayed recall at baseline (both p<0.05) and in follow up of approximately 3 years duration (both p<0.01).
Conclusions/Significance
Reduced CSF BDNF was associated with age-related cognitive decline, suggesting a potential mechanism that may contribute in part to cognitive decline in older individuals.
Introduction
Age-related cognitive decline is a complex convergent phenotype that likely derives in part from brain senescence and in part from prodromal dementing illnesses, most commonly Alzheimer's disease (AD). Brain-derived neurotrophic factor (BDNF) is an activity-dependent secreted protein that, along with its receptors, is expressed widely in the central nervous system and is critical to organization of neuronal networks and synaptic plasticity, especially in the hippocampus, in a variety of animal models and apparently in humans [1], [2]. Although controversy remains, these human data derive mostly from investigations of an allelic variant of BDNF (Val66Met or Met-BDNF), inheritance of which has been associated with poorer cognitive performance in healthy older adults [3], impaired memory in patients with schizophrenia and healthy controls [1], [2], abnormal hippocampal activation as assessed by fMRI [2], and an approximately 4% to 11% smaller hippocampal volume as determined by MRI in healthy adult volunteers [3], [4]. The mechanisms that underlie these associations of functional and structural differences with inheritance of Met-BDNF are not clear; however, one study has shown diminished depolarization-induced secretion of Met-BDNF compared to Val-BDNF, and failure of Met-BDNF to localize to secretory granules or synapses in transfected neurons [2]. Together, these findings have led to the hypothesis that reduced BDNF secretion is one mechanism of age-related cognitive decline. We are unaware of any published study that has yet tested this hypothesis.
Methods
All procedures were approved by the institutional review boards of the University of Washington; all subjects were recruited from University of Washington Alzheimer's Disease Research Center and provided written informed consent. Subjects underwent detailed clinical and laboratory evaluation and were classified as no cognitive impairment (Normals), amnestic mild cognitive impairment (MCI) [5], or probable AD [6]. Inclusion and exclusion criteria, method of CSF collection, and analysis were exactly as previously described [7].
CSF concentration of BDNF, total tau and Aβ42 was measured using an X-MAP-based assay [7]. APOE genotype was determined by a restriction digest method [8]. BDNF SNP rs6265 was genotyped using TaqMan allelic discrimination detection, as previously described [9].
Neuropsychological tests included: (i) Paragraph Recall - a test of declarative memory [10], [11]. Paragraphs were modeled after the Logical Memory subtests I and II of the WMS-R [12]. Total score for immediate recall and delayed recall (each with possible range 0–25) were used. (ii) Category Fluency - a test of semantic memory [13]. Total number of unique animals named in 60 seconds was used. (iii) Trail Making Test, Parts A and B - test of ability to adapt to shifting task demands. Time taken to complete Part B (upper bound of 300 sec), a measure of executive function [14], was used.
One-way analysis of variance (ANOVA) was used with post hoc Bonferroni multiple pair-wise comparisons for continuous variables and Fisher's exact test for categorical variables to assess differences between diagnostic and genetic groups. Linear regression models were used for associations of demographic characteristics with CSF BDNF concentration, and cross-sectional relationships between CSF BDNF concentration and coincident cognitive test performance. Raw scores were used for each test except log-transformed times for Trails B to remove skewness. We used two-stage regression (least squares slope for each test in each individual over time, then weighted regression model with slope as response variable) to assess association of baseline CSF BDNF concentration with subsequent longitudinal changes of cognitive test performance [15]. Weights were based on subjects having different numbers of follow-up visits at different times after baseline. Statistical analyses were performed using S-PLUS version 8.0 [16] and R version 2.7.1 [17].
Results
Age-matched Normals, MCI, and AD
Table 1 presents demographics and baseline CSF BDNF levels in 128 normal controls (Normals), 9 MCI and 21 AD patients. To compare measures between these three groups after adjusting for age, a subset of the Normals (n = 76) age≥50 years (Older Normals, ON) was used as a comparison group. CSF BDNF level was lower in AD patients compared to ON with a mean difference of 25 pg/ml (post-hoc Bonferroni test, p = 0.02). CSF BDNF level for the nine MCI patients was not different from either group (both p>0.05). The frequencies of Met-BDNF genotype (G/G vs. G/A or A/A) were not different between ON, MCI, and AD subjects (Fisher's Exact Test p = 0.36). Moreover, CSF BDNF concentration in AD subjects was not different among Met-BDNF genotype G/G (70%, 217±38 pg/ml), G/A (25%, 214±39 pg/ml), or A/A (5%, 213 pg/ml, n = 1) (F = 0.02, df = 2, p = 0.99).
Table 1. Subject characteristics and CSF BDNF levels at baseline.
| Normals | Normals With Follow-up | ON (Normals age≥50) | MCI | AD | p-value (ON, MCI & AD)** | |
| Number | 128 | 50 | 76 | 9 | 21 | |
| Age, years, mean±SD (Range) | 52±20 (21–100) | 72±9 (41–100) | 67±10 (50–100) | 74±8 (63–82) | 68±10 (52–87) | 0.14 |
| Gender, male % | 49 | 46 | 43 | 67 | 48 | 0.45 |
| Race, Caucasian % * | 90 | 90 | 89 | 100 | 95 | 0.29 |
| APOE Genotype, any ε4 % * | 30 | 28 | 35 | 78 | 65 | 0.004 |
| BDNF Genotype rs6265 G/A or A/A % * | 40 | 34 | 39 | 12 | 30 | 0.36 |
| Education, years mean±SD * | 16±3 | 16±3 | 16±3 | 16±3 | 15±3 | 0.33 |
| BDNF, pg/ml, mean±SD | 246±33 | 233±35 | 240±36 | 241±36 | 216±35 | 0.02 |
| MMSE, mean±SD | 29±1 | 29±1 | 29±1 | 28±1 | 20±5 |
ON: Older Normals; MCI: Mild Cognitive Impairment; AD: Alzheimer disease; MMSE: Mini-Mental State Exam.
Missing Values. Race – 1 (AD). APOE – 2 (one ON, one AD). BDNF Genotype – 4 (two ON, one MCI, one AD). Education – 1 (AD).
p-value based on one-way ANOVA for continuous variables and Fisher's exact test for categorical variables. No comparison between groups was performed for MMSE since this test is used for diagnosis.
CSF BDNF concentration in all cognitively normal subjects
Our focus was on 128 cognitively normal volunteers (Normals) in whom we measured CSF BDNF, Aβ42, and total tau, and who underwent neuropsychological evaluation ( Table 1 ). CSF BDNF concentration was not different among rs6265 genotype in Normals: G/G (60%, 242±37 pg/ml), G/A (36%, 253±26 pg/ml), or A/A (5%, 251±28 pg/ml) (F = 1.52, df = 2, p = 0.22).
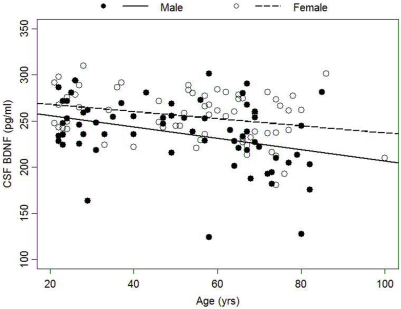
CSF BDNF concentration decreased with age among Normals and was higher in women than men (both p<0.001; Figure 1 ). In a multivariate linear regression with CSF BDNF as the dependent variable and age, gender, presence of the APOE ε4 allele, and Met-BDNF (G/G vs G/A or A/A) as independent predictors, both age and gender were significantly associated with CSF BDNF level (p<0.001). Neither presence of APOE ε4 allele (p = 0.33) nor Met-BDNF (p = 0.09) was related to CSF BDNF levels. In addition, there was no interaction between APOE ε4 allele and Met-BDNF genotype (p = 0.25), nor between age and gender (p = 0.45). Removing the three lowest values of BDNF for males did not change the results. Finally, after adjusting for age and gender, CSF BDNF levels were related inversely (slope = −0.03) to CSF levels of Aβ42 (p = 0.05) but not to CSF total tau (p = 0.34).
Figure 1. Cross-sectional relationships between age and CSF BDNF concentration by gender.
Association between CSF BDNF concentration, memory, and cognitive ability in cognitively normal subjects at baseline
Most (n = 121) Normals underwent extensive neuropsychological testing at baseline ( Table 1 ). Table 2 shows the regression coefficients and p-values associated with baseline CSF BDNF levels for each cognitive test in multivariate regression models after adjusting for age, gender, and years of education (Model 1); adjusting for the previous variables as well as concentration of CSF Aβ42 and total tau (biomarkers of latent and prodromal AD [18], [19], Model 2); and adjusting for the previous variables as well as presence of APOE ε4 and Met-BDNF (Model 3). There was an association between higher CSF BDNF concentrations and better performance on Paragraph Recall - Delayed that was independent of CSF Aβ42 and total tau concentrations (Model 2) as well as presence of APOE ε4 and Met-BDNF (Model 3); correlation with the Paragraph Recall - Immediate was significant when all predictor variables were in the model (Model 3). An association between lower CSF BDNF and poor performance on Trail Making Test Part B also was observed. There was no significant correlation between CSF BDNF concentration and performance on the Category Fluency test.
Table 2. Relationships between baseline CSF BDNF concentration with cross-sectional and longitudinal cognitive performance.
| Cross-sectional (n = 121) | Longitudinal (n = 50) | |||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
| Paragraph Recall: Immediate Recall | 0.17 (0.11); 0.13 | 0.22 (0.12); 0.06 | 0.26 (0.12); 0.03 | 0.09 (0.04); 0.02 | 0.11 (0.04); 0.01 | 0.13 (0.04); <0.01 |
| Paragraph Recall: Delayed Recall | 0.32 (0.11); <0.01 | 0.39 (0.11); <0.01 | 0.43 (0.10); <0.01 | 0.13 (0.05); <0.01 | 0.13 (0.05); 0.01 | 0.15 (0.05); <0.01 |
| Category Fluency: Animal | −0.08 (0.19); 0.67 | 0.02 (0.19); 0.93 | 0.06 (0.19); 0.73 | 0.16 (0.06); 0.01 | 0.19 (0.06); <0.01 | 0.21 (0.07); <0.01 |
| Log10 Trail Making test: Part B | −0.009 (0.005); 0.07 | −0.013 (0.005); 0.02 | −0.011 (0.005); 0.03 | −0.001 (0.001); 0.42 | −0.002 (0.001); 0.14 | −0.002 (0.001); 0.11 |
Data are: linear regression model coefficient (SE) per10 pg/ml; p-value. Models 1, 2, and 3: Cross-sectional relationships between baseline CSF BDNF concentration and coincident cognitive test scores in 121 Normals with baseline neuropsychological testing. Model 1: adjusted for age, gender, and years of education. Model 2: Model 1 plus CSF Aβ42 and total tau concentrations. Model 3: Model 2 plus APOE ε4 (ε4− vs. ε4+) and Met-BDNF genotype (G/G vs. G/A or A/A) (n = 119 because of two missing values for rs6265 genotype). Models 4, 5 and 6: longitudinal relationships between baseline CSF BDNF concentration and subsequent annual change in cognitive test scores for 50 Normals with follow-up evaluation. Model 4: adjusted for age, gender, years of education, and baseline test score. Model 5: Model 4 plus baseline CSF Aβ42 and total tau concentrations. Model 6: Model 5 plus APOE ε4 (ε4− vs. ε4+) and Met-BDNF genotype (G/G vs. G/A or A/A).
Association between baseline CSF BDNF concentration and longitudinal changes in cognitive performance
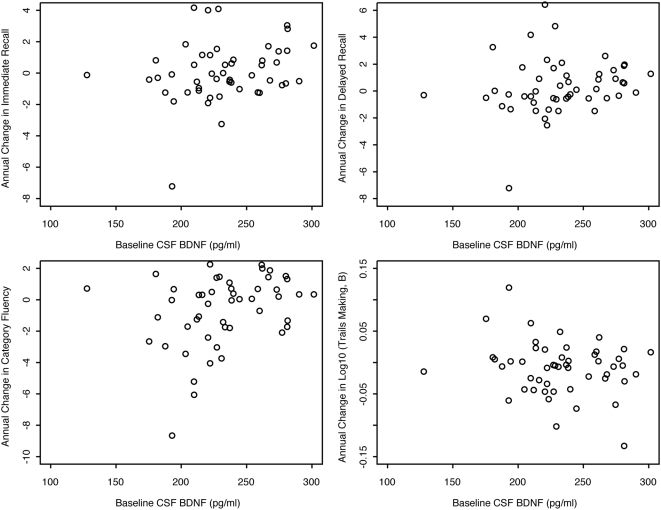
Fifty Normals (27 women), aged 40–100 years (mean±SD = 71.5±8.9) had at least one clinical follow-up visit (mean±SD 4.0±1.1, Range = 2–6) with an average length of follow-up of 3.3 years (SD = 1.2 years, Range = 0.7–5.8 years) ( Table 1 ). Figure 2 shows unadjusted annual changes in cognitive test scores versus baseline CSF BDNF concentration. After adjusting for age, gender, years of education, and baseline test score, lower values of baseline BDNF concentration were significantly associated with greater annual decline in Immediate and Delayed Recall scores on the Paragraph Recall test, and a greater annual decline in the Category Fluency test (Model 4). These relationships were unchanged after further adjusting for baseline CSF Aβ42 and total tau concentrations (Model 5), suggesting that prediction by baseline CSF BDNF concentration for subsequent cognitive changes is independent of preclinical AD. These relationships also were unchanged after further adjusting for presence of APOE ε4 and Met-BDNF (Model 6). All results were unaffected by removing the subject who had the largest annual decline for Immediate Recall, Delayed Recall, and Category Fluency ( Figure 2 ).
Figure 2. Annual change in cognitive test scores versus baseline CSF BDNF concentration.
Relationship between BDNF genotype and cognitive performance in the Normals
Among all Normals (n = 119; 2 missing values for genotype), baseline performance on each of the four cognitive tests (Immediate and Delayed Recall, Category Fluency, and Trail Making Part B) did not differ by Met-BDNF genotype (G/G vs. G/A or A/A, all p>0.05), even after adjusting for age, gender and education in the multiple regression model (all p>0.05). Similarly, the longitudinal changes in these cognitive performances (n = 50) were not associated with Met-BDNF genotype (all p>0.05).
Discussion
We made three novel observations in cognitively normal individuals. (i) CSF BDNF decreased across the human life-span in the absence of dementia or MCI and was independent of inheritance of Met-BDNF or the APOE ε4 allele. (ii) Women had higher average CSF BDNF concentrations than men. (iii) Lower CSF BDNF concentration was associated strongly with poorer memory and less so with diminished executive function; importantly, these associations were independent of CSF biomarkers of preclinical AD, suggesting that the mechanisms that contribute to early AD and to age-related decline in CSF BDNF might be independent. We must acknowledge that this study has a relatively small sample size and short duration of follow-up and further studies are needed to validate our results.
While ours is the first study of which we are aware to investigate age-related changes in CSF BDNF, other studies have investigated disease-associated changes in CSF BDNF in patients with idiopathic Parkinson disease [7], [20] or in patients with AD [7], [21], [22]. We confirmed that CSF BDNF is further reduced beyond age-related decline in patients with probable AD. CSF and serum BDNF concentrations do not correlate [23]; yet, increased and decreased serum BDNF levels are related to early and late stages, respectively, of AD [24].
The gender difference in CSF BDNF observed in this study is especially interesting, and we speculate that this may be due to hormonal effects. Animal studies have shown that estrogen receptors colocalize to cells that express BDNF and its receptor trkB, and estrogen regulates the expression of BDNF [25]. The relationship between estrogen levels and BDNF expression and secretion, and their potential effect on the cognition in humans requires further studied.
Our data showed that progressive decline in CSF BDNF concentration was a feature of advancing age independent of preclinical AD or dementia, and was associated strongly with reduced performance in declarative memory tests and less strongly with performance on tests of executive function, perhaps a reflection of the special role of BDNF in hippocampal function. Age-related reduction in CSF BDNF was independent of inheritance of Met-BDNF or APOE ε4. Further reduction in CSF BDNF occurred in AD. Our data suggest that reduced secretion of BDNF in the central nervous system is one mechanism that may contribute to age-related cognitive decline.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: National Institution on Aging: P50-AG005136, AG023185 and AG05136; the Nancy and Buster Alvord Endowment; and an anonymous foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–4. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 3.Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, et al. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7:411–7. doi: 10.1111/j.1601-183X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 4.Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, et al. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–5. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 6.McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Sokal I, Peskind ER, Quinn JF, Jankovic J, et al. CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. Am J Clin Pathol. 2008;129:526–9. doi: 10.1309/W01Y0B808EMEH12L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–8. [PubMed] [Google Scholar]
- 9.Bekris LM, Millard SP, Galloway NM, Vuletic S, Albers JJ, et al. Multiple SNPs within and surrounding the apolipoprotein E gene influence cerebrospinal fluid apolipoprotein E protein levels. J Alzheimers Dis. 2008;13:255–66. doi: 10.3233/jad-2008-13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newcomer JW, Selke G, Melson AK, Gross J, Vogler GP, et al. Dose-dependent cortisol-induced increases in plasma leptin concentration in healthy humans. Arch Gen Psychiatry. 1998;55:995–1000. doi: 10.1001/archpsyc.55.11.995. [DOI] [PubMed] [Google Scholar]
- 11.Craft S, Dagogo-Jack SE, Wiethop BV, Murphy C, Nevins RT, et al. Effects of hyperglycemia on memory and hormone levels in dementia of the Alzheimer type: a longitudinal study. Behav Neurosci. 1993;107:926–40. doi: 10.1037//0735-7044.107.6.926. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler D, Stone CP. Manual: Wechsler Memory Scale. New York: Psychological Corporation; 1983. [Google Scholar]
- 13.Gomez RG, White DA. Using verbal fluency to detect very mild dementia of the Alzheimer type. Arch Clin Neuropsychol. 2006;21:771–5. doi: 10.1016/j.acn.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Reitan RW, D. The Halstead-Reitan neuropsychological test battery. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- 15.Milliken JK, Edland SD. Mixed effect models of longitudinal Alzheimer's disease data: a cautionary note. Stat Med. 2000;19:1617–29. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1617::aid-sim450>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16. Corporation I, S-PLUS. 2008, Insightful Corporation: seattle, WA.
- 17. Team R D C, R: A language and environment for statistical computing. 2008, R Foundation for Statistical Computing: Vienna, Austria.
- 18.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–9. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 19.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, et al. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–9. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 20.Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000:277–90. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Goodlett DR, Quinn JF, Peskind E, Kaye JA, et al. Quantitative proteomics of cerebrospinal fluid from patients with Alzheimer disease. J Alzheimers Dis. 2005;7:125–33; discussion 73–80. doi: 10.3233/jad-2005-7205. [DOI] [PubMed] [Google Scholar]
- 22.Blasko I, Lederer W, Oberbauer H, Walch T, Kemmler G, et al. Measurement of thirteen biological markers in CSF of patients with Alzheimer's disease and other dementias. Dement Geriatr Cogn Disord. 2006;21:9–15. doi: 10.1159/000089137. [DOI] [PubMed] [Google Scholar]
- 23.Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, et al. BDNF serum and CSF concentrations in Alzheimer's disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–94. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, et al. Stage-dependent BDNF serum concentrations in Alzheimer's disease. J Neural Transm. 2006;113:1217–24. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- 25.Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27:404–14. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]