Abstract
In order to understand more details about the role of abscisic acid (ABA) in fruit ripening and senescence of tomato, two cDNAs (LeNCED1 and LeNCED2) which encode 9-cis-epoxycarotenoid dioxygenase (NCED) as a key enzyme in ABA biosynthesis, two cDNAs (LeACS2 and LeACS4) which encode 1-aminocyclopropane-1-carboxylic acid (ACC) synthase, and one cDNA (LeACO1) which encodes ACC oxidase involved in ethylene biosynthesis were cloned from tomato fruit using a reverse transcription-PCR (RT-PCR) approach. The relationship between ABA and ethylene during ripening was also investigated. Among six sampling times in tomato fruits, the LeNCED1 gene was highly expressed only at the breaker stage when the ABA content becomes high. After this, the LeACS2, LeACS4, and LeACO1 genes were expressed with some delay. The change in pattern of ACO activity was in accordance with ethylene production reaching its peak at the pink stage. The maximum ABA content preceded ethylene production in both the seeds and the flesh. The peak value of ABA, ACC, and ACC oxidase activity, and ethylene production all started to increase earlier in seeds than in flesh tissues, although they occurred at different ripening stages. Exogenous ABA treatment increased the ABA content in both flesh and seed, inducing the expression of both ACS and ACO genes, and promoting ethylene synthesis and fruit ripening, while treatment with fluridone or nordihydroguaiaretic acid (NDGA) inhibited them, delaying fruit ripening and softening. Based on the results obtained in this study, it was concluded that LeNCED1 initiates ABA biosynthesis at the onset of fruit ripening, and might act as an original inducer, and ABA accumulation might play a key role in the regulation of ripeness and senescence of tomato fruit.
Keywords: ABA, ethylene, fruit ripening, LeACS2, LeACO1, NCED gene, tomato
Introduction
The plant hormone abscisic acid (ABA) not only plays a regulatory role in plant growth and development, in seed dormancy, and in the adaptation of plants to stress conditions, but also displays a pattern of change similar to ethylene at late stages of fruit development. Some scholars considered that ABA had a crucial role, perhaps even more crucial role than that of ethylene, in fruit maturation and senescence (Giovannoni, 2001, 2004; Rodrigo et al., 2003). To date, the ripening mechanism of climacteric fruit, especially the effect of ethylene, has been well studied (Alexander and Grierson, 2002). However, the mechanism involved in the ripening of non-climacteric fruits remains unclear, despite similar research efforts being made. The two types of fruits exhibit the same ripening phenomena in terms of colour and texture, with only a difference in ethylene production. Therefore, it is assumed that an upstream gene for ethylene expression should exist in controlling fruit ripening of both fruit types.
On the other hand, tomato has proved to be an excellent model system for the analysis of fruit ripening and development, in part due to the availability of well characterized ripening mutants. The ripening inhibitor (rin) and non-ripening (nor) mutants are deficient in climacteric respiration and the associated burst peak in ethylene biosynthesis. Application of exogenous ethylene does not promote fruit ripening, yet it does induce expression of ethylene-regulated ripening genes (Tigchelaar et al., 1978; Yen et al., 1995), which indicated that in addition to general ethylene biosynthesis and signalling, upstream of ethylene there may be other ethylene-independent regulatory factors for climacteric fruit maturation. ABA can be considered as the other ripening control factor, because the ABA content is very low in unripe fruit but increases during the process of fruit ripening in both climacteric (Vendrell and Buesa, 1989; Buesa et al., 1994) and non-climacteric (Inaba et al., 1976; Coombe, 1989; Kojima, 1996; Kondo, 1997, 1998) fruits. At present, ABA deficiency has been thoroughly studied in mutants in various plant species (Taylor et al., 2000; Galpaz et al., 2008). Recently, it was reported (Galpaz et al., 2008) that the ABA content in ABA-deficient mutants is 75% lower than the normal level. Because of this, both the plant and fruit did not show the normal growth observed in the wild type; the total fruit weight and average fruit weight in ABA-deficient mutant fruits were reduced compared with wild-type fruit, and the plant weight was 50% lower in the ABA-deficient plant than in the wild type, indicating that ABA was not only required for plant growth, but was also indispensable for fruit development and ripening. The degreening stage of ABA-deficient orange mutants started later than that of the wild type (Rodrigo et al., 2003), and the sensitivity to ethylene of ABA-deficient orange mutants was also weaker than that of the wild type (Alferez and Zacarias, 1999), which indicated that plants could not grow normally if there was insufficient ABA available.
There is now biochemical and genetic evidence showing that 9-cis-epoxycarotenoid dioxygenase (NCED) is the key enzyme of the ABA biosynthesis pathway in higher plants (Tan et al., 1997; Burbidge et al., 1999; Iuchi et al., 2001). Since its was first isolated from the maize vp14 mutant (Tan et al., 1997), the NCED gene has been cloned and characterized in various plant species including bean (Qin and Zeevaart, 1999), cowpea (Iuchi et al., 2000), Arabidopsis (Iuchi et al., 2001), and citrus (Rodrigo et al., 2006). However, previous studies were focused on the mechanisms of drought resistance and signal transduction of the NCED gene (Thompson et al., 2000; Iuchi, 2001; Nambara and Marion-Poll, 2005). Its expression in fruits has only been studied in avocados (Chernys and Zeevaart, 2000) and citrus (Rodrigo et al., 2006). In this study, the NCED gene from tomato fruit was cloned and the expression of the NCED gene, the function of ABA, and their relationship with the mechanism of ethylene action during fruit ripening were analysed.
Materials and methods
Plant material
Tomato [Solanum lycopersicum (formerly Lycopersicon esculentum) cv. Alisa Craig] plants were grown in a greenhouse under standard culture conditions [25/20 °C, relative humidity (RH) 70%]. About 1000 flowers on 30 tomato plants were tagged at the date of anthesis. During the development and ripening stage, tomato fruits were collected on the following days (stages) after anthesis: 30 d (immature green), 40 d (mature green), 44 d (breaker 1), 45 d (breaker 2), 47 d (turning), 50 d (pink), 53 d (mature red), and 57 d (over-ripe). Twenty fruits of uniform size were selected at each sampling time (one replication). There were six replications of sampling times in total. The seeds were separated from the fruits. These seeds and fruits, used for determination of ABA and ethylene content, were cut into small cubes of 0.5–0.8 cm3, quickly frozen in liquid nitrogen, and stored at –80 °C before RNA extraction.
RNA extraction, reverse transcription- PCR (RT-PCR), and sequencing
Total RNA was extracted from 10 g of flesh or 5 g of seed using the hot borate method (Wan and Wilkins, 1994). Poly(A)+ RNA was purified using oligo(dT)30–latex (Takara, Kyoto, Japan) following the manufacturer's protocol. Synthesis of the first-strand cDNA from the purified poly(A)+ RNA was conducted using a Marathon™ cDNA Amplification Kit (Clontech). The cDNA was used as a template for amplifying NCEDs with degenerate primers (forward, 5′-TTYGAYGGIGAYGGIATGGTICA-3′; reverse, 5′-TCCCAIGCRTTCCAIARRTGRAA-3′) designed from the conserved sequences of plant NCEDs (AF224671, Z97215, DQ028471, DQ028472, and AY337613). PCR was performed under the following conditions: 94 °C for 3 min, followed by 30 cycles of 94 °C for 1 min, 50 °C for 2 min, and 72 °C for 3 min, with the final reaction being terminated at 72 °C for an additional 10 min. The PCR products were ligated into a pGEM-T easy vector and subsequently transformed into Escherichia coli DH5α. Positive colonies were selected, amplified, and then sequenced by Invitrogen China (Shanghai, China). Sequences encoding plant NCEDs were determined by a homology search of the NCBI databases using the BLAST program. Furthermore, using the same method as above mentioned, the desired genes from tomato fruit were obtained using the primers LeACS2 (5′-CGAGGATTCGGAGGTTCGTAGGTGT-3′ and 5′-GGTGAGGGAGGAATAGGTGACGAAAG-3′), LeACS4 (5′-GCAAGGATTCGGATGTTTATGGATGC-3′ and 5′-TGCTCGCACTACGAGCGAGGAATTG-3′), and LeACO1 (5′-GCTTGTGARAAYTGGGGYTTYTT-3′ and 5′-CATCGCCTCRAAYCKGGYTTCC-3′) designed from the conserved sequences of tomato plant.
Probe preparation and northern hybridization
Digoxigenin (DIG)-labelled probes were synthesized using a PCR DIG probe synthesis Kit (Roche Diagnostics), and amplified using pGEM-T Easy plasmids containing the cDNA fragments obtained from the RT-PCR as templates, and T7 and SP6 primers corresponding to vector sequences adjoining the multiple cloning sites. For northern hybridization analysis, gene probes of the cDNAs were amplified using plasmids. Aliquots of total RNA (5 μg) were separated by electrophoresis on 1% (w/v) agarose gels containing 2.2 M formaldehyde, and blotted onto nylon membranes (Hybond N+, Amersham Biosciences, UK). The filters were then hybridized with the DIG-labelled DNA probes in high SDS buffer [7% (w/v)) SDS, 5× SSC, 50 mM sodium phosphate, pH 7.0, 2% (w/v) blocking reagent, and 0.1% N-lauroylsarcosine] containing 50% (v/v) formamide overnight at 42 °C. After hybridization, filters were washed twice at 37 °C in 2× SSC and 0.1% (w/v) SDS for 15 min and twice at 55 °C in 0.1× SSC and 0.1% (w/v) SDS for 30 min. The membranes were then subjected to immunological detection according to the manufacturer's instructions using CDP-Star™ as a chemiluminescent substrate for alkaline phosphatase (Roche Diagnostics).
ABA content and LeNCED1 expression in dehydrated fruit
In order to evaluate the effect of dehydration stress, fruits were harvested 4 d before the green mature stage and then divided into two groups, with fruits and seeds included in each group. The first group (as control) was stored at 20 °C under high RH (95%) and the second group (dehydration stress) was stored at the same temperature but under low RH (45%, dehydrated fruits). ABA content and the degree of LeNCED1 expression in the fruits or seeds were determined at 0, 2, 4, 6, 8, 10 and 12 d after treatment. Each treatment included three replications. Each sample included 20 fruits or 5 g of seeds. Some samples were quickly frozen in liquid nitrogen and stored at –80 °C before RNA extraction.
Effect of exogenous ABA, fluridone, and ACC treatments on fruit ripening
In order to evaluate the effect of exogenous ABA, fluridone (an ABA synthetic inhibitor), or 1-aminocyclopropane-1-carboxylic acid (ACC) treatment, tomato fruits were harvested at the green mature stage. They were then divided into four groups and used for the following treatments: ABA (100 μM, group 1), fluridone (100 μM, group 2), ACC (100 μM, group 3), and control (distilled water, group 4). The fruits were treated with 0.5 ml each of the solutions or distilled water, respectively. Three replications were conducted for each solution, with each treatment comprising 20 tomato fruits and 5 g of seeds. Each solution was injected into the fruit from the pedicle with a medical syringe. Then the treated fruits were incubated (stored) at 20 °C and 95% RH for 0, 2, 4, 6, 8 10, and 12 h. Twenty fruits or 5 g of seeds were sampled every 2 d after treatment for measurement of the ABA content and ethylene.
Effect of repeat treatment with exogenous ABA, fluridone, and ACC on postharvest fruit firmness
As the fluridone, exogenous ABA, and ACC treatments have only a short-term effect of several days, for better determination of their effects the fruits were repeatedly treated. A total of 240 tomato fruits on the plants were selected and tagged at 1 week before the mature green stage. These fruits on the tomato plants were treated by injecting them with 0.5 ml of 100 μM ABA, fluridone, and ACC solutions, or water, following the methods described above. At the green mature stage, these fruits were harvested, held in the laboratory under ambient conditions, and repeatedly subjected to the same treatment as described above. Then the fruits were stored at 4 °C, 95% RH for 1 month. During the whole storage period, the fruits were subjected to injections at weekly intervals. Fruits were determined for firmness every week after treatment.
Effect of exogenous ABA, NDGA, and ABA+1-MCP treatments on fruit ripening
Because fluridone cannot completely block ABA accumulation, nordihydroguaiaretic acid (NDGA) (Creelman et al., 1992) was used to block ABA in this test. Preliminary tests showed that NDGA was an ideal inhibitor of the NCED enzyme with regard to its permeating speed and its ability to block ABA biosynthesis. The lowest NDGA concentration which blocks ABA accumulation completely is 100 μM as determined by preliminary tests in tomato fruit. In addition, 1-methylcyclopropene (1-MCP) (Sisler and Serek, 1997) was used to block ethylene action during the ripening stage of tomato fruit.
Tomato fruits were harvested at the mature green stage. Immediately after harvest, fruits were divided into four groups and were treated with 0.5 ml per fruit each of 100 μM ABA (group 1), 100 μM NDGA (group 2), 100 μM ABA (group 3), or distilled water (group 4, control) using the methods described above, and held at 20 °C and 95% RH. The fruits in group 3 were treated 4 d after ABA treatment with 5 μl l−1 of 1-MCP for 1 h according to Nakatsuka et al. (1997). 1-MCP (5 μl l−1) was applied by injecting a measured volume of stock gas into sealed glass jars with the fruits inside. Three replications were conducted for each treatment, using 20 fruits. Fruits were sampled every 2 d after treatment for measurement of the content of ABA and ethylene production. Some pulp tissues were frozen in liquid nitrogen and stored at –80 °C before RNA extraction.
Determination of ABA content
For ABA extraction, 1 g of flesh or 0.5 g of seed were ground in a mortar and homogenized in extraction solution (80% methanol, v/v). Extracts were centrifuged at 10 000 g for 20 min. The supernatant liquid was eluted through a Sep-Pak C18 cartridge (Waters, Milford, MA, USA) to remove polar compounds, and then stored at –20 °C for enzyme-linked immunosorbent assay (ELISA). The ELISA procedures were conducted according to the instructions provided by the manufacturer (China Agricultural University, Beijing, China). ABA was determined by Thermo Electron (labsystems) Multiskan MK3 (PIONEER Co., China).
Determination of ACC content
Samples of powdered plant material (1 g of flesh or 0.5 g of seed; three replications) were extracted with 80% aqueous ethanol (v/v). Following oxidative conversion, the ACC content was determined as ethylene by gas chromatography (Agilent, 6890N, USA) according to Lizada and Yang (1979).
Determination of ethylene production
The rate of ethylene produced by whole fruit was measured by enclosing five fruits or 3 g of seeds in 1.5 l air-tight containers for 1 h at 20 °C, withdrawing 1 ml of the headspace gas, and injecting it into a gas chromatograph (model Agilent, 6890N, USA) fitted with a flame ionization detector and an activated alumina column. The unit of ethylene production is nl g−1 FW h−1. The measurement conditions were as follows: chromatograph column, HP-5 5% phenyl methyl siloxane, 30 m capillary alumina column (Agilent 19091J-413); the temperature of the column and detector was 80 °C and 150 °C, respectively; the carrier gas flow rate was N2, 40 ml min−1; and the hydrogen pressure was 0.6 kg cm−2.
Assay of ACO activity
A 1 g aliquot of flesh tissue or 0.5 g of seed powder was added into 1 ml of a solution containing 100 mM TRIS-HCl pH 7.5, 10% glycerine, 30 mM sodium ascorbic acid, 5% polyvinylpyrrolidone (PVP), 0.1 mM FeSO4, and 5 mM dithiothreitol (DTT), and centrifuged at 12 000 g (4 °C) for 15 min. A 0.2 ml aliquot of the above solution was added to 1.8 ml of enzyme solution containing 100 mM TRIS-HCl buffer pH 7.5, 10% glycerine, 30 mM sodium ascorbic acid, 30 mM NaHCO3, 1 mM ACC, and 0.1 mM FeSO4, kept at 30 °C for 20 min, and then 1 ml of gas was injected into a gas chromatograph for measurement of ethylene. The ACO activity unit was nl (g h−1)−1, and measurement was repeated three times.
Fruit firmness measurement
Flesh firmness was measured after the removal of fruit skin on three sides of each fruit using a KM model fruit hardness tester (FUJIHARA Co., Japan). The strength of flesh firmness was recorded as kg cm−2.
Results
Expression of LeNCED1, LeACS2, LeACS4, and LeACO1 at different ripening stages
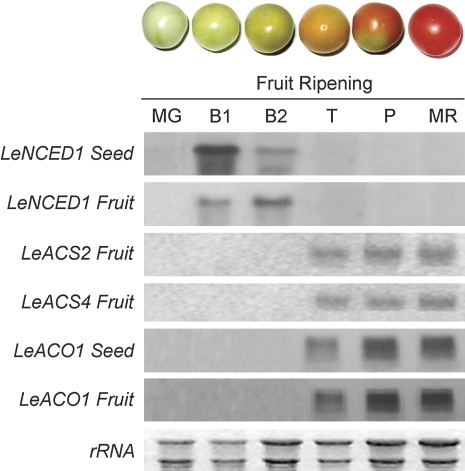
Two different NCED genes were obtained from the cDNA of tomato fruits (mix of samples collected at different stages). These cDNAs differed in the sequence of a 740 bp fragment. A Blast homology search revealed an amino acid similarity of 79.67% between the LeNCED genes. One DNA sequence showed 100% homology to LeNCED1 (located at bases 669–1408 of the LeNCED1 sequence) (Burbidge et al., 1997; GenBank accession no. Z97215) and was designated as LeNCED1 in this study. The full length of LeNCED1 is 2171 bases, with an open reading frame coding for 605 amino acids. The other DNA sequence (LeNCED2, accession no, EU912387) showed 95% homology to StNCED2 (located at bases 514–1254 of the StNCED2 sequence, AY662343), and was omitted from the study. In addition, an ACS cDNA fragment (LeACS2-like) (M34289), an ACS cDNA fragment (LeACS4-like) (AF548375), and an ACO cDNA fragment (LeACO1-like) (A35021), were cloned (Zarembinski and Theologis, 1994; Oetiker et al., 1997; Shiu et al., 1998). The expression patterns of these genes during ripening are shown in the Fig. 1. At the six time points at which tomato fruits were sampled, the LeNCED1 gene was expressed only at the breaker stage, 44–45 d after anthesis when ABA accumulation became high, and no expression was detected during the other ripening stages. The expression of LeNCED1 was earlier in seed than in fruit, and both were earlier than the expression of LeACS2, LeACS4, and LeACO1 (Fig. 1). However, in contrast to LeNCED1, the LeACS2, LeACS4 and LeACO1 genes were expressed from the turning to the over-ripe stage.
Fig. 1.
Expression of LeNCED1, LeACS2, LeACS4, and LeACO1 during ripening in tomato fruits revealed by northern analysis. Each lane contained 5 μg of total RNA, and the transcript levels of rRNA are shown as an internal loading control. MG, mature green (40 d after anthesis); B1, breaker (44 d after anthesis); B2, breaker (45 d after anthesis); T, turning (47 d after anthesis); P, pink (50 d after anthesis); MR, mature red (53 d after anthesis).
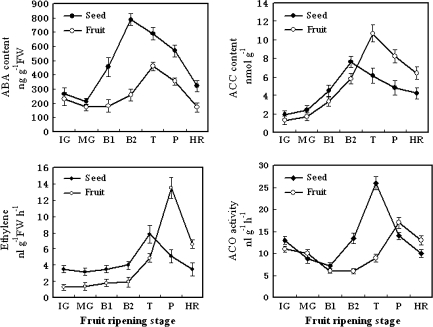
Changes in endogenous ABA, ACC content, ethylene production, and ACO oxidase activities at different ripening stages
After the LeNCED1 gene was expressed, endogenous ABA or ACC in both seeds and fruits increased rapidly to a peak at the breaker or turning stage; this high level was retained until the pink stage, and then declined rapidly at the harvest red stage (Fig. 2). The maximum ABA content preceded ethylene production in both the seeds and the flesh. However, ethylene began to increase in the turning stage and reached its peak value at the pink stage, consistent with ACC oxidase activities during fruit ripening. The ethylene production was lower in the seed than in the fruit. Although the peak value of ABA, ACC, ACC oxidase activity, and ethylene occurred at different ripening stages, they all increased first in seeds and then in flesh tissues.
Fig. 2.
Changes in the endogenous ABA content, ethylene production, ACC content, and ACO activity during fruit ripening in the seed and flesh of tomato. Fruit samples were collected from the immature green to the red ripe stage. Twenty fruits were collected for each treatment with three replications. Each point represents the mean of three replications, and vertical bars represent ±SE. IG, immature green (20 d after anthesis); MG, mature green (40 d after anthesis); B1, breaker (44 d after anthesis); B2, breaker (45 d after anthesis); T, turning (47 days after anthesis); P, pink (50 d after anthesis); MR, mature red (53 d after anthesis).
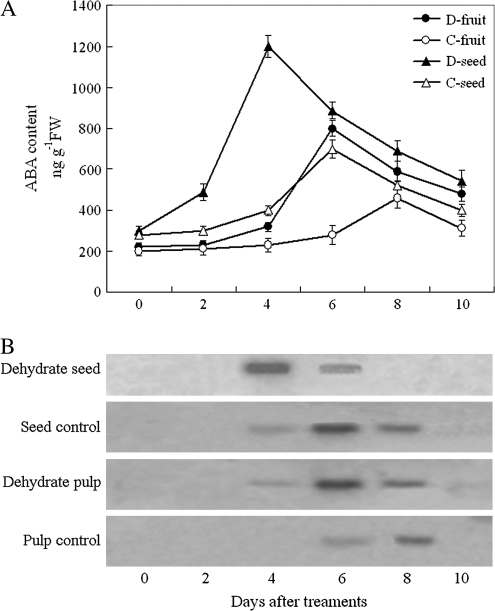
Effect of dehydration on endogenous ABA and expression of the LeNCED1 gene
When dehydration treatment was performed on both fruit and seed 4 d before the mature green stage, the endogenous ABA rapidly increased in both flesh and seed of tomato fruits (Fig. 3A).
Fig. 3.
Changes in the endogenous ABA contents (A) and LeNCED1 expression (B) in the dehydrated flesh and seed of tomato fruit held in low (45%) or high (95%) RH conditions (temperature 20 °C). Each lane in B contained 5 μg of total RNA, and the transcript levels of rRNA are shown as an internal loading control. Each point in A represents the mean of three replications and vertical bars represent ±SE. Filled triangles, dehydrated seed; open squares, control (seed); filled circles, dehydrated flesh; open circles, control (flesh)
The peak level of ABA was observed at the early mature stage, occurring 2 d earlier than that in the control. The expression of LeNCED1 was also earlier in dehydration-treated fruits than in control fruits (Fig. 3B). Seed was hypersensitive to dehydration treatment compared with the flesh, but the increased amount of endogenous ABA in the flesh was higher than that in the seed. These results suggested that water stress-induced NCED gene expression and ABA accumulation may regulate the onset of ripening.
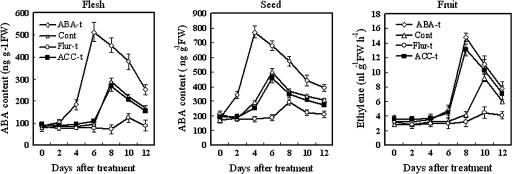
Effect of exogenous ABA, fluridone, and ACC treatments on fruit maturation
When the fruits were treated with exogenous ABA, ABA in fruits increased rapidly from 4 d to 6 d (at the maximum); the peak ABA level was found 2 d earlier than in the control fruits. In contrast, in fruits treated with fluridone, the peak ABA level was delayed by 2 d compared with that in the control (Fig. 4). The ABA peak in seed treated with fluridone appeared 2 d later than that in the flesh. In contrast, the ABA peak in seed treated with exogenous ABA occurred 2 d earlier than in the flesh. However, there was no difference in ABA content between ACC treatment and the control.
Fig. 4.
Effects of exogenous ABA, fluridone, and ACC treatments on ABA levels and ethylene production in the flesh and seed of tomato fruits during the mature green stage. Each point in A represents the mean of three replications and vertical bars represent ±SE. Open diamond, 100 μM ABA; open square, control (distilled water); open circle, 100 μM fluridone; filled square, 100 μM ACC.
On the other hand, ethylene increased remarkably in the fruits treated with exogenous ABA or ACC, from 6 d, and reached a peak at 8 d after treatments. The treatment of fruits with fluridone significantly decreased ABA levels and inhibited fruit softening. The experimental results indicated that ABA treatment induced ethylene synthesis, but ethylene (ACC) treatment had no effect on ABA.
Effects of repeatedly applied exogenous ABA, fluridone, and ACC on postharvest fruit firmness
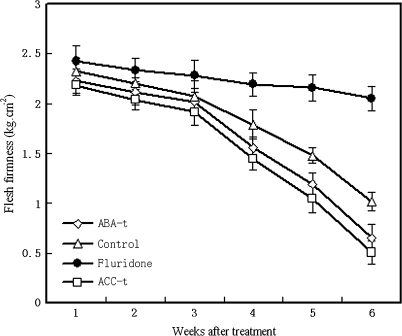
The application of ABA or ACC accelerated the rise in ethylene production and respiration associated with changes in skin colour and fruit softening compared with control fruits treated only with water (Fig. 6). Flesh firmness was higher in fruits treated repeatedly with fluridone than in fruits treated with ABA or ACC, and in control. The flesh firmness of the ABA- and ACC-treated fruits at the sixth week after treatment were ∼36.3% and 50.0% lower than that of control fruits, respectively (Fig. 5). The reduction of the ABA level in fruit resulted in delayed ripening and softening, indicating that the fruit ripening and softening were closely related to ABA accumulation; that is, ABA may play an important role in regulating the later stage of fruit ripening.
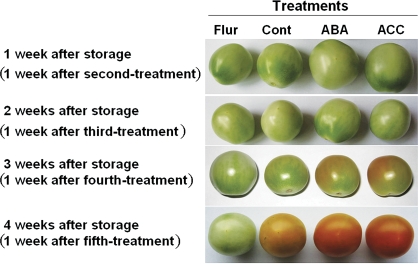
Fig. 6.
Changes of skin colour in tomato fruits treated with exogenous ABA or fluridone or ACC solution. Non-treated fruits were taken as the control. In the green mature stage, tomato fruits were harvested and treated with 0.5 ml per fruit each of 100 μM ABA, 100 μM fluridone, or 100 μM ACC, or 0.5 ml of distilled water per fruit (control).
Fig. 5.
Changes in flesh firmness in tomato fruits treated with exogenous ABA or fluridone or ACC solution. Non-treated fruits were taken as the control. Each point represents the mean of three replications and vertical bars represent ±SE. Open diamond, 100 μM ABA; open square, control (distilled water); filled circle, 100 μM fluridone; open square, 100 μM ACC.
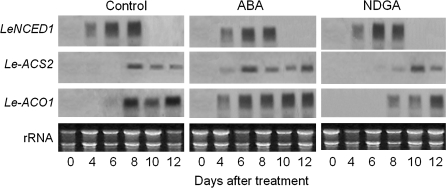
Expression of the LeACS2 and LeACO1 genes in the fruit after treatment with exogenous ABA or NDGA
Northern analysis showed that in the pulp of control fruit, lower expression of the LeNCED1 gene occurred 4 d after harvest, and peaked at 6–8 d, whereas LeACS2 and LeACO1 were expressed at 8–12 d after harvest (Fig. 7). NDGA treatment suppressed and deferred the expression of LeACS2 and LeACO1 to lower levels, and ABA treatment, in contrast, promoted them. All treatments with ABA or NDGA had no effect on expression of the LeNCED1 gene. These observations indicate that ABA may mediate the ACC synthase gene (LeACS2) and ACC oxidase gene (LeACO1) in tomato fruit during ripening.
Fig. 7.
Changes in the expression of LeNCED1, LeACS2, and LeACO1 in tomato fruit treated with exogenous ABA or NDGA. Each lane contained 5 μg of total RNA, and the transcript levels of rRNA are shown as an internal loading control.
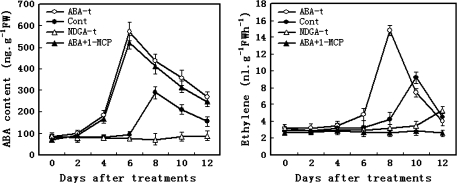
Effect of NDGA and 1-MCP on ABA accumulation and ethylene synthesis
The changes in content of ABA and ethylene production after treatment with exogenous ABA, NDGA, or 1-MCP are shown in Fig. 8. When the fruits were treated with exogenous ABA, both alone and together with 1-MCP, the ABA content in fruits increased rapidly from 4 d and reached the peak level at 6 d after treatment, which was 2 d earlier than that in the control fruits. In contrast, ABA accumulation was completely blocked when fruit was treated with NDGA, but NDGA could not completely block ethylene biosynthesis in the later ripening stage. On the other hand, ethylene increased remarkably in the fruits treated with exogenous ABA, from 6 d, and reached the peak at 8 d after treatment. The ethylene production was completely blocked by 1-MCP treatment (Fig. 8). Treatment with both NDGA and 1-MCP inhibited fruit ripening and softening. The roles of both ABA and ethylene in the later ripening of fruit are complex.
Fig. 8.
Effects of exogenous ABA, NDGA, and ABA+1-MCP treatments on ABA levels and ethylene production in tomato fruits. Each point represents the mean of three replications and vertical bars represent ±SE. Open circles, 100 μM ABA; filled circles, control (distilled water); open squares, 100 μM NDGA; filled triangles; 100 μM ABA+5 μl l−1 1-MCP.
Discussion
The initiation of ABA biosynthesis by the LeNCED1 gene at the stage of onset of fruit ripening
Cloning and characterization of NCEDs in fruits facilitate understanding of the role of ABA in fruit ripening. Two NCED genes have been isolated from avocado (Persea Americana Mill.), a climacteric fruit. PaNCED1 and PaNCED3 were expressed consistently with ethylene production during fruit ripening but earlier than ABA accumulation. Thus it has been proposed that ethylene induces NCED gene expression and ABA accumulation, which results in post-ripeness (Chernys and Zeevaart, 2000). Recently, two NCED genes, CsNCED1 and CsNCED2, have been isolated from Citrus sinensis L. Osbeck, a non-climacteric fruit (Rodrigo et al., 2006). Only CsNCED1 was expressed in fruit in accordance with ABA accumulation, suggesting that CsNCED1 may play a key role in ABA biosynthesis.
In this study, two cDNAs of NCED gene were cloned from tomato fruit, which shows the existence of at least two NCED genes. The expression of the LeNCED1 gene and ethylene-associated genes (LeACS2, LeACS4, and LeACO1) was also analysed in tomato fruit during ripening. The comparison of the expression of these genes led to the important discovery that the LeNCED1 gene was expressed only at the stage of onset of ripening in both the flesh and seed when the ABA content becomes high, in response to which the expression of LeNCED1 occurred earlier than the expression of LeACS2, LeACS4, and LeACO1. Therefore, it was proposed that the expression of the LeNCED1 gene initiated ABA biosynthesis at the beginning of fruit ripening, acting as an original inducer for maturation. On the other hand, ethylene biosynthesis during the climacteric stage has been demonstrated to be due to the accumulation of transcripts of two ACC synthase genes, LeACS2 and LeACS4 (Rottmann et al., 1991; Lincoln et al., 1993), and two ACC oxidase genes, LeACO1 and LeACO4 (Barry et al., 1996; Nakatsuka et al., 1998). Moreover, fruit ripening in both rin and nor, ripening-impaired tomato mutants, was not induced by exogenous ethylene treatment, although the expression of some ripening-associated genes is induced by ethylene treatment in both mutant fruits (Lanahan et al., 1994; Yen et al., 1995; Giovannoni, 2001). These results suggest that an ethylene-independent regulator may exist upstream of ethylene for controlling the onset of ripening in both fruit types. Exogenous ABA treatment might induce gene expression of LeACS2 and LeACO1 (Fig. 7) and initiate the ripening process in the tomato fruits (Figs 6–8), suggesting that ABA might act as an upstream regulator before ethylene in fruit ripening.
The induction of ethylene synthesis and initiation of fruit ripening by ABA accumulation
Generally, the ripeness and senescence of respiratory climacteric fruits are regulated by ethylene. However, the content of ABA increases during the ripening of respiratory climacteric fruits such as apples (Scriren and Wills, 1984; Kondo et al., 1991), peaches (Looney et al., 1974; Tsuchida, 1990), and pears (Rudnicki et al., 1968). In this study, endogenous ABA accumulated before ethylene production in tomatoes. The results suggested that it is endogenous ABA, but not ethylene, that is critical for the onset of ripening, while the role of ethylene is limited to later ripening stages. The results indicated that exogenous ABA accelerated fruit ripening and, in contrast, fluridone treatment delayed the ripening by inhibiting ABA.
The production of ethylene was triggered by applying ABA to tomato fruits during the ripening stage, which indicated that increasing ABA to a certain level in the fruit could promote the transformation of ACC to ethylene and result in a series of physiological and biochemical responses that facilitate fruit ripening. Thus, it was proposed that ABA may act at upstream metabolic events of ethylene action/perception and initiate the ripening process by inhibiting or activating general metabolic events. In addition, ABA might induce the expression of non-ethylene-dependent genes and initiate the ripening process in respiratory climacteric fruits.
Since 1-MCP effectively blocks ethylene receptors and represses ethylene-mediated effects in plant tissues (Sisler and Serek, 1997), it was used to study ethylene-regulated processes during later ripening in this study (Fig. 8). The result showed that fruit ripening and softening were effectively inhibited by 1-MCP treatment, although ABA was fully biosynthesized by exogenous ABA stimulation. These results indicate that ABA plays an important role in fruit ripening, and ethylene is more important for later ripening stages.
Effects of exogenous ABA, fluridone, and NDGA on ABA accumulation, ethylene synthesis, and fruit ripening
In higher plants, ABA is formed by oxidative cleavage of epoxicarotenoids, such as neoxanthin and violaxanthin. Fluridone specifically inhibits the biosynthesis of epoxicarotenoid, and thus is considered as an effective inhibitor of ABA biosynthesis (Bartels and Watson, 1978; Rasmussen et al., 1997). Fluridone inhibits phytoene desaturase, which converts phytoene to phytofluene in the carotenoid biosynthesis pathway. Since carotenoids are the precursors of ABA in plants, carotenoid biosynthesis inhibitors should also prevent the biosynthesis of ABA. The results in this study showed that exogenous applications of ABA to mature green fruit could induce ethylene synthesis, accelerated fruit colouring, and accelerated softening (Figs 5, 6). In contrast, treating the fruits with fluridone led to an obvious reduction of endogenous ABA and impeded fruit maturity and softening (Figs 4–6). Because colour change and fruit softening are dependent on both the presence and perception of ethylene (Blankenship and Sisler, 1993), the results suggest that ABA has a direct effect on ethylene synthesis although fluridone could not completely block ABA accumulation (Fig. 4). In order to block ABA accumulation fully, NDGA was used in the test (Figs 7, 8). NDGA is now emerging as a useful compound to study ABA function in plants (Creelman et al., 1992). Preliminary tests showed that NDGA was an ideal inhibitor of the NCED enzyme with regard to its permeating speed and ability to block ABA biosynthesis. ABA accumulation in detached mature green tomato is very sensitive to NDGA. Because a concentration of 100 μM could totally block ABA accumulation, it was then used for the assessment of the role of ABA. There are two ways for NDGA to inhibit ABA accumulation: one is to inhibit the expression of ABA synthase genes at the transcriptional level while the other is to inhibit synthase activities at the post-transcriptional level, or both. The results of northern analysis of ABA synthase genes showed that the expression of LeNCED1 was not influenced by NDGA compared with the control samples. This indicates that the blocked ABA accumulation caused by NDGA was due to its direct inhibition of the synthase activities at the post-transcriptional level (Fig. 7). As shown in Figs 7 and 8, ABA accumulation was totally blocked by 100 μM NDGA, but ethylene production increased ∼12 d after NDGA treatment, suggesting that fluridone or NDGA were acting only during a limited period of time, and the increase of ethylene and fruit ripening at later stages could be explained by a cessation of the effectiveness of fluridone or NDGA with time after treatment.
In conclusion, a relationship between ABA and ethylene during ripening and senescence was indicated in the tomato fruit: (i) the expression of the ABA biosynthetic gene (LeNCED1) occurs before that of ethylene biosynthesis genes; (ii) ABA content also preceded the climacteric increase in ethylene production; (iii) ABA may induce ethylene biosynthesis via the regulation of ACS and ACO gene expression; (iv) exogenous ABA accelerates fruit ripening, and fluridone or NDGA treatment delayed fruit ripening by inhibition of ABA; and (v) ethylene plays a key role in the later stages of fruit ripening.
Acknowledgments
This work was supported by the Beijing Natural Science Foundation (6052013) and the Beijing Municipal Science and Technology Commission (D0706002000091).
References
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. Journal of Experimental Botany. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- Alferez F, Zacarias L. Interaction between ethylene and abscisic acid in the regulation of citrus fruit maturation. In: Kanellis AK, Chang C, Klee H, Blecker AB, Pech JC, Grierson D, editors. Biology and biotechnology of the plant hormone ethylene II. Amsterdam: Kluwer Academic Publishers; 1999. pp. 183–184. [Google Scholar]
- Barry CS, Blume B, Bouzayen M, Cooper W, Hamillton AJ, Grierson D. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. The Plant Journal. 1996;9:525–535. doi: 10.1046/j.1365-313x.1996.09040525.x. [DOI] [PubMed] [Google Scholar]
- Bartels PG, Watson CW. Inhibition of carotenoid synthesis by fluridone and norflurazon. Weed Science. 1978;26:198–203. [Google Scholar]
- Blankenship SM, Sisler EC. Response of apples to diazocyclopentadiene inhibition of ethylene binding. Postharvest Biology and Technology. 1993;3:95–101. [Google Scholar]
- Buesa C, Dominguez M, Vendrell M. Abscisic acid effects on ethylene production and respiration rate in detached apple fruits at different stages of development. Revista Espanola de Ciencia y Tecnologia de Alimentos. 1994;34:495–506. [Google Scholar]
- Burbidge A, Grieve TM, Jackson A, Thompson A, McCarty DR, Taylor IB. Characterization of the ABA-deficient tomato mutant notabilis and its relationship with maize Vp14. The Plant Journal. 1999;17:427–431. doi: 10.1046/j.1365-313x.1999.00386.x. [DOI] [PubMed] [Google Scholar]
- Burbidge A, Grieve TM, Jackson A, Thompson A, Taylor T. Structure and expression of a cDNA encoding a putative neoxanthin cleavage enzyme (NCE), isolated from a wilt-related tomato (Lycopersicon esculentum Mill.) library. Journal of Experimental Botany. 1997;317:2111–2112. [Google Scholar]
- Chernys JT, Zeevaart JAD. Characterization of the 9-cisepoxycarotenoid dioxygenase gene family and the regulation of abcisic acid biosynthesis in avocado. Plant Physiology. 2000;124:343–353. doi: 10.1104/pp.124.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe BG. The grape berry as a sink. Acta Horticulturae. 1989;239:149–158. [Google Scholar]
- Creelman RA, Bell E, Mullet JE. Involvement of a lipoxygenase-like enzyme in abscisic acid biosynthesis. Plant Physiology. 1992;99:1258–1260. doi: 10.1104/pp.99.3.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galpaz N, Wang Q, Menda N, Zamir d, Hirschberg J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. The Plant Journal. 2008;53:717–730. doi: 10.1111/j.1365-313X.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- Giovannoni J. Molecular biology of fruit maturation and ripening. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Giovannoni J. Genetic regulation of fruit development and ripening. The Plant Cell. 2004;16:S170–S180. doi: 10.1105/tpc.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba A, Ishida M, Sobajima Y. Changes in endogenous hormone concentration during berry development in relation to the ripening of Delaware grape. Journal of the Japanese Society for Horticultural Science. 1976;45:245–252. [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi K, Shinozaki K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase. a key enzyme in abscisic acid biosynthesis in Arabidopsis. The Plant Journal. 2001;27:325–333. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Yamaguchi K, Shinozaki K. A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiology. 2000;123:553–562. doi: 10.1104/pp.123.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K. Distribution and change of endogenous IAA and ABA in asparagus spear and orange fruit. Chemical Regulation of Plants. 1996;31:68–71. [Google Scholar]
- Kondo S, Inoue K. Abscisic acid and 1-aminocyclopropane-1-carboxylic acid (ACC) content during growth of ‘Satohnishiki’ cherry fruit, and the effect of ABA and ethephon application on fruit quality. Journal of Horticultural Science. 1997;72:221–227. [Google Scholar]
- Kondo S, Tomiyama A. Changes of free and conjugated ABA in the fruit of Satohnishiki sweet cherry and the ABA metabolism after application of (s)-(+)-ABA. Journal of Horticultural Science and Biotechnology. 1998;73:467–472. [Google Scholar]
- Kondo S, Uthaibutra J, Gemma H. Comparison of ACC, abscisic acid and anthocyanin content of some apple cultivars during fruit growth and maturation. Journal of the Society for Horticultural Science. 1991;60:505–511. [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. The Never Ripe mutation blocks ethylene perception in tomato. The Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln JE, Campbell AD, Oetiker J, Rottmann WH, Oeller PW, Shen NF, Theologis A. LE-ACS4, a fruit ripening and wound-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum) Journal of Biological Chemistry. 1993;268:19422–19430. [PubMed] [Google Scholar]
- Lizada MCC, Yang SF. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Analytical Biochemistry. 1979;100:140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Looney NE, Mc Glasson WB, Coombe BF. Control of fruit ripening in peach, Prunus persica: action of succinic acid-2,2-dimethylhydrazide and (2-choloroethyl) phosphonic acid. Australian Journal of Plant Physiology. 1974;1:77–86. [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiology. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Shiomi S, Kubo Y, Inaba A. Expression and internal feedback regulation of ACC synthase and ACC oxidase genes in ripening tomato fruit. Plant and Cell Physiology. 1997;38:1103–1110. doi: 10.1093/oxfordjournals.pcp.a029094. [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. Abscisic acid biosynthesis and metabolism. Annual Review of Plant Biology. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- Oetiker JH, Olson DC, Shiu OY, Yang SF. Differential induction of seven 1-aminocyclopropane-1-carbocylate synthase genes by elicitor in suspension cultures of tomato (Lycopersicon esculentum) Plant Molecular Biology. 1997;34:275–286. doi: 10.1023/a:1005800511372. [DOI] [PubMed] [Google Scholar]
- Qin X, Zeevaart JAD. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proceedings of the National Academy of Sciences, USA. 1999;96:15354–15361. doi: 10.1073/pnas.96.26.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen RD, Hole D, Hess JR, Carman JG. Wheat kernel dormancy and abscisic acid level following exposure to fluridone. Journal of Plant Physiology. 1997;150:440–445. [Google Scholar]
- Rodrigo MJ, Marcos JF, Alferez F, Mallent M, Zacarias L. Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. Journal of Experimental Botany. 2003;54:727–738. doi: 10.1093/jxb/erg083. [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, Alquezar B, Zacarias L. Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck) Journal of Experimental Botany. 2006;57:633–643. doi: 10.1093/jxb/erj048. [DOI] [PubMed] [Google Scholar]
- Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. Journal of Molecular Biology. 1991;222:937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Rudnicki R, Machik J, Pieniazek J. Abscisin II in strawberry plants at two different stages of growth. Bulletin de l'Academie Polonaise des Sciences, Series des Sciences Biologiques. 1968;16:509–512. [PubMed] [Google Scholar]
- Scriren FM, Wills RBN. Postharvest changes in abscisic acid levels in flesh tissue and seeds of Jonathan apples susceptible to storage breakdown. Journal of Horticultural Science. 1984;59:171–174. [Google Scholar]
- Shiu OY, Oetiker JH, Yip WK, Yang SF. The promoter of Le-ACS7, an early flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato, is tagged by a Sol3 transposon. Proceedings of the National Academy of Sciences, USA. 1998;95:10334–10339. doi: 10.1073/pnas.95.17.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiologia Plantarum. 1997;100:577–582. [Google Scholar]
- Tan BC, Schwartz SH, Zeevaart JAD, McCarty DR. Genetic control of abscisic acid biosynthesis in maize. Proceedings of the National Academy of Sciences, USA. 1997;94:12235–12240. doi: 10.1073/pnas.94.22.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor IB, Burbidge A, Thompson AJ. Control of abscisic acid synthesis. Journal of Experimental Botany. 2000;51:1563–1574. doi: 10.1093/jexbot/51.350.1563. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Jackson AC, Symonds RC, Mulholland BJ, Dadswell AR, Blake PS, Burbidge A, Taylor IB. Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. The Plant Journal. 2000;23:363–374. doi: 10.1046/j.1365-313x.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- Tigchelaar E, McGlasson W, Buescher R. Genetic regulation of tomato fruit ripening. HortScience. 1978;13:508–513. [Google Scholar]
- Tsuchida H, Mizuno S. Changes in abscisic acid and phaseic acid during senescence of peach fruits. Journal of the Japanese Society for Horticultural Science. 1990;58:801–805. [Google Scholar]
- Vendrell M, Buesa C. Relationship between abscisic acid content and ripening of apples. Acta Horticulturae. 1989;258:389–396. [Google Scholar]
- Wan CY, Wilkins TA. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.) Analytical Biochemistry. 1994;223:7–12. doi: 10.1006/abio.1994.1538. [DOI] [PubMed] [Google Scholar]
- Woldrep WT, Taylor HM. 1-Methyl-3-phenyl-5-{-(trifluoromethyl) phenyl}-4(1H)-pyridinone, a new herbicide. Journal of Agricultural and Food Chemistry. 1976;24:1250–1251. doi: 10.1021/jf60208a047. [DOI] [PubMed] [Google Scholar]
- Yen H, Lee S, Tanksley S, Lanahan M, Klee HJ, Giovannoni JJ. The tomato Never-ripe locus regulates ethylene-inducible gene expression and is linked to a homologue of the Arabidopsis ETR1 gene. Plant Physiology. 1995;107:1343–1353. doi: 10.1104/pp.107.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarembinski TI, Theologis A. Ethylene biosynthesis and action: a case of conservation. Plant Molecular Biology. 1994;26:1579–1597. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]