Abstract
Most natural killer (NK) T cells express an invariant Vα14 T-cell receptor. To explore the contribution of NKT cells in an animal model for multiple sclerosis, Theiler’s murine encephalomyelitis virus (TMEV) infection, TMEV-infected mice were treated with Vα14 antibody. Treatment during the early stage of infection delayed the onset of demyelinating disease with higher interleukin-4 production, whereas administration during the late stage or weekly resulted in more severe demyelination with enhanced virus persistence. The effect of in vivo depletion of NKT cells differed depending on the stage of infection, suggesting contrasting roles for NKT cells over the disease course.
Keywords: CD1d, CNS autoimmune demyelinating diseases, cytokines, iNKT lymphocytes, Picornaviridae infections, Theiler’s virus
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS). Although the etiology of MS remains unclear, MS is considered to be an immune-mediated disease; CD4+ and CD8+ T cells and B cells have been shown to play potentially pathogenic as well as protective roles in MS (reviewed in Rose et al, 2004). MS has been suggested to develop in genetically susceptible individuals and requires environmental triggers, such as virus infection. Epidemiological studies and experimental models have suggested a role for viruses in MS (reviewed in Greenlee and Rose, 2000).
Natural killer (NK) T cells have both NK cell markers, such as CD161 (CD161c is known as NK1.1 in mice), and T-cell receptors (TCR) (Taniguchi et al, 1996). Most NKT cells express an invariant TCR encoded by Vα14 in combination with Vβ2, Vβ7, or Vβ8 in mice and Vα24 in combination with Vβ11 in humans (Makino et al, 1995). Vα14 TCR is expressed on murine NKT cells, but not on NK cells or conventional T cells (Apostolou et al, 1999; Dang et al, 2000; Schofield et al, 1999; Sharif et al, 2002; Makino et al, 1995). NKT cells recognize glycolipid antigens presented with CD1d (Kawano et al, 1997). NKT cells can produce large amounts of cytokines, particularly interleukin (IL)-4 and/or interferon (IFN)-γ, leading to modulation of a variety of immune cells; NKT cells can play an important role in bridging innate and acquired immunity (Yoshimoto et al, 1995; Taniguchi et al, 2003; Yamamura et al, 2007). NKT cells have been implicated in a variety of immune responses such as tumor immunity (Cui et al, 1997; Kawano et al, 1998) and immune responses against a variety of microbial pathogens, including viruses, in both humans and mice (Grubor-Bauk et al, 2003; Levy et al, 2003; Rigaud et al, 2006; Van Kaer, 2007).
It has been proposed that NKT cells play a protective role in the development of several immune-mediated diseases, such as lupus and diabetes, in humans and other animals (Miyake and Yamamura, 2005; Van Kaer, 2007; van der Vliet et al, 2001). In MS, several studies have suggested a beneficial role for NKT cells in pathogenesis, although it may be difficult to compare the studies due to the differences in the proportion of NKT cells in normal individuals among the studies. Some investigators have reported that Vα24+ NKT cells were rare in plaque lesions and reduced in the peripheral blood (Illés et al, 2000; van der Vliet et al, 2001). However, others found no difference in the frequency of CD4− CD8− Vα24+ NKT cells between MS and control subjects, whereas frequencies of IL-4 secreting NKT cell clones were lower in relapsing-remitting MS than in progressive MS and controls (Gausling et al, 2001). The CD1d-restricted NKT cells expanded from MS patients in remission produced a larger amount of IL-4 than those from patients with active MS and control individuals (Araki et al, 2003). Similarly, in experimental allergic (autoimmune) encephalomyelitis (EAE), an autoimmune model for MS, NKT cells have been shown to play a protective role against demyelination, whereas others reported no role or a pathogeneic role for NKT cells (Furlan et al, 2003; Jahng et al, 2001; Mars et al, 2002; Miyamoto et al, 2001; Singh et al, 2001; Teige et al, 2004). The discrepancies could be due to differences in mouse strains, encephalitogen, and methods of depletion/stimulation of NKT cells used in the studies.
Theiler’s murine encephalomyelitis virus (TMEV) belongs to the family Picornaviridae, and the Theiler’s original (TO) subgroup viruses include the Daniels (DA) and BeAn strains. Intracerebral injection of susceptible mice with DA virus leads to a persistent infection of the white matter in the spinal cord of susceptible mice. This leads to the development of inflammatory demyelinating lesions (Tsunoda and Fujinami, 1999), reminiscent of those observed in MS (Pirko et al, 2007). Although precise mechanisms of demyelination are unclear, direct virus infection of myelin-forming cells, oligodendrocytes, as well as immune-mediated pathomechanisms, including CD4+ and CD8+ T cells and demyelinating antibody, have been demonstrated to play important roles.
NKT cells have been shown to play a protective role in virus infection, including picornaviruses (Exley et al, 2001; Potvin et al, 2003). However, NKT cells could play a regulatory or pathogenic role in autoimmunity through the production of immunoregulatory cytokines (Yamamura et al, 2007). Thus, in TMEV infection, NKT cells could not only contribute to viral clearance but also modulate the immune-mediated demyelinating disease. It has been reported that administration of monoclonal antibody against Vα14 TCR, CMS-5 (Ito et al, 1991), to MRL lpr/lpr mice leads to a decrease in the number of Vα14+ NKT cells and acceleration of autoimmune disease (Mieza et al, 1996). Here, we examined clinically, histologically and immunologically whether NKT cells could modulate TMEV-induced demyelinating disease, using the same depletion protocol and Vα14 antibody CMS-5.
Early Vα14 antibody treatment delays the onset of chronic disease
Infection of SJL/J mice with the DA strain of TMEV causes a biphasic disease. During the acute stage, 1 week post infection (p.i.), TMEV predominantly infects neurons in the gray matter of the brain. Infected mice generally do not develop paralysis of the limbs and had only mild weight loss and alterations in the righting reflex. Infected mice survive and develop an inflammatory demyelinating disease in the white matter of the spinal cord (chronic stage). During the chronic stage, TMEV persists in macrophages and glial cells but not in neurons. The onset of the chronic stage is around 3 to 4 weeks p.i., and after this time point, infected mice develop a spastic paralysis of the hind limbs due to inflammatory demyelinating changes in the spinal cord. Effects of immunomodulation therapies, such as T-cell depletion or cytokine antibody administration, have been shown to differ depending on the timing of treatment in TMEV infection (Tsunoda and Fujinami, 1999). Thus, we decided to administer Vα14 antibody: (1) before and at the time of infection (the early treatment group); (2) at 3 and 4 weeks p.i. (the late treatment group); or (3) weekly (the weekly treatment group) in TMEV-infected mice.
Purified Vα14 antibody (1 mg/ml) from the supernatant fluid of CMS-5 cell culture (previously described as CMS-1) (Ito et al, 1991) was used for the injection of female SJL/J mice (Jackson Laboratory, Bar Harbor, ME), as described previously (Mieza et al, 1996). NK and NKT cells in SJL/J mice have been shown to be undetectable or very few (Kaminsky et al, 1983, 1985; Kaminsky et al.); we have confirmed that NK1.1+ CD3+ NKT cells were undetectable by flow cytometry in peripheral mononuclear cells (MNCs) in SJL/J mice (<0.5%). This made it difficult to assess the efficacy of NKT cell depletion by Vα14 antibody treatment in SJL/J mice. Thus, we took advantage of using A.SW mice whose NKT cells are inducible and their H-2 haplotype (H-2s) is the same as SJL/J mice (Kaminsky et al, 1983, 1985; Kaminsky et al.; Tsunoda et al, 2000). Weekly Vα14 antibody treatment of A.SW mice resulted in depletion of 96.3% of NK1.1+CD3+ NKT cells, whereas the treatment did not deplete NK1.1+CD3− NK cells. Groups of mice were injected intraperitoneally with 100 μg of Vα14 antibody: (1) on weeks −2, −1, 0, and 1 p.i. (four injections before and during the early stage of infection); (2) on 3 and 4 weeks p.i. (two injections during the late stage of infection); or (3) weekly (seven injections). Control mice received purified mouse immunoglobulin (Ig) weekly, or no injections. All mice were infected intracerebrally with 2 × 105 plaque-forming units (PFU) of the DA strain of TMEV at 7 weeks of age. Clinical signs of disease were evaluated by means of an impaired righting reflex (Tsunoda et al, 2001).
During the first 3 weeks p.i., all groups of mice had mild impairment of the righting reflex, and there were no significant differences in clinical signs among the groups (Figure 1). Beginning around 3 and 4 weeks p.i., impairment of the righting reflex, i.e., the onset of demyelinating disease, was seen in control infected mice and groups of mice receiving Vα14 antibody weekly or during the late stage of infection. Interestingly, the onset of the clinical signs was delayed about 1 week in the group receiving Vα14 antibody during the early stage. Mice treated during the early stage had significantly lower righting reflex scores than mice treated during the late stage or weekly or control mice (P < .05: day 24, early versus weekly groups; day 27, early versus late or weekly groups; day 28, early versus weekly or control groups). However, at 5 weeks p.i., all groups of mice, including the ones treated during the early stage, had similar levels of impairment of the righting reflex.
Figure 1.
Modulation of clinical disease in TMEV-infected mice by administration of Vα14 antibody. TMEV was injected on day 0. TMEV-infected mice received Vα14 antibody: weekly (○); on weeks, −2, −1, 0, and 1 (early; π); or on weeks 3 and 4 post infection (late; ▲). Control mice received mouse immunoglobulin (Ig) or no injection (●). Impaired righting reflex scores were compared between the groups. When the proximal end of the mouse’s tail is grasped and twisted to the right and then to the left, a healthy mouse resists being turned over (score of 0). If the mouse is flipped onto its back but immediately rights itself on one side or both sides, it is given a score of 1 or 1.5, respectively. If it rights itself in 1 to 5 seconds, the score is 2. If righting takes more than 5 s, the score is 3. Three to four weeks after infection, mice treated during the early stage had significantly lower righting reflex scores, comparing with the other groups of mice (*P < .05). Shown are mean righting reflex scores of a group of 5 to 10 mice.
Late or weekly Vα14 antibody administration alters neuropathology and virus persistence in TMEV infection
We compared the neuropathology 5 weeks after TMEV infection, in mice that received control antibody or Vα14 antibody. Mice were perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). Brains were coronally divided into five slabs and spinal cords were transversely divided into 12 slabs, which were embedded in paraffin. Four micrometer thick sections were stained with Luxol fast blue for myelin visualization. Histological scoring was performed as previously described (Tsunoda et al, 2001). Viral antigen–positive cells were visualized by immunohistochemistry with TMEV antiserum using the avidin-biotin-peroxidase complex (ABC) technique (Vector, Burlingame, CA) with 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich) as chromogen (Tsunoda et al, 2007a). Enumeration of viral antigen–positive cells per quadrant in the spinal cord was carried out as described previously (Tsunoda et al, 2007b). The number of TMEV antigen–positive cells in the CNS has been shown to correlate with CNS virus titers measured by a plaque assay (McCright et al, 1999).
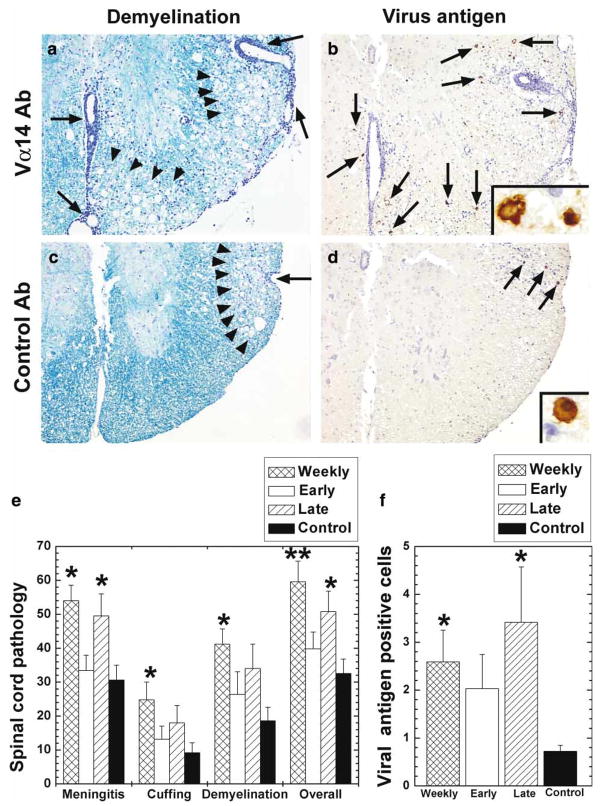
In the spinal cord, control infected mice receiving no antibody had inflammatory demyelinating lesions in the white matter, particularly in the ventral funiculus and the ventral root exit zone (Figure 2c). Using consecutive sections immunostained against viral antigen, we found small numbers of viral antigen–positive cells in the demyelinating lesions (Figure 2d). The mice receiving Vα14 antibody weekly or during the late stage had more severe inflammatory demyelination (Figure 2a) with more viral antigen–positive cells in the spinal cord (Figure 2b), compared with control mice. Spinal cord pathology scores and numbers of viral antigen–positive cells in mice treated weekly or during the late stage were significantly higher than those of control mice (Figure 2e, f). No significant difference was seen in neuropathology between control mice versus mice treated during the early stage. In contrast to the spinal cord, in the brain all groups of mice only had a mild degree of inflammation without accompanying demyelination. There was no difference in pathology scores or numbers of viral antigen–positive cells in the brain among groups (P > .05, analysis of variance [ANOVA], data not shown).
Figure 2.
Spinal cord pathology 5 weeks after TMEV infection, in mice that received either control antibody or Vα14 antibody. (a–d) TMEV-infected mice were treated with Vα14 antibody (a, b) or control antibody (c, d) weekly. Mice receiving Vα14 antibody had more severe meningitis (arrow), perivascular cuffing and demyelination (arrow head) (a) with an increase in the number of virus antigen– positive cells (arrow) (b) compared with control mice (c, d). (a, c) Luxol fast blue stain. (b, d) Immunohistochemistry for TMEV antigen. Magnifications: a–d, 48, inset, × 410. (e) Spinal cord pathology scores. Mice receiving Vα14 antibody weekly had significantly higher pathology scores in all categories: meningitis, perivascular cuffing, demyelination, and overall pathology than control mice receiving mouse Ig (**P < .01; *P < .05), whereas mice treated during the late stage had higher meningitis and overall pathology scores than control mice. (f) Numbers of viral antigen-positive cells per quadrant of the spinal cord white matter. Higher numbers of viral antigen-positive cells were detected in mice receiving Vα14 antibody weekly or during the late stage of TMEV infection, compared with control mice receiving mouse Ig (*P < .05). (e, f) Values are mean+standard error of the mean (SEM) for five mice. For scoring of spinal cord sections, each spinal cord segment was divided into four quadrants: the ventral funiculus, the dorsal funiculus, and each lateral funiculus. Any quadrant containing meningitis, perivascular cuffing, or demyelination was given a score of 1 in that pathologic class. The total number of positive quadrants for each pathologic class was determined, divided by the total number of quadrants present on the slide and multiplied by 100 to give the percent involvement for each pathologic class. An overall pathologic score was also determined by giving a positive score if any pathology was present in the quadrant. This was also presented as a percent involvement.
Effects of Vα14 antibody treatment on lymphoproliferative, antibody, and cytokine responses
We also compared cellular and humoral immune responses to TMEV, and monitored cytokine production, at 5 weeks p.i., among infected mice treated with Vα14 antibody or with control antibody. Spleen MNCs were isolated with Histopaque-1083 (Sigma-Aldrich). A volume of 100 μl of 2 × 105 MNCs was incubated with a 100-μl solution containing either live TMEV at a multiplicity of infection (MOI) of 5, 5 μg of purified ultraviolet-irradiated TMEV or 2 × 105 TMEV antigen-presenting cells (TMEV APCs). TMEV APCs were made from whole spleen cells infected in vitro with TMEV at an MOI of 1 and irradiated with 2000 rads (Tsunoda et al, 2007b). The MNCs were then cultured for 5 days and TMEV-specific lymphoproliferative responses were measured using [3H]thymidine. Regardless of the timing of Vα14 antibody administration, we found no significant differences in TMEV-specific lymphoproliferative responses. All groups showed substantial lymphoproliferation to TMEV, which was comparable to the control group (Figure 3a).
Figure 3.
Cellular and humoral immune responses at 5 weeks after TMEV infection, in mice that received Vα14 antibody weekly, during the early or late stage or control antibody. (a) Mononuclear cells (MNCs) were isolated from two to three spleens in each group and stimulated with no antigen (no Ag), live virus, UV-inactivated purified virus (virus Ag), or TMEV antigen-presenting cells (TMEV-APC) for 5 days. All groups of mice showed substantial lymphoproliferative responses to all stimuli. All cultures were performed in triplicate. Results are means of two independent experiments. (b) Serum anti-TMEV antibody titers were titrated from mice infected with TMEV using enzyme-linked immunosorbent assays (ELISAs). High antivirus antibody responses were detected in all groups of mice. There were no significant differences in antibody titers among groups. The end point of the assay was determined as the reciprocal of the highest dilution that gave an optical density reading that was two standard deviations above the control baseline from preimmune sera. Values are mean+SEM for five mice. (c) Interferon (IFN)-γ (left) and interleukin (IL)-4 (right) levels in spleen MNCs from TMEV-infected mice treated with Vα14 antibody. Cytokine concentrations of the supernatants from concanavalin A–stimulated MNCs were measured by ELISA. We detected lower IFN-γ and higher IL-4 production in the group receiving Vα14 antibody during the early time point, compared with the other groups. Each group was composed of five mice. ELISA was performed in duplicate, and shown are means of two independent experiments.
Using an enzyme-linked immunosorbent assay (ELISA), we titrated serum TMEV-specific antibodies among TMEV-infected mice treated with Vα14 antibody or control antibody, as described previously (Tsunoda et al, 2001). Ninety-six-well plates were coated overnight with TMEV antigen at 4°C. After blocking, two-fold dilutions of mouse sera beginning at 1:27 were added to the plates and incubated for 90 min. After washing, the plates were incubated with a peroxidase-labeled goat anti-mouse antibody, were colorized with o-phenylene-diamine dihydrochloride (Sigma-Aldrich), and were read at 492 nm. We found that all groups of mice had high titers of TMEV-specific antibody (Figure 3b). There were no significant differences in TMEV antibody titers among the groups (P > .05, ANOVA).
We measured the mitogen-induced production of IFN-γ versus IL-4 by spleen MNCs from TMEV-infected mice treated with Vα14 antibody, using the ELISA system, OptEIA Set (BD PharMingen, San Jose, CA), according to the manufacture’s instruction (Tsunoda et al, 2005). MNCs were cultured at 2 × 106 cells/ml in six-well plates (Corning, Corning, NY) in the presence or absence of concanavalin A (5 μg/ml) or TMEV. Culture supernatant fluids were harvested 48 h after stimulation. In cultures stimulated with concanavalin A, we detected a large amount of IFN-γ in all groups, while IL-4 was low (Figure 3c). The group receiving Vα14 antibody during the early stage produced lower levels of IFN-γ but higher levels of IL-4, compared with the other groups. Cytokine production was not seen in cultures stimulated with TMEV or no stimulation (data not shown). Because Vα14+ NKT cells have been predicted to play a role during the early phase of several infectious diseases, the modulation of cytokine production in this group is consistent with this hypothesis.
Contrasting roles for NKT cells in SJL/J mice infected with TMEV
In the present study, we found that Vα14 antibody treatment during the late stage or weekly resulted in severe neuropathology with higher numbers of virus antigen–positive cells in the CNS versus other groups. This suggests a protective role for NKT cells during TMEV infection. In contrast, treatment of mice in the early stage of TMEV infection delayed the onset of clinical disease with an alteration of the Th1/Th2 profile. At 1 month p.i., although we did not observe differences in TMEV-specific lymphoproliferative responses or serum antibody titers among any groups treated with Vα14 antibody, Vα14 antibody treatment did modulate the clinical disease, the extent of virus persistence and pathology. Further studies, including time course analyses of virus replication and anti-viral immune responses, are required to understand why Vα14 antibody treatment differs depending on the timing of antibody administration. Currently, we hypothesize that disease modulation by Vα14 antibody could be due to alterations in the cytokine profile. The cytokine profile of NKT cells is likely to change during the course of TMEV infection leading to the differential effects of Vα14 antibody treatment. Indeed in MS, CD4+ NKT cell lines from MS patients in remission have been shown to produce a larger amount of IL-4 than those from MS patients in relapse (Araki et al, 2003).
Previously, it was reported that Th1/Th2 and proinflammatory/anti-inflammatory cytokines are important in virus clearance and demyelination in TMEV infection. The modulation of cytokines at different stages in TMEV infection leads to different outcomes (Kim et al, 2005). Here, cytokines have dual functions: a protective role in the early stage of infection and a pathogeneic/regulatory role in inflammatory demyelinating disease in the late stage. Thus, the different outcomes of Vα14 antibody treatment could be due to (1) functional changes in the NKT cells during the course of TMEV infection, including cytokine production and cytotoxicity; and (2) different roles for the cytokines produced by NKT cells during the early versus the late stage of TMEV infection.
One concern in our current experiment could be that certain percentages of NKT cells have been shown to be Vα14 mRNA positive, but the cell surface expression of Vα14 TCR is negative in lymphoid organs, particularly in the thymus (Makino et al, 1995). This can be due to down-regulation of Vα14 TCR expression at a certain stage of differentiation in NKT cells. If this is the case, certain immature NKT cells would not be depleted by Vα14 antibody treatment; however, we do not know whether such a cell type is functional, or whether the cells become Vα14 TCR positive after maturation.
Another concern might be the fact that SJL/J mice have a low number of NKT cells and lack Vβ8+ NKT cells (Kaminsky et al, 1983; Kaminsky et al. 1985; Habu et al, 2007). The low NKT cell number in SJL/J mice was associated with defective early IL-4 responses to CD3 antibody challenge (Yoshimoto et al, 1995). However, despite the low number of NKT cells, in vivo and in vitro stimulation of NKT cells with a synthetic ligand of NKT cells, α-galactosylceramide (GalCer) (Kawano et al, 1997; Burdin et al, 1998), in SJL/J mice resulted in an increase of IL-4 and a decrease in IFN-γ (Serizawa et al, 2000; Singh et al, 2001). Stimulation of NKT cells in SJL mice has also been shown to modulate immune-mediated diseases including collagen-induced arthritis (Chiba et al, 2004) and pristine-induced lupus (Singh et al, 2005). When EAE was induced in SJL/J mice with myelin basic protein (Singh et al, 2001) or with myelin proteolipid protein peptide (PLP139–151) (Miyake and Yamamura, 2005), α-GalCer treatment exacerbated the morbidity and mortality, while the onset of disease was delayed. Our current depletion studies of NKT cells by Vα14 antibody administration also support the presence of functional NKT cells in vivo in SJL/J mice. We are investigating whether the relative lack of NKT cells in SJL/J mice, compared with other strains of mice, can contribute to susceptibility to demyelinating disease in TMEV infection. This can be tested by adoptive transfer of NKT cells or by α-GalCer treatment (Berzofsky and Terabe, 2008) during the course of TMEV infection.
NKT cells can play different roles depending on the mouse strain. Singh et al (Singh et al., 2005) tested whether α-GalCer could modulate pristine-induced lupus in BALB/c and SJL/J mice. They found that NKT cells in SJL/J mice exhibited a Th1-biased cytokine profile while BALB/c mice had a mixed Th1/Th2 cytokine profile. They also found that repeated injections of α-GalCer were able to prevent pristine-induced lupus in BALB/c mice that was dependent on CD1d and IL-4 expression. In contrast, such a regimen augmented disease induced in SJL mice; suggesting that NKT cells can play both suppressive and pathogenic roles in the regulation of lupus in a strain-dependent manner.
In separate experiments, we have investigated the role of CD1d-restricted NKT cells in TMEV infection using CD1d-deficient mice (CD1−/− mice) on a background of BALB/c mice, which are resistant to TMEV-induced demyelinating disease (Tsunoda et al, 2008). We found that only TMEV-infected CD1−/− mice developed demyelination with neurologic deficits 3 to 5 weeks p.i., suggesting that CD1d-restricted NKT cells may play a protective role against demyelination around 3 to 5 weeks p.i. This is consistent with our current results in SJL/J mice, where mice depleted of NKT cells at 3 and 4 weeks p.i. had an enhancement of the demyelinating disease versus control mice.
Although CD1d-restricted cells may include not only Vα14+ NKT cells but also certain T cells and Vα14− NKT cells (Amano et al, 1998; Baron et al, 2002; Huber et al, 2003), CD1d antibody treatment during the course of TMEV infection in SJL/J mice would give further insight into the role of NKT cells in TMEV-induced demyelination (Chiba et al, 2005). In addition, TMEV infection in CD1d- or Jα18-deficient mice with SJL/J background (currently not available) is an alternative method to see a role for NKT cells (Berzofsky and Terabe, 2008; Chiba et al, 2005; Cui et al, 1997), whereas it might not be the best way to see potentially contrasting roles for NKT cells, depending on the stage of TMEV infection.
Although the role of NKT cells has not been previously investigated in TMEV infection, the role of NK cells in TMEV infection was reported by Paya et al (Paya et al., 1989). In their study, DA virus-infected resistant C57BL/10 mice were treated with NK1.1 antibody or asialo GM1 antibody throughout the course of infection. Both antibody modalities resulted in exacerbation of the acute gray matter disease without an increase in viral titers in the CNS, although only asialo GM1 treated mice developed demyelination. In addition, NK-defective beige/beige mice also showed exacerbation of gray matter disease without demyelination. The authors suggested that NK cells were important for defense against early neuronal damage in the gray matter and that depletion of NK1.1+ cells was not sufficient to induce demyelination in the white matter in a demyelination resistant mouse strain. However, these results may not be solely attributed to depletion of NK cells alone because (1) both NK1.1 and asialo GM1 are expressed on NK cells, NKT cells and some other cell types, including T cells (Byrne et al, 2004; Slifka et al, 2000; Trambley et al, 1999); and (2) although beige mice seem to have functional NKT cells, beige mice have other abnormalities in immune responses in addition to deficient NK cell function (Bannai et al, 2000; Cavarra et al, 1999; Nakagawa et al, 2001). In this context, changes in pathology by asialo GM1 and NK1.1 antibody treatments in Paya’s study (Paya et al, 1989) could be attributed to depletion of not only NK cells but also NKT cells. If this is the case, NKT cells may play a protective role against demyelination during the late stage of TMEV infection in both resistant (Paya’s study) and susceptible (current study) mouse strains, because we found exacerbation of demyelination in TMEV-infected susceptible SJL/J mice treated with Vα14 antibody weekly or during the late stage.
In summary, we found that the effect of Vα14 antibody injections in TMEV infection differed depending on the timing and numbers of the treatments. Treatment of mice during the early stage delayed the onset of TMEV-induced clinical signs that were associated with an alteration of the Th1/Th2 profile. In contrast, administration during the late stage or weekly resulted in enhanced neuropathology with higher levels of virus antigen–positive cells in the CNS. The timing of NKT cell modulation as well as cytokine profiles and numbers of NKT cells in hosts should be taken into account when planning therapeutic strategies for MS.
Acknowledgments
The authors thank Jane E. Libbey, MS, and Takahisa Masaki, PhD, for helpful discussions, along with Nikki J. Kirkman, BS, Andy K. Luu, and Faris Hasanovic for excellent technical assistance. The authors are grateful to Ms. Kathleen Borick for preparation of the manuscript. This work was supported by the National Institutes of Health (R21NS059724, NS034497 and AI581501).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, Strober S. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: β2-microglobulin-dependent and independent forms. J Immunol. 1998;161:1710–1717. [PubMed] [Google Scholar]
- Apostolou I, Takahama Y, Belmant C, Kawano T, Huerre M, Marchal G, Cui J, Taniguchi M, Nakauchi H, Fournié J-J, Kourilsky P, Gachelin G. Murine natural killer T(NKT) cells [correction of natural killer cells] contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc Natl Acad Sci U S A. 1999;96:5141–5146. doi: 10.1073/pnas.96.9.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki M, Kondo T, Gumperz JE, Brenner MB, Miyake S, Yamamura T. Th2 bias of CD4+ NKT cells derived from multiple sclerosis in remission. Int Immunol. 2003;15:279–288. doi: 10.1093/intimm/dxg029. [DOI] [PubMed] [Google Scholar]
- Bannai M, Oya H, Kawamura T, Naito T, Shimizu T, Kawamura H, Miyaji C, Watanabe H, Hatakeyama K, Abo T. Disparate effect of beige mutation on cytotoxic function between natural killer and natural killer T cells. Immunology. 2000;100:165–169. doi: 10.1046/j.1365-2567.2000.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- Berzofsky JA, Terabe M. NKT cells in tumor immunity: Opposing subsets define a new immuno-regulatory axis. J Immunol. 2008;180:3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- Byrne P, McGuirk P, Todryk S, Mills KHG. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur J Immunol. 2004;34:2579–2588. doi: 10.1002/eji.200425092. [DOI] [PubMed] [Google Scholar]
- Cavarra E, Martorana PA, de Santi M, Bartalesi B, Cortese S, Gambelli F, Lungarella G. Neutrophil influx into the lungs of beige mice is followed by elastolytic damage and emphysema. Am J Respir Cell Mol Biol. 1999;20:264–269. doi: 10.1165/ajrcmb.20.2.3235. [DOI] [PubMed] [Google Scholar]
- Chiba A, Kaieda S, Oki S, Yamamura T, Miyake S. The involvement of Vα14 natural killer T cells in the pathogenesis of arthritis in murine models. Arthritis Rheum. 2005;52:1941–1948. doi: 10.1002/art.21056. [DOI] [PubMed] [Google Scholar]
- Chiba A, Oki S, Miyamoto K, Hashimoto H, Yamamura T, Miyake S. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of α-galactosylceramide. Arthritis Rheum. 2004;50:305–313. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- Dang Y, Beckers J, Wang C-R, Heyborne KD. Natural killer 1.1+ αβ T cells in the periimplantation uterus. Immunology. 2000;101:484–491. doi: 10.1046/j.1365-2567.2000.t01-1-00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley MA, Bigley NJ, Cheng O, Tahir SMA, Smiley ST, Carter QL, Stills HF, Grusby MJ, Koezuka Y, Taniguchi M, Balk SP. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. (Erratum in J Leukoc Biol 2001, 70; 340) J Leukoc Biol. 2001;69:713–718. [PubMed] [Google Scholar]
- Furlan R, Bergami A, Cantarella D, Brambilla E, Taniguchi M, Dellabona P, Casorati G, Martino G. Activation of invariant NKT cells by αGalCer administration protects mice from MOG35-55-induced EAE: critical roles for administration route and IFN-γ. Eur J Immunol. 2003;33:1830–1838. doi: 10.1002/eji.200323885. [DOI] [PubMed] [Google Scholar]
- Gausling R, Trollmo C, Hafler DA. Decreases in interleukin-4 secretion by invariant CD4−CD8− Vα24JαQ T cells in peripheral blood of patients with relapsing-remitting multiple sclerosis. Clin Immunol. 2001;98:11–17. doi: 10.1006/clim.2000.4942. [DOI] [PubMed] [Google Scholar]
- Greenlee JE, Rose JW. Controversies in neurological infectious diseases. Semin Neurol. 2000;20:375–386. doi: 10.1055/s-2000-9429. [DOI] [PubMed] [Google Scholar]
- Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant Vα14-Jα281 TCR. J Immunol. 2003;170:1430–1434. doi: 10.4049/jimmunol.170.3.1430. [DOI] [PubMed] [Google Scholar]
- Habu Y, Shinomiya N, Kinoshita M, Matsumoto A, Kawabata T, Seki S. Mice deficient in Vβ8+ NKT cells are resistant to experimental hepatitis but are partially susceptible to generalised Shwartzman reaction. Clin Exp Med. 2007;7:30–38. doi: 10.1007/s10238-007-0122-2. [DOI] [PubMed] [Google Scholar]
- Huber S, Sartini D, Exley M. Role of CD1d in coxsackievirus B3-induced myocarditis. J Immunol. 2003;170:3147–3153. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- Illés Z, Kondo T, Newcombe J, Oka N, Tabira T, Yamamura T. Differential expression of NK T cell Vα24JαQ invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J Immunol. 2000;164:4375–4381. doi: 10.4049/jimmunol.164.8.4375. [DOI] [PubMed] [Google Scholar]
- Ito T, Ishibashi K, Imai K, Koseki H, Ra C, Fernandez E, Kantake M, Saito T, Taniguchi M. Monoclonal antibody against murine T cell receptor Vα14 cross-reacts with human CD3ε and detects disulfide-linked dimeric form. Int Immunol. 1991;3:991–995. doi: 10.1093/intimm/3.10.991. [DOI] [PubMed] [Google Scholar]
- Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky SG, Nakamura I, Cudkowicz G. Selective defect of natural killer and killer cell activity against lymphomas in SJL mice: Low responsiveness to interferon inducers. J Immunol. 1983;130:1980–1984. [PubMed] [Google Scholar]
- Kaminsky SG, Nakamura I, Cudkowicz G. Genetic control of the natural killer cell activity in SJL and other strains of mice. J Immunol. 1985;135:665–671. [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, Tanaka Y, Taniguchi M. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci U S A. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Fuller AC, Koh C-S. Cytokines, chemokines and adhesion molecules in TMEV-IDD. In: Lavi E, Constantinescu CS, editors. Experimental models of multiple sclerosis. New York: Springer Science+Business Media; 2005. pp. 659–671. [Google Scholar]
- Levy O, Orange JS, Hibberd P, Steinberg S, LaRussa P, Weinberg A, Wilson SB, Shaulov A, Fleisher G, Geha RS, Bonilla FA, Exley M. Disseminated varicella infection due to the vaccine strain of varicellazoster virus, in a patient with a novel deficiency in natural killer T cells. J Infect Dis. 2003;188:948–953. doi: 10.1086/378503. [DOI] [PubMed] [Google Scholar]
- Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Predominant expression of invariant Vα14+ TCR α chain in NK1.1+ T cell populations. Int Immunol. 1995;7:1157–1161. doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- Mars LT, Laloux V, Goude K, Desbois S, Saoudi A, Van Kaer L, Lassmann H, Herbelin A, Lehuen A, Liblau RS. Vα14-Jα281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J Immunol. 2002;168:6007–6011. doi: 10.4049/jimmunol.168.12.6007. [DOI] [PubMed] [Google Scholar]
- McCright IJ, Tsunoda I, Whitby FG, Fujinami RS. Theiler’s viruses with mutations in loop I of VP1 lead to altered tropism and pathogenesis. J Virol. 1999;73:2814–2824. doi: 10.1128/jvi.73.4.2814-2824.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieza MA, Itoh T, Cui JQ, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, Koseki H, Taniguchi M. Selective reduction of Vα14+ NK T cells associated with disease development in auto-immune-prone mice. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- Miyake S, Yamamura T. Therapeutic potential of glycolipid ligands for natural killer (NK) T cells in the suppression of autoimmune diseases. Curr Drug Targets- Immune Endocr Metab Disord. 2005;5:315–322. doi: 10.2174/1568008054863772. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- Nakagawa R, Nagafune I, Tazunoki Y, Ehara H, Tomura H, Iijima R, Motoki K, Kamishohara M, Seki S. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by α-galactosylceramide in mice. J Immunol. 2001;166:6578–6584. doi: 10.4049/jimmunol.166.11.6578. [DOI] [PubMed] [Google Scholar]
- Paya CV, Patick AK, Leibson PJ, Rodriguez M. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler’s virus. J Immunol. 1989;143:95–102. [PubMed] [Google Scholar]
- Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–642. doi: 10.1212/01.wnl.0000250267.85698.7a. [DOI] [PubMed] [Google Scholar]
- Potvin DM, Metzger DW, Lee WT, Collins DN, Ramsingh AI. Exogenous interleukin-12 protects against lethal infection with coxsackievirus B4. J Virol. 2003;77:8272–8279. doi: 10.1128/JVI.77.15.8272-8279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud S, Fondanèche M-C, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P, Fischer A, de Saint Basile G, Latour S. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- Rose JW, Watt HE, White AT, Carlson NG. Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann Neurol. 2004;56:864–867. doi: 10.1002/ana.20287. [DOI] [PubMed] [Google Scholar]
- Schofield L, McConville MJ, Hansen D, Campbell AS, Fraser-Reid B, Grusby MJ, Tachado SD. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- Serizawa I, Koezuka Y, Amao H, Saito TR, Takahashi KW. Functional natural killer T cells in experimental mouse strains, including NK1.1− strains. Exp Anim. 2000;49:171–180. doi: 10.1538/expanim.49.171. [DOI] [PubMed] [Google Scholar]
- Sharif S, Arreaza GA, Zucker P, Mi Q-S, Delovitch TL. Regulation of autoimmune disease by natural killer T cells. J Mol Med. 2002;80:290–300. doi: 10.1007/s00109-002-0332-8. [DOI] [PubMed] [Google Scholar]
- Singh AK, Wilson MT, Hong S, Olivares-Villagómez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Yang J-Q, Parekh VV, Wei J, Wang C-R, Joyce S, Singh RR, Van Kaer L. The natural killer T cell ligand α-galactosylceramide prevents or promotes pristane-induced lupus in mice. Eur J Immunol. 2005;35:1143–1154. doi: 10.1002/eji.200425861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Pagarigan RR, Whitton JL. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J Immunol. 2000;164:2009–2015. doi: 10.4049/jimmunol.164.4.2009. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Makino Y, Cui J, Masuda K, Kawano T, Sato H, Kondo E, Koseki H. Vα14+ NK T cells: A novel lymphoid cell lineage with regulatory function. J Allergy Clin Immunol. 1996;98:S263–S269. doi: 10.1016/s0091-6749(96)70074-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Seino K, Nakayama T. The NKT cell system: bridging innate and acquired immunity. Nat Immunol. 2003;4:1164–1165. doi: 10.1038/ni1203-1164. [DOI] [PubMed] [Google Scholar]
- Teige A, Teige I, Lavasani S, Bockermann R, Mondoc E, Holmdahl R, Issazadeh-Navikas S. CD1-dependent regulation of chronic central nervous system inflammation in experimental autoimmune encephalomyelitis. J Immunol. 2004;172:186–194. doi: 10.4049/jimmunol.172.1.186. [DOI] [PubMed] [Google Scholar]
- Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze S-Y, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1+ CD8+ T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Fujinami RS. Theiler’s murine encephalomyelitis virus. In: Ahmed R, Chen ISY, editors. Persistent viral infections. Chichester, West Sussex, England: John Wiley & Sons; 1999. pp. 517–536. [Google Scholar]
- Tsunoda I, Kuang L-Q, Theil DJ, Fujinami RS. Antibody association with a novel model for primary progressive multiple sclerosis: Induction of relapsing-remitting and progressive forms of EAE in H2s mouse strains. Brain Pathol. 2000;10:402–418. doi: 10.1111/j.1750-3639.2000.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Libbey JE, Kuang L-Q, Terry EJ, Fujinami RS. Massive apoptosis in lymphoid organs in animal models for primary and secondary progressive multiple sclerosis. Am J Pathol. 2005;167:1631–1646. doi: 10.1016/S0002-9440(10)61247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Tanaka T, Saijoh Y, Fujinami RS. Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration. Am J Pathol. 2007a;171:1563–1575. doi: 10.2353/ajpath.2007.070147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Tanaka T, Terry EJ, Fujinami RS. Contrasting roles for axonal degeneration in an auto-immune versus viral model for multiple sclerosis: When can axonal injury be beneficial? Am J Pathol. 2007b;170:214–226. doi: 10.2353/ajpath.2007.060683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Wada Y, Libbey JE, Cannon TS, Whitby FG, Fujinami RS. Prolonged gray matter disease without demyelination caused by Theiler’s murine encephalomyelitis virus with a mutation in VP2 puff B. J Virol. 2001;75:7494–7505. doi: 10.1128/JVI.75.16.7494-7505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Tanaka T, Fujinami RS. Regulatory role of CD1d in neurotropic virus infection. J Virol. 2008;82:10279–10289. doi: 10.1128/JVI.00734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet HJJ, von Blomberg BME, Nishi N, Reijm M, Voskuyl AE, van Bodegraven AA, Polman CH, Rustemeyer T, Lips P, van den Eertwegh AJM, Giaccone G, Scheper RJ, Pinedo HM. Circulating Vα24+ Vβ11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol. 2001;100:144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Yamamura T, Sakuishi K, Illés Z, Miyake S. Understanding the behavior of invariant NKT cells in autoimmune diseases. J Neuroimmunol. 2007;191:8–15. doi: 10.1016/j.jneuroim.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Bendelac A, Hu-Li J, Paul WE. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc Natl Acad Sci U S A. 1995;92:11931–11934. doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]