Abstract
Background
IGF-1 and the major serum IGF-1 binding protein, IGFBP-3, are under extensive investigation as potential prognostic markers of specific malignancies and vascular diseases. However, there is conflicting evidence that tobacco smoking may influence systemic concentrations of IGF-1 and IGFBP-3.
Subjects and methods
Serum concentrations of IGF-1 and IGFBP-3 were measured in 20 smokers and 20 non-smokers, matched for age and gender. Serum concentrations of cotinine, the major metabolite of nicotine, and ICAM-1, known to exhibit a dose-dependent relationship with cotinine, were also assayed.
Results
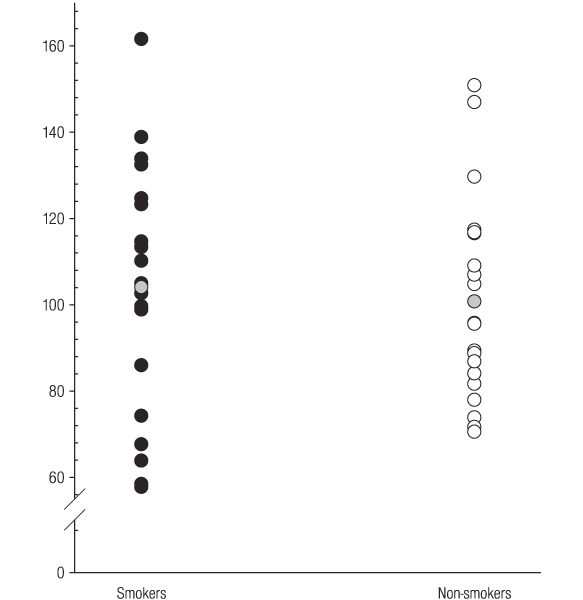
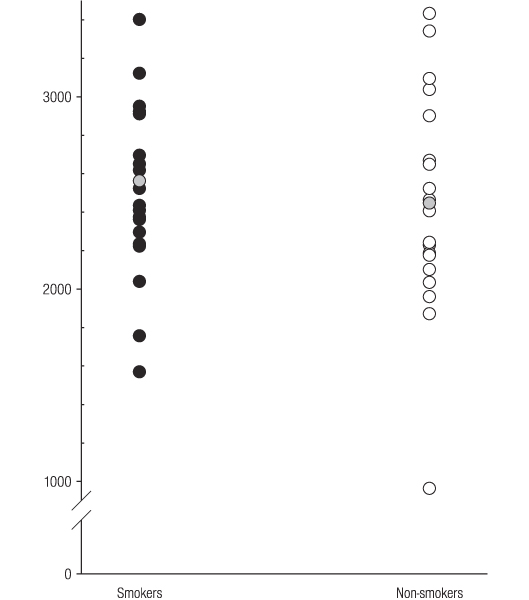
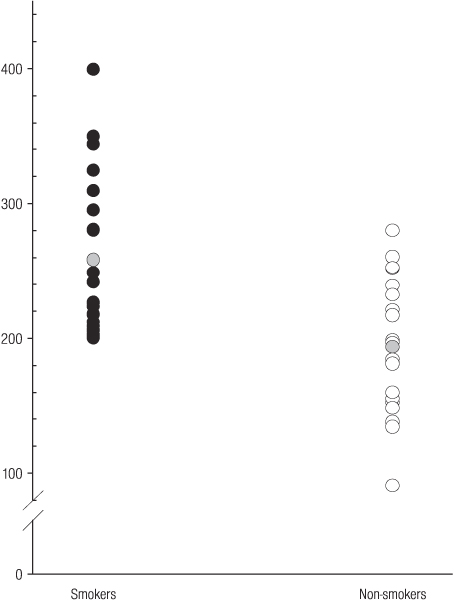
There was no difference between the systemic concentrations of IGF-1 or IGFBP-3 found in smokers and non-smokers (IGF-1: mean [s.d]; 104 [29] vs 101 [24] ng ml-1, respectively; and IGFBP-3: 2562 [522] vs 2447 [570] ng ml-1, respectively). Similarly, there was no correlation between serum cotinine and IGF-1 or IGFBP-3 concentrations in smokers. Soluble ICAM-1 concentrations were significantly increased in smokers, compared to non-smokers (mean [s.d]; 258 [60] vs 194 [50] ng ml-1, respectively; p = 0.002).
Conclusion
There was no relationship noted between tobacco smoking and either IGF-1 or IGFBP-3. These data suggest that smoking would not appear to be a major confounder of the reported clinical associations between IGF-1, IGFBP-3, or IGF-1/IGFBP-3 ratios and specific disease entities.
Introduction
Insulin-like growth factor (IGF-1) is a 70 amino acid (7.6 kDa) cytokine constitutively produced by the liver due to stimulation by insulin [1] and growth hormone [2]. IGF-1, which exhibits a high degree of structural homology to proinsulin, acts to lower glucose levels [3,4] and suppress insulin production [5].
There are at least six IGF binding proteins (IGFBPs). IGFBP-3 is the major IGFBP in serum, with more than 90% of circulating IGF-1 bound in a ternary complex with IGFBP-3 and acid-labile subunit, and only 1% of the total serum IGF-1 normally circulates in free form [4-8]. While IGFBP-3 is expressed in many tissues, non-parenchymal liver cells are considered the primary source of circulating IGFBP-3. IGFBP-3 is susceptible to cleavage by a variety of proteases, including cathepsin G [9], neutrophil elastase [9], and metalloproteases [10,11]. Proteolytic cleavage of systemic IGFBP-3 would increase the amount of free IGF-1, and be expected to alter the systemic IGF-1/IGFBP-3 ratio.
IGF-1 and IGFBP-3 both promote the differentiation of bone, with systemic levels of IGF-1 and IGFBP-3 positively associated with bone mass density [12]. Indeed, there is increasing evidence that alterations to the IGF-1/IGFBP axis are associated with osteoporosis [13-15]. IGF-1 has been shown to act as a potent mitogen, with anti-apoptotic actions, on various cancer cells [16], whereas IGFBP-3 may have IGF-1-independent pro-apoptotic activity [17,18]. An aetiological role for IGF-1 and IGFBP-3 in malignancy is supported by clinical studies that associate high IGF-1 and low IGFBP-3 levels with increased risk of several cancers, such as breast, colorectum, and lung cancer [19-21]. Dysregulation of the IGF/IGFBP system is also thought to influence the development of coronary atherosclerosis and other vascular diseases [22-26].
It should be noted that specific malignancies (including colorectal, lung and breast cancers), osteoporosis, and vascular disease are all tobacco-induced and/or – exacerbated diseases. Previous studies have come to conflicting conclusions on a potential relationship between IGF-1, IGFBP-3 and smoking status [20,21,23,27-30]. Considering the important potential of the IGF/IGFBP system as prognostic markers of malignancy and vascular disease, there is a pressing need to clarify the relationship between tobacco use and the systemic load of IGF-1 and the major IGF-1 binding protein, IGFBP-3. Age and gender have been shown to correlate negatively with serum IGF-1 and IGFBP-3 concentrations [12,20,23,27,31]. We have previously shown that circulating concentrations of soluble intercellular adhesion molecule-1 (sICAM-1; CD54) are known to be significantly elevated in smokers, in a dose-dependent manner, making this a suitable positive control serum marker [32,33]. Therefore, we examined the circulating concentrations of IGF-1, IGFBP-3, and soluble ICAM-1 in 20 smokers and 20 non-smokers, matched for age and gender, and whose smoking status was confirmed by serum cotinine analysis.
Subjects, Materials, and Methods
Subjects
Serum was obtained from 20 smokers and 20 non-smokers, matched for age and gender (12 females in each group) and stored at -80°C until required. The mean age of the smoking group was 44.4 [s.d. 6.1] years. The mean age of the non-smoking group was 44.9 [s.d. 6.5] years. Smokers were required to have smoked ≥ 10 cigarettes daily for ≥ 3 years. Those who reported to have consumed no cigarettes in the previous 10 years were considered for inclusion in the non-smoking group. Smoking status was confirmed by serum cotinine analysis (smokers ≥ 50 ng ml-1 cotinine; non-smokers ≥ 10 ng ml-1 cotinine). Exclusion criteria were pregnancy, diabetes, reported history of hypertension, angina, any inflammatory disease such as rheumatoid arthritis or eczema, use of antibiotics within the preceding 2 months, or the current use of anti-inflammatory medication, including NSAID's. Written, informed consent was obtained from each subject, following granting of ethical approval by the local ethics committee.
Measurement of cotinine
Smoking status was confirmed and tobacco smoke exposure quantified by analysis of serum cotinine, by using a capillary column gas-liquid chromatography technique, as previously described [34]. Cotinine assays were performed blind to self-reported smoking status. The mean coefficient of variation (CV) for analysis of cotinine (over the range 1.0 to 1000 ng ml-1) has been determined to be 2.2% [34]. The lower limit of detection for cotinine in this system is 100 pg ml-1. Smokers can be reliably differentiated from non-smokers with over 99% confidence using an optimal cut-off value of >13.7 ng ml-1 serum cotinine [35].
Measurement of IGF-1
IGF-1 concentrations were measured in duplicate by immunoassay (Quantikine IGF-1 Immunoassay, R&D Systems, Minneapolis, MN), using Eschericia coli-expressed recombinant human IGF-1 to generate the standard curve. The mean intra-assay CV, determined by assaying the IGF-1 concentration in three samples in replicates of twenty, is reported by the manufacturer to be 3.0%. The mean inter-assay CV, determined by assaying three samples in forty separate assays, has been determined to be 8.0%. The mean minimal detectable concentration of IGF-1 in this assay is 0.026 ng ml-1.
Measurement of IGFBP-3
IGFBP-3 concentrations were measured in duplicate by immunoassay (Quantikine IGFBP-3 Immunoassay, R&D Systems, Minneapolis, MN), using NSO-expressed recombinant human IGFBP-3 to generate the standard curve. The mean intra-assay CV, determined by the manufacturer by assaying the IGFBP-3 concentration in three samples in replicates of twenty, is reported to be 4.0%. The mean inter-assay CV, determined by assaying three samples in forty separate assays, has been determined to be 6.6%. The mean minimal detectable concentration of IGFBP-3 in this assay is 0.05 ng ml-1.
Measurement of sICAM-1
Soluble ICAM-1 concentrations were measured by ELISA (sICAM-1 Parameter Immunoassay, R&D Systems, Minneapolis, MN) in duplicate, according to the manufacturers instructions, and as previously described [36]. The mean intra-assay CV, determined by assaying the sICAM-1 concentration in three serum samples in replicates of ten, is reported to be 4.4%. The mean inter-assay CV, determined by assaying three serum samples in duplicate in 18 separate assays by four operators, has been determined to be 7.4%. The reported sensitivity of the ELISA is less than 0.35 ng ml-1.
Statistical analysis
Statistical analysis was carried out using STATA 7.0 software (Stata Corp., Texas 77845, USA). Differences between smoking groups were analyzed using a two-group t-test. Relationships between variables were assessed using Spearman correlation.
Results
The smoking status of all subjects was validated by serum cotinine analysis. The serum cotinine concentrations (mean [s.d.]) in the smoking and non-smoking groups were 251.8 [89.8] and 0.79 ng ml-1 [0.72] (p < 0.001), respectively.
Serum IGF-1 levels in individual smokers and non-smokers are shown in Figure 1. There was no significant difference in IGF-1 concentrations between smokers and non-smokers (mean [s.d]; 104 [29] and 101 [24] ng ml-1, respectively).
Figure 1.
Serum IGF-1 concentrations ng ml-1 in smokers and non-smokers. The grey circles represent mean serum concentrations.
Serum IGFBP-3 concentrations in individual smokers and non-smokers are presented in Figure 2. No significant difference in IGFBP-3 concentrations measured between smokers and non-smokers (mean [s.d]; 2562 [522] and 2447 [570] ng ml-1, respectively) was observed.
Figure 2.
Serum IGFBP-3 concentrations ng ml-1 in smokers and non-smokers. The grey circles represent mean serum concentrations.
Furthermore, there was no significant difference in circulating IGF-1: IGFBP-3 ratios between smokers and non-smokers (mean [s.d]; 0.041 [0.008] and 0.044 [0.017], respectively). Similarly, there was no correlation between serum cotinine and IGF-1 (r = -0.005, p = 0.978) or IGFBP-3 (r = 0.079, p = 0.628) concentrations in smokers.
Systemic sICAM-1 concentrations, as expected [32,33], were significantly elevated in smokers, compared to non-smokers (mean [s.d]; 258 [60] and 194 [50] ng ml-1, respectively; p = 0.002), as shown in Figure 3.
Figure 3.
Serum ICAM-1 concentrations ng ml-1 in smokers and non-smokers. The grey circles represent mean serum concentrations.
Discussion
Previous studies that have addressed the possibility of a direct relationship between tobacco smoking and the systemic load of IGF-1 and/or IGFBP-3 have been inconsistent in their conclusions. Probst-Hensch et al. [29] observed an increase in IGF-1 concentration in smokers, correlating with the number of cigarettes consumed daily, but no difference in circulating IGFBP-3 concentrations between smokers and non-smokers. Kaklamani et al [27] also reported that IGF-1 concentrations are higher in smokers, and positively associated with an index of cumulative smoke exposure (pack-years).
Elsewhere in the medical literature, a negative relationship between IGF-1 and the amount of tobacco smoked has been reported, in men only [31]. In another relevant study, Coutant et al [37] showed that IGF-1 was reduced in cord blood of neonates from smoking mothers compared to neonates from non-smoking mothers, and that this reduction was consistent with their lower birth weight percentile. Others have indicated that circulating IGF-1 levels may be lowered in smokers, compared to non-smokers [23]. Others have found a minimal or no relationship between tobacco smoking and IGF-1 concentrations [19,20,28].
Recently, Renehan et al [30] recognized that both tobacco smoking and changes in the IGF/IGFBP system have been implicated as risk factors for common epithelial cancers. These authors, therefore, examined the relationship between cigarette smoke exposure and serum IGF-1, and IGFBP-3 levels. No significant difference in mean serum IGF-1 between smokers and non-smokers was observed. Mean IGFBP-3 concentrations, on the other hand, were significantly lower in smokers. These results are in agreement with a previous study that reported serum levels of IGFBP-3 to be independently and negatively associated with the number of cigarettes smoked per day or pack-year history of smoking [27]. However, smoking status in the majority of previous studies has been determined by questionnaire, which is known to be unreliable [38]. Furthermore, subjects were often not matched for gender and/or age, both known to influence systemic IGF-1 and IGFBP-3 concentrations.
Thus, with respect to the relationship between tobacco use and IGF-1/IGFBP-3 ratios, the present study is an improvement on previous study designs. Smoking subjects have been matched for age and gender with non-smokers, smoking status validated biochemically, and tobacco smoke exposure quantified by serum cotinine analysis. Furthermore, all subjects in the present study were healthy, with no known confounding disease or condition that may influence IGF-1 or IGFBP-3 concentrations.
Unlike ICAM-1 (Figure 3), no relationship between tobacco smoking and IGF-1 (Figure 1) or IGFBP-3 (Figure 2) was observed. Therefore, tobacco smoking would not appear to be a major confounder of the reported clinical associations between IGF-1, IGFBP-3, or IGF-1/IGFBP-3 ratios and specific disease entities, including certain malignancies and vascular disease.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgements
This paper was presented, in part, at the inaugural meeting of the International Society for the Prevention of Tobacco Induced Diseases, Germany, 2002.
References
- Phillips LS, Harp JB, Goldstein S, Klein J, Pao CI. Regulation and action of insulin-like growth factors at the cellular level. Proceedings of the Nutrition on Society. 1990;49:451–458. doi: 10.1079/pns19900053. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocrine Reviews. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Boulware SD, Tamborlane WV, Matthews LS, Sherwin RS. Diverse effects of insulin-like growth factor I on glucose, lipid, and amino acid metabolism. Americal Journal of Physiology. 1992;262:E130–E133. doi: 10.1152/ajpendo.1992.262.1.E130. [DOI] [PubMed] [Google Scholar]
- Janssen JA, Lamberts SW. The role of IGF-I in the development of cardiovascular disease in type 2 diabetes mellitus: is prevention possible? European Journal of Endocrinology. 2002;146:467–477. doi: 10.1530/eje.0.1460467. [DOI] [PubMed] [Google Scholar]
- Rennert NJ, Caprio S, Sherwin RS. Insulin-like growth factor I inhibits glucose-stimulated insulin secretion but does not impair glucose metabolism in normal humans. Journal of Clinical Endocrinology and Metabolism. 1993;76:804–806. doi: 10.1210/jc.76.3.804. [DOI] [PubMed] [Google Scholar]
- Binoux M. The IGF system in metabolism regulation. Diabetes and Metabolism. 1995;21:330–337. [PubMed] [Google Scholar]
- Janssen JA, Lamberts SW. Is the measurement of free IGF-I more indicative than that of total IGF-I in the evaluation of the biological activity of the GH/IGF-I axis? Journal of Endocrinological Investigation. 1999;22:313–315. doi: 10.1007/BF03343563. [DOI] [PubMed] [Google Scholar]
- Kelley KM, Oh Y, Gargosky SE, Gucev Z, Matsumoto T, Hwa V, Ng L, Simpson DM, Rosenfeld RG. Insulin-like growth factor-binding proteins (IGFBPs) and their regulatory dynamics. International Journal of Biochemistry and Cell Biology. 1996;28:619–637. doi: 10.1016/1357-2725(96)00005-2. [DOI] [PubMed] [Google Scholar]
- Gibson TL, Cohen P. Inflammation-related neutrophil proteases, cathepsin G and elastase, function as insulin-like growth factor binding protein proteases. Growth Hormone and IGF Research. 1999;9:241–253. doi: 10.1054/ghir.1999.0115. [DOI] [PubMed] [Google Scholar]
- Fowlkes JL, Thrailkill KM, Serra DM, Suzuki K, Nagase H. Matrix metalloproteinases as insulin-like growth factor binding protein-degrading proteinases. Progress in Growth Factor Research. 1995;6:255–263. doi: 10.1016/0955-2235(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Loechel F, Fox JW, Murphy G, Albrechtsen R, Wewer UM. ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. Biochemical and Biophysical Research Communications. 2000;278:511–515. doi: 10.1006/bbrc.2000.3835. [DOI] [PubMed] [Google Scholar]
- Gillberg P, Olofsson H, Mallmin H, Blum WF, Ljunghall S, Nilsson AG. Bone Mineral Density in Femoral Neck is Positively Correlated to Circulating Insulin-Like Growth Factor (IGF)-I and IGF-Binding Protein (IGFBP)-3 in Swedish Men. Calcified Tissue International. 2002;70:22–29. doi: 10.1007/s002230020048. [DOI] [PubMed] [Google Scholar]
- Celiker R, Arslan S. Comparison of serum insulin-like growth factor-1 and growth hormone levels in osteoporotic and non-osteoporotic postmenopausal women. Rheumatology International. 2000;19:205–208. doi: 10.1007/s002960000058. [DOI] [PubMed] [Google Scholar]
- Gamero P, Sornay-Rendu E, Delmas PD. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet. 2000;355:898–899. doi: 10.1016/S0140-6736(99)05463-X. [DOI] [PubMed] [Google Scholar]
- Munoz-Torres M, Mezquita-Raya P, Lopez-Rodriguez F, Torres-Vela E, de Dios Luna J, Escobar-Jimenez F. The contribution of IGF-I to skeletal integrity in postmenopausal women. Clinical Endocrinology. 2001;55:759–766. doi: 10.1046/j.1365-2265.2001.01390.x. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Jenkins PJ. The growth hormone-insulin-like growth factor-I axis and colorectal cancer. Trends in Molecular Medicine. 2001;7:447–454. doi: 10.1016/S1471-4914(01)02104-9. [DOI] [PubMed] [Google Scholar]
- Butt AJ, Williams AC. IGFBP-3 and apoptosis–a license to kill? Apoptosis. 2001;6:199–205. doi: 10.1023/A:1011388710719. [DOI] [PubMed] [Google Scholar]
- Baxter RC. Signalling pathways involved in antiproliferative effects of IGFBP-3: a review. Molecular Pathology. 2001;54:145–148. doi: 10.1136/mp.54.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukanova A, Toniolo P, Akhmedkhanov A, Biessy C, Haley NJ, Shore RE, Riboli E, Rinaldi S, Kaaks R. A prospective study of insulin-like growth factor-I, IGF-binding proteins-1, -2 and -3 and lung cancer risk in women. International Journal of Cancer. 2001;92:888–892. doi: 10.1002/ijc.1265. [DOI] [PubMed] [Google Scholar]
- Lukanova A, Toniolo P, Akhmedkhanov A, Hunt K, Rinaldi S, Zeleniuch-Jacquotte A, Haley NJ, Riboli E, Stattin P, Lundin E, Kaaks A cross-sectional study of IGF-I determinants in women. European Journal of Cancer Prevention. 2001;10:443–452. doi: 10.1097/00008469-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. Journal of the National Cancer Institute. 1999;91:151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circulation Research. 2000;86:125–130. doi: 10.1161/01.res.86.2.125. [DOI] [PubMed] [Google Scholar]
- Janssen JA, Stolk RP, Pols HA, Grobbee DE, Lamberts SW. Serum total IGF-1, free IGF-1, and IGFB-1 levels in an elderly population: relation to cardiovascular risk factors and disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;8:277–282. doi: 10.1161/01.atv.18.2.277. [DOI] [PubMed] [Google Scholar]
- Motomura N, Lou H, Orskov H, Ramwell PW, Foegh ML. Exposure of vascular allografts to insulin-like growth factor-I (IGF-I) increases vascular expression of IGF-I ligand and receptor protein and accelerates arteriosclerosis in rats. Transplantation. 1998;65:1024–1030. doi: 10.1097/00007890-199804270-00003. [DOI] [PubMed] [Google Scholar]
- Schuler-Lüttmann S, Mönnig G, Enbergs A, Schulte H, Breithardt G, Assmann G, Kerber S, von Eckardstein A. Insulin-like growth factor-binding protein-3 is associated with the presence and extent of coronary arteriosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:10e–15e. [PubMed] [Google Scholar]
- Schwab S, Spranger M, Krempien S, Hacke W, Bettendorf M. Plasma insulin-like growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke. 1997;28:1744–1748. doi: 10.1161/01.str.28.9.1744. [DOI] [PubMed] [Google Scholar]
- Kaklamani VG, Linos A, Kaklamani E, Markaki I, Mantzoros C. Dietary fat and carbohydrates are independently associated with circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 concentrations in healthy adults. Journal of Clinical Oncology. 1999;17:813–817. doi: 10.1200/JCO.1999.17.10.3291. [DOI] [PubMed] [Google Scholar]
- Mucci LA, Tamimi R, Lagiou P, Trichopoulou A, Benetou V, Spanos E, Trichopoulos D. Are dietary influences on the risk of prostate cancer mediated through the insulin-like growth factor system? BJU International. 2001;87:814–820. doi: 10.1046/j.1464-410x.2001.02191.x. [DOI] [PubMed] [Google Scholar]
- Probst-Hensch NM, Yuan JM, Stanczyk FZ, Gao YT, Ross RK, Yu MC. IGF-1, IGF-2 and IGFBP-3 in prediagnostic serum: association with colorectal cancer in a cohort of Chinese men in Shanghai. British Journal of Cancer. 2001;85:1695–1699. doi: 10.1054/bjoc.2001.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan AG, Gleeson H, Atkin WS, O'Dwyer ST, Shalet SM. Cigarette smoking exposure, serum insulin-like growth factors, and cancer risk: A population-based study. Endocrine Abstracts. 2002;3:P128. (Abstract) [Google Scholar]
- Landin-Wilhelmsen K, Wilhelmsen L, Lappas G, Rosen T, Lindstedt G, Lundberg PA, Bengtsson BA. Serum insulin-like growth factor I in a random population sample of men and women: relation to age, sex, smoking habits, coffee consumption and physical activity, blood pressure and concentrations of plasma lipids, fibrinogen, parathyroid hormone and osteocalcin. Clinical Endocrinology. 1994;41:351–357. doi: 10.1111/j.1365-2265.1994.tb02556.x. [DOI] [PubMed] [Google Scholar]
- Scott DA, Todd DH, Coward PY, Wilson RF, Odell EW, Poston RN, Matthews JP, Palmer RM. The acute influence of tobacco smoking on adhesion molecule expression on monocytes and neutrophils and on circulating adhesion molecule levels in vivo. Addiction Biology. 2000;5:195–205. doi: 10.1080/13556210050003793. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Stapleton JA, Wilson RF, Sutherland G, Coward PY, Scott DA. Nicotine replacement therapy does not compromise a rapid recovery in circulating adhesion molecule profiles (ICAM-1, CD44v5, CD44v6) in quitting smokers. European Journal of Clinical Investigation. [DOI] [PubMed]
- Feyerabend C, Russell MA. A rapid gas-liquid chromatographic method for the determination of cotinine and nicotine in biological fluids. Journal of Pharmacy and Pharmacology. 1990;42:450–452. doi: 10.1111/j.2042-7158.1990.tb06592.x. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from non-smokers. American Journal of Public Health. 1987;77:1435–1438. doi: 10.2105/AJPH.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundouros E, Odell E, Coward PY, Wilson RF, Palmer RM. Soluble adhesion molecules in serum of smokers and non-smokers, with and without periodontitis. Journal of Periodontal Research. 1996;31:596–599. doi: 10.1111/j.1600-0765.1996.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Coutant R, Boux de Casson F, Douay O, Mathieu E, Rouleau S, Beringue F, Gillard P, Limal JM, Descamps P. Relationships between placental GH concentration and maternal smoking, newborn gender, and maternal leptin: possible implications for birth weight. Journal of Clinical Endocrinology and Metabolism. 2001;86:4854–489. doi: 10.1210/jc.86.10.4854. [DOI] [PubMed] [Google Scholar]
- Scott DA, Palmer RM, Stapleton JA. Validation of smoking status in clinical research into inflammatory periodontal disease. Journal of Clinical Periodontology. 2001;28:715–722. doi: 10.1034/j.1600-051X.2001.280801.x. [DOI] [PubMed] [Google Scholar]