Abstract
Two-dimensional infrared photon echo and pump probe studies of the OH stretch vibration provide a sensitive probe of the correlations and couplings in the hydrogen bond network of liquid water. The nonlinear response is simulated using numerical integration of the Schrödinger equation with a Hamiltonian constructed to explicitly treat intermolecular coupling and nonadiabatic effects in the highly disordered singly and doubly excited vibrational exciton manifolds. The simulated two-dimensional spectra are in close agreement with our recent experimental results. The high sensitivity of the OH stretch vibration to the bath dynamics is found to arise from intramolecular mixing between states in the two-dimensional anharmonic OH stretch potential. Surprisingly small intermolecular couplings reproduce the experimentally observed intermolecular energy transfer times.
INTRODUCTION
The special properties of liquid water ultimately originate from the correlations and intermolecular couplings in the extended hydrogen bond network. The fluctuating local electric fields and the variations in number, strength, and orientation of hydrogen bonds strongly deform the potential energy surfaces. Two-dimensional infrared photon echo (2DIR-PE) spectroscopy has been recently proven to be a very powerful tool for investigating the dynamics of local structures in water.1, 2, 3, 4 Changes in the local environments are directly reflected in modulations of the transition frequencies, dipole moments, and anharmonicities of the stretching vibrations, making the OH stretch mode the most direct probe of structural correlations and fluctuations in the liquid.
Most previous studies have focused on isotopically substituted systems HOD∕D2O and HOD∕H2O,3, 4, 5, 6 where the vibrational mode probed is localized on one bond of the molecule and no resonant vibrational energy transfer is observed due to a large separation of the chromophores. Most recently, the first experimental studies on pure H2O have shown significantly faster structural dynamics1, 2 compared to the HOD systems. A stronger coupling to librational motions was found to be the main reason for this behavior, although some contributions from resonant energy transfer (ET) and delocalization of the vibrational excitations are also expected.
Explicit treatment of intermolecular vibrational coupling in simulations of the nonlinear vibrational response of water has not yet been demonstrated. Molecular dynamics (MD) based calculations of localized stretching frequency trajectories and subsequent calculation of the nonlinear response without considering intermolecular coupling have been shown;4, 7 fluctuations of the transition dipole moments through non-Condon effects have also been included.5 Alternatively, intermolecular ET was studied by using harmonic approximations.8, 9 In this work, we use numerical integration of the Schrödinger equation10 (NISE) in a fully anharmonic nonadiabatic simulation procedure, explicitly treating the intermolecular coupling, as well as fluctuations and anharmonicities of transition frequencies and dipole moments, to calculate the 2DIR-PE and pump probe (PP) response of OH stretch vibrations in liquid water. With this new procedure, we find excellent agreement with the recent experimental results.2
METHODS
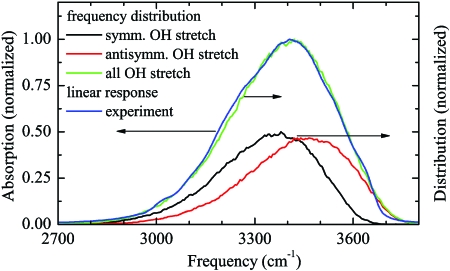
A constant volume simple point charge∕extended water MD trajectory of N=64 molecules with 0.5 fs time steps at room temperature was generated using the GROMACS-3.3.1 program.11 Transition frequencies and dipole moments between six vibrational eigenstates of the OH stretch potential were calculated at each time step and for each molecule using the ab initio electrostatic map for the OH stretch vibrations in H2O, which is equivalent to the previous work on HOD in D2O.6 The electrostatic potential in the vicinity of a single H2O molecule generated by the surrounding molecules is expanded to the second order in Cartesian coordinates. The anharmonic vibrational potential surface of H2O in the multipole electrostatic field was expanded in the three normal coordinates in the gas phase. Vibrational transition frequencies between ground state and the five eigenstates (symmetric and antisymmetric O–H stretch, their overtones, and combination) and the transition dipole moments between these states were parametrized with the electrostatic coefficients.12 To compensate for insufficient solvent shifts produced by the ab initio map, we scaled all electric fields with a factor of 2.2 to match the maximum of the fundamental frequency distribution to the maximum of the linear spectrum of H2O, as shown in Fig. 1.
Figure 1.
Fundamental frequency distribution of the symmetric (black), antisymmetric (red) OH stretch vibration, and combined symmetric and antisymmetric frequency distribution (green). Blue: Experimental linear spectrum (Ref. 13).
We employed the following time dependent effective Hamiltonian for M=128 coupled modes (64 molecules, two modes each), using the anharmonic local eigenstates provided by the electrostatic map as a basis (ℏ=1):
| (1) |
The first two terms describe the free boson system, where ωm(τ) is the fundamental transition frequency of mode m, is the boson creation (annihilation) operator for mode m. The intermolecular coupling Jm,m′, for m,m′ on different molecules, was calculated using resonant dipole-dipole coupling. Since the electrostatic map provides the instantaneous local eigenstates, we set all intramolecular couplings to 0. The last term describes the interaction with the optical fields. The third term contains both intramolecular and intermolecular anharmonicities, the latter arising from anharmonicities in the transition dipole moments, Eq. 2, affecting the dipole-dipole coupling,
| (2) |
Here, μm is the fundamental transition dipole moment of mode m and Δμ is the anharmonicity of the fundamental to overtone transition dipole moment. In Eq. 2, we neglected intermolecular anharmonicities while fully treating all intramolecular anharmonicities of the transition dipole moments.
Calculation of the 2DIR-PE and PP signal requires summation over the contributions from six Liouville pathways. They are commonly referred to as ground state bleach (S1 and S4), induced emission from the excited state (S2 and S5), and excited state absorption (S3 and S6), for the rephasing (S1,S2,S3) and nonrephasing (S4,S5,S6) phase matching conditions.14 For illustration, the nonlinear response functions for the rephasing pathways are given in Eq. 3.
| (3) |
Here, and denote dipole excitation and de-excitation operations, respectively. The propagators , , and propagate the ground, singly excited, and doubly excited state, respectively, from τa to τb.
The propagation was performed using NISE,8, 10
| (4) |
where θ(τ) is the Heaviside function, Δτ=2 fs. The matrix exponential in Eq. 4 was calculated exactly for the single particle propagations . This is not possible for propagation of the two particle excitations in diagrams S3 and S6 due to the large size of the symmetrized two-particle basis being M(M+1)∕2. These matrix exponentials were calculated by splitting the two-particle Hamiltonian into harmonic [first two terms in Eq. 1] and anharmonic [third term in Eq. 1] parts and employing the split-operator method15 to calculate the total propagator. The harmonic exponential can be calculated exactly from factorization of the single particle propagators, and the small anharmonic part is calculated by first order Taylor expansion.
The photon echo signal was calculated in the impulsive limit. The 2D spectra were obtained by double Fourier transformation with respect to t1 and t3, ti=τi−τi−1. The PP signal was also calculated in the impulsive limit setting t1=0. The polarization anisotropy (PA) r(t2) was calculated1, 16 from spectrally integrated PP signal with parallel and crossed polarization of the pump and probe pulses.
RESULTS AND DISCUSSION
The main focus of this study is on the effect of intermolecular coupling on the vibrational response when entering the fully resonant coupling regime for pure H2O. We used the dielectric constant as a scaling factor in the resonant dipole-dipole coupling to reproduce the PA decay regime of ≈80 fs observed in H2O.1, 2, 16 Statistical analysis of the intermolecular coupling for a given value of the dielectric constant allows extraction of the average next neighbor coupling strength κ=⟨∣Jmm′∣⟩ for Rmm′<3.2 Å, where Rmm′ is the distance between molecules for the local modes m and m′.
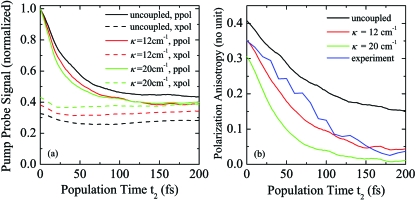
In Fig. 2, we show the spectrally integrated PP signal for parallel and crossed polarization of pump and probe pulses (a) and the PA calculated from these signals (b) for three coupling regimes. Also shown is the experimentally observed anisotropy decay in H2O.2
Figure 2.
Spectrally integrated PP and PA signal. (a) Transients for parallel (ppol) and crossed (xpol) polarization of pump and probe pulses for three coupling regimes. (b) PA calculated from (a). Blue: Experimental PA (Ref. 2).
The PA of the uncoupled system shows a fast initial decay due to strong orientational fluctuations of the transition dipole moments, accounting for ≈1∕2 of the signal decay. The remaining contributions are due to librational and slow reorientation dynamics.12 With increasing coupling strength, the anisotropy decay speeds up and the long-lived components vanish. This effect can clearly be assigned to intermolecular ET which is expected to be at least partially coherent. The anisotropy decay can now be fitted with a single exponential decay, resulting in decay times of 75 fs for κ=12 cm−1 and 40 fs for κ=20 cm−1. An additional effect is observed for the initial value of the PA, which is decreasing with increasing coupling strength. Both decay time and initial value of the PA are in excellent agreement with experiment for κ=12 cm−1. Here, we note that the pump probe cross correlation can distort the experimental anisotropy which, however, was found to not affect the observed dynamics within experimental error.
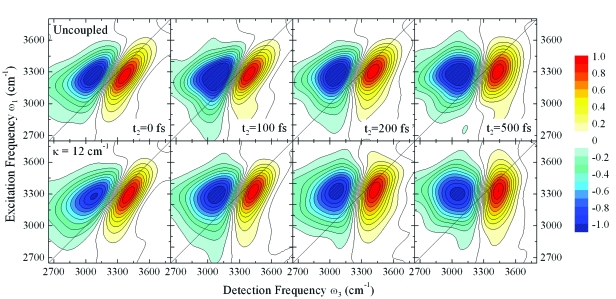
In Fig. 3, we show the 2DIR-PE spectra for two models, κ=0 cm−1 (uncoupled) and κ=12 cm−1 for population times t2=0, 100, 200, 500 fs. Two peaks corresponding to the fundamental 0→1 transition and excited state 1→2 absorption are observed. The 1→2 transition is redshifted due to anharmonicities of the vibrational frequencies. A strong interference between these two peaks leading to partial or complete cancellation of signal in the overlap region, distorts the individual peak shapes, amplitudes, and positions.
Figure 3.
2DIR-PE spectra of the OH stretch vibration in H2O for population times t2=0,100,200,500 fs. Top panel: Uncoupled system, bottom panel: κ=12 cm−1. Each spectrum is normalized to its maximum.
In both models at t2=0 fs, the fundamental peak is stretched along the diagonal, indicating some initial inhomogeneity. As a function of t2, the peaks become more vertical on similar time scales for both models, somewhat faster for the κ=12 cm−1 system. Due to the interference between the two peaks and the distribution of dynamics across the spectrum, no single time scale for loss of correlations can be extracted from the 2DIR-PE spectra. On closer inspection of the κ=12 cm−1 spectra, a bending of the fundamental peak and the nodal lines between the two peaks is observed. This is an indication of faster fluctuations and loss of inhomogeneity on the red side of the spectrum. From our results for κ=12 cm−1, any initial correlations on the red side of the spectrum have decayed by 100 fs, whereas correlations on the blue side persist beyond 200 fs. This finding is in very close agreement with the recent experimental results on the 2DIR-PE spectrum of H2O.2
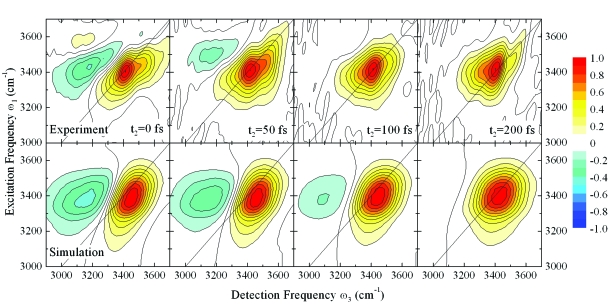
In Fig. 4, we compare our simulation results for κ=12 cm−1 with the experimental spectra.2 For better comparison, we corrected the simulated spectra for the experimental excitation pulse spectrum. Population relaxation effects are now included by applying an ad hoc relaxation factor using a population life time T1=200 fs.1 Additionally, we included a persisting ground state bleach to match the experimental conditions. For multimode nonadiabatic simulations, a strict separation of the different contributions to the nonlinear signal is not possible. We approximated the persisting ground state bleach by only considering diagonal contributions to the respective nonlinear response function S1 and S4.12 With these assumptions, our simulations are in close agreement with the experiment for peaks shapes, amplitudes, and dynamics.
Figure 4.
2DIR-PE spectra of the OH stretch vibration in H2O for population times t2=0,50,100,200 fs. Top panel: Experimental data (Ref. 2). Bottom panel: κ=12 cm−1 corrected for experimental pulse spectrum and ad hoc population relaxation (see text). Each spectrum is normalized to its maximum.
Our new simulation procedure provides the first nonlinear response treatment of highly disordered vibrational excitons, which explicitly allows multiple state crossing between vibrational energy surfaces. With no separation of time scales between the transfer and fluctuation processes, the nonadiabatic nature of the transfer process is thus fully accounted for. The explicit treatment of these processes, as well as intra- and intermolecular vibrational anharmonicities, and the fluctuations and anharmonicities of the transition dipole moments facilitated in the NISE approach provides the highest level description of the nonlinear vibrational response of water to date.
Many interesting features of the PP and 2DIR-PE response are caused by the mixing between states in the 2D anharmonic OH stretch potential. This is markedly different from the two decoupled one-dimensional stretch potentials in HOD. The fundamental transitions show an increased sensitivity to bath fluctuations, mainly caused by resonantly enhanced fluctuations in orientation and amplitudes of the transition dipole moments, i.e., non-Condon effects.5 The fluctuations in the local basis result in changes in the character and symmetry of the modes, and can, therefore, to some degree be interpreted as intramolecular ET. Mixing of the overtone states further leads to strong intramolecular anharmonicities. In particular, the harmonic selection rules are lifted, opening up additional pathways contributing to the nonlinear response. These effects manifest the increased sensitivity of the OH stretch vibrations to fluctuations in the hydrogen bond network in water, compared to isotopically substituted systems such as HOD∕D2O or HOD∕H2O.3, 4 In our results, this is evident in the fast loss of correlation in the 2DIR-PE spectra (Fig. 3) and the fast decay of PA transients (Fig. 2) even for the uncoupled system.
The main result of the intermolecular coupling is observed in the PA (see Fig. 2). The experiment is reproduced for κ=12 cm−1 which can be considered to be surprisingly small. Previous theoretical8, 9 work had to invoke much larger couplings to reproduce the extremely fast time scales of the PA decay in H2O. Here, we show that the large number of acceptor modes, as well as anharmonicities and fluctuations in the system open up many intermolecular transfer pathways, which lead to a full decay of the PA on time scales observed in the experiment1, 2, 16 even for these small average couplings. The effect of the ET on the 2DIR-PE spectra is found to be rather small (see Fig. 3). Only for large t2 (>200 fs), somewhat faster dynamics in the coupled system become apparent. We conclude that most of the fast dynamics in the 2DIR-PE spectrum of H2O are indeed caused by the increased sensitivity of the OH stretch potential to anharmonic bath coupling. Consequently, the OH stretch vibration is shown to be an excellent probe of the hydrogen bond network in H2O.
In summary, we presented simulations of the 2DIR-PE and PP response of the OH stretch vibration in liquid water. Our new technique using numerical integration of the Schrödinger equation allowed explicit treatment of intermolecular vibrational couplings and nonadiabatic effects for the first time. The 2D OH stretch potential was shown to be extremely sensitive to bath fluctuations resulting in very fast spectral diffusion dynamics. We also found surprisingly small intermolecular couplings to be fully sufficient to reproduce experimentally observed energy transfer times. The simulation results are in close agreement with our most recent experimental work.2
ACKNOWLEDGMENTS
This research was supported by the Canadian Institute of Photonics Innovation, Photonics Research Ontario, and the Natural Sciences and Engineering Research Council of Canada. S.M. gratefully acknowledges the support of NIH (Grant No. GM59230) and NSF (Grant No. CHE-0745892). A.P. thanks Thomas la Cour Jansen for helpful discussions.
References
- Cowan M. L., Bruner B. D., Huse N., Dwyer J. R., Chugh B., Nibbering E. T. J., Elsaesser T., and Miller R. J. D., Nature (London) 10.1038/nature03383 434, 199 (2005). [DOI] [PubMed] [Google Scholar]
- Kraemer D., Cowan M. L., Paarmann A., Huse N., Nibbering E. T. J., Elsaesser T., and Dwayne Miller R. J., Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0705792105 105, 437 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecko C. J., Eaves J. D., Loparo J. J., Tokmakoff A., and Geissler P. L., Science 10.1126/science.1087251 301, 1698 (2003). [DOI] [PubMed] [Google Scholar]
- Asbury J. B., Steinel T., Kwak K., Lawrence C. P., Skinner J. L., and Fayer M. D. J., J. Chem. Phys. 10.1063/1.1818107 121, 12431 (2004). [DOI] [PubMed] [Google Scholar]
- Schmidt J. R., Corcelli S. A., and Skinner J. L., J. Chem. Phys. 10.1063/1.1961472 123, 044513 (2005). [DOI] [PubMed] [Google Scholar]
- Hayashi T., Jansen T. l. C., Zhuang W., and Mukamel S., J. Phys. Chem. A 10.1021/jp046685x 109, 64 (2005). [DOI] [PubMed] [Google Scholar]
- DeVane R., Space B., Perry A., Neipert C., and Ridley C., and Keyes T., J. Chem. Phys. 10.1063/1.1776119 121, 3688 (2004). [DOI] [PubMed] [Google Scholar]
- Torii H., J. Phys. Chem. A 110, 8422 (2006). [Google Scholar]
- Poulsen J. A., Nyman G., and Nordholm S., J. Phys. Chem. A 10.1021/jp0225469 107, 8420 (2003). [DOI] [Google Scholar]
- Jansen T. l. C. and Knoester J., J. Phys. Chem. B 10.1021/jp064795t 110, 22910 (2006). [DOI] [PubMed] [Google Scholar]
- Berendsen H. J. C., van der Spoel D., and van Drunen R., Comput. Phys. Commun. 10.1016/0010-4655(95)00042-E 91, 43 (1995). [DOI] [Google Scholar]
- Paarmann A., Hayashi T., Mukamel S., and Miller R. J. D. (unpublished).
- Ashihara S., Huse N., Espagne A., Nibbering E. T. J., and Elsaesser T., J. Phys. Chem. A 10.1021/jp0676538 111, 743 (2007). [DOI] [PubMed] [Google Scholar]
- Mukamel S., Principles of Nonlinear Optical Spectroscopy (Oxford University Press, New York, 1995). [Google Scholar]
- Kosloff R., J. Phys. Chem. 10.1021/j100319a003 92, 2087 (1988). [DOI] [Google Scholar]
- Woutersen S. and Bakker H., Nature (London) 10.1038/990058 402, 507 (1999). [DOI] [Google Scholar]